Abstract
The molecular mechanism of autophagy induction following proteasome inhibition is not fully understood. We report that the proteasome inhibitor bortezomib potently induces autophagy in head and neck squamous cell carcinoma (HNSCC) cells, as demonstrated by autophagosome formation and induction of complete autophagic flux. Bortezomib treatment led to phosphorylation/activation of jun N-terminal kinase (JNK) enzymes and JNK-dependent phosphorylation of Bcl-2 on serine 70. Pharmacologic inhibition of JNK, but not p38 MAPK, dramatically inhibited bortezomib induction of autophagy regulatory proteins and autophagosome formation. These results demonstrate a key requirement for JNK signaling in the activation of autophagy by bortezomib.
Keywords: Bortezomib, Autophagy, HNSCC, JNK, Bcl-2
1. Introduction
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common form of cancer in the United States, and in some regions of the world HNSCC represents the most common human malignancy [1]. Refinements in surgical approaches and radiation and chemotherapy regimens have led to decreased morbidity in the treatment of HNSCC over the past few decades. However, success in improving survival outcomes has been very limited, with 5-year survival rates that have remained relatively unchanged at around 50% [2–5]. Thus, new therapeutic targets and strategies are needed for this disease.
An emerging field of thought suggests that the cellular process of autophagy may represent a novel therapeutic target in the treatment of cancer. Autophagy is a catabolic process regulated by a series of proteins called autophagy-regulated, or Atg proteins, wherein cellular proteins and organelles are recruited and degraded in vesicles called autolysosomes [6,7]. During the initiation of autophagy, isolated membranes begin to form in the cytoplasm via a process dependent on Atg6 (Beclin-1) [8]. The isolated membranes then elongate via an Atg7-dependent mechanism, and simultaneously recruit proteins/organelles, forming loaded vesicles called autophagosomes [7]. During this process, Atg8 (LC3) is cleaved and lipidated (now called LC3-II), then recruited to the autophagosome membrane [9]. Loaded autophagosomes fuse with lysosomes, forming autolysosomes, resulting in degradation of the captured proteins/organelles by lysosomal enzymes [7].
Recent studies have shown that the proteasome inhibitor bortezomib promotes apoptotic cell death in HNSCC [10–13]. In other cell types, bortezomib has also been shown to promote autophagy, although the mechanism of bortezomib-induced autophagy is not fully understood. Proteasome inhibition is known to lead to the accumulation/aggregation of unfolded proteins, and activation of endoplasmic reticulum (ER) stress and the unfolded protein response (UPR) [14,15]. Activation of the UPR involves activation of PKR-like endoplasmic reticulum kinase (PERK) and PERK-dependent phosphorylation of eukaryotic initiation factor 2α (EIF2α) [16,17]. Phosphorylation of EIF2α can promote autophagy induction via an Atg5-dependent process, and also via upregulation ATF4 transcription factor and subsequent upregulation of LC3 [18,19]. Bortezomib treatment is also known to activate JNK enzymes [20,21], although a link between JNK activation and bortezomib-induced autophagy has not been established. In nutrient-deprived or ceramide-treated cells, autophagy induction is associated with JNK-mediated phosphorylation of serine 70 on Bcl-2, which causes disruption of Bcl-2/Beclin-1 complexes, liberating Beclin-1 to promote autophagy [22,23].
In this study, we demonstrate that bortezomib potently induces autophagy in HNSCC cells. Bortezomib-induced HNSCC autophagy was associated with JNK activation and phosphorylation of Bcl-2. Pharmacologic inhibition of JNK enzymes markedly inhibited bortezomib-induced Bcl-2 phosphorylation and induction of autophagy, demonstrating a key role for JNK activity in autophagy resulting from proteasome inhibition.
2. Materials and methods
2.1. Reagents and cell lines
Three human HNSCC cell lines, UMSCC-22A, 1483, and UMSCC-1 were used in this study [24]. Cells were cultured in DMEM medium (Mediatech) containing 10% heat-inactivated fetal bovine serum supplemented with 1×penicillin/streptomycin. Lipofectamine-2000 was obtained from Invitrogen and G418 from Mediatech. SP600125, an inhibitor of JNK, and SB203580, an inhibitor of p38, were purchased from LC Laboratories. E64d, leupeptin and pepstatin A were from Sigma. Bortezomib was obtained from the University of Pittsburgh Cancer Institute Pharmacy. Antibody against Beclin-1 was purchased from BD Biosciences. Antibodies against total JNK, phospho-JNK (Thr183/Tyr185) and phospho-Bcl-2 (Ser70) were from Cell Signaling. Antibody against total Bcl-2 was from DAKO. Anti-β-actin was from Sigma. Horseradish peroxidase-conjugate secondary antibodies were from Promega.
2.2. Analysis of GFP-LC3 puncta
To analyze the effect of bortezomib on autophagy in HNSCC cell lines, UMSCC-22A, 1483 and UMSSC-1 cell lines were transfected using Lipofectamine-2000 with an expression construct (pCMV/GFP-LC3B) encoding GFP-LC3B (hereafter GFP-LC3; kindly provided by Dr. Xiao-Ming Yin, University of Pittsburgh [25]). Following selection in 1 mg/ml G418, single clones were isolated for further analyses. For detection of autophagasome formation, 5×104 cells/well were seeded into 24-well plates which contained sterilized circular cover slips. After 24 hours, cells were treated for 24 or 48 hours with bortezomib. The treated cells on cover slips were then washed with cold PBS and fixed in 2% paraformaldehyde for 10 minutes at room temperature. The fixed cells were rinsed twice with cold PBS, briefly dried, stained with Hoechst 33258 (Sigma) for 30 seconds at room temperature, dried for 10 minutes, then sealed with mounting medium. A confocal Olympus Flueview 1000 microscope was used to capture images, enabling detection of GFP-LC3 punctate dots. For each sample, five random fields, with a minimum of 40 cells/field, were counted to determine the average number of GFP-LC3 puncta per cell. Experiments were performed 3 times, and the mean number of puncta/cell from the 3 experiments was graphed.
2.3. Immunoblotting
Cells were washed once with cold PBS, harvested by cell scraping, centrifuged at 4°C and 1000 rpm, and resuspended in lysis buffer (50 mM Tris pH=8.0, 150 mM NaCl, 0.1% SDS 1% NP40) containing one tablet of Protease Inhibitor Cocktail (Roche Diagnostics) per 10 mls of buffer. Lysates were subjected to microcentrifugation and protein concentrations determined using Bio-Rad Protein Assay Reagent (Bio-Rad Laboratories, Inc.). Equal amounts of protein (40 μg/lane) were electrophoresed on SDS-PAGE gels, transferred to nitrocellulose, and probed with the indicated antibodies as previously described [13].
2.4. Statistical analysis
SigmaStat software was used to perform analysis of the data. One-way ANOVA and Student's-Newman-Keul's tests were applied for comparisons; P < 0.05 was considered significant.
3. Results
3.1. Bortezomib induces autophagy in HNSCC cell lines
To determine the impact of bortezomib on autophagy in HNSCC, three independent cell lines were studied, UMSCC-22A, 1483, and UMSCC-1 [24]. Each cell line was first stably transfected with an expression construct encoding GFP-LC3, to allow fluorescence visualization of LC3-II relocalization to punctate cytoplasmic dots, a measure of autophagosome formation [26]. Treatment of the transfected cells with 20 nM bortezomib for 24 hours led to a roughly 3-fold (UMSCC-22A), 5-fold (1483), or 35-fold (UMSCC-1) induction in the average number of fluorescent puncta per cell, relative to untreated cells or cells treated with vehicle (DMSO) alone (Fig. 1). The average number of puncta/cell was slightly reduced in all 3 cell lines after 48 hours of bortezomib treatment (compared to 24 hour treatment), yet remained substantially higher than in the control cells. These findings indicated that treatment of HNSCC cells with bortezomib led to formation of autophagosomes.
Fig. 1.
Bortezomib induces autophagosome formation in HNSCC cells. UMSCC-22A, 1483, and UMSCC-1 cells stably expressing GFP-LC3 were left untreated (A, E, I), or treated for 24 hours with 0.l% DMSO (B, F, J), or for 24 (C, G, K) or 48 (D, H, L) hours with 20 nM bortezomib. Following treatment, cells were fixed in 2% paraformaldehyde, and nuclei were stained with Hoechst 33258. Cells were then observed by confocal fluorescence microscopy. The number of puncta/cell was determined as described in Materials and methods. Columns represent the average number of puncta/cell from 3 independent experiments, and error bars the standard error of the means.
To confirm the induction of autophagy in bortezomib-treated HNSCC cells, we examined the expression levels of LC3-II in untransfected UMSCC-22A, 1483, and UMSCC-1 cells. During induction of autophagy, LC3 protein present in the cytoplasm is cleaved and lipidated, generating a faster migrating protein termed LC3-II; it is the LC3-II protein that is recruited to forming autophagosomes [9]. Treatment with bortezomib for 24 or 48 hours led to marked upregulation of LC3-II levels in all 3 cell lines (Fig. 2A–C). Similarly, Beclin-1, whose expression is known to be upregulated during autophagy, was found to be induced following bortezomib treatment (Fig. 2A–C). Taken together with our fluorescence detection of autophagosome formation (Fig. 1), these data strongly indicated that bortezomib induces autophagy in HNSCC cells. However, it remained possible that bortezomib might inhibit fusion of autophogasomes with autolysosomes, or a subsequent step in the complete autophagic process. To determine whether complete autophagic flux was occurring in bortezomib-treated cells we examined the expression of LC3-II in cells simultaneously treated with inhibitors of lysosomal proteases (E64d, leupeptin, and pepstatin A). In cells undergoing complete autophagic flux, induced LC3-II protein eventually is degraded by lysosomal proteases in autolysosomes, and inhibition of these proteases results in a further increase in the levels of cellular LC3-II [26,27]. As shown in Figure 2, treatment with bortezomib in the presence of lysosomal protease inhibitors led to increased levels of LC3-II relative to LC3-II levels seen in cells treated with bortezomib alone, demonstrating that bortezomib induces complete autophagic flux in HNSCC cell lines. However, despite the demonstration of complete autophagic flux in bortezomib treated cells, we cannot rule out the possibilities that bortezomib also may partially impair cellular LC3 degradation or partially block autophagosome fusion with lysosomes.
Fig. 2.
Bortezomib treatment of HNSCC cells induces complete autophagic flux. UMSCC-22A (A), 1483 (B), and UMSCC-1 (C) cells were left untreated, or treated for 24 hours with 0.1% DMSO, or for 24 or 48 hours with bortezomib, as in Figure 1. In addition, to assess for complete autophagic flux, cells treated with bortezomib for 24 hours were simultaneously treated with E64d (10 nM), leupeptin (Leu; 10 μM), and pepstatin A (PA; 10 μM). As a control, cells were also treated with the protease inhibitors alone. Following treatment, cell lysates were subjected to immunoblotting for LC3-II, Beclin-1, or β-actin. Densitometry was used to determine LC3-II/β-actin and Beclin-1/β-actin ratios. Similar results were obtained in 3 independent experiments.
3.2. Bortezomib induces HNSCC JNK activity and Bcl-2 phosphorylation
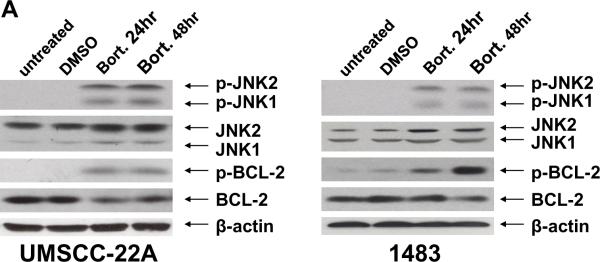
To investigate the mechanism of bortezomib-induced HNSCC autophagy, we examined the role of JNK. Treatment of cells for 24 or 48 hours with bortezomib led to increased phosphorylation of JNK1 (46 kDa) and JNK2 (54 kDa; Fig. 3A); these phosphorylation events are known to be associated with JNK activation. In addition to examining JNK activation, we also examined the phosphorylation status of anti-apoptotic Bcl-2. Recent studies have shown that in cells undergoing nutrient deprivation- or ceramide-induced autophagy, JNK1 phosphorylates serine 70 on Bcl-2, promoting disruption of Bcl-2/Beclin-1 complexes, and liberating Beclin-1 to promote autophagy [22,23]. Following treatment with bortezomib, we observed a substantial increase in the phosphorylation of Bcl-2 on serine 70 (Fig. 3A). The increase in Bcl-2 phosphorylation occurred despite a modest decline in total Bcl-2 levels (Fig. 3A). Moreover, although the antibody employed is specific for Bcl-2 phosphorylated on serine 70, we did not independently verify serine 70 phosphorylation using other biochemical methods.
Fig. 3.
Bortezomib induces JNK activation and JNK-dependent phosphorylation of Bcl-2. (A) UMSCC-22A and 1483 cells were left untreated, treated for 24 hours with 0.1% DMSO, or were treated with 20 nM bortezomib for 24 or 48 hours. Immunoblotting was performed using antibodies directed against phospho-JNK (arrows indicate the location of phospho-JNK1 and phospho-JNK2), total JNK proteins, phospho-serine 70 Bcl-2, or total Bcl-2. Similar results were obtained in 3 independent experiments. (B) Cells were treated as in (A) in the absence or presence of either 10 nM SP600125 (JNK inhibitor (JNKi)) or 10 nM SB203580 (p38 inhibitor (p38i)). Immunoblotting was performed to detect either phosphorylated (phospho-serine 70) Bcl-2 or total Bcl-2 protein. Densitometry was used to determine p-Bcl-2(phospho-Bcl-2)/β-actin and Bcl-2(total Bcl-2)/β-actin ratios.
To determine whether bortezomib-induced phosphorylation of Bcl-2 was dependent on JNK activity, cells were treated with bortezomib in the presence of SP600125, an inhibitor of JNK activity, or SB203580, an inhibitor of p38. As shown in Figure 3B, the JNK inhibitor abolished bortezomib-induced Bcl-2 phosphorylation. Little if any effect was observed with the p38 inhibitor, although in 1483 cells p38 inhibition caused a modest reduction in total, but not phosphorylated, Bcl-2 levels. Thus, serine 70 phosphorylation of Bcl-2 in bortezomib treated HNSCC cells is dependent on JNK activation.
3.3. Bortezomib-induced HNSCC autophagy is dependent on JNK
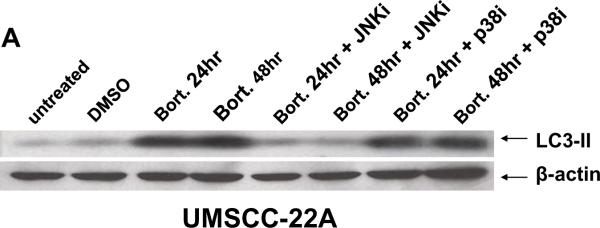
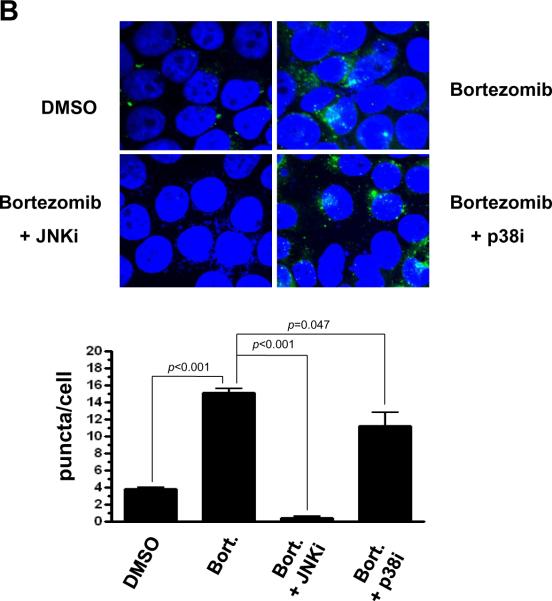
To determine the importance of JNK activation in bortezomib-induced HNSCC autophagy, we assessed LC3-II expression levels and autophagosome formation in the presence or absence of the pharmacologic inhibitors of JNK or p38. JNK inhibitor provided nearly complete inhibition of bortezomib-induced LC3-II production, while p38 inhibitor had little effect (Fig. 4A). In UMSCC-22A cells engineered to express GFP-LC3, JNK inhibitor reduced the average number of bortezomib-induced puncta/cell to levels even lower than the basal levels observed in DMSO-treated cells (Fig. 4B; p<0.001). p38 inhibitor (in combination with bortezomib), on the other hand, provided only a modest decline in the average number of puncta/cell relative to cells treated with bortezomib alone (Fig. 4B; p=0.047). These results demonstrate that bortezomib-induced autophagy in HNSCC cells is dependent on JNK. Moreover, even the low levels of basal autophagy that occur in untreated HNSCC cells may be JNK-dependent.
Fig. 4.
Bortezomib-induced autophagy is dependent on JNK. (A) UMSCC-22A cells were treated with 20 nM bortezomib for 24 or 48 hours in the absence or presence of either JNKi (SP600125, 10 nM) or p38i (SB203580, 10 nM). Following treatment, immunoblotting was performed to detect LC3-II protein or β-actin. (B) UMSCC-22A cells stably expressing GFP-LC3 were treated for 24 hours with 0.1% DMSO, or with bortezomib in the absence or presence of JNKi or p38i. Relocalization of GFP-LC3-II to punctate cytoplasmic dots was visualized as in Figure 1, and the number of puncta/per cell determined. Columns represent the average number of puncta/cell from 3 independent experiments, and error bars the standard error of the means.
4. Discussion
Although HNSCC represents the sixth most common cancer in the United States, autophagy induction and the role of autophagy in this malignancy has not been investigated. Our studies show that the proteasome inhibitor bortezomib potently induces autophagy in HNSCC cells, as demonstrated by upregulation of LC3-II and Beclin-1, and relocalization of GFP-LC3 to a punctate distribution in the cytoplasm. The enhanced production of LC3-II and Beclin-1 when cells were co-incubated with bortezomib and lysosomal protease inhibitors demonstrated that bortezomib induces complete autophagic flux in these cells.
The induction of autophagy following proteasome inhibition has been observed in other cell types, with autophagy serving a pro-survival role in colon, prostate, and ovarian cancer cells [25,28,29], and a pro-death role in MEFs, HUVECs, and multiple myeloma cells [29–31]. At present it is difficult to predict whether bortezomib-induced autophagy will play a pro-survival or pro-death role in a particular cell type. Thus, the design of clinical trials employing autophagy inhibitors is currently dependent on careful and empirical, preclinical testing in specific cell types. Better understanding of the molecular mechanisms of bortezomib-induced autophagy, as well as identification of molecular indicators of response, will also help to guide the design of clinical trials combining proteasome and autophagy inhibitors. However, at present, the molecular mechanism of bortezomib-induced autophagy is incompletely understood.
To investigate the mechanism of bortezomib-induced autophagy, we focused on the role of JNK, which has previously been shown to be activated by proteasome inhibitors. Bortezomib treatment of HNSCC cells led to phosphorylation/activation of JNK enzymes, accompanied by JNK-dependent phosphorylation of Bcl-2 on serine 70. Prior studies have shown that anti-apoptotic Bcl-2 family members, including Bcl-2, Bcl-XL, and Mcl-1L form complexes with Beclin-1 preventing Beclin-1 from promoting autophagy [8,32–34]. In the case of autophagy induced by nutrient deprivation or ceramide treatment, phosphorylation of Bcl-2 has been shown to disrupt Bcl-2/Beclin-1 complexes, liberating Beclin-1 for autophagy induction [22,23]. Although the upregulation of Beclin-1 in bortezomib-treated HNSCC cells suggests initiation of autophagy, the action of Beclin-1 may be constrained by Bcl-2. The finding that bortezomib treatment also induces phosphorylation of Bcl-2 suggests that, similar to nutrient deprivation or ceramide treatment, the bortezomib stimulus is likely to disrupt the inhibitory interactions of Bcl-2 with Beclin-1. This is further supported by our observation that inhibition of JNK enzymes resulted in abrogation of bortezomib-induced Bcl-2 phosphorylation and reduced autophagy. It also is possible that bortezomib-induced autophagy may involve disruption of Beclin-1 complexes with Bcl-XL or Mcl-1L. Bcl-XL is known to be overexpressed in a majority of HNSCC cell lines and primary specimens [35]. Moreover, although Mcl-1L does not bind as avidly as Bcl-2 or Bcl-XL to Beclin-1 [34], Mcl-1L is dramatically upregulated in cells treated with bortezomib, including HNSCC cells [12].
Additional mechanisms of JNK-mediated autophagy induction also cannot be excluded. JNK activation has been shown to mediate Beclin-1 upregulation via c-Jun transcription factor (a JNK target) binding to the beclin-1 gene promoter [36,37]. Further, JNK activation has been shown to upregulate expression of the p53 target damage-regulated autophagy modulator (DRAM), a key mediator of autophagy [38]. In our studies, the three HNSCC cell lines that were used either lack p53 expression or express mutant p53 [24]. Thus, the involvement of DRAM in JNK-mediated autophagy in bortezomib-treated HNSCC cells seems less likely.
In summary, treatment of HNSCC cells with the proteasome inhibitor bortezomib led to activation of JNK enzymes, phosphorylation of Bcl-2 on serine 70, upregulation of autophagy regulatory proteins, formation of autophagosomes, and complete autophagic flux. Phosphorylation of Bcl-2 was dependent on the cellular activity of JNK, but not p38 MAPK. Importantly, JNK activity was critically important for the onset of autophagy following bortezomib treatment, demonstrating a new mechanism of autophagy induction following proteasome inhibition.
Acknowledgements
NIH grants R01 CA137260 and P50 CA097190 supported this work. We thank Drs. Jennifer Grandis and Yan Zang for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest None declared.
References
- [1].Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- [2].Mao L, Hong WK, Papadimitrakopoulou VA. Focus on head and neck cancer. Cancer Cell. 2004;5:311–6. doi: 10.1016/s1535-6108(04)00090-x. [DOI] [PubMed] [Google Scholar]
- [3].Gibson MK, Forastiere AA. Reassessment of the role of induction chemotherapy for head and neck cancer. Lancet Oncol. 2006;7:565–74. doi: 10.1016/S1470-2045(06)70757-4. [DOI] [PubMed] [Google Scholar]
- [4].Khuri FR, Shin DM, Glisson BS, Lippman SM, Hong WK. Treatment of patients with recurrent or metastatic squamous cell carcinoma of the head and neck: current status and future directions. Semin Oncol. 2000;27:25–33. [PubMed] [Google Scholar]
- [5].Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med. 2001;345:1890–900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- [6].Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–73. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- [7].Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9:1102–9. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- [8].Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–6. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- [9].Kabeya Y, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–8. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sunwoo JB, et al. Novel proteasome inhibitor PS-341 inhibits activation of nuclear factor-kappa B, cell survival, tumor growth, and angiogenesis in squamous cell carcinoma. Clin Cancer Res. 2001;7:1419–28. [PubMed] [Google Scholar]
- [11].Fribley A, Zeng Q, Wang CY. Proteasome inhibitor PS-341 induces apoptosis through induction of endoplasmic reticulum stress-reactive oxygen species in head and neck squamous cell carcinoma cells. Mol Cell Biol. 2004;24:9695–704. doi: 10.1128/MCB.24.22.9695-9704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Li C, Li R, Grandis JR, Johnson DE. Bortezomib induces apoptosis via Bim and Bik up-regulation and synergizes with cisplatin in the killing of head and neck squamous cell carcinoma cells. Mol Cancer Ther. 2008;7:1647–55. doi: 10.1158/1535-7163.MCT-07-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li C, Zang Y, Sen M, Leeman-Neill RJ, Man DS, Grandis JR, Johnson DE. Bortezomib up-regulates activated signal transducer and activator of transcription-3 and synergizes with inhibitors of signal transducer and activator of transcription-3 to promote head and neck squamous cell carcinoma cell death. Mol Cancer Ther. 2009;8:2211–20. doi: 10.1158/1535-7163.MCT-09-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ding WX, Yin XM. Sorting, recognition and activation of the misfolded protein degradation pathways through macroautophagy and the proteasome. Autophagy. 2008;4:141–50. doi: 10.4161/auto.5190. [DOI] [PubMed] [Google Scholar]
- [15].McConkey DJ, Zhu K. Mechanisms of proteasome inhibitor action and resistance in cancer. Drug Resist Updat. 2008;11:164–79. doi: 10.1016/j.drup.2008.08.002. [DOI] [PubMed] [Google Scholar]
- [16].Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–4. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- [17].Koumenis C, Naczki C, Koritzinsky M, Rastani S, Diehl A, Sonenberg N, Koromilas A, Wouters BG. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2alpha. Mol Cell Biol. 2002;22:7405–16. doi: 10.1128/MCB.22.21.7405-7416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Park MA, Curiel DT, Koumenis C, Graf M, Chen CS, Fisher PB, Grant S, Dent P. PERK-dependent regulation of HSP70 expression and the regulation of autophagy. Autophagy. 2008;4:364–7. doi: 10.4161/auto.5593. [DOI] [PubMed] [Google Scholar]
- [19].Rzymski T, et al. Regulation of autophagy by ATF4 in response to severe hypoxia. Oncogene. 29:4424–35. doi: 10.1038/onc.2010.191. [DOI] [PubMed] [Google Scholar]
- [20].Yang Y, Ikezoe T, Saito T, Kobayashi M, Koeffler HP, Taguchi H. Proteasome inhibitor PS-341 induces growth arrest and apoptosis of non-small cell lung cancer cells via the JNK/c-Jun/AP-1 signaling. Cancer Sci. 2004;95:176–80. doi: 10.1111/j.1349-7006.2004.tb03200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dai Y, Rahmani M, Pei XY, Dent P, Grant S. Bortezomib and flavopiridol interact synergistically to induce apoptosis in chronic myeloid leukemia cells resistant to imatinib mesylate through both Bcr/Abl-dependent and -independent mechanisms. Blood. 2004;104:509–18. doi: 10.1182/blood-2003-12-4121. [DOI] [PubMed] [Google Scholar]
- [22].Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell. 2008;30:678–88. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pattingre S, Bauvy C, Carpentier S, Levade T, Levine B, Codogno P. Role of JNK1-dependent Bcl-2 phosphorylation in ceramide-induced macroautophagy. J Biol Chem. 2009;284:2719–28. doi: 10.1074/jbc.M805920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lin CJ, Grandis JR, Carey TE, Gollin SM, Whiteside TL, Koch WM, Ferris RL, Lai SY. Head and neck squamous cell carcinoma cell lines: established models and rationale for selection. Head Neck. 2007;29:163–88. doi: 10.1002/hed.20478. [DOI] [PubMed] [Google Scholar]
- [25].Ding WX, Ni HM, Gao W, Yoshimori T, Stolz DB, Ron D, Yin XM. Linking of autophagy to ubiquitin-proteasome system is important for the regulation of endoplasmic reticulum stress and cell viability. Am J Pathol. 2007;171:513–24. doi: 10.2353/ajpath.2007.070188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Klionsky DJ, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–75. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tanida I, Minematsu-Ikeguchi N, Ueno T, Kominami E. Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy. 2005;1:84–91. doi: 10.4161/auto.1.2.1697. [DOI] [PubMed] [Google Scholar]
- [28].Zhu K, Dunner K, Jr., McConkey DJ. Proteasome inhibitors activate autophagy as a cytoprotective response in human prostate cancer cells. Oncogene. 2010;29:451–62. doi: 10.1038/onc.2009.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ding WX, Ni HM, Gao W, Chen X, Kang JH, Stolz DB, Liu J, Yin XM. Oncogenic transformation confers a selective susceptibility to the combined suppression of the proteasome and autophagy. Mol Cancer Ther. 2009;8:2036–45. doi: 10.1158/1535-7163.MCT-08-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hoang B, Benavides A, Shi Y, Frost P, Lichtenstein A. Effect of autophagy on multiple myeloma cell viability. Mol Cancer Ther. 2009;8:1974–84. doi: 10.1158/1535-7163.MCT-08-1177. [DOI] [PubMed] [Google Scholar]
- [31].Belloni D, Veschini L, Foglieni C, Dell'Antonio G, Caligaris-Cappio F, Ferrarini M, Ferrero E. Bortezomib induces autophagic death in proliferating human endothelial cells. Exp Cell Res. 316:1010–8. doi: 10.1016/j.yexcr.2009.11.005. [DOI] [PubMed] [Google Scholar]
- [32].Pattingre S, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–39. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- [33].Maiuri MC, et al. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 2007;26:2527–39. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Erlich S, et al. Differential interactions between Beclin 1 and Bcl-2 family members. Autophagy. 2007;3:561–8. doi: 10.4161/auto.4713. [DOI] [PubMed] [Google Scholar]
- [35].Trask DK, Wolf GT, Bradford CR, Fisher SG, Devaney K, Johnson M, Singleton T, Wicha M. Expression of Bcl-2 family proteins in advanced laryngeal squamous cell carcinoma: correlation with response to chemotherapy and organ preservation. Laryngoscope. 2002;112:638–44. doi: 10.1097/00005537-200204000-00009. [DOI] [PubMed] [Google Scholar]
- [36].Li DD, et al. The pivotal role of c-Jun NH2-terminal kinase-mediated Beclin 1 expression during anticancer agents-induced autophagy in cancer cells. Oncogene. 2009;28:886–98. doi: 10.1038/onc.2008.441. [DOI] [PubMed] [Google Scholar]
- [37].Park KJ, Lee SH, Lee CH, Jang JY, Chung J, Kwon MH, Kim YS. Upregulation of Beclin-1 expression and phosphorylation of Bcl-2 and p53 are involved in the JNK-mediated autophagic cell death. Biochem Biophys Res Commun. 2009;382:726–9. doi: 10.1016/j.bbrc.2009.03.095. [DOI] [PubMed] [Google Scholar]
- [38].Lorin S, Borges A, Ribeiro Dos Santos L, Souquere S, Pierron G, Ryan KM, Codogno P, Djavaheri-Mergny M. c-Jun NH2-terminal kinase activation is essential for DRAM-dependent induction of autophagy and apoptosis in 2-methoxyestradiol-treated Ewing sarcoma cells. Cancer Res. 2009;69:6924–31. doi: 10.1158/0008-5472.CAN-09-1270. [DOI] [PubMed] [Google Scholar]