Abstract
Previous studies have reported that environmental lead (Pb) exposure can result in neurological alterations in children leading to reduced IQ, attention deficit hyperactivity disorder, and diminished reading and learning abilities. However, the specific alterations in neurodevelopmental morphology and the underlying genetic mechanisms of these alterations have not yet been thoroughly defined. To investigate alterations in neurologic morphology and test the hypothesis that developmental Pb neurotoxicity is partially mediated through alterations in neuronal growth and transport function of axons, the changes of specific axon tracts in the embryonic zebrafish brain were observed with anti-acetylated α-tubulin staining at several developmental time points through 36 hours post fertilization (hpf). In addition, the role of a subset of axonogenesis-related genes including shha, epha4b, netrin1b, netrin2, and noi were investigated with real-time quantitative PCR (qPCR). Pb treatment resulted in decreased axonal density at 18, 20, and 24 hpf for specific axon tracts in the midbrain and forebrain. These observations corresponded to an observed down-regulation of shha and epha4b at 14 and 16 hpf, respectively. The axonal density in Pb exposed individuals at later stages (30 and 36 hpf) was not significantly different from controls. An overexpression of netrin2 at these two developmental stages suggests a novel role for this gene in regulating axonal density specific to Pb neurotoxicity. Although no significant differences in axonal density was observed in the two later developmental stages, further studies are needed to determine if the morphologic alterations observed at the earlier stages will have lasting functional impacts.
Keywords: lead, Pb, neurodevelopment, axon tract, embryo, zebrafish, neurotoxicity
1. Introduction
Lead (Pb) exposure can result in a myriad of adverse health effects that are dependent on dose. The central nervous system is the most sensitive target of Pb toxicity and epidemiological studies have reported several neurological alterations in children including reduced IQ, attention deficit hyperactivity disorder, diminished reading and learning abilities, hearing loss and other health and behavioral disruptions [2,9,24,34,42]. Researchers have found that even low-dose Pb exposure (below 10μg/dl, the current action level of CDC) can induce neurodevelopmental alterations in children [5,6,16,33]. However, the specific alterations in neurodevelopmental morphology in the brain and the genetic mechanisms underlying these alterations are not yet completely understood.
Multiple mechanisms have been implicated in Pb neurotoxicity including cell type-specific responses and various molecular targets involving cell signaling and other functions [32]. One of the suggested mechanisms specific to developmental Pb neurotoxicity is the impairment of neuronal morphogenesis. In a study investigating the effects of Pb in the developing retinotectal system of tadpoles, all tested Pb treatments (PbCl2, ranging from 10−10 to 10−6M, surgically implanted over optic tectum) were observed to significantly reduce the area and branchtip number of retinal ganglion cell axon arborizations within the optic tectum after 6 weeks treatment [10]. In addition, Pb exposure (0.1 mM or 2 mM Pb acetate administered in drinking water) has been reported to result in neuritic beading in auditory axons of postnatal day 21 Balb/c mice and in vitro in differentiated SH-SY5Y cells [19]. These two findings suggest that developmental Pb exposure may impair axonal growth and transport function, but how these alterations progress over the developmental time course and the underlying genetic mechanisms are not well defined.
In this study to further investigate the morphologic and genetic alterations associated with developmental Pb neurotoxicity, the zebrafish vertebrate model system was used. The zebrafish model system provides an informative tool to investigate neurogenesis from the one cell stage to fully-developed organs and systems. A strength of the zebrafish vertebrate model system utilized in this study is the rapid ex utero embryonic development to elucidate brain morphological alterations associated with developmental Pb exposure. In a previous toxicogenomics study in our laboratory [37], zebrafish embryos exposed to 100 parts per billion (ppb) Pb from ~2 hours post fertilization (hpf) through 72 hpf resulted in gene alterations associated with neurodevelopment. This gene list included numerous genes involved in neuronal ontogenesis and synapse formation. Thus in this current study, the morphological alterations in axon tracts associated with the Pb exposure were investigated to link genetic and morphologic data at earlier developmental time points.
2. Methods
2.1. Zebrafish husbandry
Zebrafish used in this study were of the wild-type AB strain and were housed on a 14:10 hour light:dark cycle in a Z-Mod System (Aquatic Habitats, Apopka, FL) at 28°C. Fish were maintained and bred according to protocols approved by the university’s Institutional Animal Care and Use Committee with all fish treated humanely and with regard to alleviation of suffering [44]. Adults were bred in spawning cages to obtain staged embryos following established protocols [44]. Based on our previous study [37], a 100 ppb Pb treatment (Pb acetate, Sigma, St. Louis, MO) or a control treatment (filtered fish water) were initiated at ~2 hpf and embryos were collected at numerous developmental stages from 12 to 36 hpf for experimental procedures.
2.2 Pb concentration of tissue and treatment water determined by inductively coupled plasma - mass spectrometry (ICP-MS)
Zebrafish embryos were collected, washed, weighed, and digested for Pb analysis using an Elan DRC-e ICP-MS (PerkinElmer Sciex, Shelton, CT) at multiple developmental time points from 12 to 36 hpf. For the digestion, whole zebrafish embryos were submerged in 50 to 200 μl 50% nitric acid (volume dependent on weight/developmental stage) and digested overnight at 65°C in a water bath. Samples were diluted with deionized water to reach a final 1% HNO3 concentration and analyzed by ICP-MS following standard protocols [7]. Thallium (ICP-081, ULTRA Scientific, North Kingstown, RI) was used as an internal standard and Pb standard curves were performed simultaneously. The concentration of Pb in zebrafish tissue was calculated as ng/g tissue. In addition, Pb stock solutions and treatment water were collected and analyzed by ICP-MS following a similar procedure. To observe Pb distribution in the chorion and fish body, embryos at the 24 hpf stage were dissected using a tungsten needle and chorion and fish body processed separately.
2.3 Immunofluorescence staining
Embryos were collected at multiple developmental time points (18, 20, 24, 30 and 36 hpf) following Pb treatment, washed, and dechorionated. Embryos were fixed overnight with 4% paraformaldehyde (PFA) at 4°C. The eyes and skin in the brain region were removed and embryos were blocked overnight in blocking buffer (PBS, 1% Triton, 10% FBS, 0.5% BSA, 1% DMSO). Embryos were then incubated in 1:1000 mouse monoclonal anti-acetylated α-tubulin antibody (Catalog No.T6793; Sigma-Aldrich, St. Louis, MO) overnight at 4°C, washed four times with PBS, 1% Triton, and incubated in 1:1000 Alexa Fluor® 546 rabbit anti-mouse IgG (H+L) (Catalog No.A11060; Invitrogen, Carlsbad, CA) overnight at 4°C. Whole mount embryos were laterally oriented and scanned in serial 2 μm optical sections to observe the axon tracts using a laser scanning confocal microscope (Nikon, Japan). Z-stack images were merged using ImageJ 1.44p (NIH, Bethesda, MD). After background subtraction, the fluorescence density (in arbitrary unit, AU) of the region of interest was measured using Image-pro plus 6.0 (Media Cybernetics, Silver Springs, MD).
2.4 Real-time quantitative PCR
Embryos were collected at multiple developmental time points from 14 to 36 hpf following Pb treatment. RNA was isolated and cDNA was synthesized as described in Peterson and Freeman [36]. qPCR analysis was performed on a Stratagene MX3000P (Agilent, La Jolla, CA) using the iQ SYBR green kit (Biorad, Hercules, CA) following similar methods as described in Peterson et al. [37]. The cycling parameters included a 3 minute incubation phase at 95°C, 40 cycles of 95°C for 10 seconds, 60°C for 30 seconds and 72°C for 30 seconds with a dissociation curve to check for non-specific amplification. Efficiency and specificity were checked with melting and dilution curve analysis and no-template controls. Quantitative copies were calculated by standard curve and individual gene expression was normalized to gapdh (ratio of gene/gapdh). Details of the primers are listed in Table 1.
Table 1.
Primer sequences for axonogenesis genes for qPCR analysis
| Gene name | Primer sequence | GenBank ID | Amplicon size (bp) |
|---|---|---|---|
| shha | Forward: AGACCGAGACTCCACGACGC Reverse: TGCAGTCACTGGTGCGAACG |
NM_131063.1 | 265 |
| epha4b | Forward: TGCCGAGTGCGAAACCAGT Reverse: TCCCTCCCTCACACCGAGTC |
NM_153658.1 | 219 |
| netrin1b | Forward: TCACCTCGATGGTGTCCGG Reverse: GCAGCGTCTAGGGTTCCCGT |
NM_130998.1 | 110 |
| netrin2 | Forward: TATAGGGACATGGGCAAAG Reverse: AGCCTTTAGCACAGCGGTTA |
NM_001037415.1 | 160 |
| noi | Forward: GGGACTGAGAGACACTCGCC Reverse: ACGAAGACACGAGGCTGGTCA |
NM_131184.2 | 291 |
2.5 Statistical analysis
The quantitative data are expressed as mean ± SD. For the comparison of Pb concentration in tissue throughout development, data were analyzed with a two-way ANOVA and a post hoc least significant difference (LSD) test. A Student’s t-test was used to compare Pb concentration in chorion and embryo within treatment. Anti-acetylated α-tubulin staining data were analyzed by time point with a Student’s t-test. The gene expression data was analyzed by gene at each time point with a Student’s t-test. The level accepted for statistical significance in all cases was p<0.05.
3. Results
3.1 Time course absorption of Pb in zebrafish tissue
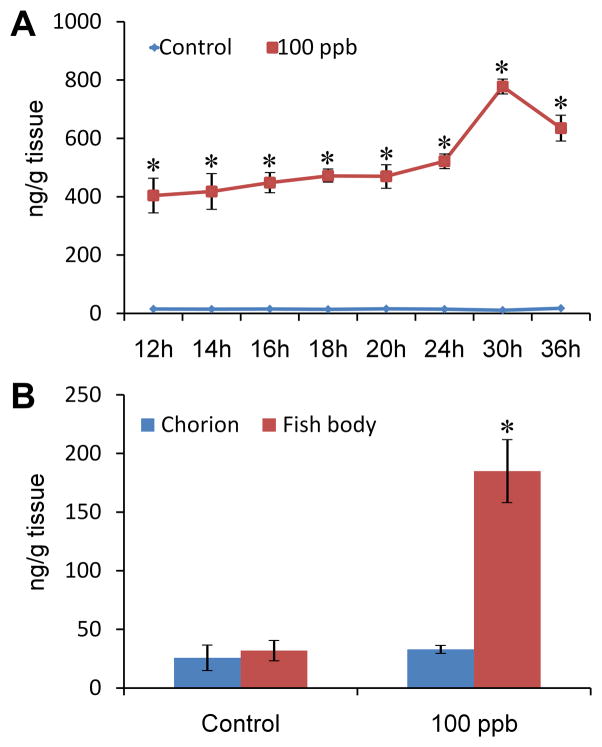
To confirm Pb exposure and estimate the actual dose of Pb in zebrafish tissue, ICP-MS was employed to analyze the Pb concentration of zebrafish tissue in a series of developmental time points (12, 14, 16, 18, 20, 24, 30 and 36 hpf). Compared to the control group at each time point, Pb treated embryos showed an obvious increase of Pb accumulation in tissue (p<0.0001, for developmental time point, Pb treatment, and the interaction between time point and Pb treatment, Fig. 1A). The values at all time points (≥404.39±57.49 ng/g) was >6-fold higher than the measured Pb concentration in the original treatment solution (65.64±1.45 ppb). From 12 to 30 hpf, Pb concentrations increased from 404.39±57.49 to 778.11±23.27 ng/g, and then decreased slightly at 36 hpf (635.25±42.14 ng/g). The comparative data among the Pb concentration in the original treatment water and the increasing Pb concentration in the embryos indicate Pb accumulates in zebrafish tissue. At 24 hpf, bodies of Pb treated embryos contained a much higher amount of Pb than the chorions (body: 184.94±26.30 ng/g; chorion: 32.93±8.09 ng/g, p=0.001, Fig. 1B). The average concentration of Pb in the body and chorion of the treatment group at 24 hpf is less than the average amount of Pb measured in the complete embryo at this same time point (Fig. 1A). This unaccounted Pb is suspected to be in the chorionic fluid and thus lost when fish body and chorion were separated for this measurement. No obvious differences for Pb distribution were found between fish bodies and chorions in the control group (p=0.549).
Figure 1. Pb accumulation in zebrafish tissue during embryonic exposure.
A: Pb concentration of zebrafish tissue from 12 to 36 hpf (with chorion). *p<0.0001, compared with control by time point; B: Pb distribution in chorion and fish body at 24 hpf. *p<0.05, fish body compared with chorion within treatment. For each concentration each time point, n=3.
3.2 Changes of specific axon tracts in forebrain and midbrain at 18, 20, 24, 30, and 36 hpf
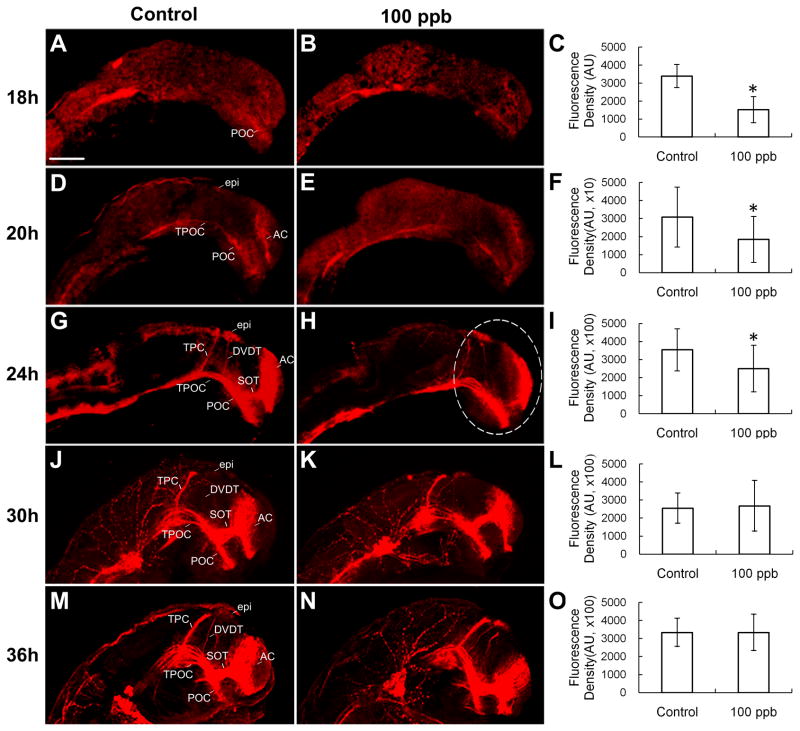
Anti-acetylated α-tubulin antibody is commonly used to stain early axon tracts. Specific axon tracts including the anterior commissure (AC), post-optic commissure (POC), supra-optic tract (SOT), tract of post-optic commissure (TPOC), tract of the posterior commissure (TPC) and dorsal-ventral diencephalic tract (DVDT) in the forebrain and midbrain in lateral view were observed in this study (Fig. 2). In the control group, the AC and POC were slightly stained at 18 hpf (Fig. 2A). These two commissures extended longer and showed brighter staining at 20 hpf, while the TPOC also appeared at this time point (Fig. 2D). At 24 hpf, considerably more axon tracts, including the SOT, TPC, DVDT, and epiphyseal cluster (epi) had formed (Fig. 2G). Further development of axon tracts was observed at 30 hpf (Fig. 2J) and 36 hpf (Fig. 2M). Density data showed that 100 ppb Pb treatment significantly decreased the density of axon tracts at 18, 20 and 24 hpf (p=0.030, 0.039 and 0.029, respectively, Fig. 2B, 2C, 2E, 2F, 2H, and 2I), but not at 30 and 36 hpf (p=0.729 and 0.996, respectively, Fig. 2K, 2L, 2N, and 2O).
Figure 2. Effect of Pb on density of axon tracts of embryonic zebrafish brain in lateral view at 18, 20, 24, 30 and 36 hpf.
Anti-acetylated α-tubulin staining clearly showed axon tracts at 18, 20, 24, 30 and 36 hpf. A, B and C: 18 hpf (n=15–18); D, E and F: 20 hpf (n=14–16); G, H and I: 24 hpf (n=14–16), J, K and L: 30 hpf (n=21–22); M, N and O: 36 hpf (n=13–14). Density data of stained region of interest (white dash line marked) were measured using Image-pro plus (C, F, I, L and O). The units for F are 10 times greater than C while the units for I, L and O are 100 times greater (denoted as ×10 and ×100). AC, anterior commissure; POC, post-optic commissure; SOT, supra-optic tract; TPOC, tract of post-optic commissure; TPC, tract of the posterior commissure; DVDT, dorsal-ventral diencephalic tract; epi, epiphyseal cluster. Scale bar=100 μm. *p<0.05.
3.3 Observation of axon tracts and neurons in hindbrain at 20 and 24 hpf
Hindbrain axon tracts were also observed in this study as anti-acetylated α-tubulin stains Mauthner neurons and other axon tracts including the dorsal longitudinal fasciculus (DLF) and ventral longitudinal fasciculus (VLF) in the zebrafish hindbrain. No significant difference between control and Pb treatment were observed at either time point (p=0.516 and 0.374, respectively).
3.4 Changes of axonogenesis genes in Pb neurotoxicity at 14, 16, 18, 20, 24, 30 and 36 hpf
The expression of a subset of genes involved in axonogenesis [sonic hedgehog a (shha), ephrin type-A receptor 4b (epha4b), netrin (netrin1b, netrin2), and no-isthmus (noi)] were evaluated by qPCR analysis at multiple developmental time points from 14 to 36 hpf. In Pb treated embryos the shha transcript level was significantly decreased at 14 hpf (decreased 30%, p=0.018, compared to control, Fig. 3A). At 16 hpf, a significant decrease in transcript level was observed for epha4b (decreased 24%, p=0.043, compared to control, Fig. 3B). The expression of netrin2 was significantly upregulated at 30 and 36 hpf (p=0.022 and 0.026, respectively, compared to control at each time point, Fig. 3C and 3D). Significant differences in expression of these genes were not observed at the intermediate developmental time points analyzed (i.e., 18, 20, or 24 hpf). Moreover, no significant difference in transcript level was observed for netrin1b or noi at any of the developmental time points assessed in this study.
Figure 3. Altered transcript level of axonogenesis genes following Pb treatment at different developmental time points.
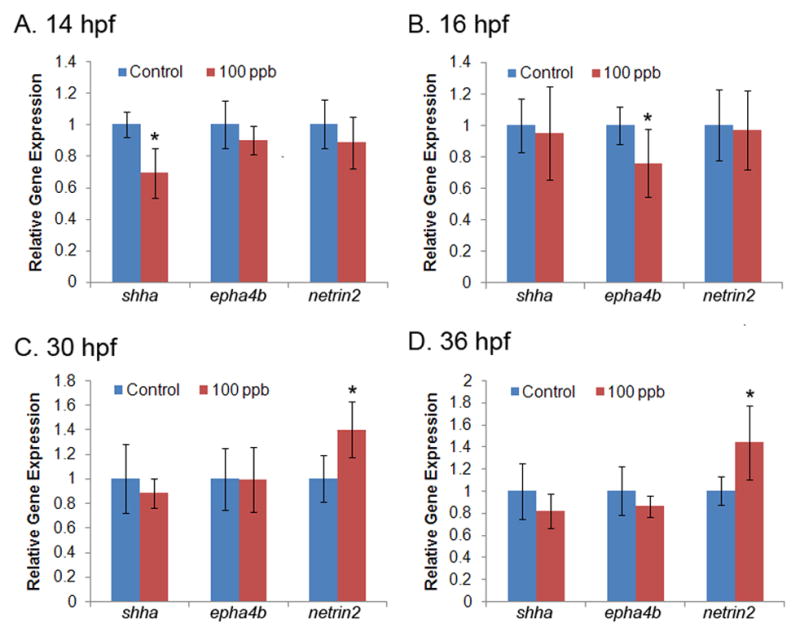
Transcript levels for five genes of interest were measured at seven developmental time points with qPCR. Data were quantified with standard curves using gapdh as a reference gene and normalized to control treatment. Transcript levels of shha and epha4b were significantly altered at 14 hpf (A) and 16 hpf (B), respectively. Netrin2 was significantly altered at 30 and 36 hpf (C and D). No differences were detected in gene expression at 18, 20 or 24 hpf for shha, epha4b or netrin2 (data not shown) or in netrin1b or noi at any time point analyzed (data not shown). n=6 (50 pooled embryos for each replicate per time point per group). *p<0.05, Pb treatment compared to control
4. Discussion
The early development of embryonic zebrafish primarily occurs in a chorionic membrane. After fertilization, embryos grow rapidly through several stages with differences in morphology denoting progression through each developmental stage. These developmental stages include the zygote period (0–0.75 hpf), cleavage period (0.7–2.2 hpf), blastula period (2.25–5.25 hpf), gastrula period (5.25–10 hpf), segmentation (10–24 hpf), pharyngula period (24–48 hpf) and hatching period (48–72 h) [21]. At 16 hpf, the first post-mitotic neurons appear in the forebrain and midbrain in three distinct clusters, including the dorsorostral cluster (drc), ventrorostral cluster (vrc) and ventrocaudal cluster (vcc) [38]. These first primary neurons extend long axons to pioneer pathways and make small local connections [18,20]. Another cluster, epi, can be found from 18 hpf [38]. By 24 hpf, these clusters have extended axon tract commissures including AC, POC, SOT, TPOC, TPC, and DVDT forming a simple scaffold of axon tracts (Fig. 2G). Specific and simple axon tracts can be studied in embryonic zebrafish and are a good model to study the axonogenesis process. By using anti-acetylated α-tubulin staining, our results indicate that 100 ppb Pb treatment decreases axon density at early embryonic stages (18, 20 and 24 hpf), but this difference is no longer observed at 30 and 36 hpf.
To investigate the genetic pathways associated with the decreased axonal density associated with developmental Pb neurotoxicity observed at 18, 20 and 24 hpf in this study, the expression level of a subset of genes expressed in the regions adjacent to the AC and POC and involved in axonal guidance and migration were evaluated [45]. These genetic targets included shha [1,4], epha4b [29,46], netrin1b [30,41], netrin2 [30,41], and noi [3,29]. Shh is a morphogen that plays an important role in the patterning of multiple organs, including the nervous system [17,26]. Shh can act as an axonal guidance cue on spinal cord commissural axons and retinal ganglion cell (RGC) axons [8,22]. In zebrafish, a previous study reported that shh is expressed at 6 hpf (embryonic shield), 11 hpf (notochord, floor plate, and ventral forebrain), 48 hpf and 3 weeks (whole nervous system) [13]. In an additional study, a decreased shh transcript level was found at 5 and 6 days post fertilization (dpf) compared to that at 1 dpf [14]. In the present study, Pb exposure down-regulated shha levels at 14 hpf. Shh is reported to collaborate with Netrin1 to guide commissural axon extension [39] and overexpression of shh impairs netrin1b expression in the ventral neural tube [41]. Although netrin1b expression was not altered in Pb treated embryos in this study, decreased shha transcript levels may relate to limited growth of axon tracts observed at 18, 20 and 24 hpf.
Epha4 is a member of the ephrin receptor subfamily of protein-tyrosine kinases and is involved in mediating developmental events, particularly in the nervous system [15]. In mouse, Epha4 has been implicated in the restriction of axons from entering the anterior branch of the AC [11]. As evidenced by an Epha4 mutant mouse, knocking out Epha4 causes failure of axon guidance of corticospinal neurons and a defect in formation of the AC [12]. Kullander et al. [23] demonstrated that Epha4 has both kinase-dependent and kinase-independent functions in the formation of major axon tracts, including the corticospinal tract and the AC. In zebrafish, epha4 has been related to the segmental restriction of gene expression in the hindbrain and is required in patterning the developing zebrafish forebrain (diencephalic territory and structures) [46]. In our study, the transcript level of epha4b following Pb treatment was significantly decreased at 16 hpf indicating epha4b may also play a role in the decreased axonal density observed at 18, 20 and 24 hpf.
The netrin family of laminin-related molecules is reported to be involved in axon guidance and cell migration during development [27,41]. In zebrafish, two netrin homologues, netrin1b and netrin2, are mainly expressed in the floor plate and the anterior ventral neural tube (including ventral to the AC) [29,30,35,41]. It has been demonstrated that netrins act to stimulate axonal outgrowth in addition to attracting and repelling growth cones in zebrafish embryos [25]. Although there is no direct evidence of netrin knockout defects in zebrafish, Serafini et al. [40] reported that Netrin1 deficient mice exhibit defects in spinal commissural axon projections and in several forebrain commissures. In the present study, Pb treatment resulted in a significant increase in expression of netrin2, but not netrin1b at 30 and 36 hpf. Considering the role of netrins on stimulating axonal outgrowth, our results indicate that netrin2 may play a role in regulating axonal growth in the response mechanism to Pb neurodevelopmental toxicity.
noi is a zebrafish gene that belongs to the Pax family of transcriptional regulators which is important in early development for differentiation of multiple tissues, including nerve development [43,47]. In the zebrafish, noi has important functions at multiple stages of embryogenesis [28,29,31] and is expressed in restricted domains of the developing zebrafish central nervous system, such as midline cells dorsal/rostral to the POC [3,29]. noi normally discourages axonal extension into the noi expressed domain [29,45]. Early decussating axon tracts are prevented from entering the territory that expresses noi protein in wild-type embryos, and noi mutant zebrafish embryos show severe defects in the rostral forebrain. These defects include axon tracts that are no longer tightly fasciculated in POC and many axon tracts that have migrated inappropriately [29,45]. Based on our data, noi expression was not significantly changed due to Pb treatment at any of the developmental time points tested in this study.
In summary, our results showed that Pb exposure (100 ppb) decreased the density of axon tracts in midbrain and forebrain at 18, 20 and 24 hpf, corresponding to the down-regulation of shha and epha4b genes at 14 hpf and 16 hpf, respectively. Alternatively, no significant difference in axon density was observed at 30 and 36 hpf. The increased expression level of netrin2 at these two developmental stages, indicates netrin2 may play a role mediating axon density alterations caused by Pb toxicity. Although no significant difference in axon density was detected at the later stages in this study, further studies need to be conducted to determine if lasting functional impacts may result from the lag in axonal development.
Highlights.
Decreased axonal density at 18, 20, and 24 hpf in the midbrain and forebrain.
Decreased axonal density corresponded to down-regulation of shha and epha4b.
Axonal density in Pb exposed individuals at later stages was not altered.
Overexpression of netrin2 observed at these later developmental stages.
Acknowledgments
We thank Wendy Jiang and Sherleen Fu for their help on the laser confocal microscopy. We also thank Dr. Linda Lee and Stephen Sassman for assistance with the ICP-MS analysis. This work was supported by a grant from the Ralph W. and Grace M. Showalter Research Trust (JLF) and by grant ES017055 from NIEHS/NIH (WZ).
Abbreviations
- AC
anterior commissure
- dpf
days post fertilization
- DLF
dorsal longitudinal fasciculus
- drc
dorsorostral cluster
- DVDT
dorsal-ventral diencephalic tract
- epha4b
ephrin type-A receptor 4b
- epi
epiphyseal cluster
- hpf
hours post fertilization
- ICP-MS
inductively coupled plasma - mass spectrometry
- noi
no-isthmus
- Pb
lead
- PFA
paraformaldehyde
- POC
post-optic commissure
- ppb
part per billion
- qPCR
real-time quantitative PCR
- RGC
retinal ganglion cell
- shha
sonic hedgehog a
- SOT
supra-optic tract
- TPC
tract of the posterior commissure
- TPOC
tract of post-optic commissure
- vcc
ventrocaudal cluster
- VLF
ventral longitudinal fasciculus
- vrc
ventrorostral cluster
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barth KA, Wilson SW. Expression of zebrafish nk2.2 is influenced by sonic hedgehog/vertebrate hedgehog-1 and demarcates a zone of neuronal differentiation in the embryonic forebrain. Development. 1995;121:1755–68. doi: 10.1242/dev.121.6.1755. [DOI] [PubMed] [Google Scholar]
- 2.Bellinger DC. Very low lead exposures and children’s neurodevelopment. Curr Opin Pediatr. 2008;20:172–7. doi: 10.1097/MOP.0b013e3282f4f97b. [DOI] [PubMed] [Google Scholar]
- 3.Brand M, Heisenberg CP, Jiang YJ, Beuchle D, Lun K, Furutani-Seiki M, Granato M, Haffter P, Hammerschmidt M, Kane DA, et al. Mutations in zebrafish genes affecting the formation of the boundary between midbrain and hindbrain. Development. 1996;123:179–90. doi: 10.1242/dev.123.1.179. [DOI] [PubMed] [Google Scholar]
- 4.Brennan C, Monschau B, Lindberg R, Guthrie B, Drescher U, Bonhoeffer F, Holder N. Two Eph receptor tyrosine kinase ligands control axon growth and may be involved in the creation of the retinotectal map in the zebrafish. Development. 1997;124:655–64. doi: 10.1242/dev.124.3.655. [DOI] [PubMed] [Google Scholar]
- 5.Canfield RL, Henderson CR, Jr, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N Engl J Med. 2003;348:1517–26. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canfield RL, Kreher DA, Cornwell C, Henderson CR., Jr Low-level lead exposure, executive functioning, and learning in early childhood. Child Neuropsychol. 2003;9:35–53. doi: 10.1076/chin.9.1.35.14496. [DOI] [PubMed] [Google Scholar]
- 7.Carmosini N, Lee LS. Partitioning of fluorotelomer alcohols to octanol and different sources of dissolved organic carbon. Environ Sci Technol. 2008;42:6559–65. doi: 10.1021/es800263t. [DOI] [PubMed] [Google Scholar]
- 8.Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 2003;113:11–23. doi: 10.1016/s0092-8674(03)00199-5. [DOI] [PubMed] [Google Scholar]
- 9.Cleveland LM, Minter ML, Cobb KA, Scott AA, German VF. Lead hazards for pregnant women and children: part 1: immigrants and the poor shoulder most of the burden of lead exposure in this country. Part 1 of a two-part article details how exposure happens, whom it affects, and the harm it can do. Am J Nurs. 2008;108:40–9. doi: 10.1097/01.NAJ.0000337736.76730.66. quiz 50. [DOI] [PubMed] [Google Scholar]
- 10.Cline HT, Witte S, Jones KW. Low lead levels stunt neuronal growth in a reversible manner. Proc Natl Acad Sci U S A. 1996;93:9915–20. doi: 10.1073/pnas.93.18.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coulthard MG, Duffy S, Down M, Evans B, Power M, Smith F, Stylianou C, Kleikamp S, Oates A, Lackmann M, et al. The role of the Eph-ephrin signalling system in the regulation of developmental patterning. Int J Dev Biol. 2002;46:375–84. [PubMed] [Google Scholar]
- 12.Dottori M, Hartley L, Galea M, Paxinos G, Polizzotto M, Kilpatrick T, Bartlett PF, Murphy M, Kontgen F, Boyd AW. EphA4 (Sek1) receptor tyrosine kinase is required for the development of the corticospinal tract. Proc Natl Acad Sci U S A. 1998;95:13248–53. doi: 10.1073/pnas.95.22.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ertzer R, Muller F, Hadzhiev Y, Rathnam S, Fischer N, Rastegar S, Strahle U. Cooperation of sonic hedgehog enhancers in midline expression. Dev Biol. 2007;301:578–89. doi: 10.1016/j.ydbio.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Fan CY, Cowden J, Simmons SO, Padilla S, Ramabhadran R. Gene expression changes in developing zebrafish as potential markers for rapid developmental neurotoxicity screening. Neurotoxicol Teratol. 2010;32:91–8. doi: 10.1016/j.ntt.2009.04.065. [DOI] [PubMed] [Google Scholar]
- 15.Flanagan JG, Vanderhaeghen P. The ephrins and Eph receptors in neural development. Annu Rev Neurosci. 1998;21:309–45. doi: 10.1146/annurev.neuro.21.1.309. [DOI] [PubMed] [Google Scholar]
- 16.Grandjean P. Even low-dose lead exposure is hazardous. Lancet. 2010;376:855–6. doi: 10.1016/S0140-6736(10)60745-3. [DOI] [PubMed] [Google Scholar]
- 17.Herzog W, Zeng X, Lele Z, Sonntag C, Ting JW, Chang CY, Hammerschmidt M. Adenohypophysis formation in the zebrafish and its dependence on sonic hedgehog. Dev Biol. 2003;254:36–49. doi: 10.1016/s0012-1606(02)00124-0. [DOI] [PubMed] [Google Scholar]
- 18.Hjorth J, Key B. Development of axon pathways in the zebrafish central nervous system. Int J Dev Biol. 2002;46:609–19. [PubMed] [Google Scholar]
- 19.Jones LG, Prins J, Park S, Walton JP, Luebke AE, Lurie DI. Lead exposure during development results in increased neurofilament phosphorylation, neuritic beading, and temporal processing deficits within the murine auditory brainstem. J Comp Neurol. 2008;506:1003–17. doi: 10.1002/cne.21563. [DOI] [PubMed] [Google Scholar]
- 20.Kimmel CB. Patterning the brain of the zebrafish embryo. Annu Rev Neurosci. 1993;16:707–32. doi: 10.1146/annurev.ne.16.030193.003423. [DOI] [PubMed] [Google Scholar]
- 21.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 22.Kolpak A, Zhang J, Bao ZZ. Sonic hedgehog has a dual effect on the growth of retinal ganglion axons depending on its concentration. J Neurosci. 2005;25:3432–41. doi: 10.1523/JNEUROSCI.4938-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kullander K, Mather NK, Diella F, Dottori M, Boyd AW, Klein R. Kinase-dependent and kinase-independent functions of EphA4 receptors in major axon tract formation in vivo. Neuron. 2001;29:73–84. doi: 10.1016/s0896-6273(01)00181-7. [DOI] [PubMed] [Google Scholar]
- 24.Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, Canfield RL, Dietrich KN, Bornschein R, Greene T, et al. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113:894–9. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lauderdale JD, Davis NM, Kuwada JY. Axon tracts correlate with netrin-1a expression in the zebrafish embryo. Mol Cell Neurosci. 1997;9:293–313. doi: 10.1006/mcne.1997.0624. [DOI] [PubMed] [Google Scholar]
- 26.Lewis KE, Eisen JS. Hedgehog signaling is required for primary motoneuron induction in zebrafish. Development. 2001;128:3485–95. doi: 10.1242/dev.128.18.3485. [DOI] [PubMed] [Google Scholar]
- 27.Livesey FJ, Hunt SP. Netrin and netrin receptor expression in the embryonic mammalian nervous system suggests roles in retinal, striatal, nigral, and cerebellar development. Mol Cell Neurosci. 1997;8:417–29. doi: 10.1006/mcne.1997.0598. [DOI] [PubMed] [Google Scholar]
- 28.Lun K, Brand M. A series of no isthmus (noi) alleles of the zebrafish pax2.1 gene reveals multiple signaling events in development of the midbrain-hindbrain boundary. Development. 1998;125:3049–62. doi: 10.1242/dev.125.16.3049. [DOI] [PubMed] [Google Scholar]
- 29.Macdonald R, Scholes J, Strahle U, Brennan C, Holder N, Brand M, Wilson SW. The Pax protein Noi is required for commissural axon pathway formation in the rostral forebrain. Development. 1997;124:2397–408. doi: 10.1242/dev.124.12.2397. [DOI] [PubMed] [Google Scholar]
- 30.Macdonald R, Xu Q, Barth KA, Mikkola I, Holder N, Fjose A, Krauss S, Wilson SW. Regulatory gene expression boundaries demarcate sites of neuronal differentiation in the embryonic zebrafish forebrain. Neuron. 1994;13:1039–53. doi: 10.1016/0896-6273(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 31.Majumdar A, Lun K, Brand M, Drummond IA. Zebrafish no isthmus reveals a role for pax2.1 in tubule differentiation and patterning events in the pronephric primordia. Development. 2000;127:2089–98. doi: 10.1242/dev.127.10.2089. [DOI] [PubMed] [Google Scholar]
- 32.McQueen CAB, James Ramos, Kenneth Lamb, James Guengerich, Peter F, Lawrence David, Walker Mary, Campen Matthew, Schnellmann Rick, Yost Garold S, Roth Robert A, Ganey Patricia, Hooser Stephen, Richburg John, Hoyer Patricia, Knudsen Thomas, Daston George, Philbert Martin, Roberts Ruth, editors. Comprehensive Toxicology. 2. 1–14. New York: Elsevier; 2010. [Google Scholar]
- 33.Min JY, Min KB, Cho SI, Kim R, Sakong J, Paek D. Neurobehavioral function in children with low blood lead concentrations. Neurotoxicology. 2007;28:421–5. doi: 10.1016/j.neuro.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Needleman HL, Schell A, Bellinger D, Leviton A, Allred EN. The long-term effects of exposure to low doses of lead in childhood. An 11-year follow-up report. N Engl J Med. 1990;322:83–8. doi: 10.1056/NEJM199001113220203. [DOI] [PubMed] [Google Scholar]
- 35.Park KW, Urness LD, Senchuk MM, Colvin CJ, Wythe JD, Chien CB, Li DY. Identification of new netrin family members in zebrafish: developmental expression of netrin 2 and netrin 4. Dev Dyn. 2005;234:726–31. doi: 10.1002/dvdy.20474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peterson SM, Freeman JL. RNA isolation from embryonic zebrafish and cDNA synthesis for gene expression analysis. J Vis Exp. 2009 doi: 10.3791/1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peterson SM, Zhang J, Weber G, Freeman JL. Global Gene Expression Analysis Reveals Dynamic and Developmental Stage Dependent Enrichment of Lead (Pb)-Induced Neurological Gene Alterations. Environ Health Perspect. 2010 doi: 10.1289/ehp.1002590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ross LS, Parrett T, Easter SS., Jr Axonogenesis and morphogenesis in the embryonic zebrafish brain. J Neurosci. 1992;12:467–82. doi: 10.1523/JNEUROSCI.12-02-00467.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salinas PC. The morphogen sonic hedgehog collaborates with netrin-1 to guide axons in the spinal cord. Trends Neurosci. 2003;26:641–3. doi: 10.1016/j.tins.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–14. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- 41.Strahle U, Fischer N, Blader P. Expression and regulation of a netrin homologue in the zebrafish embryo. Mech Dev. 1997;62:147–60. doi: 10.1016/s0925-4773(97)00657-6. [DOI] [PubMed] [Google Scholar]
- 42.Surkan PJ, Zhang A, Trachtenberg F, Daniel DB, McKinlay S, Bellinger DC. Neuropsychological function in children with blood lead levels <10 microg/dL. Neurotoxicology. 2007;28:1170–7. doi: 10.1016/j.neuro.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thanos S, Puttmann S, Naskar R, Rose K, Langkamp-Flock M, Paulus W. Potential role of Pax-2 in retinal axon navigation through the chick optic nerve stalk and optic chiasm. J Neurobiol. 2004;59:8–23. doi: 10.1002/neu.20001. [DOI] [PubMed] [Google Scholar]
- 44.Westerfield M. The Zebrafish Book: A Guide for the Laboratory use of Zebrafish (Danio rerio) 4. Eugene: Univ. of Oregon; 2000. [Google Scholar]
- 45.Wilson SW, Brennan C, Macdonald R, Brand M, Holder N. Analysis of axon tract formation in the zebrafish brain: the role of territories of gene expression and their boundaries. Cell Tissue Res. 1997;290:189–96. doi: 10.1007/s004410050922. [DOI] [PubMed] [Google Scholar]
- 46.Xu Q, Alldus G, Macdonald R, Wilkinson DG, Holder N. Function of the Eph-related kinase rtk1 in patterning of the zebrafish forebrain. Nature. 1996;381:319–22. doi: 10.1038/381319a0. [DOI] [PubMed] [Google Scholar]
- 47.Ziman MR, Rodger J, Chen P, Papadimitriou JM, Dunlop SA, Beazley LD. Pax genes in development and maturation of the vertebrate visual system: implications for optic nerve regeneration. Histol Histopathol. 2001;16:239–49. doi: 10.14670/HH-16.239. [DOI] [PubMed] [Google Scholar]