Abstract
Painful diabetic neuropathy (PDN) is a common, yet devastating complication of type 2 diabetes. At this time, there is no objective test for diagnosing PDN. In the current study, we measured the peptidergic intraepidermal nerve fiber densities (IENFD) from hind paws of the db/db mouse, an animal model for type 2 diabetes, during the period of mechanical allodynia from 6–12 wk of age. Intraepidermal nerve fibers (IENF) of the hind footpads were identified by protein gene product (PGP) 9.5 immunohistochemistry. The peptidergic IENF were determined by double immunofluorescence using anti-PGP9.5 and antibodies against tropomyosin-receptor-kinase (Trk) A. We observed a significant increase in PGP9.5-positive IENFD at 8 and 10 wk of age. Similarly, Trk A-positive peptidergic IENF, which also express substance P and calcitonin gene related peptide in db/db mice, were observed to be elevated from 1.5 to 2 fold over controls. This upregulation ended at 16 wk of age, in accordance with the reduction of mechanical allodynia. Anti-NGF treatment significantly inhibited the upregulation of peptidergic IENFD during the period of mechanical allodynia, suggesting increased neurotrophism may mediate this phenomenon. In addition, SB203580, an inhibitor of p38, blocked the increase in peptidergic IENFD in db/db mice. The current results suggest peptidergic IENFD could be a potential diagnostic indicator for PDN in type 2 diabetes. Furthermore, the inhibition of NGF-p38 signaling could be a potential therapeutic strategy for treating this painful condition.
Keywords: Diabetic pain, intraepidermal nerve fibers, type 2 diabetes, nerve growth factor, peptidergic nerve fibers, mechanical allodynia
Introduction
Diabetes mellitus affects over 20 million Americans. About 60% of patients diagnosed with diabetes develop diabetic neuropathy (DN). Painful diabetic neuropathy (PDN) is an early manifestation of DN, which frequently presents in the pre-diabetic states of impaired fasting glucose (IFG) or impaired glucose tolerance (IGT) (Boulton et al.; Feldman et al.; Ziegler et al., 2009). This devastating complication can be found in 40% to 50% of patients with diabetes that have documented neuropathies (Galer et al., 2000). Regrettably, the quality of life for diabetic patients with PDN is significantly diminished (Dworkin et al.; Dworkin et al.; Jensen et al.).
Patients with PDN experience mechanical allodynia and thermal hyperalgesia, which are frequently described as continuously burning, tingling, electric-like, crampy, or achy pain. Allodynia occurs when normally non-painful stimuli become painful, whereas hyperalgesia is an increase in sensitivity to normally painful stimuli. PDN begins in the feet and progresses proximally over time. Symptoms of neuropathic pain are generated by small-calibered Aδ and C fibers that innervate the skin.
At present, there is no available diagnostic tool to objectively determine the severity of PDN. Clinicians must rely on subjective reports from patients concerning their personal experiences of pain, which are frequently influenced by their psychosocial conditions and potential secondary gain from illness. As a result, such measures often lack credibility. In order to objectively test for DN, nerve conduction study and skin biopsy are most commonly used. Unfortunately, nerve conduction study is not a reliable method to diagnose PDN due to its inability to detect small fiber pathology. Skin biopsy can detect small fiber pathology, and thus has been widely used to determine intraepidermal nerve fiber density (IENFD) in DN (Catalan et al.), in addition to the diagnosis of other small-fiber neuropathies. However, its value for determining or predicting the development of PDN is still under debate (Sorensen et al., 2006a, b).
The current method of IENFD measurement uses protein gene product (PGP) 9.5 to identify all IENF in the skin (Johnson et al., 2008; Sullivan et al., 2007; Zandecki et al., 2008). Furthermore, PGP9.5 IENFD is considered a standard method to determine small fiber sensory neuropathies including DN (Lauria et al., 2005). Many published animal and human studies have demonstrated a decrease of PGP9.5 IENFD in DN (Kennedy et al., 1996; Shun et al., 2004). Such a decrease of PGP9.5 IENFD is detected early in the course of diabetes and has been found in patients with IGT (Sumner et al., 2003). Nevertheless, the value of PGP9.5 IENFD for the diagnosis of PDN has yet to be determined.
In the present study, our aim was to determine if there is evidence of a correlation between IENFD and mechanical allodynia specific to PDN. Additionally, we intended to further explore our hypothesis that there is enhanced axonal sprouting or regeneration present at the initial stage of PDN to mediate mechanical allodynia in the db/db mouse. Based on previous findings, which report that nerve growth factor (NGF) mediates mechanical allodynia in db/db mice, we focused on subgroups of IENF called peptidergic fibers that express neuropeptides involved in the mediation of nociception. The development of these fibers is dependent on NGF, and these peptidergic fibers are positive for tropomyosin-receptor-kinase A (Trk A), the high affinity receptor for NGF. Two of the major neuropeptides expressed by these peptidergic fibers are substance P (SP) and calcitonin gene related peptide (CGRP). In order to quantify the peptidergic IENFD, we performed double immunofluorescent staining with Trk A and either SP or CGRP on footpads from hind paws of the db/db mouse, an animal model of type 2 diabetes. Additionally, we applied anti-NGF and SB203580, a p38 inhibitor, to db/db mice to gain insight on the molecular mechanisms underlying the changes in IENFD during the early phase of mechanical allodynia.
Previously, we reported that mechanical allodynia is detected in the db/db mouse from 6-12 wk of age. Additionally, we reported an upregulation of SP and NGF at 8 and 10 wk of age in the db/db mouse (Cheng et al., 2009). Here, we observe a 1.5- to 2-fold elevation in SP and CGRP expression compared to the control values in Trk A-positive peptidergic IENF. Furthermore, we report a cessation of the increase in peptidergic IENFD at 16 wk of age, corresponding to the reduction of pain behaviors at this time point.
Anti-NGF treatment and SB203580 treatment significantly inhibit the increase in peptidergic IENFD during the period of mechanical allodynia, suggesting that increased neurotrophism may mediate PDN. These results suggest that quantification of peptidergic IENFD could be a potential diagnostic indicator for PDN in type 2 diabetes, and that inhibiting NGF-p38 signaling could be a potential therapeutic strategy for the treatment of PDN.
Materials and Methods
Animals
Male C57BLKS db/db (stock number 000642) mice were purchased from Jackson Laboratories (Bar Harbor, ME). The homozygous (Leprdb/ Leprdb, or db/db) mice were used as a model of type 2 diabetes, while heterozygous mice (Leprdb/+, or db/+) served as nondiabetic controls. Analyses and procedures were performed in compliance with protocols established by the Animal Models of Diabetic Complications Consortium (AMDCC) (http://www.amdcc.org) and were approved by the Use and Care of Animals Committee at the University of Michigan. All possible efforts were made to minimize the animals’ suffering and the number of animals used.
Intraepidermal nerve fiber (IENF) measurement
IENF quantification was performed using 4 db/+ mice and 4 db/db mice at 5, 8, 10, and 16 wk time points. Prior to perfusion, both right and left hind foot pads were collected from the plantar surface of the hind paw, immersed for 6–8 hours at 4°C in Zamboni’s fixative (2% paraformaldehyde, 0.2% picric acid in 0.1M phosphate buffer), rinsed in 30% sucrose in PBS solution overnight, cryoembedded in mounting media (OCT), and sectioned at 30μm thickness before being processed for immunohistochemistry.
Tissue sections were processed for PGP9.5, Trk A, SP, CGRP, and TNF-α immunohistochemistry (Polydefkis et al., 2001). Sections were incubated at 4°C for 16-24 h with primary antibodies: PGP9.5 (1:1000, Millipore, Billerica, MA), Trk A (1:500, R&D Systems, Minneapolis, MN), SP (1:200, Abcam, Cambridge, MA), CGRP (1:500, Sigma-Aldrich, St. Louis, MO), and TNF-α (1:1000, Abcam). Sections were then rinsed 3 times in phosphate buffered saline (PBS) and incubated with secondary antiserum conjugated with different fluorophores (AlexaFluor 488, 594, or 647, Invitrogen, Carlsbad, CA). Sections were rinsed and mounted with ProLong® Gold antifade reagent (Invitrogen). In order to confirm that there were no nonspecific immunoreactions, sections were incubated with primary or secondary antisera alone. Fluorescent images were collected on an Olympus FluoView 500 confocal microscope using a 20 × 1.2 water immersion objective at a resolution of 1024 × 1024 pixels. The optical section thickness was 0.5 μm. Approximately forty images per stack were flattened using the MetaMorph (Molecular Devices, Sunnyvale, CA, version 6.14) arithmetic option. Six sections were measured for each footpad. IENFD data were presented as the mean number of fibers per linear mm of epidermis from a total of 12 sections per animal (Christianson et al., 2003).
Anti-NGF treatment
In order to inhibit NGF action during the period of allodynia, we administered anti-NGF (10 mg/kg, mouse monoclonal antibody clone AS21, Exalpha Biologicals, Maynard, MA) or control IgG intraperitoneally. Treatment was performed once weekly at the beginning of 6 wk of age for two weeks (Wild et al., 2007). Hind foot pads were collected for immunohistochemistry at the end of treatment at 8 wk of age.
SB203580 treatment
An osmotic minipump (Alzet minipump model 1007D, Duent Corporation, Cupertino, CA) was used for continuous intrathecal infusion into the lumbar spinal cord region. The 100 μl volume minipump is designed with a 0.51 μl/hr infusion rate. The minipumps were filled with artificial cerebrospinal fluid (CSF) that contained 10% dimethyl sulfoxide (DMSO) with or without SB203580 (1 mg/ml, EMD Chemicals, Gibbstown, NJ). The minipumps were implanted into the dorsal subcutaneous space between the shoulder blades of each mouse at 7 wk of age under sterile conditions. A caudally directed polyethylene cannula (Becton Dickinson and Company, Sparks, MD) was threaded subcutaneously at the level of the L5 spinal process. The L5 spinal process was removed and the tip of the cannula was then inserted into the subarachnoid space at the L5 level. The intrathecal infusion lasted for 1 wk, after which hind foot pads were collected for immunohistochemistry.
Data presentation and statistical analyses
All data are presented as group means ± SEM. The data between db/+ and db/db mice of the same age were analyzed using the Mann-Whitney test. Statistical comparisons between different age groups were made by a one-way ANOVA test followed by a post hoc Tukey’s multiple comparison test. A p-value of less than 0.05 was considered statistically significant.
Results
PGP9.5 and Trk A-positive IENFD increase in diabetic animals during the period of mechanical allodynia
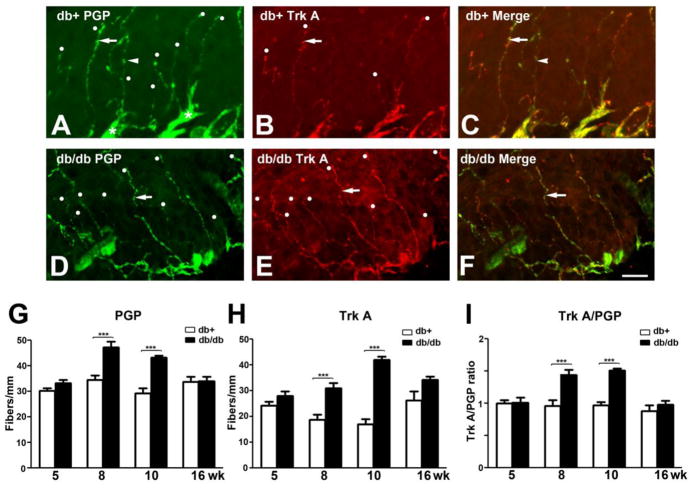
Previously, we reported that the db/db mouse, an animal model of type 2 diabetes, develops mechanical allodynia at 6–12 wk of age (Cheng et al., 2009). In our current study, we examined the temporal correlation between the change of peptidergic IENFD and the development of mechanical allodynia using the hind foot pads from animals characterized in previous studies (Cheng et al., 2009; Cheng et al., 2010). First, we performed immunohistochemistry of a pan-neuronal marker, PGP 9.5, on hind footpads from 5, 8, 10, and 16 wk of age (Fig. 1). The 5 wk group was prediabetic; 8 and 10 wk groups experienced mechanical allodynia, and the 16 wk group was diabetic with significant sensory neuropathy but no PDN (Cheng et al., 2009). As demonstrated in Fig. 1, PGP9.5 immunohistochemistry labeled the horizontal plexus of nerve fibers underneath the epidermis (Fig. 1A, asterisks). IENF originate from the dermal plexus and extend into the epidermis (Fig. 1A, arrow and arrowhead). PGP9.5 immuno-positive IENF are either Trk A-positive (Fig. 1 arrows) or Trk A-negative (Fig. 1, arrowheads). The representative confocal double immunofluorescent images from 8 wk db/+ (Fig. 1A, B, C) and db/db mice (Fig. 1D, E, F) demonstrated the presence of both PGP9.5- and Trk A-positive IENF in both animals. Quantitative analysis revealed that there was a significant 1.4-fold increase in PGP9.5-positive IENFD in db/db mice at 8 and 10 wk of age (Fig. 1G). In contrast, no significant difference was detected at 5 and 16 wk of age (Fig. G). In accordance with PGP9.5, Trk A-positive IENFD was not changed in the db/db mouse at 5 wk of age but significantly increased at 8 and 10 wk of age (Fig. 1H). There was a 1.5-fold increase of Trk A-positive IENF in db/db mice at 8 wk of age and a >2-fold increase at 10 wk of age. By 16 wk of age, the upregulation of Trk A-positive IENFD in diabetic animals diminishes and reverts to that of the control level (Fig. 1H). In order to demonstrate the fiber-type specific upregulation of Trk A-positive IENFD out of the general increase of PGP9.5-positive IENFD, we calculated the percentages of Trk A-positive / PGP9.5-positive fibers in each sample and normalized the data to the means of db/+ for each age group (Fig. 1I). We found that there was a 1.5-fold increase in the ratio of TrkA/PGP9.5 between db/db and db/+ mice at 8 and 10 wk of age.db/+
Fig. 1. Increased IENFD in db/db mice during the period of mechanical allodynia.
A-C: Immunohistochemistry studies from the hind paw skin of 8 wk-old db/+ mice (A-C) demonstrate PGP9.5 (A) and Trk A (B) -positive IENF. Each counted IENF is labeled with a white dot. A: IENF (arrow and arrowhead) extend from the dermal plexus (asterisks) into the epidermis in db/+ mice. B: Trk A immunoreactive fibers are also positive for PGP9.5 (arrow). C: The merged picture demonstrates both Trk A-positive (arrow) and -negative (arrowhead) IENF. D-F: Immunohistochemistry studies from the hind paw skin of 8 wk-old db/db mice demonstrated PGP9.5 (D) and Trk A (E)-positive IENF. D: PGP9.5-positive IENF (arrow) in epidermis of db/db mice. E: Trk A-positive IENF (arrow) in db/db mice. F: The merged picture demonstrates most PGP9.5 immunoreactive fibers are also positive for Trk A (arrow). Quantification analysis of IENFD demonstrates increased PGP9.5 (G) and Trk A (H) at 8 and 10 wk of age. I: Fold changes of the ratio of Trk A- positive fibers and PGP9.5-positive IENF in each animal to db/+ mice of the same age. There was a 1.5 fold increase of TrkA / PGP9.5-positive IENFD ratio in db/db mice when compared to db/+ mice of the same age. ***, p < 0.001, N = 4. Bar = 20 μm.
SP and CGRP-positive IENFD are increased in diabetic animals during the period of mechanical allodynia
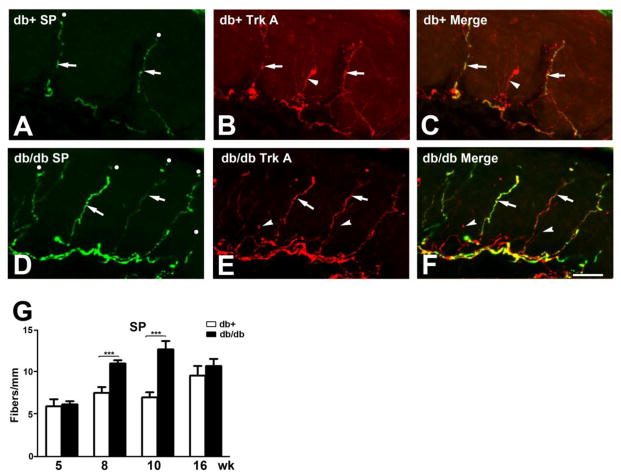
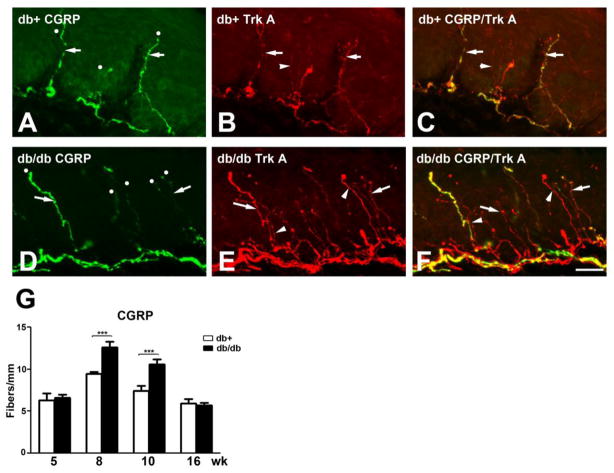
Trk A-positive IENF are peptidergic nerve fibers that express SP and CGRP. Confocal double immunofluorescent studies demonstrated most SP immunoreactivity colocalize with Trk A-positive IENF in both db/+ and db/db mice (Fig. 2A-C, D-F) respectively. Quantitative analysis demonstrated increased SP-positive IENF at 8 and 10 wk in db/db mice compared to db/+ control mice (Fig. 2G). Similar to Trk A-positive IENF, a 1.5-fold increase at 8 wk and 2-fold increase at 10 wk of SP-positive IENF were detected in db/db mice (Fig. 2G). In parallel, CGRP-positive IENF were detected in both db/+ and db/db mice (Fig. 3A-C, D-F, respectively). The CGRP IENF staining was higher in number than SP-positive IENF, but was only detected in Trk A-positive IENF (Fig. 3A-F). Up to 1.5-fold elevation of CGRP-positive IENF was detected in db/db mice at 8 and 10 wk of age (Fig. 3G). Consistent with other peptidergic IENFD, the upregulation of CGRP-positive IENF diminished by 16 wk of age (Fig. 3G).
Fig. 2. Increased SP-positive IENFD in db/db mice during the period of mechanical allodynia.
A-C: Immunohistochemistry studies from the hind paw skin of 8 wk-old db/+ mice (A-C) demonstrated SP (A) and Trk A (B)-positive IENF. Each counted IENF was labeled with a white dot. AC: SP immunoreactive fibers are also positive for Trk A (arrows). There are also SP-positive but Trk A-negative IENF (B: arrowhead). C: The merged picture demonstrates both SP-positive (arrow) and -negative (arrowhead) Trk A-positive IENF. D-F: Immunohistochemistry studies from the hind paw skin of 8 wk-old db/db mice demonstrated SP (D) and Trk A (E)-positive IENF. D: SP-positive IENF (arrows) in epidermis of db/db mice. E: Trk A-positive IENF (arrows) in db/db mice. Some Trk A-positive IENF are negative for SP (arrowheads). F: The merged picture demonstrates both SP-positive (arrows) and -negative (arrowheads) Trk A-positive IENF. G: Quantification analysis of IENFD demonstrates increased SP-positive IENFD at 8 and 10 wk of age. ***, p < 0.001, N = 4. Bar = 20 μm.
Fig. 3. Increased CGRP-positive IENFD in db/db mice during the period of mechanical allodynia.
A-C: Immunohistochemistry studies from the hind paw skin of 8 wk-old db/+ mice (A-C) demonstrated CGRP (A) and Trk A (B)-positive IENF. Each counted IENF was labeled with a white dot. A-C: CGRP immunoreactive fibers are also positive for Trk A (arrows). There are CGRP-positive but Trk A-negative IENF (B: arrowhead). C: The merged picture demonstrates both CGRP-positive (arrows) and -negative (arrowhead) Trk A-positive IENF. D-F: Immunohistochemistry studies from the hind paw skin of 8 wk-old db/db mice demonstrated SP (D) and Trk A (E)-positive IENF. D: CGRP-positive IENF (arrows) in epidermis of db/db mice. E: Trk A-positive IENF (arrow) in db/db mice. Some Trk A-positive IENF are negative for SP (arrowhead). F: The merged picture demonstrates both CGRP-positive (arrow) and -negative (arrowhead) Trk A-positive IENF. G: Quantification analysis of IENFD demonstrates increased CGRP-positive IENFD at 8 and 10 wk of age. ***, p < 0.001, N = 4. Bar = 20 μm.
Continuation of TNF-α-positive IENFD increase in diabetic animals beyond the period of mechanical allodynia
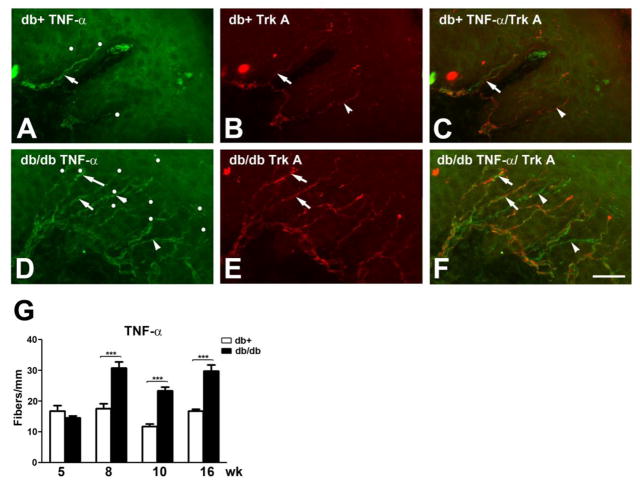
To determine whether this upregulation of peptidergic IENF was a general phenomenon involving all the IENF, we measured TNF-α-positive IENF from 5,8, 10, and 16 wk of age. In db/+ mice, TNF-α-positive IENF include both Trk A-positive and -negative fibers (Fig. 4A-C). In db/db mice, the TNF-α-positive IENF increase at 8 wk of age in both Trk A-positive and -negative fibers (Fig. 4D-F). Quantitative studies detected up to a 1.5-fold increase of TNF-α-positive IENFD at 8 and 10 wk of age (Fig. 4G). In contrast to peptidergic IENF, TNF-α-positive IENFD remain elevated at 16 wk of age (Fig. 4G).
Fig. 4. Increased TNF-α-positive IENFD in db/db mice during the period of mechanical allodynia.
A-C: Immunohistochemistry studies from the hind paw skin of 8 wk-old db/+ mice (A-C) demonstrated TNF-α (A) and Trk A (B)-positive IENF. Each counted IENF was labeled with a white dot. A-C: TNF-α immunoreactive fibers are also positive for Trk A (arrow). There are also TNF-α-positive but Trk A-negative IENF (B: arrowhead). C: The merged picture demonstrates both TNF-α-positive (arrows) and -negative (arrowhead) Trk A-positive IENF. D-F: Immunohistochemistry studies from the hind paw skin of 8 wk-old db/db mice demonstrated TNF-α (D) and Trk A (E)-positive IENF. D: TNF-α-positive IENF (arrows and arrowheads) in epidermis of db/db mice. E: Trk A-positive IENF (arrows) in db/db mice. Some TNF-α-positive IENF are negative for Trk A (arrowheads). F: The merged picture demonstrates both Trk A-positive (arrows) and -negative (arrowheads) TNF-α-positive IENF. G: Quantification analysis of IENFD demonstrates increased TNF-α-positive IENFD at 8, 10, and 16 wk of age. ***, p < 0.001, N = 4. Bar = 20 μm.
Anti-NGF inhibited the upregulation of PGP9.5-positive and peptidergic IENFD in diabetic animals
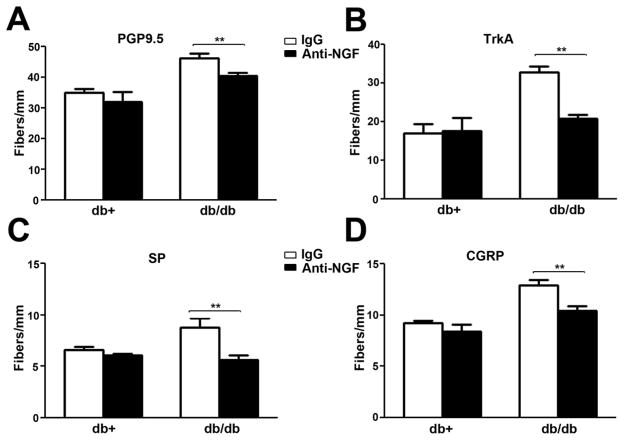
The observation of increased peptidergic IENF suggests that there is NGF-dependent neurotrophism in the skin that mediates mechanical allodynia. In order to test this hypothesis, we administered anti-NGF intraperitoneally to antagonize NGF actions weekly for 2 wk starting at 6 wk of age. Previously, we reported that this treatment effectively inhibited the development of mechanical allodynia in the db/db mouse at 8 wk of age (Cheng et al., 2009). Consistent with its analgesic effects, a two-week duration of systemic anti-NGF treatment significantly decreased PGP9.5, Trk A, SP, and CGRP-positive IENFD in db/db mice at 8 wk of age (Fig. 5). Anti-NGF treatment did not affect these IENFD in db/+ mice (Fig. 5).
Fig. 5. Anti-NGF inhibits the upregulation of PGP9.5 and peptidergic IENF in db/db mice.
A: Db/+ and db/db mice were treated with control IgG or anti-NGF for 2 wk. IENFD were measured for PGP9.5 (A), SP (B), and CGRP(C)-positive fibers. Anti-NGF, but not IgG, significantly inhibited the upregulation of PGP9.5, Trk A, SP, and CGRP-positive IENFD in db/db mice. Anti-NGF had no effect on IENFD of db/+ mice. N = 4, **, p < 0.01.
SB203580 treatment inhibited the upregulation of PGP9.5-positive and peptidergic IENFD in diabetic animals
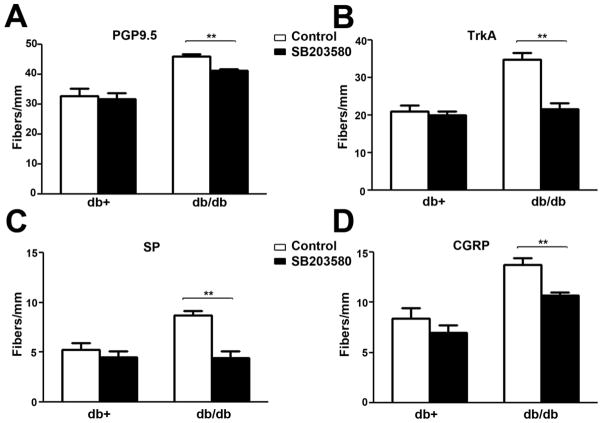
We previously reported NGF-mediated p38 activation is an important mechanism for the development of mechanical allodynia (Cheng et al., 2009). In order to further understand its role, we tested whether p38 could mediate the effects of NGF on increasing peptidergic IENFD. SB203580, a p38 inhibitor, was administered via an intrathecal minipump for 1 wk to animals at 7 wk of age as described previously (Cheng et al., 2009). SB203580 treatment significantly decreased PGP9.5, Trk A, SP, and CGRP-positive IENFD in db/db mice at 8 wk of age (Fig. 6). SB203580 treatment did not affect IENFD in db/+ mice (Fig. 6).
Fig. 6. SB203580 inhibits the upregulation of PGP9.5 and peptidergic IENF in db/db mice.
A: Db/+ and db/db mice were treated with control CSF or SB203580 intrathecally for 2 wk. IENFD were measured for PGP9.5 (A), SP (B), and CGRP(C)-positive fibers. SB203580, but not CSF, significantly inhibited the upregulation of PGP9.5, Trk A, SP, and CGRP-positive IENFD in db/db mice. SB203580 had no effect on IENFD of db/+ mice. N = 4, **, p < 0.01.
Discussion
The results from our current study support the use of peptidergic IENFD measurement for the diagnosis of PDN of type 2 diabetes in clinical practice. As was previously mentioned, PGP9.5 has been established as the best pan-neuronal marker across species to localize IENF (Karanth et al., 1991). However, PGP9.5 IENFD measurement has not been proven to be a useful method for the diagnosis of pain. Similar to the current protocol for human IENFD measurement, PGP9.5 immunohistochemistry has been used to determine IENFD in db/db mice (Polydefkis et al., 2001). We previously reported that decreased IENFD correlated with the decrease of nerve conduction velocities in db/db mice at 24 wk of age (Sullivan et al., 2007). In addition, Wright et al also reported no change of IENFD in db/db mice at 15 wk of age, similar to our findings at 16 wk of age (Wright et al., 2007). In the current study, a significant increase in PGP 9.5-positive IENFD was detected in db/db mice at 8 and 10 wk of age, during the period of mechanical allodynia. This study suggests that early pain phenotypes are associated with increased IENFD in the animal model of type 2 diabetes. This phenomenon has not been previously characterized. Consistent with our current findings, Karanth et al reported increased PGP9.5 IENFD in streptozotocin (STZ) treated rats after 12 wk of diabetes, suggesting similar mechanisms could mediate PDN of type 1 diabetes (Karanth et al., 1990).
In human studies, however, the use of PGP9.5 IENFD in the diagnosis of PDN has been controversial. Properzi et al demonstrated increased PGP9.5 IENFD in PDN patients at the early stage (< 3 years) of type 1 diabetes (Properzi et al., 1993). Similar to our current findings, they reported that PGP9.5 IENFD decreased at later stages of diabetes. Conversely, instead of an increase in IENFD, several recent reports demonstrated, a loss of PGP9.5-positive IENFD in animals and humans with PDN from both type 1 and type 2 diabetes (Holland et al., 1997; Polydefkis et al., 2001; Sorensen et al., 2006a, b). Among these studies, Holland and colleagues correlated the severity of PGP9.5-positive IENFD loss in patients with mixed groups of painful sensory neuropathy including PDN (Holland et al., 1997). Furthermore, Levy and colleagues reported decreased PGP9.5-positive IENFD in patients with mostly type 1 diabetes. However, it was still unclear if this reduction correlated with the development of PDN. In addition, Sorensen and colleagues reported a decrease of PGP9.5-positive IENFD in the distal leg of patients with and without PDN (Sorensen et al., 2006b). The majority of their patients had type 2 diabetes. They concluded that the severity of PGP9.5 IENF loss is associated with neuropathic pain only in individuals with little or no objective signs of neuropathy. They suggested that this reduction correlated with the early stage of PDN. Unlike human skin studies, our current study detected early changes of IENFD in db/db mice during the period of mechanical allodynia. This phenomenon is followed by reduction of IENFD in footpads and sensory loss. Unlike most human studies, where biopsy specimens are collected from patients who have experienced PDN for months, our experimental paradigms can truly correlate pain behavior with the initial IENF changes in footpads. The human skin studies by Sorensen et al only had the ability to reveal the reduction in IENFD at a later stage of the disease. At this later stage, mechanical allodynia could be a result of excessive firing from distal ends of the sensory axons which have withdrawn from the skin. In addition, central sensitization, which develops in the spinal cord dorsal horn, could play an important role at the later stage of PDN in spite of decreased IENFD (Ji and Woolf, 2001). We believe such chronological variables contribute to the many ambiguous results from human IENFD studies. We are currently performing studies to correlate the IENFD from both proximal thigh and distal leg biopsies of patients with type 2 diabetes and PDN. Thus far, we have observed that IENFD of skin from the proximal thigh indeed retain more early pathological changes associated with PDN (data not shown).
Our current findings suggest a greater percentage increase (up to 3 fold) of peptidergic fibers in total PGP9.5-positive IENF in db/db mice during the period of mechanical allodynia. Our data demonstrated a positive correlation between the peptidergic IENFD with PDN at the early stages of DN. Similar studies have not been reported for PDN of type 2 diabetes. Using a STZ model of type 1 diabetes, Karanth et al reported an early increase in CGRP-positive IENF in diabetic rats (Karanth et al., 1990). Unfortunately, it is unclear in this study if the upregulation is associated with pain behaviors. Apart from this inquiry, most other reports demonstrated decreased CGRP- and SP-positive IENF in STZ treated animals (Christianson et al., 2003). Johnson et al demonstrated an early loss of peptidergic IENF in the diabetic mouse following STZ injection with insensate diabetic neuropathy (Johnson et al., 2008). The peptidergic IENF loss occurs as early as 4 wk after STZ injection, without the prior development of pain behaviors. Levy et al also observed reduced CGRP immunoreactive areas in diabetic skin, suggesting that the diminution of CGRP immunopositivity is associated with neuropathy (Levy et al., 1992). However, neither of these reports correlated the loss of CGRP immunoreactivity with PDN. In general, these reports mainly focus on the late phase of DN, not the phase with PDN (Christianson et al., 2003). We believe that the upregulation of peptidergic IENFD could be a phenomenon specific to the PDN period of type 2 diabetes.
Our results demonstrated increased TNF-α-positive IENFD in db/db mice from 8 to 16 wk of age. This finding is consistent with our previous published data that there is a NGF-p38-mediated upregulation of TNF-α in DRG of db/db mice at 8 wk of age (Cheng et al., 2010). However, this increment was not correlated with the diminution of mechanical allodynia at 16 wk of age. Unfortunately, similar results have not been reported in rodents or human skin. Our data suggest that TNF-α might not be a good indicator for PDN, but rather an indicator for the progressive nerve damage from DN. A similar phenomenon was reported in a chronic nerve constriction model by Schaefers and colleagues. After sciatic nerve constriction, increased TNF-α expression was detected in the skin and muscle nerve afferents as well as their associated DRG neurons (Schafers et al., 2003). This upregulation of TNF-α in peripheral nerves is most likely associated with enhanced anterograde axonal transport after nerve injury (Schafers et al., 2002).
Based on our findings, there is no initial loss of IENFD before or during the early stages of PDN. In actuality, increased IENFD are observed. We believe that this phenomenon is most likely based on increased axonal sprouting, rather than regeneration. It is well known that unmyelinated nociceptive fibers reinnervate the skin after denervation, which is similar to the reestablishment of neuromuscular junctions after degeneration in myelinated fibers (Diamond and Foerster, 1992; Nixon et al., 1984). Similar to our model of PDN, Griffin et al detected nerve regeneration or axonal sprouting after nerve injury (Griffin et al., 2010). The current measurement of IENFD only includes axons that penetrate the dermal-epidermal junction. We believe that increased axonal sprouting likely occurs at the dermal plexus, prior to axonal innervation of the epidermis. There is a plethora of evidence that indicates the subepidermal plexus and basal skin layers are sources of neurotrophic factors (Cheng et al., 2009; English et al., 2005). Additionally, our findings from the anti-NGF experiment demonstrate that NGF could contribute to the increase of IENFD during PDN.
We observed that anti-NGF treatment significantly decreased SP and CGRP-positive IENFD. The same experimental paradigm also inhibited the development of mechanical allodynia in the db/db mouse (Cheng et al., 2009), indicating that increased neurotrophism is an important mechanism to mediate PDN in type 2 diabetes. NGF is a promoter for both SP and CGRP expression in cultured DRG neurons. Our data suggests increased NGF action during the initial period of mechanical allodynia could increase the regeneration of Trk A positive IENF which express neuropeptides such as SP and CGRP. In the literature, increased NGF action has been reported to mediate several chronic painful conditions (Pezet and McMahon, 2006). The current data are in agreement with our previous report that revealed increased cutaneous NGF action of db/db mice during mechanical allodynia (Cheng et al., 2009). In addition to our findings, Christianson and colleagues reported that exogenous NGF administration enhanced the cutaneous nerve sprouting and protected against DN, indicating NGF could promote IENF regeneration (Christianson et al., 2003). Additionally, NGF treatment enhances the expression of pain-specific sodium channel expression in peripheral nerves and DRG neurons, including SNS, a tetrodotoxin-resistant (TTX-R) sodium channel (Dib-Hajj et al., 1998). In further support of our conclusions, increased NGF gene expression has been detected in the calf skin of patients with diabetes (Diemel et al., 1999). Taken together, these enhanced NGF actions in DN of type 2 diabetes could be responsible for the increased peptidergic IENFD during the development of PDN.
We previously reported that the NGF-p38 pathway in DRG mediated the development of mechanical allodynia in db/db mice. In our current study, SB203580 inhibited the upregulation of IENFD during the period of mechanical allodynia. In support of our findings, SB203580, but not the ERK pathway blocker U0126, inhibited the ability of PC12m3 and PC12m32 cells to induce neurite outgrowth in response to osmotic shock (Kano et al., 2007). This p38-mediated neurite outgrowth requires CREB and paxillin phosphorylation (Huang et al., 2004; Kano et al., 2007). The current findings suggest similar mechanisms could also mediate increased IENFD during mechanical allodynia in db/db mice.
Finally, our current results suggest increased regeneration of peptidergic nerve fibers in the skin during the period of mechanical allodynia. Our findings suggest that there is increased innervation in skin from large-sized DRG neurons in db/db mice. Multiple studies have suggested NGF-related nerve regeneration in animal models of diabetic neuropathy (Yasuda et al., 2003). In our previous report, we demonstrated the conversion of large-sized DRG neurons to NGF-β and SP-positive phenotypes during the period of mechanical allodynia (Cheng et al., 2009). These large-sized DRG neurons normally extend myelinated A fibers, which do not innervate skin nor mediate nociception. Combining our current and previous findings, we propose a hypothesis that there could be increased skin innervation from those large DRG neurons to enhance mechanical sensitivity. This hypothesis is supported by Christianson and colleagues who reported that NGF increases myelinated cutaneous innervation in diabetic animals and restores sensory dysfunction from DN (Christianson et al., 2007).
Conclusions
In conclusion, the upregulation of peptidergic IENFD via NGF-p38 signaling is observed in an animal model of type 2 diabetes during PDN. This phenomenon is associated with and better correlated with the development of mechanical allodynia than the changes in PGP9.5-positive IENFD. These findings not only support the measurement of peptidergic IENFD for the diagnosis of PDN in type 2 diabetes, but also identify the NGF-p38 signaling pathway as a possible pharmacological target for treating this devastating condition.
Highlights.
We found increased peptidergic nerve fiber densities in diabetic skin.
Anti-nerve growth factor inhibited the increased peptidergic nerve fiber densities.
SB203580 inhibited the upregulation of peptidergic nerve fiber densities.
Nerve growth factor /p38 signaling mediates increased nerve fibers in diabetic skin.
Acknowledgments
The authors thank Carey Backus and Sang Su Oh for technical assistance, and Jennifer Cheng for help in the preparation of this manuscript. This work utilized the Morphology and Image Analysis Core of the Michigan Diabetes Research and Training Center funded by NIH (5P60 DK20572) from the National Institute of Diabetes & Digestive & Kidney Diseases.
This study is supported by National Institutes of Health [UO1-DK60994 (ELF); 1K08NS061039 (HTC)] and the Juvenile Diabetes Research Foundation Center for the Study of Complications in Diabetes.
Abbreviations
- CGRP
calcitonin gene related peptide
- CSF
cerebrospinal fluid
- DMSO
dimethyl sulfoxide
- IFG
impaired fasting glucose
- IGT
impaired glucose tolerance
- IENFD
intraepidermal nerve fiber densities
- NGF
nerve growth factor
- PDN
painful diabetic neuropathy
- PBS
phosphate buffered saline
- PGP
protein gene product
- SP
substance P
- STZ
streptozotocin
- Trk
tropomyosin-receptor-kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28:956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- Catalan V, Gomez-Ambrosi J, Ramirez B, Rotellar F, Pastor C, Silva C, Rodriguez A, Gil MJ, Cienfuegos JA, Fruhbeck G. Proinflammatory cytokines in obesity: impact of type 2 diabetes mellitus and gastric bypass. Obes Surg. 2007;17:1464–1474. doi: 10.1007/s11695-008-9424-z. [DOI] [PubMed] [Google Scholar]
- Cheng HT, Dauch JR, Hayes JM, Hong Y, Feldman EL. Nerve growth factor mediates mechanical allodynia in a mouse model of type 2 diabetes. J Neuropathol Exp Neurol. 2009;68:1229–1243. doi: 10.1097/NEN.0b013e3181bef710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HT, Dauch JR, Oh SS, Hayes JM, Hong Y, Feldman EL. p38 mediates mechanical allodynia in a mouse model of type 2 diabetes. Mol Pain. 2010;6:28. doi: 10.1186/1744-8069-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JA, Riekhof JT, Wright DE. Restorative effects of neurotrophin treatment on diabetes-induced cutaneous axon loss in mice. Exp Neurol. 2003;179:188–199. doi: 10.1016/s0014-4886(02)00017-1. [DOI] [PubMed] [Google Scholar]
- Christianson JA, Ryals JM, Johnson MS, Dobrowsky RT, Wright DE. Neurotrophic modulation of myelinated cutaneous innervation and mechanical sensory loss in diabetic mice. Neuroscience. 2007;145:303–313. doi: 10.1016/j.neuroscience.2006.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J, Foerster A. Recovery of sensory function in skin deprived of its innervation by lesion of the peripheral nerve. Exp Neurol. 1992;115:100–103. doi: 10.1016/0014-4886(92)90229-j. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj SD, Black JA, Cummins TR, Kenney AM, Kocsis JD, Waxman SG. Rescue of alpha-SNS sodium channel expression in small dorsal root ganglion neurons after axotomy by nerve growth factor in vivo. J Neurophysiol. 1998;79:2668–2676. doi: 10.1152/jn.1998.79.5.2668. [DOI] [PubMed] [Google Scholar]
- Diemel LT, Cai F, Anand P, Warner G, Kopelman PG, Fernyhough P, Tomlinson DR. Increased nerve growth factor mRNA in lateral calf skin biopsies from diabetic patients. Diabet Med. 1999;16:113–118. doi: 10.1046/j.1464-5491.1999.00035.x. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Jensen MP, Gammaitoni AR, Olaleye DO, Galer BS. Symptom profiles differ in patients with neuropathic versus non-neuropathic pain. JPain. 2007;8:118–126. doi: 10.1016/j.jpain.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- English AW, Meador W, Carrasco DI. Neurotrophin-4/5 is required for the early growth of regenerating axons in peripheral nerves. Eur J Neurosci. 2005;21:2624–2634. doi: 10.1111/j.1460-9568.2005.04124.x. [DOI] [PubMed] [Google Scholar]
- Feldman EL, Stevens MJ, Russell JW, Peltier A, Inzucchi S, Porte JD, Sherwin RS, Baron A. The Diabetes Mellitus Manual. 6. McGraw-Hill; 2005. Somatosensory neuropathy; pp. 366–384. [Google Scholar]
- Galer BS, Gianas A, Jensen MP. Painful diabetic polyneuropathy: epidemiology, pain description, and quality of life. Diabetes Res Clin Pract. 2000;47:123–128. doi: 10.1016/s0168-8227(99)00112-6. [DOI] [PubMed] [Google Scholar]
- Griffin JW, Pan B, Polley MA, Hoffman PN, Farah MH. Measuring nerve regeneration in the mouse. Exp Neurol. 2010;223:60–71. doi: 10.1016/j.expneurol.2009.12.033. [DOI] [PubMed] [Google Scholar]
- Holland NR, Stocks A, Hauer P, Cornblath DR, Griffin JW, McArthur JC. Intraepidermal nerve fiber density in patients with painful sensory neuropathy. Neurology. 1997;48:708–711. doi: 10.1212/wnl.48.3.708. [DOI] [PubMed] [Google Scholar]
- Huang C, Borchers CH, Schaller MD, Jacobson K. Phosphorylation of paxillin by p38MAPK is involved in the neurite extension of PC-12 cells. J Cell Biol. 2004;164:593–602. doi: 10.1083/jcb.200307081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MP, Dworkin RH, Gammaitoni AR, Olaleye DO, Oleka N, Galer BS. Do pain qualities and spatial characteristics make independent contributions to interference with physical and emotional functioning? J Pain. 2006;7:644–653. doi: 10.1016/j.jpain.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Ji RR, Woolf CJ. Neuronal plasticity and signal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological pain. Neurobiol Dis. 2001;8:1–10. doi: 10.1006/nbdi.2000.0360. [DOI] [PubMed] [Google Scholar]
- Johnson MS, Ryals JM, Wright DE. Early loss of peptidergic intraepidermal nerve fibers in an STZ-induced mouse model of insensate diabetic neuropathy. Pain. 2008;140:35–47. doi: 10.1016/j.pain.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano Y, Nohno T, Shimada K, Nakagiri S, Hiragami F, Kawamura K, Motoda H, Numata K, Murai H, Koike Y, Inoue S, Miyamoto K. Osmotic shock-induced neurite extension via activation of p38 mitogen-activated protein kinase and CREB. Brain Res. 2007;1154:1–7. doi: 10.1016/j.brainres.2007.03.087. [DOI] [PubMed] [Google Scholar]
- Karanth SS, Springall DR, Francavilla S, Mirrlees DJ, Polak JM. Early increase in CGRP- and VIP-immunoreactive nerves in the skin of streptozotocin-induced diabetic rats. Histochemistry. 1990;94:659–666. doi: 10.1007/BF00271994. [DOI] [PubMed] [Google Scholar]
- Karanth SS, Springall DR, Kuhn DM, Levene MM, Polak JM. An immunocytochemical study of cutaneous innervation and the distribution of neuropeptides and protein gene product 9.5 in man and commonly employed laboratory animals. Am J Anat. 1991;191:369–383. doi: 10.1002/aja.1001910404. [DOI] [PubMed] [Google Scholar]
- Kennedy WR, Wendelschafer-Crabb G, Johnson T. Quantitation of epidermal nerves in diabetic neuropathy. Neurology. 1996;47:1042–1048. doi: 10.1212/wnl.47.4.1042. [DOI] [PubMed] [Google Scholar]
- Lauria G, Cornblath DR, Johansson O, McArthur JC, Mellgren SI, Nolano M, Rosenberg N, Sommer C. EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur J Neurol. 2005;12:747–758. doi: 10.1111/j.1468-1331.2005.01260.x. [DOI] [PubMed] [Google Scholar]
- Levy DM, Terenghi G, Gu XH, Abraham RR, Springall DR, Polak JM. Immunohistochemical measurements of nerves and neuropeptides in diabetic skin: relationship to tests of neurological function. Diabetologia. 1992;35:889–897. doi: 10.1007/BF00399938. [DOI] [PubMed] [Google Scholar]
- Nixon BJ, Doucette R, Jackson PC, Diamond J. Impulse activity evokes precocious sprouting of nociceptive nerves into denervated skin. Somatosens Res. 1984;2:97–126. doi: 10.1080/07367244.1984.11800553. [DOI] [PubMed] [Google Scholar]
- Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- Polydefkis M, Hauer P, Griffin JW, McArthur JC. Skin biopsy as a tool to assess distal small fiber innervation in diabetic neuropathy. Diabetes Technol Ther. 2001;3:23–28. doi: 10.1089/152091501750219994. [DOI] [PubMed] [Google Scholar]
- Properzi G, Francavilla S, Poccia G, Aloisi P, Gu XH, Terenghi G, Polak JM. Early increase precedes a depletion of VIP and PGP-9.5 in the skin of insulin-dependent diabetics--correlation between quantitative immunohistochemistry and clinical assessment of peripheral neuropathy. J Pathol. 1993;169:269–277. doi: 10.1002/path.1711690215. [DOI] [PubMed] [Google Scholar]
- Schafers M, Geis C, Brors D, Yaksh TL, Sommer C. Anterograde transport of tumor necrosis factor-alpha in the intact and injured rat sciatic nerve. J Neurosci. 2002;22:536–545. doi: 10.1523/JNEUROSCI.22-02-00536.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafers M, Geis C, Svensson CI, Luo ZD, Sommer C. Selective increase of tumour necrosis factor-alpha in injured and spared myelinated primary afferents after chronic constrictive injury of rat sciatic nerve. Eur J Neurosci. 2003;17:791–804. doi: 10.1046/j.1460-9568.2003.02504.x. [DOI] [PubMed] [Google Scholar]
- Shun CT, Chang YC, Wu HP, Hsieh SC, Lin WM, Lin YH, Tai TY, Hsieh ST. Skin denervation in type 2 diabetes: correlations with diabetic duration and functional impairments. Brain. 2004;127:1593–1605. doi: 10.1093/brain/awh180. [DOI] [PubMed] [Google Scholar]
- Sorensen L, Molyneaux L, Yue DK. The level of small nerve fiber dysfunction does not predict pain in diabetic Neuropathy: a study using quantitative sensory testing. Clin J Pain. 2006a;22:261–265. doi: 10.1097/01.ajp.0000169670.47653.fb. [DOI] [PubMed] [Google Scholar]
- Sorensen L, Molyneaux L, Yue DK. The relationship among pain, sensory loss, and small nerve fibers in diabetes. Diabetes Care. 2006b;29:883–887. doi: 10.2337/diacare.29.04.06.dc05-2180. [DOI] [PubMed] [Google Scholar]
- Sullivan KA, Hayes JM, Wiggin TD, Backus C, Su OhS, Lentz SI, Brosius F, 3rd, Feldman EL. Mouse models of diabetic neuropathy. Neurobiol Dis. 2007;28:276–285. doi: 10.1016/j.nbd.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology. 2003;60:108–111. doi: 10.1212/wnl.60.1.108. [DOI] [PubMed] [Google Scholar]
- Wild KD, Bian D, Zhu D, Davis J, Bannon AW, Zhang TJ, Louis JC. Antibodies to nerve growth factor reverse established tactile allodynia in rodent models of neuropathic pain without tolerance. J Pharmacol Exp Ther. 2007;322:282–287. doi: 10.1124/jpet.106.116236. [DOI] [PubMed] [Google Scholar]
- Wright DE, Johnson MS, Arnett MG, Smittkamp SE, Ryals JM. Selective changes in nocifensive behavior despite normal cutaneous axon innervation in leptin receptor-null mutant (db/db) mice. J Peripher Nerv Syst. 2007;12:250–261. doi: 10.1111/j.1529-8027.2007.00144.x. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Terada M, Maeda K, Kogawa S, Sanada M, Haneda M, Kashiwagi A, Kikkawa R. Diabetic neuropathy and nerve regeneration. Prog Neurobiol. 2003;69:229–285. doi: 10.1016/s0301-0082(03)00034-0. [DOI] [PubMed] [Google Scholar]
- Zandecki M, Vanden Berghe P, Depoortere I, Geboes K, Peeters T, Janssens J, Tack J. Characterization of myenteric neuropathy in the jejunum of spontaneously diabetic BB-rats. Neurogastroenterol Motil. 2008;20:818–828. doi: 10.1111/j.1365-2982.2008.01091.x. [DOI] [PubMed] [Google Scholar]
- Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A. Neuropathic pain in diabetes, prediabetes and normal glucose tolerance: the MONICA/KORA Augsburg Surveys S2 and S3. Pain Med. 2009;10:393–400. doi: 10.1111/j.1526-4637.2008.00555.x. [DOI] [PubMed] [Google Scholar]