Abstract
Food and nutrition play an intimate and inextricable role in all aspects of drug metabolism, safety, and effectiveness. Antiretroviral therapies (ART) have assumed a preeminent position in the prevention, care, and treatment of HIV and its comorbidities. The interaction between food, nutrition, and ART has become an expanding area of interest both in terms of clinical standards of care and as a target for research. Since the original review of this topic by the WHO in 2005, much has been learned (8). This article contains a review of what is known about the general relationships between nutrition and pharmacology, as well as issues specific to ART, with particular attention to their use in low- and middle-resource settings. The importance of food and nutrition on the bioavailability of drugs and vice versa has been an area of historical interest. However, much has been learned about the importance of nutritional status on drug metabolism, distribution, and effectiveness. The impact of traditional therapies (herbal/botanical) is highlighted as an area of clinical concern and one in need of further research. Additional attention is focused on the impact of individual micronutrients on drug pharmacokinetics and pharmacodynamics. Finally, attention is given to the nutritional implications of the metabolic consequences of ART, which include the potential impact of “colliding epidemics” of infection (eg, HIV, tuberculosis) and noncommunicable diseases. Much has been learned, but much remains to be accomplished to ensure the effective integration of nutritional considerations into the effective and safe use of ART.
INTRODUCTION
Nutrition may be defined as the sum total of the processes involved in the taking in and use of food substances through which growth, repair, and maintenance of activities of the body as a whole or in any of its parts are accomplished. The processes of nutrition consist of ingestion, digestion, absorption, metabolism, functional use/activation of dependent systems, and excretion. All these processes are similarly integral to how the body takes in and uses therapeutics/drugs, which include antiretroviral therapies (ART).
Not only do drugs and nutrients share these same processes, their availability and function are also intimately and inextricably entwined. The body's ability to process foreign substances depends on metabolic systems that rely on essential nutrients (vitamins, minerals, fatty acids, and so forth) obtained through diet. Yang et al (1) offered a teleologic explanation of the synergism between diet and the detoxification of foreign substances, based on the evolutionary change to a complex diet paradoxically rich in essential nutrients but that also contained botanical sources of potentially toxic chemicals. The need to find sources of essential nutrients was linked to the need to develop mechanisms for detoxification of the accompanying toxins. These mechanisms, in turn, became dependent on many of the same essential nutrients, which created interdependence between nutrition and detoxification. In our modern world it is not just exposure to toxins in the environment, but also the response to pharmacologic substances, which, in much the same way as the early botanicals, are being used on a trial-and-error basis to improve the human condition. As is the case with exposures to potentially poisonous herbals/botanicals, exposure to modern medicines can have a healthful or hurtful outcome. The response, either therapeutic or toxic, to any foreign substance is contingent on numerous factors that include stage of development, genetics, general health, and nutritional status.
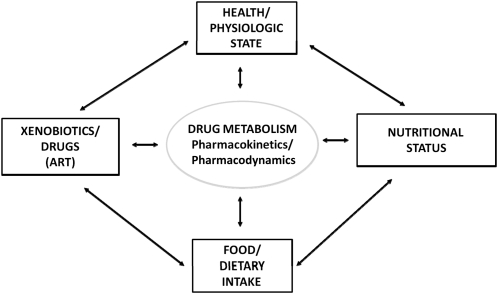
The general relation between diet, nutrition, and pharmacology is conceptualized in Figure 1. It is discussed in the context of several core concepts, which are outlined in Table 1. Within the context of pharmacokinetics and pharmacodynamics several pathways exist by which nutrition might affect drugs and vice versa (Table 2).
FIGURE 1.
Conceptual model of drug-nutrient interactions. ART, antiretroviral therapies.
TABLE 1.
Core concepts of pharmacology1
| Concept | Description |
| Pharmacodynamics | The study of the biochemical and physiologic effects of drugs on the body or on microorganisms or parasites within or on the body and the mechanisms of drug action and the relation between drug concentration and effect. A prime example is drug-receptor interactions. |
| Pharmacokinetics | The action of drugs in the body over a period of time, including the processes of absorption, distribution, localization in tissues, biotransformation, and excretion. |
| Phases of drug metabolism | |
| Phase I | Oxidation reduction reactions that result in activation, deactivation, or preparation for eventual elimination. These reactions occur primarily in the liver, but also in other tissues (eg, lungs, kidneys, gastrointestinal tract) and use the synergism between 3 primary components: MFO, which include cytochrome P450 enzymes (oxidation) NADPH-P450 reductase (reduction) Phospholipid (phosphatidylcholine or lecithin). The phospholipid component provides stability for these membrane-bound enzymes. The dependence on stable membranes introduces the potential for damage due to lipid oxidation, which implies a role for antioxidants in the protection of the integrity of the MFO system. |
| Phase II (conjugation reactions) | The attachment of substances, which yields a more polar and water-soluble substance and thereby facilitates elimination. |
MFO, mixed-function oxygenase.
TABLE 2.
Potential mechanisms to explain drug-nutrient interactions1
| Mechanism | Description |
| Ingestion | Both drugs and disease can cause changes in appetite and nutrient intake; resultant malnutrition can affect drug efficacy. |
| Absorption | Drugs and foods can have a mechanical effect, via binding or adsorption, that can increase or decrease drug and nutrient absorption. Some drugs can increase or decrease gastrointestinal motility, which may result in increased or decreased nutrient absorption. Chemical factors, in particular the pH of the stomach contents and the influence of foods therein, can affect the subsequent absorption of drugs. Nutritional status, infection, and inflammation can cause homeostatic responses, which lead to increased or decreased nutrient absorption. |
| Gastrointestinal transport | The ability of drugs and nutrients to be transported can depend on factors such as lipid solubility and competition for amino acid transport systems. |
| Metabolism | MFO and conjugase systems that convert drugs and nutrients into their active and excretory forms are nutrient/cofactor dependent. Certain drugs can increase the activity of the MFO systems required to convert nutrient precursors into their active forms. Nonnutritive components in foods/supplements can induce MFO activity and thereby affect drug metabolism. |
| Distribution | The use of both drugs and nutrients depends on body composition, the availability and functional integrity of transport proteins, receptor integrity, and intracellular metabolic machinery, all of which are sensitive to nutritional status and the impact of disease (inflammation and infection via the acute-phase response). |
| Elimination | Drugs and nutrients can synergistically and competitively interact to cause increased or decreased excretion. Systemic factors such as pH and physiologic state (eg, sweating) can dictate whether a drug or nutrient is excreted or resorbed. |
| Direct Action | The effectiveness of some drugs is directly related to their impact on nutrient metabolism (eg, antimalarial antifolate drugs, isoniazid, and vitamin B-6). |
MFO, mixed-function oxygenase.
In the context of potential food/nutrient-drug interactions, most of the available information used clinically is focused on factors that pertain to drug pharmacokinetics and, in particular, bioavailability (eg, foods that may affect drug absorption because of physicochemical solubility relationships). There is, however, a historical knowledge base with regard to the role of specific nutrients and pharmacodynamic processes [ie, those Phase I metabolic systems, mixed-function oxygenase/cytochrome P450 (CYP), responsible for the activation, transport, and excretion of drugs (2)]. Examples of these types of interactions are listed in Table 3.
TABLE 3.
Examples of the impact of specific nutrients on Phase 1/MFO metabolism1
| Nutrient | Effect on MFO metabolism | Potential mechanism(s) |
| Protein | Deficiency: ↓ rate of metabolism | ↓ Protein synthesis; ↓ in synthesis of other elements, such as hormones, involved in enzyme induction |
| Excess: can ↑ rate of metabolism | ||
| Lipids | Deficiency (or diet high in saturated fatty acids): ↓ | ↓ Activity of MFO possibly connected to the requirement for polyunsaturated fatty acid in the β-position of phosphatidylcholine (lecithin), which is an essential component of the MFO system |
| Excess (or diet high in polyunsaturated fatty acids): ↑ activity and induction of MFO enzymes | ||
| Carbohydrates | Excess: ↓ | Secondary effect due to ↓ protein or possibly inhibition of P450 via ↓ in supporting enzyme components |
| Vitamin C | Deficiency: ↓ | Alterations in activities of P450 and P450 reductase mediated via either ↑ or ↓ in the expression of specific CYP isozymes in excess or deficiency states |
| Excess: ↑ P450 activity | ||
| Vitamin B-6 | Deficiency: ↓ | ↓ Synthesis of heme; possible impairment of protein synthesis |
| Thiamine | Deficiency: ↑ activity of cytochrome P450 | ↑ Activity of specific P450 isozymes and perhaps other enzymes in deficiency by an unknown mechanism. Excess may be due to ↓ substrate binding |
| Excess: ↓ (both reductase and P450) | ||
| Riboflavin | Deficiency: ↓ or ↑ depending on the severity | ↓ Reductase activity but ↑ P450 activity, such that the metabolism of some drugs will be ↑, whereas others may be ↓ |
| Vitamin E | Deficiency: ↓ | Because activities of P450 and reductase are unaffected, it may be due to reduction in antioxidative mechanisms (eg, protection of the lecithin component): lack of effect from excess may be due to rapid metabolic clearance of vitamin E isomers via P450 (3) |
| Excess: no reported effect | ||
| Iron | Deficiency: ↓ and ↑ | Differential effects on various components of the MFO system. ↑ Lipid peroxidation could lead to damage to the integrity of the system |
| Excess: ↑ in microsomal lipid peroxidation |
Reproduced with permission (2). MFO, mixed-function oxygenase.
In addition, a greater appreciation has emerged for the interaction between genes [which includes genetic polymorphisms in mixed-function oxygenase and related systems (4)] and developmental changes [eg, in infants, pregnancy, and lactation (5, 6)]. Finally, it is also important to note that the disease process (eg, inflammation or response to infection) elicits a unique response to nutrient homeostasis that affects nutrient absorption, availability, and response to treatment (7). This is best exemplified by the anemia of infection and the impact on iron status, and the impact of inflammation via the acute-phase response on the key carriers of iron (eg, ferritin) (8). Thus, a close relation exists between the body's response to illness, drugs, nutrients, and the requisite systems involved in the functional use of the drugs and nutrients. Many of these concepts are appropriately applied to environmental toxicants as well and have been discussed in greater detail elsewhere (2).
NUTRITION AND ART
In 2003, as part of the WHO effort to develop recommendations for the nutritional care of people living with HIV and AIDS, a review of the extant evidence with regard to the role of diet and nutrition in the safe and effective use of ART was requested. The review was released in 2005 (8) and contained 2 overarching principles: 1) antiretroviral drugs are essential to prolong lives and halt the spread of HIV/AIDS, and 2) food is essential to life for all people. The challenge then was and now is how to apply sound principles of clinical care and nutrition science to the safe and efficacious implementation of ART and long-term care for people living with HIV and AIDS. Additionally, the report highlighted the importance of food and adequate dietary intake as essential to achieve optimal nutrition and health for people before and during treatment of HIV and related comorbidities. HIV-infected adults and children being considered for ART should be screened for nutritional problems, and the extent of such screening will depend on the technical capacity and level of support at the clinical care setting.
In addition to an outline of the key elements of nutrient-drug interactions, 3 core findings were highlighted and have since been reinforced with new evidence:
Certain foods affect the bioavailability of antiretroviral medications; examples included garlic (10, 11) and other traditional therapies such as African potato (12). Since the original report, other medicinal plants/herbal remedies have been implicated in drug interactions (13, 14).
Use of “traditional medicines” and complementary and alternative medicines may also affect antiretroviral use (adherence), efficacy (15), and safety (16).
A substantial body of evidence exists with regard to the impact of ART on the metabolism of adults and children; many of these effects have dietary and nutritional implications (17).
A more direct conceptualization of the model presented in Figure 1 as it specifically pertains to HIV infection and its treatment is presented in Figure 2. Aside from the mechanisms described above, a number of ART-specific interactions may occur, primarily via the key pathways responsible for drug metabolism. These include drug-drug interactions, drug-botanical/herbal interactions, and drug-nutrient interactions. Whereas the principles may differ, the core mechanisms may often be similar and be mediated via the CYP drug-metabolizing systems, primarily in the liver and gastrointestinal tract.
FIGURE 2.
Specific relationships between HIV, treatment, health, and nutrition.
ART drug-drug interactions were reviewed recently by Fletcher (18), who observed that a specific enzyme, CYP3A4, the most abundant isoform of the cytochrome system in the human liver, is responsible for the metabolism of ~60% of HIV-related drugs. Most protease inhibitors and nonnucleoside reverse transcription inhibitors are CYP3A4 substrates. Therefore, it is critical to recognize the potential for either inhibition or induction of this enzyme in the use of these drugs. A number of examples are cited in which one drug affects the use of another via this mechanism (18). Although the potential for drug interactions is recognized, what is less well acknowledged is the potential for interactions between ART and other commonly used substances through this same mechanism.
The CYP system has been shown to be the target of a number of other ART interactions with substances commonly used by HIV-infected patients in domestic/US and international settings. Mills et al (12) reported on the impact of African herbal medicines on antiretroviral metabolism and noted specifically a significant inhibition of CYP4A4 by 2 common African herbal remedies (African potato and Sutherlandia).
Flavonoids are a group of substances that occur naturally in fruit (including cocoa), vegetables, beverages (tea, wine), and many dietary supplements (eg, ginkgo biloba) or herbal remedies. They have been touted as having beneficial effects on a number of health conditions. Of particular relevance to this discussion is evidence that indicates that these compounds significantly affect the activity (induction or inhibition) of CYP isoforms and other related drug-metabolizing enzymes (19, 20).
The historical approach to micronutrients in the context of ART, and more broadly with HIV, is to limit the conversation to micronutrient insufficiency (21). However, as evidenced by the discussions above and below, both low and high micronutrient exposure and status must be considered in the context of ART use. With specific regard to nutrients, several have been the focus of investigation in this context.
Vitamin C has been shown to significantly affect the regulation of several of the key CYP enzymes, which include isoforms of CYP, family 3, and subfamily A (CYP3A), some of which were shown to be decreased by vitamin C deficiency (22). Conversely, the observation of Slain et al (23) is of particular interest in the context of ART use. Their study involved the evaluation of the pharmokinetics of a specific protease inhibitor (indinavir) in HIV-uninfected subjects who were receiving doses of vitamin C that ranged from 800 to 1000 mg/d. They reported that “concomitant administration of high doses of vitamin C can reduce steady-state indinavir plasma concentrations.” Did the excess vitamin C result in increased CYP450 metabolism of indinavir? The clinical implications of these findings have not been established.
Vitamin D has been highlighted as an important nutrient of concern for the general public (24). Of relevance here, vitamin D has also been identified as an nutrient of concern in the context of HIV, in terms of both nutritional adequacy and specific problems associated with HIV-related bone problems that are potentially associated with either nutritional deficiency and/or drug interactions (9, 25). On the other hand, other mechanism(s) by which vitamin D might have an impact on drug metabolism were described by Kutuzova and DeLuca (26), who reported that 1,25-dihydroxyvitamin D3 regulates the genes responsible for the production of enzymes (including CYP3A4) that are responsible for detoxification in the intestine. This study has been followed by numerous others that point to an important and underappreciated role for vitamin D in drug metabolism through induction of the gene expression of key drug-metabolizing enzymes. Consequently, in addition to the concerns about vitamin D insufficiency associated with poor exposure or nutrient-drug interactions, there is the potential that vitamin D supplementation can operate independently through the regulation of the drug-metabolizing enzymes to affect ART safety and efficacy. These relationships should be evaluated closely, particularly in light of the expanding interest in vitamin D supplementation across all segments of the population, including HIV-infected people (27).
Vitamin A continues to hold a position of great interest in the global health dialogue and vitamin A insufficiency continues to be a major concern (28, 29). Again, most of the focus of the public health community, generally and in the context of HIV, has been on insufficiency. Several lines of evidence have indicated that vitamin A supplementation may be an issue of concern, particularly for HIV infection (30, 31).
With specific regard to drug metabolism, evidence similar to that for vitamin D and vitamin C exists for an important role for vitamin A. Vitamin A has 3 active forms (retinal, retinol, and retinoic acid) and a storage form (retinyl ester):
Investigators have reported that 3 primary forms of vitamin A (9-cis-retinal, 9-cis-retinoic acid, and all-trans-retinoic acid) induce CYP3A expression at messenger RNA, as well as enzyme activity levels in both liver and intestinal cells (32). The study by Chen et al (33) showed that, in their model, retinoids were able to alter drug metabolism through CYP3A induction. These reports reinforce the notion that the use of vitamin A supplements may have implications mediated through this role in drug metabolism, which will require further research and clinical attention.
It is clear that in light of what we know about the processes of nutrition and pharmacology, the view of nutrition and specific nutrients must be expanded beyond the desire to prevent and treat undernutrition. Moreover, the use of dietary supplements, whether in the form of traditional therapies (herbal/botanical) or as nutrient supplements intended to correct presumed insufficiency, must be viewed in a larger context. This context must include the health of the individual [the presence or absence of active disease (either communicable and/or chronic), developmental stage, nutritional status (replete or deplete)] and the potential impact of these bioactive substances on all aspects of pharmacokinetics/pharmacodynamics.
CONCLUSIONS
This article has provided an overview of the potential role of nutrition and specific nutrients and other dietary substances in the safe and effective use of ART. A description of these phenomena is just the first step. In terms of care and treatment, what do we need to do to improve our clinical approach? What does it all mean and what can we do?
To start, we have to look more carefully at potential drug-nutrient interactions in the clinical setting and to ask the correct questions, particularly in settings in which poor nutrition might be anticipated. To support our ability to do that, we need to delineate more clearly the role of nutrients in pharmacology beyond just bioavailability. We also need to look more critically at the nutritional context in which people live and to ask some basic questions at the initial visit, such as “Are you hungry?,” ‘”What are you eating?,” and “Are you using dietary supplements and/or traditional therapies?” From a public health perspective a need exists to examine more closely the “one-size-fits-all” public health approach to nutrition, particularly as it relates to micronutrients and especially in the context of infections such as HIV and their treatment (34). We cannot limit our focus to only amelioration of undernutrition. The provision of additional micronutrients in some scenarios may, in fact, exacerbate rather than ameliorate problems. Our ability to determine when that might be the case will depend on our evolving knowledge about these complex relationships as well as our access to the tools needed to evaluate them (eg, biomarkers) for accurate and reliable assessment of nutritional status.
Much work has been done to address the important synergies between food, nutrition, and the safe and effective implementation of ART use to prevent and treat HIV. However, continued effort and vigilance is needed to ensure that these issues are fully integrated into prevention, care, and treatment programs. Only through such efforts can we achieve the goals of all the global efforts to address this compelling and ongoing global health challenge.
Acknowledgments
The author had no conflict of interest.
REFERENCES
- 1.Yang CS, Brady JF, Hong JY. Dietary effects on cytochromes P450, xenobiotic metabolism, and toxicity. FASEB J 1992;6:737–44 [DOI] [PubMed] [Google Scholar]
- 2.Raiten DJ. Nutrition, pharmacology, and toxicology: a dialectic Massaro E, Handbook of human toxicology. Boca Raton, FL: CRC Press, 1997 [Google Scholar]
- 3.Traber MG. Regulation of xenobiotic metabolism, the only signaling function of alpha-tocopherol? Mol Nutr Food Res 2010;54:661–8 [DOI] [PubMed] [Google Scholar]
- 4.Johansson I, Ingelman-Sundberg M. Genetic polymorphism and toxicology—with emphasis on cytochrome P450. Toxicol Sci 2011;120:1–13 [DOI] [PubMed] [Google Scholar]
- 5.Ward RM, Lane RH, Albertine KH. Basic and translational research in neonatal pharmacology. J Perinatol 2006;26(Suppl 2):S8–12 [DOI] [PubMed] [Google Scholar]
- 6.McCarter-Spaulding DE. Medications in pregnancy and lactation. MCN Am J Matern Child Nurs 2005;30:10–7, quiz 18–9 [PubMed] [Google Scholar]
- 7.Prentice AM, Ghattas H, Cox SE. Host-pathogen interactions: can micronutrients tip the balance? J Nutr 2007;137:1334–7 [DOI] [PubMed] [Google Scholar]
- 8.Northrop-Clewes CA. Interpreting indicators of iron status during an acute phase response–lessons from malaria and human immunodeficiency virus. Ann Clin Biochem 2008;45:18–32 [DOI] [PubMed] [Google Scholar]
- 9.Raiten DJ, Grinspoon S, Arpadi S. Nutritional considerations in the use of ART in resource-limited settings. Geneva, Switzerland: World Health Organization, 2005 [Google Scholar]
- 10.Piscitelli SC, Burstein AH, Welden N, Gallicano KD, Falloon J. The effect of garlic supplements on the pharmacokinetics of saquinavir. Clin Infect Dis 2002;34:234–8 [DOI] [PubMed] [Google Scholar]
- 11.Berginc K, Milisav I, Kristl A. Garlic flavonoids and organosulfur compounds: impact on the hepatic pharmacokinetics of saquinavir and darunavir. Drug Metab Pharmacokinet 2010;25:521–30 [DOI] [PubMed] [Google Scholar]
- 12.Mills E, Cooper C, Seely D, Kanfer I. African herbal medicines in the treatment of HIV: Hypoxis and Sutherlandia. An overview of evidence and pharmacology. Nutr J 2005;4:19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown L, Heyneke O, Brown D, van Wyk JP, Hamman JH. Impact of traditional medicinal plant extracts on antiretroviral drug absorption. J Ethnopharmacol 2008;119:588–92 [DOI] [PubMed] [Google Scholar]
- 14.Wiegman DJ, Brinkman K, Franssen EJ. Interaction of Ginkgo biloba with efavirenz. AIDS 2009;23:1184–5 [DOI] [PubMed] [Google Scholar]
- 15.Peltzer K, Friend-du Preez N, Ramlagan S, Fomundam H, Anderson J. Traditional complementary and alternative medicine and antiretroviral treatment adherence among HIV patients in Kwazulu-Natal, South Africa. Afr J Tradit Complement Altern Med 2009;7:125–37 [PMC free article] [PubMed] [Google Scholar]
- 16.Ladenheim D, Horn O, Werneke U, Phillpot M, Murungi A, Theobald N, Orkin C. Potential health risks of complementary alternative medicines in HIV patients. HIV Med 2008;9:653–9 [DOI] [PubMed] [Google Scholar]
- 17.Fitch K, Grinspoon S. Nutritional and metabolic correlates of cardiovascular and bone disease in HIV-infected patients. Am J Clin Nutr 2011;94(suppl):1721S–28S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fletcher CV. Antiretroviral drug-drug interaction considerations for HIV-infected children. Pediatr Infect Dis J 2009;28:429–30 [DOI] [PubMed] [Google Scholar]
- 19.Sergent T, Dupont I, Van der Heiden E, Scippo ML, Pussemier L, Larondelle Y, Schneider YJ. CYP1A1 and CYP3A4 modulation by dietary flavonoids in human intestinal Caco-2 cells. Toxicol Lett 2009;191:216–22 [DOI] [PubMed] [Google Scholar]
- 20.Moon YJ, Wang X, Morris ME. Dietary flavonoids: effects on xenobiotic and carcinogen metabolism. Toxicol In Vitro 2006;20:187–210 [DOI] [PubMed] [Google Scholar]
- 21.Drain PK, Kupka R, Mugusi F, Fawzi WW. Micronutrients in HIV-positive persons receiving highly active antiretroviral therapy. Am J Clin Nutr 2007;85:333–45 [DOI] [PubMed] [Google Scholar]
- 22.Mori T, Itoh S, Ohgiya S, Ishizaki K, Kamataki T. Regulation of CYP1A and CYP3A mRNAs by ascorbic acid in guinea pigs. Arch Biochem Biophys 1997;348:268–77 [DOI] [PubMed] [Google Scholar]
- 23.Slain D, Amsden JR, Khakoo RA, Fisher MA, Lalka D, Hobbs GR. Effect of high-dose vitamin C on the steady-state pharmacokinetics of the protease inhibitor indinavir in healthy volunteers. Pharmacotherapy 2005;25:165–70 [DOI] [PubMed] [Google Scholar]
- 24.Dietary Reference Intakes for Calcium and Vitamin D. Institute of Medicine, 2010. Available from: http://books.nap.edu/openbook.php?record_id=13050 [Google Scholar]
- 25.Overton ET, Yin MT. The rapidly evolving research on vitamin D among HIV-infected populations. Curr Infect Dis Rep 2011;13:83–93 [DOI] [PubMed] [Google Scholar]
- 26.Kutuzova GD, DeLuca HF. 1,25-Dihydroxyvitamin D3 regulates genes responsible for detoxification in intestine. Toxicol Appl Pharmacol 2007;218:37–44 [DOI] [PubMed] [Google Scholar]
- 27.Villamor E. A potential role for vitamin D on HIV infection? Nutr Rev 2006;64:226–33 [DOI] [PubMed] [Google Scholar]
- 28.Global prevalence of vitamin A deficiency in populations at risk 1995–2005. WHO Global Database on Vitamin A Deficiency. Geneva, Switzerland: World Health Organization, 2009. Available from: http://whqlibdoc.who.int/publications/2009/9789241598019_eng.pdf [Google Scholar]
- 29.Imdad A, Herzer K, Mayo-Wilson E, Yakoob MY, Bhutta ZA. Vitamin A supplementation for preventing morbidity and mortality in children from 6 months to 5 years of age. Cochrane Database Syst Rev 2010;CD008524. [DOI] [PubMed] [Google Scholar]
- 30.Arsenault JE, Aboud S, Manji KP, Fawzi WW, Villamor E. Vitamin supplementation increases risk of subclinical mastitis in HIV-infected women. J Nutr 2010;140:1788–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villamor E, Koulinska IN, Aboud S, Murrin C, Bosch RJ, Manji KP, Fawzi WW. Effect of vitamin supplements on HIV shedding in breast milk. Am J Clin Nutr 2010;92:881–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang K, Chen S, Xie W, Wan YJ. Retinoids induce cytochrome P450 3A4 through RXR/VDR-mediated pathway. Biochem Pharmacol 2008;75:2204–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen S, Wang K, Wan YJ. Retinoids activate RXR/CAR-mediated pathway and induce CYP3A. Biochem Pharmacol 2010;79:270–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raiten DJ. One size fits all? Complications in a complicated world. Sight and Life 2010 March:34–5. Available from: http://www.sightandlife.org/images/stories/pageimages/content/magazine/3_2010/SL_Mag_3_2010.pdf [Google Scholar]