Abstract
A role for dorsomedial hypothalamus (DMH) cholecystokinin (CCK) signaling in feeding control has been proposed. Administration of CCK into the DMH reduces food intake and OLETF rats lacking CCK1 receptors (CCK1R) become hyperphagic and obese. We hypothesized that site specific replenishment of CCK1R in the DMH of OLETF rats would attenuate aspects of their feeding deficits. Recombinant vectors of adeno-associated viral (AAV)-mediated expression of CCK1R (AAVCCK1R) were bilaterally delivered into the DMH of OLETF. OLETF rats with AAVCCK1R injections demonstrated a 65% replenishment of Cck1r mRNA expression in the DMH relative to lean LETO control rats. Although this level of replenishment did not significantly affect overall food intake or body weight through 14 weeks following viral injections, meal patterns were partially normalized in OLETF rats receiving AAVCCK1R with a significant decrease in dark cycle meal size and a small but significant decrease in daily food intake in the meal analysis chambers. Importantly, the elevation in blood glucose level of OLETF rats was attenuated by the AAVCCK1R injections (p=0.03), suggesting a role for DMH CCK signaling in glucose homeostasis. In support of this role, administration of CCK into the DMH of intact rats enhanced glucose tolerance, as this occurred through activation of CCK1R but not CCK2R signaling. In conclusion, partial replenishment of CCK1R in the DMH of OLETF rats, although insufficient for altering overall food intake and body weight, normalizes meal pattern changes and reduces blood glucose levels. Our study also shows a novel role of DMH CCK signaling in glucose homeostasis.
Keywords: Cholecystokinin 1 receptors, food intake, obesity, insulin sensitivity, adeno-associated virus, gene therapy
1. Introduction
The Otsuka Long-Evans Tokushima Fatty (OLETF) rat is an outbred animal model of obesity and type II diabetes that has been characterized as having hyperphagia, mild obesity, late onset of hyperglycemia, hyperinsulinemia and hyperleptinemia (Moran and Bi, 2006; Moran, 2008). Genetic analysis has revealed a congenital defect in the expression of the cholecystokinin (CCK) 1 receptor (CCK1R) gene in this animal model (Takiguchi et al., 1997). We have suggested that the disruption of CCK1R in OLETF rats results in deficits in the control of food intake and body weight. In support of this view, we have documented that OLETF rats do not have a feeding inhibitory response to peripheral exogenous CCK (Moran et al., 1998). Consistent with deficient CCK satiety signal, the hyperphagia of OLETF rats is characterized by a significant increase in meal size with a decrease in meal frequency that is not sufficient to compensate for the meal size increase (Moran et al., 1998).
OLETF rats also have dysregulated neuropeptide Y (NPY) signaling in the dorsomedial hypothalamus (DMH). Pair-feeding OLETF rats to the amount of food consumed by lean Long-Evans Tokushima Otsuka (LETO) rats normalizes body weight, fat mass and plasma insulin and leptin levels and results in increased Npy expression in the DMH (Bi et al., 2001). This Npy overexpression is also found in juvenile preobese OLETF rats (Bi et al., 2001; Schroeder et al., 2009). Thus, we have suggested that the elevation of DMH Npy expression contributes to the hyperphagia and obesity of OLETF rats. Moreover, CCK1R and NPY are normally co-localized in a population of neurons within the DMH and injection of CCK into the DMH reduces DMH Npy expression (Chen et al., 2008) and inhibits food intake in intact rats (Blevins et al., 2000; Chen et al., 2008). Together, these data suggest that DMH CCK signaling through CCK1R plays important roles in the control of food intake and body weight by regulating DMH Npy expression.
In addition, previous evidence has shown a role of peripheral CCK in modulating insulin release and sensitivity. Peripheral CCK administration stimulates insulin release (Rossetti et al., 1987), whereas administration of the non-selective CCK receptor antagonist proglumide impairs insulin sensitivity (Peitl and Szilvassy, 2007). However, little is known about central actions of CCK on glucose homeostasis. OLETF rats lacking functional CCK1R have impaired insulin sensitivity secondary to their obesity (Peitl et al., 2010). Chronic treatment with rosiglitazone, an insulin sensitizing drug widely used for the treatment of type II diabetes, results in increased hypothalamic Cck1r, but not Cck2r mRNA expression in LETO rats (Peitl et al., 2010). These results imply an important role for hypothalamic CCK1R in the regulation of insulin sensitivity.
In this study, we examined the role of DMH CCK signaling in the controls of meal patterns, body weight and overall glucose homeostasis and insulin sensitivity. We replenished CCK1R in the DMH of OLETF rats using adeno-associated virus (AAV)-mediated gene delivery. We found that although there were no clear effects on total food intake and body weight, OLETF rats with partially DMH CCK1R replenishment somewhat normalized meal patterns and improved insulin sensitivity. To further explore the regulation of insulin sensitivity by DMH CCK signaling, we examined the effect of DMH CCK administration on glucose tolerance in intact Long Evans rats. We found that DMH CCK administration improved glucose clearance following gastric glucose load. Thus, these data suggest that the DMH serves as a site of action for CCK’s effects on overall insulin sensitivity.
2. Materials and methods
2.1. Experiment animals
Male OLETF rats and age-matched male lean LETO rats were obtained as a generous gift from the Tokushima Research Institute, Otsuka Pharmaceuticals (Tokushima, Japan). Male Long-Evans rats weighing 225~275g were purchased from Charles River laboratories. Rats were individually housed in hanging wire mesh cages and maintained on a 12 h light/dark cycle (lights on at 4:00 A.M.) in a temperature-controlled colony room (22–23°C) with ad libitum access to tap water and standard laboratory rodent chow (Prolab), except where noted. All procedures were approved by the Institutional Animal Care and Use Committee at The Johns Hopkins University.
2.2. AAV-mediated CCK1R expression vector
As previously described (Yang et al., 2009), the AAV Helper-Free System (Stratagene) was used for viral vector preparation. Briefly, the full length cDNA encoding rat CCK1R was first cloned into a pAAV-IRES-hrGFP vector to make a recombinant CCK1R expression plasmid (pAAVCCK1R), so that the plasmid contains the Cck1r gene driven by the cytomegalovirus (CMV) promoter and the marker gene of humanized Renilla green fluorescent protein (hrGFP) translationally controlled by the internal ribosome entry site (IRES), flanked by AAV2 inverted terminal repeats (ITRs) (see Fig. 1A). For viral packaging, three plasmids of pAAV-CCK1R, pHelper (carrying adenovirus-derived genes), and pAAV-RC (carrying AAV-2 replication and capsid genes) were cotransfected into the AAV-293 cells according to the manufacturer’s protocol (Stratagene). The vector pAAV-hrGFP was used for packaging the control viral vector, AAVGFP. Three days after transfection, cells were harvested, and the recombinant viral vector AAVCCK1R (or AAVGFP) was purified using the AAV purification kit (Virapur) and concentrated using Centricon YM-100 (Millipore) according to the manufacturers’ protocols. Viral titers were determined using quantitative polymerase chain reaction (qPCR) and ~1.75 ×107 particles/site for AAVGFP and 0.65 ×107 particles/site for AAVCCK1R were used for each viral injection and the volume of each injection is 0.5μl.
Figure 1.
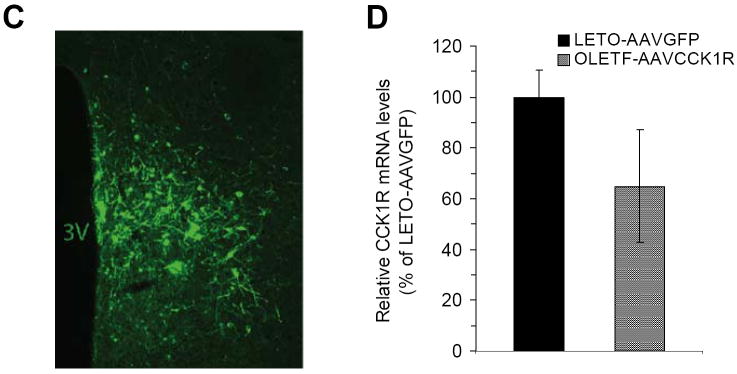
Adeno-associated virus (AAV)-mediated expression of cholecystokinin (CCK) 1 receptor (CCK1R). (A) Construct of AAV-mediated CCK1R expression vector (AAVCCK1R) containing the CCK1R gene driven by the CMV promoter and the expression marker of hrGFP translationally controlled by the IRES; (B) Both CCK1R and hrGFP produced in the AAVCCK1R-infected cells three days after viral infection as determined by Western blot; (C) Representative micrograph shows hrGFP expression in the dorsomedial hypothalamus (DMH) two weeks after DMH viral injection as examined under fluorescence microscopy; (D) Quantitative Cck1r mRNA expression in the DMH of Otsuka Long-Evans Tokushima Fatty (OLETF) receiving AAVCCK1R (OLETF-AAVCCK1R) 14 weeks post-viral injection relative to lean control Long-Evans Tokushima Otsuka (LETO) rats receiving control vector AAVGFP (LETO-AAVGFP) as determined by in situ hybridization determination. Values are means ± SEM. n= 6~8 animals in each group.
Viral product CCK1R was further evaluated by Western blot. Briefly, AAV-293 cells were cultured in DMEM growth medium containing 4.5g/L glucose, 110 mg/L sodium pyruvate, and 4mM L-glutamine (Invitrogen) supplemented with 10% (v/v) heat-inactivated fetal bovine serum. When cells reached 70% confluence, AAVGFP or AAVCCK1R were added into culture medium respectively. Three days after infection, cells were harvested and lysed for Western blot analysis. Twenty micrograms of cell lysate protein were separated using 4-12% SDS-PAGE, and transferred to a nitrocellulose membrane. The membrane was then incubated with rabbit anti-CCK1R antibody (1:2000 dilutions, Accurate Chemical & Scientific Corp.) or rabbit anti-hrGFP antibody (1:2000 dilution; Stratagene), followed by horseradish peroxidase-labeled anti-rabbit antibody (1:5000 dilutions; GE Healthcare) and detected by ECL Western blotting detection reagents and analysis system (GE Healthcare).
2.3. DMH viral injection
At 9 weeks of age, 15 male OLETF rats weighing 284–350g and 7 male age-matched LETO rats weighing 253–279g received viral injections. Rats were assigned into three groups: one group of 8 OLETF rats received bilateral DMH injections of AAVCCK1R (OLETF-AAVCCK1R), a second group of body weight matched (n=7) OLETF rats received bilateral DMH injections of AAVGFP (OLETF-AAVGFP), and the 7 LETO rats received bilateral DMH injections of AAVGFP (LETO-AAVGFP) as a normal control group. DMH viral injections were made in a stereotaxic apparatus (KOPF) under anesthesia: i.p. ketamine (100 mg/kg) mixed with xylazine (20 mg/kg), with the following coordinates: 3.1 mm caudal to bregma, 0.4 mm lateral to midline, and 8.6 mm ventral to skull surface. After viral injections, rats were maintained with ad libitum access to standard rodent chow and tap water. Body weights were monitored daily and food intake was recorded weekly. At 22 weeks of age, rats were killed between 9:00 A.M. and 11:00 A.M after food had been removed for 2 hours. The left epidydimal and inguinal subcutaneous white adipose tissue (WAT) and interscapular brown adipose tissue (BAT) were harvested and weighed. Trunk blood was taken for evaluation of blood glucose, plasma leptin and insulin levels. Blood glucose levels were determined with a FreeStyle glucometer (TheraSense), plasma leptin concentrations were determined by a rat leptin radioimmunoassay kit (Millipore) and plasma insulin concentrations were determined by a rat insulin radioimmunoassay kit (Millipore). Brains were removed and rapidly frozen in pre-cooled N-methylbutane on dry ice for subsequent studies of hypothalamic gene expression.
2.4. Analysis of meal patterns
Six weeks after virus injections (at 15 weeks of age), rats were transferred to individual test cages connected to a computerized Dietmax Analyzer (AccuScan Instruments, Inc.) for meal pattern analysis. Rats had ad libitum access to powdered chow containing the same ingredients as pelleted chow (Prolab) and a water bottle. The weight of food in the tray was recorded continuously for 22hrs/day in a compute (the first 2 hrs were used for food preparation and the program was initiated at dark onset). Rats were adapted to the testing apparatus for 3~5 days. After adaptation, data for 22 h food intake were collected and meal patterns were analyzed using the software DietMax Meals (AccuScan Instruments, Inc.). A meal was defined as the acquisition of at least 0.2g powdered chow preceded and followed by at least 20 min of no feeding. Meal size was defined as the amount of food consumed during a meal.
2.5. Locomotor activity
Seven weeks after virus injections (at 16 weeks of age), rats were transferred to open-field test chambers (Digiscan; Accuscan Instruments, Columbus, OH) for measurements of spontaneous locomotor activity. The system consists of a 40cm ×40cm×30cm Plexiglas chambers surrounded by sensor panels that emit infrared beams across the open field. Each beam interruption is detected, and patterns of beam interruptions are analyzed to yield measures of locomotor activity. Activity counts were tallied and stored on file in a computer. Rats had ad libitum access to food and water in the chambers. Locomotor activity, as the number of beam interruptions in the horizontal plane, was recorded in 11 bins of 2-hr duration, for a total of 22hrs. The first 2 hrs considered as a habituation period.
2.6. Quantitative in situ hybridization
Radioactive in situ hybridization was conducted as previously described (Bi et al., 2003). Briefly, 35S labeled antisense riboprobe of Cck1r was transcribed from rat Cck1r precursor cDNA by using the in vitro transcription system (Promega). Sections ranging from 3.0 to 3.5 mm posterior to bregma (Paxinos and Watson, 2005) were selected, anatomically matched among animals, and used for determination of Cck1r mRNA levels in the DMH. Sections were treated with acetic anhydride and incubated in our standard hybridization buffer at 58°C overnight. After hybridization, sections were washed three times with 2×SSC, treated with 20 μg/ml RNase A (Sigma-Aldrich) at 37°C for 30 min, and then rinsed in 2×SSC twice at 55°C, and washed twice in 0.1×SSC at 60°C for 30min. Slides were dehydrated in gradient ethanol, air-dried, and exposed with BMR-2 film (Eastman Kodak) for 1–3 days.
Quantitative analysis of the in situ hybridization data was done with NIH Scion image software as previously described (Bi et al., 2003). Levels of Cck1r mRNA were determined by a mean of the product of hybridization area and density (background density was subtracted) in each animal. Data from each group were normalized to the LETO control group as 100%.
2.7. DMH injection of CCK and oral glucose tolerance test (OGTT)
At the age of 10 weeks, 19 male Long-Evans rats were implanted with indwelling unilateral DMH cannulae. As previously described (Chen et al., 2008), a 26-gauge stainless-steel guide cannula (Plastics One, Wallingford, CT, USA) was implanted into the DMH under ketamine and xylazine anesthesia. A 33-gauge stainless steel obturator was inserted into the cannula to maintain patency. Animals were allowed to recover for 7 days. Before DMH administration, animals were given pseudo-injections everyday, i.e. the obturator was removed from the cannula and then reinserted so that rats were adapted to the procedure of injections. After habituation, DMH injection was made by using a 33-gauge stainless steel injector extended 1.0 mm past the tip of guide cannula (Plastics One, Inc., Wallingford, CT, USA). The opposite end of the injector tip was connected to PE-20 tubing and a 2μl Hamilton syringe loaded on a minipump (KD Scientific). 0.3μl of solvent vehicle, 500 pmol of CCK (sulfated CCK-8, Bachem, Torrance, CA, USA) or desulfated CCK-8 (dCCK) in 0.3μl of solvent was administered within 30 seconds, and the injector was left in place for 30 seconds before removal. The dose of CCK has been demonstrated to inhibit food intake at this site (Blevins et al., 2000b; Bi et al., 2004).
To examine the effects of DMH CCK on OGTT, the rats were assigned into two groups according to their body weights – one group of the rats receiving DMH CCK injection (n=10) and the other group of the rats receiving DMH vehicle injection (n=9). Following the 16 hour overnight fast, DMH injection of CCK or vehicle was made as described above and immediately followed by oral gavage of glucose at a dose of 2 g/kg. Tail blood was sampled before and 15, 30, 45, 60, and 120 min after giving glucose, and levels of blood glucose and plasma insulin were measured as described above. After 7-day recovery, rats were given a second DMH injection with vehicle solvent or 500 pmol of dCCK in counterbalanced order for an OGTT as conducted above.
2.8. Statistical analysis
Data for body weight gain and food intake of OLETF and LETO rats, and blood glucose and plasma insulin levels of Long-Evans rats during OGTT were analyzed using repeated measures ANOVA. Data for Cck1r mRNA expression in the DMH, meal patterns, fat pads weight, plasma leptin and insulin levels, blood glucose levels and locomotor activity were analyzed using one-way ANOVA. All ANOVA were followed by Fisher LSD comparisons. Data for the area under the curve (AUC) of blood glucose and plasma insulin responses to oral glucose gavage were analyzed by Student’s t-test.
3. Results
3.1. AAV-mediated expression of CCK1R in the DMH
We constructed a recombinant vector of AAV-mediated expression of CCK1R that contains the Cck1r gene and the hrGFP marker gene (Fig. 1A). We verified that the vector AAVCCK1R successfully produced CCK1R product as determined by Western blot analysis. Both CCK1R and hrGFP were detected in the AAV293 cells that were infected by AAVCCK1R three days after infection whereas hrGFP was only detected in the AAVGFP-infected cells (Fig. 1B). After verification, the vector AAVCCK1R was injected into the DMH of OLETF rats bilaterally at 9 weeks of age for determination of effects of CCK1R replacement in the DMH on food intake and body weight. Fig. 1C presents the accuracy of DMH viral injection and the expression of hrGFP within DMH neurons two weeks after viral injection. Fourteen weeks post-injection (at 22 weeks of age), we examined Cck1r mRNA expression in the DMH using in situ hybridization determination. Cck1r mRNA expression in the DMH of OLETF-AAVCCK1R rats was ~65% of that found in LETO-AAVGFP rats (Fig. 1D).
3.2. Effects of CCK1R replacement on body weight gain, food intake and meal patterns
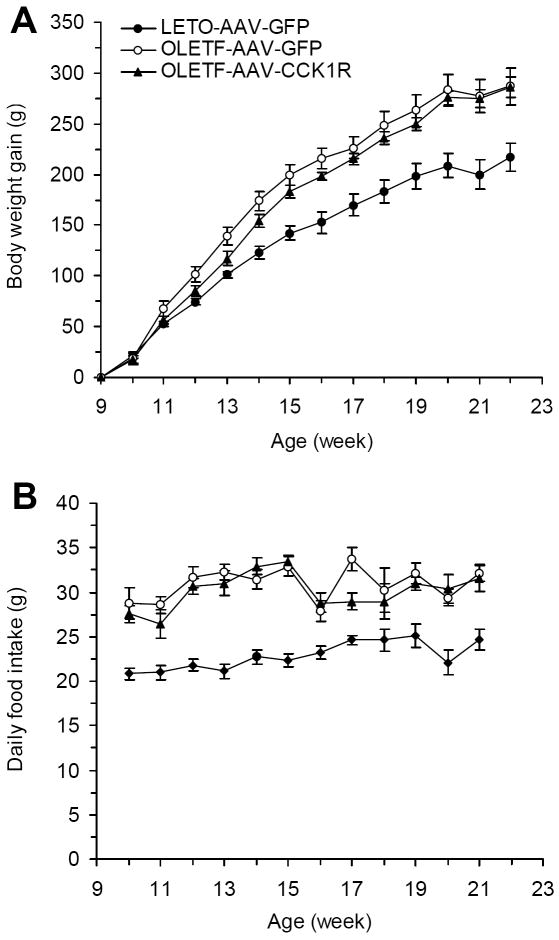
Although there was a trend toward decrease in body weight gain in OLETF-AAVCCK1R rats compared with that of OLETF-AAVGFP rats, this only reached significance 4 weeks post-viral injection or at 13 week of age (117.8±6.4 vs. 139.3±8.6, p=0.03) (Fig. 2A). There were no differences in overall daily food intake between OLETF-AAVCCK1R and OLETF-AAVGFP rats (Fig. 2B).
Figure 2.
Effects of DMH CCK1R replacement on body weight and food intake in OLETF rats. (A) Body weight in the three groups of LETO-AAVGFP, OLETF-AAVGFP (OLETF rats receiving DMH AAVGFP injection) and OLETF-AAVCCK1R rats beginning at the time of DMH injection; (B) Food intake in the three groups of the rats from the time of DMH injection. Values are means ± SEM. n= 7 animals in each group.
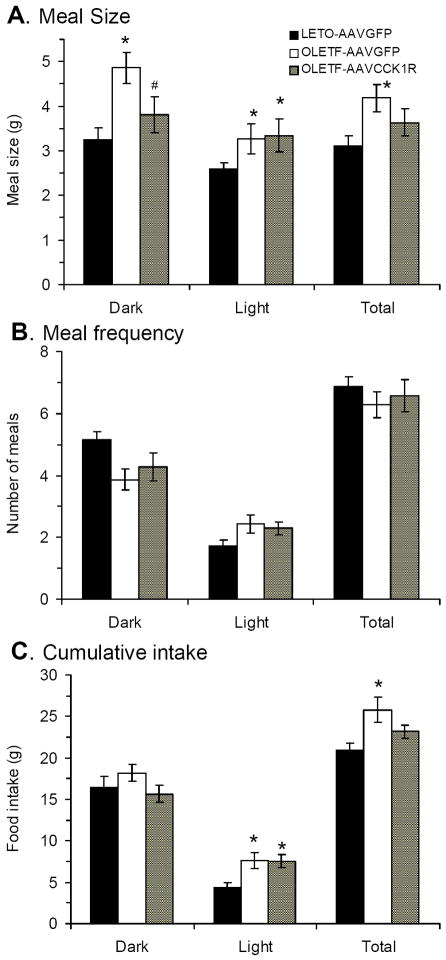
Previous studies have shown that OLETF rats have increased meal size, which has been proposed to contribute to their positive overall energy balance (Moran et al., 1998). We analyzed meal patterns six weeks after viral injection in the present study. On average for each meal, OLETF-AAVGFP rats consumed significantly more food than LETO-AAVGFP rats during dark, light period and total daily (p<0.01) (Fig. 3A). CCK1R placement in the DMH of OLETF rats produced a significant effect on meal size. Meal size during the dark period was significantly smaller in OLETF-AAVCCK1R rats compared with OLETF-AAVGFP rats (p<0.03) and not significantly different from that of LETO-AAVGFP rats (Fig. 3A). However, OLETF-AAVCCK1R rats maintained a relative increase in average meal size during light period, which was significantly different from that of LETO-AAVGFP rats (p<0.01) (Fig. 3A). Overall for the total daily, the average meal size of OLETF-AAVCCK1R was not different from that of LETO-AAVGFP rats (p=0.16).
Figure 3.
Effects of DMH CCK1R replacement on meal patterns in OLETF rats. (A) Meal size in the three groups of the rats; (B) Meal frequency in the three groups of the rats; (C) Total food intake in the three groups of the rats. Values are means ± SEM. n= 7~8 animals in each group. *p<0.05 compared with LETO-AAVGFP rats; # p<0.05 compared with OLETF-AAVGFP rats.
Meal number did not differ among any of the groups (Fig.3B). Overall, OLETF-AAVGFP rats consumed significantly more food in total 22 hrs compared with LETO-AAVGFP rats (p<0.01) and the difference was more obvious during the light period (Fig. 3C). The CCK1R replacement in OLETF rats resulted in total daily food intake that was not significantly different from that of LETO-AAVGFP rats (Fig. 3C). The light period food intake of OLETF-AAVCCK1R rats was significantly different from that of LETO-AAVGFP rats (p=0.02) (Fig. 3C), consistent with the increase in light meal size (Fig. 3A) and unchanged meal number (Fig. 3B) in these rats.
3.3. CCK1R replacement does not influence locomotor activity of OLETF rats
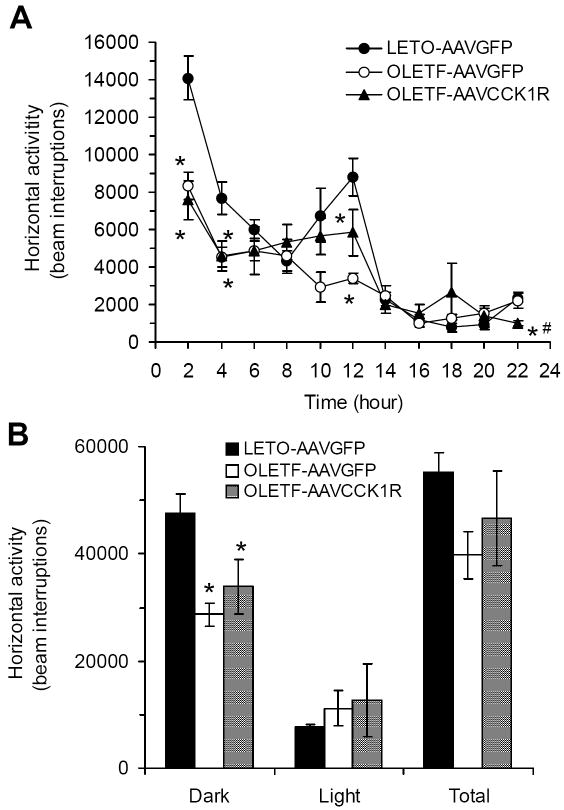
Decreased locomotor activity has also been proposed to contribute to the obesity of OLETF rats (Sei et al., 1999). We examined whether replacement of CCK1R in the DMH can attenuate the reduced locomotor activity of OLETF rats. Consistent with previous studies, OLETF-AAVGFP rats were less active than LETO-AAVGFP rats during the dark period (p=0.01) (Fig. 4A and 4B). The CCK1R replacement did not affect this alteration. Locomotor activity during the dark period remained significantly lower in OLETF-AAVCCK1R rats as compared to LETO rats (p<0.05) (Fig. 4B). During the light period, locomotor activity did not differ among the three groups (Fig. 4A and 4B). Overall, there were no statistically significant differences in total 22hr activity between any of the groups (Fig. 4B).
Figure 4.
Effects of DMH CCK1R replacement on locomotor activity in OLETF rats. (A) Locomotor activity over the 22 hrs period; (B) Locomotor activity during the dark, light and total 22hrs. Values are means ± SEM. n= 6~7 animals in each group. *p<0.05 compared with LETO-AAVGFP rats and #p<0.05 compared with OLETF-AAVGFP rats.
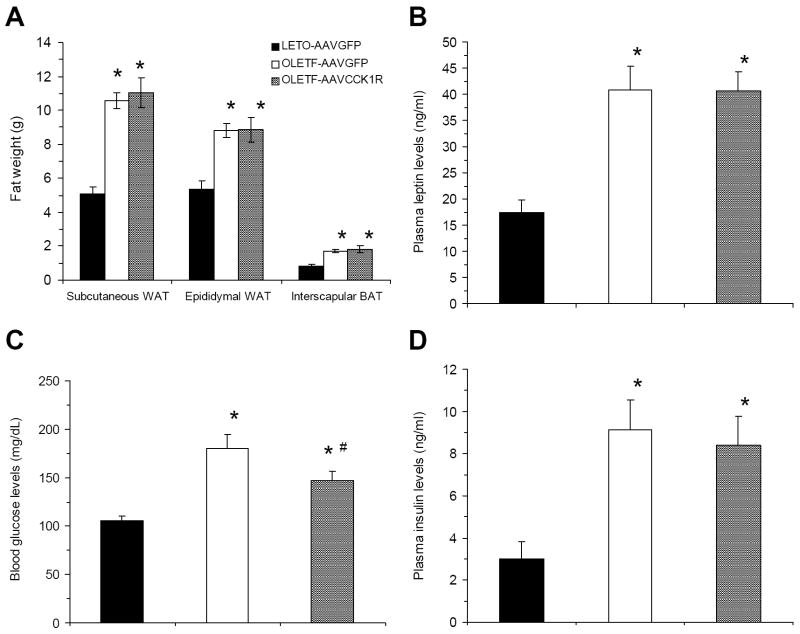
3.4. Fat pad, plasma leptin, blood glucose & plasma insulin levels: CCK1R contributes to the decrease of blood glucose level without affecting plasma insulin level
At 22 weeks of age, all three fat pads (subcutaneous WAT, epididymal WAT and interscapular BAT) from OLETF-AAVGFP and OLETF-AAVCCK1R rats weighed more than the corresponding pads in LETO rats, but none of them differed between OLETF-AAVGFP and OLETF-AAVCCK1R rats (Fig. 5A). OLETF-AAVGFP and OLETF-AAVCCK1R rats both had significantly higher (~2.35 fold) plasma leptin level compared to that of the LETO control group, but DMH CCK1R replacement did not affect plasma leptin levels (Fig. 5B).
Figure 5.
Effects of DMH CCK1R replacement on fat pads, plasma leptin, blood glucose and insulin levels in OLETF rats. (A) Fat composition among the three groups of the rats; (B) Plasma leptin levels in the three groups of the rats; (C) Blood glucose levels in the three groups of the rats; (D) Plasma insulin levels in the three groups of the rats. Values are means ± SEM. n= 5~7 animals in each group. *p<0.05 compared with LETO-AAVGFP rats and #p<0.05 compared with OLETF-AAVGFP rats.
In contrast to plasma leptin levels, although the blood glucose levels of OLETF-AAVCCK1R rats were still significantly higher than that of LETO-AAVGFP rats (p<0.01), they were significantly reduced compared to those of OLETF-AAVGFP rats at sacrifice (p<0.05) (Fig 5C). Plasma insulin levels of OLETF-AAVCCK1R rats were similar to those of OLETF-AAVGFP rats – both had increased insulin levels compared to LETO rats (Fig. 5D), suggesting that the CCK1R replacement increased relative insulin sensitivity by improving the clearing efficiency of high blood glucose.
3.5. CCK1R is involved in the increase of insulin sensitivity during OGTT
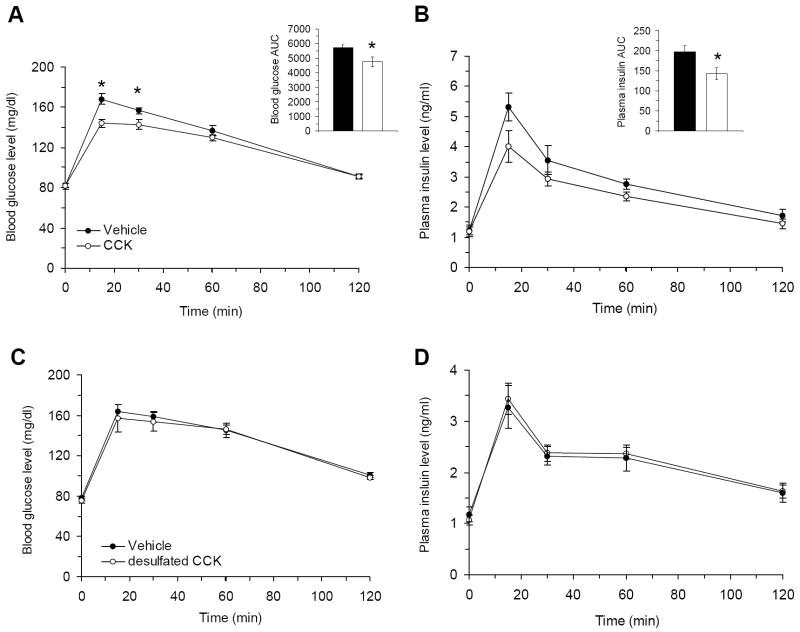
To determine roles for CCK and CCK1R in the regulation of glucose homeostasis and insulin sensitivity, we examined the effect of DMH CCK injection on OGTT in lean Long-Evans rats. Following CCK administration into the DMH, glucose was cleared more quickly especially at 15min and 30min after glucose administration (p=0.00001 and 0.009, respectively) and the AUC of blood glucose response was significantly lower in CCK injected group (p=0.033) (Fig. 6A). At each time point of OGTT, insulin levels did not differ between the two groups, but the AUC of insulin response was significantly lower in the CCK injected group (Fig. 6B), suggesting greater insulin sensitivity in the CCK treated group. This further provides support for our suggestion that CCK1R replacement in the DMH of OLETF rats increases insulin sensitivity.
Figure 6.
Effects of DMH CCK injection on blood glucose and plasma insulin levels in intact rats. (A-B) Following gastric glucose load, blood glucose levels (A) and plasma insulin levels (B) were decreased in response to administration of CCK into the DMH compared to vehicle group. (C-D) Administration of desulfated CCK (dCCK) into the DMH did not alter blood glucose levels (C) and plasma insulin levels (D) following gastric glucose load compared to vehicle group. Vehicle: vehicle treated group; CCK: CCK-treated group; dCCK: dCCK-treated group. Values are means ± SEM. n= 9~10 animals per group. *p<0.05 compared with vehicle.
Although the CCK1R is a primary receptor mediating the effects of CCK within the DMH, the ventromedial hypothalamus (VMH), a region ventral to the DMH, contains a large population of CCK2R-containing neurons (Chen et al., 2008). To exclude the possibility of leakage of exogenous DMH CCK into the VMH or VMH CCK2R mediating effects of DMH CCK injection, we administered dCCK, a CCK2R specific agonist, into the DMH of Long-Evans rats and did an OGTT following dCCK injection. The OGTT revealed that there were no any differences in the dynamic changes of blood glucose and plasma insulin levels (Fig. 6C and 6D). These data confirm that the CCK1R is the main receptor responsible for the increased insulin sensitivity during OGTT experiment after administration of CCK into the DMH.
Discussion
In the present study, we attempted to restore DMH CCK1R signaling in OLETF rats lacking functional CCK1R via AAV-mediated gene delivery. We demonstrated that the vector AAVCCK1R was capable of producing CCK1R in the viral-infected cells. Following DMH viral injection, CCK1R expression was partially restored in the DMH of OLETF rats compared to that of control LETO-AAVGFP rats. Although the DMH CCK1R replacement did not alleviate the hyperphagia and obesity of OLETF rats, this replacement did attenuate alterations in meal patterns that have been evident in OLETF rats (Moran et al., 1998). In addition, we found that the CCK1R replacement attenuated hyperglycemia of OLETF rats. Administration of CCK, but not dCCK, into the DMH of intact rats enhanced glucose clearance following gastric glucose load and increased insulin sensitivity. Overall, these results not only provide new evidence indicating the role of DMH CCK1R in the control of meal size, but also identify a novel function of DMH CCK1R in glucose homeostasis.
The hyperphagia of OLETF rats is characterized primarily by a significant increase in meal size (Moran et al., 1998). Deficits in meal size control are found in OLETF rats as young as 2 days of age as indicated by increased intake in independent ingestion tests (Blumberg et al., 2006). Pair-fed and pre-obese OLETF rats have greatly elevated Npy mRNA expression in the DMH (Bi et al., 2001; Schroeder et al., 2009). The absence of CCK-1R within the DMH may well be the cause of elevated Npy mRNA expression in the DMH. In intact rats, NPY and CCK-1R co-localize to neurons within the DMH and local CCK administration reduces food intake and decreases DMH Npy mRNA expression (Bi et al., 2004; Chen et al., 2008). It has been proposed that the absence of DMH CCK-1R and the increase in DMH Npy expression significantly contribute to the OLETF rats’ inability to compensate for their meal size control deficit leading to their overall hyperphagia (Bi et al., 2004; Moran and Bi, 2006; Bi, 2007). In support of this view, knockdown of NPY expression in the DMH of OLETF rats normalizes food intake and meal patterns (Yang et al., 2009). In the present study, we found that after DMH CCK1R delivery, the meal patterns of OLETF rats were altered, with dark period meal size significantly decreased while that of light period was relatively increased. Why the decrease in meal size was restricted to the dark cycle is not clear. However, this is consistent with what we observed in response to DMH NPY downregulation and may reflect a recognized role for the DMH in the controls of a number of diurnal variables. In spite of these changes in meal patterns, total daily food intake was not changed and there was only a trend toward a reduction in body weight after CCK1R delivery. While overall food intake in the home cage was unchanged, we did find a small but significant decrease in daily food intake in the meal pattern testing chambers. The reason for this difference is unclear. It may be a function of the accuracy of measurement in the meal pattern cages but, given that body weight was not significantly altered by the AAV-CCK1R injections, it is unlikely that such a difference was sustained in the post-injection period. Energy expenditure as assessed by locomotor activity was not significantly affected by the DMH CCK1R delivery.
The extent of viral-mediated CCK1R expression in the DMH was partial and this may explain the relatively small effects on food intake and body weight. In situ hybridization for CCK1R demonstrated mRNA expression in the OLETF-AAVCCK1R rats that was ~65% of that found in the LETO rats. This is probably due to insufficient viral delivery (~1 ×107 particles/site). It should also be stated that this was a site directed injected so it is likely that a significant part of this expression occurred in neurons that would not normally express the receptor so the functional relevance of some of the replacement was likely negligible. We have no way of quantifying the portion of NPY expressing neurons that were infected but it is likely that such infection is only a portion of the total. However, even with such a partial replacement, there was significant rescue of the meal pattern alteration, and, as discussed below, significant improvement in glucose homeostasis.
While a role for the DMH in the hyperphagia and obesity of OLETF rats has been supported from previous work (Bi., et al., 2001; Yang et al., 2009), both oral and postoral deficits in nutrient processing have also been demonstrated. Thus, OLETF rats have enhanced preference for sucrose but the ability of intestinal sucrose to reduce intake is impaired (De Jonge et al., 2005; Hajnal et al., 2005). Similarly, the ability of gastric and duodenal lipids to inhibit intake is impaired in OLETF rats (Schwartz et al., 1999). Presumably these aspects of the OLETF phenotype would not be affected by altering DMH CCK1 receptor signaling. These remaining deficits may contribute to the relatively minor effects of DMH CCK1 receptor replacement,
Surprisingly, our results did show that the partial CCK1R replenishment was sufficient to affect glucose homeostasis in the OLETF rats. Blood glucose levels of OLETF rats were significantly reduced after DMH CCK1R delivery while the plasma insulin levels were similar to those of the control OLETF-AAVGFP rats, suggesting increased insulin sensitivity after replacement of CCK1R. These data pinpointed a novel role of DMH CCK signaling in overall glucose homeostasis. In support of this view, the subsequent OGTT revealed that DMH CCK injection significantly increased the efficiency of blood glucose clearance levels after gastric glucose load. The AUC for both glucose and insulin were significantly reduced. Moreover, we examined the receptor-specific effect of DMH CCK. The data showed that administration of the CCK2R agonist dCCK into the DMH was ineffective at altering glucose tolerance, leading to the conclusion that DMH CCK modulates glucose homeostasis through CCK1R signaling.
Hypothalamic neurons have long been shown to be glucose sensitive and/or glucose responsive (Anand et al., 1962; Burdakov et al., 2005; Oomura et al., 1969) and they share similar signaling pathways as those found within the pancreas (Trapp and Ashcroft, 1997; Dunn-Meynell et al., 1998). Roles for hypothalamic signaling in overall glucose homeostasis have also been documented in multiple studies (Lam et al., 2005; Pocai et al., 2005). VMH neurons are among the main focus in the regulation of glucose metabolism (Levin et al., 1999). While peripheral CCK administration has been shown to stimulate insulin release (Rossetti et al., 1987) and, in that way, reduce blood glucose levels, whether central CCK also produces such effects is unclear. The present study demonstrated a novel role for brain CCK signaling in glucose homeostasis and insulin sensitivity and showed that rather than occurring within the VMH, its action appears to be specific to the DMH. The underlying mechanism awaits further investigation.
In summary, using AAV-medaited CCK1R delivery, we have partially replaced CCK1R in the DMH of OLETF rats. This partial replacement attenuated alterations in meal patterns of OLETF rats. Importantly, the CCK1R replacement ameliorated hyperglycemia and enhanced insulin sensitivity in OLETF rats. Consistent with the effects of DMH CCK signaling on glucose homeostasis, exogenous DMH CCK improved glucose clearance and increased insulin sensitivity through CCK1R signaling in intact rats. Together, our results demonstrate roles of DMH CCK signaling in the controls of meal patterns and glucose homeostasis.
Highlights.
Replacement of CCK1R in the DMH of OLETF rats normalizes meal pattern changes.
The DMH CCK1R replacement attenuates hyperglycemia of OLETF rats.
Actions of DMH CCK signaling in glucose homeostasis through CCK1R.
Acknowledgments
This work was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases DK057609. The OLETF and LETO rats were a generous gift from Otsuka Pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anand BK, Chhina GS, Singh B. Effect of glucose on the activity of hypothalamic “feeding centers”. Science. 1962;138:597–598. doi: 10.1126/science.138.3540.597. [DOI] [PubMed] [Google Scholar]
- Bi S. Role of dorsomedial hypothalamic neuropeptide Y in energy homeostasis. Peptides. 2007;28:352–356. doi: 10.1016/j.peptides.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Bi S, Ladenheim EE, Schwartz GJ, Moran TH. A role for NPY overexpression in the dorsomedial hypothalamus in hyperphagia and obesity of OLETF rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R254–260. doi: 10.1152/ajpregu.2001.281.1.R254. [DOI] [PubMed] [Google Scholar]
- Bi S, Robinson BM, Moran TH. Acute food deprivation and chronic food restriction differentially affect hypothalamic NPY mRNA expression. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1030–1036. doi: 10.1152/ajpregu.00734.2002. [DOI] [PubMed] [Google Scholar]
- Bi S, Scott KA, Kopin AS, Moran TH. Differential roles for cholecystokinin a receptors in energy balance in rats and mice. Endocrinology. 2004;145:3873–3880. doi: 10.1210/en.2004-0284. [DOI] [PubMed] [Google Scholar]
- Blevins JE, Stanley BG, Reidelberger RD. Brain regions where cholecystokinin suppresses feeding in rats. Brain Res. 2000;860:1–10. doi: 10.1016/s0006-8993(99)02477-4. [DOI] [PubMed] [Google Scholar]
- Blumberg S, Haba D, Schroeder M, Smith GP, Weller A. Independent ingestion and microstructure of feeding patterns in infant rats lacking CCK-1 receptors. Am J Physiol Regul Integr Comp Physiol. 2006;290:R208–18. doi: 10.1152/ajpregu.00379.2005. [DOI] [PubMed] [Google Scholar]
- Burdakov D, Luckman SM, Verkhratsky A. Glucose-sensing neurons of the hypothalamus. Philos Trans R Soc Lond B Biol Sci. 2005;360:2227–2235. doi: 10.1098/rstb.2005.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Scott KA, Zhao Z, Moran TH, Bi S. Characterization of the feeding inhibition and neural activation produced by dorsomedial hypothalamic cholecystokinin administration. Neuroscience. 2008;152:178–188. doi: 10.1016/j.neuroscience.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jonghe BC, Hajnal A, Covasa M. Increased oral and decreased intestinal sensitivity to sucrose in obese, prediabetic CCK-A receptor-deficient OLETF rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R292–300. doi: 10.1152/ajpregu.00481.2004. [DOI] [PubMed] [Google Scholar]
- Dunn-Meynell AA, Rawson NE, Levin BE. Distribution and phenotype of neurons containing the ATPsensitive K1 channel in rat brain. Brain Res. 1998;814:41–54. doi: 10.1016/s0006-8993(98)00956-1. [DOI] [PubMed] [Google Scholar]
- Hajnal A, Covasa M, Bello NT. Altered taste sensitivity in obese, prediabetic OLETF rats lacking CCK-1 receptors. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1675–86. doi: 10.1152/ajpregu.00412.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam TK, Gutierrez-Juarez R, Pocai A, Rossetti L. Regulation of blood glucose by hypothalamic pyruvate metabolism. Science. 2005;309:943–947. doi: 10.1126/science.1112085. [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA, Routh VH. Brain glucose sensing and body energy homeostasis: role in obesity and diabetes. Am J Physiol. 1999;276:R1223–1231. doi: 10.1152/ajpregu.1999.276.5.R1223. [DOI] [PubMed] [Google Scholar]
- Moran TH. Unraveling the obesity of OLETF rats. Physiol Behav. 2008;94:71–78. doi: 10.1016/j.physbeh.2007.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran TH, Bi S. Hyperphagia and obesity in OLETF rats lacking CCK-1 receptors. Philos Trans R Soc Lond B Biol Sci. 2006;361:1211–1218. doi: 10.1098/rstb.2006.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran TH, Katz LF, Plata-Salaman CR, Schwartz GJ. Disordered food intake and obesity in rats lacking cholecystokinin A receptors. Am J Physiol. 1998;274:R618–625. doi: 10.1152/ajpregu.1998.274.3.R618. [DOI] [PubMed] [Google Scholar]
- Oomura Y, Ono T, Ooyama H, Wayner MJ. Glucose and osmosensitive neurones of the rat hypothalamus. Nature. 1969;222:282–4. doi: 10.1038/222282a0. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5. Elsevier Academic Press; San Diego, California: 2005. [Google Scholar]
- Peitl B, Dobronte R, Drimba L, Sari R, Varga A, Nemeth J, Pazmany T, Szilvassy Z. Involvement of cholecystokinin in baseline and post-prandial whole body insulin sensitivity in rats. Eur J Pharmacol. 2010;644:251–256. doi: 10.1016/j.ejphar.2010.06.062. [DOI] [PubMed] [Google Scholar]
- Peitl B, Szilvassy Z. The inhibitory effect of proglumide on meal-induced insulin sensitization in rats. Metabolism. 2007;56:863–864. doi: 10.1016/j.metabol.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, Aguilar-Bryan L, Rossetti L. Hypothalamic K(ATP) channels control hepatic glucose production. Nature. 2005;434:1026–1031. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- Rossetti L, Shulman GI, Zawalich WS. Physiological role of cholecystokinin in meal-induced insulin secretion in conscious rats. Studies with L 364718, a specific inhibitor of CCK-receptor binding. Diabetes. 1987;36:1212–1215. doi: 10.2337/diab.36.10.1212. [DOI] [PubMed] [Google Scholar]
- Schroeder M, Zagoory-Sharon O, Shbiro L, Marco A, Hyun J, Moran TH, Bi S, Weller A. Development of obesity in the Otsuka Long-Evans Tokushima Fatty rat. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1749–1760. doi: 10.1152/ajpregu.00461.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz GJ, Whitney A, Skoglund C, Castonguay TW, Moran TH. Decreased responsiveness to dietary fat in Otsuka Long-Evans Tokushima fatty rats lacking CCK-A receptors. Am J Physiol. 1999;277(4 Pt 2):R1144–51. doi: 10.1152/ajpregu.1999.277.4.R1144. [DOI] [PubMed] [Google Scholar]
- Sei M, Sei H, Shima K. Spontaneous activity, sleep, and body temperature in rats lacking the CCK-A receptor. Physiol Behav. 1999;68(1-2):25–9. doi: 10.1016/s0031-9384(99)00146-8. [DOI] [PubMed] [Google Scholar]
- Takiguchi S, Takata Y, Funakoshi A, Miyasaka K, Kataoka K, Fujimura Y, Goto T, Kono A. Disrupted cholecystokinin type-A receptor (CCKAR) gene in OLETF rats. Gene. 1997;197:169–175. doi: 10.1016/s0378-1119(97)00259-x. [DOI] [PubMed] [Google Scholar]
- Trapp S, Ashcroft FM. A metabolic sensor in action: news from the ATP-sensitive K1- channel. News Physiol Sci. 1997;12:255–263. [Google Scholar]
- Yang L, Scott KA, Hyun J, Tamashiro KL, Tray N, Moran TH, Bi S. Role of dorsomedial hypothalamic neuropeptide Y in modulating food intake and energy balance. J Neurosci. 2009;29:179–190. doi: 10.1523/JNEUROSCI.4379-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]