Abstract
Developmental exposure of rats to the pesticide chlorpyrifos (CPF) causes persistent neurobehavioral impairment. In a parallel series of studies with zebrafish, we have also found persisting behavioral dysfunction after developmental CPF exposure. We have developed a battery of measures of zebrafish behavior, which are reliable and sensitive to toxicant-induced damage. This study determined the critical duration of developmental CPF exposure for causing persisting neurobehavioral effects. Tests of sensorimotor response (tap startle response and habituation), stress response (novel tank diving test) and learning (3-chamber tank spatial discrimination) were conducted with adult zebrafish after early developmental CPF exposure. The CPF exposure level was 100 ng/ml with durations of 0-1, 0-2, 0-3, 0-4 and 0-5 days after fertilization. Developmental CPF exposure had persisting behavioral effects in zebrafish tested as adults. In the tactile startle test, CPF exposed fish showed decreased habituation to startle and a trend toward increased overall startle response. In the novel tank exploration test, exposed fish showed decreased escape diving response and increased swimming activity. In the 3-chamber learning test, the 0-5 day CPF exposure group had a significantly lower learning rate. There was evidence for persisting declines in brain dopamine and norepinepherine levels after developmental CPF exposure. In all of the measures the clearest persistent effects were seen in fish exposed for the full duration of five days after fertilization. In a follow-up experiment there were some indications for persisting behavioral effects after exposure during only the later phase of this developmental window. This study demonstrated the selective long-term neurobehavioral alterations caused by exposure to CPF in zebrafish. The zebrafish model can facilitate the determination of the molecular mechanisms underlying long-term neurobehavioral impairment after developmental toxicant exposure.
Keywords: Chlorpyrifos, Neurotoxicity, Zebrafish, Learning
1. Introduction
A variety of studies have shown that exposure to the organophosphate (OP) pesticide chlorpyrifos (CPF) during development can cause persisting neurobehavioral dysfunction, even with low doses that do not elicit acute cholinergic toxicity. In mammals, developmental CPF exposure clearly alters neurochemical and neurobehavioral function. Epidemiological studies have shown significant association between developmental CPF exposure and persisting cognitive impairment. Experimental studies with rodents have demonstrated the causative nature of developmental CPF exposure being the source of cognitive impairment. Zebrafish with a clear chorion and elegant molecular reporter systems offer an important model for determining the mechanisms of neurotoxicity throughout embryonic development and into early larval growth. The zebrafish model has also been shown to be sensitive to the persistent cognitive impairment from developmental exposure to low doses of CPF. The current study examined in the zebrafish model the critical duration of CPF which was necessary to produce persisting cognitive impairment as well as other persisting neurobehavioral dysfunction.
Epidemiologic evidence supports the harmful neurodevelopmental effects of prenatal exposure to organophosphate pesticides, in particular CPF. Whyatt et al. (2004) investigated the effects of CPF exposure on development of children in New York and found a significant adverse impact of high prenatal exposure to CPF, which caused deficiencies in birth weight and birth length. The same group (Rauh et al., 2006) showed that there was an increased risk of developmental delay in behavior symptoms of attentional impairment. It was also concluded that ADHD-like problems at three years of age were due to CPF exposure in the residential area (Rauh et al., 2006). Supporting these findings, Bouchard and colleagues (2010) also found a significant relationship between pesticide exposure during development and higher ADHD rates in children. Continuing to study CPF will bring us a step closer toward finding appropriate treatment and understanding its effects on the central nervous system.
Experimental rodent studies have shown that developmental exposure to CPF and other OP pesticides causes persistent neurobehavioral impairment. Exposure to low doses of CPF during development that do not cause persistent systemic toxicity do cause neurotoxic damage in rats (Campbell et al., 1997; Roy et al., 2004; Slotkin et al., 2006a; Slotkin and Seidler, 2007; Song et al., 1997; Whitney et al., 1995), including effects on DNA synthesis (Dam et al., 1998), gene transcription (Crumpton et al., 2000), cell differentiation (Roy et al., 1998), and synaptogenesis (Dam et al., 1999). Developmental exposure to CPF in rats alters neuronal development of serotonin (5HT), acetylcholine and to a lesser degree dopamine (DA) systems (Dam et al., 1999; Slotkin, 1999; Slotkin et al., 2001; Slotkin et al., 2006a; Slotkin et al., 2002; Slotkin et al., 2006b).
Developmental CPF also causes long-term alterations in behaviors related to these neural effects (Aldridge et al., 2005a; Aldridge et al., 2005b; Icenogle et al., 2004; Levin et al., 2002; Levin et al., 2001). Studies with rats revealed that early neonatal exposure to CPF induces long-term changes in cognitive performance in the 16-arm radial maze that selectively impaired males (Levin et al., 2001). When challenged with the muscarinic antagonist scopolamine there was less of an amnestic effect in CPF-exposed rats, which indicated that muscarinic acetylcholine receptors were not as critically important for memory function in rats with a history of CPF exposure during development (Levin et al., 2001).
In addition to cognition CPF effects changes in emotional function. Increased risk taking behavior was seen in male rats treated in early postnatal periods with a low dose of CPF as indicated by significantly increased time spent in the opened arms of the elevated plus maze (Aldridge et al., 2005a). Persisting alterations in emotional function was also seen after developmental exposure to other OPs, diazinon and parathion (Roegge et al., 2008; Timofeeva et al., 2008).
Complementary models like zebrafish (Danio rerio) are increasingly becoming models of choice in studying the molecular bases of neurodevelopment. The clear chorion of zebrafish allows continual visualization of the entire development process. The rapid development of zebrafish facilitates the throughput of studies. These are just some of the qualities that make the zebrafish an excellent model for studying neurodevelopment. The extensive library of genetic mutants that are widely available in zebrafish offers the promise of determining the molecular mechanisms of neurobehavioral effects. Using the zebrafish for neurodevelopment studies has led to the identification of a variety of genes that affect various aspects of neurodevelopment and function. Zebrafish can be quite useful in looking at neurotoxic effects in overall neurodevelopment as we move toward future mammalian investigations. With a single acetylcholinesterase gene that has been show to cause lethality as a homozygous null mutant (Behra et al., 2002) and with mutant studies implying that its loss results in hyperstimulation of muscle cells, zebrafish allows one to isolate specific functional roles for acetylcholinesterase inhibition. In our current research the zebrafish model has emerged as a promising and sensitive tool enabling reliable detection of neurodevelopmental injury in response to OP pesticide exposure (Levin et al., 2003; Levin et al., 2004; Linney et al., 2004).
Past studies show that the zebrafish model has helped to discover molecular processes that underlie neurodevelopmental impairments (Levin et al., 2003). The integration of zebrafish has helped to develop more profound methods for assessing cognitive systems such as those previously mentioned including the startle response task, the novel tank swim test, and the three-chamber spatial discrimination task. In startle response and swim activity measures, methods are applied to show motor responses to stimuli and habituation over time. In spatial discrimination, tasks are more directed toward differentiating response latency from choice accuracy as seen in the three-chambered spatial learning test. Levin et al. (2003) showed that low and high doses of CPF were administered at 10 and 100 ng/ml to zebrafish embryos for the first 5 days post-fertilization. It was concluded that both doses had significant persistent effects on spatial discrimination as well as response latency. It was also found that the higher dose but not necessarily the lower dose significantly accelerated mortality rates, but the long-term mortality rate was just subthreshold for being higher than controls. The low-dose effect was observed mainly in early testing, while impairment caused by the higher dose became more pronounced as testing continued. The higher dose of CPF caused more persistent impairment in zebrafish. The two doses had opposite effects on response latency, the lower dose significantly increased discrimination and the higher dose significantly decreased response latency (Levin et al., 2003).
The study with zebrafish and CPF showed response latency over 18 weeks of testing (Levin et al., 2003). Developmental exposure to either 10 or 100 ng/ml of CPF caused significant spatial discrimination impairments in zebrafish when they were adults. Both of these effects diminished with continued testing within the study. CPF exposure during early development was said to cause clear behavioral impairments. These behavior impairments were seen throughout adulthood in zebrafish. The molecular mechanisms by which early developmental CPF exposure produces these behavioral impairments were expressed throughout adulthood and are now being studied in the current neurobehavioral studies using the zebrafish model.
The zebrafish model offers the opportunity for critical neurodevelopment analysis at a low cost in a timely and sufficient manner. Zebrafish develop outside of the mother and their clear chorion enhances its ability to be easily studied during neurodevelopment. It is known that zebrafish show the ability to learn and discriminate color difference (Arthur and Levin, 2001). Developmental CPF has shown impacts in learning in zebrafish (Levin et al., 2003). Previously, Eddins et al. assayed the effects of developmental CPF exposure on startle response, startle habituation, and recovery from habituation (Eddins et al., 2010). They found that a quick automated test of startle can detect persisting neurobehavioral impairments caused by developmental exposure to CPF. They also concluded that the finding will better determine time windows and help in screening for persisting neurobehavioral defects from a variety of toxicants to validate the model.
Our laboratory has developed a battery of methods to evaluate sensory function, motor behavior, and learning to characterize behavioral response to environmental toxicants in adult zebrafish. We have worked to increase the efficiency of throughput by developing an automated assessment of startle response and habituation as well as novel tank exploration. Startle response has previously provided a quick measure of sensory and motor integration (Eddins et al., 2010). This task detects rapid habituation curves with repeated trials and has given substantial information concerning behavior. The novel tank diving procedure, a newly developed assay, takes into consideration the advantage of the natural tendency for zebrafish to dive to the bottom of the novel tank and gradually spend more time in the entire tank over a given time period. When introduced to the tank the zebrafish dives to the bottom naturally as a measure of avoidance and predatory escape. The tank provides a novel environment where over a period of minutes their position of swimming increases to higher portions of the test tank. Spatial learning was assessed in three-chambered task (Levin et al., 2006). This method acts to differentiate response latency from choice accuracy in zebrafish. With this task it has been shown that adult zebrafish after exposure to CPF (10 or 100 ng/ml on days 0–5 post-fertilization), exhibited persisting defects (Levin et al., 2003). Therefore, zebrafish have shown to be valuable in determining abnormal developmental neurobehavioral function after CPF exposure.
The current study was conducted to determine whether the persisting neurobehavioral impairments could be seen in zebrafish after early developmental exposure at different developmental times. The 100 ng/ml dose was chosen because from previous research assessing the dose-response effect of chlorpyrifos we found this dose given over the first five days post-fertilization caused moderate inhibition of acetylcholinesterase and persistent behavioral and neurochemical effects while being at the cusp for excessive long-term mortality. The current study focused on how long the duration of CPF exposure during early development needed to be to cause these neurobehavioral effects. Thus the variable investigated was duration of exposure to a fixed dose of CPF. The critical durations of exposure at which we start to see these effects are unknown. We hypothesize the later embryonic exposure may be more sensitive because of rapid brain development. These effects impair cognitive function, as well as a cascade of adverse sensory, motor response, and learning events. We performed a battery of behavior assessments and pinpoint the duration of exposure where we start to see these cascading events of persisting and neurological deficits. These deficits will help determine the critical time windows of zebrafish over given durations of 0, 1, 1-2, 1-3, and 1-4 and 1-5, as well as 4-5, 3-5, 2-5, 1-5 and 0-5 days post fertilization, and to provide critical assessment on the involvement of monoaminergic neurotransmitters dopamine, serotonin, and norepinephrine after exposure to CPF.
2. Methods
2.1. Subjects
AB strain of zebrafish (Danio rerio) embryos was kept at approximately 28.5 °C on a 12 hour dark and a 12 hour light cycle. Embryos were fed only brine shrimp for the first two months post-fertilization. At month three adult zebrafish were fed brine shrimp and flake fish food (Tetra Fish Food, Blacksburg, VA, USA) once a day. Female and male zebrafish were housed together with like CPF-exposed groups together in 3-liter tanks. Tanks were housed on a 6-tier dual filtration and constantly aerated rack unit. The tank water consists of de-ionized H2O, sodium bicarbonate, and sea salts (Instant Ocean, 1.2 g/20 l of water). Adult behavioral testing of pre-exposed embryos took place at the third month post fertilization. Experiments were performed during the light phase between 8:00 a.m. and 5:00 p.m.
2.2. Chlorpyrifos Administration
The purity of the CPF was assayed by the manufacturer at 99.6% pure (Accustandard, Inc., New Haven, CT, USA). The dosing solutions were replaced each day. CPF was administered the first five days at either 0 ng/ml or 100 ng/ml with 0.1% dimethyl sulfoxide (DMSO), which served as the vehicle in which to administer CPF in liquid form. We have previously found 100 ng/ml CPF dose to cause significant inhibition of AChE in zebrafish embryos and larvae (Aschner et al., 2010). Embryos were exposed to CPF in 30% Danieau solution in the first experiment for durations from fertilization until 1 through 5 days after fertilization in one-day increments (0-1, 0-2, 0-3, 0-4, and 0-5 days post-fertilization exposure) and in the second experiment the effects of differential duration of CPF exposure during the later developmental stages were assessed (4-5, 3-5, 2-5, 1-5 and 0-5 days post-fertilization exposure). In both experiments controls were exposed to the vehicle. At three months, the fish were transported to the Levin laboratory for neurobehavioral testing.
2.3. Tap Startle and Habituation Test
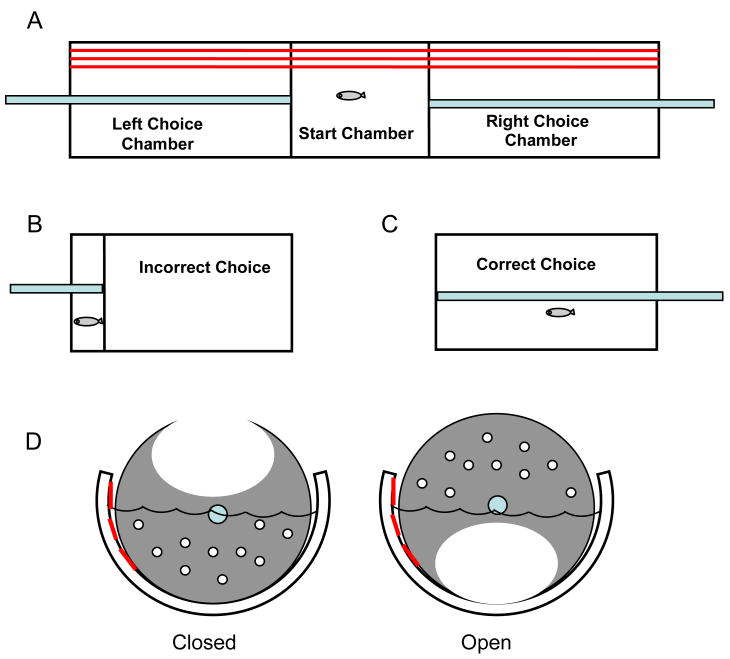
The testing apparatus (Fig. 1) consisted of flat white 20.4 cm × 38.1 cm surface with white 12.7 cm × 15.2 cm frontal and rear blocking barriers attached. On the flat surface attached eight 5.1 cm × 7.6 cm clear cylindrical arenas which were made of Plexiglas and arranged in a 2 × 4 setup. Each arena contained 30 ml of tank water. The apparatus was positioned between two 50.8 cm white opaque barriers, which faced each other and projected a bare white screen. Mechanical solenoids were positioned underneath each cylindrical arena. A Samsung 8mm camcorder was located approximately 71 cm above the apparatus. The solenoids were used to administer impulse taps to the bottom of the cylindrical arena with instruction from the computer. The zebrafish ranged from 12 to 32 per group. Eight zebrafish were taken from the holding tanks and each one was placed into a cylindrical arena on the tap apparatus. Zebrafish were allowed to acclimate in the arena for 3 min. prior to testing. After the adjustment period, the tap test was started and solenoids tapped the testing arenas at 1 min. intervals every 30 s. for 10 consecutive trials. The zebrafish response in distance traveled was recorded 5 s. before the tap and 5 s. after the tap. The total testing time for the initial phase was 10 min. The image of the fish movement in response to the repeated taps was indexed by the EthoVision™ program (Noldus, Wageningen, Netherlands).
Figure 1.
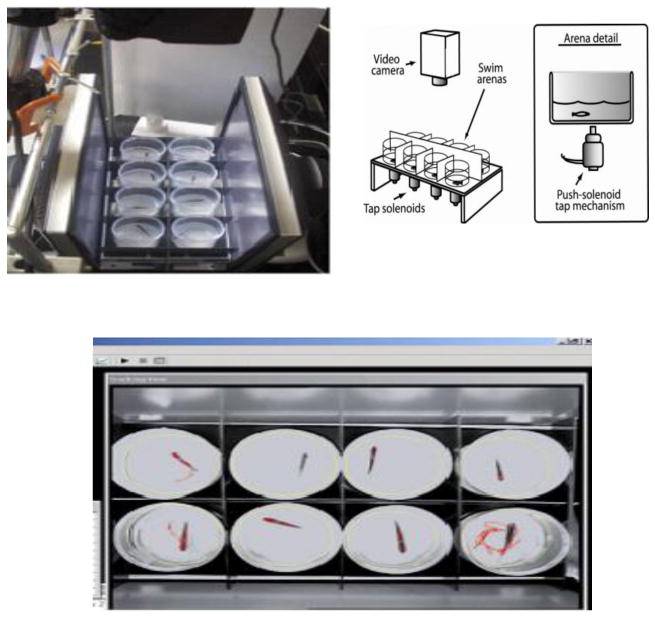
Zebrafish tactile startle apparatus: Overview of the eight test wells; Cross-section schematic of the tap solenoids, test wells, screen and digital video camera; Computer screen shot of the Noldus program recording the movement of the fish (Eddins et al., 2009).
2.4. Novel Tank Dive Test
Two zebrafish were placed separately in two 1.5-liter plastic tanks filled with 1350 ml of tank water. The tanks were trapezoidal in shape and extended 22.9 cm along the bottom and 27.9 cm in length cross the top. The diagonal side of the tank was approximately 15.9 cm in length and the opposing side was 15.2 cm long. (Fig. 2) (Levin et al., 2007). The two tanks had the short side next to each other with a barrier between them and a solid white 60 cm × 122 cm plastic board that was positioned behind the tanks. The video image was divided into lower, middle, and top swimming areas using the EthoVision™ program (Noldus Information and Technology, Wageningen, Netherlands) (Figure 4). There was a one-minute acclimation period prior to testing. After the acclimation period ended, the trial series was started with a five-minute trial duration for testing. The video signal was transmitted through an 8 mm Samsung Camcorder that was positioned approximately 88 cm away from the horizontal facing tanks. The video was then transmitted to the computer for analysis.
Figure 2.

Zebrafish novel tank diving test apparatus: A) Digital video camera and two 1.5 liter test tanks; B) Computer screen shot of the Noldus program recording the movement of the fish over the five minutes of the test.
Figure 4.
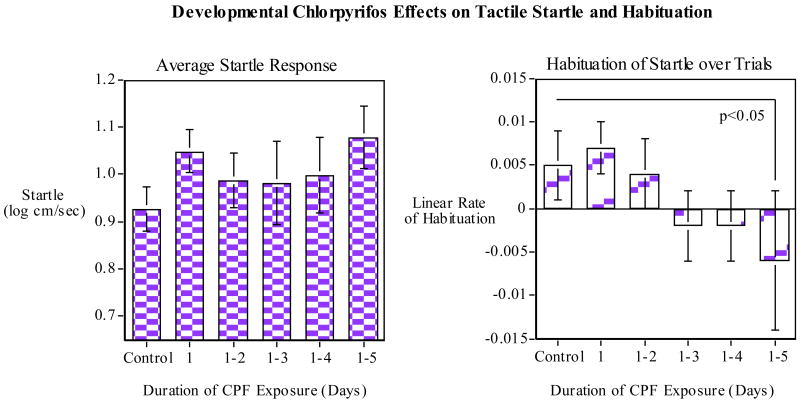
Developmental CPF (earlier vs. complete exposure) effects on tactile startle response and habituation: Left Panel) cm traveled in the five seconds after each tap, (log of cm/second); Right Panel) linear rate of habituation (slope) over the ten trials of the session (mean±sem).
2.5. Three-Chamber Learning Test
There were 19 to 32 zebrafish ran per group in this task. The testing apparatus was a half cylindrical pipe unit that was divided into three chambers, the start chamber, right-choice chamber and the left-choice chamber (Figure 3). Plexiglas rods extended through both sides of the apparatus. On the inner part of each of the two rods were rotatable plastic partitions that allowed swim entrances for the zebrafish. A choice of one side or the other was recorded when the fish swam past the threshold of the open partition from the central start chamber into one of the lateral choice chambers. The partitions were circular and stood vertical on both side within the test chamber. The partitions were attached to the 12.7 cm long rod rails. The two rails could be moved completely back toward the wall or completely forward toward the central chamber. Three thick black lines outlined the back of the chamber as a visual cue to provide an axis of orientation for right–left discrimination.
Figure 3.
Zebrafish 3-chamber learning apparatus: A) Overview of the 3-chamber tank with the central start chamber and two lateral choice chambers (the lines along the length of one wall the apparatus provide an axis of orientation for the fish); B) when the incorrect side is chosen the partition is moved to one cm of the end wall to sequester the fish in a small space; C) when the correct side is chosen the fish is left to swim in the full space of the choice chamber; D) cross-section of the apparatus showing a partition rotated in the closed and open configuration below the water surface (Eddins et al., 2009).
One zebrafish was placed in the start chamber after acclimation. After 60 s., the partitions to each of the choice chambers were simultaneously opened. If the fish swam into the correct choice chamber, the partition was closed and the fish was left alone in that chamber unpunished for 1 min. However, if the fish swim into the incorrect choice chamber, the partition was closed and then moved back to the wall 1 cm to reduce swimming space as punishment for 10 sec. If the fish did not make a choice within 20 s., a plastic beaker was dropped just above the start chamber to induce a selection. Choice accuracy and response latency were recorded. Prior to training, 5 preliminary trials were done to establish a preferred side. After preliminary testing, 10 trials were performed to test zebrafish learning against their preferred side.
2.6. Neurochemistry
Neurochemical levels of DA, 5HT, and norepinepherine were assayed after the adult behavioral task to observe long term effects of CPF exposure over 0–5 day post-fertilization period. 18 to 34 zebrafish were run per group. The zebrafish were sacrificed and brain tissues were removed for analysis. Zebrafish were anesthetized by submersion in 4 °C aquarium water and decapitated. The brains were rapidly removed and homogenized (25 × volume per weight) in a solution. The excised samples underwent column purification and were diluted in a mobile 1:10 solution. 20 μl were analyzed for neurotransmitter levels.
The methods are the same as we have used previously to determine monoamine levels in the zebrafish brain (Eddins et al., 2010; Eddins et al., 2008). The HPLC system used consists of an isocratic pump (Model LC1120, GBC Separations, Hubbardston, MA), a Rheodyne injector (Model 7725i) with a 20 μl PEEK loop, and an INTRO Amperometric detector (Antec Leyden, Zoeterwoude, Netherlands). The electrochemical flow cell (model VT 03, Antec Leyden) had a 3 mm carbon working electrode with a 25 μm spacer. An Ag/AgCl served as reference electrode. The cell potential was set at 700 mV. The signal was filtered with a low pass in-line noise killer, (LINK) set at a 14 seconds peak width and a cut off frequency of 0.086 Hz. The signal was integrated and aligned using the EZ Chrom elite chromatography software by Scientific Software Inc. The injector flow cell and analytical column were placed in the Faraday shielded compartment of the detector where the temperature was maintained at 30 °C. The stationary phase was a reverse phase BDS Hypersil C18 column often used to minimize peak tailing. The column expanded 100 mm × 2.1 mm, with 5 μm particle size and 120 Å pore size (Keystone Scientific). The mobile solution for experimental phase consist of 50 mM H3PO4, 50 mM citric acid, 100 mg/l 1-octanesulfonic acid (sodium salt), 40 mg/l EDTA, 2 mM KCl and 3% methanol. pH was obtained to 3.0 with NaOH. The mobile phase was continually degassed with a Degasys Populaire, an on-line degasser (Sanwa Tsusho Co., Tokyo, Japan.). Delivery was set at a flow rate of 0.26 ml/min. The limit of quantitation was approximately 1.56 pg/mg tissue. The limit of detection was approximately 1.07 pg/mg tissue. There were external standards ran with this procedure. The standard curve was run at concentrations of 2.5, 10, 40 and 160 pg/20 μl. Data was stored and profile by computerized software.
2.7. Statistical Analysis
Data was assessed by analysis of variance. The factors included were exposure of chlorpyrifos and repeated measures of time that were assessed by using a statistical model of mixed design analysis between variance and subjects. Trials were used for the tap test and the 3-chamber learning test and minutes were used for the novel tank dive test. Linear trend analyses across repeated measures and CPF exposure duration were performed to evaluate the progressive effects of these factors across the levels of each factor. Planned comparisons were used to evaluate the effect of each CPF dosed group vs. vehicle treated controls.
3. Results
3.1. Effects of Chlorpyrifos on Tap Startle and Habituation
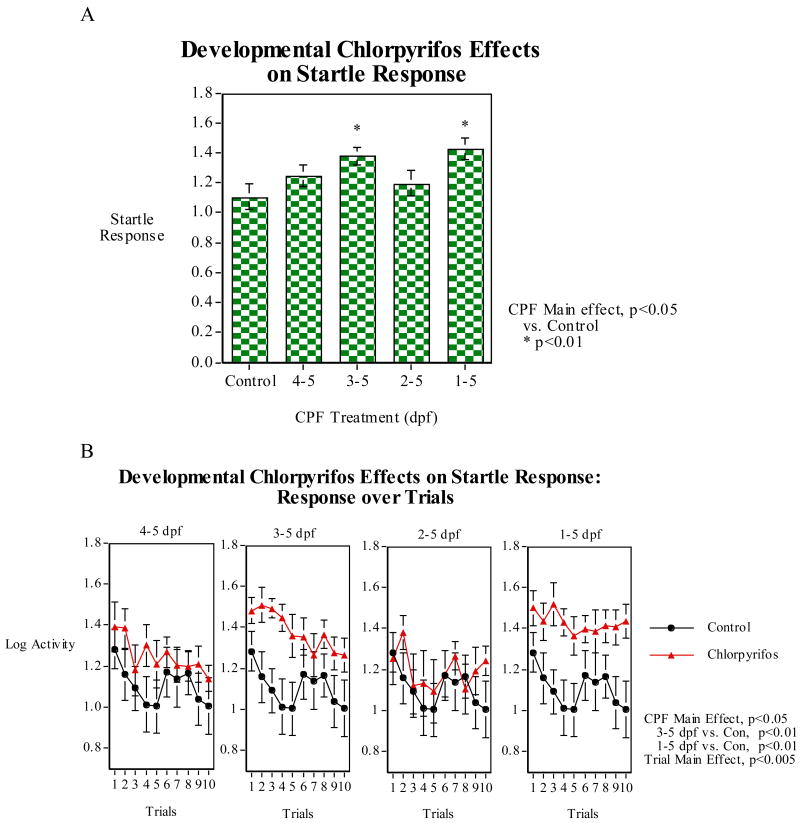
CPF effects on startle response and habituation were measured over ten trials (1/min) in the tap startle test. CPF exposure to zebrafish had persisting effects on startle response only in the group exposed to the full five days after fertilization (Fig. 4). In terms of comparing mean startle response, there was no significant effect but there was a trend toward increase startle response. The effect of the five-day CPF exposure was seen with significantly (p<0.05) lower habituation of the startle response compared with controls over the ten trials of the session. The second experiment compared the effects of CPF exposure during the full first five days after fertilization vs. the later stages of development. This study also detected an effect of the five-day CPF exposure. In this case it was the mean startle response which was significantly (p<0.01) increased in the five-day exposure group relative to controls. A significant (p<0.01) elevation in mean startle across the session was also seen with the group exposed to CPF on days 3-5. Curiously, the group exposed to CPF on days 2-5 did not show a significant increase in startle relative to control. As shown in figure 5B, there was a significant (p<0.005) effect of trial reflecting the habituation of response over the ten-trial session. No significant differential habituation between CPF treated groups and controls was seen in this experiment though there was a trend toward a decreased habituation in the five-day CPF exposure group compared with controls (Fig 5B, right panel). In the two studies the full length CPF exposure caused either an increase in startle or a decrease in habituation over the session.
Figure 5.
Developmental CPF (later vs. complete exposure) effects on tactile startle response and habituation: A) cm traveled in the five seconds after each tap (log of cm/second); B) Response on individual trials in the 10-trial session (mean±sem)
3.2. Effects of Chlorpyrifos on the Novel Tank Diving Test
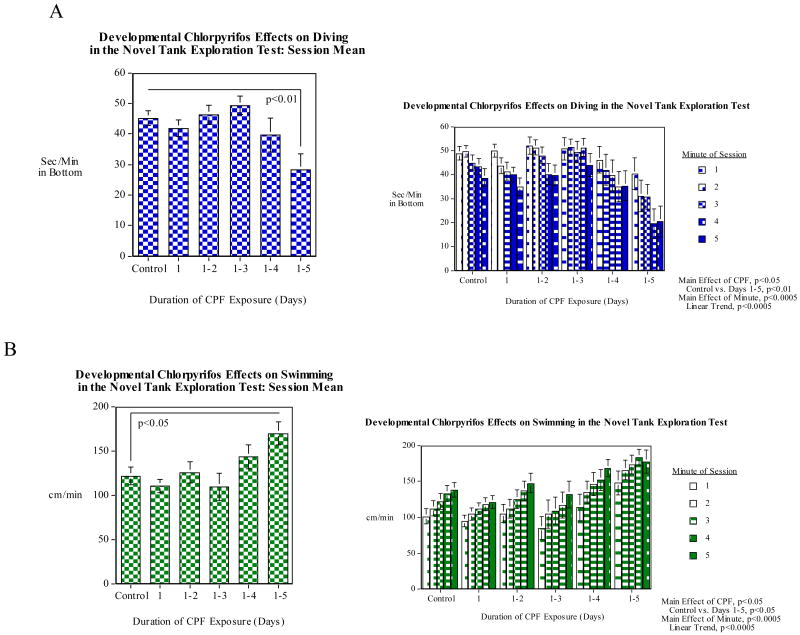
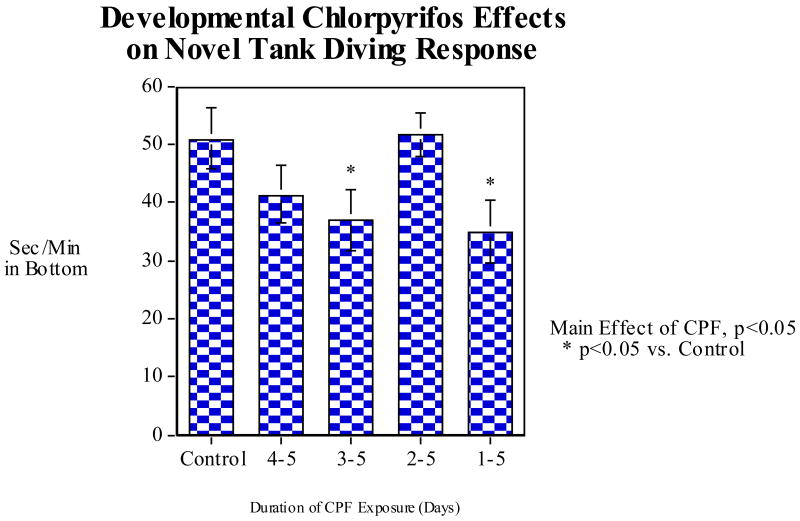
To assess whether CPF had effects on diving response and swimming activity the zebrafish were assayed on the novel tank diving task. CPF exposure over the full five days of development caused a significant (p<0.01) decrease in diving response (Fig. 6A). There were no significant effects seen with lesser durations of exposure. As also shown in figure 6A, there was a significant (p<0.005) linear decrease in diving over the five-minute test session. No interaction of minute within session with CPF exposure was seen. There was a significant (p<0.05) increase in swimming activity with CPF exposure during the full first five days after fertilization (Fig. 6B). Swimming activity also showed a significant (p<0.005) linear increase in swim speed over the five-minute session (Fig. 6B). There was no indication of a differential effect of minute across CPF treatment groups. In the second experiment (Fig. 7) comparing the full duration exposure with exposures during the later phases of development, zebrafish exposed to CPF for the first five days also showed a significant (p<0.05) decrease in the normal novel tank diving response compared with the controls, replicating the first experiment. The group exposed for only days 3-5 also showed a significant (p<0.05) decrease. Curiously, no significant effect was seen in the group exposed to CPF for days 2-5 post fertilization.
Figure 6.
Developmental CPF (earlier vs. complete exposure) effects on diving in the novel tank diving test: A) seconds/minute spent in the bottom third of the tank; B) Swimming speed (cm/min) (mean±sem)
Figure 7.
Developmental CPF (later vs. complete exposure) effects on diving in the novel tank diving test: A) seconds/minute spent in the bottom third of the tank; B) Swimming speed (cm/min) (mean±sem)
3.3. Effects of CPF on Spatial Discrimination
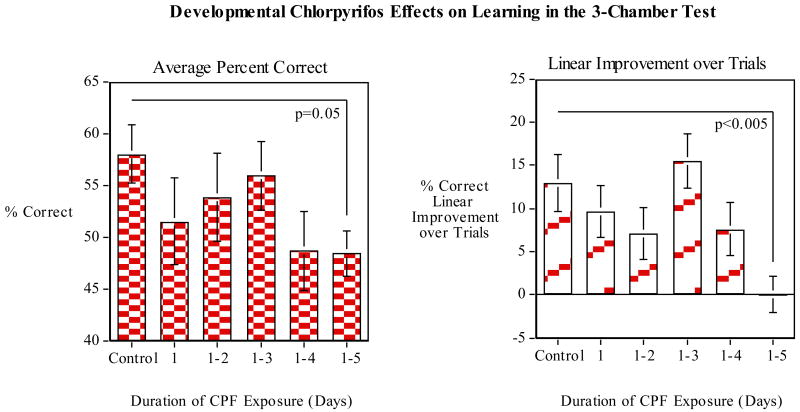
As shown in figure 8 average percent correct after exposure to CPF during the full five days after fertilization was not significantly different from control (p=0.05). None of the other groups showed significant deficits relative to control either. The learning rate as indicated in Figure 8 showed linear improvements over trials. In contrast to the control group, a significant (p<0.005) decline at was seen in group 1-5. There was no evidence of learning in this group over the ten trials of training. There were no effects on response latency in this task.
Figure 8.
Developmental CPF (later vs. full exposure) effects on spatial learning in the 3-chamber test: Left Panel) percent correct; Right Panel) Linear improvement (slope) in percent correct over the training trials (mean±sem).
3.4. Effects of CPF on Monoamine Transmitters
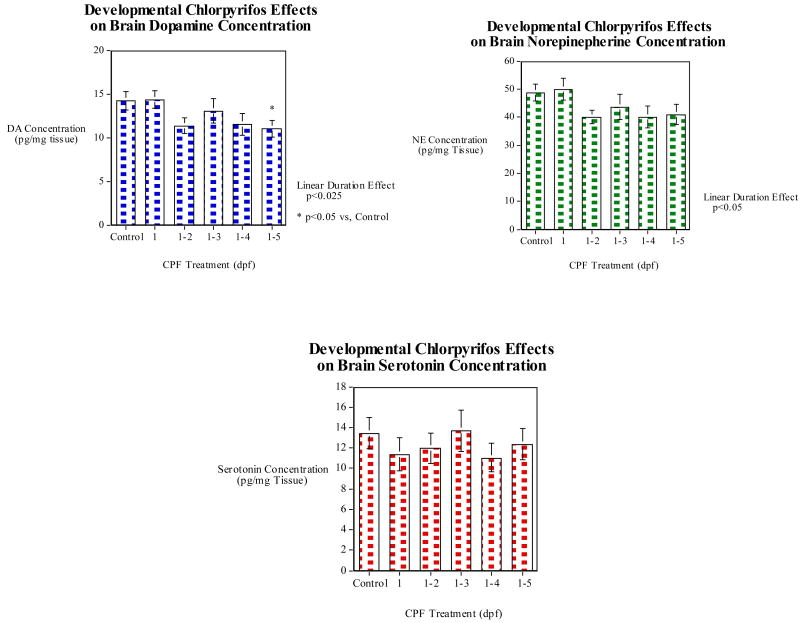
Whole brain DA concentration was significantly (p<0.025) decreased in a linear duration effect manner by CPF (Fig. 9). Specifically, the full duration exposure of the first five days after fertilization caused a significant (p<0.05) decrease in dopamine levels relative to vehicle-treated controls. Brain norepinepherine showed a similar significant (p<0.05) linear decrease over the increasing durations of CPF exposure. However none of the individual CPF treated groups showed significant decreases relative to controls. 5HT concentration in the brain was not found to be significantly affected by CPF.
Figure 9.
Developmental CPF (later vs. full exposure) effects on the monoamine neurotransmitters, dopamine, norepinepherine and serotonin brain concentration (mean±sem).
4. Discussion
CPF exposure during early development in zebrafish caused long-lasting neurobehavioral deficits. These deficits in the zebrafish model are related to OPs and are detrimental to humans. OP exposures during development pose a substantial risk to produce developmental neurotoxicity (Slotkin et al., 2006a). Since these compounds are widely used, it is imperative that we investigate the use of pesticides and the risk they pose to neurobehavioral development. Rodents were traditionally used to study the neurobehavioral effects of these environmental toxicants. Extensive studies have shown that early developmental exposure to CPF caused persisting impairment in cognition and prenatal deficits that persisted long after the initial exposure period (Aldridge et al., 2005a; Icenogle et al., 2004; Levin et al., 2002; Levin et al., 2001; Slotkin, 1999; Slotkin, 2004; 2005; Slotkin and Seidler, 2007). The effect of CPF in zebrafish has provided similar behavioral deficits and to further develop the zebrafish model we must examine deficits in the rat model that advanced neurotoxicity. This project examines whether zebrafish like rats are impaired by developmental exposure to OPs and develop persistent neurotoxicity.
In this study we investigated the critical durations of CPF exposure necessary to induce significant persisting impairment. Exposure was administered during the first five days to produce the effects. This study showed that developmental CPF exposure during the first five days significantly altered behavior function in a battery of test. A significant decrease in habituation was produced in startle response in adult zebrafish during the first ten trials at the full five day exposure. In the second startle experiment there was a significant main effect in startle response. Startle effects were seen with increased average startle response and attenuated habituation across repeated trials. This effect was also seen in previous studies by Eddins et al. where CPF exposure had similar effects (Eddins et al., 2010). We found that for the full effect of startle response the first five days were necessary. However, we did see changes in startle response from an exposure of days 3-5. In terms of the novel tank diving task, developmental exposure during the first days after exposure caused a significant decrease in normal diving response. In the second experiment we also found decreased diving caused by CPF exposure. For the first time we demonstrated that CPF exposure after fertilization days 1-5 caused persisting impairment in diving response. This was not seen in CPF exposure, as with the startle response there were indications at days 3-5, in the novel tank CPF also caused similar effects. However all CPF groups showed the normal habituation over the five day period. In the novel tank study swim speed was significantly increased over days 1-5 and there was also a significant increase in bottom dwelling by the adult zebrafish across all groups tested. These effects were replicated in our second experiment. The results from both studies indicated that it was the full five-day exposure that significantly affected overall response.
In terms of the three-chamber learning test we performed the task to assess the full five days of exposure. There was a nearly significant effect and a decrease in percent correct. There was a significant decrease effect in learning rate. Zebrafish with the complete CPF exposure during the first five days showed no learning at all when tested as adults. Previous studies from Levin et al. (2003) showed these impairments. There were strong trending possibilities that suggest an effect; however, due to variability it is not significant. There are trends with shorter exposure also of decrease accuracy however not significant in comparison to the control. All three of the behavioral tasks showed significant impairments caused by CPF exposure from fertilization through day five, indicating that the neurobehavioral toxicity was not focal.
At the end of behavioral task assessment, the adult zebrafish were euthanized and the whole brain neurochemical levels were determined. In this study there were no significant effects in whole brain DA, 5HT, norepinepherine or 5HT turnover concentrations. In previous studies Slotkin and colleagues (Slotkin et al., 2006a) stated that through a diverse combination of mechanisms adverse effects are rendered in synaptic activity and patterns of innervations from CPF exposure. In this study the neurochemical aspect was important because it allows for the observation of what other systems are interrelated that may affect the basis of neurobehavioral teratology. In recent studies Eddins et al. (2010) tested these conclusions and found that in adult zebrafish, developmental CPF exposure had significant effects on neurochemical levels.
Eddins et al. showed a significant decrease in whole brain activity but not in DA turnover of zebrafish after exposure to CPF (Eddins et al., 2010). Significant effects in DA turnover were seen in all five groups. Exposure from day 1-3 had an even greater decrease when compared to the control group. Decreases in DA turnover of adult zebrafish can be important in short and long term behavior defects. In accordance with Eddins et al., our data showed that developmental CPF exposure had no significant effect on 5HT, 5HT turnover, or norepinephrine concentrations. There are persisting behavioral defects after early developmental exposure to CPF but the cause of the effect is unclear. The brain is very complex, especially the frontal cortex which within itself presents challenges when determining systemic behavioral effects. Through more research of monoamines and mechanistic approaches we may better determine causes for altered behaviors from CPF exposure.
The current study found persisting neurobehavioral effects of developmental CPF exposure in zebrafish as has also been seen in rodent model (Aldridge et al., 2004; Icenogle et al., 2004; Levin et al., 2001; Slotkin and Seidler, 2007; Slotkin et al., 2002). These effects provide broad deficits in increase startle response, decrease predatory avoidance, increase hyper-activity, and impairment in learning. Similarly these are the types of impairments that are seen in epidemiological studies. The zebrafish model can be a useful screening tool for persisting adverse effects in developmental exposure to CPF and other toxicants. The model is economical and its rapid throughput provides a distinct advantage over other models. The zebrafish model will be useful in future studies that aim to discovering molecular and cellular activity that are associated with behavioral these effects.
Highlights.
Embryonic exposure of zebrafish to the pesticide chlorpyrifos causes persisting neurobehavioral impairment.
Chlorpyrifos exposure for the first five days after fertilization caused significant increase in tactile startle and a decrease in habituation.
Chlorpyrifos exposure for the first five days after fertilization caused significant impairment in the normal novel tank diving response and locomotor hyperactivity.
Chlorpyrifos exposure for the first five days after fertilization caused significant impairment in spatial learning.
Chlorpyrifos exposure for the first five days after fertilization caused significant decrease in brain dopamine concentration.
Embryonic chlorpyrifos exposure of shorter durations had much-attenuated effect.
Acknowledgments
Supported by the Duke University Superfund Research Center ES10356 and NIEHS ARRA grant ES016554.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldridge JE, Levin ED, Seidler FJ, Slotkin TA. Developmental exposure of rats to chlorpyrifos leads to behavioral alterations in adulthood, involving serotonergic mechanisms and resembling animal models of depression. Environ Health Perspect. 2005a;113:527–531. doi: 10.1289/ehp.7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Meyer A, Seidler FJ, Slotkin TA. Alterations in central nervous system serotonergic and dopaminergic synaptic activity in adulthood after prenatal or neonatal chlorpyrifos exposure. Environ Health Perspect. 2005b;113:1027–1031. doi: 10.1289/ehp.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Seidler FJ, Slotkin TA. Developmental exposure to chlorpyrifos elicits sex-selective alterations of serotonergic synaptic function in adulthood: critical periods and regional selectivity for effects on the serotonin transporter, receptor subtypes, and cell signaling. Environ Health Perspect. 2004;112:148–155. doi: 10.1289/ehp.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur D, Levin ED. Spatial and non-spatial discrimination learning in zebrafish (Danio rerio) Animal Cognition. 2001;4:125–131. [Google Scholar]
- Aschner M, Levin ED, Sunol C, Olopade JO, Helmcke KJ, Avila DS, Sledge D, Ali RH, Upchurch L, Donerly S, Linney E, Forsby A, Ponnuru P, Connor JR. Gene-environment interactions: neurodegeneration in non-mammals and mammals. Neurotoxicology. 2010;31:582–588. doi: 10.1016/j.neuro.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behra M, Cousin X, Bertrand C, Vonesch JL, Biellmann D, Chatonnet A, Strahle U. Acetylcholinesterase is required for neuronal and muscular development in the zebrafish embryo. Nature Neuroscience. 2002;5:111–118. doi: 10.1038/nn788. [DOI] [PubMed] [Google Scholar]
- Bouchard MF, Bellinger DC, Wright RO, Weisskopf MG. Attention-Deficit/Hyperactivity Disorder and Urinary Metabolites of Organophosphate Pesticides. Pediatrics. 2010;125 doi: 10.1542/peds.2009-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell CG, Seidler FJ, Slotkin TA. Chlorpyrifos interferes with cell development in rat brain regions. Brain Research Bulletin. 1997;43:179–189. doi: 10.1016/s0361-9230(96)00436-4. [DOI] [PubMed] [Google Scholar]
- Crumpton TL, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos in vivo and in vitro: effects on nuclear transcription factors involved in cell replication and differentiation. Brain Res. 2000;857:87–98. doi: 10.1016/s0006-8993(99)02357-4. [DOI] [PubMed] [Google Scholar]
- Dam K, Garcia SJ, Seidler FJ, Slotkin TA. Neonatal chlorpyrifos exposure alters synaptic development and neuronal activity in cholinergic and catecholaminergic pathways. Dev Brain Res. 1999;116:9–20. doi: 10.1016/s0165-3806(99)00067-x. [DOI] [PubMed] [Google Scholar]
- Dam K, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: Delayed targeting of DNA synthesis after repeated administration. Dev Brain Res. 1998;108:39–45. doi: 10.1016/s0165-3806(98)00028-5. [DOI] [PubMed] [Google Scholar]
- Eddins D, Cerutti D, Williams P, Linney E, Levin ED. Developmental chlorpyrifos causes behavioral and neurochemical defects in zebrafish. Neurotoxicology and Teratology. 2010;32:99–108. doi: 10.1016/j.ntt.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddins D, Petro A, Pollard N, Freedman JH, Levin ED. Mercury-induced cognitive impairment in metallothionein knockout mice. Neurotoxicology and Teratology. 2008;30:88–95. doi: 10.1016/j.ntt.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddins D, Petro A, Williams P, Cerutti DT, Levin ED. Nicotine effects on learning in zebrafish: the role of dopaminergic systems. Psychopharmacology. 2009;202:103–109. doi: 10.1007/s00213-008-1287-4. [DOI] [PubMed] [Google Scholar]
- Icenogle LM, Christopher NC, Blackwelder WP, Caldwell DP, Qiao D, Seidler FJ, Slotkin TA, Levin ED. Behavioral alterations in adolescent and adult rats caused by a brief subtoxic exposure to chlorpyrifos during neurulation. Neurotoxicol Teratol. 2004;26:95–101. doi: 10.1016/j.ntt.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Levin ED, Addy N, Baruah A, Elias A, Christopher NC, Seidler FJ, Slotkin TA. Prenatal chlorpyrifos exposure in rats causes persistent behavioral alterations. Neurotoxicology and Teratology. 2002;24:733–741. doi: 10.1016/s0892-0362(02)00272-6. [DOI] [PubMed] [Google Scholar]
- Levin ED, Addy N, Nakajima A, Christopher NC, Seidler FJ, Slotkin TA. Persistent behavioral consequences of neonatal chlorpyrifos exposure in rats. Dev Brain Res. 2001;130:83–89. doi: 10.1016/s0165-3806(01)00215-2. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bencan Z, Cerutti DT. Anxiolytic effects of nicotine in zebrafish. Physiology and Behavior. 2007;90:54–58. doi: 10.1016/j.physbeh.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Levin ED, Chrysanthis E, Yacisin K, Linney E. Chlorpyrifos exposure of developing zebrafish: effects on survival and long-term effects on response latency and spatial discrimination. Neurotoxicol Teratol. 2003;25:51–57. doi: 10.1016/s0892-0362(02)00322-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, Limpuangthip J, Rachakonda T, Peterson M. Timing of nicotine effects on learning in zebrafish. Psychopharmacology. 2006;184:547–552. doi: 10.1007/s00213-005-0162-9. [DOI] [PubMed] [Google Scholar]
- Levin ED, Swain HA, Donerly S, Linney E. Developmental chlorpyrifos effects on hatchling zebrafish swimming behavior. Neurotoxicol Teratol. 2004;26:719–723. doi: 10.1016/j.ntt.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Linney E, Upchurch L, Donerly S. Zebrafish as a neurotoxicological model. Neurotoxicol Teratol. 2004;26:709–718. doi: 10.1016/j.ntt.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Rauh VA, Garfinkel R, Perera FP, Andrews H, Barr D, Whitehead D, Tang D, Whyatt RM. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first three years of life among inner-city children. Pediatrics. 2006;118:1845–1859. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roegge CS, Timofeeva OA, Seidler FJ, Slotkin TA, Levin ED. Developmental diazinon neurotoxicity in rats: Later effects on emotional response. Brain Research Bulletin. 2008;75:166–172. doi: 10.1016/j.brainresbull.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy T, Seidler F, Slotkin T. Morphologic effects of subtoxic neonatal chlorpyrifos exposure in developing rat brain: regionally-selective alterations in neurons and glia. Dev Brain Res. 2004;148:197–206. doi: 10.1016/j.devbrainres.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Roy TS, Andrews JE, Seidler FJ, Slotkin TA. Chlorpyrifos elicits mitotic abnormalities and apoptosis in neuroepithelium of cultured rat embryos. Teratology. 1998;58:62–68. doi: 10.1002/(SICI)1096-9926(199808)58:2<62::AID-TERA7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Developmental cholinotoxicants: Nicotine and chlorpyrifos. Environ Health Perspect. 1999;107 1:65–69. doi: 10.1289/ehp.99107s171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol. 2004;198:132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Developmental neurotoxicity of organophosphates: a case study of chlorpyrifos. In: Gupta RC, editor. Toxicity of Organophosphate and Carbamate Pesticides. Elsevier Academic Press; San Diego: 2005. pp. 293–314. [Google Scholar]
- Slotkin TA, Cousins ML, Tate CA, Seidler FJ. Persistent cholinergic presynaptic deficits after neonatal chlorpyrifos exposure. Brain Res. 2001;902:229–243. doi: 10.1016/s0006-8993(01)02387-3. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Levin ED, Seidler FJ. Comparative developmental neurotoxicity of organophosphate insecticides: Effects on brain development are separable from systemic toxicity. Environ Health Perspect. 2006a;114:746–751. doi: 10.1289/ehp.8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Prenatal chlorpyrifos exposure elicits presynaptic serotonergic and dopaminergic hyperactivity at adolescence: critical periods for regional and sex-selective effects. Reprod Toxicol. 2007;23:421–427. doi: 10.1016/j.reprotox.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Tate CA, Cousins MM, Seidler FJ. Functional alterations in CNS catecholamine systems in adolescence and adulthood after neonatal chlorpyrifos exposure. Dev Brain Res. 2002;287:163–173. doi: 10.1016/s0165-3806(02)00284-5. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Tate CA, Ryde IT, Levin ED, Seidler FJ. Organophosphate insecticides target the serotonergic system in developing rat brain regions: Disparate effects of diazinon and parathion at doses spanning the threshold for cholinesterase inhibition. Environ Health Perspect. 2006b;114:1542–1546. doi: 10.1289/ehp.9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Seidler FJ, Saleh JL, Zhang J, Padilla S, Slotkin TA. Cellular mechanisms for developmental toxicity of chlorpyrifos: Targeting the adenylyl cyclase signaling cascade. Toxicology and Applied Pharmacology. 1997;145:158–174. doi: 10.1006/taap.1997.8171. [DOI] [PubMed] [Google Scholar]
- Timofeeva OA, Sanders D, Seemann K, Yang L, Hermanson D, Regenbogen S, Agoos S, Kallepalli A, Rastogi A, Braddy D, Wells C, Perraut C, Seidler FJ, Slotkin TA, Levin ED. Persistent behavioral alterations in rats neonatally exposed to low doses of the organophosphate pesticide, parathion. Brain Research Bulletin. 2008;77:404–411. doi: 10.1016/j.brainresbull.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney KD, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: Cellular mechanisms. Toxicology and Applied Pharmacology. 1995;134:53–62. doi: 10.1006/taap.1995.1168. [DOI] [PubMed] [Google Scholar]
- Whyatt RM, Rauh V, Barr DB, Camann DE, Andrews HF, Garfinkel R, Hoepner LA, Diaz D, Dietrich J, Reyes A, Tang D, Kinney PL, Perera FP. Prenatal insecticide exposures and birth weight and length among an urban minority cohort. Environ Health Perspect. 2004;112:1125–1132. doi: 10.1289/ehp.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]