Abstract
Mitochondrial dysfunction plays an important role in the pathogenesis of neurodegenerative diseases, numerous other disease states and senescence. The ability to monitor reactive oxygen species (ROS) within tissues and over time in animal model systems is of significant research value. Recently, redox-sensitive fluorescent proteins have been developed. Transgenic flies expressing genetically encoded redox-sensitive GFPs (roGFPs) targeted to the mitochondria function as a useful in vivo assay of mitochondrial dysfunction and ROS. We have generated transgenic flies expressing a mitochondrial-targeted roGFP2, demonstrated its responsiveness to redox changes in cultured cells and in vivo and utilized this protein to discover elevated ROS as a contributor to pathogenesis in a characterized neurodegeneration mutant and in a model of mitochondrial encephalomyopathy. These studies identify the role of ROS in pathogenesis associated with mitochondrial disease and demonstrate the utility of genetically encoded redox sensors in Drosophila.
Keywords: Drosophila melanogaster, ATP6, ATPalpha, mitochondrial dysfunction, ROS, redox sensor
Introduction
An increase in the level of reactive oxygen species (ROS) is known to contribute to the pathogenesis of several neurodegenerative diseases, including Alzheimer’s, Parkinson’s and Huntington’s diseases, as well Amyotrophic lateral sclerosis and premature aging (Emerit et al., 2004; Halliwell, 2006). Natural antioxidant systems serve as a defense against oxidative stress. When the production of ROS overwhelms antioxidant mechanisms damage to cellular macromolecules can occur resulting in accumulation of unfolded and misfolded proteins, membrane instability and DNA mutations. All of these are implicated in aging/senescence and in various pathological processes resulting from neurodegenerative diseases. In addition to direct oxidation, ROS can also activate numerous signaling pathways that increase apoptosis (Allen and Tresini, 2000; Li et al., 2008; Temkin and Karin, 2007).
Since ROS are involved in a broad range of physiological and pathological processes, the measurement of ROS is critical for the investigation of the occurrence, development and outcome of diseases. Thus, numerous assays have been developed to measure ROS, each having distinct advantages and limitations. Among the most promising are genetically encoded redox sensors (Hanson et al., 2004), which have recently proven functional in mammalian cells (Dooley et al., 2004), Arabidopsis (Maughan et al., 2010; Meyer et al., 2007; Rosenwasser et al., 2010; Schwarzlander et al., 2009), and in mice (Guzman et al., 2010). Redox sensors were engineered with pairs of cysteine residues on adjacent surfaces of the fluorophore barrel structure such that excitation ratios are affected by the formation of disulfide linkages; therefore, the population of expressed roGFPs serve as a ratiometric probe of the redox status (Cannon and Remington, 2008; Dooley et al., 2004; Hanson et al., 2004). Here, we report the generation and characterization of transgenic flies expressing a genetically expressed roGFP probe to measure ROS. The principal advantages of using such an approach are: 1) the probe is genetically encoded, 2) ratiometric imaging allows one to assess redox status accurately regardless of the absolute levels of probe concentration due to expression, photobleaching or variation of tissue thickness, and 3) this method allows real time detection of redox status in different live cells and animal tissues without the permeation and preincubation of exogenous probes.
Studies of aging and various disease models in Drosophila would benefit from a genetically encoded means of measuring ROS within various tissues over a range of animal ages. We have generated roGFP transgenic flies bearing a mitochondrial or matrix target signal (MTS) fused to roGFP2 expressed in mitochondria (UAS-MTSroGFP2) and a cytosolic version (UAS-roGFPR12). Expression is conditional upon GAL4, allowing expression in various tissues using available GAL4 strains. We have used MTSroGFP2 to examine changes in redox status over time in several mutant and control animals. Our results demonstrate the role of progressive ROS accumulation in the pathogenesis of neurodegeneration and mitochondrial encephalomyopathy and document an increase in mitochondrial ROS observed with normal senescence of wild type animals. These data demonstrate that MTSroGFP2 is an excellent indicator for the redox status within Drosophila.
Materials and methods
Drosophila strains, locomotor function and pharmacology
For experiments all fly stocks were maintained on standard cornmeal–molasses agar media at 25°C. For drug treatment, groups of ~15–20 newly eclosed flies were placed in a vial containing a half circle filter paper containing the drug in DMSO vehicle. Vials and drug were changed every other day. The ATP61 and ATPalphaDTS1 strains have previously been reported (Ashmore et al., 2009; Celotto et al., 2006a; Palladino et al., 2003). Locomotor function stress-sensitivity (a.k.a. bang-sensitivity) was assayed as previously(Celotto et al., 2006a; Celotto et al., 2006b; Hrizo and Palladino, 2010). The elav-Gal4 strain is from the Bloomington Drosophila stock center. Transgenes were made using the pUASTattB construct (Bischof et al., 2007). pUASTattB-MTSroGFP2 and pUASTattB-roGFPR12 transgenic constructs were generated using Bgl II and Not I from MTSroGFP2 and roGFPR12 clones, respectively, kindly provided by James Remington (Hanson et al., 2004). Transgenic flies were generated using standard ΦC31 integrase transgenesis with insertion into the attP18 site. pUASTattB-roGFPR12 transgene fluorescence was dim in neurons and was not used further in these studies.
Primary Drosophila neuronal culture
Primary neural cultures were prepared as described by O’Dowd with minor modifications (Sicaeros et al., 2007). Briefly, heads were removed from animals at 55–78h after pupation and were placed in sterile dissecting saline containing 137 mM NaCl, 5.4 mM KCl, 0.17 mM NaH2PO4, 0.22 mM KH2PO4, 33.3 mM glucose, 43.8 mM sucrose, and 9.9 mM HEPES, pH 7.4. Central brain regions were obtained and incubated in dissecting saline containing 50 U/ml papain activated by L-cysteine (1.32 mM) for 15 min at room temperature. Tissues were washed with dissecting saline and Drosophila-defined culture medium composed of Ham’s F-12 DMEM (high glucose; Irvine Scientific, Santa Ana, CA) supplemented with 1 mg/ml sodium bicarbonate, 20mM HEPES, 100 μM putrescine, 30 nM sodium selenite, 20 ng/ml progesterone, 50 μg/ml insulin, 100 μg/ml transferrin, and 1 μg/ml of 20-hydroxyecdysone. Each culture was prepared from a single brain transferred to a 5 μl drop of Drosophila-defined culture medium on a ConA/laminin-coated glass coverslip. The tissue was mechanically dissociated with dissecting needles and repeated pipetting, and the cells were allowed to settle for 30 min. Culture dishes were flooded with 1.5 ml of Drosophila-defined culture medium and maintained in a 23°C humidified 5% CO2 incubator.
In vitro characterization of MTSroGFP2 in cultured neurons
The UAS-MTSroGFP2 transgene was expressed in Drosophila brains using the pan neural driver elav-GAL4. The progeny pupa was used to generate primary neurons cultured on 10mm coverslips. On day ~4–5, coverslips were transferred to a perfusion chamber (DH35, Warner Instruments) and positioned on the movable stage of an Olympus BX50W1 epifluorescence microscope. The cells were perfused with PBS and the baseline was measured for 4 minutes before 30 minutes application of oxidizing agent H2O2 (at 1mM, 10mM, or 50mM concentrations). After H2O2 treatments, the reducing agent dithiothreitol (DTT) at 10mM was applied for 10 minutes. Images were acquired using a 60X objective at 535nm emission wavelength every minute using Hamamatsu C4742-95 with excitation wavelengths (Polychrome V) at 405nm and 488 nm for oxidized and reduced roGFP2, respectively. Analysis was performed using Simple PCI6 software (Compix Inc.) and 405nm/488nm fluorescent ratios were calculated.
In vivo adult brain redox status analysis
elav-Gal4; UAS-MTSroGFP animals were generated with and without the ATP6 or ATPalpha mutations. Brains were dissected in PBS from mutant and control animals on day 3, 15 and 30. After dissection, brains were placed in mounting medium (Victor, H-1000) on a thin cover slip attached to a petri dish. An Olympus IX 81 inverted laser scanning Fluoview 1000 confocal microscope (Olympus, Tokyo, Japan) was used for imaging. Images were acquired by using a BA 510–540 emission filter for following excitation at 405 and 488 nm. The UPLSAPO 20X objective (NA 0.75, WD 0.6mm, and cover glass thickness 0.17) and Z-scan with a 10um step size were used to obtain whole brain fluorescence. At least 5 brains were imaged for each group and 4 equal sized images of neuropil area of every brain were analyzed. The 405nm/488nm fluorescence was obtained using FV10-ASW2.0 software and ImageJ (NIH) was used to subtract background and perform image analyses. Statistical analysis was performed by student’s t-test.
MTSroGFP localization
Primary neural cultures were attached to glass bottom petri dishes and examined on day 5. MitoTracker Red (Invitrogen, M7521) 1 mM stock solution was diluted to the final working concentration 250 nM in neuronal culture medium DDM2. Prewarmed (23°C) staining solution containing MitoTracker Red probe was added to the culture, incubated for 30 minutes under growth conditions, cells were washed 3 times with prewarmed PBS and mounted in medium (Victor, H-1000). The cells were imaged on an Olympus IX 81 inverted laser scanning Fluoview 1000 confocal microscope (Olympus, Tokyo, Japan). The Plan Apo 60X objective (NA 1.42, WD 0.15 mm) and zoom 5.0X were used to obtain MTSroGFP2 and MitoTracker Red fluroecence images of whole neurons using 488nm excitation laser with 510–540 emission filter and 543 nm excitation laser with 555–655 emission filter, respectively.
Results
Expression of roGFP in Drosophila neurons
Redox sensing GFPs have previously been described including the roGFP2 (C48S/T65S/S147C/Q204C) variant (Cannon and Remington, 2008; Dooley et al., 2004; Hanson et al., 2004). Owing to the T65S substitution, roGFP2 has a much larger dynamic range and we selected this variant to target to the mitochondria in Drosophila using the pyruvate dehydrogenase MTS (mitochondria or matrix targeting signal), similar to previously described studies (Hanson et al., 2004). We generated UAS-attB transgenic flies expressing MTSroGFP2 in a GAL4-dependent manner.
Using elav-Gal4, we expressed MTSroGFP2 and examined fluorescence in cultured primary neurons. MTSroGFP2 gave bright punctate expression consistent with mitochondrial localization. To examine the specificity of the localization we used mitotracker red to examine colocalization, which was significant (Supplemental Figure 1). These data suggest that the elav-Gal4; UAS-MTSroGFP2 will be suitable to measure neural mitochondrial ROS.
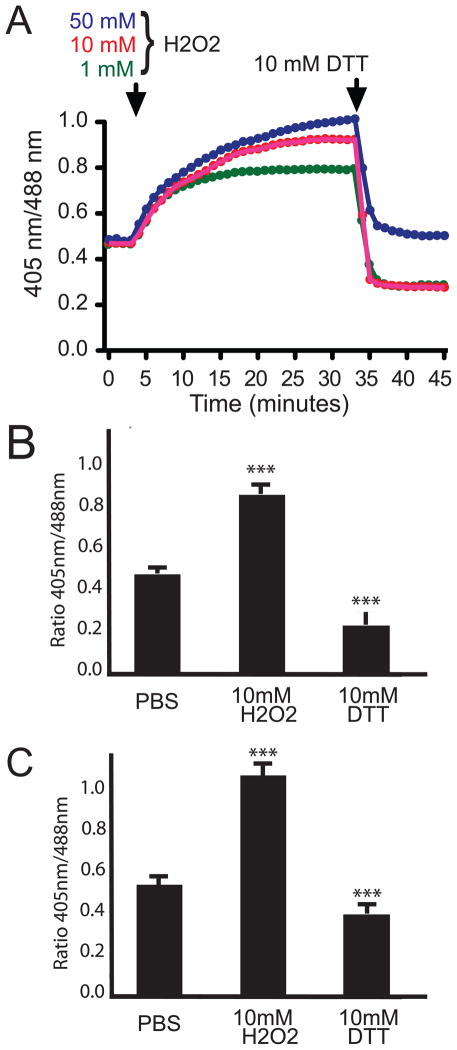
To ensure the MTSroGFP2 is dynamically reporting the redox status within Drosophila neurons, we performed pilot experiments using live cell imagining and pharmacologic treatments to alter cellular redox state. We used H2O2 followed by DTT to cause changes in redox state. The data demonstrate that MTSroGFP2 was responsive to extracellular H2O2 in a dose dependent manner (Figure 1A, 1B). The observed change in redox status was readily reversible with 10 mM DTT treatment. We also examined the responsiveness to redox changes using confocal microscopy and similar results were obtained (Figure 1C). These data suggest that MTSroGFP2 will likely function well as a redox sensor within Drosophila tissues.
Figure 1. Dynamic change in redox-dependent fluorescence of MTSroGFP2.
A) Representative time course of redox changes in primary neurons after the addition of H2O2 at indicated concentrations and then 10mM DTT. The increase in oxidative status observed was dose-responsive and immediately reversible upon DTT treatment. B & C) Responsiveness of MTSroGFP2 expressed within primary neurons to redox changes imaged via epifluorescence (B) or confocal microscopy (C). *** indicates a significant change (Student’s t-test, p<0.001) from base line (PBS). For DTT treatment significance is compared to H2O2 levels.
Validating altered redox status in mitochondria in vivo
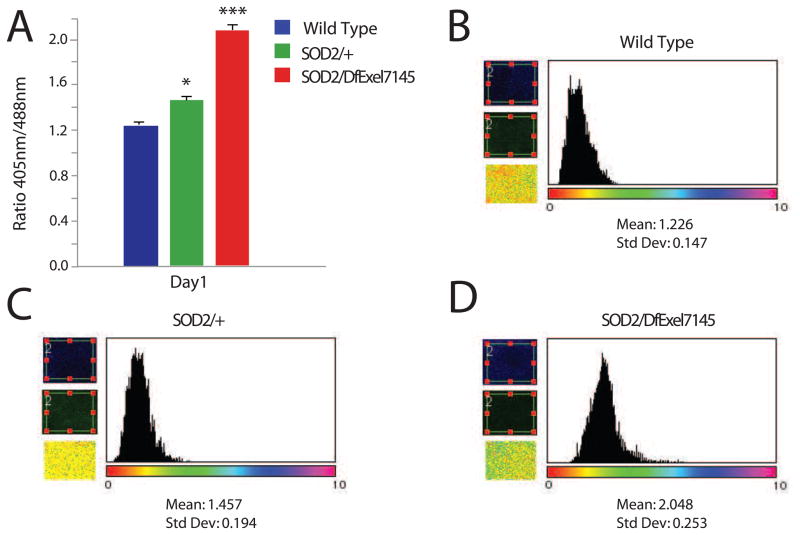
To validate whether MTSroGFP2 was responsive to physiologically relevant mitochondrial ROS associated with dysfunction/disease states in vivo we examined the redox status in brains from mutants of a well-characterized gene known to encode MnSOD/SOD2 in Drosophila (Belton et al., 2006; Duttaroy et al., 2003; Kirby et al., 2002; Martin et al., 2009; Palladino et al., 2002; Paul et al., 2007a). In young SOD2 mutants a modest elevation in redox status is observed in heterozygotes and a marked increase is observed in trans-heterozygous animals (mutants with an established SOD2 deficiency), both compared to age-matched wild type controls (Figure 2).
Figure 2. Increased redox status in SOD2 mutant flies.
MTSroGFP2 fluorescence was examined in brains of SOD2/DfExl7145 mutants, heterozygotes (SOD2/+) and wild type controls. A) The SOD2 mutant flies exhibited an elevated redox status from age-matched controls (Student’s T test, * is p<0.05, *** is p<0.001, n= 5 for each genotype). B-D) Ratio image calculations and histograms were obtained using ImageJ for each genotype indicated. Representative histograms are provided.
Altered redox status in a neurodegenerative fly mutant
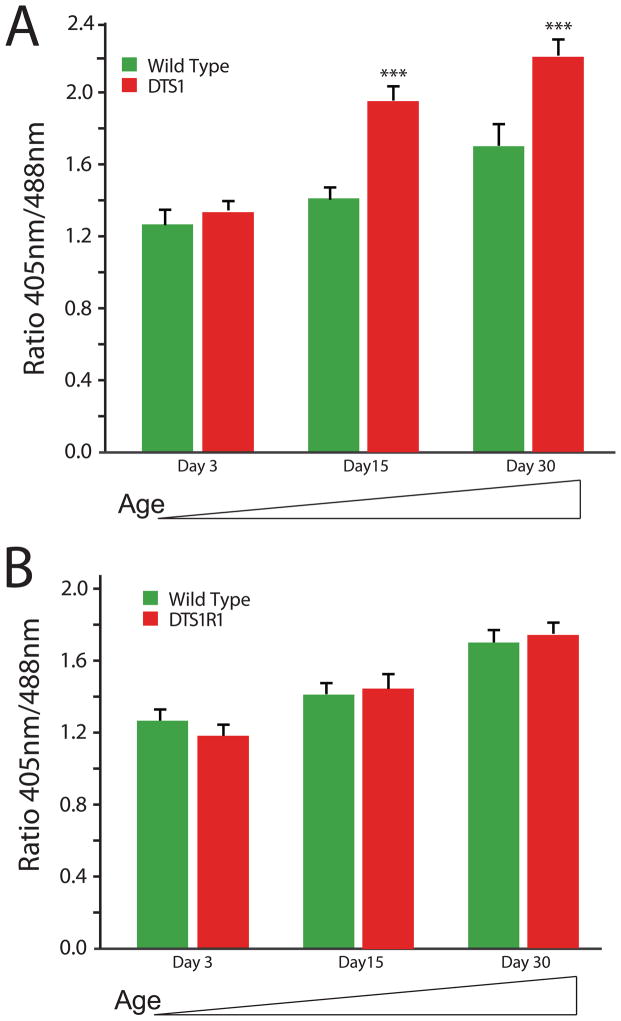
Specific mutations in the ATPalpha gene have previously been shown to result in progressive neuropathology. ATPalpha encodes the catalytic subunit of the Na+, K+ ATPase and specific dominant gain-of-function mutants exhibit reduced longevity and severe progressive neuropathogy (Ashmore et al., 2009; Fergestad et al., 2006; Palladino et al., 2003). We hypothesized that increased ROS might contribute to pathogenesis in ATPalphaDTS1 and that ROS may be increased in an age-dependent manner. We examined the redox status of ATPalphaDTS1 and age-matched control brains as a function of age using the genetically encoded MTSroGFP2. There was no difference in redox status in young animals; however, in an age-dependent manner, mitochondrial ROS was significantly elevated in ATPalphaDTS1 animals from that of wild type animals (Figure 3A, Supplemental Figure 2A). Importantly, there was a significant and detectable increase in redox status observed over time in wild type animals.
Figure 3. Progressive redox status in aged ATPalpha mutant flies.
A) Both wild type and ATPalphaDTS1 mutant flies exhibit an age-dependent increase in neural ROS as measured using MTSroGFP2. At day 3, there is no difference between mutant and control brains (Student’s t-test, P>0.05). At day 15 and day 30 the ROS levels are significant increased in ATPalphaDTS1 over age-matched control animals P<0.001(***). Wild type at day 30 are also increased from day 3 wild type (p<0.05). B) Wild type and ATPalphaDTS1R1 mutant flies exhibit an age-dependent increase in neural ROS as measured using MTSroGFP2. At all time points examined there is no difference between mutant and control brains (Student’s t-test, P>0.05). Wild type at day 30 are also increased from day 3 wild type (p<0.05).
Genetic revertants of ATPalphaDTS1 have been isolated and extensively characterized (Ashmore et al., 2009; Fergestad et al., 2006; Palladino et al., 2003; Paul et al., 2007b). The first such revertant, ATPalphaDTS1R1, has been the focus of considerable study and the basis of the reversion is known to be a 4 base pair deletion causing a premature termination codon that prevents expression of the protein (Ashmore et al., 2009; Palladino et al., 2003). This mutation reverts the dominant gain-of-function aspects of ATPalphaDTS1, exhibits a striking increase in longevity and is an ideal control for the ATPalphaDTS1 mutant. Thus, we asked whether ATPalphaDTS1R1 exhibited altered ROS using MTSroGFP2 fluorescence as an assay. Specifically, we hypothesized that ROS would be normal in ATPalphaDTS1R1 animals consistent with reversion of the gain-of-function pathogenesis of ATPalphaDTS1. Alternatively, it is possible that ROS would be significantly lower in ATPalphaDTS1R1 animals than wild type, in part explaining the robust increase in longevity. Our results show that ROS progressively increases with time as is seen in wild type controls, is not elevated dramatically as is the case for ATPalphaDTS1, and that ROS is not significantly decreased (Figure 3B, Supplemental Figure 2B). These data further demonstrate the utility of a genetically encoded redox sensor in the fruit fly.
Altered redox status in a fly model of mitochondrial encephalomyopathy
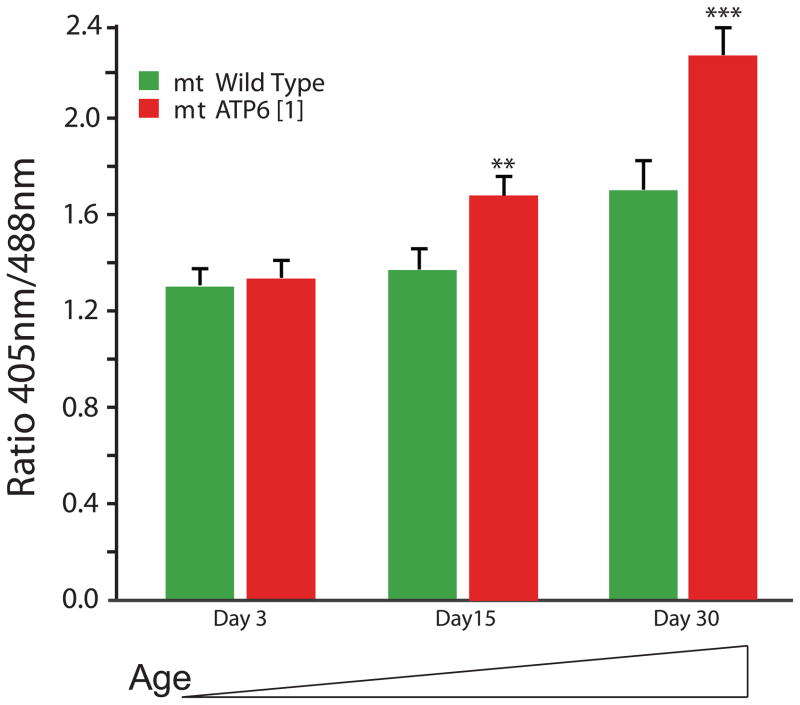
An endogenous mitochondrial mutation affecting ATP6, encoding a subunit of complex V, has been shown to model mitochondrial encephalomyopathy (Celotto et al., 2006a; Palladino, 2010). We hypothesized that increased mitochondrial ROS may be instrumental in pathogenesis of ATP61 and may contribute to the progressive onset of disease phenotypes including locomotor impairment, reduced longevity or myodegeneration. To investigate this hypothesis we examined the redox status of ATP61 and control brains over a time course using genetically encoded MTSroGFP2. Consistent with previous data (Figure 3A, 3B), control animals exhibited a significant increase in ROS with age (Figure 4). The redox status of ATP61 mitochondria were not significantly changed in young animals; however, in aged animals (day 15 and 30) the redox status was significantly increased (Figure 4, Supplemental Figure 2C). ROS appeared elevated at times coinciding with the onset of phenotypes in ATP61 mutants, suggesting that ROS may contribute significantly to pathogenesis.
Figure 4. Progressive altered redox status in aged ATP61 mutant flies.
With age (day 15 and 30) ATP61 mutants exhibit increased ROS compared to age-matched control animals that was not evident in young mutants. ** is p <0.01 and *** is p <0.001, Student’s t-test. Wild type at day 30 are also increased from day 3 wild type (Student’s t-test, p<0.05).
Mitochondrial-targeted antioxidants improve ATP61 pathogenesis
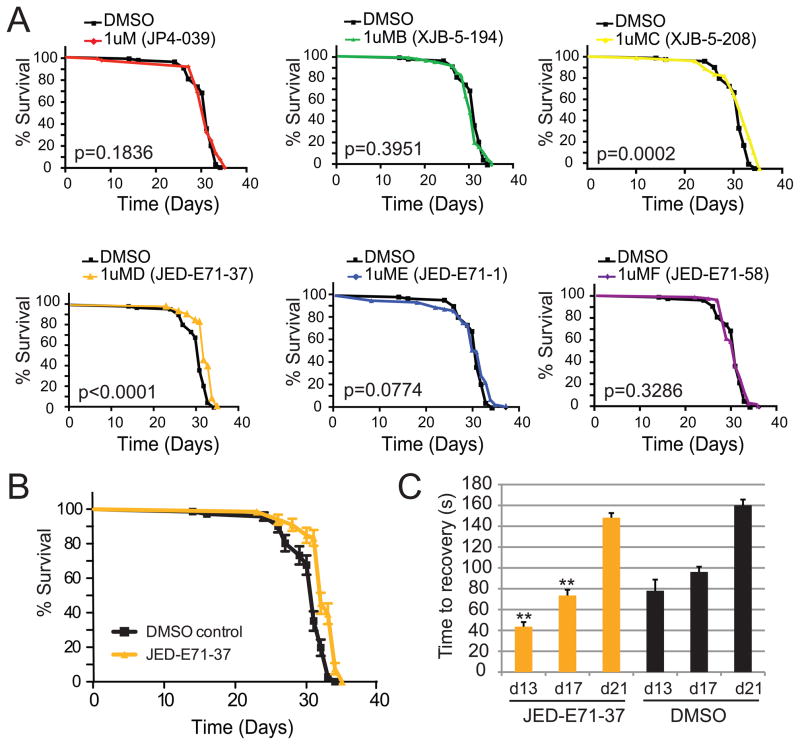
Mitochondria are a significant source of oxidative damage. Mitochondrial dysfunction is believed to trigger increased ROS production, contributing further to pathogenesis in mitochondrial encephalomyopathy and progressive neurological diseases (Addabbo et al., 2009). To determine the extent to which ROS contributes to pathogenesis, we screened a series of well-characterized mitochondrial-targeted ROS scavengers (Frantz and Wipf, 2010; Hoye et al., 2008; Jiang et al., 2007; Kagan et al., 2009; Macias et al., 2007; Wipf et al., 2005) for those that improve ATP61 phenotypes. Initially we examined five ROS scavengers and a control XJB-5-194 with a related backbone but lacking the nitroxide ROS scavenging subunit for their ability to improve longevity in ATP61. We tested all six compounds in parallel in a blinded manner at 0.1 μM, 1 μM and 10 μM concentrations using identical delivery methods (Supplemental Figure 3). Only JED-E71-37 showed a significant and reproducible increase in longevity, with 1 μM providing the most significant result (Figure 5A). Importantly, the control compound XJB-5-194 did not improve longevity. The experiment was independently replicated with 1 μM JED-E71-37 (Figure 5B). XJB-5-208 significantly altered longevity by log-rank analysis but did not significantly improve median lifespan and thus was not pursued further. We thus asked whether 1 μM JED-E71-37 would effectively improve locomotor function in ATP61 mutants. Consistent with the modest improvement in longevity, we observed a significant improvement in locomotor function early in pathogenesis (day 13 and 17) but not later in pathogenesis (day 21). Importantly, ROS scavenger treatment did not fully ameliorate locomotor function at any time point, as wild type animals at all three time points did not paralyze in response to mechanical stress (data not shown).
Figure 5. Mitochondrial targeted antioxidants improve ATP61 animals.
A) Five mitochondrial-targeted ROS scavengers and a control (XJB-5-194) were tested for efficacy at improving longevity in ATP61 animals. Only JED-E71-37 significantly improved longevity. B) Treatment with JED-E71-37 results in a statistically significant and reproducible increase in lifespan. Shown are the combined independent replicates of JED-E71-37 and DMSO control treatments (log-rank test, p <0.0001). C) Treatment with JED-E71-37 improves stress-sensitive locomotor function at day 13 and 17 but not significantly at day 21. ** p<0.01, student’s t-test.
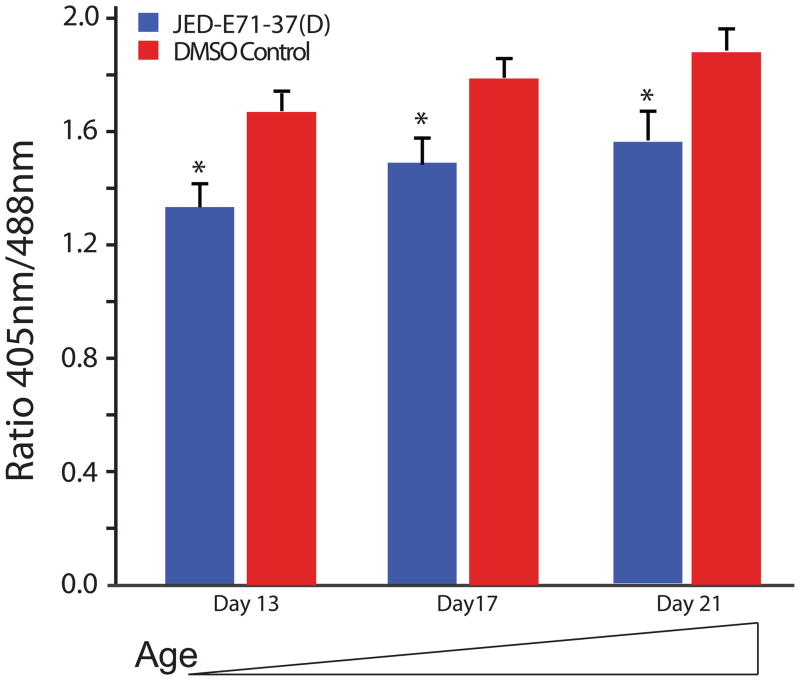
The improvement in phenotypes observed with antioxidant treatment demonstrates mitochondrial ROS is an important part of mitochondrial encephalomyopathy pathogenesis. The modest improvement observed suggests that ROS is a minor part of pathogenesis or that the antioxidant treatment only minimally improved ROS. To better understand the extent to which ROS contributes to mitochondrial encephalomyopathy pathogenesis, we examined ROS at days 13, 17 and 21 following chronic treatment with 1 μM JED-E71-37. The mitochondrial-targeted antioxidant was able to very significantly reduce ROS at all time points examined (Figure 6).
Figure 6. The mitochondrial-targeted antioxidant JED-E71-37 significantly reduces ATP61 ROS.
ATP61 mutant flies chronically treated with JED-E71-37 have significantly reduced mitochondrial oxidative stress at all time points examined relative to age-matched vehicle only treated ATP61 animals (*p<0.05).
Discussion
Mitochondrial dysfunction and ROS have proven key to the pathogenesis of numerous disease conditions including AD, PD, HD, and ALS (Halliwell, 2006). The extent to which an altered redox status contributes to pathogenesis is actively being studied in various model systems. Although there are numerous neurodegenerative and mitochondrial disease models in Drosophila, assays to accurately measure ROS and mitochondrial dysfunction are limited. roGFPs have proven useful in other systems suggesting they would be valuable in Drosophila. We developed cytosolic and mitochondrial targeted versions and our initial studies suggest the latter is more useful, especially within neurons. MTSroGFP2 was detectable, responsive to changes in redox state in cells and was used to examine redox changes with age in vivo. Our data suggest that brain mitochondria in vivo are a much more oxidizing environment than mitochondria within cultured primary neurons (Figure 1C and 2A wild type). These data are consistent with cultured neurons being much less reliant upon OXPHOS for energy and exemplify the importance of in vivo studies of mitochondrial function.
Mitochondrial redox levels were examined using MTSroGFP2 in two well-characterized mutants with progressive and degenerative phenotypes. Both the ATP6 and ATPalpha mutants examined showed increase in ROS in vivo. Importantly, ROS was not elevated in young animals but was observed to be elevated in a progressive manner in accord with phenotypes observed in these mutants. These data strongly support the assertion that a progressive increase in mitochondrial ROS may underlie the progressive nature of disease pathogenesis associated with these degenerative conditions.
Several strains of wild type flies exhibited a steady increase in redox status with age as measured by MTSroGFP2, demonstrating the importance of examining ROS over a time course and suggesting that increased mitochondrial redox status or mitochondrial dysfunction are instrumental to normal senescence. ROS has been extensively implicated in aging and previous data have led others to hypothesize that cellular and or mitochondrial dysfunction with age may lead to age-related increases in ROS (Kregel and Zhang, 2007). Although there is extensive literature to suggest ROS may underlie aging, much of the data in support of this are based upon the notion that the damage caused by ROS may be cumulative. That said there is some evidence that ROS may increase with age within various tissues including muscle, heart, liver, and brain (Bejma and Ji, 1999; Bejma et al., 2000; Driver et al., 2000). Our data elegantly demonstrate a significant change in mitochondrial redox status with age consistent with the hypothesis that mitochondrial dysfunction and subsequent increases in ROS generation may underlie mechanisms of senescence.
ATPalphaDTS1 is a gain-of-function mutation in the gene encoding the Na+/K+ ATPase alpha subunit, previously shown to cause marked progressive neuropathology (Palladino et al., 2003). Although pathogenesis of ATPalphaDTS1 is not known, ion dyshomeostasis, altered neural signaling and elevated ROS are hypothesized to contribute to pathogenesis. Our studies using MTSroGFP2 reveal a marked elevation in redox status in an age-dependent manner. Interestingly, if we had examined only young adults we would have concluded that ROS likely did not contribute to pathogenesis, demonstrating the importance of examining adults in a time course to elucidate mechanisms of pathogenesis. It is important to note that phenotypes emerge with time and that previous histopathology revealed neuropathology only in aged animals (Fergestad et al., 2006; Palladino et al., 2003), suggesting a relationship might exist between elevated ROS and the emergence of neuropathology in ATPalphaDTS1 animals. Importantly, a revertant of the temperature-sensitive paralysis of ATPalphaDTS1 has been described and this mutant, ATPalphaDTS1R1, does not have elevated ROS. Also, the ATPalphaDTS1R1 mutant has been shown to have a surprising ~25% increase in longevity over wild type animals (Ashmore et al., 2009). Interestingly, our data revealed that ROS is not significantly altered in ATPalphaDTS1R1animals from controls. These data demonstrate that the gain-of-function associated with temperature-sensitive paralysis in ATPalphaDTS1results in elevated ROS and that the surprising increase in longevity in ATPalphaDTS1R1 is associated with normal levels of ROS.
Mitochondrial encephalomyopathy is associated with mitochondrial dysfunction in oxidative phosphorylation (complex I-V) either resulting from an endogenous mitochondrial mutation or mutation of a nuclear encoded subunit of complex I – V (Leonard and Schapira, 2000a; Leonard and Schapira, 2000b). We have previously isolated an endogenous mitochondrial mutation affecting ATP6, an essential component of complex V (Celotto et al., 2006a). We have previously shown that ATP61 causes a dramatic loss of mitochondrial ATP synthase activity (Celotto et al., 2006a; Palladino, 2010). We hypothesized that the mitochondrial dysfunction resulting from ATP61 might cause elevated ROS that contribute to pathogenesis. Our studies using MTSroGFP2 demonstrate a progressive increase in ROS that parallels the onset of pathogenic phenotypes in ATP61.
Mitochondrial-targeted ROS scavengers are potentially an effective treatment for various mitochondrial and neurodegenerative diseases (Frantz and Wipf, 2010; Hoye et al., 2008; Kagan et al., 2009; Macias et al., 2007; Wipf et al., 2005). The finding that a mitochondrial-targeted ROS scavenger was able to improve ATP61 phenotypes in vivo is promising. Furthermore, these data demonstrate ROS has an important role in pathogenesis of mitochondrial encephalomyopathy. Our data show that JED-E71-37 was able to fully ameliorate ROS at a time when locomotor function was partially improved. Furthermore, ROS was reduced at later time points but was not lowered to completely wild type levels. Consistent with the interpretation that ROS are one important aspect of pathogenesis, ATP61 animals treated with mitochondrial-targeted ROS scavengers exhibit a modest but significantly improved longevity. It is possible that improvements in ROS observed with ROS scavenger treatment lead to the phenotypic improvements, however, it is also feasible that this complex structure could lead to phenotypic improvements by some independent mechanism of action. Five putative ROS scavengers and a control compound were examined for efficacy in vivo, and one was able to significantly improve longevity and locomotor function in ATP61 animals. These compounds have not yet been extensively tested in vivo and the selectivity may derive simply from better pharmacokinetic properties or lower toxicity. Alternatively, the activity of compound JED-E71-37 could be due to its improved mitochondrial delivery properties. The alkene peptide isosteres JP4-039, XJB-5-208, and their dimeric congeners JED-E71-1 and JED-E71-58 do not contain the full sequence of the lead structure XJB-5-131, which has been shown to be a highly effective mitochondrial targeting system (Jiang et al., 2007; Wipf et al., 2005). In contrast, JED-E71-37 represents a flexibly linked homodimer of XJB-5-131. Further studies will be needed to completely resolve the specific mechanism of action.
Conclusions
The data strongly support the conclusion that genetically encoded redox sensors are a valuable tool for examining mitochondrial dysfunction and ROS in vivo. Studies of specific Drosophila mutants with neurological dysfunction demonstrate their utility within neural tissues and the importance of examining ROS over a relevant time course. These studies also demonstrate that an increase in ROS is associated with normal aging, suggesting this may be integral to mechanisms of senescence. Our data support this hypothesis but further studies, possibly using an assay capable of specifically measuring mitochondrial redox status such as MTSroGFP2, will be needed to examine mitochondrial ROS over the full lifespan to better appreciate its role in the aging process.
Supplementary Material
Primary elav-Gal4; MTSroGFP2 neurons treated with mitotracker red were examined for fluorescence using confocal microscopy. There was a high degree of cololocalization, consistent with efficient targeting of the roGFP to mitochondria.
A-C) Fluorescence was examined in brains of ATPalphaDTS1 (A), ATPalphaDTS1R1 (B) ATP61(C) and the appropriate wild type controls (A–C). Shown here are example ratio image analyses from day 30 animal brains. After background subtraction of subsaturation images, ratio fluorescence from 3000 pixels per image was calculated, analyzed, and displayed in graphical form using the colorimetric scale shown. The mean pixel ratio and standard deviation are shown for each analysis.
Structures, chemical formulae and molecular weights of the mitochondrial targeted ROS scavengers and control compounds examined.
Highlights.
Genetically encoded mitochondrial redox sensors in vivo.
Mitochondrial redox status increases with age suggesting a role in aging/senescense.
Progressive mitochondrial redox changes in mitochondrial encephalopathy in vivo.
Mitochondrial ROS associated with Na/K ATPase neurodegeneration in vivo.
Acknowledgments
We thank Dr. James Remington for sharing the roGFP clones, Dr. Ed Levitan for advise with imaging, Eric Kelley for critical review of the manuscript, the Bloomington stock center for fly strains, Dr. Konrad Basler for the pUASTattB vector, and Dr. Jennifer Davoren for preparation of the ROS scavenger compounds. This work could not have been completed without funding from NSF 0910560 (PW), NIH R56DK079864 (GR), NIH R01AG025046 (MJP), and NIH R01AG027453 (MJP) grants.
List of Abbreviations
- ROS
reactive oxygen species
- roGFP
redox-sensitive green fluorescent protein
- MTS
mitochondrial or matrix target signal
- DTT
dithiothreitol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addabbo F, et al. Mitochondria and reactive oxygen species. Hypertension. 2009;53:885–92. doi: 10.1161/HYPERTENSIONAHA.109.130054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RG, Tresini M. Oxidative stress and gene regulation. Free Radic Biol Med. 2000;28:463–99. doi: 10.1016/s0891-5849(99)00242-7. [DOI] [PubMed] [Google Scholar]
- Ashmore LJ, et al. Novel mutations affecting the Na, K ATPase alpha model complex neurological diseases and implicate the sodium pump in increased longevity. Hum Genet. 2009;126:431–47. doi: 10.1007/s00439-009-0673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejma J, Ji LL. Aging and acute exercise enhance free radical generation in rat skeletal muscle. J Appl Physiol. 1999;87:465–70. doi: 10.1152/jappl.1999.87.1.465. [DOI] [PubMed] [Google Scholar]
- Bejma J, et al. Free radical generation and oxidative stress with ageing and exercise: differential effects in the myocardium and liver. Acta Physiol Scand. 2000;169:343–51. doi: 10.1046/j.1365-201x.2000.00745.x. [DOI] [PubMed] [Google Scholar]
- Belton A, et al. Deletions encompassing the manganese superoxide dismutase gene in the Drosophila melanogaster genome. Genome. 2006;49:746–51. doi: 10.1139/g06-029. [DOI] [PubMed] [Google Scholar]
- Bischof J, et al. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci U S A. 2007;104:3312–7. doi: 10.1073/pnas.0611511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon MB, Remington SJ. Redox-sensitive green fluorescent protein: probes for dynamic intracellular redox responses. A review. Methods Mol Biol. 2008;476:51–65. doi: 10.1007/978-1-59745-129-1_4. [DOI] [PubMed] [Google Scholar]
- Celotto AM, et al. Mitochondrial encephalomyopathy in Drosophila. J Neurosci. 2006a;26:810–20. doi: 10.1523/JNEUROSCI.4162-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celotto AM, et al. Drosophila model of human inherited triosephosphate isomerase deficiency glycolytic enzymopathy. Genetics. 2006b;174:1237–46. doi: 10.1534/genetics.106.063206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley CT, et al. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J Biol Chem. 2004;279:22284–93. doi: 10.1074/jbc.M312847200. [DOI] [PubMed] [Google Scholar]
- Driver AS, et al. Age-related changes in reactive oxygen species production in rat brain homogenates. Neurotoxicol Teratol. 2000;22:175–81. doi: 10.1016/s0892-0362(99)00069-0. [DOI] [PubMed] [Google Scholar]
- Duttaroy A, et al. A Sod2 null mutation confers severely reduced adult life span in Drosophila. Genetics. 2003;165:2295–9. doi: 10.1093/genetics/165.4.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerit J, et al. Neurodegenerative diseases and oxidative stress. Biomed Pharmacother. 2004;58:39–46. doi: 10.1016/j.biopha.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Fergestad T, et al. Neuropathology in Drosophila membrane excitability mutants. Genetics. 2006;172:1031–42. doi: 10.1534/genetics.105.050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz MC, Wipf P. Mitochondria as a target in treatment. Environ Mol Mutagen. 2010;51:462–75. doi: 10.1002/em.20554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman JN, et al. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature. 2010;468:696–700. doi: 10.1038/nature09536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006;97:1634–58. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- Hanson GT, et al. Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J Biol Chem. 2004;279:13044–53. doi: 10.1074/jbc.M312846200. [DOI] [PubMed] [Google Scholar]
- Hoye AT, et al. Targeting mitochondria. Acc Chem Res. 2008;41:87–97. doi: 10.1021/ar700135m. [DOI] [PubMed] [Google Scholar]
- Hrizo SL, Palladino MJ. Hsp70- and Hsp90-mediated proteasomal degradation underlies TPI sugarkill pathogenesis in Drosophila. Neurobiol Dis. 2010;40:676–83. doi: 10.1016/j.nbd.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, et al. Structural requirements for optimized delivery, inhibition of oxidative stress, and antiapoptotic activity of targeted nitroxides. J Pharmacol Exp Ther. 2007;320:1050–60. doi: 10.1124/jpet.106.114769. [DOI] [PubMed] [Google Scholar]
- Kagan VE, et al. Mitochondrial targeting of electron scavenging antioxidants: Regulation of selective oxidation vs random chain reactions. Adv Drug Deliv Rev. 2009;61:1375–85. doi: 10.1016/j.addr.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby K, et al. RNA interference-mediated silencing of Sod2 in Drosophila leads to early adult-onset mortality and elevated endogenous oxidative stress. Proc Natl Acad Sci U S A. 2002;99:16162–7. doi: 10.1073/pnas.252342899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kregel KC, Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol. 2007;292:R18–36. doi: 10.1152/ajpregu.00327.2006. [DOI] [PubMed] [Google Scholar]
- Leonard JV, Schapira AH. Mitochondrial respiratory chain disorders I: mitochondrial DNA defects. Lancet. 2000a;355:299–304. doi: 10.1016/s0140-6736(99)05225-3. [DOI] [PubMed] [Google Scholar]
- Leonard JV, Schapira AH. Mitochondrial respiratory chain disorders II: neurodegenerative disorders and nuclear gene defects. Lancet. 2000b;355:389–94. doi: 10.1016/s0140-6736(99)05226-5. [DOI] [PubMed] [Google Scholar]
- Li W, et al. Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem Pharmacol. 2008;76:1485–9. doi: 10.1016/j.bcp.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias CA, et al. Treatment with a novel hemigramicidin-TEMPO conjugate prolongs survival in a rat model of lethal hemorrhagic shock. Ann Surg. 2007;245:305–14. doi: 10.1097/01.sla.0000236626.57752.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin I, et al. Sod2 knockdown in the musculature has whole-organism consequences in Drosophila. Free Radic Biol Med. 2009;47:803–13. doi: 10.1016/j.freeradbiomed.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan SC, et al. Plant homologs of the Plasmodium falciparum chloroquine-resistance transporter, PfCRT, are required for glutathione homeostasis and stress responses. Proc Natl Acad Sci U S A. 2010;107:2331–6. doi: 10.1073/pnas.0913689107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AJ, et al. Redox-sensitive GFP in Arabidopsis thaliana is a quantitative biosensor for the redox potential of the cellular glutathione redox buffer. Plant J. 2007;52:973–86. doi: 10.1111/j.1365-313X.2007.03280.x. [DOI] [PubMed] [Google Scholar]
- Palladino MJ. Modeling mitochondrial encephalomyopathy in Drosophila. Neurobiol Dis. 2010;40:40–5. doi: 10.1016/j.nbd.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino MJ, et al. Neural dysfunction and neurodegeneration in Drosophila Na+/K+ ATPase alpha subunit mutants. J Neurosci. 2003;23:1276–86. doi: 10.1523/JNEUROSCI.23-04-01276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino MJ, et al. Temperature-sensitive paralytic mutants are enriched for those causing neurodegeneration in Drosophila. Genetics. 2002;161:1197–208. doi: 10.1093/genetics/161.3.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A, et al. Reduced mitochondrial SOD displays mortality characteristics reminiscent of natural aging. Mech Ageing Dev. 2007a;128:706–16. doi: 10.1016/j.mad.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul SM, et al. A pump-independent function of the Na,K-ATPase is required for epithelial junction function and tracheal tube-size control. Development. 2007b;134:147–55. doi: 10.1242/dev.02710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser S, et al. A fluorometer-based method for monitoring oxidation of redox-sensitive GFP (roGFP) during development and extended dark stress. Physiol Plant. 2010;138:493–502. doi: 10.1111/j.1399-3054.2009.01334.x. [DOI] [PubMed] [Google Scholar]
- Schwarzlander M, et al. Monitoring the in vivo redox state of plant mitochondria: effect of respiratory inhibitors, abiotic stress and assessment of recovery from oxidative challenge. Biochim Biophys Acta. 2009;1787:468–75. doi: 10.1016/j.bbabio.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Sicaeros B, et al. Primary neuronal cultures from the brains of late stage Drosophila pupae. J Vis Exp. 2007:200. doi: 10.3791/200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temkin V, Karin M. From death receptor to reactive oxygen species and c-Jun N-terminal protein kinase: the receptor-interacting protein 1 odyssey. Immunol Rev. 2007;220:8–21. doi: 10.1111/j.1600-065X.2007.00560.x. [DOI] [PubMed] [Google Scholar]
- Wipf P, et al. Mitochondrial targeting of selective electron scavengers: synthesis and biological analysis of hemigramicidin-TEMPO conjugates. J Am Chem Soc. 2005;127:12460–1. doi: 10.1021/ja053679l. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primary elav-Gal4; MTSroGFP2 neurons treated with mitotracker red were examined for fluorescence using confocal microscopy. There was a high degree of cololocalization, consistent with efficient targeting of the roGFP to mitochondria.
A-C) Fluorescence was examined in brains of ATPalphaDTS1 (A), ATPalphaDTS1R1 (B) ATP61(C) and the appropriate wild type controls (A–C). Shown here are example ratio image analyses from day 30 animal brains. After background subtraction of subsaturation images, ratio fluorescence from 3000 pixels per image was calculated, analyzed, and displayed in graphical form using the colorimetric scale shown. The mean pixel ratio and standard deviation are shown for each analysis.
Structures, chemical formulae and molecular weights of the mitochondrial targeted ROS scavengers and control compounds examined.