Abstract
Zebrafish are increasingly used for developmental neurotoxicity testing because early embryonic events are easy to visualize, exposures are done without affecting the mother and the rapid development of zebrafish allows for high throughput testing. We used zebrafish to examine how exposures to three different organophosphorus pesticides (chlorpyrifos, diazinon and parathion) over the first five days of embryonic and larval development of zebrafish affected their survival, acetylcholinesterase (AChE) activity and behavior. We show that at non-lethal, equimolar concentrations, chlorpyrifos (CPF) is more effective at equimolar concentrations than diazinon (DZN) and parathion (PA) in producing AChE inhibition. As concentrations of DZN and PA are raised, lethality occurs before they can produce the degree of AChE inhibition observed with CPF at 300nM. Because of its availability outside the mother at the time of fertilization, zebrafish provides a complementary model for studying the neurotoxicity of very early developmental exposures.
Keywords: chlorpyrifos, zebrafish, AChE, behavior, neurodevelopment, organophosphates
1. Introduction
Zebrafish is a vertebrate model increasingly used to examine toxicant effects and underlying mechanisms that impact environmental and human populations (Law 2003; Spitsbergen and Kent 2003; Teraoka et al. 2003). Its usefulness in toxicological studies has been reviewed by several groups (Augustine-Rauch et al. 2010; Hill et al. 2005; Linney et al. 2004; Peterson et al. 2008; Scholz et al. 2008; Teraoka et al. 2003). Zebrafish is an oviparous species, which allows one to expose from shortly after fertilization and visually follow early effects upon development that are otherwise restricted with mammalian models. Such aqueous exposure regimens can closely mimic environmental exposures. During early development zebrafish acquire simple behaviors that can easily be tested. The embryo’s transparency, small size, and ease of use make zebrafish an excellent candidate for high-throughput approaches in exposure studies and behavioral analysis. A major, complementary strength of this vertebrate model is the access it provides, both visually and through direct exposures, for studying the very earliest effects on neurodevelopment. Our objective in this study is to use zebrafish to compare how early exposures to different organophosphorus pesticides (OPs) could affect its development and subsequent behavior.
OPs are used as pesticides to increase agricultural yields, but its use also presents a threat to human and environmental health. They are manufactured in large amounts and applied widely to food crops and in household environments to control insect pests. As much as 500 million kg of pesticides are applied annually in the US (Timchalk et al. 2002) and China, India and other developing countries have substantially increased production of pesticides. Two of the most common organophosphorous pesticides are chlorpyrifos (CPF) and diazinon (DZN) (Cong et al. 2009; Zaim and Jambulingam 2009). Parathion (PA) is banned worldwide. In 2001, the United States Environmental Protection Agency (EPA) banned CPF application for household use after studies indicated that children were put at risk by unintended exposures (Interim Reregistration Eligibility Decision for Chlorpyrifos, US EPA, 2001). Studies conducted in New York City determined that children had neurological and developmental impairments after indoor application of OPs (Landrigan et al. 1999; Rauh et al. 2006; Whyatt et al. 2004). DZN use was also restricted by the EPA due to developmental risks. These regulatory actions have resulted in both CPF and DZN levels falling in urban areas as measured by detection of metabolites of both chemicals in air and plasma blood samples (Whyatt et al. 2005). At this time, the EPA has not extended the OP ban to agricultural and industrial use. Despite the limitations on OP use in the United States and Europe, many other countries still employ CPF and other OPs for pest control needs and there is still much to be learned about how to measure the risks of OP use to human and environmental health.
CPF, DZN and PA have a thiophosphate backbone that is metabolized by cytochrome p450s into their respective OP-oxon forms that inhibit acetylcholinesterase (AChE). One model suggests that the result of AChE inhibition is an accumulation of the neurotransmitter acetylcholine (ACh) in the synapse which causes hyperstimulation of ACh receptors (AChR) (Chiappa et al. 1995; Fukuto 1990). This is consistent with the phenotype of a zebrafish ache mutant (Behra et al. 2002) in which axial musculature was disrupted due to hyperstimulation of cholinergic receptors. We observed a similar phenotype after embryonic exposure to 500 ng/mL (1.43µM) CPF exposure (Linney et al. 2004). Since AChE is highly conserved across species, OP exposure becomes a problem for humans despite its effectiveness on pests (Bertrand et al. 2001).
Our laboratory and collaborators have studied OP toxicity to zebrafish and have found effects on neurochemistry and behavior (Eddins et al. 2010; Levin et al. 2003; Levin et al. 2004; Linney et al. 2004). 100 ng/mL (285nM) CPF exposure from 0-5 days post fertilization (dpf) inhibits AChE by the end of the exposure by at least 80% resulting in hypoactive fish when tested at 6 dpf (Levin et al. 2004; Linney et al. 2004). The fish in that study had spatial discrimination and startle response impairments when tested as adults (Eddins et al. 2010; Levin et al. 2003). There has not been a comprehensive study comparing three major OPs and their effects on early zebrafish development measuring lethality, AChE activity and simple behavior. A recent study examining DZN exposure on early zebrafish development evaluated mortality and found that a 3-day exposure at 9µM DZN results in approximately 35% mortality with some fish experiencing developmental malformations (Osterauer and Kohler 2008). A 5-day exposure of 6µM DZN resulted in significantly reduced locomotor activity (Scheil et al. 2009). However, neither study measured AChE inhibition in larvae. PA has also been studied in zebrafish, but with a 28- or 250-day sublethal exposure, and do not address the question of how an exposure early in development might result in a later behavioral alteration (Roex et al. 2002; Roex et al. 2003). Two recent studies have examined the effects of CPF-oxon exposure on embryonic zebrafish (Jacobson et al. 2010; Yang et al. 2011).
In the current study, we investigate how CPF, DZN and PA affect zebrafish by 1) determining what dose of each of the three chosen OPs would result in substantial AChE inhibition without significantly changing viability and 2) comparing whether developmental OP exposure affects zebrafish motility at 6 dpf. Earlier studies from our laboratory used 100 ng/mL (285 nM) CPF exposure as a standard. In order to make comparisons between different OPs, we are using 300nM as our standard dose.
2. Materials and Methods
2.1 Reagents
Chlorpyrifos (CPF, P-094N), diazinon (DZN, P-033N) and parathion (PA, P-070N) were purchased from Accustandard Inc. (New Haven, CT, USA). All chemicals were stored according to manufacturer’s instructions. Test concentrations were prepared fresh using 100% DMSO at the beginning of a 5 day exposure series.
2.2 Animals
All experiments were carried out with protocols approved by the Duke University Institutional Animal Care and Use Committee. Adult wild-type zebrafish (AB* strain) were bred in our facility maintained in deionized water containing final concentrations of 0.013% SeaChem (SeaChem Laboratories Inc., Madison, GA, USA) and 0.05% Instant Ocean (Instant Ocean, Cincinnati, OH, USA). Fish were kept on a 14/10 h light/dark cycle at 28.5°C, with continuous fluid recirculation. In each experiment, embryos were collected by placing glass dishes covered by a plastic mesh into tanks the evening prior to embryo collection and removing the dishes from tanks no later than 4 h after the start of embryo fertilization. After embryos were collected, they were rinsed thoroughly with, and maintained in 30% Danieau’s Solution (58 mM NaCl, 0.7 mM KCl, 0.4 mM MgSO4, 0.6 mM Ca(NO3)2, 5 mM HEPES, pH 7.2). Embryos were checked under a light microscope for proper cell division and general health before use in an experiment. Embryos and larval fish were kept under the same temperature and lighting conditions as adults.
2.3 Exposures
Exposures to determine survival were carried out by exposing sets of 10 healthy 6 hpf (shield stage) embryos in 150-mL glass beakers with 10-mL of exposure solution consisting of 30% Danieau’s solution with the desired OP that achieved a final concentration of DMSO of 0.1%. For AChE inhibition and larval motility studies, we exposed sets of 30 healthy 6 hpf (shield stage) embryos in 150-mL glass beakers to 10-mL of previously described exposure solution. A lid is kept on each beaker to minimize volatilization of the OPs from the beaker. The exposure solution was changed daily to provide the embryos with a constant concentration of chemical. At 5 dpf, larvae were rinsed twice with 10-mL of 30% Danieau’s solution and released from the exposure regimen.
2.4 AChE Activity Assay (Ellman assay)
AChE activity was measured using the modified Ellman assay (Ellman et al. 1961). Each sample consisted of ten larvae homogenized in 100 mM Tris-Cl, pH 7.4 and 1% Triton X-100. To an aliquot of the homogenate, we added 5,5'-dithiobis-2-nitrobenzoic acid (DTNB) and acetylthiocholine iodide (ATChI) to final concentrations of 0.33 mM and 0.8 µM respectively. Spectrophotometric measurements were made at 412-nm after 15 minutes. The BioRad DC Protein assay (Hercules, CA, USA), a modified Lowry assay, was used to determine the total protein concentration in the homogenate for determination of specific activity.
2.5 Larval Behavior
6 dpf larvae in 100mm petri dishes were allowed to acclimate to the light on the presentation platform in a dark room for at least 15 minutes. 6 dpf larvae were placed in individual wells of a 9 well clear spot plate with 1 mL of 30% Danieau. Fish were allowed to acclimate to the well for 2 minutes and their motility was tracked for 2 minutes by Noldus Ethovision 3.0 Wageningen, The Netherlands. Tracks were visualized and analyzed for total distance moved over 2 minutes. Incomplete tracks were filled in by interpolation between the two nearest points.
2.6 Data Analysis
Survival was assessed using χ2 analysis for observed vs. expected frequencies for all comparisons where the expected frequency was at 5 or greater; where the expected frequency was less than 5, we used the Fisher’s Exact Test. The expected frequency was determined from the DMSO controls. Significance for these parameters was assessed one-tailed, since exposures were expected only to contribute to lethality.
AChE activity for Figure 2 and 3 was assessed using ANOVA with differences among groups assessed using Fisher’s Least Significant Differences with control group defined as DMSO exposed.
2.
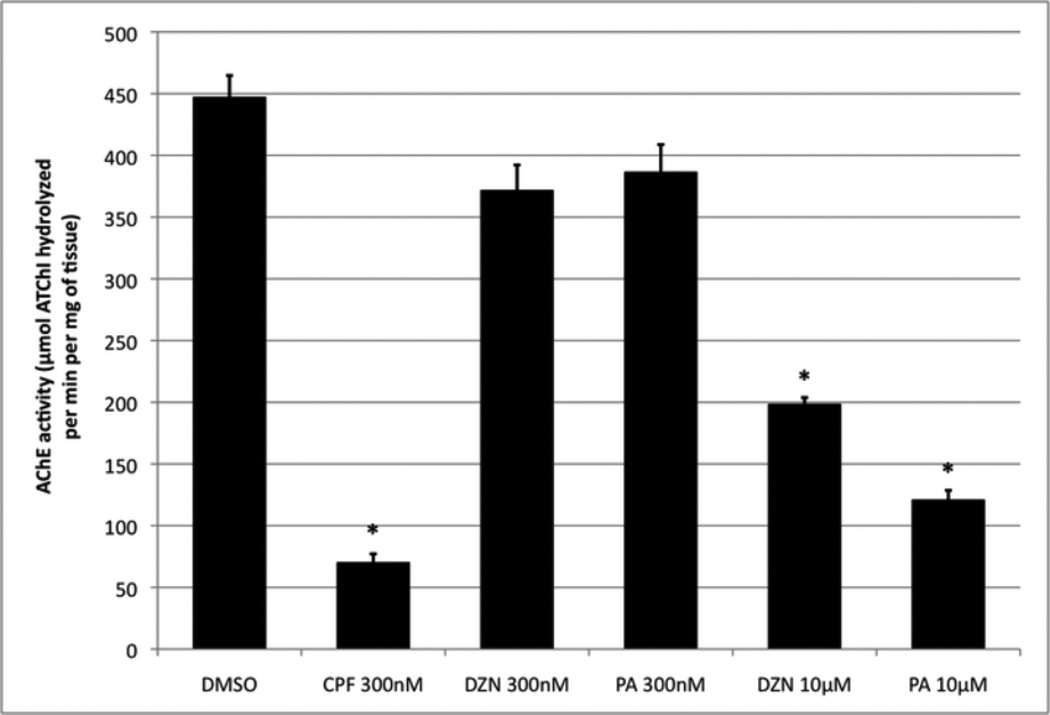
Effects of CPF, DZN and PA on AChE activity measured at 5 dpf after an exposure from 0-5 dpf renewed fresh daily. n > 4 per treatment with each sample having 10 fish. ANOVA: Treatments, p < 0.0001; asterisks indicate treatments significantly different from DMSO.
3.
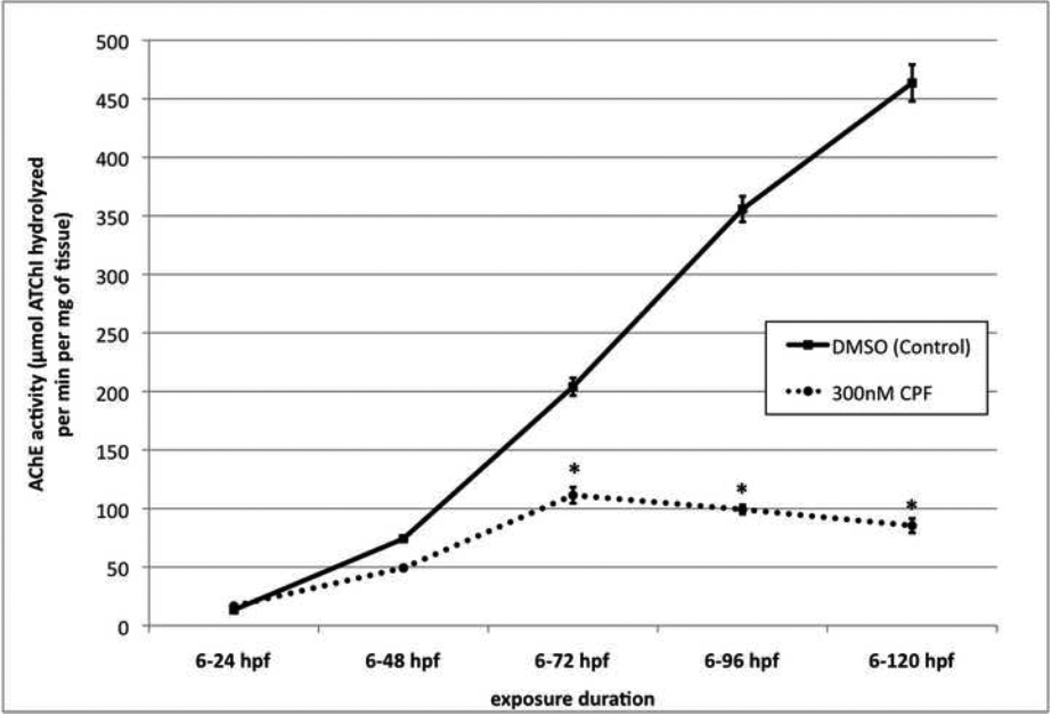
AChE activity of control DMSO zebrafish larvae measured daily throughout 0-5 dpf exposure and effects of 300 nM CPF on AChE activity measured daily throughout 0-5 dpf exposure with exposure renewed fresh daily. n > 4 per treatment with each sample having 10 fish. ANOVA: Treatments, p < 0.0001; asterisks indicate treatment was significantly different from DMSO control.
Larval motility for Figure 4 was assessed using ANOVA with differences among groups assessed using Fisher’s Least Significant Differences with control group defined as DMSO exposed. For all statistical tests, significance was assumed at p<0.05.
4.
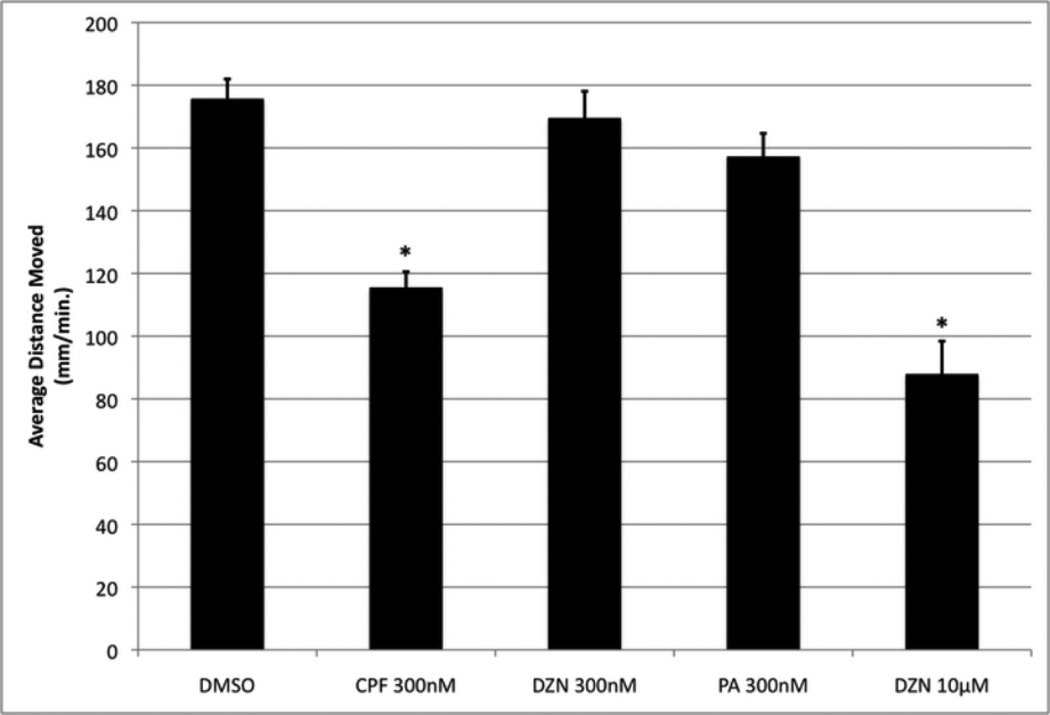
Effect of CPF, DZN and PA on larval motility (distance swum per minute) measured at 6 days using Noldus Ethovision video-tracking after exposure from 0-5 dpf with exposure renewed fresh daily. n > 34 per treatment. ANOVA: Treatments, p < 0.0001; asterisks indicate treatments significantly different from DMSO.
3. Results
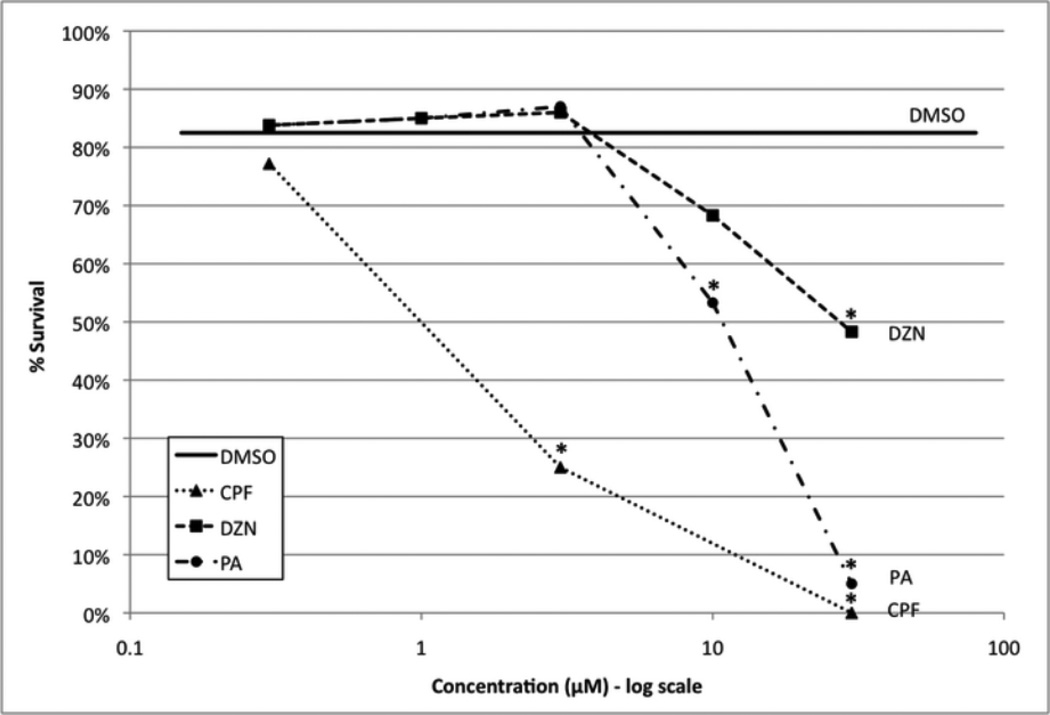
To compare the effectiveness between each OP (CPF, DZN, PA), we determined how different concentrations of each OPs affected zebrafish survival over the first 5 days post fertilization. For larval zebrafish exposed to 300nM CPF, survival was not significantly affected when compared to control DMSO exposed zebrafish (Fig. 1). However, a 3µM and 30µM exposure to CPF over 5 days resulted in 75% and 100% mortality, respectively. DZN and PA had milder effects compared to CPF at equimolar concentrations. There was no significant effect on mortality up to 10µM DZN and 3µM PA exposure for 5 days when compared to DMSO control. At 30µM DZN exposure over 5 days, the highest concentration examined, there was only 50% mortality, which is significant compared to DMSO but not as lethal as equimolar CPF and PA. 10µM PA exposure achieved a similar mortality as 30µM DZN whereas 30µM PA nearly reduced survival to zero. Therefore at equimolar concentrations, CPF is much more lethal to larval zebrafish than PA and DZN. Given the lack of significant mortality and no detectable effect upon morphogenesis at 300nM for all three OPs, we used this concentration for comparison of effects on AChE activity and larval motility.
1.
Effects of CPF, DZN and PA on embryonic survival after exposure from 0-5 dpf with exposure renewed fresh daily. The number of fish used in each group: 560 for 0.1% DMSO; CPF, 320 at 0.3 µM, 100 at 3 µM, 40 at 30 µM; DZN and PA, 160 at 0.3 µM, 40 at 1µM, 100 at 3 µM, 120 at 10 µM, 60 at 30 µM. The solid line shows the survival for control embryos (DMSO); treatments that are significantly different from control (χ2 analysis) are marked with asterisks.
Previous studies have shown that AChE inhibition plays an important role to larval zebrafish survival (Behra et al. 2002); therefore we compared the effect of all three OPs on AChE activity and whether it correlated to mortality (Fig. 2). For DMSO exposed fish at 5 dpf, there is robust AChE activity at 447±18 µM ATChI hydrolyzed per min. per mg of tissue. 300nM CPF exposure over 5 days inhibited AChE activity by over 80% when compared to DMSO controls. Both DZN and PA exposures did not have as potent of an effect on AChE activity as CPF. 300nM DZN and 300nM PA exposures did not significantly inhibit AChE activity. 10µM DZN exposure inhibited AChE activity over 50% and 10µM PA exposure inhibited AChE activity by almost 70% confirming that PA is more potent than DZN based on mortality. In conclusion, we found that 300nM CPF is much more effective than equimolar DZN and PA at inhibiting AChE. Up to 10µM of DZN and PA was not able to inhibit AChE as much as 300nM CPF without causing a significant increase in mortality.
We examined the effect of CPF exposure upon AChE activity daily. Over a 5 dpf exposure, 300nM CPF exposure began to inhibit AChE at 2 dpf and steadily increased day after day until it culminated with a 80% inhibition at 5 dpf when compared to DMSO control (Fig. 3).
As shown in Figure 5, we examined the motility of 6 dpf larvae that had been exposed for 5 days to 300nM of the 3 different OPs, which had not caused significant mortality. Using a Noldus video tracking/software interrogation system, we tracked the movement of 6 dpf zebrafish that were one day removed from their 0-5 dpf exposure. DMSO exposed controls swam 175 mm/min (Fig. 4). 300nM CPF exposures reduced locomotor activity by 35%. 300nM DZN and PA did not significantly affect locomotor activity when compared with DMSO. However, 10µM DZN exposure reduced locomotor activity by 50%. 10µM PA exposure resulted in larval fish unable to move because they did not hatch out of the chorion and had developed a number of developmental malformations.
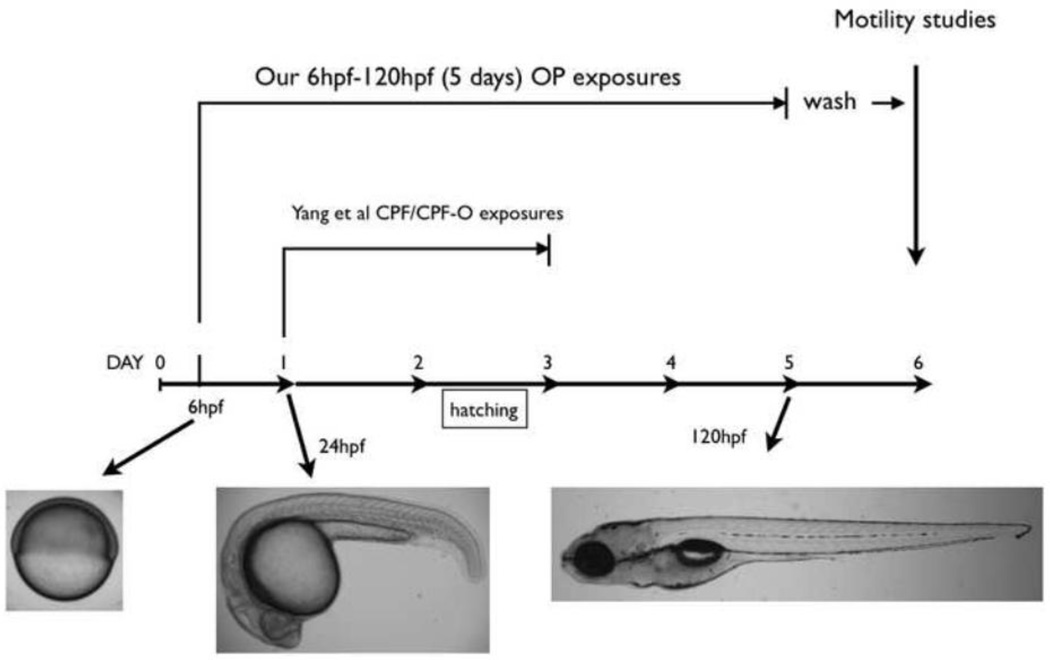
5.
Our exposure regimen for 0-5 dpf exposure using CPF, DZN and PA along with normal development of zebrafish. Compared to Yang et al. (2011) whose exposures start at 24 hpf, our exposures begin at 6 hpf. At 6 hpf, gastrulation has just begun and neurons are not formed. At 24 hpf, somitogenesis is ending and a nervous system is rapidly developing. After a 0-5 day exposure, larvae are washed and tested for motility at 6 dpf.
4. Discussion
In this study we compared the effects of DZN and PA with CPF during the first 5 days of zebrafish development. In Figure 1 using the criterion of lethality, the developmental sensitivity to OPs for zebrafish is CPF > PA > DZN. In Figure 2, the reduction in AChE activity at the nonlethal concentration of 300nM was significant for CPF exposed fish, but not for DZN and PA exposed fish. At 33x higher concentration (10µM), DZN and PA exposures do not result in as much inhibition as 300nM CPF exposure. In Figure 3, we show the large increase in AChE activity from 2 dpf through 5 dpf for control DMSO exposed fish, and also describe increasing AChE inhibition with continued exposure from 6–120 hpf with 300nM CPF. Small changes in AChE levels are difficult to determine before 2 dpf due to low absolute values of AChE activity at 1 dpf. AChE activity is inhibited by 300nM CPF beginning at 3 dpf. In Figure 4, using Noldus video tracking and Ethovision software interpretation to analyze distance moved, we show that after 5 days of exposure to each of the three OPs at 300nM, only CPF exposed fish show a significant difference in distance moved in time. At the higher concentration of 10uM DZN, exposed fish showed a significant difference in motility.
Table 1 summarizes survival, AChE activity and larval motility after a 5 day exposure to different concentrations of CPF, DZN and PA. At 300nM none of the three OPs studied affected survival and there was no detectable evidence of morphological changes in larvae. However, 300nM CPF exposure reduced AChE activity by 81% and motility by 35%. In comparison, 300nM DZN or PA did not have a significant effect on AChE activity or motility, and even 10µM DZN or PA did not result in as much AChE inhibition as 300nM CPF. At a concentration that does not affect survival (300nM), only CPF has a significant effect on AChE activity and larval motility.
Table 1.
Comparison of effects from CPF, DZN and PA on survival, AChE activity and larval mobility
| 300nM CPF | 300nM DZN | 300nM PA | 10µM DZN | 10µM PA | |
|---|---|---|---|---|---|
| Survival (Fig. 1) |
NS | NS | NS | NS | 55% |
| AChE activity (Fig. 2) |
−81% | NS | NS | −55% | −73% |
| Larval behavior (Fig. 4) |
−35% | NS | NS | −50% | Does not move, not out of chorion |
Survival measured at 5 dpf after 0-5d exposure
AChE activity is specific activity measured at 5 dpf after 0-5d exposure
Larval behavior is change in motility measured at 6 dpf after 0-5d exposure
NS = not significant difference from DMSO control exposed fish
10µM PA exposure resulted in 45% lethality by 5 dpf, and of the fish that survived, there was 73% AChE inhibition and most did not hatch out of the chorion by 5 dpf. In rodents, PA is the most potent of the OPs we tested in inhibiting AChE (Chambers and Carr 1993). However, in exposing young channel catfish to CPF and PA, one set of investigators found that as the fish aged, the previously CPF exposed fish had an increase in AChE inhibition in the brain compared to those exposed with PA (Carr et al. 1995). Increased lipophilicity of CPF versus PA was suggested to play a role in the differential toxicities between rodents and fish. Also, the authors later published reviews suggesting that the different affinities of AChE for CPF and PA play a bigger role in their toxicities for fish (Carr and Chambers 1996; Chambers and Carr 1995).
10µM DZN exposures did not result in a significant effect on survival, and exposed fish had over 50% AChE inhibition. Despite not having as large an AChE inhibition as fish exposed with 300nM CPF, 10µM DZN exposed fish moved less than 300nM CPF exposed fish, indicating that AChE activity is not the only factor in determining behavioral outcomes with this assay.
Each OP has its own phenotypic profile where some inhibit AChE more effectively than others, but the final larval behavioral outcome is most likely due to a combination of various factors. From our studies, at the non-lethal concentration of 300nM the efficiency of inhibiting AChE for the 3 compounds was CPF > PA, DZN. While we cannot tell from this study why there is a difference in the molar effectiveness among the OPs, there could be several possible explanations: i) a different efficiency in forming the OP-oxon form for each OP (Buratti et al. 2003; Ma and Chambers 1994, 1995; Sams et al. 2000); ii) a difference in how effective each OP is at entering the different developmental compartments of the developing embryo; or iii) a difference in the effectiveness of each OP–oxon form at inhibiting AChE (Katz et al. 1997). For further discussion we refer the reader to the discussion section of Yang et al. (2011).
Our previous collaborative work with the Levin laboratory has shown that non-lethal 5 day exposures of zebrafish with CPF results in adults with learning and behavioral differences (Levin et al. 2003). Previous research has also shown significant locomotor hypoactivity at 6 dpf and 10 dpf after 0-5 dpf exposure to 285nM CPF (Levin et al. 2004). Correlative with these exposures was an inhibition of AChE (Linney et al. 2004). In those studies we mimicked environmental exposures by exposing shortly after fertilization through 5 days of development. Also in collaboration with the Levin laboratory, we injected embryos with an ache morpholino, which resulted in a knock down of AChE activity and behavioral changes in adults (Aschner et al. 2010).
We were interested in CPF exposures and its effect on the developing nervous system, because it is designed specifically to inhibit AChE. One clue to what we might expect comes from genetic studies with zebrafish. The first zebrafish AChE mutant, ache, abolished AChE activity and its phenotype involved disruptions in both neural and muscle fiber development (Behra et al. 2002). By crossing the ache mutant with a mutant for the α-subunit of the zebrafish nicotinic acetylcholine receptor (nic1) the muscle phenotype was eliminated. This suggested that losing AChE activity caused hyperstimulation of the muscle fibers, which lead to fiber disruption. In our initial CPF dose response work we observed deterioration of muscle cells at 4 days from a 500 ng/ml CPF exposure (see Figure 4 in Linney et al. 2004). Therefore, we have continued to look at the possibility that one of the effects of OP exposure on the developing embryo involves hyperstimulation at cholinergic synapses.
Two recent CPF related studies (Yang et al. 2011 and Jacobson et al. 2010) have provided useful information in understanding its effects on developing zebrafish. In Figure 5, we contrast our exposure times with Yang et al. (2011). While Yang et al. showed that from 6 hpf to 24 hpf CPF is incorporated into the embryo, their exposure study started at 24 hpf and concluded at either 48 hpf or 72 hpf. They did not detect AChE inhibition using 300nM CPF from 24 hpf to 48 hpf or from 24 hpf to 72 hpf exposures. In our study of exposures starting at 6 hpf we did measure significant AChE inhibition. At 6 hpf the embryo has not completed epiboly, so immersion exposure to compounds does not require partitioning or complex transfer through multiple layers of tissue. Also, at 6 hpf gastrulation has just begun; neurons have not been born; organs have not yet formed. In contrast, at 24 hpf somitogenesis has occurred; the embryo has a spinal cord, a five-lobed brain and musculature; and it exhibits tail movement. One possible explanation for the difference seen in AChE inhibition between an exposure that starts at 6 hpf and an exposure that starts at 24 hpf is the clear anatomical differences between 6 hpf and 24 hpf zebrafish embryos and the length of time CPF is allowed to be taken up. Another possible explanation for the difference between exposures with different start times might involve a transient level of cytochrome p450 activity inherited maternally. A recent study of the zebrafish cytochrome p450 family and its expression in zebrafish embryos (Goldstone et al, 2010) suggests the possibility that cytochrome p450 mRNAs may be maternally inherited in the embryos. In the absence of identifying which cytochrome p450s are involved in producing the OP–oxon form, this remains to be determined.
In Jacobson et al. (2010) the focus was on a one-time 300nM exposure of embryos to CPF-oxon and observing up to 3 days of age (Jacobson et al. 2010). They described effects on Rohon-Beard neurogenesis and described severe phenotypes in up to 14.3% of the fish by days 1 through 3. They showed that the half-life of CPF-oxon in egg water alone was 1 day. In Yang et al. (2011) exposing with 100nM CPF-oxon from day 1 to day 3 resulted in effects on axonal growth and motor behavior in 3 day old zebrafish. The CPF-oxon studies provide useful information regarding direct effects of the OP-oxon to a developing vertebrate embryo at the time of neural connectivity. However, exposure to the OP is more environmentally relevant, because the parent compound must first be metabolized by cytochrome p450s into the OP-oxon form. An OP-oxon exposure will not accurately reflect what may result from the parent compound exposure if the enzyme activity is not present at the time of exposure. In this context, the comparison of these three OPs (CPF, DZN and PA) provides one with a description of their effects on lethality and the sublethal effects on larval motility.
While it would be convenient and fortunate to identify a single mechanism for CPF effects on neural development, the mature nervous system and subsequent behavior, it is clear from mammalian and zebrafish studies that mechanisms behind differential lethality and behavioral/learning differences from CPF exposure are complex and that they may be different for CPF, CPF-oxon, and for the developmental stage of exposure.
Highlights.
-
>
Developing zebrafish were exposed to three different organophosphorus pesticides.
-
>
Chlorpyrifos inhibited acetylcholinesterase activity more than diazinon
-
>
or parathion 300nm exposed larvae, chlorpyrifos motility < diazinon or
-
>
parathion larvae motility
Acknowledgements
This research was supported by PHS grant ES 016554 (E. Linney) and ES 10356 (E. Levin).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aschner M, Levin ED, Sunol C, Olopade JO, Helmcke KJ, Avila DS, et al. Gene-environment interactions: neurodegeneration in non-mammals and mammals. Neurotoxicology. 2010;31:582–588. doi: 10.1016/j.neuro.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine-Rauch K, Zhang CX, Panzica-Kelly JM. In vitro developmental toxicology assays: A review of the state of the science of rodent and zebrafish whole embryo culture and embryonic stem cell assays. Birth Defects Res C Embryo Today. 2010;90:87–98. doi: 10.1002/bdrc.20175. [DOI] [PubMed] [Google Scholar]
- Behra M, Cousin X, Bertrand C, Vonesch JL, Biellmann D, Chatonnet A, et al. Acetylcholinesterase is required for neuronal and muscular development in the zebrafish embryo. Nat Neurosci. 2002;5:111–118. doi: 10.1038/nn788. [DOI] [PubMed] [Google Scholar]
- Bertrand C, Chatonnet A, Takke C, Yan YL, Postlethwait J, Toutant JP, et al. Zebrafish acetylcholinesterase is encoded by a single gene localized on linkage group 7. Gene structure and polymorphism; molecular forms and expression pattern during development. J Biol Chem. 2001;276:464–474. doi: 10.1074/jbc.M006308200. [DOI] [PubMed] [Google Scholar]
- Buratti FM, Volpe MT, Meneguz A, Vittozzi L, Testai E. CYP-specific bioactivation of four organophosphorothioate pesticides by human liver microsomes. Toxicol Appl Pharmacol. 2003;186:143–154. doi: 10.1016/s0041-008x(02)00027-3. [DOI] [PubMed] [Google Scholar]
- Carr RL, Chambers JE. Kinetic analysis of the in vitro inhibition, aging, and reactivation of brain acetylcholinesterase from rat and channel catfish by paraoxon and chlorpyrifos-oxon. Toxicol Appl Pharmacol. 1996;139:365–373. doi: 10.1006/taap.1996.0177. [DOI] [PubMed] [Google Scholar]
- Carr RL, Straus DL, Chambers JE. Inhibition and aging of channel catfish brain acetylcholinesterase following exposure to two phosphorothionate insecticides and their active metabolites. J Toxicol Environ Health. 1995;45:325–336. doi: 10.1080/15287399509531999. [DOI] [PubMed] [Google Scholar]
- Chambers JE, Carr RL. Inhibition patterns of brain acetylcholinesterase and hepatic and plasma aliesterases following exposures to three phosphorothionate insecticides and their oxons in rats. Fundam Appl Toxicol. 1993;21:111–119. doi: 10.1006/faat.1993.1079. [DOI] [PubMed] [Google Scholar]
- Chambers JE, Carr RL. Biochemical mechanisms contributing to species differences in insecticidal toxicity. Toxicology. 1995;105:291–304. doi: 10.1016/0300-483x(95)03225-5. [DOI] [PubMed] [Google Scholar]
- Chiappa S, Padilla S, Koenigsberger C, Moser V, Brimijoin S. Slow accumulation of acetylcholinesterase in rat brain during enzyme inhibition by repeated dosing with chlorpyrifos. Biochem Pharmacol. 1995;49:955–963. doi: 10.1016/0006-2952(95)00004-j. [DOI] [PubMed] [Google Scholar]
- Cong NV, Phuong NT, Bayley M. Effects of repeated exposure of diazinon on cholinesterase activity and growth in snakehead fish (Channa striata) Ecotoxicol Environ Saf. 2009;72:699–703. doi: 10.1016/j.ecoenv.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Eddins D, Cerutti D, Williams P, Linney E, Levin ED. Zebrafish provide a sensitive model of persisting neurobehavioral effects of developmental chlorpyrifos exposure: comparison with nicotine and pilocarpine effects and relationship to dopamine deficits. Neurotoxicol Teratol. 2010;32:99–108. doi: 10.1016/j.ntt.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Fukuto TR. Mechanism of action of organophosphorus and carbamate insecticides. Environ Health Perspect. 1990;87:245–254. doi: 10.1289/ehp.9087245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AJ, Teraoka H, Heideman W, Peterson RE. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol Sci. 2005;86:6–19. doi: 10.1093/toxsci/kfi110. [DOI] [PubMed] [Google Scholar]
- Jacobson SM, Birkholz DA, McNamara ML, Bharate SB, George KM. Subacute developmental exposure of zebrafish to the organophosphate pesticide metabolite, chlorpyrifos-oxon, results in defects in Rohon-Beard sensory neuron development. Aquat Toxicol. 2010;100:101–111. doi: 10.1016/j.aquatox.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz EJ, Cortes VI, Eldefrawi ME, Eldefrawi AT. Chlorpyrifos, parathion, and their oxons bind to and desensitize a nicotinic acetylcholine receptor: relevance to their toxicities. Toxicol Appl Pharmacol. 1997;146:227–236. doi: 10.1006/taap.1997.8201. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Claudio L, Markowitz SB, Berkowitz GS, Brenner BL, Romero H, et al. Pesticides and inner-city children: exposures, risks, and prevention. Environ Health Perspect. 1999;107 Suppl 3:431–437. doi: 10.1289/ehp.99107s3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law JM. Issues related to the use of fish models in toxicologic pathology: session introduction. Toxicol Pathol. 2003;31 Suppl:49–52. doi: 10.1080/01926230390174922. [DOI] [PubMed] [Google Scholar]
- Levin ED, Chrysanthis E, Yacisin K, Linney E. Chlorpyrifos exposure of developing zebrafish: effects on survival and long-term effects on response latency and spatial discrimination. Neurotoxicol Teratol. 2003;25:51–57. doi: 10.1016/s0892-0362(02)00322-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, Swain HA, Donerly S, Linney E. Developmental chlorpyrifos effects on hatchling zebrafish swimming behavior. Neurotoxicol Teratol. 2004;26:719–723. doi: 10.1016/j.ntt.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Linney E, Upchurch L, Donerly S. Zebrafish as a neurotoxicological model. Neurotoxicol Teratol. 2004;26:709–718. doi: 10.1016/j.ntt.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Ma T, Chambers JE. Kinetic parameters of desulfuration and dearylation of parathion and chlorpyrifos by rat liver microsomes. Food Chem Toxicol. 1994;32:763–767. doi: 10.1016/s0278-6915(09)80009-4. [DOI] [PubMed] [Google Scholar]
- Ma T, Chambers JE. A kinetic analysis of hepatic microsomal activation of parathion and chlorpyrifos in control and phenobarbital-treated rats. J Biochem Toxicol. 1995;10:63–68. doi: 10.1002/jbt.2570100202. [DOI] [PubMed] [Google Scholar]
- Osterauer R, Kohler HR. Temperature-dependent effects of the pesticides thiacloprid and diazinon on the embryonic development of zebrafish (Danio rerio) Aquat Toxicol. 2008;86:485–494. doi: 10.1016/j.aquatox.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Peterson RT, Nass R, Boyd WA, Freedman JH, Dong K, Narahashi T. Use of non-mammalian alternative models for neurotoxicological study. Neurotoxicology. 2008;29:546–555. doi: 10.1016/j.neuro.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Garfinkel R, Perera FP, Andrews HF, Hoepner L, Barr DB, et al. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118:e1845–e1859. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roex EW, de Vries E, van Gestel CA. Sensitivity of the zebrafish (Danio rerio) early life stage test for compounds with different modes of action. Environ Pollut. 2002;120:355–362. doi: 10.1016/s0269-7491(02)00118-5. [DOI] [PubMed] [Google Scholar]
- Roex EW, Keijzers R, van Gestel CA. Acetylcholinesterase inhibition and increased food consumption rate in the zebrafish, Danio rerio, after chronic exposure to parathion. Aquat Toxicol. 2003;64:451–460. doi: 10.1016/s0166-445x(03)00100-0. [DOI] [PubMed] [Google Scholar]
- Sams C, Mason HJ, Rawbone R. Evidence for the activation of organophosphate pesticides by cytochromes P450 3A4 and 2D6 in human liver microsomes. Toxicol Lett. 2000;116:217–221. doi: 10.1016/s0378-4274(00)00221-6. [DOI] [PubMed] [Google Scholar]
- Scheil V, Kienle C, Osterauer R, Gerhardt A, Kohler HR. Effects of 3,4-dichloroaniline and diazinon on different biological organisation levels of zebrafish (Danio rerio) embryos and larvae. Ecotoxicology. 2009;18:355–363. doi: 10.1007/s10646-008-0291-0. [DOI] [PubMed] [Google Scholar]
- Scholz S, Fischer S, Gundel U, Kuster E, Luckenbach T, Voelker D. The zebrafish embryo model in environmental risk assessment--applications beyond acute toxicity testing. Environ Sci Pollut Res Int. 2008;15:394–404. doi: 10.1007/s11356-008-0018-z. [DOI] [PubMed] [Google Scholar]
- Spitsbergen JM, Kent ML. The state of the art of the zebrafish model for toxicology and toxicologic pathology research--advantages and current limitations. Toxicol Pathol. 2003;31 Suppl:62–87. doi: 10.1080/01926230390174959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teraoka H, Dong W, Hiraga T. Zebrafish as a novel experimental model for developmental toxicology. Congenit Anom (Kyoto) 2003;43:123–132. doi: 10.1111/j.1741-4520.2003.tb01036.x. [DOI] [PubMed] [Google Scholar]
- Timchalk C, Nolan RJ, Mendrala AL, Dittenber DA, Brzak KA, Mattsson JL. A Physiologically based pharmacokinetic and pharmacodynamic (PBPK/PD) model for the organophosphate insecticide chlorpyrifos in rats and humans. Toxicol Sci. 2002;66:34–53. doi: 10.1093/toxsci/66.1.34. [DOI] [PubMed] [Google Scholar]
- Whyatt RM, Camann D, Perera FP, Rauh VA, Tang D, Kinney PL, et al. Biomarkers in assessing residential insecticide exposures during pregnancy and effects on fetal growth. Toxicol Appl Pharmacol. 2005;206:246–254. doi: 10.1016/j.taap.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Whyatt RM, Rauh V, Barr DB, Camann DE, Andrews HF, Garfinkel R, et al. Prenatal insecticide exposures and birth weight and length among an urban minority cohort. Environ Health Perspect. 2004;112:1125–1132. doi: 10.1289/ehp.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Lauridsen H, Buels K, Chi LH, La Du J, Bruun DA, et al. Chlorpyrifos-oxon disrupts zebrafish axonal growth and motor behavior. Toxicol Sci. 2011;121:146–159. doi: 10.1093/toxsci/kfr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaim M, Jambulingam P. Global Insecticide Use for Vector-Borne Disease Control. World Health Organization; 2009. [Google Scholar]