Abstract
Early life stress can elicit long-lasting changes in gene expression and behavior. Recent studies on rodents suggest that these lasting effects depend on the genetic background. Whether epigenetic factors also play a role remains to be investigated. Here we exposed the stress-susceptible mouse strain Balb/c and the more resilient strain C57Bl/6 to a powerful early life stress paradigm, infant maternal separation. In Balb/c mice, infant maternal separation led to decreased expression of mRNA encoding the histone deacetylases (HDACs) 1, 3, 7, 8, and 10 in the forebrain neocortex in adulthood, an effect accompanied by increased expression of acetylated histone H4 proteins, especially acetylated H4K12 protein. These changes in HDAC expression and histone modifications were not detected in C57Bl/6 mice exposed to early life stress. Moreover, a reversal of the H4K12 hyperacetylation detected in infant maternally separated Balb/c mice (achieved with chronic adolescent treatment with a low dose of theophylline that only activates HDACs) worsened the abnormal emotional phenotype resulting from this early life stress exposure. In contrast, fluoxetine, a drug with potent antidepressant efficacy in infant maternally separated Balb/c mice, potentiated all histone modifications triggered by early life stress. Moreover, in non-stressed Balb/c mice, co-administration of an HDAC inhibitor and fluoxetine, but not fluoxetine alone, elicited antidepressant effects and also triggered changes in histone H4 expression that were similar to those provoked by fluoxetine treatment of mice exposed to early life stress. These results suggest that Balb/c mice develop epigenetic modifications after early life stress exposure that, in terms of the emotive phenotype, are of adaptive nature, and that enhance the efficacy of antidepressant drugs.
Keywords: early life stress, mouse, adolescent development, frontal cortex, histone deacetylases, histone H4 acetylation
Introduction
Early life stress is a prominent risk factor for several psychiatric illnesses, including mood and anxiety disorders (Holmes et al., 2005). More than 30% of mental disorders are directly related to early life stress (Afifi et al., 2008; Green et al., 2010). Early life stress (childhood abuse and neglect, loss of parents, or extreme poverty) occurs worldwide and cannot be eliminated. Hence, developing therapies that prevent the long-term consequences of early life stress is of utmost importance, and necessitates a better understanding of the mechanisms by which early life stress triggers long-lasting alterations in gene expression and behavior.
While early-life stress effects on adult psychopathology may depend upon genetic risk, it is thought that the nature of gene and environment interaction is a critical determinant of outcome. In animal studies on the effect of early life stress, inbred strains of mice proved particularly useful because of their natural genetic variability and their stable behavioral differences at baseline and after stress exposure. This is best documented for the isogenic strains C57Bl/6 and Balb/c that differ not only in their sensitivity to early life and adult stress (Holmes et al., 2005; Millstein and Holmes, 2007) but also in their susceptibility to develop frontal cortical gene expression changes (Bhansali et al., 2007; Navailles et al., 2010; Schmauss et al., 2010) and deficits in cognitive functions governed by the frontal cortex (Mehta and Schmauss, 2011) after early life stress exposure. Originally, such differences were widely assumed to relate to genetic differences between both strains. However, it has now been shown that the behavioral traits of both strains (Francis et al., 2003) as well as the neuroendocrine abnormalities that result from early life stress exposure (Murgatroyd et al., 2009) are also influenced by epigenetic mechanisms. This has led to the hypothesis that the influence of early environmental factors on the chromatin structure of certain genes is critical in the development of stable changes in gene expression and behavior (Dulac, 2010).
Here we asked whether either the mouse strain responsive to early life stress (Balb/c) or the resilient strain (C57Bl/6) exhibits changes in chromatin modifications during adolescent development that influence the severity of the impact of early life stress on adult psychopathology. We used a powerful paradigm of early life stress for rodents, infant maternal separation, to test whether this stress exposure triggers persistent changes in chromatin structure. We focused on histone acetylation, a dynamic process that is controlled by the antagonistic actions of histone acetyltransferases and histone deacetylases (HDACs). The balance between the activities of these enzymes serves as a key regulatory mechanism for gene expression. Histone tail acetylation neutralizes the basic charge of lysine residues and, thereby, unfolds chromatin and almost invariantly activates gene transcription. HDACs remove acetyl groups of histone tails and they can silence transcriptional activity (Kouzarides, 2007; Haberland et al., 2009).
In the present study, we examined the expression of class I and II HDACs and acetylated histone H3 and histone H4 proteins during postnatal development of mice exposed to early life stress. In addition, we used pharmacological tools to manipulate HDAC activity during adolescence, and measured the effects of these treatments on emotive behavior and responsiveness to adolescent antidepressant treatment with fluoxetine.
Materials and methods
Animals
Balb/cJ and C57Bl/6J mice were housed in a temperature-controlled (26 ± 2°C) barrier facility with a 12-hour light/dark schedule (lights on at 6:00 AM) and had free access to food and water. All experiments involving animals were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23; revised 1996) and approved by the Institutional Animal Care and Use Committees at Columbia University. During the course of the study, adequate measures were taken to minimize the number of animals used and their suffering.
Infant maternal separation (IMS)
We employed the IMS protocol as previously described (Bhansali et al., 2007). Briefly, offspring of first-time mothers were separated from their dam daily for three hours (from 1:00 to 4:00 PM) from postnatal age day 2 (P2) until P15. Control animals were standard-facility-reared (SFR) pups of first-time mothers. Housing and husbandry conditions were identical for IMS and SFR mice. Pups were weaned at P28 and group housed by sex (five animals randomly selected from at least five different litters). Since this study involved behavioral tests of emotive phenotypes that are sensitive to differences in the estrus cycle of females, we conducted all studies described below on male mice.
Drug treatments
For chronic drug treatment, drugs were administered via the drinking water. Fluoxetine and theophylline (10−4M) were dissolved in water, and mice consumed about 16 mg/kg and 32 mg/kg, respectively, per day. Suberoylanidide hydroxamic acid (SAHA) was dissolved in DMSO and subsequently diluted ~100 fold in water containing 0.2% sucrose. Mice consumed ~200mg/kg/day of SAHA (Butler at al., 2000) and their controls received drinking water supplemented with the same amount of DMSO and sucrose that SAHA-treated animals received. Some mice were treated with both SAHA and fluoxetine. In these experiments, mice received a single intraperitoneal injection of SAHA (100 mg/kg) at P35 followed by SAHA treatment via the drinking water for the next 3 days. Then, fluoxetine (16 mg/kg/day) was added to the drinking water and the combined SAHA and fluoxetine treatment was continued until P60.
Behavioral tests
Mice were exposed to the Elevated Plus Maze (EPM) for 5 min as previously described (Mehta and Schmauss, 2011). Their times spent in open arms and the number of arm crossings was recorded. Three days later, mice were tested in a modified version of Forced Swim Test (FST), i.e., a 6 min exposure on day 1 followed by another 6 min exposure on day 2. On both days, the number of passive episodes and their duration (in sec) were recorded during the last 4 min of FST exposure (see Bhansali et al., 2007), and the results obtained from the day 2 exposure were compared between the different treatment groups.
Real-time RT- PCR
Total RNA, extracted from freshly dissected neocortical tissue of the forebrain (whose caudal border is the mesodiencephalic junction), hippocampi, and striatae via guanidine/cesium chloride ultracentrifugation, served as a template for first-strand cDNA synthesis using Murine Moloney Leukemia Virus reverse transcriptase (USB, Cleveland, OH). Real time PCR was performed using the iQ Real Time PCR detection System (Bio-Rad, Hercules, CA) and SYBR Green (Bio-Rad). The primers for amplification of HDAC mRNAs, shown in Table 1, were designed such that they fit a single PCR protocol with a transcript length of 50–100 base pairs. Cycle thresholds (Ct) of amplification (normalized to β-actin whose Ct values did not differ between groups) were expressed as 1/2ΔCt values, i.e., higher numbers reflect higher expression.
Table 1.
List of primers used for real-time quantitative RT-PCRa
| mRNA | Accession # | Forward primer | Reverse primer |
|---|---|---|---|
| HDAC1 | NM_008228 | 5’-CACGCCAAGTGTGTGGAGTT-3’ | 5’-CATCAGCATGGGCAAGTTGA-3’ |
| HDAC2 | NM_008229 | 5’-GCTGCGACTGGCTTCAACTAC-3’ | 5’-GCTGCTTGGCTTCACTAGGC-3’ |
| HDAC3 | NM_010411 | 5’-GTGATCGATTAGGCTGCT-3’ | 5’-ATTCCCCATGTCCTCGAATG-3’ |
| HDAC4 | NM_207225 | 5’-CCTCGAGAATGTGATCAGGGA-3’ | 5’-GGCCTTGACGTTTGAGAGCA-3’ |
| HDAC5 | NM_010412 | 5’-GGAGGACTGCATTCAGGTCAA-3’ | 5’-TCATCAGGACCACTCTCGCC-3’ |
| HDAC7 | NM_019572 | 5’-CTGGGCAGGTAGTCAGGTCC −3’ | 5’-TTGGGATAGCCGTCAGGGT-3’ |
| HDAC8 | NM_027382 | 5’-GTGCCTGATTGACGGGAAGT-3’ | 5’-CCACCCTCCAGACCAGTTGAT-3’ |
| HDAC9 | NM_024124 | 5’-TCAGCTGAGAGCAGGCTGTG-3’ | 5’-TCAGAAGGGCTGACGGTTG-3’ |
| HDAC10 | NM_199198 | 5’-TGGAGGGTTTCTGAGCCTCA-3’ | 5’-CCATAGGCCAAGGGCAGTAC-3’ |
The nucleotide sequences for the targeted mRNA sequences were derived from the NCBI Data Base using the accession numbers listed here. Details of the RT-PCR experiments are described in the Materials and Methods section.
Western blotting
Immunoblotting was performed as described previously (Levine et al., 2005) with some modifications. Forebrain neocortical tissue was dissected in phosphate-buffered saline supplemented with protease inhibitors and homogenized in 0.5% SDS, protease inhibitor cocktail (Complete Mini, Roche, Germany), 10 mM EDTA, and 10 mM Tris·HCl (pH 8.0). Protein concentration was measured using the NanoDrop instrument (Thermo Scientific, Wilmington, DE). For each sample, fifty micrograms of protein were separated on 15% SDS/PAGE gels, transferred to nitrocellulose membranes (BioRad) and incubated overnight at 4°C with one of the following rabbit polyclonal antibodies from Millipore (Billerica, MA): anti-acetyl histone H4K5 (1:1,000,000), anti-acetyl histone H4K8 (1:100,000), anti-acetyl histone H4K12 (1:20,000), anti-acetyl histone H4K16 (1:100,000), pan histone H4 (1:10,000), pan histone H3 (1:500,000), anti-dimethyl-histone H3 (Lys9) (1:1,000), and anti-trimethyl-histone H3 (Lys4) (1:100,000). For loading controls, an anti-GAPDH antibody was used (Abcam, Cambridge, MA; 1:100,000). After incubation with primary antibody, membranes were incubated with a horseradish-peroxidase-conjugated secondary antibody (anti-rabbit IgG-HRP; Sigma, St. Louis, MO; dilution: 1:5,000) for 1 h at room temperature. SuperSignal West Dura (Thermo Scientific) was used to visualize bound antigen. Optical densities (OD) of histone protein signals were determined using NIH ImageJ software. ODs were normalized to corresponding ODs obtained for GAPDH.
Statistical analysis
For comparisons between two groups of animals (SFR and IMS), two-tailed Student’s t tests were used. For experiments involving multiple groups, one-way analysis of variance ANOVA (effect of postnatal age or treatment) was used, and statistical differences were resolved post hoc using Tukey-Kramer Multiple Comparisons tests. All statistical analyses were carried out using Graph Pad InStat Version 3.0 (GraphPad Software, San Diego, CA).
Results
Biphasic changes in HDAC mRNA expression in the forebrain neocortex of Balb/c mice exposed to early life stress
We used real-time PCR to compare the forebrain neocortical expression levels of mRNA encoding class I and class II HDACs (de Ruitjer et al., 2003) between IMS Balb/c and their SFR controls during postnatal development. At P15 (the end of IMS), none of the HDACs mRNA expression levels examined differed between IMS Balb/c mice and their SFR controls. For the individual HDACs, the 1/2ΔCt values determined in real-time PCR experiments for SFR controls and IMS mice, respectively, were as follows: HDAC 1 (means ± sem): 0.007± 0.002 and 0.008 ± 0.0005; HDAC2: 0.0015 ± 0.0004 and 0.0011 ± 0.0001; HDAC3: 0.043 ± 0.01 and 0.038 ± 0.0016; HDAC4: 0.015 ± 0.004 and 0.014 ± 0.001; HDAC5: 0.028 ± 0.004 and 0.028 ± 0.006; HDAC7: 0.0033 ± 0.001 and 0.0037 ± 0.001; HDAC8: 0.0027 ± 0.001 and 0.0020 ± 0.0001; HDAC9: 0.0027 ± 0.0008 and 0.0026 ± 0.0004; HDAC10: 0.0038 ± 0.001 and 0.0049 ± 0.001).
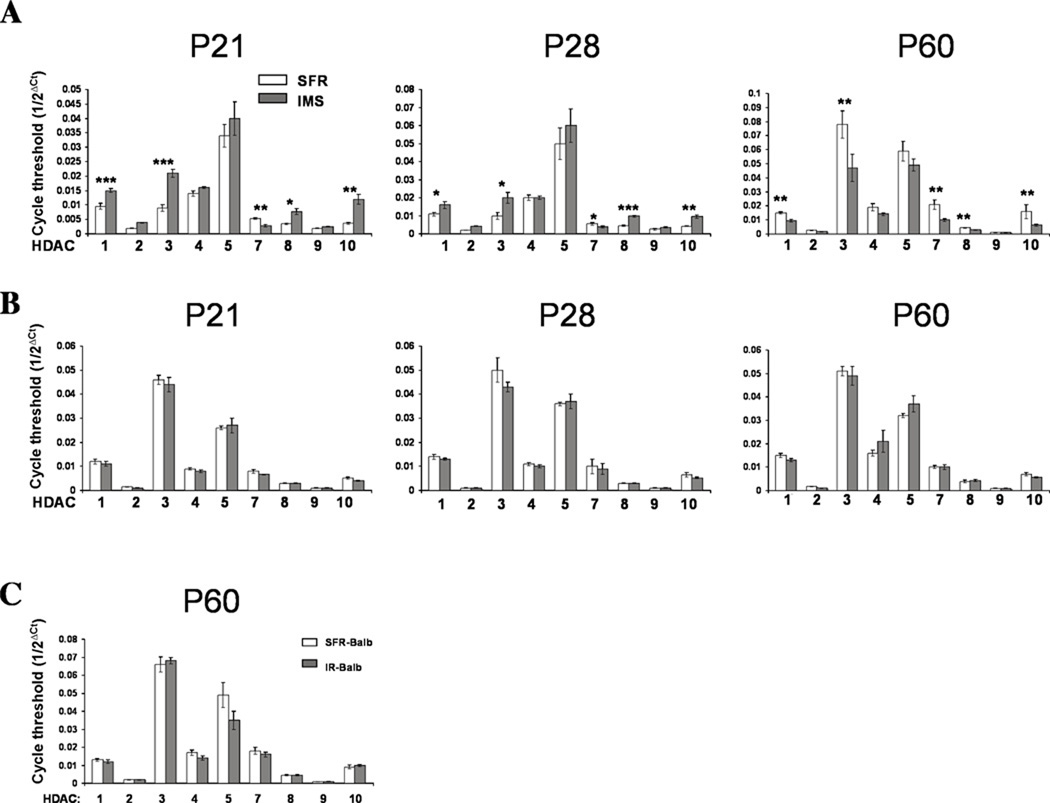
However, significant differences in HDAC mRNA expression emerged at P21 (early adolescence): While the expression of HDACs 2, 4, 5, and 9 did not differ between SFR controls and IMS Balb/c mice, HDACs 1, 3, 8 and 10 were expressed at significantly higher levels in IMS mice relative to SFR controls, an effect that was still detected at P28 (Fig. 1A). In addition, HDAC7 mRNA expression was also affected, but its expression was significantly lower in IMS Balb/c mice at these postnatal ages (Fig. 1A).
FIGURE 1. Altered forebrain neocortical HDAC mRNA expression in IMS Balb/c mice.
(A), Comparison of HDAC mRNA expression in the forebrain neocortex of SFR and IMS Balb/c mice at P21, P28 and P60. (B), HDAC mRNA expression in the hippocampus of SFR and IMS Balb/c mice at P21, P28, and P60. (C), HDAC mRNA expression in the forebrain neocortex of SFR Balb/c mice and Balb/c mice reared in isolation (IR) during adolescent development. mRNA expression levels were determined by real-time PCR. Data are mean ± sem of 5 to 7 animals per group and were compared by two-tailed Student’s t test. *p<0.03, **p<0.003, ***p<0.001.
A different result was obtained at later postnatal ages. At P35 (the end of early adolescence), the expression levels of several HDAC mRNAs became lower in IMS mice compared with SFR controls. Specifically, two-tailed Student’s t tests revealed lower expression of HDAC1 (p<0.01), HDAC3 (p<0.03), HDAC7 (p<0.03) and HDAC8 (p<0.03) and also decreased expression of HDAC 10 that did not reach significance (p=0.096). This change in HDAC mRNA expression persisted into adulthood. As shown in Fig. 1A, P60 IMS Balb/c mice exhibited significantly lower mRNA expression of HDACs 1, 3, 7, 8, and 10 than SFR controls, but, like at P21, the expression of HDACs 2, 4, 5, and 9 was unaltered (Fig. 1A).
Interestingly, for SFR controls, a comparison of the mRNA expression levels of all 9 HDACs at defined postnatal ages (P15, P21, P28, P35, and P60) by one-way ANOVA revealed significant developmental changes in expression of the 5 HDACs affected by early life stress (HDAC1: F(4,22)=6.712, p=0.0011; HDAC3: F(4,22)=8.94, p=0.0002; HDAC7: F(4,22)=6.306, p=0.0015; HDAC8: F(4,22)=8.34, p=0.0003; HDAC10: F(4,22)=6.305, p=0.0015). Specifically, HDAC1 and HDAC8 mRNA levels increased gradually after P15 to reach mature levels at P35 (mid-adolescence). HDAC3 mRNA was expressed at the highest levels at P15 and P60, but exhibited significantly lower expression between P21 and P35 (early to mid-adolescence). HDAC7 and HDAC10 reached their highest expression levels only by P60. In contrast, while all HDACs affected by IMS exposure undergo expression changes during normal postnatal development, the majority of the unaffected HDACs do not. One exception is HDAC5 mRNA (ANOVA, F(4,22)=9.574, p=0.0001) which, like HDACs 7 and 10, reached highest expression levels at P60.
In IMS Balb/c mice, altered HDAC mRNA expression did not occur throughout the brain: In contrast to the expression changes in the forebrain neocortex (Fig. 1A), none of the nine HDACs examined had altered expression in the hippocampus, neither between P21 and P28 nor at P60 (Fig. 1B), and the same was found for the striatum (not shown). Moreover, even in the forebrain neocortex, HDAC mRNA expression was unaffected in Balb/c mice that were exposed to a powerful adolescent stressor, namely isolation rearing from P28 to P60 (Fig. 1C) indicating that the timing of stress exposure during postnatal development (i.e., infancy versus adolescence) is a critical determinant of the effect of stress on forebrain neocortical HDAC mRNA expression.
Altered histone modifications in IMS Balb/c mice
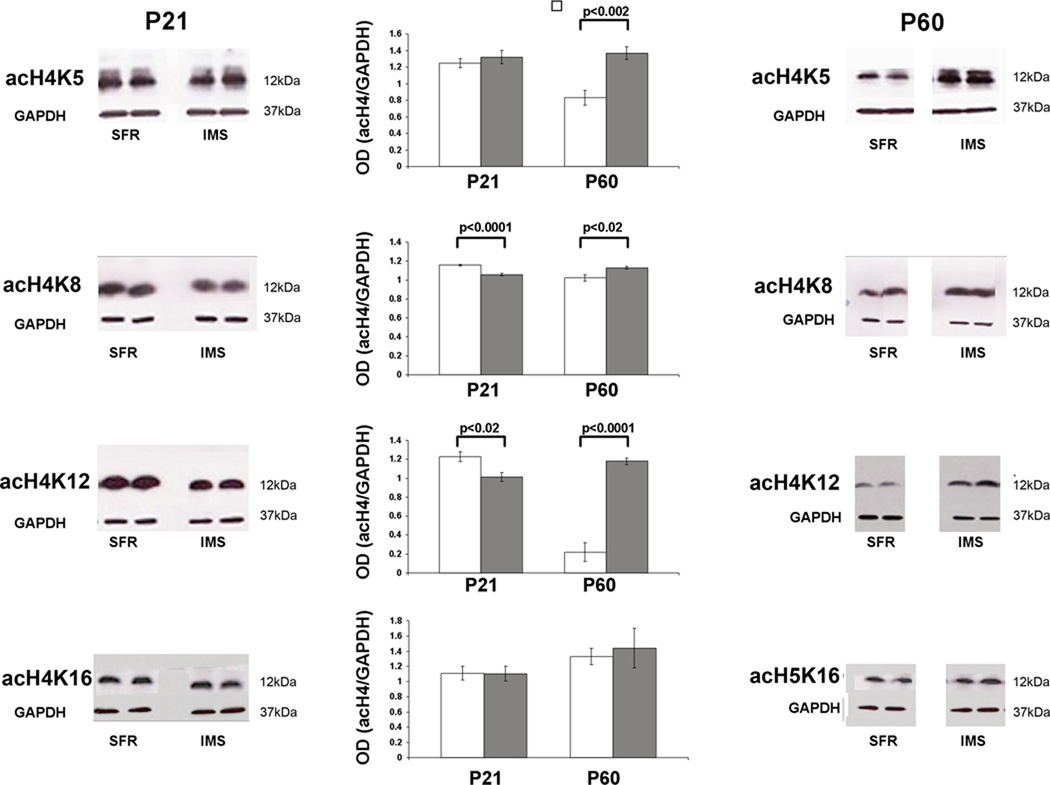
If changes in HDAC mRNA expression lead to changes in HDAC activity that is functionally relevant, changes in histone acetylation must occur. To test this, we used Western blots to measure the expression of acetylated histone H4 and H3 protein in the forebrain neocortex. As expected, no differences were found between SFR controls and IMS Balb/c mice at P15 (not shown), but altered histone modifications became evident at P21. As shown in Fig. 2, like the biphasic changes in HDAC mRNA expression in IMS Balb/c mice, the expression of histone H4 protein acetylated at lysine (K) 8 and K12 was also altered in the biphasic manner: Expression significantly dropped at P21 and significantly increased at P60 relative to SFR controls. Moreover, at P60, H4 protein acetylated at K5 was also increased, but the expression of H4 protein acetylated at K16 was unaffected (Fig. 2).
FIGURE 2. Expression of acetylated histone H4 protein at P21 and P60.
Representative Western blots are shown that were probed with antibodies directed against the indicated histone H4 modifications. The bar graphs summarize results of densitometry measures of optical densities (OD) of enhanced luminescent signals that were normalized to corresponding ODs of GAPDH signals. White bars: SFR controls; gray bars: IMS mice. Data are mean ± sem of 5 animals per group and were compared by two-tailed Student’s t test.
The changes in histone H4 acetylation in IMS Balb/c mice occurred in the absence of changes in total H4 protein expression, and neither the expression of total histone H3 protein nor the expression of H3 protein acetylated at K9 were altered in these mice (Table 2). In addition, since histone H3 methylation could facilitate H4 acetylation (Wang et al., 2009), we also measured expression levels of di- and tri-methylated H3 protein at P21 and P60. As shown in Table 2, there was no change in either form of methylated H3 protein expression at P21, and at P60, the trimethylated H3 protein expression was also not significantly altered in IMS mice. Only the expression of dimethylated H3 was increased in P60 IMS Balb/c mice.
TABLE 2.
Expression of total histone H3 and H4 protein and acetylated or methylated H3 protein in the forebrain neocortex of SFR and IMS Balb/c mice at postnatal ages P21 and P60a.
| P21 | P60 | |||
|---|---|---|---|---|
| SFR | IMS | SFR | IMS | |
| H4 | 0.92 ± 0.03 | 0.80 ± 0.06 | 0.78 ± 0.09 | 0.83 ± 0.05 |
| H3 | 1.50 ± 0.11 | 1.74 ± 0.11 | 0.90 ± 0.13 | 0.83 ± 0.06 |
| acH3K9 | 0.56 ± 0.06 | 0.67 ± 0.02 | 0.79 ± 0.02 | 0.76 ± 0.02 |
| H3me2 | 0.88 ± 0.03 | 0.89 ± 0.03 | 0.75 ± 0.02 | 0.88 ± 0.01* |
| H3me3 | 1.57 ± 0.09 | 1.59 ± 0.10 | 1.11 ± 0.03 | 1.19 ± 0.05 |
Data are mean ± sem of optical densities on Western blots (n=6 per group) that were normalized to GAPDH optical densities and compared by two-tailed Student’s t test.
p<0.002 compared to SFR P60 mice.
In summary, in the forebrain neocortex of IMS Balb/c mice, changes in histone H4 acetylation follow the biphasic developmental changes that we observed for the expression of HDAC mRNA. This was most evident for the expression of acetylated histone H4K12 protein. In contrast to the biphasic changes in histone H4 acetylation, increased dimethylation of H3 protein was only observed at P60.
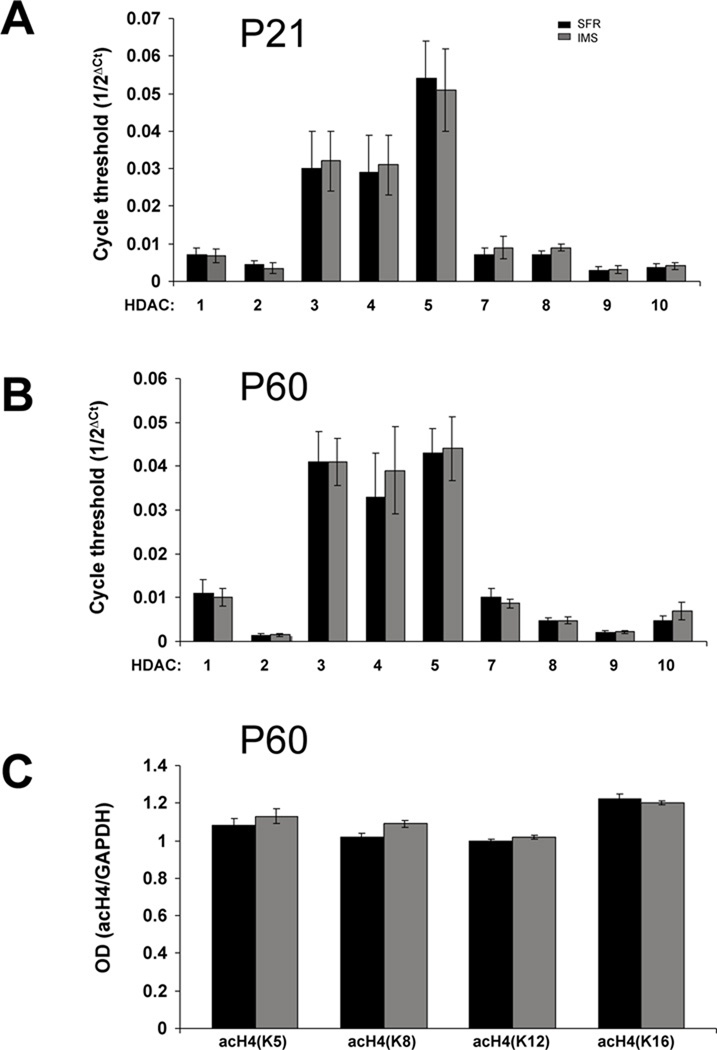
Unaltered HDAC expression and histone modifications in the forebrain neocortex of IMS C57Bl/6 mice
In contrast to the biphasic changes in HDAC expression detected in P21 and P60 IMS Balb/c mice, HDAC mRNA expression was unaltered in IMS C57Bl/6 mice at these ages (Fig. 3A,B). Moreover, none of the changes in histone modifications detected in IMS Balb/c mice were detected in C57Bl/6 mice (Fig. 3C, Table 3). Thus, HDAC-triggered changes in post-translational histone modifications are strain-specific and, interestingly, occur in the stress-susceptible strain and not in the resilient strain.
FIGURE 3. HDAC mRNA expression in the forebrain neocortex of SFR and IMS C57Bl/6 mice.
(A), HDAC mRNA expression at P21. (B), HDAC mRNA expression at P60. (C), Expression of acetylated histon H4 proteins at P60. Data are mean ± sem of 5–6 animals per group. mRNA expression levels were determined by real-time PCR. Two-tailed Student’s t tests revealed no significant differences between groups.
TABLE 3.
Expression of total histone H3 and H4 protein and acetylated or methylated H3 protein in the forebrain neocortex of SFR and IMS C57Bl/6 mice at P60a.
| SFR | IMS | |
|---|---|---|
| H4 | 0.98 ± 0.02 | 1.00 ± 0.03 |
| H3 | 0.97 ± 0.02 | 0.98 ± 0.02 |
| acH3K9 | 1.00 ± 0.02 | 1.00 ± 0.03 |
| H3me2 | 0.85 ± 0.02 | 0.89 ± 0.03 |
| H3me3 | 0.90 ± 0.032 | 0.94 ± 0.02 |
Data are mean ± sem of optical densities on Western blots (n=5 per group) that were normalized to GAPDH optical densities. Two-tailed Student’s t tests revealed no significant differences between groups.
There are two notable differences between SFR control mice of both strains at P60: One is that, although the majority of HDAC mRNAs was expressed at equal levels, HDAC3 mRNA was roughly twice as abundant in Balb/c mice. In P60 IMS Balb/c mice, however, the reduction of HDAC3 mRNA expression led to mRNA levels that were similar to those found in SFR and IMS C57Bl/6 mice (Figs. 1 and 3). In addition, while the majority of the histone variants were expressed at equal levels in SFR controls of both strains, there was a difference in the levels of acetylated H4K12 protein: Its expression was 3-fold lower in Balb/c mice and, strikingly, only the increased expression of acetylated H4K12 in IMS Balb/c mice led to expression levels comparable to that of C57Bl/6 mice. Thus, in IMS Balb/c mice, changes in expression of two epigenetic modulators (HDAC3 and acetylated H4K12) abolished the differences in expression normally found between SFR controls of both strains. These data suggest that the resilience of C57Bl/6 mice to early life stress exposure could, at least in part, be due to their lower levels of HDAC3 expression and their higher levels of acetylated H4K12. However, further studies are needed to test this directly.
Chronic activation of HDACs in adolescent IMS Balb/c mice decreases the adult histone H4K12 hyperacetylation phenotype
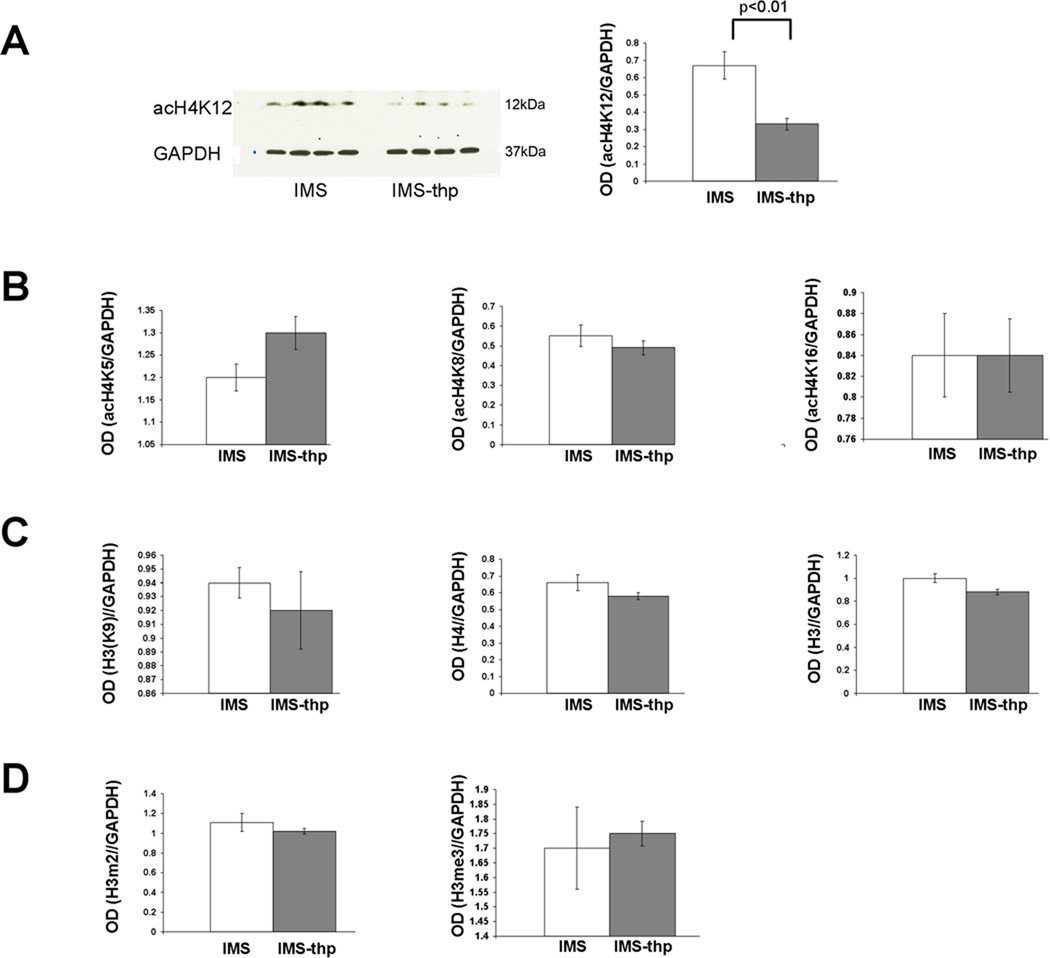
To test whether the increased histone H4 acetylation found in IMS Balb/c mice can be reversed by stimulating HDACs during mid- to late adolescence, we treated IMS Balb/c mice chronically with theophylline (10−4M in drinking water) from P35 to P60. Thus, over 24 h of theophylline-supplemented drinking water consumption, the plasma concentrations of theophylline are below 10−4M, i.e., a concentration at which theophylline activates HDACs (specifically HDAC1 and HDAC3, but not HDAC2) but exerts no antagonist effect on adenosine receptors or inhibition of phosphodiesterases (Ito et al., 2002).
In IMS Balb/c mice, this theophylline treatment did not alter the expression levels of any of the nine HDAC mRNAs studied here (not shown). However, it significantly reduced expression of acetylated H4K12 protein compared to non-treated IMS controls (Fig. 4). The expression of acetylated histone H4K5, H4K8, H4K16 (Fig. 4B), acetylated histone H3K9 and total H3 and H4 protein (Fig. 4C), and the expression of di- and trimethylated H3 protein remained unaltered (Fig. 4D). Thus, theophylline reversed the most prevalent histone modification resulting from early life stress exposure, namely increased acetylation of histone H4K12.
FIGURE 4. Effect of theophylline on histone acetylation in IMS Balb/c mice.
(A), Effect of theophylline on histone H4K12 acetylation. A representative Western blot is shown on the left. (B), Corresponding effect on histone H4K5, K8, and K16 acetylation. (C), Effect of theophylline on total H3 and H4 protein. The bar graphs show ODs of enhanced luminescent signals that were normalized to corresponding ODs of GAPDH signals. White bars: SFR controls; gray bars: IMS mice. Data are mean ± sem of 4 animals per group and were compared by two-tailed Student’s t test. tph= theophylline.
The emotional phenotype of theophylline-treated IMS Balb/c mice
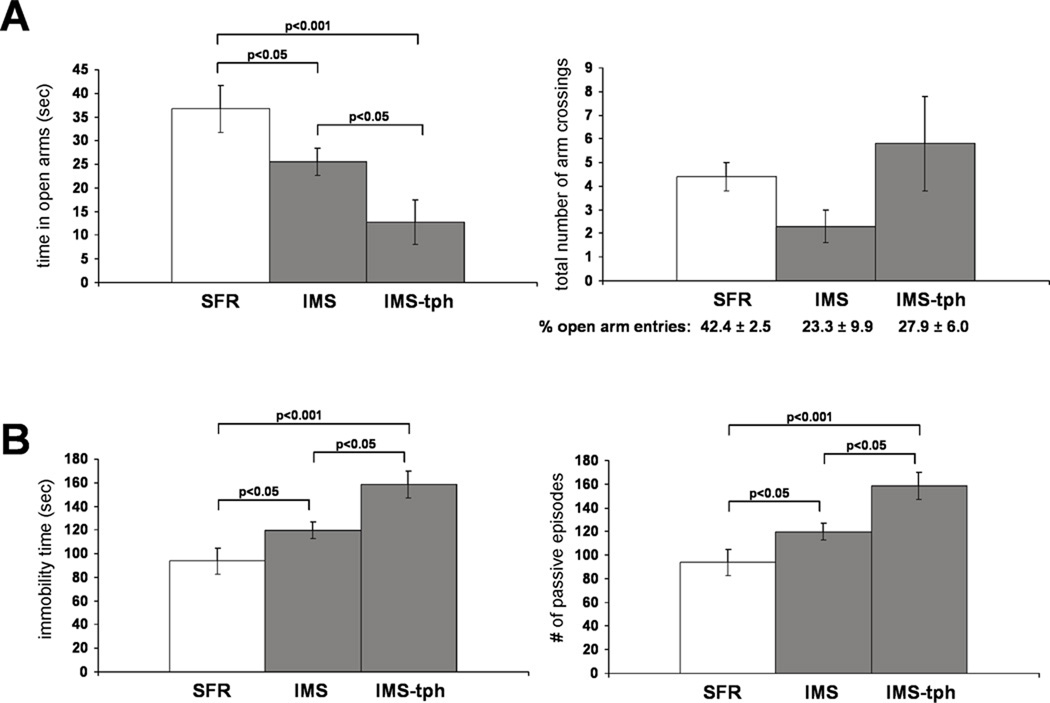
The reversibility of the most prominent IMS-induced histone modification by adolescent theophylline treatment enabled us to ask whether the hyperacetylated histone H4K12 phenotype is an adaptive or mal-adaptive epigenetic process in terms of the behavioral phenotype. Since we have previously shown that adult IMS Balb/c mice exhibit increased anxiety-like behavior in the Elevated Plus Maze (EPM) test as well as depression-like behavior in the Forced Swim test (FST) (Bhansali et al., 2007, Mehta and Schmauss, 2011), we tested whether IMS Balb/c mice treated with theophylline during mid- to late adolescence (P35 to P60) exhibit differences in these behavioral phenotypes when compared to non-treated IMS mice. A one-way ANOVA revealed significant differences in the total time spent in open arms of the EPM between SFR controls, IMS mice, and theophylline-treated IMS Balb/c mice (F(2,17)=7.288, p=0.0052). Consistent with our previous findings, IMS Balb/c mice spent significantly less time in the open arms compared with SFR controls (Fig. 5A). Strikingly, theophylline-treated IMS Balb/c mice exhibited a more severe anxiety-like phenotype, i.e., they spent significantly less time in the open arms than non-treated IMS mice (Fig. 5A). There was no significant difference in the total number of arm crossings between the three group of mice (ANOVA, F(2,20)=1.74, p=0.20) indicating that the difference in times spend in open arms is not due to decreased locomotor activity of non-treated and theophylline-treated IMS Balb/c mice (Fig. 5A). Moreover, although non-treated and theophylline-treated IMS Balb/c mice exhibited lower percentages of crossings into open arms compared with SFR controls, this difference did not reach statistical significance (ANOVA, F(3,20)=3.28; p=0.06) (Fig. 5A). Thus, the main difference between the groups of mice is the total time spent in open arms.
FIGURE 5. The performance of SFR and IMS Balb/c mice and theophylline-treated IMS Balb/c mice in the EPM (A) and FST (B).
Data are mean ± sem of 8 to 10 animals per group and were compared by one-way ANOVA. Statistical differences were resolved post hoc using Tukey-Kramer Multiple Comparisons tests as indicated. For the EPM results shown in A, the total number of open and closed arm entries are illustrated in the graph and the corresponding percentages of open arm entries are listed underneath the graph.
A similar result was obtained in the FST. Although there were no significant differences in the number of passive episodes between SFR controls, IMS mice, and theophylline-treated IMS mice, ANOVA revealed significant differences in the total immobility time (i.e., time spent passively floating) between the three groups of mice F(2,16)=10.26, p=0.0014). As shown in Fig. 5B, IMS Balb/c mice exhibited greater immobility compared with SFR controls, a phenotype that was also significantly potentiated after theophylline treatment.
These data illustrate that the theophylline-induced decrease in acetylated H4K12 expression in IMS Balb/c mice worsened their emotional behavioral phenotype, suggesting that the H4K12 hyperacetylation triggers distinct changes in gene expression that ameliorate the severity of the emotive phenotype resulting from early life stress.
The effect of adolescent fluoxetine treatment on histone modifications in IMS Balb/c mice
We have previously shown that adolescent treatment with the antidepressant drug fluoxetine (a selective serotonin re-uptake inhibitor) effectively reversed the abnormal behavior of IMS Balb/c mice in the FST (Bhansali et al., 2007). Since this is in contrast to the effects of theophylline treatment shown above, we asked whether adolescent treatment with fluoxetine affects either HDAC mRNA expression and/or histone modifications in IMS Balb/c mice in a manner opposite to our theophylline-treatment paradigm. Indeed, although fluoxetine treatment did not alter HDAC mRNA expression at P60 (real-time PCR cycle thresholds (1/2ΔCt (IMS/IMS-fluoxetine, mean ± sem)); HDAC1: 0.0094 ± 0.0001/0.011 ± 0.0006, HDAC2: 0.0017 ± 0.0001/0.0016 ± 0.0001, HDAC3: 0.047 ± 0.006/0.055 ± 0.004, HDAC4: 0.014 ± 0.001/0.011 ± 0.002, HDAC5: 0.049 ± 0.004/0.044 ± 0.006, HDAC7: 0.011 ± 0.001/0.011 ± 0.001, HDAC8: 0.0032 ± 0.0001/0.0035 ± 0.0004, HDAC9: 0.001 ± 0.0002/0.001 ± 0.0002, HDAC10: 0.0065 ± 0.0008/0.0074 ± 0.0006), it triggered changes in histone modifications in the forebrain neocortex of IMS Balb/c that were strikingly different: Fluoxetine increased total histone H3 and H4 protein expression and significantly increased the amounts of acetylated histones H3K9, H4K8, and H4K12 compared to non-treated IMS mice (Table 4). Thus, in contrast to the effects of adolescent theophylline treatment, adolescent fluoxetine treatment globally augmented histone H3 and H4 expression and further elevated the expression of the acetylated histone H4 proteins that were already increased after early life stress exposure alone. While the fluoxetine-induced increase in total histone H3 and H4 protein expression was 60 and 13%, respectively, the increase in acetylated H3K9 and H4K12 proteins was even larger (55 and 230%, respectively). Thus, fluoxetine treatment also altered the ratio of acetylated to non-acetylated protein in favor of increased histone acetylation. Finally, adolescent fluoxetine also increased the expression of trimethylated histone H3 protein, but did not change the expression of dimethylated histone H3 protein (Table 4) that was elevated in IMS Balb/c mice (Table 2).
TABLE 4.
Expression of total and post-translationally modified histone H3 and H4 proteins in the forebrain neocortex of Balb/c mice treated with fluoxetine during adolescencea.
| IMS | IMS-fluoxetine | |
|---|---|---|
| H4 | 0.62 ± 0.05 | 0.97 ± 0.05** |
| acH4K5 | 0.92 ± 0.01 | 0.94 ± 0.02 |
| acH4K8 | 1.10 ± 0.02 | 1.51 ± 0.06* |
| acH4K12 | 0.50 ± 0.11 | 1.16 ± 0.04*** |
| acH4K16 | 0.96 ± 0.03 | 1.04 ± 0.04 |
| H3 | 1.16 ± 0.01 | 1.31 ± 0.03** |
| acH3K9 | 0.78 ± 0.03 | 1.21 ± 0.02*** |
| H3me2 | 1.00 ± 0.08 | 0.93 ± 0.03 |
| H3me3 | 0.61 ± 0.03 | 0.94 ± 0.07* |
Data are mean ± sem of optical densities on Western blots (n=5 per group) that were normalized to GAPDH optical densities and compared by two-tailed Student’s t test.
p<0.02;
p<0.001;
p<0.0007.
Inhibiting HDAC activity increases the antidepressant effects of fluoxetine
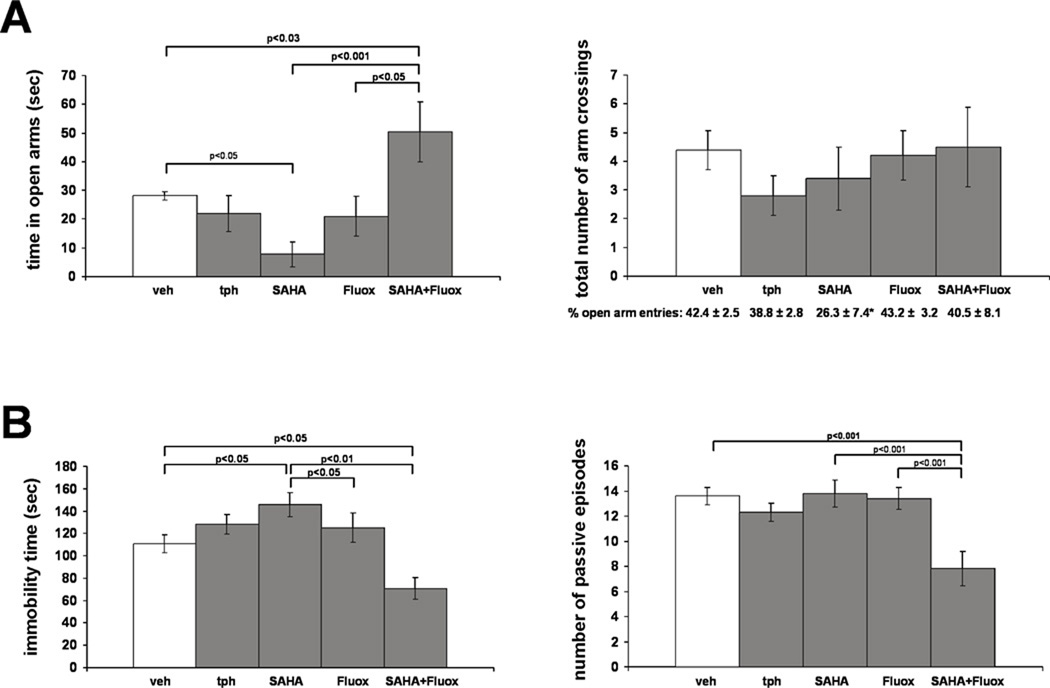
In contrast to the potent antidepressant effects of adolescent fluoxetine treatment detected in IMS Balb/c mice, we have previously shown that the same treatment had no effect on the FST behavior of SFR controls (Bhansali et al., 2007). Since our data from IMS Balb/c mice suggest that their reduced HDAC activity and the resultant histone modifications enhance the antidepressant efficacy of fluoxetine, we asked whether co-treating SFR mice with an HDAC inhibitor and fluoxetine would also enhance the effects of fluoxetine. Thus, we treated SFR mice with the class I/II HDAC inhibitor SAHA (200 mg/kg/day) between P35 and P60 (a treatment known to increase both H3 and H4 acetylation (Butler et al., 2000; Hockly et al., 2003)) either alone or in combination with fluoxetine. We first measured the effects of these treatments on EPM and FST behavior. For comparison, we also included SFR mice treated only with theophylline or fluoxetine during adolescence. Although a one-way ANOVA revealed no significant differences for the total number of arm crossings between non-treated SFR controls and the 4 groups of treated mice (F(4,37)=1.062; p=0.39), there were significant differences in the times spent in the open arms of the EPM (F(4,29)=6.934; p=0.0005): While SFR mice treated only with theophylline or fluoxetine did not differ from non-treated controls and, while SFR Balb/c mice treated only with SAHA spent significantly less time in the open arms, SFR mice treated with SAHA and fluoxetine spent significantly more time in the open arms (Fig. 6A). Moreover, ANOVA revealed no significant differences in the percentages of open arm entries between the different treatment groups (F(2,37)=1.835; p=0.15). It is, however, noted that SAHA-treated SFR mice did exhibited the lowest percentage of open arm entries (see Fig. 6A). Nevertheless, similar to the results shown in Fig. 5, the main difference between the treatment groups resides in the total time spent in open arms.
FIGURE 6. The effect of adolescent SAHA and fluoxetine treatment on the performance of SFR Balb/c mice in the EPM (A) and FST (B).
Data are mean ± sem of 5 to 7 animals per group and were compared by one-way ANOVA. Statistical differences were resolved post hoc using Tukey-Kramer Multiple Comparisons tests as indicated. For the EPM results shown in A, the total number of open and closed arm crossings are illustrated in the graph and the corresponding percentages of open arm entries are listed underneath the graph. Note that SAHA-treated SFR mice exhibit the lowest percentage of open arm entries. When compared to non-treated SFR mice, this difference is significant (two-tailed Student’s t test, p<0.04). tph= theophylline, fluox=fluoxetine.
A similar result was obtained for the FST. ANOVA revealed significant differences between the five treatment groups for both the total time spent in immobility (F(4,42)=3.91; p=0.0087) and the number of passive episodes (F(4,42)=11.481, p<0.0001). Similar to the results obtained from the EPM test, SFR mice treated only with theophylline or fluoxetine did not differ from SFR controls, and SFR Balb/c mice treated only with SAHA exhibited significantly increased immobility in the FST (Fig. 6B). However, compared with all other groups, mice treated with SAHA and fluoxetine exhibited not only significantly decreased immobility but also a significantly reduced number of passive episodes (Fig. 6B).
Altogether, these data illustrate that HDAC inhibition also enhances the antidepressant efficacy of adolescent fluoxetine treatment in non-stressed Balb/c mice.
Increased expression of histone H4 protein in SFR mice treated with SAHA and fluoxetine during adolescence
The enhanced behavioral responsiveness of SAHA-treated SFR mice to fluoxetine treatment prompted us to test whether the SAHA-induced decrease in HDAC activity in SFR mice also affected the expression of histone H3 and H4 proteins in response to fluoxetine treatment, i.e., an effect detected in fluoxetine-treated IMS mice with naturally occurring reduced HDAC activity (Table 4). As shown in Table 5, in SAHA-treated SFR mice, fluoxetine also altered histone modifications, and there is substantial overlap between the fluoxetine-induced changes in SAHA-treated SFR mice and fluoxetine-treated IMS mice: Compared to SFR mice treated only with fluoxetine, mice co-treated with SAHA exhibited significantly increased total histone H4 expression along with increased expression of acetylated H4K5 and H4K12 proteins. Also the expression of acetylated histone H4K8 was increased, but only a trend towards significance (p=0.07) was found for this protein. Thus, while there are subtle differences, in general, fluoxetine affected histone H4 expression similarly in SAHA-treated SFR mice and in IMS mice (Tables 4 and 5) and, also in SAHA-treated SFR mice, the increase in acetylated H4K5 and H4K12 expression (93 and 81%, respectively) was larger than the increase in total H4 expression (31%). In contrast to fluoxetine-treated IMS mice, however, in SAHA-treated SFR mice, fluoxetine did not affect the expression of total H3 and acetylated H3K9 proteins. However, like in fluoxetine treated IMS mice, SAHA-treated SFR mice also exhibited increased expression of trimethylated histone H3 protein (Table 5). Thus, these data suggest that increased expression of acetylated histone H4 and trimethylated histone H3 play a functional role in mediating the behavioral responsiveness to adolescent fluoxetine treatment.
TABLE 5.
Expression of total and post-translationally modified histone H3 and H4 proteins in the forebrain neocortex of SFR Balb/c mice treated with fluoxetine alone or in combination with SAHAa.
| SFR-fluox | SFR-SAHA-fluox | |
|---|---|---|
| H4 | 1.10 ± 0.04 | 1.44 ± 0.11* |
| acH4K5 | 0.45 ± 0.06 | 0.87 ± 0.06*** |
| acH4K8 | 0.86 ± 0.06 | 1.06 ± 0.08 |
| acH4K12 | 0.80 ± 0.03 | 1.45 ± 0.15** |
| acH4K16 | 0.70 ± 0.06 | 0.71 ± 0.09 |
| H3 | 0.96 ± 0.01 | 0.97 ± 0.01 |
| acH3K9 | 0.86 ± 0.03 | 1.15 ± 0.18 |
| H3me2 | 1.08 ± 0.04 | 1.07 ± 0.03 |
| H3me3 | 1.12 ± 0.02 | 1.33 ± 0.03*** |
Data are mean ± sem of optical densities on Western blots (n=5 per group) that were normalized to GAPDH optical densities and compared by two-tailed Student’s t test.
p<0.03
p<0.008
p<0.002.
Discussion
Studies on mice exposed to early life stress and raised to adulthood without further stress exposure can provide valuable insight into the development of adaptive and maladaptive processes that ultimately shape the adult phenotype. We found that, in the stress-susceptible mouse strain Balb/c (but not in the resilient strain C57Bl/6), early life stress elicits biphasic changes in HDAC expression and histone modifications during postnatal development that trigger several post-translational modifications of histone proteins. From those, increased expression of histone H4 acetylated at lysine residue K12 is the most prominent epigenetic phenotype in adulthood. Low doses of the HDAC activator theophylline effectively reduced the expression of acetylated H4K12 protein when administered during adolescence, and worsened two prominent behavioral phenotypes that characterize IMS Balb/c mice. In contrast, the antidepressant drug fluoxetine did not only ameliorate the severity of these behavioral phenotypes resulting from IMS exposure (Bhansali et al., 2007; the present study) but also augmented the histone modifications elicited by early life stress. These findings indicate that the reduced HDAC activity in IMS Balb/c mice is a positive adaptive process that ameliorates the severity of abnormal emotional behaviors.
In the forebrain neocortex of IMS Balb/c mice, changes in HDAC expression and histone modifications emerge in early adolescence (P21), but then exhibit a biphasic developmental pattern. Expression of HDACs 1, 3, 8, and 10 was increased during early (P21–P28) adolescence, but persistently decreased afterwards. Although HDAC7 was consistently decreased between P21 and P60, the increased expression of the HDACs 1, 3, 8, and 10 predominated in modifying the histone H4 acetylation phenotype measured at P21, i.e., acetylation of histone H4K12 and H4K8 was decreased and H4K5 and H4K16 acetylation was unaltered. Conversely, at P60, the expression of all 5 HDACs was lower compared to controls, and acetylation of histone H4K5, H4K8, and H3K12 was significantly increased. Nevertheless, at both developmental ages (P21 and P60), the effect of IMS was largest for the expression of acetylated histone H4K12, which is thought to play a unique role in orchestrating gene expression (Kwang et al., 2007) and has already been shown to be crucially involved in regulating the expression of hippocampal genes that are required for the formation of memories (Peleg et al., 2010). Although we have not measured the developmental profile of modified histone expression between P21 and P59, the data shown in Figs. 1 and 2 clearly show that HDAC expression and expression levels of acetylated histone H4 parallel each other.
The mechanism by which early life stress affects only distinct HDACs is presently unknown and future studies will have to investigate whether the expression of the affected HDACs is also influenced by epigenetic mechanisms. Equally unknown is the reason for the biphasic nature of the HDAC-triggered histone modifications in IMS Balb/c mice. Of note, a similar biphasic change in gene expression has also been found for the glucocorticoid receptor that, in the forebrain neocortex of IMS Balb/c mice, is expressed at higher levels during adolescence but at reduced levels in adulthood (Navailles et al., 2010). Although one possibility could be that early-life stress-induced changes in gene expression during early to mid-adolescence affect critical developmental processes of the forebrain neocortex that ultimately lead to the establishment of the adult phenotype precipitated by early life stress, we found that treating IMS Balb/c mice with the HDAC inhibitor SAHA between P21 and P35 (which prevented the decreased acetylation of histone H4 proteins) did not prevent the development of the adult epigenetic phenotype (A.L. and C.S., unpublished observation) thus, making a dependence of the developing adult phenotype from the P21 phenotype unlikely.
IMS Balb/c mice also exhibited increased expression of dimethylated histone H3. In contrast to the biphasic changes in histone H4 acetylation, however, increased dimethylation of H3 protein was only observed in adulthood. Although dimethylated histone H3 is considered a marker of transcriptional repression, there is also evidence that histone H3 methylation facilitates histone acetylation (Wang et al., 2009; Zhang et al., 2004). Whether there is indeed a functional link between histone H4 acetylation and histone H3 dimethylation, especially between histone H4K12 acetylation and histone H3K9 dimethylation in the adult brain, remains to be demonstrated.
Although we found altered expression of three class I and two class II HDACs in IMS Balb/c mice, the weight of the evidence suggest that the IMS-triggered changes in histone modifications can largely be accounted for by the class I HDACs 1, 3, and 8. The most predominant IMS-specific change in histone H4K12 acetylation was reversible by a low dose of theophylline that has been shown to activate HDAC1 and HDAC3, but not HDAC2 (Ito et al., 2002) without exerting other, possibly confounding, effects on adenosine receptors or phosphodiesterase inhibitors. In addition, HDAC8 has been shown to be crucial in deacetylating histone H4 (and also H3; see Lee et al., 2004). However, whether class I-selective HDAC inhibitors can recapitulate the entire chromatin modification phenotype resulting from IMS remains to be tested. Of note, we also found that, despite their high degree of sequence similarity (de Ruijter et al., 2003), HDAC1, but role of HDAC2 in hippocampal memory and synaptic plasticity have already shown that HDAC1 and HDAC2 are not functionally redundant (Guan et al., 2009).
There are two major roles of HDACs: One is to remove the acetyl groups added by histone acetyltransferases at active genes during transcriptional initiation and elongation, the other is to maintain a reduced level of histone acetylation at, and to prevent RNA polymerase II from binding to, silent genes (Wang et al., 2009). In adult IMS Balb/c mice, histone H4K12 was hyperacetylated, and increased acetylation also occurred for histones H4K5 and H4K8. Such epigenetic marks suggest increased transcriptional activity, and the here-identified histone modifications open the door for chromatin immunoprecipitation-guided identification of the affected genes.
Do the changes in histone modifications described here contribute to the IMS-specific behavioral phenotype or do they ameliorate the severity thereof? The latter possibility is supported by results shown in Fig. 5 indicating that two prominent behavioral phenotypes found in IMS Balb/c mice, namely increased anxiety and increased passivity in stressful environments (Mehta and Schmauss, 2011), are potentiated by a concentration of theophylline that, although effectively reversing the effect of IMS on histone H4K12 acetylation, has no demonstrated effects on adenosine receptors or phosphodiesterase inhibition (Fig. 4) and, as further shown in Fig. 6, had no behavioral effects in SFR controls. In contrast, adolescent fluoxetine has not only been shown to be effective in improving the emotional phenotype of IMS Balb/c mice (Bhansali et al., 2007), it also increased the expression of total H3 and H4 histones, an effect that resulted in a further increase of acetylated H4 (and also histone H3) protein expression in IMS Balb/c mice (Table 4). This finding led us to test directly whether, in non-stressed (SFR) mice, the antidepressant efficacy of fluoxetine can also be increased when HDAC activity is pharmacologically reduced. Indeed, when SFR mice were only treated with fluoxetine during adolescence, their behavioral responses to EPM and FST exposure were unaltered. In contrast, SFR mice treated with the HDAC inhibitor SAHA and fluoxetine during adolescence exhibited significantly less anxiety-like and depression-like behaviors in the EPM and FST tests, respectively (Fig. 6). Moreover, the combined treatment of SAHA and fluoxetine also recapitulated most of the effects of fluoxetine on the expression of histone proteins detected in IMS Balb/c mice, namely increased expression of total histone H4 protein along with increased expression of acetylated histone H4K12, and increased expression of trimethylated histone H3 protein, indicating that these changes in histone expression are critically involved in potentiating the behavioral responsiveness to fluoxetine treatment.
Altogether, our data suggest that reduced HDAC activity and the resultant histone modification are a positive adaptation developing in IMS Balb/c mice, at least in terms of the severity of the emotional phenotype that results from early life stress exposure. Moreover, this epigenetic adaptation enhances the antidepressant effects of adolescent fluoxetine treatment.
The present study is the first to demonstrate a global increase in histone acetylation in the forebrain neocortex of a stress-susceptible strain of mice exposed to early life stress that is paralleled by reduced HDAC expression found in this anatomic region but not in the hippocampus or striatum. It is, at present, not clear whether these epigenetic changes are specific for the forebrain neocortex or whether other anatomic regions implicated in stress responses, such as the amygdala or the hypothalamus, also exhibit decreased HDAC expression after early life stress exposure. Nevertheless, it is evident that HDACs affected by early life stress as well as the anatomic region prominently affected by reduced HDAC expression differ from those previously linked to adult stress (for review see Fischer et al., 2010). This raises several interesting questions: 1. Do early life stress and adult stress affect different HDACs? 2. Do early life and adult stress predominantly affect histone H4 and histone H3 acetylation, respectively? 3. Does stress affect different HDACs in different brain regions, i.e., is the type of HDAC affected determined by the specific function of the brain region? Regardless of the answers to these questions, there is one important common theme to the effects of early life and adult stress on HDAC activity, namely that reducing HDAC activity is an adaptive phenomenon with antidepressant effects. This is supported by the present findings as well as by earlier studies that employed chronic adult stressors and examined the effect of adult antidepressant treatment (Tsankova et al., 2006; Wilkinson et al., 2009). However, it must be stressed that reduced HDAC activity has positive adaptive effects on the behavioral phenotype only in chronically stressed animals. In fact, our data indicate that reducing HDAC activity has deleterious effects on the behavior of SFR control mice, i.e., SFR mice treated with SAHA during adolescence (without fluoxetine co-treatment) exhibited increased anxiety-like behavior in the EPM test and increased depression-like behavior in the FST (Fig. 6). Thus, equal behavioral responses of SFR and IMS mice to EPM and FST exposure depend upon different epigenetic landscapes, with IMS mice requiring a greater ratio of expression of acetylated over non-acetylated H4 histones. Interestingly, the same holds true for the behavioral responses to fluoxetine treatment that are enhanced when increased expression of acetylated histones occurs. Yet, disruption of either the epigenetic phenotype of stressed animals (Fig. 5) or the normal epigenetic signature of non-stressed animals (Fig. 6) leads to the same abnormal anxiety- and depression-like behavioral phenotype.
Conclusions
The present study identified for the first time persistent changes in histone modifications that result from early life stress and that influence emotive behavior and antidepressant treatment response. Balb/c mice exposed to early life stress develop epigenetic modifications that are further augmented by antidepressant treatment with fluoxetine during adolescence. These findings support the emerging concept that, in animal models of chronic stress, HDAC inhibitors have antidepressant effects. They also suggest that subjects with low responsiveness to antidepressant drugs could benefit from a combined treatment with HDAC inhibitors. Importantly, this type of combined treatment – initiated during adolescence – could be the most effective treatment for psychiatric patients with a history of early life stress.
Research Highlights.
Early life stress leads to changes in histone modifications in adulthood
These histone modifications are predominant in the frontal cortex of young adults
The histone modifications blunt the severity of abnormal emotive behavior
The histone modifications enhance responses to adolescent antidepressant treatment
Acknowledgements
We thank Stephen J. Haggarty for his generous gift of SAHA. This work was supported by a grant from the National Institutes of Health MH078993 and Whitehall Foundation grant 2007-12-77 to C.S.
Abreviations
- HDAC
histone deacetylase
- IMS
infant maternal separation
- SFR
standard facility reared
- EPM
Elevated Plus Maze
- FST
Forced Swim Test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afifi TO, Enns MW, Cox BJ, Asmundson GJG, Stein MB, Sareen J. Population attributable fractions of psychiatric disorders and suicide ideation and attempts associated with adverse childhood experiences. Am J Public Healths. 2008;98:946–52. doi: 10.2105/AJPH.2007.120253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhansali P, Dunning J, Singer SE, David L, Schmauss C. Early life stress alters adult serotonin 2C receptor pre-mRNA editing and expression of the alpha subunit of heterotrimeric G protein Gq. J Neurosci. 2007;27:1467–73. doi: 10.1523/JNEUROSCI.4632-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler LM, Agus DB, Scher HI, Higgins B, Rose A, Cordon-Cardo C, et al. Suberoylanidide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res. 2000;60:5165–70. [PubMed] [Google Scholar]
- de Ruijter AJM, van Gennip AH, Caron HN, Kemp S, van Kuilenburg ABP. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003;370:737–749. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulac C. Brain function and chromatin plasticity. Nature. 2010;465:728–35. doi: 10.1038/nature09231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Sananbebesi F, Mungenast A, Tsai L-H. Targeting the correct HDAC(s) to treat cognitive disorders. Trends Pharmacol Sci. 2010;31:605–17. doi: 10.1016/j.tips.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Francis DD, Szegda K, Campell G, Martin WD, Insel TR. Epigenetic sources of behavioral differences in mice. Nat Neurosci. 2003;6:445–46. doi: 10.1038/nn1038. [DOI] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, et al. Childhood adversities and adult psychiatric disorders in the National Comorbidity Survey Replication I: associations with first onset of DSM-IV disorders. Arch Gen Psychiatry. 2010;67:113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan J-S, Haggarty SJ, Giacometti E, Dannenberg J-H, Joseph N, Gao, et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland M, Montgomery RL, Olson EN. The many roles of histone deactylases in development and physiology: Implications for disease and therapy. Nat Rev Gen. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockly E, Richon VM, Woodman B, Smith DL, Zhou X, Rosa E, et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, ameliorates motor deficits in a mouse model of Huntington’s disease. Proc Natl Acad Sci USA. 2003;100:2041–46. doi: 10.1073/pnas.0437870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, le Guisquet AM, Vogel E, Millstein RA, Leman S, Belzung C. Early life genetic, epigenetic, and environmental factors shaping emotionality in rodents. Neurosci Biobehav Rev. 2005;29:1335–46. doi: 10.1016/j.neubiorev.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Ito K, Lim K, Caramori G, Cosio B, Chung F, Adcock IM, Barnes PJ. A molecular mechanism of action of theophylline: Induction of histone deacetylase activity to decrease inflammatory gene expression. Proc Natl Acad Sci USA. 2002;99:8921–26. doi: 10.1073/pnas.132556899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuozarides T. Chromatin Modifications and their Function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Kwan T, Benovoy D, Dias C, Gurd S, Serre D, Zuzan H, Clark, et al. Heritability of alternative splicing in the human genome. Genome Res. 2007;17:1210–18. doi: 10.1101/gr.6281007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Rezai-Zadeh N, Seto E. Negative regulation of histone deacetylase 8 activity by cyclic AMP-dependent protein kinase A. Mol Cell Biol. 2004;24:765–73. doi: 10.1128/MCB.24.2.765-773.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AA, Guan Z, Barco A, Xu S, Kandel ER, Schwartz JH. CREB-binding protein controls response to cocaine by acetylating histones at the fosB promoter in the mouse striatum. Proc Natl Acad Sci USA. 2005;102:19186–91. doi: 10.1073/pnas.0509735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta M, Schmauss C. Strain-specific cognitive deficits in mice exposed to early life stress. Beh Neurosci. 2011;125:29–36. doi: 10.1037/a0021952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millstein RA, Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci Biobehav Rev. 2007;31:3–17. doi: 10.1016/j.neubiorev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Murgatroyd C, Patchev A, Wu Y, Micale V, Bockmühl Y, Fischer D, et al. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. 2009;12:1559–66. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- Navailles S, Zimnisky R, Schmauss C. Expression of glucocorticoid receptor and early growth response gene 1 during postnatal development of two inbred strains of mice exposed to early life stress. Dev Neurosci. 2010;32:139–48. doi: 10.1159/000293989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg S, Sananbenesi F, Zovoitis A, Burkhardt S, Bahari-Javan S, Agis-Balboa RC, et al. Altered histone acetylation is associated with memory impairment in mice. Science. 2010;328:753–56. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- Schmauss C, Zimnisky R, Mehta M, Shapiro LP. The roles of phospholipase C activation and ADAR1 and ADAR2 pre-mRNA splicing in modulating serotonin 2C-receptor editing in vivo. RNA. 2010;16:1779–85. doi: 10.1261/rna.2188110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatine regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–25. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zang C, Cui K, Schones DE, Barski A, Peng W, Zhao K. Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell. 2009;138:1019–31. doi: 10.1016/j.cell.2009.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson MB, Xiao G, Kumar A, LaPlant Q, Renthal W, Sikder D, et al. Imipramine treatment and resiliency exhibit similar chromatin regulation in the mouse nucleus accumbens in depression models. J Neurosci. 2009;29:7820–32. doi: 10.1523/JNEUROSCI.0932-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Siino JS, Jones PR, Yau PM, Bradbury EM. A mass-spectrometric Western blot to evaluate the correlation between histone methylation and histone acetylation. Proteomics. 2004;4:3765–75. doi: 10.1002/pmic.200400819. [DOI] [PubMed] [Google Scholar]