Abstract
D-cycloserine, the glutamate N-methyl-D-aspartate receptor partial agonist, has been reported to facilitate the extinction of learned fears acquired in both naturalistic and laboratory settings. The current study extended this literature by evaluating the ability of either chronic or acute administrations of DCS to modulate the extinction and spontaneous recovery of a conditioned taste aversion (CTA).
Twenty-three hour fluid-deprived Sprague-Dawley rats acquired a strong CTA following 3 pairings of a conditioned stimulus (CS; 0.3% oral saccharin) + unconditioned stimulus [US; 81 mg/kg (i.p.) lithium chloride (LiCl)]. In separate groups of rats, we then employed 2 different extinction paradigms: (1) CS-only (CSO-EXT) in which saccharin was presented every-other day, or (2) Explicitly Unpaired (EU-EXT) in which both saccharin and LiCl were presented but on alternate days. Previous studies have indicated that the EU-EXT procedure speeds up the extinction process. Further, spontaneous recovery of a CTA emerges following CSO-EXT but the EU-EXT paradigm causes a suppression of spontaneous recovery. DCS (15 mg/kg, i.p.) was administered immediately after daily liquid presentations (saccharin or water, alternate days) during the extinction period. In an acute drug manipulation, DCS (15 mg/kg, i.p.) or saline control injections were administered for 4 days only. This was done during one of 3 different phases of extinction [i.e., static (2–5%), early dynamic (8–16%), or middle dynamic (20–40%) saccharin reacceptance]. Other animals assigned to the chronic DCS condition received daily DCS (15 mg/kg, i.p.) throughout extinction. Changes in saccharin drinking in these animals were compared to the data from rats that received no drug (saline controls). Once rats met our criterion for asymptotic extinction (90% reacceptance of the CS) they entered a 30-day latency period during which they received water for 1 hr/day. The day after the completion of the latency period, a final opportunity to drink saccharin was provided (spontaneous recovery test).
Saline-treated control rats that went through the EU-EXT procedure achieved asymptotic extinction more quickly than did the CSO-EXT rats and did not exhibit a spontaneous recovery of the CTA. Chronic DCS treatments did not significantly reduce the time to achieve asymptotic CTA extinction in rats exposed to either CSO or EU extinction methods. Further, animals treated with DCS throughout EU-EXT exhibited a spontaneous recovery of the CTA whereas the saline-treated, EU-EXT rats did not. Thus, chronic DCS treatment did not shorten the time to extinguish a CTA and this treatment eliminated the ability of EU-EXT to block spontaneous recovery of the CTA. Acute DCS treatments were more effective in reducing the time required to extinguish a CTA than were chronic drug treatments. Moreover, the timing of these acute DCS treatments affected spontaneous recovery of the CTA depending on the extinction method employed. Acute DCS administrations later in extinction were more effective in reducing spontaneous recovery than were early administrations if the rats went through the CSO-EXT procedure. However, late-in-extinction administrations of DCS facilitated spontaneous recovery of the CTA in rats that experienced the EU-EXT method.
These data agree with other findings suggesting that DCS treatments are more effective when administered a limited number of times. Our data extend these findings to the CTA paradigm and further suggest that, depending on the extinction paradigm employed, acute exposure to DCS can speed up CTA extinction and reduce spontaneous recovery of the aversion. The timing of the acute DCS treatment during extinction is generally less important than its duration in predicting the rate of CTA extinction. However, the timing of acute DCS treatments during extinction and the method of extinction employed can interact to affect spontaneous recovery of a CTA.
Keywords: Conditioned Taste Aversion, D-Cycloserine, Extinction, Spontaneous Recovery, Chronic, Acute, DCS, CTA
1. Introduction
Fears and defensive reactions to fears may be acquired through the well-known processes of classical conditioning [1]. Our recent enhanced understanding of not only how fears are acquired but also how they are extinguished continues to inform clinical practice in the treatment of phobias and post-traumatic stress disorder (PTSD). However, the specific conditioned stimulus (CS)/unconditioned stimulus (US) dynamics of extinction are still being debated. Extinction may be an unlearning of a CS+US pairing [2] or a devaluation of a US [3] such that the CS activates a conditioned response that is too weak to produce fear. However, the most widely-supported hypothesis is that a new type of learning occurs, which supplements the original CS+US association [4,5,6,7]. During extinction, an inhibitory CS+no US connection is created which competes with the original CS+US association. This inhibitory CS+no US memory temporarily suppresses the CS+US memory, which also temporarily suppresses the fear response [4,5,6]. However, a CS presented later on, or in a different context, may result in spontaneous recovery or relapse of the fear or defensive reaction to the fear [1,8].
Several biological mechanisms have been shown to subserve the CS+no US learning that produces extinction of the conditioned response (CR). Extinction learning depends on glutamate N-methyl-D-aspartate (NMDA) receptor activation in the ventromedial prefrontal cortex, hippocampus, and basolateral amygdala [9,10,11,12]. Administration of NMDA antagonists impairs fear conditioning as well as extinction learning when injected either directly into the brain [13] or systemically [14]. On the other hand, administration of the NMDA receptor agonist D-serine has been shown to compensate for memory loss in rats following cortical damage [15] or aging [16]. Unfortunately, competitive NMDA agonists like D-serine can lead to excitotoxicity, inducing cellular apoptosis [17]. The potentially toxic effects of D-serine make clinical application of this drug unlikely.
Partial NMDA receptor agonists, unlike competitive agonists, have been shown to produce the memory benefits of competitive agonists without excitotoxicity. D-Cycloserine (DCS), one such partial agonist, enhances excitatory NMDA receptor neurotransmission by binding to glycine NMDA receptor sites [18]. However, DCS’s modulatory actions on NMDA receptors are complex. When NMDA receptor glycine levels are low, DCS facilitates NMDA receptor activation. However, when glycine levels are high, DCS can have an antagonizing effect and reduce NMDA receptor functioning by up to 50% [19, 20]. These findings are important as decisions are made about dosing and timing of DCS administration. Therefore, within clinical practice DCS is most beneficial when glycine levels at the synaptic cleft are low [21].
DCS has been shown to be an effective memory enhancer in both preclinical and clinical studies [22,23,24]. Clinical studies have demonstrated that if DCS is given in conjunction with exposure therapy, participants show less acrophobia [18], social anxiety [25] and obsessive compulsive behaviors [26]. But note also that these benefits in humans have not been reported universally [27,28]. In non-human animals, DCS administration leads to improved water maze learning [29], enhanced extinction in cocaine-induced place preference [30], and a facilitation of extinction in light/shock models of fear conditioning [23,24,31]. A number of excellent review papers have appeared that discuss the pre-clinical work with DCS and the potential of the drug for clinical applications (32,33).
However, the timing of DCS administration during extinction has been subject to debate. Richardson, Ledgerwood, and Cranney [34] found that DCS is best administered immediately just before or after extinction treatments. Langton and Richardson [35], as well as Parnas, Weber, and Richardson [36] found it’s best to only administer DCS directly after extinction treatments to extinguish a conditioned fear. The benefits of DCS administration are linearly time sensitive so that DCS is most efficacious when given less than four hours after extinction treatments. As time between extinction treatments and drug administration increases, efficacy of DCS decreases [34].
The frequency of DCS administration needed for peak efficacy has also been investigated. Parnas, Weber, and Richardson [36] showed that 5 DCS exposures over a 10-day period prior to fear conditioning created no enhancing effect on extinction compared with significant facilitation of extinction with acute dosing. Similarly, Quartermain et al. [37] found that a single dose of DCS before training in a maze enhanced learning whereas 15 days of drug exposure had no beneficial effect. Human studies also indicate that acute DCS administration has been found to enhance treatment outcomes for acrophobia, suggesting that short periods of therapy may be the most effective [18].
Various laboratories have used the conditioned taste aversion (CTA) paradigm [38,39,40] to create a robust, aversive memory that causes the animal to refuse the CS of saccharin [7,41]. Animals learn an aversion to saccharin if ingested before administration of the malaise-inducing US, lithium chloride (LiCl) [42]. CTAs are extremely robust and extinction is very slow, making it an interesting model of other hard-to-extinguish defensive reactions to conditioned fears [7,43]. Based on the literature cited above, DCS may be expected to facilitate the extinction of a CTA as it has done so in other paradigms.
The nootropic properties of DCS have been studied in CTA paradigms during both conditioning and extinction. The literature consistently shows that DCS administration results in enhanced conditioning of the CTA [22,43] although DCS (at the dose commonly employed, i.e., 15 mg/kg, i.p.) does not interfere with a rat’s ability to experience either the CS or US nor is it an effective US in the context of a CTA paradigm [4,44,45]. For example, Land and Riccio [22] demonstrated that DCS administration prior to pairing the CS with a low dose of LiCl resulted in a stronger CTA as compared to saline controls. Further, Davenport and Houpt [44] showed that DCS only enhanced CTA learning when administered before a short-delay CTA protocol and had no effect on CTA learning when there was a long delay between saccharin drinking and LiCl administration. However DCS’s effect on CTA extinction is less understood. For example, Yu et al. [46] reported impaired extinction learning by knock-in mice that were genetically altered to have a polymorphism of the brain derived neurotrophic factor (BDNF) gene found only in humans. When the mice were administered DCS these mice extinguished like wild-type mice, suggesting that DCS can also facilitate extinction in a CTA paradigm [46]. However, Akirav et al. [47] found that animals showing a stress-induced impairment in CTA extinction did not recover when DCS was infused into the basolateral amygdala. The authors also noted that the controls animals (i.e., those that did not undergo a stress procedure) did not show a facilitation of extinction after DCS infusions as compared to control animals that received the vehicle. This suggests that DCS infusions do not facilitate extinction of a CTA [47]. The inconsistent findings between these two studies indicate that DCS could facilitate extinction under certain conditions, but more research is needed to understand these parameters.
The way in which a fear is extinguished has much to do with whether or not a relapse or spontaneous recovery of the fear will occur. We have explored two different paradigms in which extinction of an established CTA occurs: CS-only (CSO), which involves the presentation of only the CS every other day, and explicitly unpaired (EU), in which the CS and US are given, unpaired, on alternating days. Our previous research has shown that exposure to the EU paradigm produces a more-rapid reacceptance of the once-aversive CS (saccharin) and makes spontaneous recovery of the CTA less potent [48]. The effects of DCS on spontaneous recovery have supported the notion that DCS decreases the likelihood of fear re-emergence [31], although results from other labs have not always demonstrated this effect [49]. The EU paradigm has also been shown to resist a context-renewal effect often seen following extinction [50]. These findings are important as one of the greatest post-therapy difficulties for PTSD and phobia patients is a relapse to original fearful thoughts and behaviors [50].
Previous research suggests that acute DCS administration facilitates fear extinction more effectively than chronic administration [18]. The timing of the DCS treatments during extinction may also be important [36]. Moreover, the behavioral methods employed are also important predictors of the effectiveness of extinction in regards to spontaneous recovery of a CTA [48] or relapse of a conditioned fear [50]. Therefore, the goal of the current study was to investigate the effects of chronic DCS treatments given throughout extinction and compare them to the effects of acute DCS treatments, given during different phases of extinction. We studied how these different dosing parameters affected the progress of 2 different types of CTA extinction (CSO and EU). We also studied the effects of these factors on the spontaneous recovery of a CTA.
2. Methods
2.1 Animals
One hundred and ten adult male Sprague-Dawley Rats (Mean weight = 349.55g; SEM = 34.09g) were purchased from Charles River Laboratories (Wilmington, MA) and used in this study. All animals were maintained and used in accordance with the Animal Welfare Act and The Guide for the Care and Use of Laboratory Animals [51]. Throughout the experiment the animals were housed in plastic tub cages (20 cm × 22cm × 20 cm deep) with wire top lids. The bottoms of the cages contained corncob bedding (The Andersons, Inc., Maumee, OH). Rats lived in a temperature-controlled room under a 12-hr light/dark cycle (lights on at 06:00 hrs; off at 1800 hrs). Rats had free access to food (Purina Rodent Chow, No. 5001, PMI Nutrition International, Brentwood, MO).
2.2 Overview of Drug Treatments and Groups
Animals were randomly assigned to one of ten groups (see Table 1). All acquired a CTA and then underwent extinction (via 1 of 2 different methods) while receiving acute or chronic DCS (15 mg/kg, i.p.) or physiological saline control injections (see procedural descriptions below). Once they reached 90% of baseline saccharin consumption, rats were either sacrificed for immunohistological analyses (not reported here) or they entered a 30-day latency period that preceded a spontaneous recovery test. See the distribution of N/group at each stage of the study in Table 1.
Table 1. Treatment groups and numbers of animals.
| Group | Sub-Group | N through EXT | Conditioning | Extinction (EXT) | N at SR | SR Test Solution | ||
|---|---|---|---|---|---|---|---|---|
| Days 1,3, 5 | Days 2, 4, 6 | Odd Days | Even Days | |||||
| Acute DCS | CSO (2–5%) | 11 | SAC1+ LiCl2 | Water | SAC+ SAL/DCS3 | Water+ (SAL+SAL/DCS)4 | 6 | SAC |
| CSO (8- 16%) | 10 | SAC+ LiCl | Water | SAC+ SAL/DCS | Water+ (SAL+SAL/DCS) | 5 | SAC | |
| CSO (20–40%) | 9 | SAC+ LiCl | Water | SAC+ SAL/DCS | Water+ (SAL+SAL/DCS) | 7 | SAC | |
| EU (2–5%) | 12 | SAC+ LiCl | Water | SAC+ SAL/DCS | Water+ (LiCl+SAL/DCS)5 | 7 | SAC | |
| EU (8–16%) | 13 | SAC+ LiCl | Water | SAC+ SAL/DCS | Water+ (LiCl+SAL/DCS) | 9 | SAC | |
| EU (20–40%) | 15 | SAC+ LiCl | Water | SAC+ SAL/DCS | Water+ (LiCl+SAL/DCS) | 13 | SAC | |
| Chronic DCS | CSO | 11 | SAC+ LiCl | Water | SAC+ DCS | Water+ (SAL+DCS)6 | 6 | SAC |
| EU | 10 | SAC+ LiCL | Water | SAC+DCS | Water + (LiCl+DCS)7 | 6 | SAC | |
| Saline Control | CSO | 10 | SAC + LiCl | Water | SAC+SAL | Water + (SAL+SAL)8 | 6 | SAC |
| EU | 9 | SAC+ LiCl | Water | SAC+SAL | Water+ (SAL+LiCl)9 | 6 | SAC | |
SAC= saccharin dissolved in deionized water (0.3%), presented in 30 minute exposures.
LiCl= intraperitoneal injection, 81 mg/kg at a volume of 1 ml/kg, lithium chloride dissolved in physiological saline.
SAL/DCS = .9% physiological saline intraperitoneal injection (NaCl dissolved in deionized water) give at 1 ml/kg or DCS intraperitoneal injection (15 mg/kg given at 1 ml/kg, dissolved in physiological saline). Rats are given SAL control injections everyday excluding the four days they received their DCS treatment injections.
(SAL+SAL/DCS) = Two injections given within 30 seconds of each other, one SAL i.p. 1ml/kg and then one i.p. injection of either SAL 1 mg/kg or DCS 15 mg/kg at 1 ml/kg, depending on whether in DCS treatment or not.
(LiCl+SAL/DCS) = Two injections given within 30 seconds of each other, one LiCl i.p 81mg/kg at a volume of 1 ml/kg and one i.p injection of either SAL 1 mg/kg or DCS 15 mg/kg at 1 ml/kg, depending on whether in DCS treatment or not.
(SAL+DCS) = Two injections given within 30 seconds of each other, one SAL i.p. 1ml/kg and then one i.p. injection of DCS 15 mg/kg at 1 ml/kg.
(LiCL+DCS) = Two injections given within 30 seconds of each other, one LiCl i.p 81mg/kg at a volume of 1 ml/kg and one i.p injection of DCS 15 mg/kg at 1 ml/kg.
(SAL+SAL) = Two injections SAL i.p. 1 ml/kg given within 30 seconds of each other
(SAL+LiCl) = Two injections given within 30 seconds of each other, one SAL i.p. 1 ml/kg and then one LiCl i.p. 81 mg/kg at a volume of 1 ml/kg
2.3 CTA Acquisition
Two days prior to the first conditioning trial, animals were introduced to a 23hr fluid deprivation schedule during which they were given two 30-min presentations of tap water each day (1200–1230 hrs and 1245–1315 hrs). CTA acquisition began following this period of acclimation. On experimental days 1, 3 and 5 of the study all animals were presented with sodium saccharin (0.3%; %w/v) for a 30-min period (1200–1230hrs) and then injected with lithium chloride (LiCl; 81mg/kg, i.p.) [7]. Fifteen min after the injections rats were given another 30-min presentation of water to prevent dehydration (1245–1315hrs). On the rest days (days 2, 4 & 6) animals were not given any drug injections and were presented with water for two, 30-min sessions (with a 15 min period between sessions) to mimic the timing of liquid availability on the LiCl-injection days. A high dose of LiCl was employed and 3 CS+ US pairings were conducted to ensure a strong CTA was established. This promoted a lengthy period of extinction that aimed to reveal subtle group differences over time. This robust CTA was necessary to examine the different phases of extinction, as described by Nolan et al. [52]. It should be noted that the concentration of LiCl employed is hyperosmotic. However, this dose does not enhance drinking and, in fact, can suppress it [53]. Moreover, drinking measures were taken 24 or 48 hours following LiCl treatments. Given the 6-hour half-life of the drug [54], it is unlikely that its hyperosmotic feature affected drinking measures a day or 2 later.
2.4 CTA Extinction
Following CTA acquisition, rats were randomly assigned to one of two extinction groups: conditioned stimulus only (CSO-EXT) or explicitly unpaired (EU-EXT). All animals were maintained on the 23-hr fluid deprivation schedule, but presented with saccharin (0.3%) for 30 min every-other day (1200–1230hrs) followed by water (1245–1315hrs). On alternate days animals were given two, 30-min presentations of water only during the same time period.
Following the first daily drinking period (1200–1230hrs) on every day of the extinction phase of the study, animals were given an injection of DCS or saline (depending on group assignment, see details below). On water-only days animals were also given an injection of LiCl if they were assigned to an EU group. If they were assigned to a CSO group, they received saline (i.p.) (refer to Table 1 for group assignments and corresponding treatments throughout extinction). The animals were maintained on this regimen until they reached the asymptotic extinction criterion of ≥ 90% reacceptance of saccharin (i.e., ≥ 90% of baseline saccharin drinking) [53]. Nolan et al., [52] have described the shape of CTA extinction curves as reflecting 3 phases: static (during which rats abstain or drink little of the CS, i.e., less than or equal to 10% baseline saccharin consumption); dynamic (CS sampling grows and, over a few days, increases dramatically; 10–80% baseline saccharin consumption), and asymptotic (animals reaccept the CS at levels approaching baseline; 80–100% baseline saccharin consumption).
As a first step in evaluating the degree to which the rats in this study had extinguished their CTA, we needed to estimate levels of baseline familiar saccharin drinking. However, recording several days of baseline saccharin pre-exposure in our animals would have impeded future CTA training, due to latent inhibition effects [55]. Moreover, we also wanted to record saccharin consumption over several days to avoid the bias associated with the rat’s initial hesitation to consume novel substances (neophobia) [56]. Therefore, baseline saccharin consumption was determined by averaging saccharin consumption on the third day of exposure from a separate group (N = 10) of similarly-sized rats maintained on the same fluid restriction schedule as the rats in the studies reported here (see CTA Acquisition section, above). This produced a mean saccharin consumption (± SEM) = 17.57 ± 1.29ml) [7]. In order to confirm that this method of determining baseline saccharin consumption was consistent with other ways to estimate familiar saccharin drinking, we also measured the saccharin consumption of a group of rats (N = 24; also maintained on the same fluid restriction schedule as the rats in the studies reported here) that were exposed to saccharin and LiCl but did not have the US and CS paired. Saccharin or LiCl were available/administered on alternate days. The saccharin consumption of this group represented normal enhanced acceptance of the sweet tasting liquid in the absence of conditioned avoidance. The animals that had these explicitly unpaired CS-US exposures over 3 saccharin-exposure days drank amounts of the sweet liquid (Mean ± SEM = 18.2 ± 2.8ml) not significantly different from those animals that only drank saccharin over the same time period (see data above). In a final pilot study, we employed 7 fluid-restricted rats on the same 23-hr fluid deprivation schedule. Like the rats in the main study that went through CTA acquisition, these pilot animals were offered saccharin every-other day but, instead of receiving LiCl immediately after the saccharin, these rats received an equal volume of physiological saline (i.p.). On their third day of saccharin drinking, these rats drank 17.10 ± 1.38ml (Mean ± SEM) of the sweet liquid - an amount very similar to the baseline saccharin consumption estimates from the other methods described above. These data validated our method of estimating baseline saccharin consumption as a comparison point to determine 90% reacceptance of saccharin as asymptotic extinction.
Once they reached 90% of baseline saccharin consumption, rats were either sacrificed for immunohistological analyses (not reported here) or they entered a 30-day latency period that preceded a spontaneous recovery test. See the distribution of N/group at each stage of the study in Table 1 and the number of rats that contributed to our dataset at each of 2 behavioral time points (achieving asymptotic extinction and spontaneous recovery test).
2.5 Spontaneous Recovery
After reaching the asymptotic extinction criterion, animals were maintained on the 23-hour fluid deprivation schedule but given water-only for 29 days (at 1200 hours and 1245 hours). During this latency period leading up to the spontaneous recovery test day, rats received no injections. On the 30th day after the last day of extinction animals were given a spontaneous recovery test (at 1200 hours) which consisted of a single 30-min exposure to 0.3% saccharin. Rats were sacrificed following this exposure.
2.6. DCS or control Treatments
Rats in each of the 2 extinction treatment groups (CSO or EU) were randomly divided into chronic-DCS administration, acute-DCS administration, or saline-control groups. See group distribution in Table 1. All chemicals were purchased from Sigma Aldrich (St. Louis, MO) and DCS was mixed immediately prior to injection.
2.6.1 Chronic DCS treatments
Those rats in the chronic-DCS treatment group received DCS injections (15 mg/kg i.p., dissolved in sterile physiological saline; 1 ml/kg) [40] following their first fluid exposure every day during extinction. On odd days both CSO and EU rats received just this DCS injection following their 30 min saccharin exposure. On even days during extinction EU rats also received one LiCl injection (i.p.; 81 mg/kg, 81 mg/ml; dissolved in sterile saline) following water exposure. CSO extinction rats received one saline control injection (1ml/kg, i.p.; in lieu of LiCl) along with their DCS injection during this time.
2.6.2 Acute DCS treatments
Each CSO or EU extinction group was randomly divided into three additional subgroups (6 groups total; see Table 1). The three subgroups represented various stages in extinction when the animals began DCS treatment. The static stage of extinction is defined in the literature as <10% baseline CS consumption and the dynamic stage is between 10–80% baseline CS consumption [52]. Thus, to see when DCS treatment was most effective, rats were randomly assigned to the three subgroups, static (2–5% baseline saccharin consumption), early dynamic (8–16% baseline saccharin consumption), or middle dynamic (20–40% baseline saccharin consumption). Rats began DCS treatment when their saccharin consumption reached its designated level during the extinction procedure. Rats received DCS injections (15 mg/kg i.p., dissolved in sterile saline; 1 ml/kg) [43] for four consecutive days.
2.6.3 General features of DCS treatments
Once chronic or acute DCS treatments began, animals received daily injections of DCS - even on the animals’ “rest days” when no CS was presented. This regimen was implemented because the EU extinction paradigm presents the US to the animals on their “rest day”, when no saccharin was offered. As indicated earlier, animals that undergo EU extinction show a facilitation of extinction and an attenuation of spontaneous recovery [48], and these results could indicate that the learning that occurs on the days when the US is presented is just as important as the learning that occurs when the CS is presented. Likewise, recent data from studies that employed a conditioned fear paradigm suggest that presentation of the US during extinction triggers the consolidation of its associated predictor representation in the lateral amygdala [57]. In an attempt to “facilitate” all the learning that may contribute to the extinction procedure, a DCS injection was given following both the CS and US presentations.
DCS was administered after the animals received these stimuli in order to reduce the chance of state-dependent learning. The timing of our DCS administration was also motivated by previous research indicating that DCS injections, given immediately following each day of extinction, facilitated learning [23,31].
The dose of DCS (15 mg/kg, i.p.) was carefully selected based on a pilot study performed in our lab [45] indicating that DCS doses ranging from 3–15 mg/kg (i.p.) did not disrupt rats’ taste (i.e., the ability to discriminate between 0.3% and 0.6% saccharin). This finding is consistent with the data of Davenport and Houpt [44] who reported that DCS (15 mg/kg) does not cause gustatory problems or interfere with a rat’s ability to develop a preference for saccharin. The results of our pilot study also indicated that 15 mg/kg DCS (i.p.) did not significantly change LiCl-induced drinking suppression. These results are consistent with those of Nunnink et al. [43] who showed that 15 mg/kg of DCS does not possess US properties in the context of a CTA paradigm nor does the drug affect LiCl-induced malaise. Thus, 15 mg/kg DCS (i.p.) does not interfere with a rat’s ability to experience either the CS or US employed in the current study nor is it an effective US in the context of a CTA paradigm. In addition, this dose of DCS has facilitated extinction in a conditioned emotional response paradigm [24,31] and enhanced CTA learning [43].
2.6.4 Saline control treatments
Those rats in the saline control groups did not receive any DCS injections during extinction. Instead they received 1 ml/kg physiological saline (i.p.) injections only. On odd days, rats in both CSO and EU groups received one saline injection following saccharin exposure. On even days, after water exposure CSO rats received two saline injections while EU rats received one saline injection paired with one LiCl injection (i.p.; 81 mg/kg, 81 mg/ml; dissolved in sterile saline). A pilot study involving 6 rats indicated that acute injections of saline over a 4-day period (parallel to the timing of the acute DCS treatments) produced behavioral results (i.e., days to achieve asymptotic extinction; Mean ± SEM = 31.66 ± 3.66 days) that were not significantly different from the chronic saline treatments (Mean ± SEM = 34.80 ± 3.86 days). Therefore, for simplicity of presentation, the data from only the rats given chronic saline injections are presented here and represent the saline control manipulation.
2.7 Data analysis
Analyses of variance (ANOVAs) and (when appropriate) Bonferroni-corrected t-test were used to determine the reliability of group differences. An α = 0.05 was adopted throughout these studies.
3. Results
3.1 CTA acquisition
The amount of saccharin consumed over the three-day conditioning period indicated that all groups acquired a strong taste aversion (see Figure 1). Saccharin consumption decreased steadily over these three conditioning days and the first day of extinction for all animals and all groups. A repeated measures ANOVA (Treatment Group × Trial) revealed that the decrease in saccharin consumption over trials was statistically significant [F(3,297)=228.433, p<0.001]. Initial exposure to 0.3% saccharin produced relatively low levels of consumption. Higher saccharin concentrations may be borderline aversive [53] and may help explain this phenomenon.
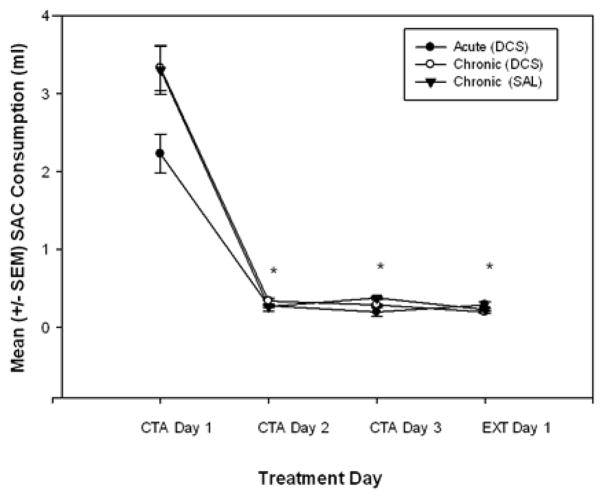
Figure 1.
Mean (± SEM) volume of saccharin (SAC) consumed by rats during CTA acquisition. CTA Day 1 represents the first day of saccharin exposure before the first LiCl injection. Saccharin consumed on conditioning days 2 and 3 and extinction (EXT) day 1represent the formation of the CTA after three CS+US pairings. All animals in all treatment groups formed a strong taste aversion following these three SAC+LiCl pairings. Note: These measurements were taken before any DCS/Saline (SAL) or extinction treatments began. Since all the rats received the same initial treatment, they are combined into 3 groups for simplicity of presentation (see Table 1). * indicates a significant decrease in saccharin consumed as compared to conditioning day 1 (before administration of LiCl)(p < 0.001).
To verify that the rats in our treatment groups had acquired the same level of aversion to saccharin during the CTA acquisition phase of the study, the saccharin consumption on the first day of extinction was compared using a two-way ANOVA [Drug Treatment (DCS or saline) × Extinction (EU or CSO)] among the treatment groups. The mean volumes of saccharin consumed on the first day of extinction by all the groups were not significantly different from one another.
3.2 CTA Extinction – Chronic DCS treatment
All groups in this study achieved the same levels of asymptotic extinction (≥90% of baseline). However, the mean days to reach asymptotic extinction for these groups were significantly different (See Figure 2A). A two-way ANOVA [Drug Treatment (DCS or saline) × Extinction (EU or CSO)] revealed a significant effect of extinction treatment [F(1,38)=24.58. p<0.05] but no main effect of drug treatment [F(1,38)=0.30. p>0.05] and no interaction effect[F(1,38)=0.81. p>0.05]. Specifically this analysis indicated that animals that underwent the EU procedure extinguished the CTA significantly faster than animals that experienced the CSO procedure. These data corroborate previous findings from our laboratory [48]. However, unlike other published research [24], the administration of DCS following CSO-extinction did not reduce the time required to achieve asymptotic extinction.
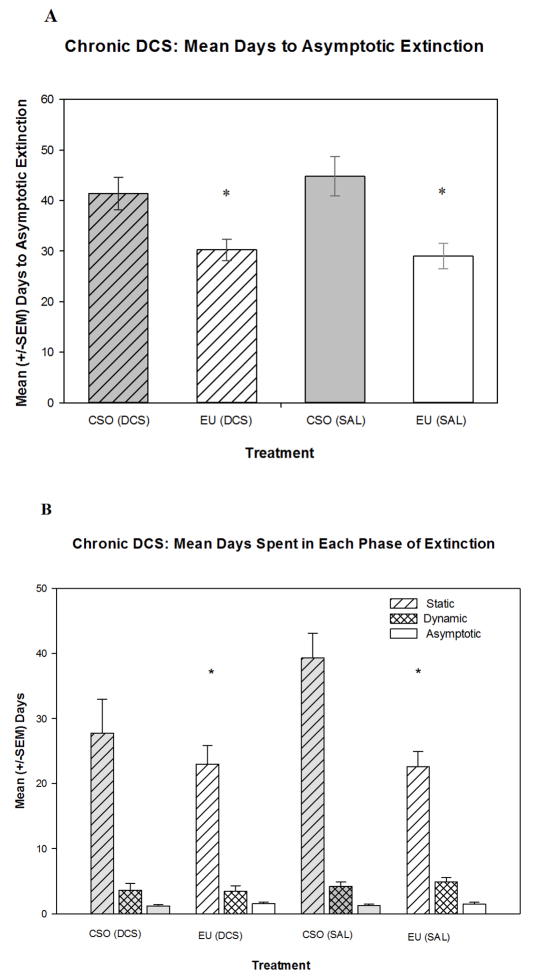
Figure 2.
(A) Mean days for rats to reach asymptotic extinction, operationally defined as ≥90% reacceptance of baseline saccharin consumption. Animals that underwent EU extinction [EU (DCS) group, EU (saline; SAL) group] extinguished significantly faster than animals that experienced the CSO extinction. (B) Mean days spent in each phase of extinction (first defined by Nolan et al. [52]). Rats that underwent the EU extinction procedure spent significantly fewer days in the static phase than the animals that experienced CSO extinction. However, all groups spent a comparable number of days in both the dynamic and asymptotic phases. * indicates significantly (p<0.05) different compared to the CSO (saline) group.
To further explore drug effects on the rate of extinction, the lengths of each of the phases of extinction (as described by Nolan et al. [52]; see Methods for description) were determined and compared. No significant effects were found between groups for either time spent in the dynamic or asymptotic stages. However a two-way ANOVA [Drug Treatment (DCS or saline) × Extinction (EU or CSO)] showed a significant main effect of extinction method [F(1,42)=7.63, p<0.05] but no effect of drug treatment and no interaction effect when examining the static phase. Rats that went through the EU extinction procedure took significantly fewer days to complete the static phase of extinction (i.e., to return to10% of baseline saccharin drinking) than did the CSO groups (see Figure 2B).
3.3 CTA Extinction – Acute DCS treatment
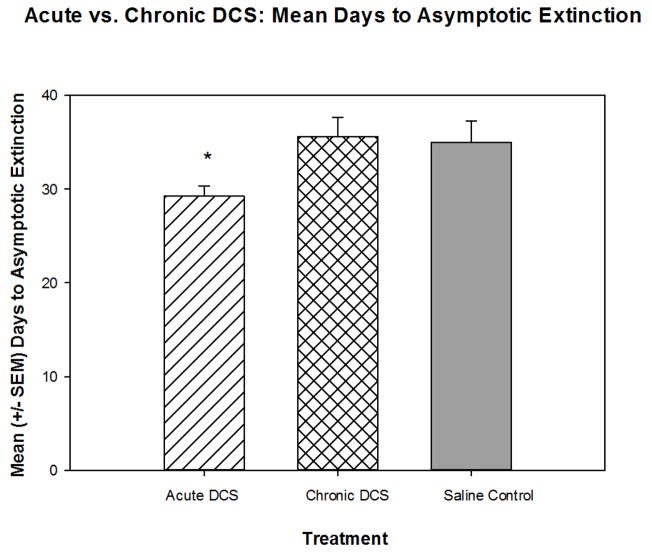
Chronic treatment with DCS does not facilitate the extinction of a CTA (see above). In the following initial analysis, we combined the rats into the 3 main treatment groups (Acute DCS, Chronic DCS, or saline controls) and investigated whether acute DCS treatment was more effective than chronic DCS treatment. A one-way analysis of variance [Group Treatment (Acute DCS, Chronic DCS, or saline)] revealed a significant main effect [F(2,107)=5.338, p<0.01] and post-hoc comparisons indicated that acute DCS animals extinguished significantly faster than both chronic DCS and saline control animals (Figure 3).
Figure 3.
Overall, acute exposure to DCS significantly decreased the time to reach asymptotic extinction compared to both chronic DCS exposure, as well as saline control animals. * indicates significantly less than the Chronic DCS group and saline Control group (p< 0.05).
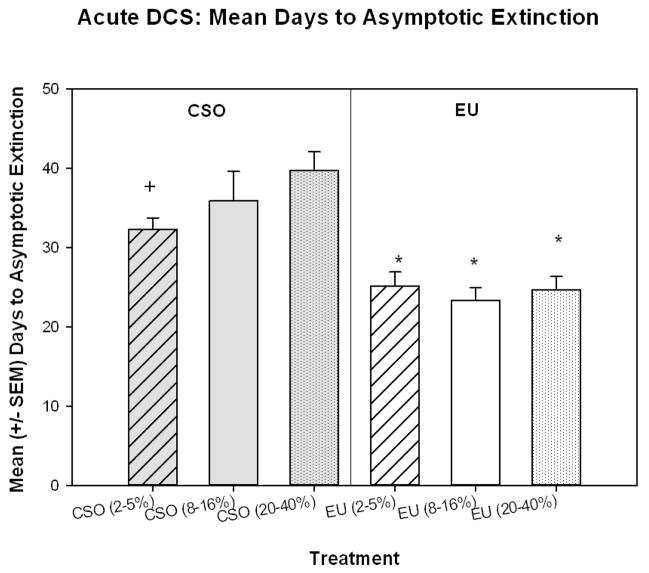
One of the main goals of the current study was to determine if the timing of acute DCS administration would have an effect on the facilitation of extinction in CSO or EU rats. A two-way analysis of variance [Extinction (EU or CSO) × DCS Group (2–5%, 8–16%, or 20–40%)] revealed a significant main effect of Extinction method [F(1,64)=44.546, p<0.001], indicating that the EU rats took significantly less time to reach asymptotic extinction than CSO rats (Figure 4). Post-hoc comparisons revealed that this was true for rats in all of the DCS treatment phases. EU (2–5%, 8–16%, & 20–40%) rats extinguished significantly faster than the corresponding rats in the CSO-EXT groups [2–5% (p<0.01), 8–16% (p<0.01), & 20–40% (p<0.001)]. Additionally, rats in the CSO (2–5%) group extinguished significantly faster than rats in the CSO (20–40%) group (p<0.02) (Figure 4).
Figure 4.
The EU-EXT (EU) procedure reduced the time to CTA extinction in rats that received acute 4-day exposure to DCS. Additionally, rats in the CSO (2–5%) group extinguished significantly faster than rats in the CSO (20–40%) group. * indicates significantly less than the corresponding CSO extinction groups (p< 0.01). * indicates significantly less than CSO (20–40%) group (p<0.02).
3.4 Spontaneous recovery of the CTA – Chronic DCS treatment
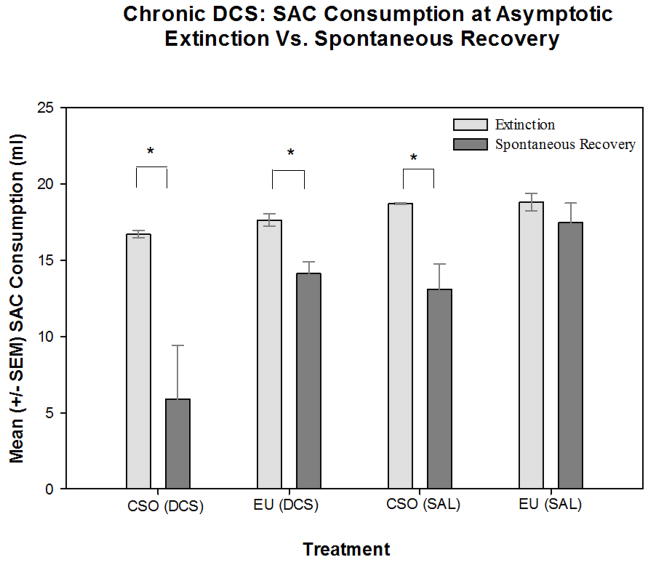
Animals chronically treated with DCS did not show a reduced spontaneous recovery of the CTA. In an analysis of saccharin consumption, a two-way ANOVA [Drug Treatment (DCS or saline) × Extinction method (EU or CSO)] with repeated-measures [saccharin drinking test time (saccharin consumption at asymptotic extinction; saccharin consumption at spontaneous recovery test)], revealed a significant main effect for drug treatment [F(1,19)=10.064, p<0.05] and a significant main effect of extinction method [F(1,19)=8.724, p<0.05]. In addition, we found a significant main effect of drinking test time [F(1,19)=23.468, p<0.001] and a significant interaction effect between drinking test time and extinction method [F(1,19)=9.368, p<0.05]. Post hoc analyses revealed that the CSO (saline) animals drank significantly less saccharin on the day of the spontaneous recovery test, than on the day of extinction, thus indicating that CSO saline control animals exhibited a spontaneous recovery of the previously extinguished CTA (see Figure 5). Similarly the CSO (DCS) and EU (DCS) animals showed a significant spontaneous recovery by drinking significantly less saccharin on the day of the spontaneous recovery test, than on the day they met the criterion for asymptotic extinction. However, the EU (saline) animals drank equivalent amounts of saccharin on day of the spontaneous recovery test as they did at asymptotic extinction. These data are consistent with a previous report [48] and indicate that when saline control rats experience the EU extinction procedure there is a significant attenuation of spontaneous recovery. However, when animals were treated with DCS, the DCS treatment eliminated the EU-induced attenuation of the spontaneous recovery. CSO animals, whether treated with saline or DCS, showed a strong spontaneous recovery when presented with saccharin after a 30-day latency. This indicates the DCS (given during either extinction method) did not accelerate extinction learning nor did it attenuate spontaneous recovery of the CTA.
Figure 5.
Mean (± SEM) volume of saccharin (SAC) consumed by rats in the chronic DCS study on the day of asymptotic extinction and on the subsequent spontaneous recovery (SR) test day. Animals that underwent the CSO [both CSO (saline: SAL) and CSO (DCS)] extinction procedure drank significantly more saccharin on the last day of extinction than on the day of the SR test, indicating a spontaneous recovery of the CTA. The EU (SAL) group did not show a significant spontaneous recovery but the EU (DCS) animals did exhibit spontaneous recovery of the CTA. * indicates a significant (p<0.05) difference between saccharin consumption on the day of extinction and the day of the spontaneous recovery test.
3.5 Spontaneous recovery of the CTA – Acute DCS treatment
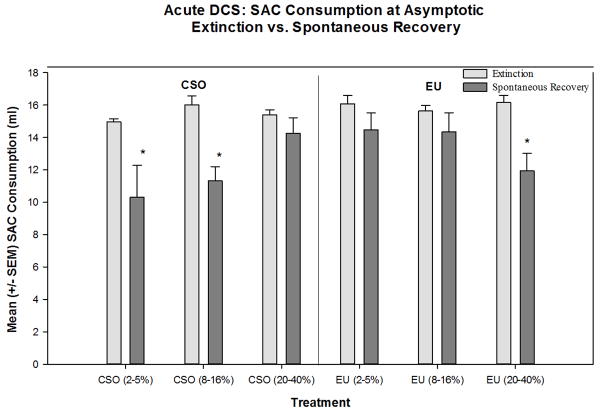
The final aspect of this study aimed to determine if the timing of acute DCS would have an effect on the spontaneous recovery of the CTA. A two-way analysis of variance [Extinction Method (EU or CSO) × DCS Group (2–5%, 8–16%, or 20–40%)] with repeated measures [Test Day (extinction or spontaneous recovery)] evaluated the influence of these factors on saccharin consumption. This test revealed a significant main effect for Extinction Method [F(1,39)=7.036, p<0.02] and a significant interaction effect between Extinction Method and DCS Group [F(2,39)=5.542, p<0.01]. In addition, we found a significant main effect of Test Day [F(1,39)=31.608, p<0.001] and a significant interaction effect between Test Day, Extinction, and DCS Group [F(2,39)=5.368, p<0.01]. Post-hoc analyses revealed that the CSO (2–5%), CSO (8–16%), and EU (20–40%) rats drank significantly less saccharin on the day of the spontaneous recovery test than on the day of asymptotic extinction, indicating that these rats had a spontaneous recovery of the CTA (Figure 6). However, CSO (20–40%), EU (2–5%), and EU (8–16%) rats drank equivalent amounts of saccharin on the day of the spontaneous recovery test as they did at asymptotic extinction, indicating that there was not a spontaneous recovery of the CTA. These data indicate that acute DCS given later to rats in the CSO paradigm may be beneficial in reducing the spontaneous recovery of a CTA. Like saline-treated rats, animals that were treated with DCS acutely as they went through the early stages of EU-EXT did not exhibit a spontaneous recovery of the CTA. However, DCS given later in EU-EXT produced a spontaneous recovery of the CTA. See a general overview of these findings in Table 2.
Figure 6.
Spontaneous recovery of the CTA occurred in rats that received acute DCS treatments early during CSO extinction (when rats reached 2–5% or 8–16% of baseline saccharin drinking) and later (when rats reached 20–40% of baseline saccharin drinking) during EU extinction. However, administration of DCS later in CSO extinction eliminated spontaneous recovery of the CTA.* = Significant spontaneous recovery of a CTA, i.e., saccharin consumed at the spontaneous recovery test is significantly less than saccharin consumed at asymptotic extinction (p < 0.01).
Table 2. Summary of experimental findings.
| Drug Treatment and timing1 | Relative rate of Extinction2 [Sub-groups combined for acute DCS treatments] | Behavioral Treatments and Tests1 | |||
|---|---|---|---|---|---|
| CS-Only Extinction (CSO) | Explicitly Unpaired Extinction (EU) | ||||
| Relative rate of Extinction2 | SR test3 | Relative rate of Extinction2 | SR Test3 | ||
| Controls | +4 | +5 | SR7 | +++5 | No SR7 |
| Chronic DCS | +4 | +5 | SR7 | +++5 | SR7 |
| Acute DCS (static: 2–5%) | +++4 | ++6 | SR8 | +++6 | No SR8 |
| Acute DCS (early dynamic: 8–16%) | +6 | SR8 | +++6 | No SR8 | |
| Acute DCS (late dynamic: 20–40%) | +6 | No SR8 | +++6 | SR8 | |
See Table 1 for group nomenclature and procedures
Days to achieve asymptotic CTA extinction. Rates are relative to other groups in the same column. + = Slow rate of extinction; ++ = Medium rate of extinction; +++ = Fast rate of extinction.
Comparison of saccharin drunk at asymptotic CTA extinction vs. saccharin drunk at the spontaneous recovery (SR) test 30 days later. SR indicates a suppression of SAC drinking relative to the amount consumed at the end of CTA extinction.
See Figure 3
See Figure 2A
See Figure 4
See Figure 5
See Figure 6
4. Discussion
Consistent with a previous report [48], our data indicate that animals in the EU-EXT training groups extinguished more quickly than animals in the CSO-EXT groups. Spontaneous recovery of the CTA was also significantly reduced in the saline-treated rats that went through the EU extinction procedure. Chronic DCS treatments did not significantly decrease the time to reach asymptotic extinction in either the CSO-EXT or EU-EXT groups. Furthermore, the spontaneous recovery test showed that levels of saccharin consumed by animals in the CSO and EU groups treated with DCS throughout extinction were significantly lower than animals receiving saline. This indicates that rats treated with DCS chronically during extinction demonstrated a significant memory of the CTA during the spontaneous recovery test, regardless of extinction method employed. Further, our data suggest that chronic DCS treatments prevent the attenuation of spontaneous recovery of a CTA in animals that experienced the EU extinction procedure.
On the other hand, acute (4-day) DCS administration was generally effective in reducing the time to extinguish the CTA in rats that went through either the EU or CSO procedures. The timing of the 4 DCS injections during the course of extinction was not an important factor in producing a reduction in the days to reach asymptotic extinction. However, the spontaneous recovery test demonstrated that the timing of acute DCS exposure and the method of extinction employed interact to affect spontaneous recovery of a CTA. Acute DCS treatments that were given later in the extinction process to rats in the CSO paradigm were beneficial in reducing the spontaneous recovery of a CTA. However, acute DCS given late in the EU-EXT process produced a spontaneous recovery of the CTA. See Table 2 for a general overview of the major findings from this study.
These data are consistent with other findings suggesting that acute DCS treatments can generally facilitate learning [43,44,58]. More specifically, the current study builds on the work of other labs that have demonstrated that acute DCS exposure can rescue an extinction deficit in CTA memory experienced by mice with a BDNF polymorphism [46]. Similarly, acute administration of DCS can reverse an impairment in CTA extinction caused by microinfusion of the gamma-Aminobutyric acid-A (GABAA) agonist muscimol into the amygdala [59]. Together these findings are building a case that acute (but not chronic) DCS exposure can facilitate extinction of a CTA.
The effects of DCS on spontaneous recovery, renewal, relapse, and reinstatement are more complex [for review see 32]. Within conditioned fear paradigms DCS has been reported to protect the extinction memory from spontaneous recovery [23,24,60] and reinstatement [31] but not renewal [61] or relapse [49]. Within the context of the CTA paradigm, our data suggest that the effects of DCS on spontaneous recovery depend on the duration of the drug administration, the extinction method employed, and the timing during extinction when the drug is administered.
Inconsistent reports in the literature regarding the effectiveness of DCS treatments may be due to varying effectiveness between chronic and acute treatment procedures, an idea first proposed by Quartermain et al. [37; also, for review see reference 25]. For example, Parnas et al. [36] explored the effects of one DCS injection in comparison to five in the context of a light-shock paradigm. Subjects that received five DCS pre-treatments failed to demonstrate significant differences in extinction of conditioned fears in comparison to saline-treated animals. However, the animals that were administered one DCS injection demonstrated significant enhancements in extinction learning. These early data suggest that the number of DCS treatments influences the potency of the fear extinction method and lead to our exploration of this issue in the context of a different learning paradigm – the CTA.
As suggested by Groblewski et al. 2009 [62], there is a benefit in the study of multiple behavioral indicators of learning as we assess the pharmacological effects of DCS on extinction. This group investigated the effects of DCS on the extinction of alcohol-mediated conditioned place preference in mice and they reported paradoxical effects. DCS did not affect the rate of extinction but it interfered with subsequent reconditioning of the place preference – suggesting that DCS did enhance some aspects of the extinction process. Similar to our findings, Groblewski et al. also report differences in the benefits of acute and chronic DCS treatments. Thus, going beyond classic conditional emotional responses may reveal some common features of how DCS affects learning more generally. The CTA has been described as a defensive reaction to a learned fear [63] but the extent to which fear mediates the aversion is not settled [47]. Still, it is a form of aversive learning that is biologically meaningful and has distinct characteristics (e.g., rapid acquisition and resistance to extinction) that may make it a useful model as we seek therapies for anxiety disorders such as phobias and PTSD.
The neural mechanisms that underlie these behavioral differences evoked by acute and chronic DCS exposure almost certainly involve glutamate NMDA receptors. In order to activate the NMDA receptor complex, the presence of glutamate and a co-agonist (glycine or D-serine) is required [64,65,66,67]. DCS has been shown to have partial agonist action on the strychnine-insensitive glycine-recognition site of the NMDA receptor complex [19]. However, when endogenous levels of glycine are high, DCS has been shown to behave as a partial antagonist and has also been associated with a significant decrease in the synthesis of d-serine through alterations in enzymatic action [66]. Thus, the potential for DCS to enhance learning and/or the induction of long-term potentiation (LTP) is affected by glycine levels [68]. If the glycine receptor is already saturated, then no exogenous activity would increase the chance of LTP occurring and it may decrease the probability of NMDA receptor-associated channel activation. It may be that, through prolonged use, DCS causes alterations in the levels of endogenous ligands that are saturating the glycine receptor complex. This could cause decreased receptor effectiveness since DCS acts with less strength than the endogenous ligands, and/or by rendering the complex unaffected by exogenous chemical activity [68]. Lanthorn [68] discusses how the chronic use of DCS may inadvertently cause the inhibition or alteration of several secondary messenger pathways, including the nitric oxide pathway. Thus, chronic DCS may cause the drug to act as an antagonist of the NMDA glycine binding site or antagonize populations of NMDA receptors under certain stressful conditions [69]. This inhibition may lead to changes in the induction of LTP and the ineffectiveness of DCS after chronic use.
Although the duration of exposure to DCS, either chronic or acute, elicited changes in average time to extinction, the timing of the 4 acute DCS exposure during the course of extinction did not impact the rate of extinction. Rather, the timing of the 4 DCS exposures impacted spontaneous recovery of the CTA. Of the EU groups, only rats injected with DCS when they reached 20–40% of baseline saccharin consumption (i.e., late in the extinction process) exhibited spontaneous recovery. However, animals that went through the CSO extinction procedure and were exposed to DCS early in extinction (having reached 2–5% of saccharin consumption baseline) demonstrated a spontaneous recovery of a CTA. Why might this difference have occurred?
The CTAs in our studies represent associations between saccharin and LiCl. During extinction, these associations may be weakened by presenting the CS without the US (the traditional CSO procedure). Alternatively, if the US is given without the CS, the bond between the 2 stimuli may be undermined as well. We have noticed that during the static stage of CTA extinction rats sample little or no saccharin [48] (See also Figure 2B). Effectively, they are experiencing the US alone, which would weaken the CS+US bond established during CTA acquisition. (See also recent data suggesting that this same principle applies to conditioned fear paradigms.) [57]. Perhaps this is why acute exposure to DCS early in the EU-EXT process is most effective in reducing spontaneous recovery of the CTA. On the other hand, rats undergoing the CSO procedure will not begin to learn that the previous CS+US contingency is no longer valid until they begin tasting the saccharin and fail to experience the LiCl-induced malaise. Since CSO rats take more days to begin sampling the saccharin, the learning that the CS no longer predicts the US takes place later in the extinction process. Perhaps this is why the DCS inhibits the spontaneous recovery of a CTA if it is given later.
5. Conclusions
The main findings of these experiments may be summarized as follows. Chronic DCS exposures throughout CTA extinction failed to speed up this process whereas acute (N = 4) DCS exposures generally accelerated extinction. Chronic DCS exposure during extinction failed to inhibit spontaneous recovery of a CTA in rats that went through the CSO extinction procedure. Further, it produced a spontaneous recovery in EU-extinguished rats that normally do not show re-emergence of the CTA. The ability of acute DCS treatments to inhibit spontaneous recovery of the CTA was dependent on the timing of the drug administration and extinction method employed. Rats in the EU and CSO extinction procedures exhibited a different pattern of CS reacceptance that may help explain why acute DCS treatments are most effective in suppressing spontaneous recovery of a CTA depending on when the drug is given. Our pilot studies and published literature [43,44] indicate that these effects were not attributable to DCS-induced changes in gustatory sensation of saccharin nor did DCS act as a US or significantly change LiCl-induced malaise.
The exact neural alterations that are produced by acute vs. chronic DCS treatments remain to be fully elucidated but the effects of chronic administration of DCS may cause a variety of changes at the neuronal level that may be consistent with glutamate antagonism [68]. Our data may help guide the methods used to explore these brain changes as DCS treatment parameters are refined in the clinic.
Highlights.
Effects of DCS on extinction (EXT) & spontaneous recovery (SR) of conditioned taste aversion (CTA)
Chronic DCS fails to speed EXT and enhances SR of a CTA
Effects of acute DCS depend on the method of CTA EXT employed
Acute DCS generally shorted the time to reach asymptotic EXT
The timing of acute DCS during EXT & method of EXT interact to affect SR of CTA
Acknowledgments
Supported by NIMH grants: 2-R15-MH063720-03 and 3-R15-MH063720-03S1. The authors would like to acknowledge the following students, research associates, and technicians for their excellent contributions to this work: Islam Ayad, Katie Burke, Kenneth Bisson, Jennifer Duman, Jasmina Ellis, Chris Gutoskey, Mike Hanna, Natalie Hogan, Nita Hoxha, Sarah Hummel, Mariana Iskander, Ivan Islamaj, Pallavi Iyer, Ashlee Jeter, Ye-Hyun Kim, Joseph Luchsinger, Nick McGinty, Mike McGinty, Aaron McNair, Henry Morchak, John Norbert, Kim Parkinson, Doug Placko, Ginger Portman, Suzie Prodan, Marcial Rodriguez, Morgan Rogers, James Romanchik, Ashley Sandmann, Andi Sulovari, Faith Tandoc, Stephen Toth, Dave Revta, and Julianne Wetula.
Abbreviations
- BDNF
brain derived neurotrophic factor
- CR
conditioned response
- CS
conditioned stimulus
- CSO
CS only
- CSO-EXT
CS-only extinction procedure
- CTA
conditioned taste aversion
- DCS
D-cycloserine
- EU
explicitly unpaired
- EU-EXT
explicitly unpaired extinction procedure
- EXT
extinction
- GABA
gamma-Aminobutyric acid
- LiCl
lithium chloride
- LTP
long-term potentiation
- NMDA
N-methyl-D-aspartate
- PTSD
post-traumatic stress disorder
- SAC
saccharin
- SR
spontaneous recovery
- US
unconditioned stimulus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
G. Andrew Mickley, Email: amickley@bw.edu.
Jennifer L. Remus, Email: JenniferLRemus@Gmail.com.
Linnet Ramos, Email: lramos1984@gmail.com.
Gina N. Wilson, Email: ginanwilson@gmail.com.
Orion R. Biesan, Email: orionbiesan@gmail.com.
Kyle D. Ketchesin, Email: kketches@umich.edu.
References
- 1.Pavlov IP. Translated and edited by Anrep GV. London: Oxford University Press; 1927. Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex. [Google Scholar]
- 2.Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical Conditioning II. New York: Appleton-Century-Crofts; 1972. [Google Scholar]
- 3.Rescorla R. Effect of US habituation following conditioning. J Comp Physiol Psychol. 1973;82:137–43. doi: 10.1037/h0033815. [DOI] [PubMed] [Google Scholar]
- 4.Bouton ME. A contextual analysis of fear extinction. In: Martin PR, editor. Handbook of behavior therapy and psychological science: An integrative approach. New York: Pergamon Press; 1991. pp. 435–53. [Google Scholar]
- 5.Bouton ME. Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–86. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- 6.Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–94. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- 7.Mickley GA, Kenmuir CL, McMullen CA, Yocom AM, Valentine EL, Dengler-Crish CM, et al. Dynamic processing of taste aversion extinction in the brain. Brain Res. 2004;1016:79–89. doi: 10.1016/j.brainres.2004.04.071. [DOI] [PubMed] [Google Scholar]
- 8.Rescorla R, Heth C. Reinstatement of fear to an extinguished conditioned stimulus. J Exp Psychol Anim Behav Process. 1975;1:88–96. [PubMed] [Google Scholar]
- 9.Royer S, Pare D. Bidirectional synaptic plasticity in intercalated amygdala neurons and the extinction of conditioned fear responses. Neuroscience. 2002;115:455–62. doi: 10.1016/s0306-4522(02)00455-4. [DOI] [PubMed] [Google Scholar]
- 10.Langton J, Richardson R. D-cycloserine facilitates extinction the first time but not the second time: An examination of the role of NMDA across the course of repeated extinction sessions. Neuropsychopharmacology. 2008;33:3096–102. doi: 10.1038/npp.2008.32. [DOI] [PubMed] [Google Scholar]
- 11.Langton J, Richardson R. The role of context in the re-extinction of learned fear. Neurobiol Learn Mem. 2009;92:496–503. doi: 10.1016/j.nlm.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Kim JH, Richardson R. New findings on extinction of conditioned fear early in development: Theoretical and clinical implications. Biol Psychiatry. 2010;67:297–303. doi: 10.1016/j.biopsych.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Falls WA, Miserendino MJD, Davis M. Extinction of fear-potentiated startle: Blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci. 1992;12:854–63. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan WY, McNally GP. Conditioned stimulus familiarity determines effects of MK-801 on fear extinction. Behav Neurosci. 2009;123:303–14. doi: 10.1037/a0014988. [DOI] [PubMed] [Google Scholar]
- 15.Andersen JM, Fonnum F, Myhrer T. D-serine alleviates retrograde amnesia of a visual discrimination task in rats with a lesion of the perirhinal cortex. Brain Res. 2003;979:240–4. doi: 10.1016/s0006-8993(03)02894-4. [DOI] [PubMed] [Google Scholar]
- 16.Mothet JP, Rouaud E, Sinet PM, Potier B, Jouvenceau A, Dutar P, et al. A critical role for the glial-derived neuromodulator D-serine in the age-related deficits of cellular mechanisms of learning and memory. Aging Cell. 2006;5:267–74. doi: 10.1111/j.1474-9726.2006.00216.x. [DOI] [PubMed] [Google Scholar]
- 17.Baran H, Gramer M, Loscher W. Alterations in plasma and brain amino acids after administration of the glycine/NMDA receptor partial agonists, d-cycloserine, to mice and rats. Eur J Pharmacol. 1995;273:197–201. doi: 10.1016/0014-2999(94)00745-s. [DOI] [PubMed] [Google Scholar]
- 18.Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, et al. Cognitive enhances as adjuncts to psychotherapy: Use of d-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–44. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- 19.Hood WF, Compton RP, Monahan JB. D-cycloserine: A ligand for the N-methyl-D-aspartate coupled glycine receptor has partial agonist characteristics. Neurosci Lett. 1989;98:91–5. doi: 10.1016/0304-3940(89)90379-0. [DOI] [PubMed] [Google Scholar]
- 20.Watson GB, Bolanowski MA, Baganoff MP, Deppeler CL, Lanthorn TH. D-cycloserine acts as a partial agonist at the glycine modulatory site of the NMDA receptor expressed in Xenopus oocytes. Brain Res. 1990;510:158–60. doi: 10.1016/0006-8993(90)90745-w. [DOI] [PubMed] [Google Scholar]
- 21.Norberg MM, Krystal JH, Tolin DF. A meta-analysis of d-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63:1118–26. doi: 10.1016/j.biopsych.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Land C, Riccio DL. D-cycloserine, a positive modulator of the NMDA receptor, enhances acquisition of a conditioned taste aversion. Psychobiology. 1997;25:210–16. [Google Scholar]
- 23.Ledgerwood L, Richardson R, Cranney J. Effects of D-cycloserine on extinction of conditioned freezing. Behav Neurosci. 2003;117:341–49. doi: 10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- 24.Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine assessed with fear potentiated startle in rats. J Neurosci. 2002;22:2343–51. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffmann SG, Meuret AE, Smits JA, Simon NM, Pollack MH, Eisenmenger K, et al. Augmentation of exposure thereapy with d-cycloserine for social anxiety disorder. Arch Gen Psychiatry. 2006;63:298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- 26.Kushner MG, Kim SW, Donahue C, Thuras P, Adson D, Kotlyar M, et al. D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biol Psychiatry. 2007;62:835–38. doi: 10.1016/j.biopsych.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 27.Guastella AJ, Dadds MR, Lovibond PF, Mitchell P, Richardson R. A randomized controlled trial of the effect of D-cycloserine on exposure therapy for spider fear. J Psychiatr Res. 2007;41:466–71. doi: 10.1016/j.jpsychires.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Guastella AJ, Lovibond PF, Dadds MR, Mitchell P, Richardson R. A randomized controlled trial of the effect of D-cycloserine on extinction and fear conditioning in humans. Behav Res Ther. 2007;45:663–72. doi: 10.1016/j.brat.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Lelong V, Duphin F, Boulouard M. RS 67333 and D-cycloserine accelerate learning acquisition in the rat. Neuropharmacology. 2001;41:517–22. doi: 10.1016/s0028-3908(01)00085-5. [DOI] [PubMed] [Google Scholar]
- 30.Botreau F, Paolone G, Stewart J. D-Cycloserine facilitates extinction of a cocaine-induced conditioned place preference. Behav Brain Res. 2006;172:173–78. doi: 10.1016/j.bbr.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Ledgerwood L, Richardson R, Cranney J. D-cycloserine and the facilitation of extinction of conditioned fear: consequences for reinstatement. Behav Neurosci. 2004;118:505–13. doi: 10.1037/0735-7044.118.3.505. [DOI] [PubMed] [Google Scholar]
- 32.Vervliet B. Learning and memory in conditioned fear extinction: Effects of D-cycloserine. Acta Psychol. 2008;127:601–13. doi: 10.1016/j.actpsy.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of D-cycloserine on extinction: Translation from preclinical to clinical work. Biol Psychiatry. 2006;60:369–75. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 34.Richardson R, Ledgerwood L, Cranney J. Facilitation of fear extinction by D-cycloserine: Theoretical and clinical implications. Learn Mem. 2004;11:510–16. doi: 10.1101/lm.78204. [DOI] [PubMed] [Google Scholar]
- 35.Langton J, Richardson R. The effect of D-cycloserine on immediate vs. delayed extinction of learned fear. Learn Mem. 2010;17:547–51. doi: 10.1101/lm.1927310. [DOI] [PubMed] [Google Scholar]
- 36.Parnas AS, Weber M, Richardson R. Effects of multiple exposures to D-cycloserine on extinction of conditioned fear in rats. Neurobiol Learn Mem. 2005;83:224–31. doi: 10.1016/j.nlm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Quartermain D, Mower J, Rafferty MF, Hertin RL, Lanthorn TH. Acute but not chronic activation of the NMDA-coupled glycine receptor with D-cycloserine facilitates learning and retention. Environ Toxicol Pharmacol. 1994;257:7–12. doi: 10.1016/0014-2999(94)90687-4. [DOI] [PubMed] [Google Scholar]
- 38.Garcia J, Kimeldorf DJ, Knelling RA. Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science. 1955;122:157–8. [PubMed] [Google Scholar]
- 39.Garcia J, Kimeldorf DJ, Hunt EL. The use of ionizing radiation as a motivating stimulus. Radiat Res. 1961;12:719–727. doi: 10.1037/h0038361. [DOI] [PubMed] [Google Scholar]
- 40.Garcia J, McGowan BK, Ervin FR, Koelling RA. Cues: Their relative effectiveness as a function of the reinforcer. Science. 1968;160:794–95. doi: 10.1126/science.160.3829.794. [DOI] [PubMed] [Google Scholar]
- 41.Houpt TA, Philopena JM, Joh TH, Smith GP. c-Fos induction in the rat nucleus of the solitary tract correlates with the retention and forgetting of a conditioned taste aversion. Learn Mem. 1996;3:25–30. doi: 10.1101/lm.3.1.25. [DOI] [PubMed] [Google Scholar]
- 42.Berman DE, Dudai Y. Memory extinction, learning anew, and learning the new: Dissociations in the molecular machinery of learning in cortex. Science. 2001;291:2417–19. doi: 10.1126/science.1058165. [DOI] [PubMed] [Google Scholar]
- 43.Nunnink M, Davenport RA, Orega B, Houpt TA. D-cycloserine enhances conditioned taste aversion learning in rats. Pharmacol Biochem Behav. 2007;87:321–30. doi: 10.1016/j.pbb.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davenport RA, Houpt TA. D-cycloserine enhances short-delay, but not long-delay, conditioned taste aversion learning in rats. Pharmacol Biochem Behav. 2009;4:596–603. doi: 10.1016/j.pbb.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mickley GA, Wilson GN, Remus J, Bieson O. Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience online; 2009. D-Cycloserine fails to facilitate extinction of a conditioned taste aversion and potentiates spontaneous recovery. http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=0991bd41-75da-4bc5-bf93-005824de969a&cKey=daa81dd0-7366-40c1-8947-a5b27e1dfb03&mKey=%7b081F7976-E4CD-4F3D-A0AF-E8387992A658%7d. [Google Scholar]
- 46.Yu H, Wang Y, Pattwell S, Jing D, Liu T, Zhang Y, et al. Variant BDNF Val66Met polymorphism affects extinction of conditioned aversive memory. J Neurosci. 2009;29:4056–64. doi: 10.1523/JNEUROSCI.5539-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akirav I, Segev A, Motanis H, Maroun M. D-cycloserine into the BLA reverses the impairing effects of exposure to stress on the extinction of contextual fear, but not on conditioned taste aversion. Learn Mem. 2009;16:682–86. doi: 10.1101/lm.1565109. [DOI] [PubMed] [Google Scholar]
- 48.Mickley GA, DiSorbo A, Wilson GN, Huffman J, Bacik S, Hoxha Z, et al. Explicit disassociation of a conditioned stimulus and unconditioned stimulus during extinction training reduces both time to asymptotic extinction and spontaneous recovery of a conditioned taste aversion. Learn Motiv. 2009;40:209–20. doi: 10.1016/j.lmot.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woods AM, Bouton ME. D-cycloserine facilitates extinction but does not eliminate renewal of the conditioned emotional response. Behav Neurosci. 2006;120:1159–62. doi: 10.1037/0735-7044.120.5.1159. [DOI] [PubMed] [Google Scholar]
- 50.Rauhut A, Thomas B, Ayres J. Treatments that weaken Pavlovian conditioned fear and thwart its renewal in rats: implications for treating human phobias. J Exp Psychol Anim B. 2001;27:99–114. [PubMed] [Google Scholar]
- 51.National Research Council. Guide for the care and use of laboratory animals. Washington, D.C: National Academy Press; 1996. [Google Scholar]
- 52.Nolan LJ, McCaughy SA, Giza BK, Rhinehart-Doty JA, Smith JC, et al. Extinction of a conditioned taste aversion in rats: I. behavioral effects. Physiol Behav. 1997;61:319–23. doi: 10.1016/s0031-9384(96)00411-8. [DOI] [PubMed] [Google Scholar]
- 53.Mickley GA, Remmers-Roeber DR, Dengler CM, McMullen CA, Kenmuir CL, Girdler B, et al. Simple behavioral methods to assess the effect of drugs or toxins on sensory experience. J Neurosci Methods. 2002;115:85–92. doi: 10.1016/s0165-0270(02)00005-5. [DOI] [PubMed] [Google Scholar]
- 54.Wood AJ, Goodwin GM, DeSouza RD, Green AR. The pharmacokinetic profile of lithium in rat and mouse: An important factor in psychopharmacological investigations of the drug. Neuropharmacology. 1986;25:1285–88. doi: 10.1016/0028-3908(86)90149-8. [DOI] [PubMed] [Google Scholar]
- 55.Bakner L, Strohen K, Marvin N, Riccio DC. Postconditioning recovery from the latent inhibition effect in conditioned taste aversions. Physiol Behav. 1991;50:1269–72. doi: 10.1016/0031-9384(91)90595-f. [DOI] [PubMed] [Google Scholar]
- 56.Gillan DJ, Domjan M. Taste-aversion conditioning with expected versus unexpected drug treatment. J Exp Psychol Anim B. 1977;3:297–309. doi: 10.1037//0097-7403.3.4.297. [DOI] [PubMed] [Google Scholar]
- 57.Diaz-Mataix L, Debiec J, LeDoux JE, Doyere V. Sensory-specific associations stored in the lateral amygdala allow for selective alteration of fear memories. J Neurosci. 2011;31:9538–43. doi: 10.1523/JNEUROSCI.5808-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bouton ME, Vurbic D, Woods AM. D-cycloserine facilitates context-specific fear extinction learning. Neurobiol of Learn Mem. 2008;90:504–10. doi: 10.1016/j.nlm.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Akirav I. NMDA partial agonist reverses blocking of extinction of aversive memory by GABAA agonist in the amygdala. Neuropsychopharamacology. 2007;32:542–50. doi: 10.1038/sj.npp.1301050. [DOI] [PubMed] [Google Scholar]
- 60.Lee JLC, Milton AL, Everitt BJ. Reconsolidation and extinction of conditioned fear: inhibition and potentiation. J Neurosci. 2006;26:10051–56. doi: 10.1523/JNEUROSCI.2466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thomas BL, Novak C, Radic T, Goodwin S. MK-801 blocks fear expression and increases fear renewal in rats. Poster presented at the Western Psychological Association conference; Cancun, Mexico. April 2010. [Google Scholar]
- 62.Groblewski PA, Lattal MK, Cunningham CL. Effects of D-cycloserine on extinction and reconditioning of ethanol-seeking behavior in mice. Alcohol Clin Exp Res. 2009;33:772–82. doi: 10.1111/j.1530-0277.2009.00895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parker LA. Taste avoidance and taste aversion: Evidence for two different processes. Learn Behav. 2003;31:165–72. doi: 10.3758/bf03195979. [DOI] [PubMed] [Google Scholar]
- 64.Johnson J, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–31. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- 65.Goff DC, Henderson DC, Evins AE, Amico E. A placebo-controlled crossover trial of D-cycloserine added to clozapine in patients with schizophrenia. Biol Psychiatry. 1999;45:512–14. doi: 10.1016/s0006-3223(98)00367-9. [DOI] [PubMed] [Google Scholar]
- 66.Coyle JT, Tsai G. The NMDA receptor glycine modulatory site: A therapeutic target for improving cognition and reducing negative symptoms of schizophrenia. Psychopharmacology. 2004;174:32–38. doi: 10.1007/s00213-003-1709-2. [DOI] [PubMed] [Google Scholar]
- 67.Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of D-cycloserine on extinction: Translation from preclinical to clinical work. Biol Psychiatry. 2006;60:369–75. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 68.Lanthorn TH. D-cycloserine: agonist turned antagonist. Amino Acids. 1994;6:247–60. doi: 10.1007/BF00813745. [DOI] [PubMed] [Google Scholar]
- 69.Yamamoto S, Morinobu S, Fuchikami M, Kurata A, Kozuru T, Yamawaki S. Effects of single prolonged stress and D-cycloserine on contextual fear extinction and hippocampal NMDA receptor expression in a rat model of PTSD. Neuropsychopharmacology. 2008;33:2108–16. doi: 10.1038/sj.npp.1301605. [DOI] [PubMed] [Google Scholar]