Abstract
Estradiol’s inhibitory effect on food intake is mediated, in part, by its ability to increase the activity of meal-related signals, including serotonin (5-HT), which hasten satiation. The important role that postsynaptic 5-HT2C receptors play in mediating 5-HT’s anorexigenic effect prompted us to investigate whether a regimen of acute estradiol treatment increases the anorexia associated with increased 5-HT2C receptor activation in ovariectomized (OVX) rats. We demonstrated that intraperitoneal and intracerebroventricular (i.c.v.) administration of low doses of the 5-HT2C receptor agonist meta-chlorophenylpiperazine (mCPP) decreased 1-h dark-phase food intake in estradiol-treated, but not oil-treated, OVX rats. During a longer feeding test, we demonstrated that i.c.v. administration of mCPP decreased 22-h food intake in oil-treated and, to a greater extent, estradiol-treated OVX rats. In a second study, we demonstrated that estradiol increased 5-HT2C receptor protein content in the caudal brainstem, but not hypothalamus, of OVX rats. We conclude that a physiologically-relevant regimen of acute estradiol treatment increases sensitivity to mCPP’s anorexigenic effect. Our demonstration that this same regimen of estradiol treatment increases 5-HT2C protein content in the caudal hindbrain of OVX rats provides a possible mechanism to explain our behavioral findings.
Keywords: estrogen, serotonin, mCPP, caudal brainstem, food intake
1. Introduction
Estrogens exert a range of behavioral effects that are mediated primarily by estradiol, the most abundant circulating form of the naturally occurring estrogens. One well-established behavioral action of estradiol is its potent anorexigenic effect. Cycling female rats display a decrease in dark-phase feeding during estrus [1,2], and recent studies have confirmed that the peri-ovulatory increase in estradiol secretion plays a causal role in mediating this cyclic change in feeding behavior [3,4]. It has also been shown that ovariectomy-induced estradiol deficiency promotes a sustained increase in daily food intake [5,6]. Taken together, these studies suggest that endogenous estradiol plays an important role in regulating food intake and body weight in female rats. In order to facilitate mechanistic investigations of estradiol’s anorexigenic effect, Geary and Asarian [7] developed a regimen of acute estradiol treatment for ovariectomized (OVX) rats that normalizes daily food intake and mimics the estrous-related decrease in dark-phase food intake that occurs in cycling rats. Unlike models of continuous estradiol replacement (via chronic injection or implants), this provides an important, physiologically-relevant model for investigating the mechanism underlying estradiol’s inhibitory effect on feeding behavior.
It is well established that estradiol decreases food intake via a selective decrease in meal size [1,8], and a growing body of literature suggests that estradiol exerts this effect indirectly, by increasing the activity of meal-related signals that hasten satiation (reviewed in [9,10]). Studies from our lab and others implicate the serotonin (5-HT) system as an important estrogenic target in this regard. First, a role for central 5-HT in the direct control of meal size is well established. In rats, feeding promotes rapid increases in extracellular concentration of 5-HT in the lateral hypothalamus [11], and systemic administration of drugs that increase 5-HT neurotransmission inhibit food intake by decreasing meal size, not meal number [12–14]. Second, we have shown that the anorexigenic effect of the serotonergic agonist fenfluramine (FEN) is increased during estrus in cycling rats [15], and by a regimen of acute estradiol treatment in OVX rats [16]. Interpretation of our findings is limited, however, by FEN’s complex pharmacological profile. FEN increases 5-HT neurotransmission by increasing both the presynaptic release and reuptake of 5-HT, and by activating excitatory, postsynaptic 5-HT2C receptors [17], which play an important role in mediating 5-HT’s inhibitory effect on feeding [18–20]. Although FEN increases 5-HT neurotransmission via multiple mechanisms, its anorexigenic effect appears to be mediated primarily by its postsynaptic action. This view is supported by studies showing that FEN’s anorexigenic effect is attenuated by selective blockade or targeted deletion of 5-HT2C receptors [21,22], but is unaffected by drugs that decrease 5-HT synthesis, deplete 5-HT storage, or block 5-HT reuptake [23–25]. Thus, the most parsimonious interpretation of our findings is that estradiol increases FEN’s anorexigenic effect by augmenting its postsynaptic action at 5-HT2C receptors.
In the current study, we examined estradiol’s ability to increase the anorexia associated with increased 5-HT2C receptor activation. Food intake was monitored in oil- and estradiol-treated OVX rats following acute administration of meta-chlorophenylpiperazine (mCPP). Although mCPP is characterized as a mixed 5-HT2C/1B receptor agonist, it has a 15-fold higher binding affinity for 5-HT2C receptors over 5-HT1B receptors [26,27], it functions as a more potent agonist at the 5-HT2C receptor subtype than does FEN [17], and its anorexigenic effect is abolished in transgenic mice that lack 5-HT2C receptors and rats pretreated with 5-HT2C receptor antagonists [20,26,28,29]. Thus, mCPP’s anorexigenic effect appears to be mediated exclusively via activation of 5-HT2C receptors. The results of our first experiment confirmed our hypothesis that a regimen of acute estradiol treatment increases the anorexigenic effect of mCPP. In order to investigate a possible mechanism to account for our behavioral findings, we conducted a second experiment that examined whether a similar regimen of acute estradiol treatment increases 5-HT2C receptor protein content in brain areas that receive afferent projections from 5-HT neurons. We demonstrated that estradiol increased 5-HT2C receptor protein content in the caudal brainstem, but not hypothalamus, of OVX rats.
2. Materials and methods
2.1. Animals and surgery
Female Long-Evans rats (Charles River Breeding Laboratories, Raleigh, NC; 200–250 g body weight) were individually housed either in custom cages equipped to accurately measure food intake or in standard, plastic tub cages. The testing room was maintained at 20 ± 2°C and on a reverse 12:12 h lighting cycle (dark onset at 1300 h). Rat chow (Purina 5001) and tap water were freely available unless otherwise specified. All animal procedures were approved by the Florida State University Institutional Animal Care and Use Committee.
Rats were anesthetized with intraperitoneal (i.p.) injections of a mixture of 50 mg/kg ketamine (Ketaset, Fort Dodge Inc., Fort Dodge, IA) and 4.5 mg/kg xylazine (Rompun, Mobay, Shawnee, KS) and then bilaterally OVX using an intra-abdominal approach. Immediately after ovariectomy surgery, a subset of rats were implanted with 26 gauge stainless-steel guide cannulas (Plastics One, Roanoke, VA) aimed at the right lateral ventricle (stereotaxic coordinates: caudal 0.6 mm, lateral 1.7 mm, and ventral 3.5 mm relative to bregma [30]). Following surgery, all rats received i.p. injections of butorphanol (0.5 mg/kg, Fort Dodge, Fort Dodge, IA) to minimize postoperative pain and the subset of rats implanted with ventricular cannulas also received i.p. injections of gentamicin (10 mg/ml; ProLabs Ltd., St. Joseph, MO) to minimize risk of infection. After 7 days of postoperative recovery, a behavioral assay was performed to verify cannula placement [31]. Light-phase water intake was monitored in individual rats following intracerebroventricular (i.c.v.) infusions of 50 ng of angiotensin II (Sigma-Aldrich, St. Louis, MO) delivered in 5 μl of saline vehicle over a period of 1 min. All rats consumed at least 5 ml of water in the 20 min period following angiotensin II infusion and were, therefore, included in the study. Behavioral testing in this group commenced 1 week later.
2.2. Experiment 1: Feeding tests following peripheral and central administration of mCPP
Estradiol’s ability to increase the anorexigenic effect of peripherally-injected mCPP was assessed during brief feeding tests conducted over a two-week period. On days 1 and 2 of each week, OVX rats received s.c. injections of either 0.1 ml sesame oil vehicle (oil group, n = 5) or 1 μg of 17β-estradiol benzoate (Sigma-Aldrich, Boston, MA) delivered in 0.1 ml oil (estradiol group, n = 5). Injections were administered 4 h prior to dark onset. As shown previously by Geary and Asarian [7], this regimen of acute estradiol treatment increases plasma estradiol to the level observed in proestrous rats, and promotes a transient decrease in dark-phase food intake (on test day 4) that mimics the estrous-related decrease in food intake in cycling rats. Accordingly, dark-phase feeding tests were conducted on day 4 of each week. On test days, food was removed from the rats’ cages during the last 2-h of the light phase. Although rats do not typically initiate meals during this period [1], we took this precaution in order to prevent any possibility that rats might consume a meal just prior to the feeding test. Using a within-subject design, estradiol- and oil-treated rats received i.p. injections of either 1.0 mg/kg mCPP (Tocris Bioscience, Ellisville, MO) dissolved in physiological saline vehicle or 1 ml/kg saline vehicle 30 min prior to dark onset, in random order across the two weeks. Food was returned to the rats’ cages at dark onset and food intake was measured during the first hour of the dark phase. The duration of the feeding test was chosen because mCPP induces a brief anorexigenic effect [32,33], consistent with its short half-life [34]. The dose of mCPP was chosen on the basis of previous research, which has been conducted almost exclusively in food-deprived male rats entrained to consume large test meals [e.g., 32,35]. Because our study involved a 1-h feeding test conducted in free-fed female rats, we anticipated that the dose of mCPP administered under our test conditions would be in the threshold, or perhaps subthreshold, anorexigenic range. Here, female rats were expected to consume ~ 1–3 g of chow during the feeding test, so a low dose of mCPP was necessary to avoid the possibility of a floor effect.
Our first feeding study involved peripheral administration of mCPP in order to be consistent with the bulk of the mCPP feeding literature. Because 5-HT2C receptors are expressed almost exclusively in the brain [36,37], we conducted a second feeding study to assess estradiol’s ability to increase the anorexigenic effect of centrally-administered mCPP over a four-week period. Using a within-subjects design (to minimize the number of rats subjected to the more invasive i.c.v. surgical procedure), OVX rats (n = 7) received s.c. injections of 1 μg estradiol (weeks 1 and 3) and oil (weeks 2 and 4) 4 h prior to dark onset on days 1 and 2 of each week. On tests days (day 4 of each week), rats received i.c.v. infusions of either 250 μg mCPP delivered in 2.5 μl artificial cerebral spinal fluid (aCSF) or aCSF alone. The dose of mCPP was chosen based on a pilot study that revealed it to be the lowest dose to exert an anorexigenic effect in estradiol-treated OVX rats. Infusions were administered over a period of 1 min, beginning 30 min prior to dark onset, and aCSF and mCPP were presented in a random order across the four weeks. Food was returned to the rats’ cages at dark onset and food intake was measured at the end of the first hour of the dark phase. In this study, we also measured food intake at 22-h after aCSF/mCPP infusions (i.e., the following morning when daily maintenance was conducted) in order to determine whether an extended feeding period would reveal an anorexigenic effect of mCPP in oil-treated rats and to confirm that our regimen of acute estradiol treatment produced a reliable decrease in dark-phase food intake.
2.3. Experiment 2: 5-HT2C receptor protein content in the brains of estradiol-treated OVX rats
2.3.1. Collection of brain tissue
OVX rats received s.c. injections of either 0 or 4 μg estradiol in sesame oil vehicle (n = 4/group) on two consecutive days (day 1 and day 2), 4-h prior to dark onset. In order to maximize our ability to detect a change in 5-HT2C receptor protein content, we used a slightly larger (but still physiologically relevant) dose of estradiol than was used in our behavioral studies. On day 4 (the day that mimics behavioral estrus), vaginal cytology samples were collected to confirm that estradiol treatment produced vaginal epithelial cell cornification (i.e., non-nucleated, cornified cells), which correlates with behavioral estrus. All rats were anesthetized with i.p. injections of Nembutal (65 mg/kg; sodium pentobarbital; Henry Schein, Mellville, NY). When a surgical plane of anesthesia was achieved, rats were rapidly decapitated and the brains were dissected into caudal brainstem and hypothalamic blocks (Fig. 1). To obtain the caudal brainstem block, the cerebellum was removed and coronal cuts were made on the ventral surface of the brain at the trapezoid body (−10.04 mm from bregma) and the spinal cord (−14.6 mm from bregma). The dorsal one-third of the remaining brainstem block (that included the nucleus of the solitary tract/area postrema region) was retained. To obtain the hypothalamic block, coronal cuts were made on the ventral surface of the brain at the optic chiasm (−0.4 mm from bregma) and the dorsal portion of the mammillary bodies (−4.3 mm from bregma). The left and right one-third lateral portions of the resulting forebrain block of tissue were cut off (including the amygdala), and the ventral one-third of the remaining forebrain block (that included the hypothalamic region) was retained. Tissue blocks were wrapped in aluminum foil, placed on dry ice, and stored at −80°C until Western blot processing.
Fig. 1.
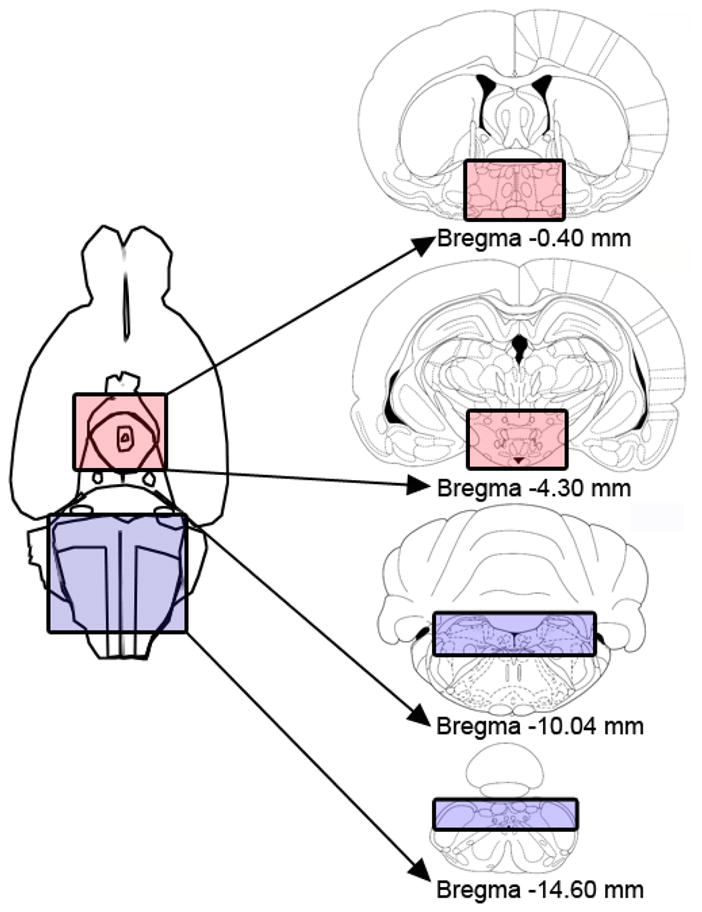
Illustration of the ventral surface of the brain and the stereotaxic coordinates used to obtain caudal brainstem and hypothalamic tissue blocks for subsequent analysis of 5-HT2C receptor protein content in oil- and estradiol-treated OVX rats. The box shaded in blue illustrates the caudal brainstem block and the box shaded in red illustrates the hypothalamic block.
2.3.2. Tissue sample preparation
Caudal brainstem and hypothalamic blocks of tissue were homogenized in 800 μl RIPA/DOC lysis buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1 mM EDTA, 0.1 % SDS, 1 % Nonidet P-40, and 0.25 % sodium deoxycholate) containing Halt protease/phosphatase inhibitor cocktail (Thermo Fisher Scientific, Rockford, IL). Tubes containing the homogenate were placed on wet ice for 30 min and then centrifuged at 15,000 × g for 30 min. Protein concentrations in the supernatant were determined by a protein assay kit (Pierce Biotechnology, Rockford, IL).
2.3.3. SDS Page and Immunoblotting
Extracts containing equal amounts of protein (8 μg total protein) were denatured at 95°C in 25% 4X sample buffer (Invitrogen, Carlsbad, CA) for 10 min. Samples were loaded onto a 12% SDS-PAGE protein gel (Thermo Fisher Scientific) and transferred onto Hybond-P Western blot membranes (VWR, Radnor, PA). Membrane blots were washed with Tris-buffered saline (TBS), incubated in blocking buffer (2% ECL advanced blocking solution dissolved in TBS containing Tween 20 (TBS-T); GE Healthcare, Waukesha, WI) for 1 hr at RT, and then incubated with a primary polyclonal rabbit antibody (ab32172; Abcam, Cambridge, MA; 1:10,000 dilution) directed against the 5-HT2C receptor overnight at 4°C. The next day, membrane blots were washed in TBS-T and incubated in horseradish peroxidase-conjugated secondary antibody (goat anti-rabbit antibody; ab7171; 1:20,000 dilution) for 1 hr at RT. After probing blots with the 5-HT2C receptor antibody, blots were stripped and re-probed with the protein loading control, β-actin (monoclonal mouse antibody; sc69879; Santa Cruz Biotechnology Inc., Santa Cruz, CA; 1:2,000 dilution) followed by incubation with a horseradish peroxidase-conjugated secondary sheep anti-mouse antibody (AP326P; Millipore, Billerica, MA; 1:20,000 dilution). The immunosignal on membrane blots was detected using an enhanced chemiluminescent kit (GE Healthcare). Following development of chemiluminescence film, images of protein bands were captured using a Kodak Imaging Station 440 CF (Kodak, Rochester, New York). Densitometric analysis was performed using Kodak 10 Imaging Analysis software (Kodak) that measures the optical density of the immunosignal.
2.4. Data analysis
In Experiment 1, feeding data were analyzed via a two-factor, mixed-design ANOVA (1-h food intake after peripheral administration of mCPP) and two-factor repeated-measures ANOVAs (1- and 22-h food intake after central administration of mCPP). Post-hoc and planned comparisons were made with Newman-Keuls tests. P values lower than 0.05 were considered statistically significant. The anorexigenic effect of centrally infused mCPP was further characterized by determining the percent suppression in 22-h food intake following mCPP infusion, relative to aCSF infusion, in oil- and estradiol-treated rats. The resulting suppressions scores were analyzed via a dependent t-test. In Experiment 2, optical densities for 5-HT2C receptor and β-actin were analyzed as the ratio of 5-HT2C receptor/β-actin. The effects of hormone treatment on caudal brainstem and hypothalamic 5-HT2C receptor protein content were analyzed via independent t-tests.
3. Results
3.1. Experiment 1: Feeding tests
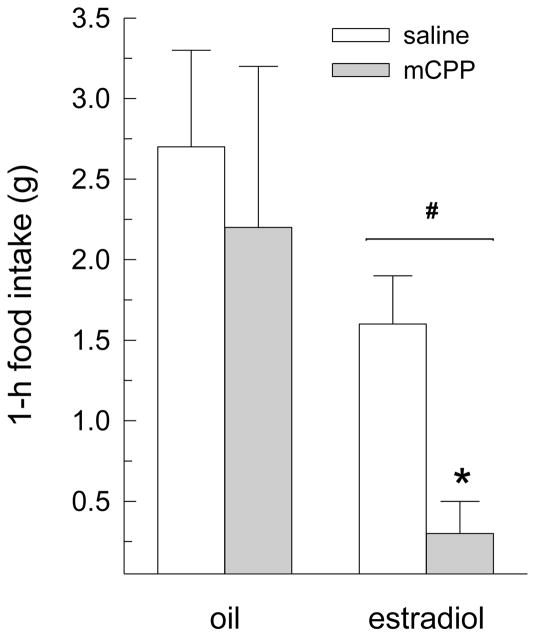
Peripheral injection of mCPP influenced 1-h dark-phase food intake (main effect of drug treatment, F(1,8) = 8.34, P < 0.05, Fig. 2). Group comparisons revealed that mCPP decreased food intake in estradiol-treated rats (P < 0.05), but not in oil-treated rats. Although the main and interactive effects of hormone treatment did not reach statistical significance (F(1,8) = 2.54 and 1.90, respectively, n.s.), planned comparisons revealed that estradiol-treated rats consumed less than oil-treated rats following injections of saline and mCPP (Ps < 0.05).
Fig. 2.
Peripheral injection of a low (1 mg/kg) dose of mCPP decreases dark-phase food intake in estradiol-treated OVX rats. Dark-phase food intake was measured for 1 h after i.p. injection of saline and mCPP in oil- and estradiol-treated OVX rats. An anorexigenic effect of mCPP was detected only in estradiol-treated rats. Data are means ± SEMs. *Less than estradiol/saline, P < 0.05. #Less than oil-treated rats, P < 0.05.
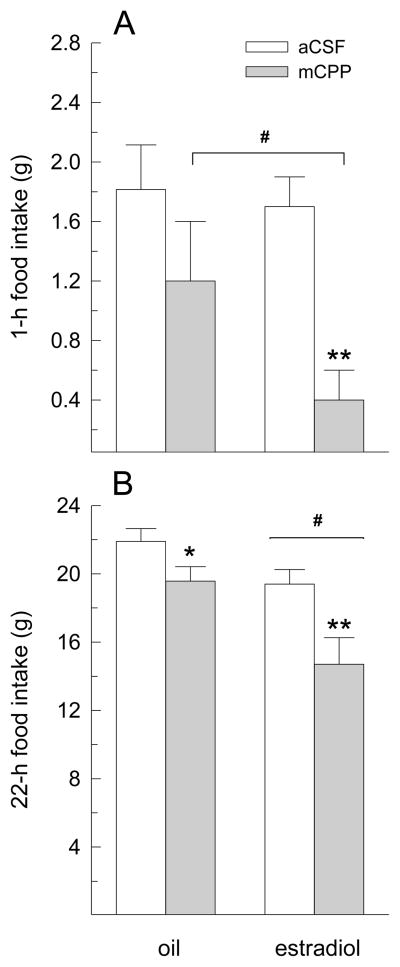
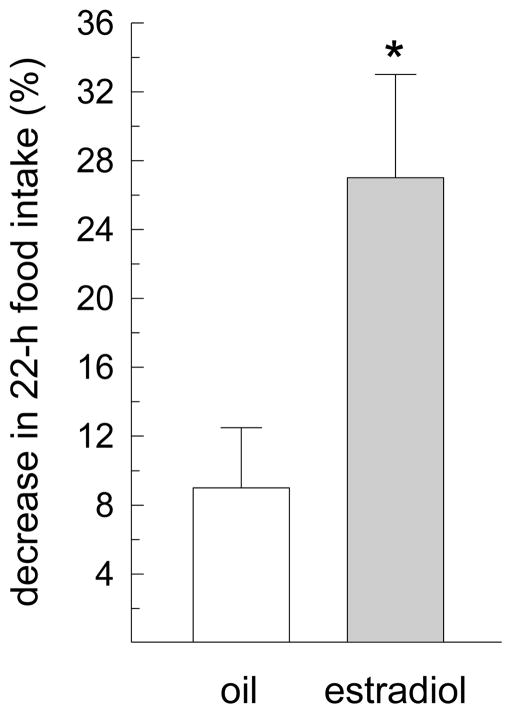
Central infusion of mCPP also influenced 1-h dark-phase food intake (main effect of drug treatment, F(1,6) = 25.23, P < 0.005, Fig. 3A). Group comparisons revealed that mCPP decreased food intake in estradiol-treated rats (P < 0.05), but not in oil-treated rats. Although the main and interactive effects of hormone treatment did not reach statistical significance (F(1,6) = 1.02 and 1.23, respectively, n.s.), planned comparisons revealed that estradiol-treated rats consumed less than oil-treated rats following infusions of mCPP (P < 0.05), but not following infusions of aCSF. Central infusion of mCPP also influenced 22-h food intake (main effect of drug treatment, F(1,6) = 14.39, P < 0.01, Fig. 3B). Group comparisons revealed that mCPP decreased food intake in oil-treated rats (P < 0.05) and, to a greater extent, in estradiol-treated rats (P < 0.01). Daily food intake was also decreased by a main effect of hormone treatment, F(1,6) = 11.93, P < 0.05. Estradiol-treated rats consumed less than oil-treated rats following infusion of aCSF and mCPP (Ps < 0.05). Although the interaction between drug and hormone treatment failed to reach statistical significance, F(1,6) = 3.06, P =0.13, an analysis of the percent suppression in 22-h food intake following infusion of mCPP, relative to aCSF, revealed a larger anorexigenic effect in estradiol-treated rats, relative to oil-treated rats, t(9) = 2.71, P < 0.05 (Fig. 4).
Fig. 3.
The anorexigenic effect of centrally infused mCPP is increased by estradiol treatment in OVX rats. Dark-phase food intake was measured at 1 and 22 h after i.c.v. infusion of aCSF and 250 μg mCPP in oil- and estradiol-treated OVX rats. (A) mCPP decreased 1-h dark-phase food intake in estradiol-treated, but not oil-treated, OVX rats. (B) mCPP decreased 22-h dark-phase food intake in oil- and estradiol-treated rats, however, the magnitude of this effect was increased by estradiol treatment. Data are means ± SEMs. *Less than oil/aCSF, P < 0.05. **Less than estradiol/aCSF, P < 0.01. #Less than oil-treated rats, P < 0.05.
Fig. 4.
Estradiol increases the suppressive effect of mCPP on 22-h food intake. The percent suppression in 22-h food intake following mCPP infusion, relative to aCSF infusion, was determined in oil- and estradiol-treated rats. Data are means ± SEMs. *Greater than oil-treated rats, P < 0.05.
3.2. Experiment 2: 5-HT2C receptor protein content
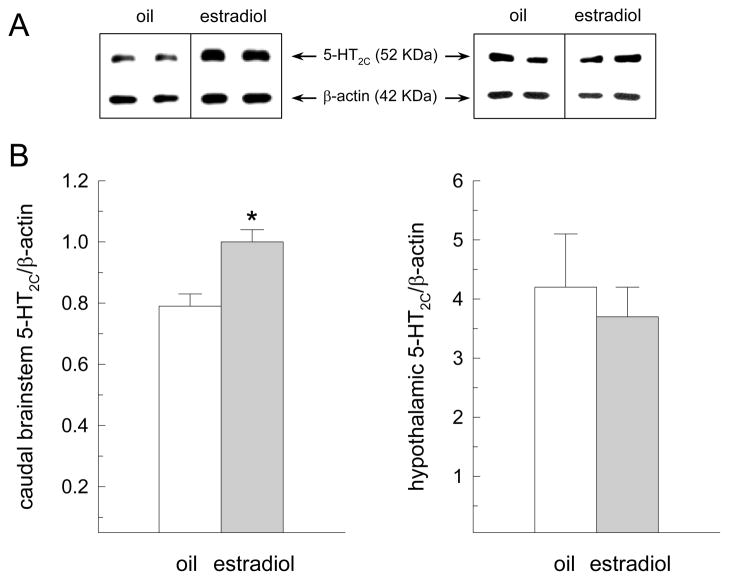
As depicted in representative immunoblots, the 5-HT2C receptor subtype was detected as a 52 kDa peptide and β-actin was detected as a 42 kDa peptide (Fig. 5A). Estradiol treatment increased 5-HT2C receptor protein content in tissue extracts from the caudal brainstem, t(6) = 3.03, P < 0.05, but not hypothalamus, t(6) = 1.10, n.s. (Fig. 5B).
Fig. 5.
Estradiol increases 5-HT2C receptor protein content in the caudal brainstem of OVX rats. OVX rats received acute s.c. injections of 1 μg estradiol or oil on tests days 1 and 2 (n = 4 per group). On test day 4 (the day in which food intake is decreased in estradiol-treated rats) caudal brainstem and hypothalamic tissue extracts were obtained for subsequent analysis of 5-HT2C receptor protein content. (A) Representative immunoblots of 5-HT2C receptor and β-actin from a subset of individual oil- and estradiol-treated rats. (B) Quantification of the optical density of the protein bands expressed as means ± SEMs. Estradiol treatment increased 5-HT2C receptor protein content in the caudal brainstem, but not hypothalamus, of OVX rats. *Greater than oil-treated rats, P < 0.05.
4. Discussion
The current findings demonstrate that acute estradiol treatment alters the anorexia associated with increased 5-HT2C receptor activation in OVX rats. Peripheral and central administration of low doses of mCPP that did not alter 1-h dark-phase food intake in oil-treated rats produced robust anorexigenic effects in estradiol-treated rats. We also observed that central infusion of mCPP decreased 22-h food intake in oil- and estradiol-treated rats, however, the magnitude of this effect was increased by estradiol treatment. These results suggest that our earlier demonstration of estradiol’s ability to increase the anorexia associated with increased 5-HT neurotransmission [15,16] is mediated, at least in part, by augmenting the activation of postsynaptic 5-HT2C receptors. This idea is further supported by our demonstration that caudal brainstem 5-HT2C receptor protein content is increased by estradiol treatment in OVX rats at a time when our regimen of acute estradiol treatment has been shown to decrease food intake in OVX rats [7]. This association raises the possibility that caudal brainstem 5-HT2C receptors may play an important role in mediating estradiol’s anorexigenic effect.
The short-term (1-h) feeding tests conducted in the current study support our hypothesis that estradiol increases the anorexigenic response to mCPP. We showed that peripheral and central administration of mCPP produced robust decreases in 1-h dark-phase food intake in estradiol-treated, but not oil-treated, OVX rats. We believe that two factors may have contributed to our inability to detect a short-term anorexigenic effect of mCPP in oil-treated rats. First, the doses of mCPP administered here are among the lowest of the anorexigenic doses reported in the literature (e.g., [(32,33,35]) and identified in our pilot study. We chose low doses of mCPP in order to minimize the possibility of a floor effect in our short-term feeding tests, which were conducted during the first hour of the dark phase when female rats typically consume no more than 3 g of chow [1]. Second, our study involved free-fed rats. This is in contrast to the bulk of the mCPP feeding literature, which has been conducted in rats adapted to prolonged (12–24 h) food-deprivation schedules (reviewed in [38]). Previous research has shown that the anorexigenic effect of mCPP is increased in food-deprived rats that have been entrained to consume large test meals [35]. Thus, under our experimental test conditions, mCPP may have been administered in doses that were very near or perhaps subthreshold to suppress 1-h food intake in oil-treated rats, which we predicted would be less sensitive to the anorexigenic effect of mCPP than estradiol-treated rats. This idea is supported by our observation that the individual variability in mCPP’s anorexigenic effect was greater in the absence of estradiol (i.p. and i.c.v. mCPP decreased 1-h food intake in 40% and 57% of oil-treated rats, respectively), than in the presence of estradiol (i.p. and i.c.v. mCPP decreased food intake in 100% of estradiol-treated rats).
Our assessment of 22-h food intake provides additional support for our hypothesis that estradiol increases the anorexigenic response to mCPP. Although central infusion of mCPP induced a reliable decrease in 22-h food intake in oil- and estradiol-treated rats, the magnitude of this anorexigenic response was greater in estradiol-treated rats. These data extend the findings of our short-term feeding tests by showing that the low dose of mCPP used in the current study can suppress food intake in oil-treated rats, but that this occurs sometime after the first hour of the dark phase. These data also confirm that our regimen of acute estradiol treatment produces a reliable decrease in 22-h food intake, which is consistent with previous studies using very low (1–2 μg) doses of estradiol [7,39–42]. In contrast, our short-term feeding tests revealed modest anorexigenic effects of estradiol treatment during the first hour of the dark phase in all but one test condition (estradiol did not decrease 1-h food intake in rats receiving i.c.v. infusions of aCSF). This is also consistent with previous studies which have shown that either higher doses of estradiol (4–10 μg) or food-deprivation is necessary to reveal a reliable anorexigenic effect of estradiol within the first few hours of spontaneous or scheduled test meals [16,39].
Previous studies have shown that the anorexia associated with 5-HT2C receptor signaling is sexually dimorphic in rats. First, both acute and chronic administration of FEN (presumably acting on 5-HT2C receptors) produced larger decreases in food intake in cycling females, relative to males [15,43]. Second, the anorexigenic effect of mCPP during a test meal in food-deprived rats was greater in cycling females, relative to males [14,35]. One interpretation of these findings is that endogenous estradiol mediates the sex- and estrous-related changes in feeding behavior induced by drugs that increase 5-HT neurotransmission. Our current findings, that mCPP’s anorexigenic effect is increased by a regimen of acute estradiol treatment that mimics the changes in endogenous estradiol concentration in cycling rats [7], provide additional support for this hypothesis.
As a first step towards investigating the mechanism underlying estradiol’s ability to increase mCPP’s anorexigenic effect, we examined 5-HT2C receptor protein content in the brains of oil- and estradiol-treated OVX rats. The caudal brainstem and hypothalamus were targeted because they contain neuronal populations implicated in both the estrogenic and serotonergic control of food intake [28,31,44,45]. We found that acute estradiol treatment increased 5-HT2C receptor protein content in the caudal brainstem, but not hypothalamus, of OVX rats. To the best of our knowledge, this represents the first demonstration that a physiologically-relevant regimen of estradiol treatment is sufficient to increase caudal brainstem 5-HT2C receptor protein. Previous studies have shown that chronic estradiol treatment increases 5-HT2C receptor protein content in the midbrain of OVX monkeys [46] and 5-HT2C receptor mRNA expression in the midbrain and hypothalamus of OVX rats [47]. The present findings extend these reports by showing that acute estradiol treatment is sufficient to increase caudal brainstem, but not hypothalamic, 5-HT2C receptor protein content in OVX rats. The reasons for the differential localization of estradiol-mediated increases in 5-HT2C receptor expression in the present and previous studies are unclear, but may be related to the regimen of estradiol treatment (acute versus chronic). For example, in the study involving monkeys, 5 months, but not 1 month, of chronic estradiol treatment was required to increase midbrain 5-HT2C receptor protein [46]. Likewise, 4 weeks of chronic estradiol treatment was required to increase hypothalamic 5-HT2C receptor mRNA expression in OVX rats [47]. Since estradiol levels are not typically elevated for weeks or months at a time, the functional significance of these previous studies is unclear.
Converging evidence suggests that the caudal brainstem is an important neural substrate for mediating the anorexigenic effects of both mCPP and estradiol. First, Grill et al [28] have shown that mCPP decreases intra-oral glucose intake in chronic decerebrate rats (which lack direct neural connections with the forebrain), and fourth ventricular infusions of the 5-HT2C receptor antagonist mesulergine abolishes the anorexigenic effect of i.p. injected mCPP. These findings suggest that 5HT2C receptors located in the caudal brainstem are both sufficient and necessary for mediating mCPP’s anorexigenic effect. Second, direct application of estradiol to the dorsal surface of the caudal brainstem, within the region of the nucleus of the solitary tract (NTS), is sufficient to decrease food intake in OVX rats [45]. Third, a regimen of acute estradiol treatment increases feeding-induced neuronal activation within the NTS of OVX rats [48]. Fourth, estradiol’s ability to increase the satiating effect of cholecystokinin (CCK), a meal-related signal that is similar to 5-HT in that both hasten satiation [49], is mediated, in part, via neurons within the NTS [50]. Finally, 5-HT2C receptor signaling appears necessary for the full expression of CCK’s anorexigenic effect [51,52], and serotonergic input to 5-HT2C receptors in the NTS may be necessary for CCK-induced anorexia [51]. Thus, our current finding that estradiol increases caudal brainstem (but not hypothalamic) expression of 5-HT2C receptor protein is particularly interesting in light of these previous reports. Taken together, the previous and current findings present a novel mechanism by which estradiol may function to increase the anorexigenic effects of both CCK and 5-HT.
In summary, we have shown that a regimen of acute estradiol treatment increases the anorexia associated with 5-HT2C receptor activation following peripheral and central administration of mCPP. Because mCPP readily crosses the blood-brain-barrier [34], and there is little evidence that 5-HT2C receptors are expressed outside of the brain [36,37], it is likely that the anorexia we observed in estradiol-treated rats following peripheral injection of mCPP resulted from the activation of central, rather than peripheral, 5-HT2C receptors. Based on our recent demonstration that central, but not peripheral, estrogen receptors are necessary for estradiol’s anorexigenic effect in OVX rats [41], it is likely that estradiol acted within the brain to increase the anorexia associated with increased 5-HT2C receptor signaling in our first experiment. The results of our second experiment suggest that the site of this estradiol/5-HT2C interaction may lie somewhere in the caudal brainstem. It will be important in future studies to examine whether estradiol’s anorexigenic effect is attenuated by 5-HT2C receptor blockade in targeted regions of the caudal brainstem, including the NTS, and to determine the phenotype and precise locations of the estradiol-responsive neurons expressing 5-HT2C receptors.
Highlights.
Estradiol increases the anorexigenic effect of the 5-HT2C receptor agonist, mCPP.
Estradiol increases 5-HT2C receptor protein in the caudal brainstem of OVX rats.
Estradiol does not increase hypothalamic 5-HT2C receptor protein in OVX rats.
Acknowledgments
This work was supported by grants from the NIH: DK-073936 and MH-063932 (LAE), T32 DC-00044 (HMR) and F31 NS-062667 (JS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eckel LA, Houpt TA, Geary N. Spontaneous meal patterns in female rats with and without access to running wheels. Physiol Behav. 2000;70:397–405. doi: 10.1016/s0031-9384(00)00278-x. [DOI] [PubMed] [Google Scholar]
- 2.Drewett RF. Oestrous and dioestrous components of the ovarian inhibition on hunger in the rat. Anim Behav. 1973;21:772–80. doi: 10.1016/s0003-3472(73)80103-4. [DOI] [PubMed] [Google Scholar]
- 3.Santollo J, Eckel LA. Effect of a putative ERα antagonist, MPP, on food intake in cycling and ovariectomized rats. Physiol Behav. 2009;97:193–8. doi: 10.1016/j.physbeh.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santollo J, Katzenellenbogen BS, Katzenellenbogen JA, Eckel LA. Activation of ERα is necessary for estradiol’s anorexigenic effect in female rats. Horm Behav. 2010;58:872–7. doi: 10.1016/j.yhbeh.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wade GN. Some effects of ovarian hormones on food intake and body weight in female rats. J Comp Physiol Psychol. 1975;88:183–93. doi: 10.1037/h0076186. [DOI] [PubMed] [Google Scholar]
- 6.McElroy JF, Wade GN. Short- and long-term effects of ovariectomy on food intake, body weight, carcass composition, and brown adipose tissue in rats. Physiol Behav. 1987;39:361–5. doi: 10.1016/0031-9384(87)90235-6. [DOI] [PubMed] [Google Scholar]
- 7.Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav. 2002;42:461–71. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- 8.Blaustein JD, Wade GN. Ovarian influences on the meal patterns of female rats. Physiol Behav. 1976;17:201–8. doi: 10.1016/0031-9384(76)90064-0. [DOI] [PubMed] [Google Scholar]
- 9.Eckel LA. Estradiol: a rhythmic, inhibitory indirect control of meal size. Physiol Behav. 2004;82:35–41. doi: 10.1016/j.physbeh.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 10.Butera PC. Estradiol and the control of food intake. Physiol Behav. 2010;99:175–80. doi: 10.1016/j.physbeh.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz DH, McClane S, Hernandez L, Hoebel BG. Feeding increases extracellular serotonin in the lateral hypothalamus of the rat as measured by microdialysis. Brain Res. 1989;479:349–54. doi: 10.1016/0006-8993(89)91639-9. [DOI] [PubMed] [Google Scholar]
- 12.Clifton PG, Lee MD, Dourish CT. Similarities in the action of Ro 60–0175, a 5-HT2C receptor agonist, and d-fenfluramine on feeding patterns in the rat. Psychopharmacology. 2000;152:256–67. doi: 10.1007/s002130000504. [DOI] [PubMed] [Google Scholar]
- 13.Simansky KJ. Serotonergic control of the organization of feeding and satiety. Behav Brain Res. 1996;73:37–42. doi: 10.1016/0166-4328(96)00066-6. [DOI] [PubMed] [Google Scholar]
- 14.Clifton PG, Barnfield AM, Curzon G. Effects of food deprivation and mCPP treatment on the microstructure of ingestive behaviour of male and female rats. J Psychopharmacol. 1993;7:257–64. doi: 10.1177/026988119300700304. [DOI] [PubMed] [Google Scholar]
- 15.Eckel LA, Rivera HM, Atchley DPD. The anorectic effect of fenfluramine is influenced by sex and stage of the estrous cycle in rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1486–91. doi: 10.1152/ajpregu.00779.2004. [DOI] [PubMed] [Google Scholar]
- 16.Rivera HM, Eckel LA. The anorectic effect of fenfluramine is increased by estradiol treatment in ovariectomized rats. Physiol Behav. 2005;86:331–7. doi: 10.1016/j.physbeh.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Rothman RB, Baumann MH. Serotonin releasing agents: neurochemical, therapeutic and adverse effects. Pharm Biochem Behav. 2002;71:825–36. doi: 10.1016/s0091-3057(01)00669-4. [DOI] [PubMed] [Google Scholar]
- 18.Fletcher PJ. Increased food intake in satiated rats induced by the 5-HT antagonists methysergide, metergoline, and ritanserin. Psychopharmacology. 1988;96:237–42. doi: 10.1007/BF00177567. [DOI] [PubMed] [Google Scholar]
- 19.Heisler LK, Chu HM, Tecott LH. Epilepsy and obesity in serotonin 5-HT2C receptor mutant mice. Ann N Y Acad Sci. 1998;861:74–8. doi: 10.1111/j.1749-6632.1998.tb10175.x. [DOI] [PubMed] [Google Scholar]
- 20.Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, et al. Eating disorder and epilepsy in mice lacking 5-HT2C serotonin receptors. Nature. 1995;374:542–6. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- 21.Vickers SP, Dourish CT, Kennett GA. Evidence that hypophagia induced by d-fenfluramine and d-norfenfluramine in the rat is mediated by 5-HT2C receptors. Neuropharmacology. 2001;41:200–9. doi: 10.1016/s0028-3908(01)00063-6. [DOI] [PubMed] [Google Scholar]
- 22.Vickers SP, Clifton PG, Dourish CT, Tecott LH. Reduced satiating effect of d-fenfluramine in serotonin 5-HT2C receptor mutant mice. Psychopharmacology. 1999;143:309–4. doi: 10.1007/s002130050952. [DOI] [PubMed] [Google Scholar]
- 23.Gibson EL, Kennedy AJ, Curzon G. d-Fenfluramine- and d-norfenfluramine-induced hypophagia: differential mechanisms and involvement of postsynaptic 5-HT receptors. Eur J Pharmacol. 1993;242:83–90. doi: 10.1016/0014-2999(93)90013-8. [DOI] [PubMed] [Google Scholar]
- 24.Oluyomi AO, Gibson EL, Barnfield AM, Curzon G. d-Fenfluramine and d-norfenfluramine hypophagias do not require increased hypothalamic 5-hydroxytryptamine release. Eur J Pharmacol. 1994;264:111–5. doi: 10.1016/0014-2999(94)90646-7. [DOI] [PubMed] [Google Scholar]
- 25.Raiteri M, Bonanno G, Vallebuona F. In vitro and in vivo effects of d-fenfluramine: no apparent relation between 5-hydroxytryptamine release and hypophagia. J Pharmacol Exp Ther. 1995;273:643–9. [PubMed] [Google Scholar]
- 26.Kennett GA, Curzon G. Evidence that hypophagia induced by mCPP and TFMPP requires 5-HT1C and 5-HT1B receptors; hypophagia induced by RU 24969 only requires 5-HT1B receptors. Psychopharmacology. 1988;96:93–100. doi: 10.1007/BF02431539. [DOI] [PubMed] [Google Scholar]
- 27.Simansky KJ, Dave KD, Inemer BR, Nicklous DM, Padron JM, Aloyo VJ, et al. A 5-HT2C agonist elicits hyperactivity and oral dyskinesia with hypophagia in rabbits. Physiol Behav. 2004;82:97–107. doi: 10.1016/j.physbeh.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan JM, Song S, Grill HJ. Serotonin receptors in the caudal brainstem are necessary and sufficient for the anorectic effect of peripherally administered mCPP. Psychopharmacology. 1998;137:43–9. doi: 10.1007/s002130050591. [DOI] [PubMed] [Google Scholar]
- 29.Prinssen EP, Koek W, Kleven MS. The effects of antipsychotics with 5-HT2C receptor affinity in behavioral assays selective for 5-HT2C receptor antagonist properties of compounds. Eur J Pharmacol. 2000;388:57–67. doi: 10.1016/s0014-2999(99)00859-6. [DOI] [PubMed] [Google Scholar]
- 30.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. San Diego: Academic Pres; 1998. [Google Scholar]
- 31.Santollo J, Torregrossa A-M, Eckel LA. Estradiol acts in the medial preoptic area, arcuate nucleus, and dorsal raphe nucleus to reduce food intake in ovariectomized rats. Horm Behav. 2011;60:86–93. doi: 10.1016/j.yhbeh.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schreiber R, Selbach K, Asmussen M, Hesse D, De Vry J. Effects of serotonin1/2 receptor agonists on dark-phase food and water intake in rats. Pharmacol Biochem Behav. 2000;67:291–305. doi: 10.1016/s0091-3057(00)00357-9. [DOI] [PubMed] [Google Scholar]
- 33.Schreiber R, De Vry J. Role of 5-HT2C receptors in the hypophagic effect of m-CPP, ORG 37684 and CP-94,253 in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:441–9. doi: 10.1016/s0278-5846(01)00284-6. [DOI] [PubMed] [Google Scholar]
- 34.Fuller RW, Snoddy HD, Mason NR, Owen JE. Disposition and pharmacological effects of m-Chlorophenylpiperazine in rats. Neuropharmacology. 1981;20:155–62. doi: 10.1016/0028-3908(81)90198-2. [DOI] [PubMed] [Google Scholar]
- 35.Haleem DJ. Function specific supersensitivity of m-chlorophenyl piperazine-induced serotonergic neurotransmission in female compared to male rats. Life Sci. 1993;52:PL279–84. doi: 10.1016/0024-3205(93)90693-w. [DOI] [PubMed] [Google Scholar]
- 36.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 37.Julius D, MacDermott AB, Axel R, Jessell TM. Molecular characterization of a functional cDNA encoding the serotonin 1c receptor. Science. 1988;241:558–64. doi: 10.1126/science.3399891. [DOI] [PubMed] [Google Scholar]
- 38.De Vry J, Schreiber R. Effects of selected serotonin 5-HT1 and 5-HT2 receptor agonists on feeding behavior: possible mechanisms of action. Neurosci Biobehav Rev. 2000;24:341–53. doi: 10.1016/s0149-7634(99)00083-4. [DOI] [PubMed] [Google Scholar]
- 39.Geary N, Asarian L. Cyclic estradiol treatment normalizes body weight and test meal size in ovariectomized rats. Physiol Behav. 1999;67:141–7. doi: 10.1016/s0031-9384(99)00060-8. [DOI] [PubMed] [Google Scholar]
- 40.Rivera HM, Oberbeck DR, Kwon B, Houpt TA, Eckel LA. Estradiol increases Pet-1 and serotonin transporter mRNA in the midbrain raphe nuclei of ovariectomized rats. Brain Res. 2009;1259:51–8. doi: 10.1016/j.brainres.2008.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rivera HM, Eckel LA. Activation of central, but not peripheral, estrogen receptors is necessary for estradiol’s anorexigenic effect in ovariectomized rats. Endocrinology. 2010;151:5680–8. doi: 10.1210/en.2010-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thammacharoen S, Geary N, Lutz TA, Ogawa S, Asarian L. Divergent effects of estradiol and the estrogen receptor-α agonist PPT on eating and activation of PVN CRH neurons in ovariectomized rats and mice. Brain Res. 2009;1268:88–96. doi: 10.1016/j.brainres.2009.02.067. [DOI] [PubMed] [Google Scholar]
- 43.Rowland NE. Effect of continuous infusions of dexfenfluramine on food intake, body weight and brain amines in rats. Life Sci. 1986;39:2581–6. doi: 10.1016/0024-3205(86)90112-8. [DOI] [PubMed] [Google Scholar]
- 44.Leibowitz SF, Alexander JT. Hypothalamic serotonin in control of eating behavior, meal size, and body weight. Biol Psychiatry. 1998;44:851–64. doi: 10.1016/s0006-3223(98)00186-3. [DOI] [PubMed] [Google Scholar]
- 45.Thammacharoen S, Lutz TA, Geary N, Asarian L. Hindbrain administration of estradiol inhibits feeding and activates estrogen receptor-α-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology. 2008;149:1609–17. doi: 10.1210/en.2007-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Henderson JA, Bethea CL. Differential effects of ovarian steroids and raloxifene on serotonin 1A and 2C receptor protein expression in macaques. Endocrine. 2008;33:285–93. doi: 10.1007/s12020-008-9087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou W, Cunningham KA, Thomas ML. Estrogen regulation of gene expression in the brain: a possible mechanism altering the response to psychostimulants in female rats. Mol Brain Res. 2002;100:75–83. doi: 10.1016/s0169-328x(02)00134-1. [DOI] [PubMed] [Google Scholar]
- 48.Eckel LA, Geary N. Estradiol treatment increases feeding-induced c-Fos expression in the brains of ovariectomized rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R738–46. doi: 10.1152/ajpregu.2001.281.3.R738. [DOI] [PubMed] [Google Scholar]
- 49.Smith GP, Gibbs J. The development and proof of the CCK hypothesis of satiety. In: Dourish CT, Cooper SJ, Iversen SD, Iversen LL, editors. Multiple cholecystokinin receptors in the CNS. Oxford: Oxford University Press; 1992. pp. 166–82. [Google Scholar]
- 50.Eckel LA, Houpt TA, Geary N. Estradiol treatment increases CCK-induced c-Fos expression in the brains of ovariectomized rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1378–85. doi: 10.1152/ajpregu.00300.2002. [DOI] [PubMed] [Google Scholar]
- 51.Asarian L. Loss of cholecystokinin and glucagon-like peptide-1-induced satiation in mice lacking serotonin 2C receptors. Am J Physiol Regul Integr Comp Physiol. 2009;296:R51–6. doi: 10.1152/ajpregu.90655.2008. [DOI] [PubMed] [Google Scholar]
- 52.Poeschla B, Gibbs J, Simansky KJ, Greenberg D, Smith GP. Cholecystokinin-induced satiety depends on activation of 5-HT1C receptors. Am J Physiol Regul Integr Comp Physiol. 1993;264:R62–4. doi: 10.1152/ajpregu.1993.264.1.R62. [DOI] [PubMed] [Google Scholar]