Abstract
Recent evidence that the heart is not a terminally-differentiated organ has provided more credence to investigations of pathways involved in inducing cardiomyocyte (CM) hyperplasia as a therapy for heart disease. Here, we leveraged zebrafish as a novel vertebrate model of cardiomyopathy to explore the therapeutic potential based on the Wnt/β-catenin signaling. In the anemia-induced zebrafish model of cardiomyopathy (tr265), we detected differently regulated CM hyperplasia and CM hypertrophy in the compact region and the trabecular region. To assess the effects of the Wnt/β-catenin pathway on these two regions, the anemia line was crossed with heat shock-inducible transgenic fish to upregulate or downregulate the pathway. Upregulation resulted in increased cardiomyocyte hyperplasia in the heart and increased cardiomyocyte hypertrophy in the trabecular region, while downregulation resulted in reduced cardiomyocyte hyperplasia in the heart and reduced cardiomyocyte hypertrophy in the trabecular region. Importantly, upregulation of the pathway resulted in improved fish survival, while downregulation decreased it. In summary, our data suggested that 1) the compact region and the trabecular region respond differently during cardiac remodeling; 2) activation of the Wnt/β-catenin pathway might exert a cardioprotective function via promoting cardiomyocyte hyperplasia.
Keywords: Wnt/β-Catenin Pathway, Anemia, Cardiomyopathy, zebrafish
Introduction
The human heart originally had been thought to have no regeneration potential. However, recent evidence is changing that paradigm. In 2009, Bergmann et al. revealed the human heart exchanges nearly half of its cardiomyocytes over a lifetime [1]. Others have shown the existence of cardiac stem cells [2,3,4,5,6]. Such evidence is giving credence to investigations of pathways involved in cardiomyocyte hyperplasia as a therapeutic strategy for heart disease. One pathway that has the potential to induce cardiomyocyte hyperplasia is the Wnt/β-catenin pathway [7]. Upregulation of the signaling results in cardiomyocyte proliferation in cell culture [8], while both in cell culture and in vivo, the pathway has also been shown to be important for specification and expansion of cardiac progenitor cells [9,10]. In addition to its multifaceted functions during cardiogenesis as well as its potential to induce cardiomyocyte hyperplasia [7,11], the Wnt/β-catenin pathway has been found to be involved in the hypertrophic response in mammalian models [12]. Humans with cardiac hypertrophy have increased levels of β-catenin in their hearts [13], and aortic banding (i.e., pressure overload) of rat hearts leads to increased frizzled expression [14]. Furthermore, downregulation of the pathway attenuates cardiac hypertrophy. Mice lacking disheveled-1 have an attenuated hypertrophic response after aortic banding [15]; cardiac-specific overexpression of a constitutively-active GSK-3β in mice prevents hypertrophic growth in response to calcineurin activation, beta-adrenergic stimulation, and aortic constriction [16]; and a mouse model with a cardiomyocyte-specific deletion of β-catenin has an attenuated hypertrophic response upon aortic banding [17].
Understanding how the cardiomyocyte hyperplasia and hypertrophy functions of the Wnt/β-catenin pathway are modulated, as well as when, where, and how to modulate the pathway, may allow for the development of therapeutic strategies for heart disease. Because of the low proliferative capacity of their cardiomyocytes, contributions from cardiomyocyte hyperplasia have been difficult to study in mammalian models. Here, we explored tr265, the first adult zebrafish cardiomyopathy model recently reported by our group [18]. Generated in an ENU screen, the tr265 line contains a mutation in the band 3 protein that prevents red blood cells from dividing during differentiation, thus causing the red blood cells to die, the fish to become anemic, and the heart to become enlarged [19]. The model exhibits phenotypes that resemble those of human heart failure, such as an enlarged heart, cardiomyocyte hypertrophy, increased cardiomyocyte death, muscular disarray, and activated fetal gene expression [18]. Importantly, cardiomyocyte hyperplasia contributes to the pathogenesis. While both cardiomyocyte hypertrophy and hyperplasia are evident at week 6, only cardiomyocyte hyperplasia is detected by week 12.
In this study, we discovered that the cardiomyocyte hyperplasia is predominately located in the compact region, while cardiomyocyte hypertrophy in the trabecular region is evident at week 6. To glean insight on the functions of the Wnt/β-catenin pathway on these two regions, the tr265 line was crossed with heat shock-inducible transgenic fish. Intriguingly, the functions of the pathway on cardiomyocyte hypertrophy and hyperplasia are location-dependent in the model. In addition, upregulation of the pathway accelerates the onset of cardiomyocyte hyperplasia, as well as improves fish survival.
Materials and Methods
Zebrafish Husbandry
The care and use of the fish adhered to the IACUC protocol A24010 approved by the Mayo Clinic Foundation. At day four post fertilization, tr265 and the wild-type (WT) siblings were manually sorted under a dissecting microscope. Fifty fish each were placed in a 0.125-L rearing container until week 4, when they were transferred to a 2.5-L tank.
Immunostaining of Tissue Sections
Immunostaining was conducted on 14-μm frozen sections adhered to Poly-Prep slides (Sigma, St. Louis, MO). The sections were fixed with 4% paraformaldehyde (Polysciences, Warrington, PA) in PBS, permeabilized with 0.1% Tween-20 for MEF2/β-catenin staining or 0.05% Triton X-100 / 0.5% sodium dodecyl sulfate for MEF2/PCNA in PBD (1% albumin bovine serum, 1% dimethyl sulfoxide, 1X PBS, 2% sheep serum), incubated with primary antibody for two hours and secondary antibody for thirty minutes, and imaged with a Zeiss Axioplan 2 microscope equipped with ApoTome and AxioVision software (Carl Zeiss, Thornwood, NY). Primary antibodies included β-catenin (1:200, mouse, Sigma), MEF2C (1:50 dilution, rabbit, Santa Cruz Biotechnology, Santa Cruz, CA), and PCNA (1:3000 dilution, mouse, Sigma). Secondary antibodies included AlexFluor-conjugated anti-mouse or anti-rabbit IgG (1:50 dilution, Invitrogen, Carlsbad, CA)[20].
Heat Shock Lines and Protocol
The anemia-induced zebrafish cardiomyopathy model (tr265) was crossed with the heat shock-inducible Tg(hsp70: ΔTCF-GFP) line[21] to downregulate the pathway and the Tg(hsp70:Wnt3a-GFP) line (described below) to upregulate the pathway (Figure 2A). Constructs are depicted in Figures 1B and 1C. Fish were heat shocked for one hour daily at 37°C from week 4 to week 6.
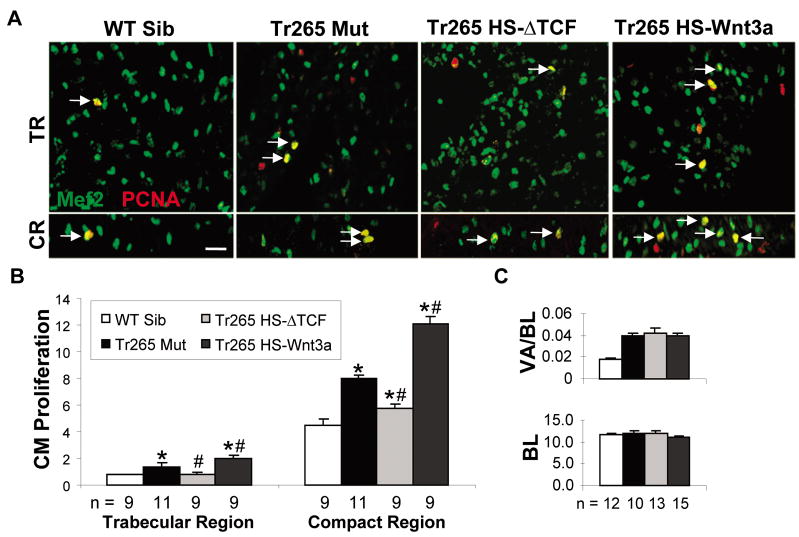
Figure 2. The Wnt/β-catenin pathway affects cardiomyocyte hyperplasia in the compact region and trabecular region.
(A) Proliferating cardiomyocytes (yellow MEF2 + PCNA overlay) in the trabecular region (TR) and compact region (CR). (B) Quantification of cardiomyocyte proliferation (%) in the trabecular region and compact region. Bar = 20 μm (C) Top panel: quantification of ventricle area (VA) over body length (BL) (in mm). Bottom panel: quantification of the BL (in mm). (B and C) mean ± SEM; * or # = P < 0.05 compared to the WT sibling or tr265, respectively. n = number of fish examined.
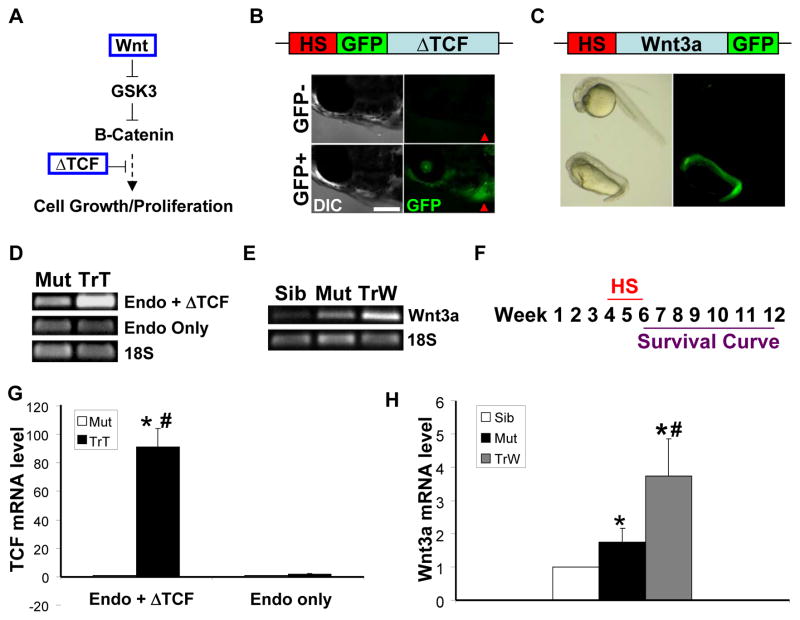
Figure 1. Modulating the Wnt/β-catenin pathway with heat shock-inducible transgenics.
(A) The anemia-induced zebrafish model of cardiomyopathy (tr265) was crossed with Tg(hsp70:Wnt3a-GFP) to upregulate the pathway and Tg(hsp70: ΔTCF-GFP) to downregulate the pathway. (B) One hour of heat shock at 37°C induces GFP expression throughout the fish, including the heart region depicted by the red arrowhead. Bar = 0.5 mm. (C) Verification that the newly generated Tg(hsp70:Wnt3a-GFP) line exhibits anterior head truncation when heat shocked at the dome stage. To confirm that the heat shock protocol induces gene expression at week 6 in the newly generated lines, RT-PCR of (D) total TCF (endogenous plus ΔTCF) and TCF (endogenous only) and (E) Wnt3a was conducted. Mut = tr265; TrT = tr265;Tg(hsp70: ΔTCF-GFP); TrW = tr265;Tg(hsp70:Wnt3a-GFP). (F) Fish were heat shocked from week 4 to week 6, and fish survival was tracked from week 6 to week 12. (G) Real-time PCR revealed that the mRNA expression level of total TCF (endogenous + ΔTCF) was upregulated in zebrafish lines tr265;Tg(hsp70: ΔTCF-GFP), but not the mRNA level of endogenous TCF only. (H) Real-time PCR revealed that the mRNA expression level of Wnt3a was upregulated in tr265 and further activated in tr265;Tg(hsp70:Wnt3a-GFP).
To generate the Tg(hsp70:Wnt3a-GFP) line, approximately 1.6 kb of upstream sequence of zebrafish hsp70-4 [22] and full-length zebrafish Wnt3a encoding sequence were amplified using primers designed with the restriction enzyme cutting sites Sal1 and Xho1 or BamH1, respectively, and inserted in-frame into the tol2 GFP vector. The resulting plasmid was co-injected with tol2 transposase RNA into one-cell embryos. Adults were outcrossed and the offspring heat shocked to identify stable transgenic founders. As would be expected, heat shock at 40°C for 30 minutes at the dome stage resulted in anterior head truncation [23] (Figure 1C).
RT - PCR
Ventricles were removed half an hour after heat shock, and pooled (6 sibling or 4 mutant ventricles) in 100 μL of TRIzol (Invitrogen). After homogenizing the ventricles with a pestle (Kimble Chase, Vineland, NJ) and 28-gauge, 1/2-cc insulin syringe (Becton, Dickinson, and Company, Franklin Lakes, NJ), Invitrogen’s instructions were followed to extract the RNA. SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen) was used to make the cDNA. 18S was used as the internal control. 18S (forward primer: 5′-cacttgtccctctaagaagttgca, reverse primer: 5′-ggttgattccgataacgaacga). Total TCF (endogenous + ΔTCF) (forward primer: 5′-ctgcactctcagctttatcc, reverse primer: 5′-tcagatgacagatacgggac). Endogenous TCF (forward primer: 5′-tggacgaggtcaaatcctcac, reverse primer: 5′-tgtcgtctcaaagcttccgc). Wnt3a (forward primer: 5′-tacgccttcttcaagcatcc, reverse primer: 5′-ctctttgcgcttttctgtcc; 35 cycles)[24]. Real-time PCR was performed in a MyiQ Single-Color Real-Time PCR Detection System (Bio-Rad) using iQ SYBR Green supermix (Bio-Rad). 18S rRNA was used as an internal control to normalize TCF or wnt3a levels.
Cardiomyocyte Density and Proliferation Calculation
Unlike in our previous paper [18], cardiomyocyte density and proliferation were calculated in both the trabecular and compact regions. As was done previously, a square/rectangle was drawn using AxioVision software (Carl Zeiss) in the trabecular/compact region. Cardiomyocytes were counted in the square/rectangle and divided by the sampled area to get cardiomyocytes per mm2. For cardiomyocyte proliferation, the total number of proliferating cardiomyocytes (PCNA+ MEF2+) in each region was divided by the estimated total number of cardiomyocytes in that region (based on multiplying the cardiomyocyte density by the total trabecular or compact region area).
Cardiomyocyte Size Quantification
In our previous paper [18], we dissociated the cardiomyocytes before measuring them. For this study, however, we measured the size of the cardiomyocytes within the 14-μm tissue sections. The β-catenin borders of MEF2-stained cells were measured with AxioVision software (Carl Zeiss).
Ventricle Area to Body Length and Body Length
Ventricle area to body length and body length were found as previously described [18].
Survival Curve
Fish that had undergone heat shock from week 4 to week 6 were identified as GFP positive or negative. Approximately, ten fish per group were placed in a 2.5-L tank. At the week-6 time point, the survival was set at 100%. Every seven days, the number of fish remaining in each tank was counted. Three independent survival curve experiments were conducted per group of fish.
Statistical Methods
Significance was determined with a Student’s t-test by comparing the WT sibling group to each of the other groups, as well as the tr265 group to each of the transgenic groups. For the survival curve, a log-rank test was used. P values less than 0.05 were deemed significant (* from the WT sibling or # from tr265, respectively). Reported are mean ± standard deviation.
Results
The Wnt/β-catenin pathway affects cardiomyocyte hyperplasia similarly in both the compact region and the trabecular region
Previously [18], we had reported the presence of both cardiomyocyte hyperplasia and hypertrophy in anemia-induced zebrafish cardiomyopathy model -tr265. To assess functions of the Wnt/β-catenin pathway in this model, we crossed the anemia line with heat shock-inducible transgenic lines. The Tg(hsp70: ΔTCF-GFP) [21] line was used to downregulate the pathway and the Tg(hsp70:Wnt3a-GFP) line to upregulate the pathway (Figures 1A - 1C). Upon generating the Tg(hsp70:Wnt3a-GFP) line, we confirmed that heat shock at the dome stage could induce the expected anterior head truncation phenotype [23] (Figure 1C). The induction of gene expression at week 6 in the new zebrafish lines tr265;Tg(hsp70: ΔTCF-GFP) and tr265;Tg(hsp70:Wnt3a-GFP) is validated by real-time RT-PCR of total TCF (endogenous + ΔTCF), TCF (endogenous only), and Wnt3a (Figures 1D-1E and 1G-1H).
Upon closer observation of the tr265 model, we found most of the hyperplasia occurred in the outer region of the ventricle (7.96 ± 0.27% in the outer compact region vs. 1.40 ±0.26% in the inner trabecular region; Figure 2B). Upregulation of the Wnt/β-catenin pathway further increased the cardiomyocyte hyperplasia (12.11 ± 0.55% vs. 7.96 ± 0.27% in tr265), while downregulating it decreased the cardiomyocyte hyperplasia (5.74 ± 0.34%) in the compact region (Figures 2A bottom panel and 2B). To a lesser extent, modulation affected proliferation in the trabecular region in a similar manner as in the compact region (2.01 ± 0.22% and 0.82 ± 0.16%, respectively, vs. 1.40 ±0.26% in tr265, respectively; Figures 2A top panel and 2B). Despite the pathway’s effect on cardiomyocyte hyperplasia, the ventricle area to body length index was not affected (Figure 2C). In summary, the Wnt/β-catenin pathway modulates cardiomyocyte proliferation similarly in both the compact region and the trabecular region.
The Wnt/β-catenin pathway affects cardiomyocyte hypertrophy oppositely in the compact region and the trabecular region
In our previous paper [18], we had measured cardiomyocyte size by dissociating the ventricle into individual cardiomyocytes and then quantifying the cardiomyocytes’ cell size. Two limitations of such a method were 1) the cardiomyocyte was taken out of context (i.e., dissociated from the rest of the cardiomyocytes) and 2) the specific location of cardiomyocyte hypertrophy within the ventricle could not be determined. To overcome these limitations, we utilized an antibody against β-catenin to outline the cardiomyocytes (MEF2-positive) in the tissue sections. Quantification of the outlined area validated that the cardiomyocytes (CMs) were larger in the trabecular region of tr265 (52.32 ± 2.16 μm2 vs. 22.93 ± 0.37 μm2 in the WT sibling; Figures 3A top panel and 3C), which is consistent with the reduced CM density (5,624 ± 212 CMs/mm2 vs. 7,495 ± 213 CMs/mm2 in the WT sibling; Figures 3A top panel and 3B).
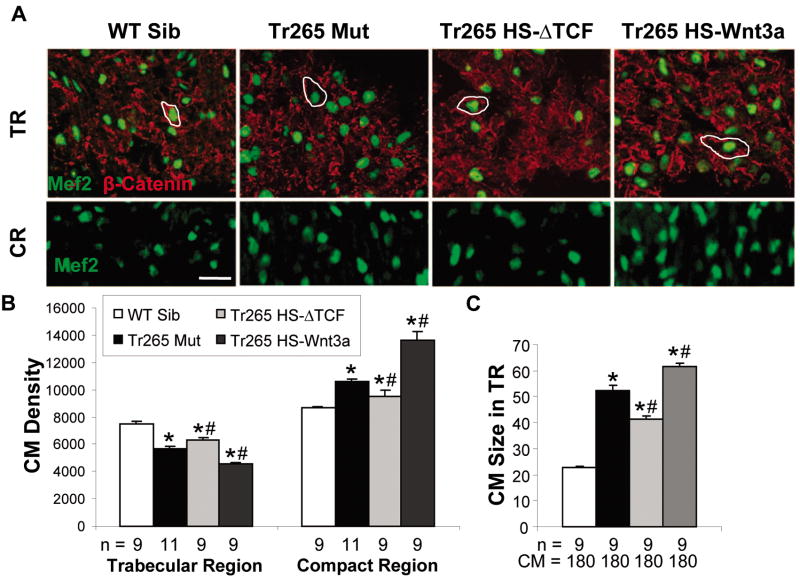
Figure 3. The Wnt/β-catenin pathway affects cardiomyocyte hypertrophy in the compact and trabecular region.
(A) Top panel: Cardiomyocytes (green MEF2) are outlined by β-catenin (red staining) in 14-μm ventricular sections of the trabecular region (TR) to quantify cardiomyocyte size. Bar = 20 μm. Bottom panel: like the top panel but without β-catenin for clarity in the compact region (CR). (B) Quantification of cardiomyocyte density (CMs/mm2) in the trabecular and compact regions. (C) Quantification of cardiomyocyte size (μm2) in the trabecular region. (B and C) mean ± SEM; * or # = P < 0.05 compared to the WT sibling or tr265, respectively. n = number of fish examined. CM = the number of cardiomyocytes analyzed.
We noted different cardiomyocyte density in the inner trabecular region and the outer compact region in the tr265 mutant (5,624 ± 212 CMs/mm2 in trabecular region vs. 10,603 ± 72 CMs/mm2 in compact region). Upregulation of the Wnt/β-catenin pathway in the trabecular region decreased cardiomyocyte density (4,540 ± 108 CMs/mm2; Figures 3A top panel and 3B) and increased cardiomyocyte size (61.49 ± 1.26 μm2 vs. 52.32 ± 2.16 μm2 in WT; Figures 3A top panel and 3C), while downregulation increased cardiomyocyte density (6,272 ± 242 CMs/mm2; Figures 3A top panel and 3B) and decreased cardiomyocyte size (41.50 ± 0.99 μm2; Figures 3A top panel and 3C). Conversely, upregulation of the pathway resulted in increased cardiomyocyte density in the compact region (13,663 ± 582 CMs/mm2 vs. 10,603 ± 72 CMs/mm2 in tr265; Figures 3A bottom panel and 3B), while downregulation decreased it (9,511 ± 410 CMs/mm2; Figures 3A bottom panel and 3B). Thus, cardiomyocyte size in the trabecular region and the compact region are oppositely regulated by the Wnt/β-catenin pathway in the anemia model.
Modulation of the Wnt/β-catenin pathway affects the onset of cardiomyocyte hyperplasia in the trabecular region and fish survival
It was noted in our previous paper that only cardiomyocyte hyperplasia is evident by week 12 and that the fish that live the longest have resorted to cardiomyocyte hyperplasia [18]. To assess how the early modulation of the Wnt/β-catenin pathway affects the onset of cardiomyocyte hyperplasia in the trabecular region, we measured cardiomyocyte density and size six weeks after heat shock (Figure 1F). In contrast to the week-6 results, the week-12 tr265;Tg(hsp70:Wnt3a-GFP) fish had an even higher cardiomyocyte density in the trabecular region than the week-12 tr265 (7,514 ± 85 CMs/mm2 vs. 6,098 ± 125 CMs/mm2, respectively; Figures 4A top panel and 4B) and even smaller-sized cardiomyocytes (40.62 ± 0.85 μm2 vs. 55.89 ± 0.65 μm2, respectively; Figures 4A top panel and 4C). A similar pattern was seen in the cardiomyocyte density in the compact region (8,795 ± 122 CMs/mm2 vs. 7,708 ± 155 CMs/mm2, respectively; Figures 4A bottom panel and 4B). The opposite occurred in tr265;Tg(hsp70: TCF-GFP): decreased cardiomyocyte density in the trabecular region (5,178 ± 85 CMs/mm2; Figures 4A top panel and 4B); increased cardiomyocyte size in the trabecular region (65.99 ± 0.77 μm2; Figures 4A top panel and 4C); and reduced cardiomyocyte density in the compact region (7,041 ± 59 CMs/mm2; Figures 4A bottom panel and 4B). Like at week 6, the ventricle area to body length indices of the transgenics were no different than that of tr265 (data not shown). In summary, these data suggested that upregulation of the Wnt/β-catenin pathway in the anemia model accelerates the onset of cardiomyocyte hyperplasia in the trabecular region, which leads to more cardiomyocytes of smaller size. In contrast, downregulation of the Wnt/β-catenin pathway delays it.
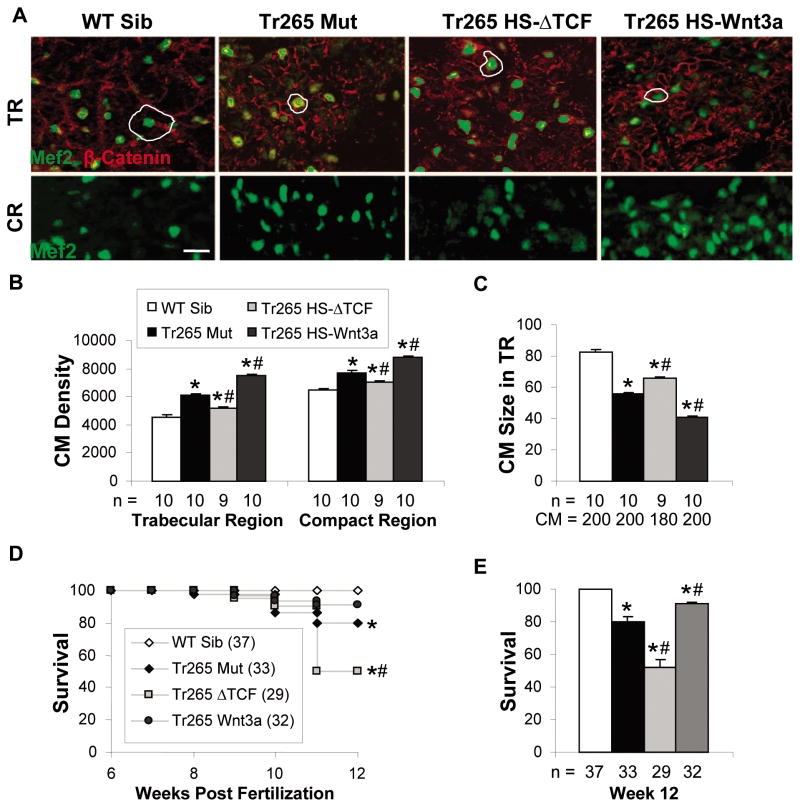
Figure 4. Modulation later affects cardiomyocyte hyperplasia in the trabecular and compact region and fish survival.
(A) Top panel: Cardiomyocytes (green MEF2) are outlined by β-catenin (red staining) in 14-μm ventricular sections of the trabecular region (TR) to quantify cardiomyocyte size. Bar = 20μm. Bottom panel: like the top panel but without β-catenin for clarity in the compact region (CR). (B) Quantification of cardiomyocyte density (CMs/mm2) in the trabecular and compact regions. (C) Quantification of cardiomyocyte size (μm2) in the trabecular region. (D) Percent survival from week 6 to week 12, with week-6 survival set at 100%. (E) Percent survival at week 12. (B, C, and E) mean ± SEM; * or # = P < 0.05 compared to the WT sibling or tr265, respectively. n = number of fish examined. CM = the number of cardiomyocytes analyzed.
Finally, we tracked survival of the anemia model from week 6 to week 12 post fertilization (Figure 1F). As previously shown [18], tr265 had a reduced lifespan compared to the WT sibling (Figure 4D). Downregulation of the Wnt/β-catenin pathway dramatically decreased fish survival, while upregulation improved the survival (Figures 4D and 4E). At week 12, 91.0 ± 1.0% of the tr265;Tg(hsp70:Wnt3a-GFP) group were alive compared to 80.0 ± 3.2% of the tr265 group and 52 ± 4.12% of the tr265;Tg(hsp70: ΔTCF-GFP) group (Figure 4E). Hence, these data suggest that upregulating the Wnt/β-catenin pathway improves fish survival.
Discussion
Through detailed characterization of a zebrafish anemic model of cardiomyopathy, the present manuscript highlights an important concept that cardiomyocytes (CMs) in different heart regions can exhibit distinct pathological changes during cardiac remodeling. The concept is supported by the observation that the Wnt/β-catenin signaling exerts opposite effects on CM hypertrophy in the trabecular region and the compact region. The underlying molecular mechanism for this location-dependent function of the Wnt/β-catenin pathway needs to be further investigated. Of note, the effects of the epicardial region which borders the compact region warrant further investigation, since epicardial-derived cells have been shown to contribute to the compact region in both zebrafish and mammals [3,4,5,25,26,27].
Due to the highly proliferative nature of CMs in the compact region of a zebrafish heart [20,25,26,28,29], it is not surprising that the Wnt/β-catenin pathway imposes a more profound effect on CM hyperplasia in the compact region. A similar effect on CM hyperplasia also occurs in the trabecular region, although to a lesser degree. We reasoned that CM hyperplasia creates a compensational cellular change, which is different from CM hypertrophy, a pathological cellular event during anemia-induced cardiomyopathy. This hypothesis was originally raised based on the observation that the tr265 fish that live the longest have resorted to CM hyperplasia [18]. It is possible that most hypertrophied CMs die at a later stage, and are replaced by smaller-sized, newly-generated CMs via CM hyperplasia. In the present manuscript, we showed that upregulation of the Wnt/β-catenin pathway promotes CM hyperplasia (Figure 2A and 2B), while downregulation of the Wnt/β-catenin pathway inhibits CM hyperplasia in both regions. We also found that CM hypertrophy is activated upon upregulation of Wnt, but suppressed upon downregulation of Wnt in the trabecular region. As a combined consequence of these cellular changes, upregulation of the Wnt/β-catenin pathway attenuates the increased CM size and improves the survival, while downregulation of the Wnt/β-catenin pathway increases CM size and decreases the survival rate at later stages. Together, these data suggest that the functions of the Wnt/β-catenin pathway on CM hyperplasia impose a more dominant role than CM hypertrophy in modulating the pathogenesis of the anemia-induced cardiomyopathy, supporting the concept that CM hyperplasia can be a therapeutic strategy for cardiomyopathy.
Together, our data emphasize the unique capacity of the zebrafish model to study both CM hypertrophy and hyperplasia. It is highly possible that the location-dependent functions uncovered here in the zebrafish model also exist in mammals for either Wnt/β-catenin or other signaling pathways, and therefore deserves consideration before developing therapeutic strategies based on these signaling pathways.
Highlights.
A zebrafish model of cardiomyopathy was used to assess Wnt/β-catenin signaling.
Wnt/β-catenin signaling exerts location-dependent effects on the heart.
Upregulation of the Wnt signaling promotes CM hypertrophy and increases survival.
Acknowledgments
We thank Beninio Tombe Jomok and Mayo’s Zebrafish Core Facility for the care of the zebrafish.
Abbreviations
- WT
Wild type
- CMs
Cardiomyocytes
- TR
Trabecular region
- CR
Compact region
Footnotes
Financial Disclosure
This study was funded by the Mayo Foundation and NIH RO1 (HL107304) to X. Xu.,
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisén J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellison GM, Torella D, Karakikes I, Nadal-Ginard B. Myocyte death and renewal: modern concepts of cardiac cellular homeostasis. Nat Clin Pract Cardiovasc Med. 2007;4(Suppl 1):S52–59. doi: 10.1038/ncpcardio0773. [DOI] [PubMed] [Google Scholar]
- 3.Cai CL, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, Yang L, Bu L, Liang X, Zhang X, Stallcup WB, Denton CP, McCulloch A, Chen J, Evans SM. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454:104–108. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, von Gise A, Ikeda S, Chien KR, Pu WT. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454:109–113. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russell JL, Goetsch SC, Gaiano NR, Hill JA, Olson EN, Schneider JW. A dynamic notch injury response activates epicardium and contributes to fibrosis repair. Circulation research. 2011;108:51–59. doi: 10.1161/CIRCRESAHA.110.233262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Nardo P, Forte G, Ahluwalia A, Minieri M. Cardiac progenitor cells: potency and control. J Cell Physiol. 2010;224:590–600. doi: 10.1002/jcp.22165. [DOI] [PubMed] [Google Scholar]
- 7.Gessert S, Kuhl M. The multiple phases and faces of wnt signaling during cardiac differentiation and development. Circ Res. 2010;107:186–199. doi: 10.1161/CIRCRESAHA.110.221531. [DOI] [PubMed] [Google Scholar]
- 8.Bicknell KA, Brooks G. Reprogramming the cell cycle machinery to treat cardiovascular disease. Curr Opin Pharmacol. 2008;8:193–201. doi: 10.1016/j.coph.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, Reinecke H, Moon RT, Murry CE. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:9685–9690. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qyang Y, Martin-Puig S, Chiravuri M, Chen S, Xu H, Bu L, Jiang X, Lin L, Granger A, Moretti A, Caron L, Wu X, Clarke J, Taketo MM, Laugwitz KL, Moon RT, Gruber P, Evans SM, Ding S, Chien KR. The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/beta-catenin pathway. Cell Stem Cell. 2007;1:165–179. doi: 10.1016/j.stem.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 11.Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, Martin JF. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–461. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blankesteijn WM, van de Schans VAM, ter Horst P, Smits JFM. The Wnt/frizzled/GSK-3 beta pathway: a novel therapeutic target for cardiac hypertrophy. Trends Pharmacol Sci. 2008;29:175–180. doi: 10.1016/j.tips.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Rezvani M, Liew CC. Role of the adenomatous polyposis coli gene product in human cardiac development and disease. J Biol Chem. 2000;275:18470–18475. doi: 10.1074/jbc.M000870200. [DOI] [PubMed] [Google Scholar]
- 14.Blankesteijn WM, Essers-Janssen YP, Ulrich MM, Smits JF. Increased expression of a homologue of drosophila tissue polarity gene "frizzled" in left ventricular hypertrophy in the rat, as identified by subtractive hybridization. J Mol Cell Cardiol. 1996;28:1187–1191. doi: 10.1006/jmcc.1996.0109. [DOI] [PubMed] [Google Scholar]
- 15.van de Schans VAM, van den Borne SWM, Strzelecka AE, Janssen BJA, van der Velden JLJ, Langen RCJ, Wynshaw-Boris A, Smits JFM, Blankesteijn WM. Interruption of Wnt signaling attenuates the onset of pressure overload-induced cardiac hypertrophy. Hypertension. 2007;49:473–480. doi: 10.1161/01.HYP.0000255946.55091.24. [DOI] [PubMed] [Google Scholar]
- 16.Antos CL, McKinsey TA, Frey N, Kutschke W, McAnally J, Shelton JM, Richardson JA, Hill JA, Olson EN. Activated glycogen synthase-3 beta suppresses cardiac hypertrophy in vivo. Proc Natl Acad Sci USA. 2002;99:907–912. doi: 10.1073/pnas.231619298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qu J, Zhou J, Yi XP, Dong B, Zheng H, Miller LM, Wang X, Schneider MD, Li F. Cardiac-specific haploinsufficiency of beta-catenin attenuates cardiac hypertrophy but enhances fetal gene expression in response to aortic constriction. J Mol Cell Cardiol. 2007;43:319–326. doi: 10.1016/j.yjmcc.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun X, Hoage T, Bai P, Ding Y, Chen Z, Zhang R, Huang W, Jahangir A, Paw B, Li YG, Xu X. Cardiac hypertrophy involves both myocyte hypertrophy and hyperplasia in anemic zebrafish. PLoS ONE. 2009;4:e6596. doi: 10.1371/journal.pone.0006596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paw BH, Davidson AJ, Zhou Y, Li R, Pratt SJ, Lee C, Trede NS, Brownlie A, Donovan A, Liao EC, Ziai JM, Drejer AH, Guo W, Kim CH, Gwynn B, Peters LL, Chernova MN, Alper SL, Zapata A, Wickramasinghe SN, Lee MJ, Lux SE, Fritz A, Postlethwait JH, Zon LI. Cell-specific mitotic defect and dyserythropoiesis associated with erythroid band 3 deficiency. Nat Genet. 2003;34:59–64. doi: 10.1038/ng1137. [DOI] [PubMed] [Google Scholar]
- 20.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 21.Lewis JL, Bonner J, Modrell M, Ragland JW, Moon RT, Dorsky RI, Raible DW. Reiterated Wnt signaling during zebrafish neural crest development. Development. 2004;131:1299–1308. doi: 10.1242/dev.01007. [DOI] [PubMed] [Google Scholar]
- 22.Halloran MC, Sato-Maeda M, Warren JT, Su F, Lele Z, Krone PH, Kuwada JY, Shoji W. Laser-induced gene expression in specific cells of transgenic zebrafish. Development. 2000;127:1953–1960. doi: 10.1242/dev.127.9.1953. [DOI] [PubMed] [Google Scholar]
- 23.Lekven AC, Thorpe CJ, Waxman JS, Moon RT. Zebrafish wnt8 encodes two wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev Cell. 2001;1:103–114. doi: 10.1016/s1534-5807(01)00007-7. [DOI] [PubMed] [Google Scholar]
- 24.Clements WK, Ong KG, Traver D. Zebrafish wnt3 is expressed in developing neural tissue. Dev Dyn. 2009;238:1788–1795. doi: 10.1002/dvdy.21977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lepilina A, Coon AN, Kikuchi K, Holdway JE, Roberts RW, Burns CG, Poss KD. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell. 2006;127:607–619. doi: 10.1016/j.cell.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 26.Wills AA, Holdway JE, Major RJ, Poss KD. Regulated addition of new myocardial and epicardial cells fosters homeostatic cardiac growth and maintenance in adult zebrafish. Development. 2008;135:183–192. doi: 10.1242/dev.010363. [DOI] [PubMed] [Google Scholar]
- 27.Kikuchi K, Holdway JE, Major RJ, Blum N, Dahn RD, Begemann G, Poss KD. Retinoic acid production by endocardium and epicardium is an injury response essential for zebrafish heart regeneration. Dev Cell. 2011;20:397–404. doi: 10.1016/j.devcel.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, MacRae CA, Stainier DYR, Poss KD. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464:601–605. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jopling C, Sleep E, Raya M, Martí M, Raya A, Belmonte JCI. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464:606–609. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]