Abstract
The keratocytes are specialized mesenchymal cells that produce and maintain the extracellular matrix of the corneal stroma. With a typical dendritic and flattened appearance, these cells can morph into fibroblasts and myofibroblasts upon injury, and produce abnormal or fibrotic extracellular matrices detrimental to corneal transparency. Insights into mechanisms that regulate these phenotypic switches and optimal culture conditions that preserve the keratocyte phenotype are important for tissue engineering of the corneal stroma. Like other cell types with self-renewing capacity, keratocytes can form spheres in culture. Here we investigated human and bovine keratocytes with respect to their sphere forming capabilities, and sought to identify potentially distinguishing markers for the keratocyte and fibroblast phenotype. Keratocytes, isolated from bovine and human corneas, cultured in serum-free medium supplemented with insulin, selenium and transferrin, assumed typical keratocyte morphology, converted to fibroblasts in serum-containing medium and reverted to keratocytes after serum-deprivation. The bovine keratocytes produced spheres under adherent or low attachment conditions, while the human keratocytes produced spheres under low attachment conditions only. The primary keratocytes and fibroblasts expressed vimentin, confirming their mesenchymal origin. Keratocan, considered to be a marker for keratocytes, was also detected in early passage bovine fibroblasts. BMP3 was expressed in keratocytes and keratocyte-derived spheres, while cadherin 5 in keratocytes only, suggesting these as potential keratocyte markers.
Keywords: cornea, keratocyte, fibroblasts, spheres, BMP3, Cdh5, keratocan, lumican
1. Introduction
The cornea is a transparent, avascular protective barrier that also provides 70% of the refractive power of the eye. It is comprised of three layers; an outer stratified epithelium, a collagen-rich middle stromal layer and an inner single-cell layered endothelium (Hassell and Birk, 2010). This stroma is a connective tissue that constitutes about 90% of the cornea and consists of a transparent extracellular matrix (ECM) deposited by the keratocytes during late embryonic development (Hassell and Birk, 2010; Hay, 1980). The keratocytes are neural crest derived mesenchymal cells that reside in the corneal stroma as flattened, quiescent cells with a typical dendritic morphology (Berlau et al., 2002). They form a three-dimensional network of cells interconnected by gap-junctions (Watsky, 1995). During corneal development these cells are biosynthetically active, producing fibrillar collagens and the small leucine-rich repeat proteoglycans (SLRP) that assemble into a highly organized ECM of collagen fibrils of uniform diameter and interfibrillar spacing, required for a transparent cornea (Chakravarti et al., 1998; Hassell and Birk, 2010; Hay, 1980; Maurice, 1957; Quantock et al., 2001).
Injury and infections perturb the stromal organization; keratocytes undergo apoptosis, while peripheral keratocytes become fibroblastic and migrate to the site of injury and can form haze-causing myofibroblasts (Helena et al., 1998; Netto et al., 2006; Zieske et al., 2001). Significant efforts are underway to elucidate regulatory mechanisms that control these cellular phenotypes for a better understanding of wound healing in the cornea. Furthermore, corneal tissue engineering efforts also require better characterization of the corneal keratocytes and the ability to propagate these in culture for extended periods of time without extensive loss of their in situ properties. In culture, the corneal stromal cells can be driven to a keratocyte phenotype in serum-free medium supplemented with insulin, selenium and transferrin, and in the presence of phosphoascorbic acid these keratocytes produce and accumulate collagens and proteoglycans mimicking their functions in the native cornea (Long et al., 2000; Musselmann et al., 2005; Musselmann et al., 2006). Exposure to fetal bovine serum causes these cells to become fibroblastic, while TGFβ_1 treatment resulted in a myofibroblastic phenotype (He and Bazan, 2008; Jester et al., 1996; Jester et al., 1999; Mohan et al., 2003). Reversal of the myofibroblast to the fibroblast phenotype occurred on treatment of the cells with low levels of FGF1 or FGF2 (Maltseva et al., 2001). Current studies are geared towards a molecular characterization of these cellular phenotypes by focusing on distinctive markers.
Here we investigated long term culturing of primary human and bovine keratocytes, their differentiation to fibroblasts, and reversal to the keratocyte phenotype upon serum – deprivation. We further demonstrated sphere formation from adherent keratocytes and expansion of the sphere cultures in serum-free and serum-containing medium. In an attempt to fully characterize these cell types and identify markers that can distinguish them, we assessed the expression of a number of genes. The results suggest that the keratocytes, fibroblasts and spheres can be distinguished from each other based on expression of specific developmental and progenitor related genes.
2. Materials and Methods
2.1 Keratocyte Isolation and Cell Culture
Fresh bovine corneas were purchased from Pel Freez® Biologicals (Rogers, AR). Human corneas, deemed unsuitable for use in cornea transplantation, were obtained from Tissue Banks International (Baltimore, MD), in Optisol-GS Corneal Storage Media (Bausch & Lomb, NY). Keratocytes were isolated as described before (Funderburgh et al., 2008). Whole corneas were rinsed in cold Hanks Balanced Salt Solution (HBSS; CellGro) supplemented with antibiotics (100 IU/ml Penicillin and 100μg/ml Streptomycin). Central corneal buttons were rinsed twice in HBSS, incubated at 37°C in 1mg/ml Collagenase Type L (Sigma Aldrich, St. Louis, MO) in DMEM/F12 (Invitrogen, Carlsbad, CA) with shaking at 125 rpm. The corneal buttons were scraped gently to remove the epithelium and endothelium, rinsed, quartered and digested in fresh collagenase (1mg/ml), rinsed (3X) and centrifuged at 1400 rpm for 5 minutes, resuspended in 1mg/ml collagenase solution and incubated on ice overnight at 4°C. The following day the corneas were centrifuged at 1400 rpm for 5 minutes and the pellet resuspended in a 10mg/ml collagenase solution for a final digestion at 37°C with shaking at 150 rpm for 2 hours. The digest was filtered through a 70μm cell strainer (BD Falcon, Bedford, MA) and the isolated keratocytes centrifuged at 2000 rpm for 10 minutes. The cell pellet was washed twice in DMEM/F12 supplemented with antibiotics; cell viability was determined using trypan blue exclusion.
To establish monolayer cultures, the primary keratocytes were plated in adherent tissue culture flasks in keratocyte plating medium (KPM) consisting of serum-free DMEM/F12 supplemented with 1mM phosphoascorbic acid (Sigma-Aldrich, St. Louis, MO). After overnight attachment, the plating medium was replaced with keratocyte growth medium (KGM) of DMEM/F12 with FGF-2 (10ng/ml), insulin, transferrin and selenium (ITS; Sigma-Aldrich) and 1mM phosphoascorbic acid. The fibroblast growth medium (FGM) was DMEM/F12 with 10% FBS (Invitrogen).
For sphere formation keratocytes were trypsinized (TrypLE™, Invitrogen) and seeded at a density of 2.5 ×105 cells per well in 6-well ultra low attachment tissue culture plates (Corning, Lowell, MA) for 8 days in KPM, KGM or FGM. The growth of spheres was monitored by measuring the average diameter of the spheres (n=5). All media were supplemented with penicillin/streptomycin and cultured at 37°C in a humidified atmosphere with 5% CO2.
2.2 Immunofluorescence of spheres
The spheres were fixed in 4% Paraformaldehyde (Sigma-Aldrich) in phosphate buffered saline (PBS) at room temperature for 20 minutes, washed twice in PBST (PBS and 0.1% Triton X-100) and incubated in blocking buffer containing 10% Fetal Bovine Serum (FBS) (Invitrogen) and PBST for 1 hour at room temperature. After washing three times the spheres were incubated overnight with primary antibodies diluted in 1% FBS/PBST. The following antibodies were used goat anti- vimentin antibody (1:100; Millipore, Billerica, MA) and rabbit anti- β1 Integrin (1:100; Santa Cruz Biotechnology, Santa Cruz, CA). After washing in PBST (3X), the spheres were incubated for 1 hour at room temperature with the appropriate secondary antibodies diluted in 5%FBS/PBST. The following secondary antibodies were used: Cy2 conjugated donkey anti- goat and Cy3 conjugated goat anti- rabbit (1:250; Jackson ImmunoResearch, West Grove, PA). Nuclei were counterstained with DAPI (1:1000; Molecular Probes, Carlsbad, PA) and mounted in fluorescent mounting media (Dako, Carpinteria, CA) and examined with a laser scanning confocal microscope (LSM 510 META; Zeiss, Thornwood, NY).
For preparing paraffin sections of the spheres, adherent cells and spheres were collected in 5% formalin, pelleted by centrifugation at 1000 rpm, 2 min. The pellets were further fixed overnight in 10% formalin at 4°C, serially dehydrated, hydrated, paraffin embedded and sectioned into 4μm thick sections. Adjacent sections were stained with hematoxylin and eosin, Periodic Acid-Schiff and Masson’s trichrome (all from Sigma- Aldrich).
2.3 RNA isolation and Semi-Quantitative RT-PCR
Pellets of keratocytes, fibroblasts and spheres were resuspended in Trizol (Invitrogen). Total RNA was isolated using the RNAeasy Mini Kit (Qiagen, Valencia, CA). For semiquantitative RT-PCR, 0.5μg of RNA was subjected to cDNA synthesis using SuperScript III (Invitrogen). All primers (see Table 1) were designed using the Primer 3 software and obtained from Integrated DNA Technologies. Target genes were amplified in a 25μl reaction volume for 35 cycles using the following settings: 1 min at 94°C followed by 35 cycles of 30 sec at 94°C, 30 sec at 58°C, 1 min at 72°C and a final extension step of 10 min at 72°C on a PTC-100 Programmable Thermal Controller (MJ Research, Ramsey, MN). The PCR products were resolved on a 1.5% agarose gel.
Table 1. PCR primer sequences.
| Gene | Primers | Size (bp) | |
|---|---|---|---|
| Bovine | Decorin | F - 5′ AGCTGAAGGAATTGCCAGAA 3′ R - 5′ GCCATTGTCAACAGCAGAGA 3′ |
371 |
| Lumican | F - 5′ CCAAGCACTGTATGGGAGGT 3′ R - 5′ TCCACCAGAGATTTGGGAAG 3′ |
345 | |
| Biglycan | F - 5′ CTCCAAGATCCACGAGAAGG 3′ R - 5′ TCTAGCTCGATTGCCTGGAT 3′ |
363 | |
| Col1a1 | F - 5′ TGGGTTGATCCTAACCAAGG 3′ R - 5′ CCCAGTGTGTTTTGTGCAAC 3′ |
435 | |
| Keratocan | F - 5′ TTCAGCAATCTGGAGAACCTG 3′ R - 5′ GTTAGATTGTTGTGTTGTCATGC 3′ |
953 | |
| αSMA | F - 5′ CCTCGACATCAGGGAGTGAT 3′ R - 5′ AGGGCGTAGCCCTCATAGAT 3′ |
401 | |
| Vimentin | F - 5′GAAGTTGCAGGAGGAGATGC 3′ R - 5′ CAGCCTCCTGAAGGTTCTTG 3′ |
302 | |
| Integrin B1 | F - 5′ AGAGAAGCTGCAGCCAGAAG 3′ R - 5′ AAACGATGCCACCAAGTTTC 3′ |
575 | |
| CK12 | F - 5′ GAGGCCTGGTTCATTGAAAA 3′ R - 5′ GGTCTCAATTTCCAGCTCCA 3′ |
336 | |
| BMP3 | F - 5′ ATCATGCCCAGAGGAAACAC 3′ R - 5′ CATCAAACTGGAGCGTCTGA 3′ |
600 | |
| Pax6 | F - 5′ GTCCATCTTTGCTTGGGAAA 3′ R - 5′ CATTTGGCCCTTCGATTAGA 3′ |
510 | |
| Nestin | F - 5′CTTCTCCACCCTCTGTGCTC 3′ R - 5′ AAGGTTGGCACAGGTGTTTC 3′ |
487 | |
| CD133 | F - 5′ TTCCAGGTTTTGGACACTCC 3′ R - 5′ CAGCTGGTTGGCTTTTCTTC 3′ |
414 | |
| Aldh1a1 | F - 5′ ACCCCTCTGACTGCTCTTCA 3′ R - 5′ AACACTGGCCCTGGTGATAG 3′ |
316 | |
| CSF1 | F - 5′ GTCTCCTGGTGAGCAATGGT 3′ R - 5′ GGCTGGAGCATTTAGCAAAG 3′ |
471 | |
| CD14 | F - 5′ TCCGTAACGTATCGTGGACA 3′ R - 5′ CAGCGAACGACAAATTGAGA 3′ |
411 | |
| TLR4 | F - 5′ AGGCAGCCATAACTTCTCCA 3′ R - 5′ GGTTGAGTAGGGGCATTTGA 3′ |
410 | |
| β actin | F- 5′ TGGCACCACACCTTCTACAATGAGC 3′ R- 5′ GCACAGCTTCTCCTTAATGTCACGC 3' |
396 | |
| Human | Biglycan | F- 5′ GGACTCTGTCACACCACCT 3′ R- 5′ AGCTCGGAGATGTCGTTGTT 3′ |
159 |
| Decorin | F- 5′ TGCTGTTGACAATGGCTCTC 3′ R- 5′ GCCTTTTTGGTGTTGTGTCC 3′ |
192 | |
| Keratocan | F- 5′ ATCTGCAGCACCTTCACCTT 3′ R- 5′ TTCCATCCAGACGGAGGTAG 3′ |
141 | |
| Lumican | F- 5′ AGCCAGACTGCCTTCTGGTCTCC 3′ R -5′ GGACAGATCCAGCTCAACCAGGG 3′ |
199 | |
| BMP3 | F-5′ GCTCTACTGGGGTCTTGCTG 3′ R- 5′ ATGAGGCCCCTTTCTCTGTT 3′ |
164 | |
| Gapdh | F- 5′ GAGTCAACGGATTTGGTCGT 3′ R’ 5′ GACAAGCTTCCCGTTCTCAG3 ′ |
185 |
3. Results
3.1. Phenotypic plasticity of keratocytes in culture
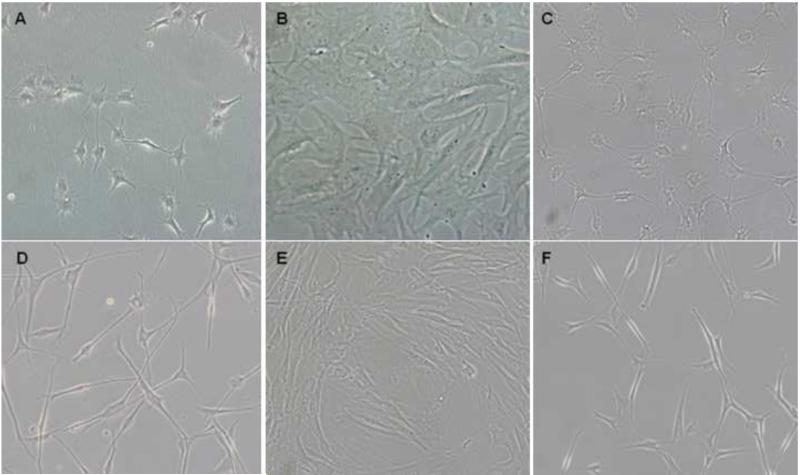
We examined the morphology of primary bovine and human corneal keratocytes under different culture conditions. Freshly isolated bovine and human keratocytes were first allowed to attach in keratocyte plating medium (KPM) consisting of serum-free DMEM/F12 supplemented with 1mM phosphoascorbic acid and subsequently grown in keratocyte growth medium (KGM), which is KPM supplemented with FGF2 and ITS. In KGM the bovine keratocytes assumed the typical dendritic morphology seen previously in bovine and rabbit keratocyte cultures (He and Bazan, 2008; Jester and Ho-Chang, 2003; Lakshman et al., 2010) (Fig. 1A). The human keratocytes were dendritic but the cell bodies were small and overall had a more neural cell phenotype (Fig. 1D). Upon changing the medium to fibroblast growth medium (FGM), the cells appeared as fibroblasts within 2 days (Fig. 1B and E). Sub-culturing the fibroblasts in KGM for 2 weeks prompted their transition back to the keratocyte morphology (Fig. 1C and F).
Figure 1. Reversible conversion of keratocytes to fibroblasts.
Bovine (A-C) and human (D-F) corneal stromal cells were plated in KPM and then transferred to KGM where they assumed the dendritic keratocyte morphology (A and D). Within 48 hrs of transfer to FGM the keratocytes display a fibroblast-like morphology (B and E) and switch back to keratocytes after maintenance in KGM for 2 weeks (C and F). Scale bar = 50 μm
3.2. Sphere formation from adherent monolayer keratocytes
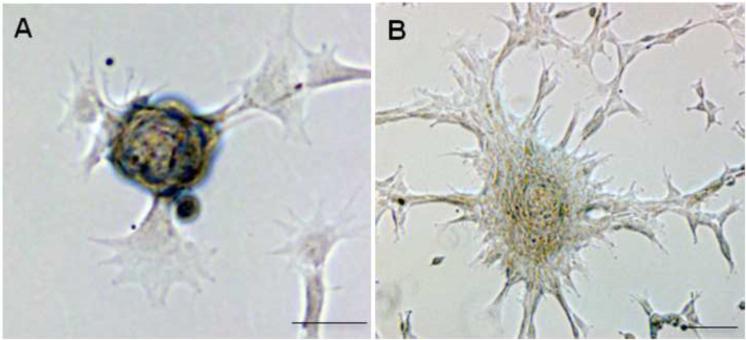
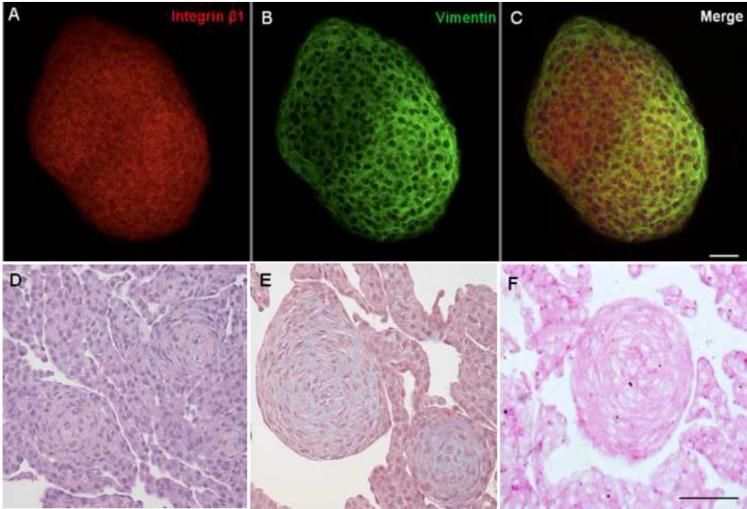
Bovine keratocytes cultured in adherent plates for seven days formed small tethered spheres that detached and floated in the medium (Fig. 2A). However, these were clearly not dying cells as transferring to fresh adherent plates prompted the cells to grow out of the spheres and resume monolayer growth (Fig. 2B). Integrin β1, known to be expressed by keratocytes in the cornea (Stepp, 2006) was present in the spheres (Fig. 3A). The expression of vimentin confirmed the mesenchymal nature of the cells in the sphere (Fig. 3B). Monolayer cultures of bovine keratocytes were scraped, pelleted, fixed in formalin, embedded in paraffin and sectioned Fig. 3 D-F). The H and E staining of these pellets showed concentric patterns indicating the formation of spheres from the adherent bovine keratocyte monolayer. Positive staining for trichrome and PAS confirmed the presence of collagen and glycosaminoglycans, respectively, further supporting the keratocyte nature of these cells (Fig. 3F). We failed to detect spontaneous sphere formation in adherent cultures of human keratocytes. However, under non-adherent conditions human keratocytes were also able to form spheres as shown below.
Figure 2. Sphere formation in adherent bovine keratocytes.
Spheres formed spontaneously from a few adherent keratocytes in monolayer cultures in KGM (A). Within 48 hrs of transferring to fresh tissue culture plates the spheres show outgrowth of adherent cells from the spheres (B). Scale bar = 50 μm.
Figure 3. Cell surface, cytoskeletal and ECM proteins in bovine keratocyte-derived spheres.
Spheres were immunostained for Integrin β1 (A) and Vimentin (B); punctate areas of yellow in the merged image (C) suggest colocalization. Pelleted spheres were H and E stained (D). Trichrome staining shows the presence of collagen (E) and Periodic acid-schiff staining shows the presence of carbohydrates primarily associated with proteoglycans (F). Scale bar = 50 μm.
3.3 Sphere formation under low attachment conditions
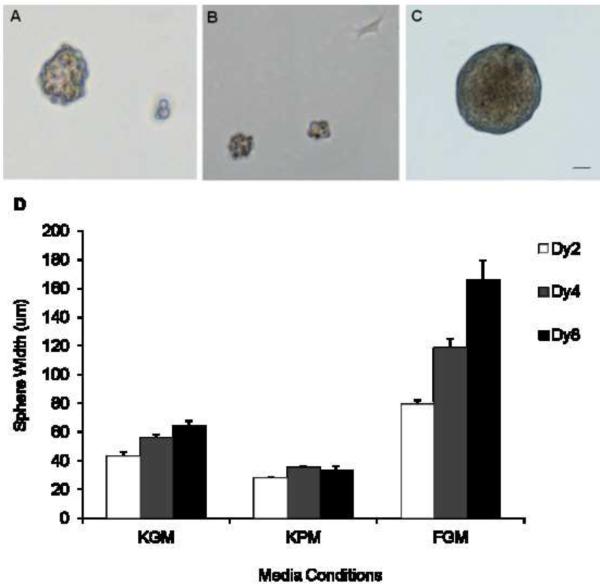
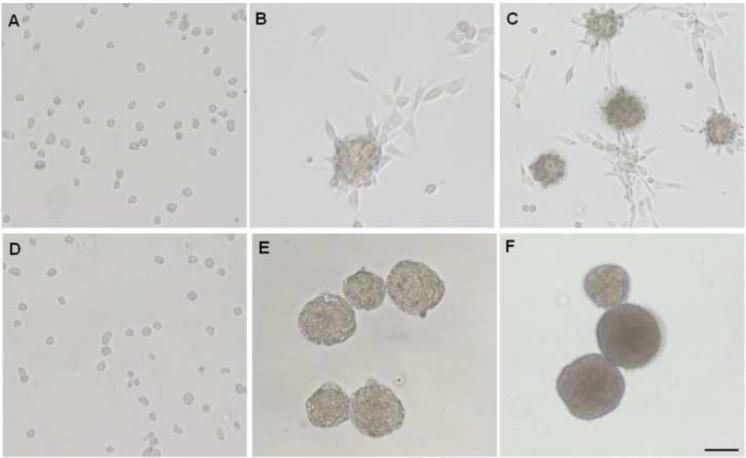
Previous studies reported the formation of spheres from aggregates of bovine keratocytes grown in suspension in KGM (Funderburgh et al., 2005). We further investigated if similar spheres would form when cells were maintained under low attachment conditions in either in KPM or FGM. In KGM a majority of the keratocytes aggregated to form small sized clusters that grew to an average diameter of 64 μm over an eight-day period (Fig. 4A and D). However in KPM the cells formed aggregates that did not increase in size, and a few cells attached to the plastic (Fig. 4B). Keratocyte cell suspensions seeded in FGM also formed spheres. Unlike KPM and KGM, cells in FGM formed spheres with very smooth contours and these grew markedly larger, 79 – 166 μm in diameter, over the eight-day observation period (Fig. 4C and D).
Figure 4. Growth of bovine keratocyte-derived spheres in different culture media.
Keratocytes plated in low- attachment tissue culture plates in KGM (A), KPM (B) and serum containing FGM (C) form spheres as shown after eight days. Mean diameter of the spheres (n=5) ± 1SD after 2, 4 and 8 days (D). Scale bar = 50 μm.
Under low attachment conditions, human keratocytes also produced spheres by 48 hours in KGM (Fig. 5B) and FGM (Fig. 5E) and these grew larger by 96 hours (Fig. 5F). Those in FGM had a smooth contour as seen for the bovine keratocyte-spheres. We counted the number of spheres derived from human keratocytes in a microscope field, and based on the total number of cells in a field, at initial plating, under the same magnification, determined that~4-5% of the keratocytes produced spheres (Table 2).
Figure 5. Growth of human keratocyte-derived spheres in KGM and FGM culture media.
Keratocytes plated in low- attachment tissue culture plates in KGM (A-C), and serum containing FGM (D-F) were observed by phase contrast microscopy after 0hr (A, D), 48h (B, E) and 96 h (C, F).
Table 2. Sphere formation frequency in human keratocytes.
| Time (hrs) | Cells | Spheres | Mean# spheres/starting #cells |
Sphere frequency (%) |
|---|---|---|---|---|
| 0 | 264 | 0 | 0/249 | 0 |
| 234 | 0 | |||
| 48 | - | 13 | 12/249 | 4.8 |
| - | 11 | |||
| 96 | - | 9 | 11/249 | 4.4 |
| - | 13 |
3.4. Gene expression in keratocytes fibroblasts and keratocyte-derived spheres
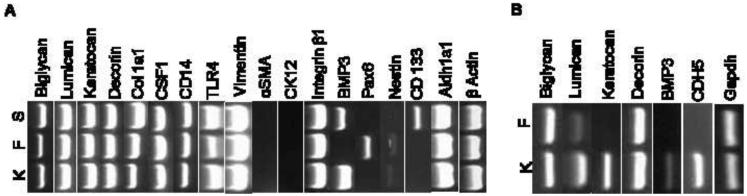
We investigated the expression of genes encoding previously known and novel ECM and cellular proteins by semi-quantitative RT- PCR using total RNA from low passage keratocytes, fibroblasts and spheres derived from bovine keratocytes, and maintained in KGM. Subsequently, we also cultured primary keratocytes and fibroblasts from human corneas and tested expression of a smaller set of genes. Of the known stromal ECM proteins, lumican, decorin and biglycan were expressed in bovine keratocytes, fibroblasts and spheres (Fig. 6A). Keratocan considered as being a marker for keratocytes, was also expressed in low passage bovine fibroblasts. In contrast, in the human cells only keratocytes expressed keratocan, while lumican was expressed in keratocytes and at very low levels in fibroblasts as well (Fig. 6B). The Col1a1 transcript for collagen type I was expressed in the bovine keratocytes and fibroblasts, and at a much higher level in the spheres. All three bovine culture types were found to express markers of hematopoietic lineage (Fig. 6A). These include CSF1, a marker of macrophage and dendritic cells, and CD14 and TLR4 that regulate innate immune response to bacterial lipopolysaccharide endotoxins. All three samples were negative for αSMA and K12 that are known to be expressed by myofibroblasts and epithelial cells, respectively. Aldh1a1, encoding one of several soluble enzymes that comprise the “corneal crystallins,” (Jester, 2008) was expressed strongly in keratocytes, fibroblasts and the keratocyte-derived spheres (Fig. 6A). The three culture conditions displayed unique patterns of gene expression for developmental, neuronal and stem cell markers. Only fibroblasts expressed the master developmental gene Pax6, whereas BMP3, a bone and cartilage developmental regulator, as well as a stimulator of mesenchymal stem cell proliferation, was detected in bovine and human keratocytes (Fig. 6A and B), and in the bovine keratocyte-derived spheres (Fig. 6A). The neuronal gene nestin was expressed weakly in keratocytes and fibroblasts. Nestin was earlier reported in a subset of cells differentiating into neural cells within spheres derived from human corneal stromal cells (Uchida et al., 2005). CD133, an adult stem cell marker, was detected in the bovine keratocyte-derived spheres only (Fig. 6A). However, we did not detect CD133 in human keratocyte-derived spheres (data not shown). We had earlier shown that cadherin 5 (Cdh5) was uniquely present in mouse corneal keratocytes (Chakravarti et al., 2004). Here we also found Cdh5 to be expressed in human keratocytes only and not fibroblasts (Fig. 6B).
Figure 6. Gene expression by semi-quatitative RT-PCR.
Total RNA was isolated from bovine (A) and human (B) keratocytes, K, fibroblasts F, bovine keratocyte-derived spheres, S. Gene expression was tested by semi-quantitaive RT-PCR using the primers presented in Table 1. Gapdh and β actin were used as controls.
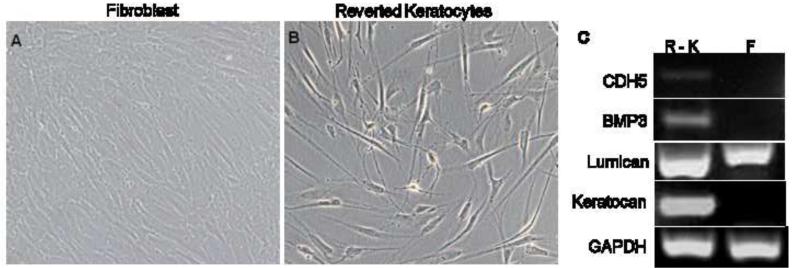
To determine if fibroblast-reverted keratocytes that regain the keratocyte morphology recreate the keratocyte molecular pattern as well, we tested the expression of key markers in human keratocytes derived from fibroblasts by switching the cells to the keratocyte growth medium. As shown (Fig, 7), the fibroblast-reverted keratocytes express CDH5, BMP3 and keratocan. While lumican is expressed weakly in fibroblasts, and strongly in the reverted keratocytes, as we saw before for cells maintained as keratocytes all along.
Figure 7. Expression of keratocyte - specific markers in fibroblast reverted keratocytes.
Human corneal keratocytes were cultured in FGM to generate fibroblasts (A). Fibroblasts were re-plated in KGM to revert to keratocytes (B). Expression of keratocyte markers in fibroblast - reverted keratocytes (R - K).
4. Discussion
Our studies show that human and bovine keratocytes grown in culture can undergo phenotypic conversion to fibroblasts in serum containing medium and revert to the typical keratocyte morphology once deprived of serum, as reported by others for rabbit and bovine corneas (Beales et al., 1999; Jester and Ho-Chang, 2003; Long et al., 2000). We detected the expression of novel and previously reported genes in these cell types. BMP3 and Cdh5 were uniquely expressed in keratocytes and may become important distinguishing markers for keratocytes. Keratocytes, fibroblasts and keratocyte-derived spheres - all express the small leucine rich repeat proteoglycan (SLRP) genes lumican, biglycan and decorin. Keratocan, considered to be a keratocyte marker (Carlson et al., 2005), was specifically expressed in human keratocytes and not fibroblasts. However, our early passage bovine fibroblasts also expressed keratocan. Earlier studies also reported expression of keratocan in early passage fibroblasts that diminished in long term fibroblast cultures (Garagorri et al., 2008). The detection of hematopoietic markers in the bovine keratocytes, spheres, and fibroblasts concur with our earlier transcript profiles of mouse keratocytes (Chakravarti et al., 2004). Detection of macrophage related markers in mouse keratocytes in culture had prompted us to suggest a dual role for keratocytes in corneal homeostasis and injury/repair (Chakravarti et al., 2004). CD14 was also detected in human corneal keratocytes by another study that proposed keratocytes to assume a repair phenotype under certain conditions (Thill et al., 2007).
The keratocytes, fibroblasts or the keratocyte-derived spheres could not be distinguished from each other based on the expression of the stromal ECM SLRP genes or the immune markers. However, they showed distinct patterns of expression when probed for developmental and stem cell related genes. BMP3 was detected specifically in the bovine and human keratocytes. BMP3 promotes mesenchymal stem cell proliferation through the TGF-β signaling pathway (Stewart et al., 2010) and its expression in the keratocytes and spheres may relate to TGF-β signaling that can drive proliferation without tipping the balance to differentiation. The Pax6 transcription factor is important to ocular development, loss of its expression leads to the poor development or complete absence of eyes (Collinson et al., 2004; Macdonald et al., 1995); it was detected in early passage bovine fibroblasts but not in the keratocyte or the spheres. In one study Pax6 expression was detected in a population of progenitor cells derived from the adult corneal keratocytes (Funderburgh et al., 2005), while in another study it was reported to be absent in mouse corneal keratocyte-derived spheres (Yoshida et al., 2005). We did not detect Pax6 in the spheres; as to why Pax6 is up regulated in the bovine fibroblast pool is unclear at this time, but it could be involved in a process akin to epithelial-to-mesenchymal transition as seen in development of lens fibers from epithelial cells during lens development (Martinez and de Iongh, 2010). The presence of CD133 in the bovine keratocyte-derived spheres underscores their connection to stem cells. CD133 is considered to be a common marker for adult (Rountree et al., 2007) and cancer stem cells (Yi et al., 2008), and reported in umbilical mesenchymal stem cells as well (Liu et al., 2010). Interestingly, we had earlier detected CD133/prominin transcript and the protein in the mouse cornea; here its presence in bovine, and not human keratocyte-derived spheres may indicate some species-specific differences in either the quantity or quality of stem cells in the corneal stroma.
Bovine keratocytes form spheres spontaneously when grown in suspension and a small proportion of the cells in adherent monolayers also form spheres. We further show that keratocytes seeded in serum containing medium can also form spheres which grow in size quite rapidly. Sphere formation was reported earlier from keratocyte aggregates grown in suspension on plastic coated with polyHEMA (Funderburgh et al., 2008) or from adherent cells on plastic coated with chitosan (Chen et al., 2009). The spheres we observed in serum-containing medium were larger than those in serum free conditions and on average increased more than 50% of their original size. The growth of the spheres may be related to increased proliferation of the cells in serum-containing medium, as these spheres appeared to contain many more cells than those grown in KGM or KPM. Whether the spheres in serum-containing medium behave more as fibroblasts than keratocytes remains to be seen. The spheres in the keratocyte growth medium maintain their mesenchymal identity as indicated by the presence of vimentin. Furthermore, the spheres contained an ECM that is rich in collagen and proteoglycans, and express collagen type I and the SLRP genes normally expressed in the cornea. The β1 integrin subunit, known to be present on corneal keratocytes (Stepp, 2006), was present in the spheres; it is likely that the spheres form through integrin-mediated attachment of the cells to the ECM they produce. The β1 integrin subunit is also present in neurospheres and in stem cells within the basal layer of the epidermis (Campos et al., 2004; Jensen et al., 1999).
Sphere formation from single cells was originally used to identify adult neural stem cells to test the capacity of a single cell to self-renew and the resulting clonal sphere to have the capacity to produce many cell types (Pastrana et al., 2011). This is now being used retrospectively to validate stem cells in various organs. This approach has been used on the mouse, rabbit and the human cornea (Mimura et al., 2008; Uchida et al., 2005; Yoshida et al., 2005). The murine study, although did not report an actual number, suggests a fairly high yield of self-renewing progenitor cells. The rabbit study reported ~ 40 spheres/10,000 cells or 0.4%. In our study, we derived spheres from cells that were sub-cultured from keratocytes maintained in monolayer for several weeks. Although, we did not use serial dilutions, the number of spheres per field for the human keratocytes indicate that ~5% of the cells have sphere formation abilities. An earlier study using serial dilution of human keratocytes, reported a slightly lower sphere formation frequency of ~ 1.5% (Uchida et al., 2005). The culture conditions in the Uchida study were different from ours in that they used B27 and epidermal growth factor as additional supplements in the medium. We did not determine sphere formation frequencies in the bovine keratocytes, and others have only examined sphere formation from aggregates of bovine keratocytes in suspension cultures (Funderburgh et al., 2005).
In summary, a significant number of the corneal stromal keratocytes have self-renewal capacity based on the sphere formation frequency reported in the current study and those by others. Our results further suggest that BMP3 and Cdh5 may serve as novel markers for keratocytes. Further elucidation of culture-conditions for expansion of keratocyte progenitor cells and appropriate distinguishing markers for each cellular phenotype will lead to significant advances in corneal cell therapies.
Research Highlights.
Approximately 5% of the human keratocytes produce spheres.
Fibroblasts revert to keratocytes after serum-deprivation for 2 weeks.
BMP3 and Cdh5 are novel keratocyte markers.
Acknowledgements
This work was supported by NIH/NEI (Ey11654 to SC). We thank Dr. John R. Hassell for critical reading of the manuscript and helping us with the immunohistochemistry of the spheres.
Abbreviations
- ECM
Extracellular matrix
- KPM
keratocyte plating medium
- KGM
keratocyte growth medium
- FGM
fibroblast growth medium
- SLRP
small leucine - rich repeat proteoglycans
- Cdh5
cadherin 5
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beales MP, Funderburgh JL, Jester JV, Hassell JR. Proteoglycan synthesis by bovine keratocytes and corneal fibroblasts: maintenance of the keratocyte phenotype in culture. Invest Ophthalmol Vis Sci. 1999;40:1658–1663. [PubMed] [Google Scholar]
- Berlau J, Becker HH, Stave J, Oriwol C, Guthoff RF. Depth and age-dependent distribution of keratocytes in healthy human corneas: a study using scanning-slit confocal microscopy in vivo. J Cataract Refract Surg. 2002;28:611–616. doi: 10.1016/s0886-3350(01)01227-5. [DOI] [PubMed] [Google Scholar]
- Campos LS, Leone DP, Relvas JB, Brakebusch C, Fassler R, Suter U, ffrench-Constant C. Beta1 integrins activate a MAPK signalling pathway in neural stem cells that contributes to their maintenance. Development. 2004;131:3433–3444. doi: 10.1242/dev.01199. [DOI] [PubMed] [Google Scholar]
- Carlson EC, Liu CY, Chikama T, Hayashi Y, Kao CW, Birk DE, Funderburgh JL, Jester JV, Kao WW. Keratocan, a cornea-specific keratan sulfate proteoglycan, is regulated by lumican. J Biol Chem. 2005;280:25541–25547. doi: 10.1074/jbc.M500249200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarti S, Magnuson T, Lass JH, Jepsen KJ, LaMantia C, Carroll H. Lumican regulates collagen fibril assembly: skin fragility and corneal opacity in the absence of lumican. J Cell Biol. 1998;141:1277–1286. doi: 10.1083/jcb.141.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarti S, Wu F, Vij N, Roberts L, Joyce S. Microarray Studies Reveal Macrophage-like Function of Stromal Keratocytes in the Cornea. Invest Ophthalmol Vis Sci. 2004;45:3475, 3484. doi: 10.1167/iovs.04-0343. [DOI] [PubMed] [Google Scholar]
- Chen YH, Wang IJ, Young TH. Formation of keratocyte spheroids on chitosan-coated surface can maintain keratocyte phenotypes. Tissue Eng Part A. 2009;15:2001–2013. doi: 10.1089/ten.tea.2008.0251. [DOI] [PubMed] [Google Scholar]
- Collinson JM, Hill RE, West JD. Analysis of mouse eye development with chimeras and mosaics. Int J Dev Biol. 2004;48:793–804. doi: 10.1387/ijdb.041885jc. [DOI] [PubMed] [Google Scholar]
- Funderburgh ML, Du Y, Mann MM, SundarRaj N, Funderburgh JL. PAX6 expression identifies progenitor cells for corneal keratocytes. FASEB J. 2005;19:1371–1373. doi: 10.1096/fj.04-2770fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funderburgh ML, Mann MM, Funderburgh JL. Keratocyte phenotype is enhanced in the absence of attachment to the substratum. Mol Vis. 2008;14:308–317. [PMC free article] [PubMed] [Google Scholar]
- Garagorri N, Fermanian S, Thibault R, Ambrose WM, Schein OD, Chakravarti S, Elisseeff J. Keratocyte behavior in three-dimensional photopolymerizable poly(ethylene glycol) hydrogels. Acta Biomater. 2008;4:1139–1147. doi: 10.1016/j.actbio.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell JR, Birk DE. The molecular basis of corneal transparency. Exp Eye Res. 2010;91:326–335. doi: 10.1016/j.exer.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay ED. Development of the vertebrate cornea. Int Rev Cytol. 1980;63:263–322. doi: 10.1016/s0074-7696(08)61760-x. [DOI] [PubMed] [Google Scholar]
- He J, Bazan HE. Epidermal growth factor synergism with TGF-beta1 via PI-3 kinase activity in corneal keratocyte differentiation. Invest Ophthalmol Vis Sci. 2008;49:2936–2945. doi: 10.1167/iovs.07-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helena MC, Baerveldt F, Kim WJ, Wilson SE. Keratocyte apoptosis after corneal surgery. Invest Ophthalmol Vis Sci. 1998;39:276–283. [PubMed] [Google Scholar]
- Jensen UB, Lowell S, Watt FM. The spatial relationship between stem cells and their progeny in the basal layer of human epidermis: a new view based on whole-mount labelling and lineage analysis. Development. 1999;126:2409–2418. doi: 10.1242/dev.126.11.2409. [DOI] [PubMed] [Google Scholar]
- Jester JV. Corneal crystallins and the development of cellular transparency. Semin Cell Dev Biol. 2008;19:82–93. doi: 10.1016/j.semcdb.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Barry-Lane PA, Cavanagh HD, Petroll WM. Induction of alpha-smooth muscle actin expression and myofibroblast transformation in cultured corneal keratocytes. Cornea. 1996;15:505–516. [PubMed] [Google Scholar]
- Jester JV, Ho-Chang J. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp Eye Res. 2003;77:581–592. doi: 10.1016/s0014-4835(03)00188-x. [DOI] [PubMed] [Google Scholar]
- Jester JV, Huang J, Barry-Lane PA, Kao WW, Petroll WM, Cavanagh HD. Transforming growth factor(beta)-mediated corneal myofibroblast differentiation requires actin and fibronectin assembly. Invest Ophthalmol Vis Sci. 1999;40:1959–1967. [PubMed] [Google Scholar]
- Lakshman N, Kim A, Petroll WM. Characterization of corneal keratocyte morphology and mechanical activity within 3-D collagen matrices. Exp Eye Res. 2010;90:350–359. doi: 10.1016/j.exer.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Zhang J, Liu CY, Wang IJ, Sieber M, Chang J, Jester JV, Kao WW. Cell therapy of congenital corneal diseases with umbilical mesenchymal stem cells: lumican null mice. PLoS One. 2010;5:e10707. doi: 10.1371/journal.pone.0010707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long CJ, Roth MR, Tasheva ES, Funderburgh M, Smit R, Conrad GW, Funderburgh JL. Fibroblast growth factor-2 promotes keratan sulfate proteoglycan expression by keratocytes in vitro. J Biol Chem. 2000;275:13918–13923. doi: 10.1074/jbc.275.18.13918. [DOI] [PubMed] [Google Scholar]
- Macdonald R, Barth KA, Xu Q, Holder N, Mikkola I, Wilson SW. Midline signalling is required for Pax gene regulation and patterning of the eyes. Development. 1995;121:3267–3278. doi: 10.1242/dev.121.10.3267. [DOI] [PubMed] [Google Scholar]
- Maltseva O, Folger P, Zekaria D, Petridou S, Masur SK. Fibroblast growth factor reversal of the corneal myofibroblast phenotype. Invest Ophthalmol Vis Sci. 2001;42:2490–2495. [PubMed] [Google Scholar]
- Martinez G, de Iongh RU. The lens epithelium in ocular health and disease. Int J Biochem Cell Biol. 2010;42:1945–1963. doi: 10.1016/j.biocel.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Maurice DM. The structure and transparency of the cornea. J. Physiology. 1957;136:263–286. doi: 10.1113/jphysiol.1957.sp005758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura T, Amano S, Yokoo S, Uchida S, Usui T, Yamagami S. Isolation and distribution of rabbit keratocyte precursors. Mol Vis. 2008;14:197–203. [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Hutcheon AE, Choi R, Hong J, Lee J, Ambrosio R, Jr., Zieske JD, Wilson SE. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp Eye Res. 2003;76:71–87. doi: 10.1016/s0014-4835(02)00251-8. [DOI] [PubMed] [Google Scholar]
- Musselmann K, Alexandrou B, Kane B, Hassell JR. Maintenance of the keratocyte phenotype during cell proliferation stimulated by insulin. J Biol Chem. 2005;280:32634–32639. doi: 10.1074/jbc.M504724200. [DOI] [PubMed] [Google Scholar]
- Musselmann K, Kane B, Alexandrou B, Hassell JR. Stimulation of collagen synthesis by insulin and proteoglycan accumulation by ascorbate in bovine keratocytes in vitro. Invest Ophthalmol Vis Sci. 2006;47:5260–5266. doi: 10.1167/iovs.06-0612. [DOI] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Sinha S, Sharma A, Dupps W, Wilson SE. Stromal haze, myofibroblasts, and surface irregularity after PRK. Exp Eye Res. 2006;82:788–797. doi: 10.1016/j.exer.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastrana E, Silva-Vargas V, Doetsch F. Eyes wide open: a critical review of sphere-formation as an assay for stem cells. Cell Stem Cell. 2011;8:486–498. doi: 10.1016/j.stem.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quantock AJ, Meek KM, Chakravarti S. An x-ray diffraction investigation of corneal structure in lumican-deficient mice. Invest Ophthalmol Vis Sci. 2001;42:1750–1756. [PubMed] [Google Scholar]
- Rountree CB, Barsky L, Ge S, Zhu J, Senadheera S, Crooks GM. A CD133-expressing murine liver oval cell population with bilineage potential. Stem Cells. 2007;25:2419–2429. doi: 10.1634/stemcells.2007-0176. [DOI] [PubMed] [Google Scholar]
- Stepp MA. Corneal integrins and their functions. Exp Eye Res. 2006;83:3–15. doi: 10.1016/j.exer.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Stewart A, Guan H, Yang K. BMP-3 promotes mesenchymal stem cell proliferation through the TGF-beta/activin signaling pathway. J Cell Physiol. 2010;223:658–666. doi: 10.1002/jcp.22064. [DOI] [PubMed] [Google Scholar]
- Thill M, Schlagner K, Altenahr S, Ergun S, Faragher RG, Kilic N, Bednarz J, Vohwinkel G, Rogiers X, Hossfeld DK, Richard G, Gehling UM. A novel population of repair cells identified in the stroma of the human cornea. Stem Cells Dev. 2007;16:733–745. doi: 10.1089/scd.2006.0084. [DOI] [PubMed] [Google Scholar]
- Uchida S, Yokoo S, Yanagi Y, Usui T, Yokota C, Mimura T, Araie M, Yamagami S, Amano S. Sphere formation and expression of neural proteins by human corneal stromal cells in vitro. Invest Ophthalmol Vis Sci. 2005;46:1620–1625. doi: 10.1167/iovs.04-0288. [DOI] [PubMed] [Google Scholar]
- Watsky MA. Keratocyte gap junctional communication in normal and wounded rabbit corneas and human corneas. Invest Ophthalmol Vis Sci. 1995;36:2568–2576. [PubMed] [Google Scholar]
- Yi JM, Tsai HC, Glockner SC, Lin S, Ohm JE, Easwaran H, James CD, Costello JF, Riggins G, Eberhart CG, Laterra J, Vescovi AL, Ahuja N, Herman JG, Schuebel KE, Baylin SB. Abnormal DNA methylation of CD133 in colorectal and glioblastoma tumors. Cancer Res. 2008;68:8094–8103. doi: 10.1158/0008-5472.CAN-07-6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Shimmura S, Shimazaki J, Shinozaki N, Tsubota K. Serum-free spheroid culture of mouse corneal keratocytes. Invest Ophthalmol Vis Sci. 2005;46:1653–1658. doi: 10.1167/iovs.04-1405. [DOI] [PubMed] [Google Scholar]
- Zieske JD, Hutcheon AE, Guo X, Chung EH, Joyce NC. TGF-beta receptor types I and II are differentially expressed during corneal epithelial wound repair. Invest Ophthalmol Vis Sci. 2001;42:1465–1471. [PubMed] [Google Scholar]