Abstract
Genetic factors strongly influence the intake and preference for sugar and saccharin solutions in inbred mouse strains. The present study determined if genetic variance also influences the learned preferences for flavors added to sugar solutions. Conditioned flavor preferences (CFP) are produced in rodents by adding a flavor (CS+) to a sugar solution and a different flavor (CS−) to a saccharin solution (CS−) in one-bottle training trials; the CS+ is subsequently preferred to the CS− when both are presented in saccharin solutions in two-bottle tests. With some sugars (e.g., sucrose), flavor preferences are reinforced by both sweet taste and post-oral nutrient effects, whereas with other sugars (e.g., fructose), sweet taste is the primary reinforcer. Sucrose and fructose were used in three experiments to condition flavor preferences in one outbred (CD-1) and eight inbred strains which have “sensitive” (SWR/J, SJL/J, C57BL/10J, C57BL/6J) or “sub-sensitive” (DBA/2J, BALB/cJ, C3H/HeJ, 129P3/J) sweet taste receptors (T1R2/T1R3). Food-restricted mice of each strain were trained (1 h/day) to drink flavored 16% sucrose (CS+16S, Experiment 1), 16% fructose (CS+16F, Experiment 2) or 8% fructose + 0.2% saccharin (CS+8F, Experiment 3) solutions on five alternate days and a differently flavored saccharin solution (0.05% or 0.2%, CS−) on the other five alternating days. The CS+ and CS− flavors were presented in 0.2% saccharin for two-bottle testing over six days. All strains preferred the CS+16S to CS− although there were significant strain differences in the magnitude and persistence of the sucrose preference. The strains also differed in the magnitude and persistence of preferences for the CS+16F and CS+8F flavors over the CS− with two strains failing to prefer the fructose-paired flavors. Sucrose conditioned stronger preferences than did fructose which is attributed to differences in the taste and post-oral actions of the sugars. These differential training intakes may not have influenced the sucrose-CFP because of the post-oral reinforcing actions of sucrose. Overall, sweet sensitive and sub-sensitive mice did not differ in sucrose-CFP, but unexpectedly, the sub-sensitive mice displayed stronger fructose-CFP. This may be related to differential training intakes of CS+ and CS− solutions: sweet sensitive mice consumed more CS− than CS+ during training while sub-sensitive mice consumed more CS+.
Keywords: Flavor-flavor conditioning, Flavor-nutrient conditioning, Sugar, Saccharin, Classical conditioning
1. Introduction
Systematic analyses of inbred mouse strain differences have emerged as important sources of information regarding the genetic control of all aspects of ingestive behavior, including those involving sweet taste preferences (see reviews: Bodnar et al., 2011; Reed et al., 1997; West & York, 1998). Mouse strain differences have been observed for intake and sensitivity to both sugars and artificial sweeteners in many studies (Bachmanov et al., 2001; Blizard et al., 1999; Fuller, 1974; Lewis et al., 2005; Lush, 1989; Pelz et al., 1973; Reed et al., 2004; Sclafani, 2006a; Stockton & Whitney, 1974). Studies of these strain differences led to the discovery of two genes (Tas1r2, Tas1r3) that code for the T1R2 and T1R3 proteins that form the sweet taste receptor (Bachmanov & Beauchamp, 2007). Furthermore, mouse strain differences in sweetener preference were found to reflect different alleles of the Tas1r3 gene which resulted in “sensitive” and “subsensitive” forms of the T1R2/T1R3 sweet taste receptor (Bachmanov & Beauchamp, 2007).
Allelic variation of the Tas1r3 gene, however, does not account for all of the differences in sweetener intake, and other genes are implicated (Boughter & Bachmanov, 2007). In addition, experiential factors can greatly influence sugar intake and preference of inbred mouse strains (Sclafani, 2006a). For example, naïve 129P3/J (129) mice, which have the sub-sensitive sweet receptor, display weaker preferences for dilute sucrose solutions than do sweet sensitive C57BL/6J (B6) mice, but after experience with concentrated sugar solutions, the sugar preferences of the two strains are indistinguishable (Sclafani, 2006a). This experiential influence was attributed to the post-oral actions of sucrose enhancing sweet taste preference. Consistent with this interpretation, Sclafani and Glendinning (2005) reported that intragastric (IG) sucrose infusions conditioned preferences for flavored saccharin solutions in both B6 and 129 strains.
Conditioned flavor preference (CFP) produced by sucrose has also been reported in rats that are trained with an arbitrary flavor mixed directly into the sugar solution (Mehiel & Bolles, 1988). In this case, however, the sweet taste of sucrose as well as its post-oral effects can reinforce the flavor preference. The potency of sweet taste alone to produce CFP was first demonstrated in an early study in which rats were trained with one flavor (the conditioned stimulus, CS+) added to a concentrated saccharin solution and a different flavor (the CS−) added to a less preferred dilute saccharin solution (Holman, 1975). In subsequent choice tests, the rats preferred the CS+ to the CS− flavor when both were presented in solutions containing the same amount of saccharin. This form of learning is referred to as flavor-flavor (or flavor-taste) conditioning to distinguish it from the flavor-nutrient conditioning produced exclusively by the post-oral effects of sugars (Sclafani, 1995). A special case of flavor-flavor learning was reported by Sclafani and Ackroff (1994) in which rats acquired a preference for a CS+ flavor added to an 8% fructose solution over a CS− flavor added to a less preferred saccharin solution. Although a sugar with post-oral nutrient effects, fructose, unlike sucrose or glucose does not condition flavor preferences in rats when infused IG during short-term training sessions (Sclafani et al., 1999; Ackroff et al., 2001). Similarly, B6 mice fail to acquire a preference for a CS+ flavor paired with IG fructose infusion although they display strong preferences for a CS+ flavor paired with IG sucrose or glucose infusion (Sclafani & Glendinning, 2005; Sclafani, unpublished data). Thus, the preference for a flavor mixed into a fructose solution is attributed to the sweet taste rather than the post-oral action of the sugar (Ackroff & Sclafani, 1991; Ackroff et al., 2009; Baker et al., 2003; Dwyer & Quirk, 2008; Golden & Houpt, 2007; Sclafani & Ackroff, 1994).
While mouse strain differences in sweetener preference have been extensively investigated, learned preferences for flavors added to sweetener solutions have not been examined. The present study, therefore, investigated sugar-CFP in one outbred strain (CD-1) and eight inbred strains, four of which have the sensitive sweet taste receptor (C57BL/6J, C57BL/10J, SJL/J, SWR/J) and four of which have the sub-sensitive receptor (BALB/cJ, C3H/HeJ, DBA/2J, 129P3/J). These strains are a subset of 12 mouse strains investigated in our laboratories for 24-h sugar and fat preferences (Lewis et al., 2005; Lewis et al., 2007), and were selected because they consumed a criterion level of sucrose (1 ml/1 h) in pharmacological studies performed in food-restricted mice (Dym et al., 2007; Dym et al., 2009). This criterion is important for the present study in that these strains display a level of drinking necessary to demonstrate a significant preference in short-term (1 h) tests, and minimize the possibility of “floor effects” associated with minimal intake. Flavor conditioning was investigated in three experiments using sucrose, fructose and saccharin. In the first experiment, mice were trained with CS+ flavored 16% sucrose and CS− flavored 0.05% saccharin solutions, and preferences were evaluated in two-bottle tests with both flavors presented in 0.2% saccharin solutions. A similar design was used in Experiment 2 except that the sugar was 16% fructose. Both the oral (sweet taste) and post-oral actions of sucrose contribute to the sugar’s conditioning effects, whereas fructose conditioning is based primarily on the oral properties of this sugar (Sclafani & Ackroff, 1994; Sclafani et al., 1999). It was predicted that strain differences in fructose-CFP would be more closely associated with the sweet taster status of the inbred strains than sucrose-CFP. A third experiment was conducted with mice trained with a combined 8% fructose + 0.2% saccharin CS+ solution relative to a 0.2% saccharin CS− solution in a paradigm which has been extensively used in flavor-flavor preference studies conducted with rats (Baker et al., 2004; Bernal et al., 2008; Dwyer, 2009; Golden & Houpt, 2007; Sclafani & Ackroff, 1994). A comparison of the results obtained in Experiments 2 and 3 would reveal which conditioning procedure was more effective in producing fructose-based CFP in mice.
2. Methods
2.1. Subjects
The eight inbred strains were purchased from Jackson Laboratories (Bar Harbor, ME, male, 6–8 weeks of age, group sizes summarized in Table 1): BALB/cJ (BALB), C3H/HeJ (C3H), C57BL/6J (B6), C57BL/10J (B10), DBA/2J (D2), SJL/J (SJL), SWR/J (SWR), 129P3/J (129). Outbred CD-1 mice were obtained from Charles River Laboratories (Wilmington, MA, male, 8 weeks of age). The mice were housed individually in plastic cages (30 × 20 × 15 cm) with stainless steel tops, and were maintained on a 12:12 h light:dark cycle at a constant temperature of 22°C. The mice were allowed to acclimate to the vivarium for at least one week before beginning experimental procedures. The experimental protocols in the three experiments were approved by the Queens College Institutional Animal Care and Use Committee certifying that all subjects and procedures are in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Table 1.
Mean CS+ and CS− solution intakes (ml, ±SEM) of nine mouse strains during one-bottle training in CS+ 16% Sucrose, 16% Fructose and 8% Fructose + 0.2% saccharin experiments.
| Experiment 1 | Experiment 2 | Experiment 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Strain | n | CS+ 16%Sucrose | CS− .05%saccharin | n | CS+ 16%Fructose | CS− .05%saccharin | n | CS+8%Fructose + .2%saccharin | CS− .2%saccharin |
| SWR | 7 | 2.04 (0.16)* a | 2.91 (0.38) a | 8 | 0.66 (0.06)* b | 1.15 (0.18) a | 10 | 1.26 (0.12)* b | 2.04 (0.15) a |
| SJL | 9 | 1.82 (0.17) a | 1.62 (0.08) bc | 7 | 0.58 (0.06)* bc | 0.94 (0.07) a | 7 | 1.04 (0.08)* b | 1.41 (0.13) b |
| B10 | 9 | 1.26 (0.05)* c | 1.50 (0.09) bc | 10 | 0.45 (0.03)* c | 0.91 (0.07) a | 10 | 1.20 (0.07)* b | 1.91 (0.09) a |
| B6 | 10 | 1.21 (0.09)* c | 2.04 (0.20) b | 10 | 0.55 (0.02)* bc | 1.21 (0.07) a | 10 | 1.11 (0.09)* b | 1.74 (0.11) a |
| D2 | 10 | 1.09 (0.05)* c | 0.45 (0.05) d | 7 | 0.57 (0.04)* bc | 0.30 (0.06) b | 9 | 0.62 (0.06)* c | 0.32 (0.06) e |
| BALB | 6 | 1.90 (0.12)* a | 1.00 (0.10) c | 6 | 0.81 (0.05)* a | 0.47 (0.07) b | 9 | 1.63 (0.13)* a | 0.95 (0.10) cd |
| C3H | 10 | 1.88 (0.09)* a | 1.21 (0.11) c | 10 | 0.84 (0.04)* a | 0.53 (0.04) b | 10 | 1.16 (0.07)* b | 0.66 (0.03) d |
| 129 | 10 | 1.68 (0.11)* a,b | 1.10 (0.13) c | 10 | 0.44 (0.04) c | 0.56 (0.09) b | 10 | 1.17 (0.03) b | 1.21 (0.09) bc |
| CD-1 | 19 | 1.44 (0.04) b,c | 1.32 (0.13) c | 10 | 0.82 (0.06) a | 0.95 (0.08) a | 10 | 1.12 (0.08) b | 1.20 (0.11) bc |
The asterisk (*) denotes a significant difference (p<0.05 or greater) between intakes of training solutions within an experiment for a given strain.
Between-strain differences in CS+ and CS− training intakes within an experiment are indicated by letter superscripts; intakes that do not share a common letter (a–d) differ significantly from each other (p < 0.05).
2.2. Solutions
Initial training solutions were unflavored sodium saccharin (0.2%, Sigma Chemical Co., St. Louis, MO). The experimental training solutions consisted of 16% sucrose (Domino Foods, Yonkers, NY) and 0.05% saccharin (Experiment 1), 16% fructose (Sigma Chemical Co.) and 0.05% saccharin (Experiment 2), and a mixture of 8% fructose + 0.2% saccharin and 0.2% saccharin only (Experiment 3). Each of these solutions was flavored with 0.05% unsweetened grape or cherry Kool-Aid (General Foods, White Plains, NY) with half of the mice in each strain having the cherry flavor added to the sugar solution and the grape flavor added to the saccharin group; the flavors were reversed for the remaining mice. In all two-bottle preference tests, the cherry and grape flavors were each presented in 0.2% saccharin solutions. The flavored sucrose, fructose and fructose+saccharin solutions used in training are referred to as the CS+16% Sucrose, CS+16% Fructose, and CS+8% Fructose, respectively. The saccharin solutions with the sugar-paired flavors used in two-bottle tests are referred to as the CS+16S, CS+16F, and CS+8F. The flavored saccharin-only solutions used in training and testing are referred to as the CS−. Solutions were presented in 10 ml plastic syringes fitted with stainless steel sipper tubes, and intakes were recorded to the nearest 0.1 ml. The sipper tubes were firmly secured to the cage tops with a metal spring.
2.3. Initial Training
The mice were food-restricted to 85–90% of body weight seven days prior to initial training and were maintained at that weight throughout the experiment. Food rations (2.5–3.0 g, Lab Diet Mouse Chow 5015, PMI Nutritional International, Brentwood, MO) were provided 30 min after the daily test sessions; water was available ad libitum throughout the study except during training and test sessions. All animals consumed their food rations each day. The mice were initially trained to drink an unflavored 0.2% saccharin solution during daily 1-h sessions over three days.
2.4. CFP Training and Testing
After initial training, the mice in Experiment 1 received one-bottle training (1 h/day) with the CS+16% Sucrose on days 1, 3, 5, 7 and 9 and with the CS− (0.05% saccharin) on days 2, 4, 6, 8 and 10 during the mid-light phase of the light:dark cycle. On training days 9 and 10, a second drinking tube containing water was available to acclimate the mice to two-bottle testing with the positions of the CS and water tubes counterbalanced over the two days. The mice were then given a series of two-bottle choice tests (1 h/day) with the CS+16S and CS− flavors presented in 0.2% saccharin solutions for six sessions. The left (L)-right (R) position of the CS+ and CS− solutions were counterbalanced throughout testing resulting in three pairs of preference tests that controlled for position effects with half of the animals of each strain in each condition receiving the CS+ bottle in a L-R-R-L-L-R order and the remainder receiving the CS+ bottle in a R-L-L-R-R-L order.
The mice in Experiments 2 and 3 received identical training except with the CS+16% Fructose and CS− (0.05% saccharin) solutions and the CS+8% Fructose +0.2% saccharin and CS− (0.2% saccharin) solutions.
2.5. Statistics
To evaluate within-strain differences in training intakes, separate two-way analyses of variance tests (ANOVA) were conducted within each strain with Experiment (1 – 3) as a between-subject variable and CS (CS+, CS−) as a within subject variable. Initially, the two-bottle CS+ vs. CS− intakes were evaluated within each strain and experiment with Tests 1–3 as within subject variable. With one exception (see Results) there were no systematic changes in CS intakes over tests, and therefore the two-bottle intake data were averaged across the three tests. Separate analyses evaluated the mean two-bottle intakes within each strain with Experiment (1–3) and CS (CS+ vs. CS−) as between- and within-subject variables, respectively The percent CS+ preferences for the CS+16S, CS+16F, and CS+8F were evaluated by separate one-way repeated ANOVAs for each strain.
To evaluate between-strain differences in training intakes, separate one-way ANOVAs compared the CS+ and CS− intakes in each experiment. Between-strain differences in CS+ preferences were evaluated in separate one-way ANOVAs comparing the percent CS+ intakes. Selected two-way ANOVAs were also conducted to compare strain differences in the CS+ preferences in Experiments 1 and 2 that involved isocaloric concentrations of different sugars (sucrose vs. fructose), and strain differences in Experiments 2 and 3 that involved different concentrations of fructose (16% vs. 8%). Separate analyses compared the one-bottle training intakes of the sugar solutions in Experiments 1 vs. 2, and 2 vs. 3.
The one-bottle training and two-bottle test intakes of the inbred mice were further analyzed as a function of sweet taster status. In this case, all of the mice with the sensitive form of the sweet receptor (B6, B10, SJL, SWR) were treated as one group and all of the mice with the sub-sensitive form of the receptor (BALB, C3H, D2, 129) were treated as another group. For each experiment, separate two-way analyses (group × CS solution) evaluated the one-bottle training data and two-bottle intake data averaged over the three tests. Percent CS+ intakes were compared using t-tests.
In the presence of significant ANOVA F scores, individual comparisons were evaluated with simple main effect tests or Neuman-Keuls comparisons (p<0.05).
3. Results
3.1. Within-Strain Differences in Sucrose- and Fructose-Conditioning
The evaluation of sucrose- and fructose-CFP was examined within each strain for each of the three experiments. The training (Table 1) and test data (Figure 1) are presented in order of the sweet sensitive inbred (SWR, SJL, B10, B6), sweet sub-sensitive inbred (D2, C3H, D2, 129), and outbred (CD-1) strains.
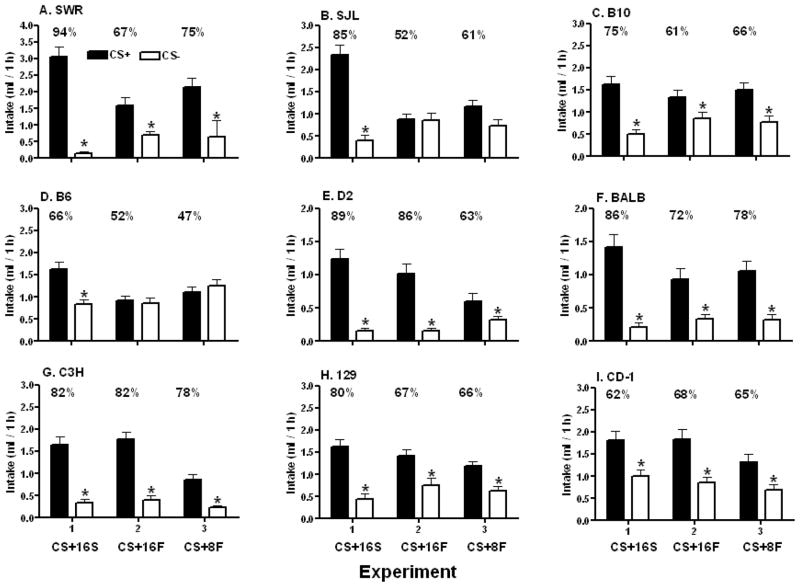
Figure 1.
Intakes (mean +SEM) of CS+ (black bars) and CS− (white bars) flavored saccharin solutions in two-bottle tests in eight inbred (SWR, SJL, B10, B6, D2, BALB, C3H, 129) and one outbred (CD-1) strains of mice. Different animals from each strain participated in three experiments in which mice were trained in one-bottle sessions with differently flavored CS+16% Sucrose and CS− 0.05% saccharin solutions (Experiment 1), with CS+16% Fructose and CS− 0.05% saccharin solutions (Experiment 2), and with CS+8% Fructose + 0.2% saccharin and CS −0.2% saccharin solutions (Experiment 3). In the two-bottle preference tests, the CS+ flavors associated with 16% sucrose, 16% fructose and 8% fructose + 0.2% saccharin (CS+16S, CS+ 16F and CS+8F respectively) as well as the CS− flavors associated with 0.05% (Experiments 1 and 2) and 0.2% (Experiment 3) saccharin were presented in 0.2% saccharin solutions. The percent CS+ intake in each experiment appears above the CS+ bar. The asterisks (*) indicate significant differences between corresponding CS+ and CS− intakes.
SWR Mice
During one-bottle training, the SWR mice consumed significantly more CS− than CS+ in all three experiments (F(2,22)= 58.34, p<0.0001), and their total intakes in the three experiments were ordered as follows: 16% Sucrose > 8% Fructose > 16% Fructose (F(2,22)= 19.85, p<0.0001; Table 1). In the two-bottle tests, significant differences were observed between CS+ and CS− intakes (F(1,22)= 110.99, p<0.0001), among the three experiments (F(2,22)= 4.53, p<0.023) and for the interaction between conditions and experiments (F(2,22)= 11.84, p<0.0003). Intakes of the CS+16S, CS+16F and CS+8F solutions were significantly higher than the intakes of their corresponding CS− solutions (Figure 1A). Overall, the SWR mice displayed significantly (F(2,22)= 11.45, p<0.0004) greater percent CS+ preferences for the CS+16S (94%) than for the CS+16F (67%) and CS+8F (75%) (Figure 1A).
SJL Mice
During one-bottle training, the SJL mice consumed significantly more CS− than CS+ in the two Fructose experiments, but CS intakes failed to differ in the CS+16% Sucrose experiment (CS × Experiment interaction, F(2,27)= 5.20, p<0.05; Table 1). Overall CS intakes differed across experiments: CS+16% Sucrose > CS+8% Fructose > CS+16% Fructose (F(2,20)= 30.55, p<0.0001). In the two-bottle tests, significant differences were observed between CS+ and CS− intakes (F(1,20)= 42.75, p<0.0001), among the three experiments (F(2,20)= 13.64, p<0.0002) and for the interaction between conditions and experiments (F(2,20)= 25.05, p<0.0001). Intake of the CS+16S solution was significantly higher than the CS− solution (Figure 1B). In contrast, intakes of the CS+16F and CS+8F solutions failed to differ significantly from their corresponding CS− solutions (Figure 1B). Overall, the SJL mice displayed significantly (F(2,20)= 16.31, p<0.0001) greater percent CS+ preference for CS+16% Sucrose (85%) than for CS+16% Fructose (52%) and CS+8% Fructose (61%) (Figure 1B).
B10 Mice
In one-bottle training sessions, the B10 mice consumed significantly more CS− than CS+ overall (F(2,26)= 132.92, p<0.0001), and significantly more of the solutions in the 16% Sucrose and 8% Fructose experiments than in the 16% Fructose experiment (F(2,26)= 59.18 p<0.0001; Table 1). In the two-bottle tests, significant differences were observed between CS+ and CS− intakes (F(1,26)= 49.68, p<0.0001), but not among the three experiments or for the interaction between conditions and experiments. Intakes of the CS+16S, CS+16F and CS+8F solutions were significantly higher than the intakes of their corresponding CS− solutions (Figure 1C). Overall, percent intake of CS+16S (75%) was significantly greater (F(2,26)= 3.26, p<0.05) than that for CS+16F (61%) and CS+8F (66%), but post-hoc tests did not reveal significant differences among the individual experiments (Figure 1C).
B6 Mice
Overall, the B6 mice consumed significantly more CS− than CS+ in all three experiments (F(2,27)= 120.44, p<0.001), and more total solution in the 16% Sucrose and 8% Fructose experiments than in the 16% Fructose experiment (F(2,27)= 16.11, p<0.001). In the two-bottle tests, significant differences were observed between CS+ and CS− intakes (F(1,27)= 9.49, p<0.005), among the three experiments (F(2,27)= 6.44, p<0.005) and for the interaction between conditions and experiments (F(2,27)= 13.58, p<0.0001). Intake of the CS+16S solution was significantly higher than the CS− solution (Figure 1D). In contrast, intakes of the CS+16F and CS+8F solutions failed to differ significantly from their corresponding CS− solutions (Figure 1D). Overall, the B6 mice displayed significantly (F(2,27)= 11.08, p<0.0002) greater percent CS+ preference for CS+16S (66%) than for CS+16F (52%) and CS+8F (47%) (Figure 1D).
D2 Mice
During one-bottle training, the D2 mice consumed significantly more CS+ than CS− in all experiments (F(2,23)=17.43, p<0.0001), and consumed significantly more in the 16% Sucrose experiment than in the 16% Fructose and 8% Fructose experiments (F(2,23)= 149.63, p<0.0001; Table 1). In the two-bottle tests, significant differences were observed between CS+ and CS− intakes (F(1,23)= 142.21, p<0.0001), among the three experiments (F(2,23)= 4.87, p<0.017) and for the interaction between conditions and experiments (F(2,23)= 15.70, p<0.0001). Intakes of the CS+16S, CS+16F and CS+8F solutions were significantly higher than the intakes of their corresponding CS− solutions (Figure 1E). D2 mice displayed significantly (F(2,23)= 21.87, p<0.0001) stronger percent CS+ preferences for the CS+16S (89%) and CS+16F (86%) than for the CS+8F (63%) (Figure 1E).
BALB Mice
During one-bottle training sessions, the BALB mice consumed significantly more CS+ than CS− overall (F(2,18)= 63.60, p<0.0001), and significantly more of the solutions in the 16% Sucrose and 8% Fructose experiments than in the 16% Fructose experiment (F(2,18)= 28.37, p<0.0001; Table 1). In the two-bottle tests, significant differences were observed between CS+ and CS− intakes (F(1,18)= 174.82, p<0.0001) and for the interaction between conditions and experiments (F(2,18)= 7.62, p<0.004), but not among the three experiments. Intakes of the CS+16S, CS+16F and CS+8F solutions were significantly higher than the intakes of their corresponding CS− solutions (Figure 1F). Overall, the BALB mice displayed a significantly greater preference (F(2,18)= 6.34, p<0.008) for the CS+16S (86%) than for CS+16F (72%) and CS+8F (78%), but post-hoc tests did not reveal significant individual differences across individual test pairs (Figure 1F).
C3H Mice
During one-bottle training, the C3H mice consumed significantly more CS+ than CS− (F(2,27)= 85.32, p<0.0001), and their total intakes in the three experiments were ordered as follows: 16% Sucrose > 8% Fructose > 16% Fructose (F(2,27)= 69.40, p<0.0001). In the two-bottle tests, significant differences were observed between CS+ and CS− intakes (F(1,27)= 176.73, p<0.0001), among the three experiments (F(2,27)= 38.72, p<0.0001) and for the interaction between conditions and experiments (F(2,27)= 8.18, p<0.002). Intakes of the CS+16S, CS+16F and CS+8F solutions were significantly higher than the intakes of their corresponding CS− solutions (Figure 1G). The C3H mice in the three experiments failed to differ in their percent CS+ preferences for the CS+16S (82%), CS+16F (82%), or CS+8F (78%).
129 Mice
During one-bottle training, the 129 mice consumed significantly more CS+ than CS− in the CS+16% Sucrose experiment but not in the two Fructose experiments (CS × Experiment interaction, F(2,27)=15.74, p<0.0001; Table 1). Overall CS intakes were higher in the 16% Sucrose and 8% Fructose experiments than in the 16% Fructose experiment (F(2,27)= 38.14, p<0.0001). In the two-bottle tests, significant differences were observed between CS+ and CS− intakes (F(1,27)= 74.28, p<0.0001), among the three experiments (F(2,27)= 4.94, p<0.015) and for the interaction between conditions and experiments (F(2,27)= 4.55, p<0.02). Intakes of the CS+16S, CS+16F and CS+8F solutions were significantly higher than the intakes of their corresponding CS− solutions (Figure 1H). Overall, the 129 mice displayed significantly (F(2,27)= 5.26, p<0.012) greater percent CS+ preference for CS+16S (80%) than for CS+16F (66%) and CS+8F (66%) (Figure 1H).
CD-1 Mice
The one-bottle training intakes of CS+ and CS− failed to differ significantly, but overall the CD-1 mice consumed significantly more CS in the 16% Sucrose and 8% Fructose experiments than in the 16% Fructose experiment (F(2,36)= 11.75, p<0.001; Table 1). In the two-bottle tests, significant differences were observed between CS+ and CS− intakes (F(1,36)= 53.69, p<0.0001) and among the three experiments (F(2,36)= 7.28, p<0.002), but not for the interaction between conditions and experiments. Intakes of the CS+16S, CS+16F and CS+8F solutions were significantly higher than the intakes of their corresponding CS− solutions (Figure 1I). Although the CD-1 mice consumed more CS+16S than CS− overall, there was a CS × Test interaction (F(2,36)= 8.85, p<0.0004) and CS+ intakes exceeded CS− intakes in Tests 1 and 2 (2.4 vs. 0.7, 1.8 vs. 1.2 g/h), but not Test 3 (1.2 vs. 1.1 g/h). The percent CS+ intakes also declined over tests (F(2,36)= 7.65, p<0.002) with the preference in Test 1 being higher than that in Test 3 (76 vs. 49%). This loss of CS+16S preference was observed in a first cohort of 10 CD-1 mice, and replicated in a second cohort of nine CD-1 mice, which explains the large sample size in this group. Overall, the percent CS+ preferences in the three experiments failed to differ (Figure 1I), but there was an Experiment × Test interaction (F(4,72)= 5.34, p<0.0008), and in Test 3 the CS+16S (49%) preference was significantly lower than that for CS+16F (69%) and CS+8F (72%).
3.2. Strain Differences in Flavor Conditioning
Table 1 summarizes the detailed strain differences in the intakes of training solutions in the three experiments. In Experiment 1, CS+16S and CS− intakes differed across strains (F(8,81) = 12.38 and 16.41, p<0.001). The D2 mice consumed the least amount of CS+16%S and CS−, whereas the SWR mice consumed the most CS+16%S and CS− of all the strains. In Experiment 2, there were significant strain differences in CS+16F and CS− intakes (F(8,69) = 11.57 and 12.66, p<0.0001). In this case, the B10 and C3H mice respectively consumed the least and most of the CS+16F solution, whereas the D2 and B6 mice consumed the least and most of the CS− solution. There were also significant differences in the CS+8F and CS− intakes in Experiment 3. The D2 mice consumed the least amount of the CS+8F and CS− solutions whereas the BALB mice consumed the most CS+8F, and the SWR mice consumed the most CS− solutions. Given these significant differences in training intakes as well as two-bottle test intakes (data not presented), strain differences in the two-bottle tests were evaluated using the percent CS+ intake scores averaged over the three tests within each experiment.
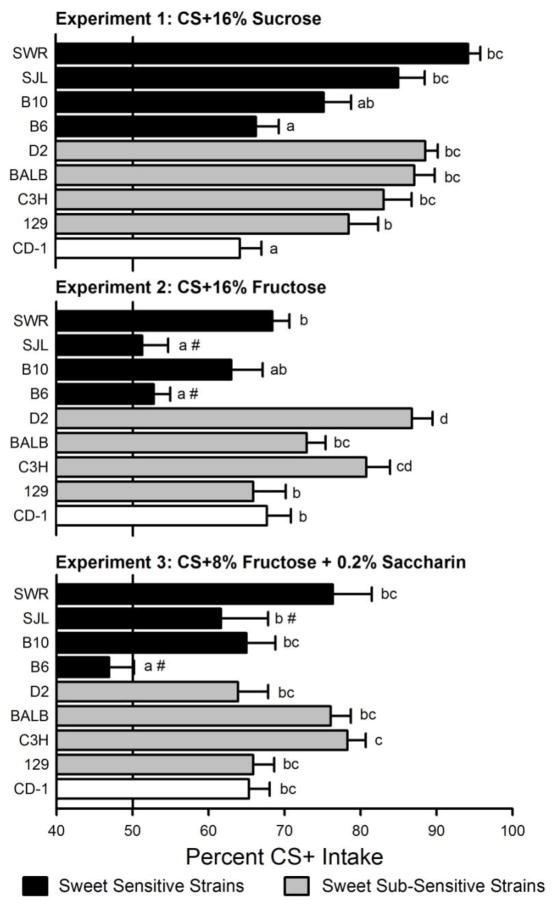
As indicated in Figure 2, there were significant strain differences in the percent intake of the CS+16S (F(8,81)= 11.12, p<0.0001). Several strains (SWR, D2, BALB, SJJ, C3H, 129) displayed robust CS+16S preferences of 80% or greater, whereas one strain (B10) exhibited an intermediate (~75%) preference and two strains (CD-1, B6) had modest (~65%) preferences (Figure 2, upper panel). Significant strain differences were also observed in percent CS+16F intake (F(8,69)= 11.72, p<0.0001). The strain preference profile differed from that observed in the CS+16% Sucrose experiment. In experiment 2, only two strains (C3H, D2) displayed a robust (>80%) CS+16F preference, whereas several others (B10, 129, CD-1, SWR, BALB) exhibited modest (~65–75%) preferences, and two strains (SJL, B6) failed to prefer the CS+16F flavor (Figure 2, middle panel). Finally, there were also significant strain differences in the preference for the CS+8F (F(8,76)= 7.13, p<0.001). In this case, only three strains (BALB, SWR, C3H) displayed preferences greater than 75%, whereas several strains (D2, B6, CD-1, 129) displayed modest (~65%) preferences, and two strains (B6, SJL) failed to prefer the CS+8F flavor (Figure 2, lower panel). The strains also differed in the consistency of their preferences. Thus, some strains showed highly similar conditioned preferences for the CS+16S and CS+16F solutions (e.g., D2: 89% vs. 86%), whereas other strains showed more disparate preferences (e.g., SJL: 85% vs. 51%).
Figure 2.
Percent CS+ intakes (mean +SEM) averaged over the three two-bottle preference tests of the sweet sensitive inbred, sweet sub-sensitive inbred and outbred mice in the three experiments. In Experiment 1 (top panel), mice received one-bottle training with flavored CS+16% Sucrose and differently-flavored CS-0.05% saccharin solutions; in Experiment 2 (middle panel) with flavored CS+16% Fructose and differently-flavored 0.05% saccharin solutions; and in Experiment 3 (bottom panel) with flavored CS+8% Fructose + 0.2% saccharin and differently-flavored CS-0.2% saccharin solutions. In the two-bottle preference tests, the CS+ flavors associated with 16% sucrose, 16% fructose and 8% fructose + 0.2% saccharin (CS+16S, CS+ 16F and CS+8F respectively) as well as the CS− flavors associated with 0.05% (Experiments 1 and 2) and 0.2% (Experiment 3) saccharin were presented in 0.2% saccharin solutions. Percent CS+ preferences were then calculated for each strain in each experiment. Bars that do not share a common letter (a-d) indicate means that differ significantly from each other (p < 0.05) within a particular experiment. A number sign (#) next to the letter indicates that the preference was not significant, i.e., CS+ intake failed to differ from CS− intake.
The conditioned preferences produced by the different sugars were compared in the nine mouse strains. Overall, 16% sucrose conditioned a stronger CS+ preference than did 16% fructose (78% vs. 67%; F(1,8) = 63.92 p<0.001), although three strains (CD-1, D2, C3H) failed to differ in the magnitude of their CS+16S and CS+16F preferences (Sugar × Strain interaction, F(8,150) = 6.33, p<0.0001). The mice also consumed significantly more CS+16% Sucrose than CS+16% Fructose during one-bottle training (1.6 vs. 0.6 ml/hr, F(1,150) = 649.2, p < 0.001), and this was true for all nine strains although the strains differed in their individual intakes (F(1,150) = 7.9, p < 0.001; Table 1). The mice also consumed more of the CS− solution in Experiment 1 than in Experiment 2 even though the CS− solutions in the two experiments were identical (1.4 vs. 0.8 ml/h; F(1,8) = 111.06, p<0.001).
In contrast to the results obtained in Experiments 1 and 2, overall differences in the CS+ preferences conditioned by the 16% and 8% fructose solutions failed to occur (both 67%) in Experiments 2 and 3. The mice did consume significantly more CS+8% Fructose than CS+16% Fructose during one-bottle training (1.2 vs. 0.6 ml/h, F(1,145) = 219.0, p<0.0001), but sugar intakes failed to differ when expressed as calories (0.38 kcal/h). There was one exception, however, in that the D2 mice displayed a stronger preference for CS+16F than CS+8F (87% vs. 64%), and consumed significantly more CS+16% Fructose, in calories, than CS+8% fructose during training (0.36 vs. 0.20 kcal/h). The between strain preference profiles for the CS+16F and CS+8F were more similar than for the CS+16F and CS+16S, but in neither case was there a significant correlation between the percent CS+ scores (Exp. 2 vs. 3, (r= 0.55, ns; Exp. 2 vs. 1, r=0.40, ns).
3.3. Sugar Conditioning in Sweet Sensitive and Sub-Sensitive Strains
Four of the inbred mouse strains (SWR, SJL, B10, B6) included in the present study have the sensitive form of the T1R3 sweet receptor, while the remaining strains (D2, BALB, C3H, 129) have the sub-sensitive form of the receptor. To determine the relationship between T1R3 receptor sensitivity and sugar conditioning, the data from mice with each receptor type were combined and between-group analyses were performed on the one-bottle intake data and two-bottle intake data averaged over the three preference tests within each experiment. A limitation of this approach is that there were significant strain differences within each group (sensitive or sub-sensitive), and the number of mice varied from strain to strain.
Figure 3 summarizes the training and test data obtained with the Sensitive and Sub-Sensitive groups. In Experiment 1, the two groups consumed similar amounts of CS+16% Sucrose during one-bottle training, but the Sensitive group drank significantly more (p<0.01) CS− than did the Sub-Sensitive group (Group × CS interaction, F(1,69)= 79.12, p<0.0001). In addition, whereas the Sensitive mice drank significantly more CS− than CS+ during training, the Sub-Sensitive mice drank significantly more CS+ than CS− (p<0.001) (Figure 3, upper left panel). The mice of both genotypes preferred the CS+ to CS− in the two-bottle tests (F(1,69)= 231.54, p<0.0001) although the Sensitive mice drank significantly more (p<0.001) CS+ than did the Sub-Sensitive mice (Group × CS interaction, F(1,69)= 4.72, p<0.05) (Figure 3, upper right panel). The groups failed to differ in their percent CS+16S intake.
Figure 3.
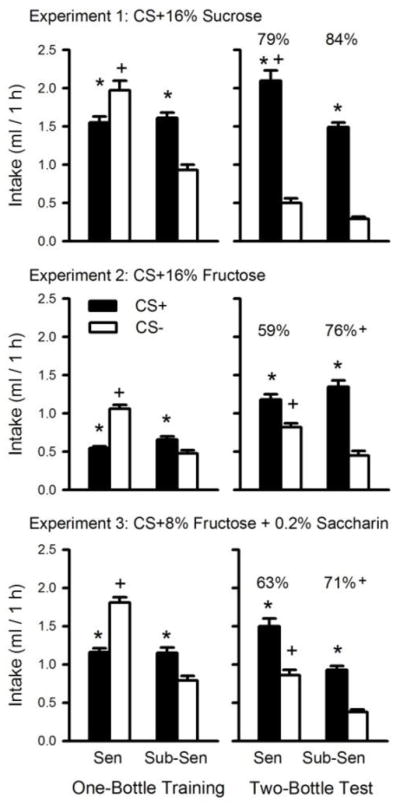
One-bottle training intake (left panels) and two-bottle intakes (mean +sem) averaged over three preference tests (right panel) of sweet sensitive (SEN: SWR, SJL, B10, B6) and sub-sensitive (SUB-SEN: D2, BALB, C3H, 129) inbred mouse strains in the three experiments. In Experiment 1 (top panel), mice received one-bottle training with flavored CS+16% Sucrose and differently-flavored CS-0.05% saccharin solutions; in Experiment 2 (middle panel) with flavored CS+16% Fructose and differently-flavored 0.05% saccharin solutions; and in Experiment 3 (bottom panel) with flavored CS+8% Fructose + 0.2% saccharin and differently-flavored CS-0.2% saccharin solutions. In the two-bottle preference tests, the CS+ flavors associated with 16% sucrose, 16% fructose and 8% fructose + 0.2% saccharin (CS+16S, CS+ 16F and CS+8F, respectively) as well as the CS− flavors associated with 0.05% (Experiments 1 and 2) and 0.2% (Experiment 3) saccharin were presented in 0.2% saccharin solutions. The percent CS+ intake appears above the CS+ bar. The asterisk (*) indicates significant difference between CS+ and CS− intakes within a strain; the plus sign (+) indicates significant difference in absolute CS+ or CS+ intake or percent CS+ intake between SEN and SUB-SEN strains.
In the second experiment, the groups failed to differ in their CS+16% Fructose training intake, but the Sensitive mice drank significantly more (p<0.001) CS− than did the Sub-Sensitive mice (Group × CS interaction, F(1,66)= 92.10, p<0.001). In addition, the Sensitive mice consumed significantly more CS− than CS+16F while the Sub-Sensitive mice consumed significantly more CS+16F than CS− during training (p<0.001) (Figure 3, middle left panel). In the two-bottle tests, both groups consumed significantly more CS+16F than CS− (F(1,66)= 92.58, p<0.001), but the Sensitive mice consumed significantly more (p<0.001) CS− and somewhat less CS+16F than did the Sub-Sensitive mice (Group × CS interaction, F(1,66)= 17.55, p<0.0001). As a result, Sub-Sensitive mice displayed a significantly stronger CS+16F preference than did the Sensitive mice (76 vs. 59%, t(66)= 6.09, p<0.0001) (Figure 3, middle right panel).
The training data of Experiment 3 indicated that the two groups consumed similar amounts of CS+8% Fructose, but that the Sensitive group consumed significantly more CS− than did the Sub-Sensitive group (Group × CS interaction, F(1,73)= 167.00, p<0.0001). Also, whereas the Sensitive mice consumed significantly more CS− than CS+8F, the Sub-Sensitive mice showed the reverse pattern (p<0.001) (Figure 3, lower left panel). Both groups preferred the CS+8F to CS− in the two-bottle tests (F(1,73)= 55.46, p<0.001) but the Sensitive group consumed significantly more of each solution than did the Sub-Sensitive groups (F(1,73)=105.33, p<0.001). Yet, the percent CS+8F intake was greater in the Sub-Sensitive mice than in the Sensitive mice (71 vs. 63%, t(73)= 2.58, p<0.05) (Figure 3, lower right panel). Note that, while as a group, the Sub-Sensitive mice displayed greater percent CS+16F and CS+8F intakes than did the Sweet-Sensitive mice, not all of the individual Sub-Sensitive strains had greater percent intakes than all of the Sensitive strains (Figure 2). However, there was not one Sensitive strain that had a greater percent intake of CS+16F or CS+8F than a Sub-Sensitive strain.
4. Discussion
The present study revealed that mice, like rats, learn to prefer flavors added to sugar solutions over flavors added to nonnutritive saccharin solutions. Marked strain differences were observed in the magnitude of the sugar-CFP as well as in the one-bottle training intakes. Further, in most strains, sucrose conditioned stronger flavor preferences than did fructose, which may be due to differences in the taste and post-oral effects of the two sugars. Finally and surprisingly, mouse strains possessing the sub-sensitive form of the T1R3 sweet receptor displayed stronger fructose-CFP than did sweet sensitive mice. These three major clusters of findings are discussed.
4.1. Strain and Sugar Differences in Conditioned Flavor Preferences
Sucrose was very effective in conditioning flavor preferences, and all nine strains consumed significantly more CS+16S than CS− in the two-bottle tests. Nevertheless, there were significant strain differences with some strains displaying strong (>80%), intermediate (>70%) and moderate (>60%) CS+16S preferences. The strains also differed significantly in their intakes of the CS+16% Sucrose and CS− solutions during one-bottle training, but this was not clearly related to the magnitude of their conditioned preferences. In particular, D2 mice consumed the least amount of CS+16% Sucrose in training, yet displayed the second highest CS+16S preference (89%). Sweet taster status was also not related to the magnitude of the CS+ preference, although, as discussed below, it was related to CS− training intakes.
Marked strain differences were also observed in the two fructose conditioning experiments although the pattern of the strain differences differed from that observed in the sucrose experiment (see Figure 2). As discussed below, the fructose-CFP was related to the sweet taster status of the mice. Overall, 16% sucrose conditioned stronger flavor preferences than did 16% fructose solution (78% vs. 67% CS+ preference) and two of the nine strains (SJL, B6) failed to display a preference for the CS+16F. Two factors may have contributed to the stronger flavor conditioning produced by sucrose. First, prior work indicates that the post-oral action of fructose is less effective than that of sucrose or glucose in conditioning flavor preferences in rats and B6 mice (Sclafani et al., 1999; Ackroff et al., 2001; Sclafani, unpublished data). Second, recent findings indicate that, unlike humans, mice appear to taste sucrose as sweeter than fructose. This is indicated by the finding that several inbred mouse strains (AKR, 129, FVB, B6) licked more for 10% sucrose than 10% fructose during 5-sec tests that measure taste palatability with post-oral effects minimized (Glendinning et al., 2010b). These differences in sugar taste and post-oral actions may also explain why all nine strains in the present experiment drank more CS+16% Sucrose than CS+16% Fructose during 1-bottle training. There was no overall difference in the flavor conditioning response to 16% and 8% fructose solutions (67% vs. 67% CS+ preference) or in the 1-bottle training intakes of the two sugars when expressed as calories to account for the different energy densities of the solutions. The similar flavor conditioning effects of CS+8% Fructose and CS+16% Fructose solutions for most mouse strains may have been due to the addition of 0.2% saccharin to the CS+8% fructose training solution. Prior work indicates that adding saccharin to relatively dilute sugar solutions enhances the palatability of the solution for mice and rats (Glendinning et al., 2010a; Smith et al., 1982). Therefore, the palatability of the CS+8% Fructose + 0.2% saccharin solution may have matched that of the CS+16% Fructose solution. This was not the case for the D2 mice, however, which conditioned a stronger CS+ preference with the 16% Fructose solution.
For most strains, the flavor preferences conditioned by sucrose and fructose were relatively stable with repeated two-bottle testing. One notable exception, however, is that CD-1 mice lost their CS+16S preference by the third preference test whereas their CS+16F and CS+8F preferences did not decline over the repeated tests. Why the CS+16S preference extinguished in the outbred strain but not in any of the inbred strains is not certain. However, the CD-1 mouse findings are similar to prior results obtained with outbred Sprague-Dawley rats (Sclafani & Ackroff, 1994). In this case, rats trained with a flavored CS+8% glucose solution lost their CS+8%G preference with repeated testing whereas rats trained with a CS+8% Fructose solution displayed a persistent CS+8F preference. The authors hypothesized that extinction of the CS+ may be more pronounced in glucose-trained animals because the reward discrepancy between their training and test solutions was more pronounced than that experienced by the fructose-trained animals. That is, when tested with the CS+ flavor presented in a saccharin solution, the glucose-trained animals are missing both the sweet taste and the post-oral reinforcing actions of glucose, whereas the fructose-trained animals are missing only the sweet taste of the fructose.
4.2. Differences in Sugar-Conditioned Flavor Preferences in Sweet-Sensitive and Sweet-Sub-sensitive Strains
Four of the inbred mouse strains (SWR, SJL, B10, B6) used in this study possessed the sensitive form of the sweet receptor while four other strains (D2, BALB, C3H, 129) had the sub-sensitive receptor form. Analysis of the data as a function of sweet receptor status indicated no overall difference between the groups in their CS+16% Sucrose training intakes or their CS+16S preference (79% vs. 84%). Yet, the sensitive strains drank more of the CS− flavored saccharin solution during training than did the sub-sensitive strains consistent with prior reports of differential saccharin preferences in these strains (Reed et al., 2004). In addition, the sensitive strains drank more CS− than CS+ during training while the sub-sensitive strains showed the reverse pattern.
The training intake profiles were the same in the two fructose experiments with the sweet sensitive mice overconsuming the CS− during training compared to the sub-sensitive strains. In contrast, the sweet sensitive and sub-sensitive mice failed to differ in their training intakes of the CS+8% Fructose or CS+16% Fructose solutions. Yet, the sub-sensitive strains displayed stronger preferences for the CS+8F and CS+16F than did the sensitive strains. Since fructose-CFP is thought to be due primarily to the sweet taste of the sugar, it was expected that the sweet sensitive mice would show the stronger fructose-CFP. It may be, however, that the enhanced saccharin preference of the sweet sensitive mice reduced the palatability difference between flavored fructose and saccharin training solutions for the sweet sensitive mice compared to the sub-sensitive mice. According to this interpretation, sweet sensitive mice may display stronger fructose-CFP than sub-sensitive mice if the animals are trained with the CS− flavor added to plain water rather than a saccharin solution. Such conditioning is not feasible when food-restricted animals are given short daily training sessions (e.g., 1 h/day) because they fail to drink the unsweetened CS− solution. However, unsweetened CS− solutions have been used with 23 h/day training sessions because such animals are compelled to drink the solution given that it is the only fluid source (Bonacchi et al., 2008; Fedorchak & Bolles, 1987).
Among the inbred strains included in this study, the conditioning results obtained with the B6 and 129 mice are of particular interest because of their well-documented differences in sweetener intakes and because they are the only two mouse strains evaluated for post-oral sugar conditioning (Sclafani & Glendinning, 2005). In 24-h sweetener vs. water choice tests, B6 mice consumed more sugar (sucrose, maltose) and noncaloric sweetener (saccharin, sucralose, acesulfame) than did 129 mice (Bachmanov et al., 2001; Lewis et al., 2005; Sclafani, 2006a). Consistent with the saccharin results, the B6 mice in the present study drank more of the CS− saccharin solution than did the 129 mice in all three experiments. However, the two strains did not differ in their intake of 8% or 16% fructose, and the 129 mice actually consumed more 16% sucrose than did the B6 mice in the 1 h/day one-bottle training sessions. The latter finding is not unprecedented; we previously observed food-restricted 129 mice to drink more 10% sucrose than B6 mice in 2 h/day tests (Dym et al., 2007; Dym et al., 2009). Furthermore, the 129 mice displayed a stronger CS+16S preference than did B6 mice in the two-bottle tests (see Figure 2). Conceivably, sucrose may have a more potent post-oral conditioning action in 129 mice than B6 mice. However, this is not supported by the results of an IG conditioning study (Sclafani & Glendinning, 2005). When trained with flavored 0.2% saccharin solutions, B6 mice developed a stronger preference for the CS+ paired with IG infusions of 16% sucrose than did 129 mice. However, the B6 also consumed more of the CS solutions during training. When training intakes were equated by adjusting the sweetener concentrations of the CS solutions to create “isosweet” solutions for the two strains, then B6 and 129 mice acquired equally robust CS+ preferences. Nevertheless, the 129 mice displayed a stronger preference than B6 mice in “extinction” tests in which the CS+ flavor was no longer paired with IG sucrose infusions. This is relevant to the present study given that the two-bottle preference tests were extinction tests with the CS+ flavor no longer paired with the sugar solutions.
The B6 and 129 strains also differed in that only the 129 mice displayed conditioned preferences for the fructose-paired CS+ flavors in Experiments 2 and 3. Given that the B6 mice consumed substantially more CS− saccharin solution than CS+ fructose solution in these experiments, it could be that they did not perceive the CS+ fructose solutions as being the sweeter option. However, two-bottle tests (1 h/day) conducted with B6 mice revealed significant preferences for 16% fructose over 0.05% saccharin and for 8% fructose + 0.2% saccharin over 0.2% saccharin (Sclafani, unpublished data). Thus, it is not obvious why B6 mice displayed weaker sugar-conditioned preferences than did 129 mice in the present study. Other findings indicate that, despite their less sensitive sweet taste receptor, 129 mice are more motivated to drink a 16% sucrose solution than are B6 mice as measured by a progressive ratio operant task (Sclafani, 2006b). Thus, motivational factors not directly related to sweet taste sensitivity may contribute to the differential conditioning results obtained with 129 and B6 mice.
4.3. Strain differences in Preference and Aversion Learning
While the above discussion has focused on strain differences in sweet taste sensitivity, obviously other possible strain differences, such as in sensitivity to the odor stimuli in the CS flavors, food motivation, and general learning ability, would influence the ability of mice to acquire sugar-CFP. Of particular relevance to the present study are mouse experiments on conditioned taste aversion which represents another form of flavor learning (Cunningham et al., 2009). Although several inbred strains have been investigated, the most extensively studied are the D2 and B6 strains. As reviewed by Cunningham et al. (2009), several studies report stronger taste aversion conditioning in D2 mice than in B6 mice which would appear to mirror the stronger flavor preferences observed in the present study with D2 mice. However, this is not a universal finding and some studies reported similar taste aversions in B6 and D2 mice trained with sucrose or saccharin solutions paired with LiCl injections (Belknap et al., 1977; Belknap et al., 1978; Dudek & Fuller, 1978). Furthermore, in one case in which B6 mice displayed a weaker conditioned flavor aversion, this was attributed to a reduced sensitivity to the toxic treatment (LiCl) rather than impaired learning ability (Risinger & Cunningham, 2000). Other studies reported stronger or weaker drug-induced conditioned place preferences in B6 mice than D2 mice depending upon the type of drug used (see Risinger & Cunningham, 2000). The variable results obtained with B6 and D2 mice argue against a generalized difference in their learning abilities but rather point to specific differences in their response to the conditioned and unconditioned stimuli employed. This does not exclude the possibility that the differential flavor conditioning observed with other strains may be related to differences in learning ability or motivational processes.
4.4. Conclusion
In conclusion, the present findings revealed significant differences in sugar-based flavor preference conditioning among inbred and outbred mouse strains, and between sucrose and fructose. The sucrose-CFP did not vary as a function of sweet taster status in the inbred strains. In contrast, fructose-CFP did vary as a function of taster status but not in the expected direction. The fructose results, however, may be related to the use of saccharin in the CS− solutions; different findings may be obtained with mice tested with unsweetened CS− solutions. Nevertheless, the current findings demonstrate that sweet taste sensitivity does not necessarily predict sugar-reinforced behavior in mice. Other recent data also show that the sweet taste sensitivity of inbred mouse strains does not predict their short-term licking response to concentrated sugar solutions (Glendinning et al., 2005), their progressive ratio operant response to sucrose solutions (Sclafani, 2006b), or their weight gain response to long-term access to sugar solutions (Glendinning et al., 2010b). Thus, taste sensitivity may determine the ingestive response to non-nutritive sweeteners and dilute sugar solutions, but does not fully account for the intake of sugars or sugar-motivated behavior.
Highlights.
Murine strain differences were found in flavor preferences conditioned by sugars.
Sucrose-conditioned flavor preferences were present, but differed across strains.
Fructose-conditioned flavor preferences occurred in some, but not other strains.
Mice sub-sensitive for sweet taste receptors had a stronger fructose preference.
Thus, genetic variance modulates both innate and conditioned intakes of sugars.
Acknowledgments
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK071761 and DK031135.
The authors thank Karen Ackroff for her helpful comments on this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackroff K, Dym C, Yiin YM, Sclafani A. Rapid acquisition of conditioned flavor preferences in rats. Physiology and Behavior. 2009;97:406–413. doi: 10.1016/j.physbeh.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackroff K, Sclafani A. Flavor preferences conditioned by sugars: Rats learn to prefer glucose over fructose. Physiology and Behavior. 1991;50:815–824. doi: 10.1016/0031-9384(91)90023-h. [DOI] [PubMed] [Google Scholar]
- Ackroff K, Touzani K, Peets TK, Sclafani A. Flavor preferences conditioned by intragastric fructose and glucose: differences in reinforcement potency. Physiology and Behavior. 2001;72:691–703. doi: 10.1016/s0031-9384(01)00442-5. [DOI] [PubMed] [Google Scholar]
- Bachmanov AA, Beauchamp GK. Taste receptor genes. Annual Review of Nutrition. 2007;27:389–414. doi: 10.1146/annurev.nutr.26.061505.111329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Tordoff MG, Beauchamp GK. Sweetener preference of C57BL/6ByJ and 129P3/J mice. Chemical Senses. 2001;26:905–913. doi: 10.1093/chemse/26.7.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker RM, Shah MJ, Sclafani A, Bodnar RJ. Dopamine D1 and D2 antagonists reduce the acquisition and expression of flavor-preferences conditioned by fructose in rats. Pharmacology Biochemistry and Behavior. 2003;75:55–65. doi: 10.1016/s0091-3057(03)00039-x. [DOI] [PubMed] [Google Scholar]
- Baker RW, Li Y, Lee M, Sclafani A, Bodnar RJ. Naltrexone does not prevent acquisition or expression of flavor preferences conditioned by fructose in rats. Pharmacology Biochemistry and Behavior. 2004;78:239–246. doi: 10.1016/j.pbb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Belknap ND, Berg JH, Coleman R. Preabsorptive vs. postabsorptive control of ethanol intake in C57BL/6J and DBA/2J mice. Behavior Genetics. 1977;7:413–425. doi: 10.1007/BF01066776. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Coleman RR, Foster K. Alcohol consumption and sensory threshold differences between C57BL/6J and DBA/2J mice. Physiological Psychology. 1978;6:71–74. [Google Scholar]
- Bernal SY, Dostova I, Kest A, Abayev Y, Kandova E, Touzani K, Sclafani A, Bodnar RJ. Role of dopamine D1 and D2 receptors in the nucleus accumbens shell on the acquisition and expression of fructose-conditioned flavor-flavor preferences in rats. Behavioural Brain Research. 2008;190:59–66. doi: 10.1016/j.bbr.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blizard DA, Kotlus B, Frank ME. Quantitative trait loci associated with short-term intake of sucrose, saccharin and quinine solutions in laboratory mice. Chemical Senses. 1999;24:373–385. doi: 10.1093/chemse/24.4.373. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ, Lewis SR, Kest B. Feeding and drinking. In: Crusio WE, Sluyter F, Gerlai RT, editors. Handbook of Behavioral Genetics in the Mouse, Volume 1: Genetics of Behavioral Phenotypes. Cambridge: Cambridge University Press; 2011. in press. [Google Scholar]
- Bonacchi KB, Ackroff K, Sclafani A. Sucrose taste but not Polycose taste conditions flavor preferences in rats. Physiology and Behavior. 2008;95:235–244. doi: 10.1016/j.physbeh.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughter J, Bachmanov A. Behavioral genetics and taste. BMC Neuroscience. 2007;8:S3. doi: 10.1186/1471-2202-8-S3-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. In: Genetic influences on conditioned taste aversion. Reilly S, Schachtman TR, editors. Oxford: Oxford University Press; 2009. pp. 387–421. [Google Scholar]
- Dudek BC, Fuller JL. Task-dependent genetic influences on behavioral response of mice (Mus musculus) to acetaldehyde. Journal of Comparative and Physiological Psychology. 1978;92:749–758. doi: 10.1037/h0077506. [DOI] [PubMed] [Google Scholar]
- Dwyer DM. The effects of midazolam on the acquisition and expression of fructose- and maltodextrin-based flavour preferences. Pharmacology Biochemistry and Behavior. 2009;91:503–510. doi: 10.1016/j.pbb.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Dwyer DM, Quirk RH. Context conditional flavor preferences in the rat based on fructose and maltodextrin reinforcers. Journal of Experimental Psychology: Animal Behavior Processes. 2008;34:294–302. doi: 10.1037/0097-7403.34.2.294. [DOI] [PubMed] [Google Scholar]
- Dym CT, Pinhas A, Ginzberg M, Kest B, Bodnar RJ. Genetic variance contributes to naltrexone-induced inhibition of sucrose intake in inbred and outbred mouse strains. Brain Research. 2007;1135:136–145. doi: 10.1016/j.brainres.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Dym CT, Pinhas A, Robak M, Sclafani A, Bodnar RJ. Genetic variance contributes to dopamine receptor antagonist-induced inhibition of sucrose intake in inbred and outbred mouse strains. Brain Research. 2009;1257:40–52. doi: 10.1016/j.brainres.2008.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorchak PM, Bolles RC. Hunger enhances the expression of calorie- but not taste-mediated conditioned flavor preferences. Journal of Experimental Psychology: Animal Behavior Processes. 1987;13:73–79. [PubMed] [Google Scholar]
- Fuller JL. Single-locus control of saccharin preference in mice. Journal of Heredity. 1974;65:33–36. doi: 10.1093/oxfordjournals.jhered.a108452. [DOI] [PubMed] [Google Scholar]
- Glendinning JI, Beltran F, Benton L, Cheng S, Gieseke J, Gillman J, Spain HN. Taste does not determine daily intake of dilute sugar solutions in mice. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2010a;299:R1333–R1341. doi: 10.1152/ajpregu.00331.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glendinning JI, Breinager L, Kyrillou E, Kacuna K, Rocha R, Sclafani A. Differential effects of sucrose and fructose on dietary obesity in four mouse strains. Physiology and Behavior. 2010b;101:331–343. doi: 10.1016/j.physbeh.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glendinning JI, Chyou S, Lin I, Onishi M, Patel P, Zheng KH. Initial licking responses of mice to sweeteners: effects of Tas1R3 polymorphisms. Chemical Senses. 2005;30:601–614. doi: 10.1093/chemse/bji054. [DOI] [PubMed] [Google Scholar]
- Golden GJ, Houpt TA. NMDA receptor in conditioned flavor-taste preference learning: Blockade by MK-801 and enhancement by D-cycloserine. Pharmacology Biochemistry and Behavior. 2007;86:587–596. doi: 10.1016/j.pbb.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman EW. Immediate and delayed reinforcers for flavor preferences in the rat. Learning and Motivation. 1975;6:91–100. [Google Scholar]
- Lewis SR, Ahmed S, Dym C, Khaimova E, Kest B, Bodnar RJ. Inbred mouse strain survey of sucrose intake. Physiology and Behavior. 2005;85:546–556. doi: 10.1016/j.physbeh.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Lewis SR, Dym C, Chai C, Singh A, Kest B, Bodnar RJ. Genetic variance contributes to ingestive processes: A survey of eleven inbred mouse strains for fat (Intralipid) intake. Physiology and Behavior. 2007;90:82–94. doi: 10.1016/j.physbeh.2006.08.028. [DOI] [PubMed] [Google Scholar]
- Lush IE. The genetics of tasting in mice. VI. Saccharin, acesulfame, dulcin and sucrose. Genetical Research. 1989;53:95–99. doi: 10.1017/s0016672300027968. [DOI] [PubMed] [Google Scholar]
- Mehiel R, Bolles RC. Learned flavor preferences based on calories are independent of initial hedonic value. Animal Learning and Behavior. 1988;16:383–387. [Google Scholar]
- Pelz WE, Whitney G, Smith JC. Genetic influences on saccharin preference of mice. Physiology and Behavior. 1973;10:263–265. doi: 10.1016/0031-9384(73)90308-9. [DOI] [PubMed] [Google Scholar]
- Reed DR, Bachmanov AA, Beauchamp GK, Tordoff MG, Price RA. Heritable variation in food preferences and their contribution to obesity. Behavior Genetics. 1997;27:373–387. doi: 10.1023/a:1025692031673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed DR, Li S, Li X, Huang L, Tordoff MG, Starling-Roney R, Taniguchi K, West DB, Ohmen JD, Beauchamp GK, Bachmanov AA. Polymorphisms in the taste receptor gene (Tas1r3) region are associated with saccharin preference in 30 mouse strains. Journal of Neuroscience. 2004;24:938–946. doi: 10.1523/JNEUROSCI.1374-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risinger FO, Cunningham CL. DBA/2J mice develop stronger lithium chloride-induced conditioned taste and place aversions than C57BL/6J mice. Pharmacology Biochemistry and Behavior. 2000;67:17–24. doi: 10.1016/s0091-3057(00)00310-5. [DOI] [PubMed] [Google Scholar]
- Sclafani A. How food preferences are learned - laboratory animal models. Proceedings of the Nutrition Society. 1995;54:419–427. doi: 10.1079/pns19950011. [DOI] [PubMed] [Google Scholar]
- Sclafani A. Enhanced sucrose and Polycose preference in sweet “sensitive” (C57BL/6J) and “subsensitive” (129P3/J) mice after experience with these saccharides. Physiology and Behavior. 2006a;87:745–756. doi: 10.1016/j.physbeh.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Sclafani A. Sucrose motivation in sweet “sensitive” (C57BL/6J) and “subsensitive” (129P3/J) mice measured by progressive ratio licking. Physiology and Behavior. 2006b;87:734–744. doi: 10.1016/j.physbeh.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K. Glucose- and fructose-conditioned flavor preferences in rats: Taste versus postingestive conditioning. Physiology and Behavior. 1994;56:399–405. doi: 10.1016/0031-9384(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Fanizza LJ, Azzara AV. Conditioned flavor avoidance, preference and indifference produced by intragastric infusions of galactose, glucose and fructose in rats. Physiology and Behavior. 1999;67:227–234. doi: 10.1016/s0031-9384(99)00053-0. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Glendinning JI. Sugar and fat conditioned flavor preferences in C57BL/6J and 129 mice: Oral and postoral interactions. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2005;289:R712–R720. doi: 10.1152/ajpregu.00176.2005. [DOI] [PubMed] [Google Scholar]
- Smith JC, Foster DF, Bartoshuk LM. The synergistic properties of pairs of sweeteners. In: Barker LM, editor. The Psychobiology of Human Food Selection. Westport CT: Avi Publishing Co Inc; 1982. pp. 123–138. [Google Scholar]
- Stockton MD, Whitney G. Effects of genotype, sugar, and concentration on sugar preference of laboratory mice (Mus musculus) Journal of Comparative and Physiological Psychology. 1974;86:62–68. doi: 10.1037/h0035929. [DOI] [PubMed] [Google Scholar]
- West DB, York B. Dietary fat, genetic predisposition, and obesity: lesions from animal models. American Journal of Clinical Nutrition. 1998;67(Suppl):505S–512S. doi: 10.1093/ajcn/67.3.505S. [DOI] [PubMed] [Google Scholar]