Abstract
Previous studies have shown that the Ron receptor is overexpressed in prostate cancer and Ron expression increases with disease severity in humans and the mouse TRAMP model. Here, the causal role of Ron overexpression in the murine prostate was examined in the development and progression of prostate cancer. Transgenic mouse strains were generated which selectively overexpressed Ron in the prostate epithelium and prostate histopathology was evaluated and compared to wild type controls. Ron overexpression led to the development of prostate intraepithelial neoplasia (mPIN) with local invasion and was associated with increases in prostate cell proliferation and decreases in cell death.
Keywords: Ron receptor, prostate cancer, mouse prostate intraepithelial neoplasia, receptor tyrosine kinase, Met receptor
1. Introduction
Prostate cancer is the second leading cause of cancer-related death among men in the United States with a 5-year survival rate of less than 31% in patients with metastatic disease [1]. While current treatments such as radiation therapy and radical prostatectomy prove useful in the early stages of the disease, the long-term prognosis for prostate cancer is poor, given that many tumors will reoccur following a post-treatment remission period. It is therefore critical to understand the biological and physiological mechanisms that govern the initiation of prostate tumor growth and metastasis. One such mechanism involves the role of aberrant receptor tyrosine kinase signaling, which is emerging as a potential therapeutic target in prostate cancer.
Inhibition of receptor tyrosine kinases such as the insulin-like growth factor 1 receptor [2], Her2/neu receptor [3] and the epidermal growth factor receptor [4] reduces both tumor growth in mouse xenograft models as well as prostate cancer cell proliferation, in vitro. In addition, use of the tyrosine kinase inhibitor gefitinib has shown some efficacy in clinical trials when used alone, or in combination with the anti-androgen bicalutamide [5], suggesting that receptor tyrosine kinase signaling is important in both androgen-dependent and androgen-independent prostate cancers.
The Ron receptor tyrosine kinase belongs to a family of tyrosine kinases of which the hepatocyte growth factor receptor (c-Met) is also a member [6]. Activation of Ron involves the binding of its ligand, hepatocyte growth factor-like protein (HGFL), which induces receptor dimerization and phosphorylation of intracellular tyrosine residues. Phosphorylation of the C-terminal tyrosine residues of Ron leads to the recruitment of downstream signaling molecules such as PI3-K/Akt, MAPK, Ras, Src and β-catenin [6] and activation of numerous signaling paradigms. Ron has been shown to be overexpressed in several cancers including breast, ovarian, colon, pancreas and prostate [7; 8; 9; 10; 11] where it regulates a variety of cellular functions relevant to cancer including invasion, angiogenesis and proliferation.
With regard to prostate cancer, our laboratory has shown previously, using a panel of human prostate tissues, that Ron is highly expressed in human prostate adenocarcinoma and metastatic lymph nodes compared to normal prostate or benign prostate hyperplasia [11]. In addition, we have also demonstrated that Ron promotes the production of angiogenic chemokines in an NF-κB-dependent manner which leads to endothelial cell migration and tumor vascularization [11]. Moreover, we have also shown using the TRAMP model of prostate cancer that not only is Ron expression elevated in the prostates at 30 weeks of age, but that deleting Ron signaling in these mice leads to a decrease in prostate tumor size, vascularization and NF-κB activation compared to age-matched wild-type TRAMP mice (23). Given this data, we therefore hypothesized that overexpression of the Ron receptor tyrosine kinase in the mouse prostate plays a role in the development and progression of prostate cancer. To test this hypothesis, we generated transgenic mice that selectively overexpress Ron in the prostate epithelium and evaluated prostate histopathology temporally.
2. Materials and Methods
2.1. Cloning and Generation of a Prostate Specific Ron Transgene
The murine Ron minigene plasmid was created as previously published [12]. Briefly, 5′ genomic DNA and 3′ cDNA fragments of the mouse (m) Ron gene were utilized (accession numbers U65949 and X74736, respectively; see Figure 1A) [13; 14; 15]. A 3.2-kb SpeI/EcoRI fragment of mRon genomic DNA encompassing exon 1 and intron 1 was cloned into pBluescript. Subsequently, a 3.2-kb EcoRI mRon genomic DNA fragment encompassing exons 2 to 6 was cloned into the EcoRI-site of this plasmid. A 2.8-kb AgeI/XhoI fragment of mRon cDNA encoding exons 4 to 19 was directionally cloned into AgeI/XhoI-digested plasmid, generating a vector harboring a full-length mRon minigene. The rat short probasin promoter [16] was cloned into the pIND vector at the KpnI and EcoR1 sites and the murine Ron minigene was cloned into the same vector at the NotI site. The transgene was removed by digestion with PmeI and the resulting mice are referred to as Pb-Ron mice. A second construct was created in a similar manner by inserting the ARR2Pb promoter [17] into the pIND vector at the BamH1 and Nhe1 sites. The mouse Ron minigene was then cloned into the same vector at the Not1 site. The promoter-gene section of the vector was then cut out with Pme1. These animals are referred to as ARR2Pb-Ron mice.
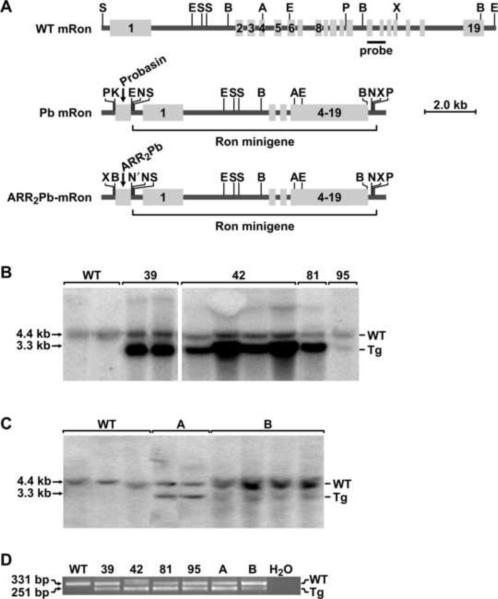
Figure 1. Generation of transgenic mice overexpressing Ron driven by rat probasin promoters.
A, Schematic representation of the Ron expression cassette utilized for the generation of the transgenic mice. The top diagram represents the endogenous (WT) wild type mouse (m) Ron gene. The middle diagram represents the Ron minigene construct driven by the rat probasin promoter (Pb mRon). The bottom diagram depicts the Ron minigene driven by ARR2Pb promoter as described in Materials and Methods. The mRon transgene harbors the first three exons and introns of WT mRon genomic DNA followed by, in the correct reading frame, exons 4 to 19 of WT mRon cDNA. The location of a 706-bp genomic DNA probe utilized for Southern analyses to differentiate transgenic from endogenous WT mRon is noted (probe). Restriction enzyme sites are as follows: A, AgeI; B, BamHI; E, EcoRI; K, Kpn1, N', Nhe1, N, NotI; P, PmeI; S, SpeI; X, XhoI. B and C, Southern analysis of the founder transgenic lines demonstrating the endogenous Ron gene and transgenic construct. Each lane represents an independent sample of genomic DNA. D, PCR analysis of genomic DNA from wild type mice and the transgenic lines depicting the endogenous Ron gene (top band) and the transgenic allele (bottom band).
2.2. Generation and Identification of Transgenic animals
Both constructs were injected into the pronucleus of fertilized eggs from FVB/N mice. Transgenic founder mice were crossed with wild type (WT) FVB/N mice to create the F1 generation which were then crossed with WT littermates in order to generate mice for analysis. Transgene confirmation was conducted by both PCR and Southern blotting analyses. For PCR analysis, previously published primers (forward: 5′-TGGGTGGTGAGGTCTGCCAACATGAGCTCC-3′ and reverse: 5′-CCGTCTTCGGGAGTTAAAGATCAGGGCAAC-3′) were used to generate a 331-bp product corresponding to the endogenous mRon allele and a 251-bp product corresponding to the mRon minigene [12]. Transgene transmission was further confirmed by Southern Blot using a 708-bp probe as previously described [12]. Briefly, DNA was digested with BamHI and the Southern membrane was probed with a PCR-generated fragment (5′-TCCCCAACAACACTCTCTGACATCA-3′ and 5′-ACAAAGGACCTGCAGCCTGAGGTCA-3′). This probe generates bands of 4.4 kb and 3.3 kb corresponding to the endogenous allele and transgenic construct, respectively. Copy number was estimated by densitometric analysis of the endogenous Ron allele compared to the transgenic band from the Southern blot analyses.
2.3. Mice
Transgenic positive and WT controls were allowed to age from 365 days to 720 days. At the appropriate time point animals were euthanized and the prostate was removed, along with a variety of other organs to examine metastasis, and either fixed for histological examination or frozen for further protein and RNA analysis. Tissues were processed and paraffin embedded as previously described [15] and cut into 4 micron sections for further staining and analysis. All animal procedures were approved by the University of Cincinnati Animal Care and Use Committee.
2.4. Quantitative Real-Time (qRT-)PCR
RNA was isolated from whole prostates from mice utilizing Trizol Reagent (Invitrogen, Carlsbad, CA) according to manufacturer's directions. Complimentary DNA was created using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA) according to manufacturer's directions and SYBR green (Roche, Indianapolis, IN) incorporation was used to measure mRon mRNA levels in each founder line. Primers specific to mouse Ron [18], mouse c-Met (foward: CATTTTTACGGACCCAACCA, reverse: TGTCCGATACTCGTCACTGC) and mouse β-glucuronidase (Gus) (forward: TTGAGAACTGGTATAAGACGCATCAG, reverse: TCTGGTACTCCTCACTGAACATGC) were utilized.
2.5. Western Analysis
Prostate, bladder, testes, lung, kidney and spleen were isolated from approximately 365 day old mice and homogenized in RIPA buffer and then briefly sonicated. Protein concentrations were determined using the MicroBCA kit (Pierce Biotechnology, Rockford, IL) per manufacturer's instructions. 50–200ug of protein extracts were loaded onto an 8% polyacrylamide gel and run for approximately 2 hours at 60–100 volts. The protein was then transferred onto a PVDF membrane by submersible transfer overnight at 30 volts. Membranes were probed with antibodies against Ron (1:400, Santa Cruz Biotechnology, Santa Cruz, CA), phospho(p)-ERK1/2 (1:2,000, Cell Signaling, Boston, MA), total ERK1/2 (1:1,000, Cell Signaling, Boston, MA), β-Catenin (1:1,000, Cell Signaling, Boston, MA), or Actin (1:40,000 a gift from Dr. James Lessard, Cincinnati Children's Hospital Medical Center, Cincinnati, OH) overnight followed by either peroxidase-conjugated Goat Anti-Rabbit or peroxidase-conjugated Donkey Anti-Mouse (Jackson ImmuoResearch Laboratories, West Grove, PA). Final detection was conducted with the ECL reagent (GE Healthcare, Piscataway, NJ) followed by visualization of the proteins by exposure to film.
2.6. Prostate Histology and Immunohistochemistry
Formalin-fixed paraffin embedded prostate sections from all lines and time points were stained with Hematoxylin and Eosin and then evaluated for specific histological abnormalities. Prostates were graded into one of 5 categories: typical, atypical hyperplasia, mPIN (mouse Prostate Intraepitheial Neoplasia), mPIN with invasion [19], and adenocarcinoma (as defined in Table I and displayed in Figure 3C). All lobes of the prostate were examined for each mouse. No pathology was observed in the anterior prostate lobes. In order to analyze proliferation, two hours before sacrifice, transgenic positive and WT control mice were injected intraperitoneally with bromodeoxyuridine (BrdU) and paraffin embedded prostate sections were stained using a BrdU staining kit (Amersham, Piscataway, NJ). Manufacturer's directions were followed except for using a sub-boiling antigen retrieval modification. The percent of positive cells was determined by blind count for each section and related to histological grade. From WT mice, 53.15 ± 5.1% of the prostate epithelial cells from glands classified as typical were positive for BrdU staining per 0.25mm2 area. To monitor changes in BrdU incorporation versus prostate pathology, the % BrdU positive cells from typical glands was normalized to 1 for each genotype. The fold change in BrdU incorporation for each transgenic line for each pathological classification is provided. For TUNEL analyses, prostate sections from transgenic and WT control mice were stained using the In Situ Cell Death Detection Kit, AP (Roche Applied Science, Indianapolis, IN) according to the manufacturer's directions, with a 1:2 dilution of TdT enzyme. The number of TUNEL-positive cells was blindly counted and changes were analyzed at 720 days. Ron immunohistochemistry was performed essentially as previously described [11; 20].
Table I.
Prostate Histology Grading System.
| Histological classification | Prominent nuclei | Atypical cells |
Invasion | Disrupted Gland Profile | |
|---|---|---|---|---|---|
| Intact Stroma | Large Foci | ||||
| Typical | |||||
| Atypical Hyperplasia | × | × | |||
| Mouse Prostate Intraepithelial | × | × | |||
| Neoplasia (mPIN) | |||||
| mPIN with invasion | × | × | × | ||
| Adenocarcinoma | × | × | × | ||
The histological classification of prostate tissue was based on examining prominent nuclei, atypical cells, invasion and disrupted glandular profiles. Prominent nuclei are defined as nuclei that stand out in the cell, contain mostly pronounced nucleoli, and may contain mitotic bodies. Atypical cells refer to the morphology of the cells which are more columnar and start to stack upon one another as compared to typical cells which are rounder and are arranged in a single layer. Invasion refers to a spot where the atypical foci infiltrate into the surrounding basal membrane. Disrupted gland profile refers to the lack of obvious prostate gland structure existing in the said gland.
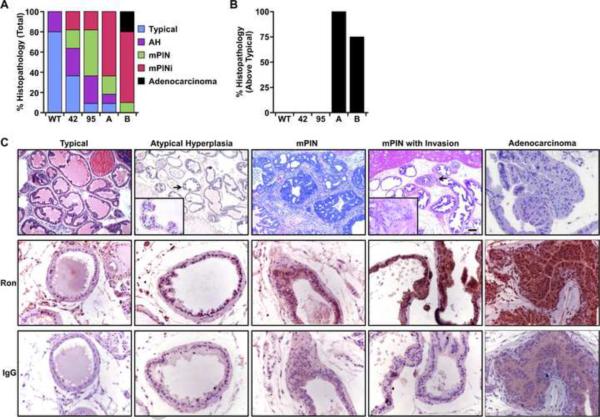
Figure 3. Prostate pathology in probasin driven Ron transgenic mice.
A, Prostate histology of wild type mice compared to that of mice overexpressing Ron in the prostate. The percent of each type of prostate pathology is pooled and noted after 450 days. Of note, the extent of neoplasia worsens in all of the transgenic lines compared to WT prostates. n=16–20 prostates evaluated per line. B, The percent of prostates observed at 365 days with histopathology over typical. Of note, the ARR2Pb-Ron transgenic lines displayed a marked increase in prostate pathology over the Pb-Ron mice at this time point. All mice in the A line exhibited pathology over typical at 365 days. n=4–6 mice per line evaluated at 365 days. In comparison with Figure 3A, there was one animal that had typical prostate pathology at the later time point. C, Representative pictures of the five histopathological prostate grades observed and subsequent Ron immunohistochemical staining. Immunohistochemical staining suggests increased Ron expression as pathology worsens. All images were captured at the original magnification of 20× or 40× and insets are at 63×.
2.7. Statistical Analyses
Data are expressed as mean ± standard error (SE). Statistical significance for BrdU staining, TUNEL staining and qRT-PCR analyses were determined by Student's t-tests with a Welch correction applied to comparisons with unequal variances. All analyses were performed utilizing GraphPad Prism software (San Diego, CA). Differences between groups were accepted as significant when P<0.05.
3. Results
3.1. Generation and characterization of transgenic animals expressing Ron in the prostate epithelium
The Ron receptor tyrosine kinase has recently been shown to play an integral role in prostate cancer with Ron being highly expressed in human prostate adenocarcinomas and playing an important role promoting prostate tumor growth in the TRAMP model of prostate cancer in mice (11, 23). To directly test the significance of Ron receptor overexpression in prostate cancer, transgenic mice that selectively overexpress Ron in the prostate epithelium were generated. To accomplish this, two independent transgene constructs were generated (Pb-Ron and ARR2Pb-Ron) and are depicted in Figure 1A. One construct utilized the rat short probasin promoter (Pb) to drive Ron expression in the prostate and the second construct contained a prostate-specific promoter with two androgen response elements from the probasin promoter arranged back to back (ARR2Pb). Each transgene was subsequently injected into blastocysts of FVB/N mice (Figure 1A). Founder mice were crossed with wild type (WT) FVB/N mice to generate offspring for analyses. Only male mice were utilized in subsequent experiments. Four independent transgenic lines were generated from the Pb-Ron construct (lines 39, 42, 81 and 95) and two founder lines were derived from the ARR2Pb-Ron construct (lines A and B). Transgene integration was confirmed by Southern blotting (Figure 1B and 1C) and PCR (Figure 1D) analyses. The Southern analysis was also used to estimate the number of transgene copies which ranged from approximately 2–4 copies of the Pb-Ron transgene (39-3; 42-3; 81-4; 95-2) and 1–2 copies of the ARR2Pb-Ron transgene (A-2; B-1). This estimation was accomplished by comparison of the endogenous Ron bands from the Southern analysis to the transgenic bands by densitometry analysis (data not shown).
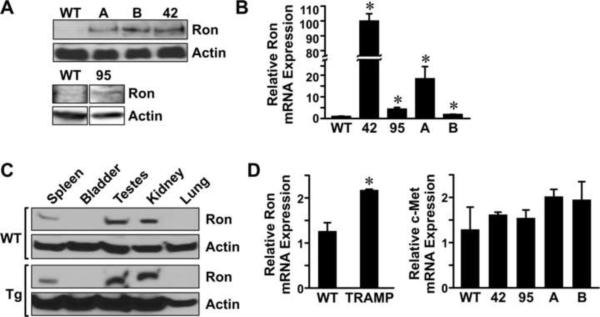
To examine Ron expression in the prostate, Western analysis and quantitative RT-PCR were performed. Figure 2A depicts the levels of Ron protein expression observed in the prostates of mice containing transgene A, B, 42 and 95 compared to prostates of wild type mice. Figure 2B shows the relative levels of Ron mRNA expression detected in all lines by qRT-PCR. Western analysis and qRT-PCR failed to detect Ron expression in lines 39 and 81 (data not shown) and these animals were eliminated from further analyses. Thus, compared to wild type mouse prostate, Ron expression is dramatically increased in the prostates of mice containing transgenes A, B, 42 and 95. To examine selectivity of transgene expression, we ascertained Ron expression in a variety of organs of the transgenic mice compared to wild type animals. As illustrated in Figure 2C, endogenous Ron expression is present in the spleen, testes and kidney of wild type mice (WT) by Western analysis and the levels of Ron expression in these tissues were not significantly altered in the transgenic mice. Ron levels were not detectable in the bladder or lungs of any of the mice analyzed by Western analysis. Shown is a representative Western analysis depicting Ron expression in tissues derived from the 42 line with similar results observed in the other transgenic lines (data not shown). The testes, spleen and mesenteric lymph nodes of the transgenic mice were also examined and compared to wild type mice by histological analyses. No differences were observed in the testes, spleen or lymph nodes of the transgenic mice or wild type at any time point analyzed (data not shown). Representative histological sections are depicted in Supplementary Figure S1. For comparison, we also examined Ron expression in a separate murine model of prostate tumorigenesis as well as examined the levels of the c-Met receptor, a promiment member of the Ron receptor tyrosine kinase family, in our transgenic lines. As depicted in Figure 2D (left), Ron expression is significantly increased in prostates taken from 30-week-old TRAMP mice compared to age-matched controls. In addition, the levels of the closely related c-Met receptor, do not change significantly in the probasin driven Ron transgenic lines (Figure 2D, right). This data demonstrate that Ron is selectively overexpressed in the prostates of our transgenic mice, with similar compensation of the related c-Met receptor, and that Ron is also observed to be upregulated in other murine models of prostate cancer, consistent with previous data [21].
Figure 2. Transgenic Ron expression.
A, Western analysis depicting Ron expression in the prostates of the transgenic lines listed compared to undetectable levels of Ron from WT prostates. Actin serves as a loading control. B, Quantitative Real-Time (qRT)-PCR shows elevated levels of Ron mRNA in the transgenic lines compared to WT controls. C, Ron is endogenously expressed in spleen, testes and kidneys of WT and transgenic mice. Representative data is shown from the 42 line. Actin serves as a loading control. D, (Left panel) qRT- PCR comparing Ron expression in the TRAMP model of prostate cancer to wild type prostates from 30-week-old mice. (Right panel), qRT-PCR was performed for c-Met expression on prostates isolated from WT mice and the transgenic lines relative. Levels of the Ron related receptor, c-Met, are not significantly altered following Ron overexpression. All qRT-PCR data are expressed as mean ± standard error from at least 3 independent samples per groups analyzed in duplicate. *P<0.05 compared to WT.
3.2. Ron overexpression in the prostate epithelium induces prostatic intraepithelial neoplasia (PIN)
To determine the role of Ron overexpression in mediating prostate tumorigenesis, we examined the histopathology of transgenic lines 42, 95, A and B temporally at 365, 450, 540 and 720 days. Prostate histopathology was graded according to characteristics observed as outlined in Table I. Five levels of histopathology were scored: Typical, atypical hyperplasia, mouse prostate intraepithelial neoplasia (mPIN), mPIN with invasion (mPINi), and adenocarcinoma and are depicted in Figure 3. Figure 3A demonstrates the combined histopathology observed as a percentage in the transgenic lines 42, 95, A and B compared to that observed in WT mice at 450 days and over. The percentage of histology is representative of the most severe phenotype observed in a given prostate for each mouse with each individual prostate represented once. The most phenotypically dramatic line with respect to prostate histology was the B line. This line was the only one that displayed prostate adenocarcinoma in 20% of the prostates with every other animal in this line containing either mPIN (10%) or mPINi (70%). In line A, only 10% of prostates were typical with the remainder displaying an abnormal histopathology. Lines 42 and 95 did not exhibit the same level of pathology as that derived from lines A and B, but still progressed to the category of mPINi (18% in both lines). By 365 days, 100% of the A line had measurable histopathology (i.e., atypical hyperplasia, mPIN, mPINi, and adenocarcinoma) and the B line had 75% with histopathology over typical (Figure 3B). This is a marked increase over the Pb-Ron lines 42 and 95, wherein none of the mice had any histopathology at this same time frame similar to WT controls. Figure 3C depicts the various levels of histopathology observed in the transgenic mice (top) along with the expression levels of Ron as detected by immunohistochemistry in each of these stages. Of note, the expression of Ron increases in accordance with the severity of pathology.
3.3 Ron overexpression is associated with temporal increases in prostate pathology and downstream signaling
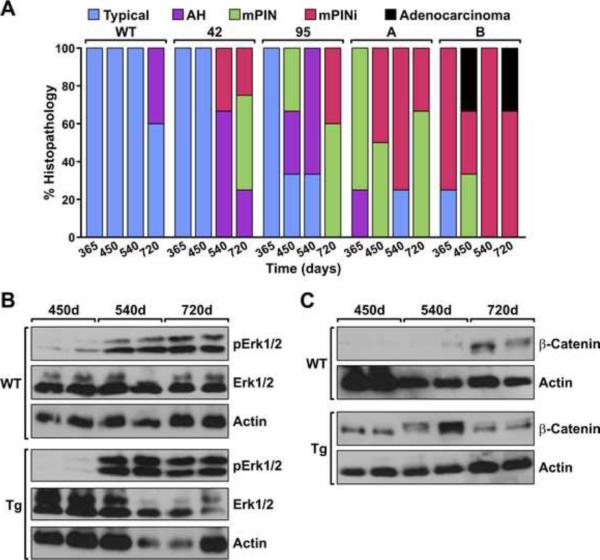
To examine the development of prostate pathology over time, prostates from wild type mice and the transgenic lines were evaluated histologically at specific time points. Figure 4A depicts the percent prostate pathology observed per animal per genotype at the times noted. WT prostates displayed typical histology through 520 days and 40% of the mice developed atypical hyperplasia by 720 days. Prostates from lines 42 and 95 displayed typical histology at 365 days whereas the A and B lines displayed considerable pathology at this time frame which progressed in the B line to adenocarcinoma. To examine signaling cascades that are associated with the increased pathology and increased Ron expression in the transgenic lines, Western analysis was performed on prostates isolated at 450, 540 and 720 days. As depicted in Figure 4B and 4C, increases in the phosphorylation of Erk (p42/p44) and increase in the levels of β-catenin were observed in the prostate from the transgenic lines compared to wild type prostates at the corresponding time points. Similar increases were observed in all of the lines (data not shown).
Figure 4. Temporal comparison of pathology and signaling factors in prostates of WT and Ron transgenic mice.
A, The transgenic lines have increases in prostate pathology over time compared to WT prostates. Of note, the Pb derived lines exhibited only typical pathology at 365 days and only progressed to mPINi stages at the latter time points. The ARR2Pb derived lines exhibited the more severe prostate pathology at earlier time points compared to WT prostates and the 42 and 95 lines with the majority of ARR2Pb lines exhibiting mPINi or adenocarcinoma by the end stage time point. (n=3–5 prostates per group/time point). B and C, Prostate lysates isolated from WT and the transgenic lines at the time points noted were evaluated by Western analysis. Increases in the phosphorylation of ERK1/2 (pERK1/2) per total ERK (B) as well as increases in β-catenin expression (C) were observed in the 95 transgenic line. Samples are shown from two separate mice for each time point. Similar data was obtained for all the transgenic lines. Actin serves as a loading control.
3.4. Ron overexpression increases prostate epithelial cell proliferation and reduces cell death
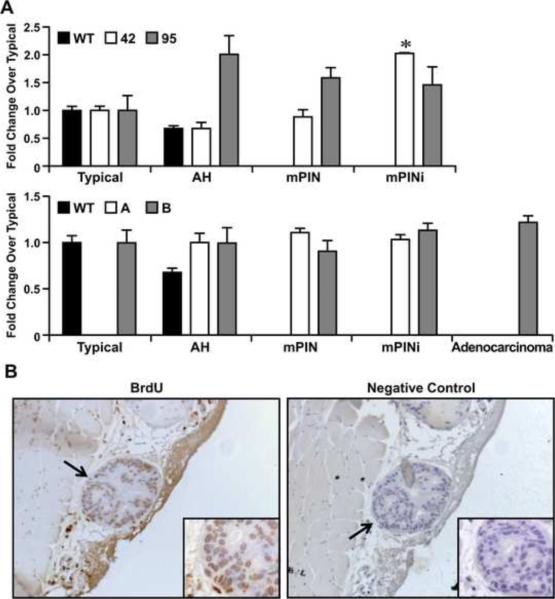
Based on the prostate histology observed in the transgenic lines, we next examined whether Ron overexpression in the prostate would lead to changes in cell turnover. To accomplish this, we evaluated prostate epithelial cell proliferation in histological sections of prostates taken from mice with varying pathologies utilizing BrdU immunohistochemistry (Figure 5). As shown in Figure 5A and 5B, a trend toward increases in cell proliferation that correlated with the severity of the pathology was observed in the prostates of mice overexpressing Ron. The 42 line exhibited the most proliferation at its highest pathological level. Cell death was evaluated utilizing TUNEL staining on sections of prostates. As depicted in Figure 6, Ron overexpression also correlated with a decrease in TUNEL staining.
Figure 5. Ron overexpression in the prostate increases cell proliferation.
A, The extent of cell proliferation in prostates containing each pathology [Typical, atypical hyperplasia (AH), mPIN, mPINi, and adenocarcinoma] for the select transgenic line is depicted. The extent of proliferation observed in WT prostates with a typical pathology was normalized to 1 and the fold-change over typical pathology is depicted. Of note, as the severity of pathology increases, the extent of cell proliferation increases in the prostates of transgenic mice compared to wild type prostates. *P<0.05 compared to typical. B, Pictures showing representative staining of a transgenic prostate and a corresponding negative control. All four transgenic lines demonstrate increased proliferation at all histological stages when compared to wild type. Original magnification was taken at 40× and insets at 63×.
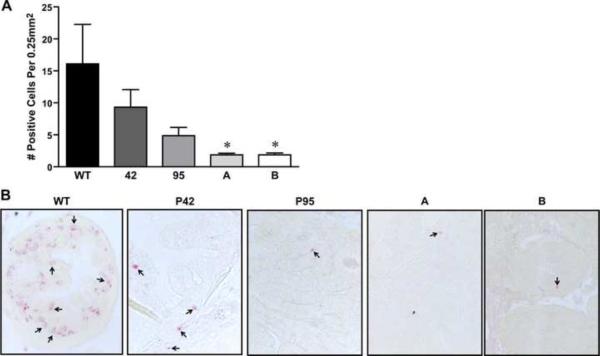
Figure 6. Ron overexpression in the prostate decreases prostate cell death.
A, TUNEL data depicting increased cell death in wild type prostates compared to prostates taken from Ron overexpressing transgenic lines at 720 days. Prostates from the ARR2Pb-Ron animals displayed even further decreases in the amount of cell death over the Pb-Ron mice. Data is expressed as mean ± standard error. *P<0.05 compared to WT. B, Pictures showing TUNEL staining in all transgenic lines evaluated compared to wild type. Original magnification was taken at 40×.
4. Discussion
Previous studies have shown that the Ron receptor tyrosine kinase is highly expressed in approximately 90% of human prostate cancers compared to non-diseased prostate tissue [11; 22]. Moreover, we have also shown that Ron is overexpressed in tumor bearing prostates from TRAMP mice compared to age matched prostates from wild type mice and that loss of Ron in the TRAMP model leads to decreases in prostate tumor mass. Combined, these studies suggest that Ron overexpression contributes to the growth of prostate tumors in both mice and humans. To elucidate the role of Ron overexpression in potentiating prostate cancer, we generated transgenic mice that selectively overexpress Ron in this organ through the use of two prostate epithelial specific promoters. Analyses of these transgenic mice demonstrated that Ron overexpression, under the control of the ARR2Pb promoter, leads to the development of prostate adenocarcinoma and also microinvasive mPIN to a high degree when compared to wild-type control mice. Similarly, Ron overexpression under control of the Pb promoter induced a high level of both mPIN and microinvasive mPIN when compared to wild-type controls but unlike the ARR2Pb-Ron mice, prostate adenocarcinomas were not observed. In addition, Ron overexpression was associated with increases in both prostate epithelial cell proliferation and decreases in cell death in vivo with these changes coinciding with severity of prostate pathology. In the study presented here, we report an increase in both the phosphorylation of ERK and in the level of β-catenin in prostates of the transgenic animals compared to controls. Interestingly, these same signaling pathways were associated with breast tumor formation in transgenic mice which overexpress Ron in the breast epithelium [12] and suggests a common signaling paradigm utilized by this receptor in vivo. Several recent studies have also reported an increase in pERK and β-catenin in relation the Ron receptor [15; 20; 22; 23; 24; 25]. Both ERK and β-catenin are well established inducers of cellular proliferation and survival of which both processes may play an important role in this model of Ron-induced pr ostate cancer. In total, the data herein supports the hypothesis that Ron overexpression in the prostate is important in the development of prostate cancer and that Ron may be an important new target for prostate cancer therapy.
Several in vivo murine models of prostate cancer have been developed but the development of prostate adenocarcinoma and mPIN have proven difficult to induce through the overexpression of endogenous genes. Based on this difficulty, our studies suggest that the models presented in this report represent effective new in vivo models to examine the development of both prostate cancer (ARR2Pb-Ron) as well as precancerous lesions (Pb-Ron). In the Pb-Ron transgenic mice, microinvasive mPIN was the most severe pathology observed. The AAR2Pb-Ron transgenic mice also developed prostate pathology over a long time frame but the development of adenocarcinoma was further observed. This slow progression of prostate tumor formation in this model is amenable for further studies in combination of genes that may associate with Ron overexpression.
Along with the long term progression of tumors in the Ron overexpressing models, the TUNEL data on prostates taken at 720 days show dramatic decreases in the death of prostate epithelial cells compared to age matched control prostates. Interestingly, however, the TUNEL positive cells in the Ron overexpressing prostates are not confined to a specific area of the gland but are sporadically spread throughout the prostate. Similarly, wild type glands exhibit increased TUNEL staining distributed throughout the gland. This data suggest that the prostates from the transgenic Ron overexpressing animals, even in areas that do not show aberrant pathology, are different from wild type cells with respect to overall cell turnover. The new transgenic Ron overexpressing murine lines also replicate aspec ts of the human histology and represent a considerable difference between murine prostate cancer models that involve the overexpression of viral oncogenes. For example, TRAMP mice, wherein the simian virus (SV) 40 early genes are driven by the rat probasin promoter, develop prostate tumors which contain a neuroendrocrine phenotype that is not observed in the Ron overexpressing models in this report [26]. The parallel to human morphology may allow further studies to identify Ron-mediated target genes that may also be important factors in human disease.
Another model that also closely replicates human prostate cancer was recently generated through the overexpression of the transcription factor Myc in the prostate epithelium [27]. This model is very similar to the data obtained from Ron overexpression. Both the Myc and Ron models utilize the same promoters to drive overexpression and both models develop similar endpoints. Interestingly, there are also differences between these models. First, the Myc model has a higher penetrance and shorter time frame for the development of prostate tumorigenesis than the Ron overexpression models. Myc overexpression leads to mPIN by 6 months of age and adenocarcinoma by 12 months in 100% of the animals. Ron overexpression leads to mPIN with close to 100% penetrance (depending on the transgenic line) but the latency for this phenotype is approximately 12 months. Similar to the long duration of prostate tumor development in our model, men develop prostate cancer later in life and not at 100% penetrance. Second, while the Myc model has a higher percentage of animals with prostate disease, this molecule may not be as amenable to targeting as the cell surface receptor tyrosine kinase Ron, which is emerging as a new cancer target.
The studies in the report are novel in that they represent the first report to examine the role of Ron overexpression in the development and progression of prostate cancer. These results support the role of the Ron receptor as an oncogene in prostate cancer, as suggested by our previous studies (11, 23). Our data show that Ron overexpression is sufficient to induce prostate cancer in mice. Several studies support the contention that tyrosine kinase inhibitors are effective as chemotherapeutic agents in prostate cancer which raises the possibility that Ron may be an important new drug target for prostate cancer. Moreover, recent studies by O'Toole et al. demonstrated that a Ron receptor blocking antibody is an effective inhibitor against various protumorigenic properties mediated by Ron overexpression including tumor growth and invasion [22]. Combined with the studies present in this report, Ron may not only be a targetable protein in prostate cancer but may represent an important protein with significance in promoting prostate tumorigenesis.
In conclusion, selective overexpression of the Ron receptor tyrosine kinase in the murine prostate leads to the development of prostate intraepithelial neoplasia (mPIN) with and without invasion as well as the development of adenocarcinomas. Ron overexpression is associated with increases in prostate cell proliferation that coincide with severity of prostate histopathology. Ron overexpression also leads to decreases in prostate cell death. This is the first report which demonstrates that Ron overexpression is sufficient to induce prostate cancer in mice. Given that tyrosine kinases are attractive targets for cancer therapy, the potential therapeutic utility of inhibiting the Ron receptor in prostate cancer may provide important new advancements for treatment of this disease.
Supplementary Material
Acknowledgements
This work was supported by Public Health Service Grant R01CA100002 (SEW) from the National Institutes of Health. We would like to thank Glenn Doerman for his contributions to the art work and Sarah Kader for technical contributions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement The authors declare no conflict of interest.
References
- [1].Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- [2].de Bono JS, Attard G, Adjei A, Pollak MN, Fong PC, Haluska P, Roberts L, Melvin C, Repollet M, Chianese D, Connely M, Terstappen LW, Gualberto A. Potential applications for circulating tumor cells expressing the insulin-like growth factor-I receptor. Clin Cancer Res. 2007;13:3611–3616. doi: 10.1158/1078-0432.CCR-07-0268. [DOI] [PubMed] [Google Scholar]
- [3].Agus DB, Scher HI, Higgins B, Fox WD, Heller G, Fazzari M, Cordon-Cardo C, Golde DW. Response of prostate cancer to anti-Her-2/neu antibody in androgen-dependent and -independent human xenograft models. Cancer Res. 1999;59:4761–4764. [PubMed] [Google Scholar]
- [4].Vicentini C, Festuccia C, Gravina GL, Angelucci A, Marronaro A, Bologna M. Prostate cancer cell proliferation is strongly reduced by the epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 in vitro on human cell lines and primary cultures. J Cancer Res Clin Oncol. 2003;129:165–174. doi: 10.1007/s00432-003-0420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sirotnak FM, She Y, Lee F, Chen J, Scher HI. Studies with CWR22 xenografts in nude mice suggest that ZD1839 may have a role in the treatment of both androgen-dependent and androgen-independent human prostate cancer. Clin Cancer Res. 2002;8:3870–3876. [PubMed] [Google Scholar]
- [6].Wagh PK, Peace BE, Waltz SE. Met-related receptor tyrosine kinase Ron in tumor growth and metastasis. Adv Cancer Res. 2008;100:1–33. doi: 10.1016/S0065-230X(08)00001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Maggiora P, Marchio S, Stella MC, Giai M, Belfiore A, De Bortoli M, Di Renzo MF, Costantino A, Sismondi P, Comoglio PM. Overexpression of the RON gene in human breast carcinoma. Oncogene. 1998;16:2927–2933. doi: 10.1038/sj.onc.1201812. [DOI] [PubMed] [Google Scholar]
- [8].Maggiora P, Lorenzato A, Fracchioli S, Costa B, Castagnaro M, Arisio R, Katsaros D, Massobrio M, Comoglio PM, Flavia Di Renzo M. The RON and MET oncogenes are co-expressed in human ovarian carcinomas and cooperate in activating invasiveness. Exp Cell Res. 2003;288:382–389. doi: 10.1016/s0014-4827(03)00250-7. [DOI] [PubMed] [Google Scholar]
- [9].Chen YQ, Zhou YQ, Angeloni D, Kurtz AL, Qiang XZ, Wang MH. Overexpression and activation of the RON receptor tyrosine kinase in a panel of human colorectal carcinoma cell lines. Exp Cell Res. 2000;261:229–238. doi: 10.1006/excr.2000.5012. [DOI] [PubMed] [Google Scholar]
- [10].Thomas RM, Jaquish DV, French RP, Lowy AM. The RON tyrosine kinase receptor regulates vascular endothelial growth factor production in pancreatic cancer cells. Pancreas. 2010;39:301–307. doi: 10.1097/mpa.0b013e3181bb9f73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Thobe MN, Gurusamy D, Pathrose P, Waltz SE. The Ron receptor tyrosine kinase positively regulates angiogenic chemokine production in prostate cancer cells. Oncogene. 2010;29:214–226. doi: 10.1038/onc.2009.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zinser GM, Leonis MA, Toney K, Pathrose P, Thobe M, Kader SA, Peace BE, Beauman SR, Collins MH, Waltz SE. Mammary-specific Ron receptor overexpression induces highly metastatic mammary tumors associated with beta-catenin activation. Cancer Res. 2006;66:11967–11974. doi: 10.1158/0008-5472.CAN-06-2473. [DOI] [PubMed] [Google Scholar]
- [13].Waltz SE, Eaton L, Toney-Earley K, Hess KA, Peace BE, Ihlendorf JR, Wang MH, Kaestner KH, Degen SJ. Ron-mediated cytoplasmic signaling is dispensable for viability but is required to limit inflammatory responses. J Clin Invest. 2001;108:567–576. doi: 10.1172/JCI11881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Iwama A, Yamaguchi N, Suda T. STK/RON receptor tyrosine kinase mediates both apoptotic and growth signals via the multifunctional docking site conserved among the HGF receptor family. EMBO J. 1996;15:5866–5875. [PMC free article] [PubMed] [Google Scholar]
- [15].Peace BE, Toney-Earley K, Collins MH, Waltz SE. Ron receptor signaling augments mammary tumor formation and metastasis in a murine model of breast cancer. Cancer Res. 2005;65:1285–1293. doi: 10.1158/0008-5472.CAN-03-3580. [DOI] [PubMed] [Google Scholar]
- [16].Greenberg NM, DeMayo FJ, Sheppard PC, Barrios R, Lebovitz R, Finegold M, Angelopoulou R, Dodd JG, Duckworth ML, Rosen JM, et al. The rat probasin gene promoter directs hormonally and developmentally regulated expression of a heterologous gene specifically to the prostate in transgenic mice. Mol Endocrinol. 1994;8:230–239. doi: 10.1210/mend.8.2.8170479. [DOI] [PubMed] [Google Scholar]
- [17].Zhang J, Thomas TZ, Kasper S, Matusik RJ. A small composite probasin promoter confers high levels of prostate-specific gene expression through regulation by androgens and glucocorticoids in vitro and in vivo. Endocrinology. 2000;141:4698–4710. doi: 10.1210/endo.141.12.7837. [DOI] [PubMed] [Google Scholar]
- [18].Meyer SE, Waltz SE, Goss KH. The Ron receptor tyrosine kinase is not required for adenoma formation in Apc(Min/+) mice. Mol Carcinog. 2009;48:995–1004. doi: 10.1002/mc.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shappell SB, Thomas GV, Roberts RL, Herbert R, Ittmann MM, Rubin MA, Humphrey PA, Sundberg JP, Rozengurt N, Barrios R, Ward JM, Cardiff RD. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res. 2004;64:2270–2305. doi: 10.1158/0008-5472.can-03-0946. [DOI] [PubMed] [Google Scholar]
- [20].Wagh PK, Gray JK, Zinser GM, Vasiliauskas J, James L, Monga SP, Waltz SE. beta-Catenin is required for Ron receptor-induced mammary tumorigenesis. Oncogene. 2011;30:3694–3704. doi: 10.1038/onc.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Thobe MN, Gray JK, Gurusamy D, Paluch AM, Wagh PK, Pathrose P, Lentsch AB, Waltz SE. The Ron receptor promotes prostate tumor growth in the TRAMP mouse model. Oncogene. 2011 doi: 10.1038/onc.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].O'Toole JM, Rabenau KE, Burns K, Lu D, Mangalampalli V, Balderes P, Covino N, Bassi R, Prewett M, Gottfredsen KJ, Thobe MN, Cheng Y, Li Y, Hicklin DJ, Zhu Z, Waltz SE, Hayman MJ, Ludwig DL, Pereira DS. Therapeutic implications of a human neutralizing antibody to the macrophage-stimulating protein receptor tyrosine kinase (RON), a c-MET family member. Cancer Res. 2006;66:9162–9170. doi: 10.1158/0008-5472.CAN-06-0283. [DOI] [PubMed] [Google Scholar]
- [23].Park JS, Park JH, Khoi PN, Joo YE, Jung YD. MSP-induced RON activation upregulates uPAR expression and cell invasiveness via MAPK, AP-1 and NF-kappaB signals in gastric cancer cells. Carcinogenesis. 2011;32:175–181. doi: 10.1093/carcin/bgq241. [DOI] [PubMed] [Google Scholar]
- [24].Xu XM, Zhou YQ, Wang MH. Mechanisms of cytoplasmic {beta}-catenin accumulation and its involvement in tumorigenic activities mediated by oncogenic splicing variant of the receptor originated from Nantes tyrosine kinase. J Biol Chem. 2005;280:25087–25094. doi: 10.1074/jbc.M414699200. [DOI] [PubMed] [Google Scholar]
- [25].Wang D, Shen Q, Chen YQ, Wang MH. Collaborative activities of macrophage-stimulating protein and transforming growth factor-beta1 in induction of epithelial to mesenchymal transition: roles of the RON receptor tyrosine kinase. Oncogene. 2004;23:1668–1680. doi: 10.1038/sj.onc.1207282. [DOI] [PubMed] [Google Scholar]
- [26].Kaplan-Lefko PJ, Chen TM, Ittmann MM, Barrios RJ, Ayala GE, Huss WJ, Maddison LA, Foster BA, Greenberg NM. Pathobiology of autochthonous prostate cancer in a pre-clinical transgenic mouse model. Prostate. 2003;55:219–237. doi: 10.1002/pros.10215. [DOI] [PubMed] [Google Scholar]
- [27].Ellwood-Yen K, Graeber TG, Wongvipat J, Iruela-Arispe ML, Zhang J, Matusik R, Thomas GV, Sawyers CL. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell. 2003;4:223–238. doi: 10.1016/s1535-6108(03)00197-1. [DOI] [PubMed] [Google Scholar]
- [28].Nikolaidis NM, Gray JK, Gurusamy D, Fox W, Stuart WD, Huber N, Waltz SE. Ron receptor tyrosine kinase negatively regulates TNFalpha production in alveolar macrophages by inhibiting NF-kappaB activity and Adam17 production. Shock. 2010;33:197–204. doi: 10.1097/SHK.0b013e3181ae8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.