Abstract
Individuals who abuse methamphetamine (MA) perform at levels below those of healthy controls on tests that require cognitive control. As cognitive control deficits may influence the success of treatment for addiction, we sought to help clarify the neural correlates of this deficit. MA-dependent (n=10, abstinent 4–7 days) and control subjects (n=18) performed a color-word Stroop task, which requires cognitive control, during functional MRI (fMRI). The task included a condition in which participants were required to respond to one stimulus dimension while ignoring another conflicting dimension, and another condition without conflict. We compared the groups on performance and neural activation in the two conditions. MA-dependent subjects made more errors and responded more slowly than controls. Controlling for response times in the incongruent condition, voxel-wise mixed effects analyses (whole-brain corrected) demonstrated that MA-dependent subjects had less activation than control subjects in the right inferior frontal gyrus, supplementary motor cortex/anterior cingulate gyrus and the anterior insular cortex during the incongruent condition only. MA-dependent subjects did not exhibit greater activation in any brain region in either of the Stroop conditions. These preliminary findings suggest that hypofunction in cortical areas that are important for executive function underlies cognitive control deficits associated with MA dependence.
Keywords: Methamphetamine, Stroop, fMRI, cognitive control, prefrontal cortex, insula
1. Introduction
Individuals who chronically use methamphetamine (MA) exhibit cognitive impairments (Scott et al., 2007; Simon et al., 2010) that may contribute to continued drug use (Lyvers 2000; Goldstein 2002; Lubman et al., 2004) and relapse (Paulus et al., 2005). MA-associated impairments have been observed on tests of inhibitory control (Salo et al., 2002; Monterosso et al., 2005), learning (Volkow et al., 2001b), and decision-making (Paulus et al., 2002; 2003). These deficits persist during abstinence from MA (Chang et al., 2002; Nordahl et al., 2003) with no significant improvement during the first month (Simon et al., 2010). As most current therapies for MA dependence involve behavioral interventions (Lee and Rawson, 2008; Smout et al., 2010), cognitive deficits may interfere with the engagement and completion of a course of treatment for stimulant dependence (Vocci, 2008).
Cognitive deficits may reflect MA-related abnormalities in brain circuits that are important for executive functions. For example, MA-dependent individuals exhibit abnormalities in gray matter structure of cingulate, limbic and paralimbic regions of cortex (Thompson et al., 2004; Berman et al., 2008a; Salo et al., 2009a;). In parallel, functional abnormalities, indexed by regional cerebral glucose metabolism, in the anterior cingulate cortex (ACC), lateral prefrontal cortex (PFC), and parietal cortex have been observed in individuals who had chronically abused MA ( Volkow et al., 2001a; London et al., 2004; Wang et al., 2004; Berman et al., 2008b).
The switch from voluntary to compulsive drug abuse has been hypothesized to reflect a transition from healthy executive functioning, largely mediated by the prefrontal cortex (PFC), to striatal control over drug-taking behaviors (Everitt et al., 2008). According to this view, a loss of cognitive control is fundamental to the development of addiction. This concept is supported by research with animal models (Everitt et al., 2007; Olausson et al., 2007) as well as human volunteers (Garavan et al., 2008). Cognitive control can be viewed as flexible, goal-directed behavior that requires a mechanism for guidance to allow for appropriate actions given contextually relevant information (Ridderinkhof et al., 2004b).
One aspect of cognitive control is the ability to resolve conflicting responses to environmental stimuli which may affect behavior when abstinent drug users perceive stimuli that might precipitate drug relapse (Garavan and Stout, 2005). The detection of response conflict involves the ACC (Bush et al., 2000; Botvinick et al., 2004; Ridderinkhof et al., 2004a; Wendelken et al., 2009; Yeung and Nieuwenhuis, 2009), and fMRI studies have identified the ACC, lateral PFC and parietal cortex as being important with respect to the resolution of response conflict (Peterson et al., 2002; Liu et al., 2004). As noted above, these regions exhibit structural or functional abnormalities associated with MA abuse.
The Stroop task (Stroop 1935; MacLeod 1991) requires cognitive control in that optimal performance on the task requires subjects to respond to one dimension of a stimulus while ignoring another conflicting dimension. We selected the Stroop task for this study because it may measure a function that is important to the success of treatment for MA dependence. Indeed, several prior studies have shown a relationship between Stroop performance and substance-abuse treatment. Performance on the Stroop task predicted compliance with treatment among cocaine-dependent individuals, suggesting that this task may be used to identify cocaine-dependent subjects at risk for dropout from treatment (Streeter et al., 2008). In an fMRI study of cocaine-dependent individuals, activation during the Stroop task predicted self-reported duration of abstinence (Brewer et al., 2008). Another study has shown activation deficits in the PFC of abstinent MA-dependent subjects performing a version of the Stroop task that measures the ability to regulate behavior as a result of exposure to previous conflict (Salo et al., 2009c). MA-dependent subjects in that study were abstinent 2 – 12 months. As engagement in treatment during the first weeks of abstinence from MA may be important to the ultimate success of treatment, we studied MA-dependent subjects during early abstinence (4 – 7 days) and hypothesized that, during this time, subjects would show activation deficits in the ACC and PFC when faced with cognitive conflict as presented in the Stroop task.
2. Methods
2.1 Subjects
Ten MA-dependent participants (20–46 years of age; mean ± SD = 33.5 ± 9.3; abstinent 4–7 days) and 18 control participants (20–55 years of age; mean ± SD = 36.4 ± 10.4) completed the study. Potential subjects were recruited from the greater Los Angeles metropolitan area through flyers and newspaper advertisements. Those passing an initial telephone screening were invited to our laboratory where they received a detailed explanation of the study and provided written informed consent, as approved by the Institutional Review Board at the University of California, Los Angeles (UCLA). All participants completed questionnaires covering demographic, medical and psychiatric histories. English language fluency and right-handedness, as indicated by the Edinburgh Handedness Questionnaire (Oldfield, 1971), were inclusion requirements. The primary exclusion criteria were: current use of psychotropic medications or other medications that affect cognitive functioning, prior hospitalization for psychiatric illness and head trauma involving loss of consciousness and/or requiring hospitalization.
The Structured Clinical Interview for DSM-IV was used to diagnose MA-dependence, which was required for the MA group, and to exclude participants from either group who met criteria for any Axis I psychiatric disorder other than nicotine dependence (or MA dependence for the MA group). Detailed drug use histories (see Table 1) were obtained using the Addiction Severity Index (McLellan et al., 1992). Although none of the participants met criteria for current abuse or dependence on an illicit drug of abuse other than MA (MA group only), seven of the ten MA subjects had lifetime histories of other drug abuse. These diagnoses included Δ9-tetrahydrocannabinol (THC) abuse (1 subject), THC dependence (3 subjects), alcohol abuse (2 subjects), alcohol dependence (1 subject), opioid abuse (1 subject), and cocaine dependence (2 subjects).
Table 1.
Demographic and drug use measures in healthy control and methamphetamine-dependent subjects.a
| MA (n=10) | Control (n=18) | |
|---|---|---|
| Age | 33.5 ± 9.3 | 36.4 ± 10.4 |
| Years of Education | 12.9 ± 1.7 | 13.8 ± 1.9 |
| Depression (BDI) | 3.4 ± 3.7 | 1.2 ± 2.2 |
| Males/Females | 5/5 | 11/7 |
| Cigarette Smoking (years) | 17.3 ± 11.3 | 18.2 ± 10.1 |
| Cigarettes Smoked (per day) | 21.0 ± 3.2 | 18.8 ± 4.5 |
| Alcohol Use (years) | 16.0 ± 3.8 | 19.2 ± 10.1 |
| Alcohol Use (days in last 30) | 2.5 ± 4.9 | 6.7 ± 8.7 |
| Marijuana Use (years) | 8.9 ± 12.8 | 7.9 ± 10.1 |
| Marijuana Use (days in last 30) | 3.2 ± 6.4 | 1.0 ± 2.4 |
| MA use (years) | 8.3 ± 3.7 | |
| MA use (days in last 30) | 18.5 ± 9.6 | |
| Average (gm/week) | 8.4 ± 7.3 |
Values shown are means ± SEM for each group.
Beck Depression Inventory (BDI) taken on day of scan.
Urine toxicology during the screening process and on the day of admission confirmed recent MA use by all of the MA subjects, and they all tested negative for other drugs of abuse (i.e., cocaine, methamphetamine, opioids, THC and benzodiazepines). Urine toxicology screening of potential control subjects was also conducted, with any positive test for illicit drugs of abuse resulting in exclusion from study participation. All participants tested negative for illicit drugs of abuse and alcohol (using a breath-based assessment; Alco-Sensor FST®, Intoximeters, Inc., St. Louis, MO) on the day of testing.
2.2 Stroop Color-Word Interference Task
The stimuli were four color words (“RED”, “BLUE”, “GREEN”, and “YELLOW”) which were presented in congruent (e.g., the word “RED” displayed in red) and incongruent (e.g., the word “RED” displayed in blue) conditions. Stimuli were presented via magnet-compatible VGA goggles (Resonance Technology, Northridge, CA), which have a field view of approximately 20° vertically and 30° horizontally, and display computer images at 800 × 600 pixel resolution. Words were presented individually at the center of the screen in Helvetica style font, size 72.
This study used a block design with congruent, incongruent and rest blocks, presented over two runs (runs counterbalanced across subjects). Each run consisted of 8 congruent, 8 incongruent and 15 rest blocks (12 trials per block) with the congruent and incongruent blocks presented alternately during each run. During congruent/incongruent blocks, subjects were to identify the font color of each word presented. The instruction was to respond as quickly as possible by pressing a button using either their right index, middle, ring, or baby finger, corresponding to red, blue, green, and yellow, respectively. Responses were registered using a magnetic-compatible four-button response box. Participants were trained on the correct finger positions and were tested for verification before the first run of the task. During rest blocks, subjects viewed a fixation cross at the center of the screen. Before each block, instructions (“Identify the Color” or “Rest”) were presented for a 2-sec period. Within a block, each stimulus was presented for 1200 msec with an inter-stimulus interval of 300 msec. Each task block lasted 18 sec, and each rest block lasted 9 sec. Each run of the task was approximately 7 min in duration. Dependent measures were the mean percentage of errors and the mean response time (RT) for the congruent and incongruent conditions. As an index of behavioral response conflict, the Stroop effect was calculated from these measures (incongruent RT - congruent RT). To ensure that the Stroop effect did not merely represent an overall increased RT in either group, we calculated the percentage increase from congruent to incongruent trials [(incongruent RT - congruent RT)/congruent RT] in an additional analysis.
2.3 Scanning parameters
Functional images were acquired on a 3T Siemens Allegra (Erlangen, Germany) head-only MRI scanner. Localizing scans were acquired to verify the head position and to identify the AC-PC line for establishing the acquisition plane. We then acquired a T2-weighted, high-resolution, echo-planar anatomical image (26 axial-oblique slices, aligned to the AC-PC line, 4 mm thick with a 1.0-mm inter-slice interval, 1.56 mm2 in-plane pixel resolution) covering the entire brain volume and used for spatial alignment. Functional images with the same slice positioning as the T2-weighted image were acquired using a gradient-echo echo-planar image (EPI) sequence (1500 msec TR, 30 msec TE, 80 degree flip angle, 26 slices, 4 mm slice thickness with a 1.0 mm inter-slice interval, 64 × 64 matrix, and 3.12 mm2 in-plane pixel resolution). During each run of the task, 282 brain volumes were collected.
2.4 Data analyses
Image pre-processing and statistical analyses were conducted using the FMRIB Software Library (FSL 4.1, http://www.fmrib.ox.ac.uk/fsl). Image pre-processing steps were as follows: motion correction utilizing FMRIB’s Linear Image Registration Tool (MCFLIRT) (Jenkinson and Smith, 2001); non-brain matter removal using Brain Extraction Tool (BET) (Smith, 2002); spatial smoothing with a 6-mm full-width half maximum Gaussian kernel; mean-based intensity normalization; nonlinear high-pass temporal filtering (Gaussian-weighted least squares straight line fit, with sigma = 25.0 sec). We also used FSL’s multivariate exploratory linear optimized decomposition into independent components (MELODIC) tool to decompose the 4D fMRI data into spatial and temporal components (Beckman and Smith, 2004). We manually delineated artefactual components (e.g., scanner-related or physiological noise) using a set of heuristics (Tohka et al., 2008) and reconstructed the fMRI data after removing them. Whole-brain fixed-effects statistical analyses were then performed by modelling the congruent, incongruent and incongruent>congruent contrast (boxcar functions convolved with the hemodynamic response function) as explanatory variables within the context of the general linear model on a voxel-by-voxel basis. Registration was conducted through a two-step procedure, whereby EPI images were first registered to the high-resolution T2 structural image, then into standard (Montreal Neurological Institute, MNI avg152 template) space, with 12-parameter affine transformations (Jenkinson and Smith, 2001). Statistical analyses were performed in native space, and the statistical maps were normalized to standard space prior to higher-level analyses. Higher-level analyses were carried out using FLAME (FMRIB’s Local Analysis of Mixed Effects) (Beckmann et al., 2003; Woolrich et al., 2004). For voxel-by-voxel one-sample (within groups) and two-sample (between groups) t-tests, resulting Z (Gaussianised T) statistic images were thresholded using a cluster extent determined by p<0.05 (corrected for multiple comparisons across the whole brain) with a voxel-wise height threshold of Z>2.3 (Worsley et al., 1992). Two sets of two-sample (between groups) t-test analyses were performed on the congruent, incongruent and Stroop Effect conditions. In the first set of analyses, individual RT values were not included in the analysis model, but in the second set of analyses, the mean RT of each subject for the each condition was included as a covariate of no interest to remove the possible confounding effect of performance and correct for differences in time-on-task across subjects (see Carp et al., 2010 for discussion on covarying with RT).
3. Results
3.1 Demographics
The MA and control groups did not differ significantly on age, Beck Depression Inventory (BDI) scores, years of education, gender distribution and histories of tobacco, alcohol, or marijuana use (Table 1).
3.2 Behavioral results
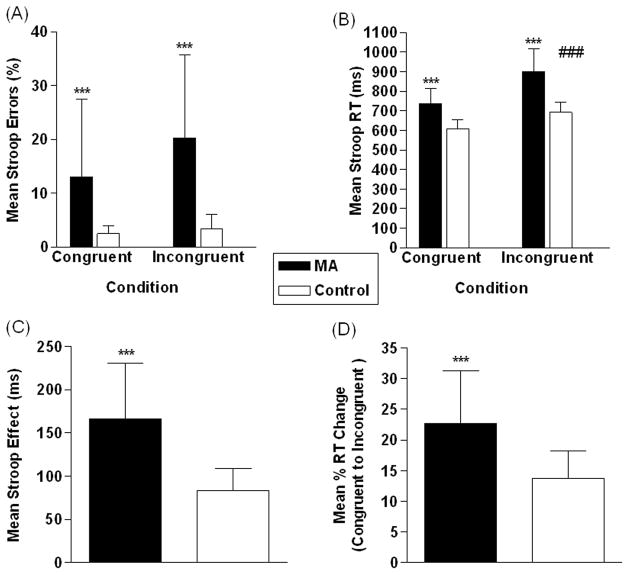
Percentage of trials in which participants made errors for both congruent and incongruent conditions are shown in Figure 1A. There was no significant effect of condition (F=2.6, df=1,52, p=0.1), a significant effect of group (F=29.7, df=1,52, p<0.001 - MA>control), but no significant condition x group interaction (F=1.5, df=1,52, p=0.2). Analysis of RTs (Figure 1B) revealed significant effects of condition (F=38.0, df=1,52, p<0.001 - incongruent>congruent, group (F=68.6, df=1,52, p<0.001 - MA>control), and a condition x group interaction (F=4.2, df=1,52, p<0.05). Planned group comparisons showed a significant group difference in RT for congruent (t=4.8, df=26, p<0.001 - MA>control) and incongruent stimuli (t=4.5, df=26, p<0.001 - MA>control). There was a significant group difference for the Stroop effect when examining raw RT values (t=4.8, df=26, p<0.001 - MA>control) (Figure 1C). We also found a significant group difference in RT change (as a percentage of baseline) from congruent to incongruent trials (t=3.6, df=26, p<0.001 - MA>control) (Figure 1D).
Figure 1.
3.3 fMRI results
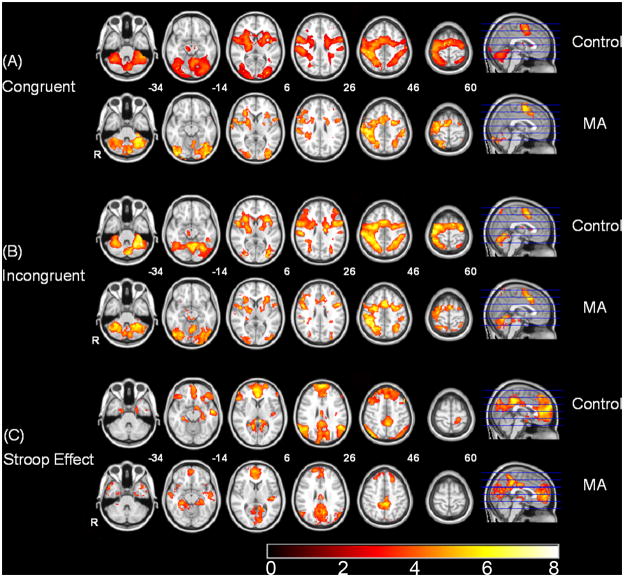
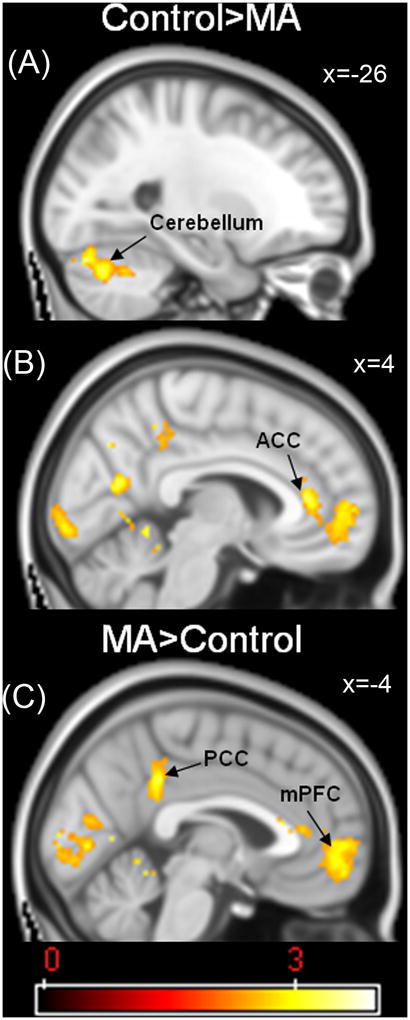
Initial one-sample t-test analyses in both groups were conducted to reveal areas of activation across the three contrasts of interest. For the congruent (Figure 2A) and incongruent (Figure 2B) conditions, both groups demonstrated robust activations in whole brain analyses with effects in the bilateral anterior cingulate cortex, insula, and lateral prefrontal cortex, as well as the temporal, parietal and occipital lobes. For the Stroop Effect (Figure 2C), both groups demonstrated activations in the bilateral anterior and posterior cingulate cortices, inferior frontal gyrus and dorso-lateral prefrontal cortex (DLPFC, Brodmann areas 9 and 46). Initially, we performed simple two-sample (between groups) t-test analyses to reveal group differences in the three conditions of interest (congruent, incongruent, and Stroop Effect). Table 2 shows the results of a mixed-effects whole-brain group analysis. The two groups significantly differed only on the Stroop Effect with MA-dependent subjects exhibiting less activation than controls in the left cerebellum (x=-26, y=−76, z=−26; p<0.01 - Figure 3A) and greater activation in the posterior cingulate cortex (PCC; x =−4; y=−42; z=36; p<0.001 - Figure 3C), right precuneus (p<0.001), left and right occipital poles (p<0.001), intracalcarine cortex (p<0.001), posterior parahippocampal gyrus (p<0.001), left anterior cingulate cortex (p<0.05), right left anterior cingulate cortex (ACC; x=4; y=36; z=10; p<0.05 - Figure 3B), right paracingulate cortex (p<0.05), right frontal pole (p<0.05), and left medial frontal cortex (mPFC; x =−4; y=54; z=−6; p<0.05 - Figure 3C).
Figure 2.
Table 2.
Mixed-effects whole-brain analysis for group (Control versus MA) on the congruent and incongruent conditions, and for the Stroop Effect. Statistical images were first thresholded using a voxel-wise threshold of Z>2.3 and a (corrected) cluster significance threshold of p=0.05. Co-ordinates represented are in Montreal Neurological Institute (MNI) space. The P value corresponding to the maximum Z-statistic within each cluster is shown.
| Region | HS | x(mm) | y(mm) | z(mm) | Z statistic | P |
|---|---|---|---|---|---|---|
| Congruent - Control>MA | - | - | - | - | - | - |
| Congruent - MA>Control | - | - | - | - | - | - |
| Incongruent - Control>MA | - | - | - | - | - | - |
| Incongruent - MA>Control | - | - | - | - | - | - |
| Stroop Effect - Control>MA | - | - | - | - | - | - |
| Cerebellum | L | −26 | −76 | −26 | 3.91 | <0.01 |
| Stroop Effect - MA>Control | - | - | - | - | - | - |
| Posterior Cingulate Gyrus | L | −4 | −42 | 36 | 3.74 | <0.001 |
| Precuneus | R | 14 | −68 | 28 | 3.85 | <0.001 |
| Occipital Pole | L | −12 | −90 | 8 | 3.6 | <0.001 |
| Occipital Pole | R | 4 | −100 | −2 | 3.46 | <0.001 |
| Intracalcarine Cortex | L | −4 | −80 | 4 | 3.47 | <0.001 |
| Posterior Parahippocampal Gyrus | L | −10 | −40 | −14 | 3.45 | <0.001 |
| Anterior Cingulate Cortex | L | 0 | 28 | 12 | 3.14 | <0.05 |
| Anterior Cingulate Cortex | R | 4 | 36 | 10 | 3.57 | <0.05 |
| Paracingulate | R | 2 | 52 | 0 | 3.16 | <0.05 |
| Frontal Pole | R | 8 | 60 | 4 | 3.13 | <0.05 |
| Medial Frontal Cortex | L | −4 | 54 | −6 | 2.85 | <0.05 |
Figure 3.
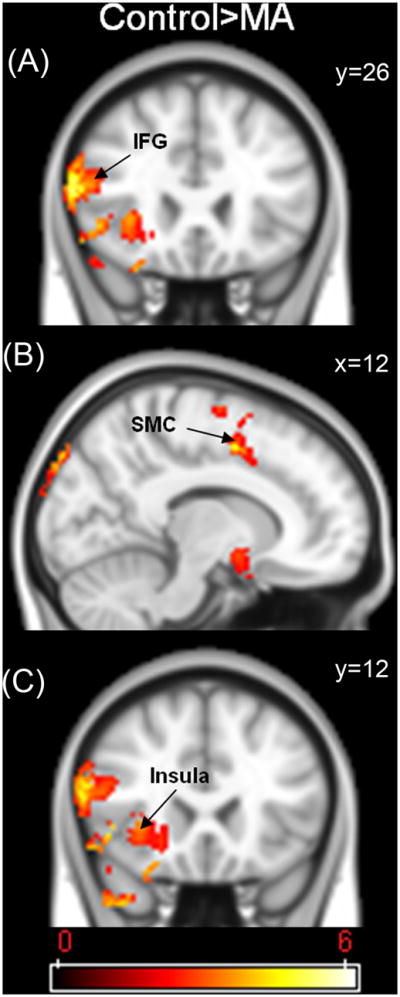
Due to the behavioural performance differences between the two groups, further between group fMRI analyses were conducted controlling for RT since the initial results in the MA group may have been confounded by effects of performance or time-on-task. Time-on-task confounds represented by RT have been observed before in fMRI studies of cognitive conflict (e.g., Carp et al., 2010), and the aim of this analysis was to examine activation related to cognitive control independent of this confound. Table 3 shows the results of this analysis. The two groups significantly differed only on the incongruent condition, with the MA group exhibiting less activation in the right inferior frontal gyrus (IFG; x=60, y=26, z=16; p<0.0001 - Figure 4A), right supramarginal gyrus (p<0.0001), right precentral gyrus (p<0.0001), right supplementary motor cortex/anterior cingulate cortex (SMC; x=12, y=2, z=48; p<0.0001 - Figure 4B), right occipital pole (p<0.001), right lateral occipital cortex (p<0.001), right inferior temporal gyrus (p<0.05), right temporal-occipital fusiform cortex (p<0.05), right middle temporal gyrus (p<0.05), right frontal operculum cortex (p<0.05), right temporal pole (p<0.05), and the anterior insular cortex (x=38, y=12, z=−2; p<0.05 - Figure 4C).
Table 3.
Mixed-effects whole-brain analysis for group (Control versus MA) on the congruent and incongruent conditions, and for the Stroop Effect, covarying for congruent, incongruent and Stroop Effect response times, respectively. Statistical images were first thresholded using a voxel-wise threshold of Z>2.3 and a (corrected) cluster significance threshold of p=0.05. Co-ordinates represented are in Montreal Neurological Institute (MNI) space. The P value corresponding to the maximum Z-statistic within each cluster is shown.
| Region | HS | x(mm) | y(mm) | z(mm) | Z statistic | P |
|---|---|---|---|---|---|---|
| Congruent - Control>MA | - | - | - | - | - | - |
| Congruent - MA>Control | - | - | - | - | - | - |
| Incongruent - Control>MA | ||||||
| Inferior Frontal Gyrus | R | 60 | 26 | 16 | 6.14 | <0.0001 |
| Supramarginal Gyrus | R | 68 | −20 | 36 | 5.44 | <0.0001 |
| Precentral Gyrus | R | 52 | 4 | 54 | 5.09 | <0.0001 |
| SMC/ACC | R | 12 | 2 | 48 | 4.83 | <0.0001 |
| Occipital Pole | R | 22 | −100 | 20 | 6.18 | <0.001 |
| Lateral Occipital Cortex | R | 30 | −66 | 46 | 4.96 | <0.001 |
| Inferior Temporal Gyrus | R | 50 | −56 | −6 | 4.29 | <0.05 |
| Temporal Occipital Fusiform Cortex | R | 42 | −52 | −14 | 4.22 | <0.05 |
| Middle Temporal Gyrus | R | 54 | −54 | 0 | 3.9 | <0.05 |
| Frontal Operculum Cortex | R | 48 | 22 | −4 | 4.49 | <0.05 |
| Temporal Pole | R | 56 | 20 | −10 | 4.26 | <0.05 |
| Insular Cortex | R | 38 | 12 | −2 | 4.15 | <0.05 |
| Incongruent - MA>Control | - | - | - | - | - | - |
| Stroop Effect - Control>MA | - | - | - | - | - | - |
| Stroop Effect - MA>Control | - | - | - | - | - | - |
Figure 4.
4. Discussion
MA-dependent subjects during the first week of abstinence exhibited a performance deficit relative to healthy controls on a color-word Stroop task. This observation is consistent with prior findings in currently using and recently abstinent MA-dependent individuals compared with control and long-term abstinent MA subjects (Simon et al., 2000; Salo et al., 2009a; 2009b). Behavioral evidence for greater response conflict has also been observed in individuals with a history of alcohol and cocaine abuse (Tedstone and Coyle, 2004; Verdejo-Garcia and Perez-Garcia, 2007) and attention-deficit/hyperactivity disorder and schizophrenia (Henik and Salo, 2004; Albrecht et al., 2008) compared with healthy control subjects. Such differences suggest that these populations may share a common neural deficit with respect to cognitive control, possibly in the processing of stimuli that produce response conflict. Despite findings of deficits in Stroop task performance in MA dependence, potentially reflecting problems in cognitive control, stimulant-dependent individuals have been shown to exhibit greater response latencies than healthy control subjects on a Stroop task that used drug-related words, but not in the incongruent condition of a color-word Stroop task (Ersche et al., 2010). Given that these individuals were currently using cocaine or d-amphetamine, it is likely that the stimulants provided cognitive enhancing effects that were not experienced by our abstinent MA subjects in this study.
Greater activation in the right anterior cingulate cortex (ACC) in MA subjects than controls associated with the Stroop effect in initial between-group analyses was of interest as ACC activity and cognitive conflict appear to be correlated. ACC activity increases when “top-down” control is compromised (e.g., Botvinick et al., 1999). Previous research in MA users has demonstrated reduced Stroop-related activation in the right prefrontal cortex (Salo et al., 2009) when BOLD activation was measured after prior exposure to conflict trials. Abstinent cocaine users also exhibit greater Stroop-related activation in the right ACC, but less activation in the left ACC (Bolla et al., 2004). Greater activation in the right ACC has also been observed in depressed and schizophrenic patients relative to control groups ( Wagner et al., 2006; Nordahl et al., 2001). Therefore, exaggerated right ACC activity in MA-dependent subjects may reflect a compensatory response supporting selective attention processes – an effect which has also been observed in abstinent cigarette smokers (Azizian et al., 2009).
Other regions where MA-dependent subjects showed a greater activation than control subjects were the left posterior cingulate cortex (PCC) and occipital cortex. Anatomically linked to the prefrontal cortex (PFC) and striatum, the PCC responds under conditions of sensory arousal (Garavan et al., 2000; Kosten et al., 2006), motivationally-linked attention (Mohanty et al., 2008), evaluation of emotional memories (Maddock, 1999), and following response errors (Menon et al., 2001). Abnormally high glucose metabolism in this region in MA-dependent subjects during error processing has previously been reported (London et al., 2005). It has also been reported that pre-treatment activation in this region during the Stroop task is a significant predictor of self-reported abstinence duration in cocaine-dependent individuals (Brewer et al., 2008), suggesting that it may influence treatment outcome in stimulant dependence.
Greater activation in the right paracingulate and left rostral medial prefrontal cortex of the MA group than the control group during incongruent trials is consistent with the literature on the functions of these regions. The paracingulate cortex participates in higher-order cognitive processing, such as planning (Baker et al., 1996; Dagher et al., 1999), while the medial frontal cortex is involved in monitoring of ongoing actions and performance outcomes (Ridderinkhof et al., 2004a). Moreover, adolescents with severe substance and conduct problems perform similarly on the Stroop task as healthy control subjects, but they exhibit greater PFC and parahippocampal activation on incongruent trials of the task (Banich et al., 2007). While MA subjects in the present study were adults without the added diagnosis of conduct disorder, exaggerated activity in similar regions suggests a common abnormality of drug-abusing populations. In addition to greater activity within regions traditionally associated with response conflict, early abstinence from chronic MA use may elicit a disproportionate response reflecting compensatory change within a more extended assemblage of neural networks.
Given the complexity of neural functions operating during the incongruent condition of the Stroop test, it is difficult to say with certainty that activation was linked to conflict resolution or other aspects of cognitive control. For example, greater activation in MA-dependent subjects may represent neural responses to errors within the aforementioned networks. For example, the ACC has been implicated in detecting cognitive response conflict (Bush et al., 2000; Botvinick et al., 2004; Ridderinkhof et al., 2004a), particularly during situations involving the high likelihood of making an error (Scheffers et al., 1996; Coles et al., 2001; Magno et al., 2006). Therefore, in order to control for the potential confound of performance-related effects (e.g., error-related activity, time on task) during group comparisons of neural activity related to performing the Stroop task (Murphy and Garavan, 2004), response time during each condition was used as a covariate in the fMRI analyses. The results of these analyses demonstrated that the two groups significantly differed only on the incongruent condition in which MA-dependent subjects exhibited less activation in the right inferior frontal gyrus than control subjects. Previous imaging studies of healthy control subjects have implicated the PFC in response conflict (MacDonald et al., 2000; Kerns et al., 2004), with evidence for reduced functioning in the right middle frontal gyrus of abstinent MA users following prior exposure to conflict-related stimuli (Salo et al., 2009c). The inferior frontal gyrus plays an important role in cognitive control (Aron et al., 2003; Ridderinkhof et al., 2004b), and the current findings suggest a deficit in conflict-related neural activity of this region.
Error-related activity in the insular cortex has also been demonstrated in healthy control subjects (Garavan et al., 2002; Klein et al., 2007), with a view that insular cortex activity reflects interoceptive (i.e., bodily) awareness (Critchley et al., 2004). When controlling for incongruent RT, we observed that MA-dependent subjects showed less activation in the right anterior insular cortex compared to the control group. Research suggests that the insular cortex and interoceptive awareness are critical to drug craving and dependence (Gray and Critchley 2007; Naqvi et al., 2007; Paulus, 2007; Naqvi and Bechara, 2008) whereby this region monitors interoceptive “urges” for rewarding stimuli such as drugs. The insular cortex is well positioned to integrate and to link affective value with adaptations in behavior, possibly through its bidirectional connections with regions, such as the orbitofrontal cortex, amygdala, ACC and ventral striatum, which have been implicated in reward-processing and decision-making, (Reynolds and Zahm, 2005).
The MA group also showed less activation in the right supplementary motor cortex (SMC)/ACC during the incongruent condition as compared with the control group. The SMC responds during conditions involving conflict (Garavan et al., 2002; 2003; Fassbender et al., 2004; Hester et al., 2004) while the ACC is involved in monitoring performance, especially during situations where risky decision-making, high response conflict or the high likelihood of error are involved ( Scheffers et al., 1996; Carter et al., 1998; Botvinick et al., 2001; Coles et al., 2001; Magno et al., 2006; Paulus and Frank, 2006). The ACC has been associated with addiction and its cognitive sequelae (Goldstein, 2002; Peoples, 2002; Volkow et al., 2002), with previous research (Peoples, 2002) demonstrating abnormal activity in this region in participants who use cocaine (Kaufman et al., 2003; Bolla et al., 2004), cannabis (Eldreth et al., 2004), opiates (Forman et al., 2004) or MA (London et al., 2004; 2005). The SMC also plays an integral role in the planning of motor actions (Thaler et al., 1995; Lau et al., 2004; Campos et al., 2005; Seo et al., 2007; Wunderlich et al., 2009) and is closely related to the primary motor cortex (Romo et al., 2004), which in the current study, showed lower activation in MA-dependent than control subjects. Therefore, neuronal deficits observed during exposure to conflict-related stimuli in MA-dependent subjects may represent abnormalities in brain areas that participate in action-outcome representations, which help guide action-based choices during instances of cognitive response conflict and its resolution.
Reduced brain activation during early MA abstinence may not just be restricted to regions typically associated with cognitive control, as lower activation (vs. control group) was observed in other areas, including the occipital and temporal cortices during the incongruent condition. During performance of the Stroop task the cognitive control system deploys selective attention mechanisms to bias perceptual processing toward the task-relevant stimulus properties, and away from task-irrelevant, distracting stimuli. This process then modulates activity in the visual pathways, which are involved in extracting target and distracter features of the stimulus (Egner and Hirsch, 2005). Thus, lower activity in the lateral occipital cortex of MA-dependent subjects during the incongruent Stroop condition may signify an inability of the cognitive control network to bias perceptual processing in the visual system towards task-relevant stimulus properties during conflict resolution. Reduced neural functioning in the temporal gyrus of MA users during the incongruent condition may represent problems with conflict resolution, as adaptation to conflict has previously been demonstrated in this region in healthy control subjects (Egner and Hirsch, 2005).
5. Limitations
There were a number of potential limitations of the present study. The small sample size in the MA group was one limitation; however, the use of a blocked fMRI design offered greater signal detection power than event-related designs, potentially requiring fewer subjects than would have been needed for an event-related study (Birn et al., 2002). Although the blocked design we used is in parallel with standard neuropsychological tests of the Stroop effect in which congruent and incongruent stimuli are presented in separate blocks, the design had several disadvantages that have been noted in the literature (Salo et al., 2001; Salo et al., 2002). For example, comparisons of blocked versus randomly-presented stimuli in the Stroop task have shown different performance effects, mainly driven by RT differences in the congruent condition across the two task designs (Salo et al., 2001). Although we did not have the advantage to assess these design differences in our sample, the interaction we observed between condition and group suggests that the Stroop effect was likely due to performance differences in incongruent trials. Use of a blocked design also precluded the separation of brain activity related to trial-to-trial adjustments following previous exposure to cognitive conflict (Kerns et al., 2004), preventing us from analyzing activation separately related to successful and unsuccessful conflict resolution. There is also evidence that the Stroop effect decreases with an increasing level of incongruity (greater proportion of incongruent trials) together with increased ACC BOLD activity (Mitchell, 2010), which has been suggested to reflect a “conflict adaptation effect” within the cognitive control system (Egner, 2008). Such adaptation effects may be driven by expectation of conflict (Aarts and Roelofs, 2011). Since the stimuli in our Stroop paradigm were presented in blocks with sequentially presented incongruent trials conferring greater levels of conflict over the course of a block, this conflict adaptation may explain the absence of difference in response accuracy we observed between conditions.
As the MA-dependent subjects were studied at 4–7 days of abstinence, one concern was the possible impact of MA withdrawal on the assessments taken. MA withdrawal symptoms, consisting primarily of increased appetite and sleep, as well as dysphoria and fatigue, can persist for approximately the first week of abstinence (London et al., 2004; Newton et al., 2004; McGregor et al., 2005; Zorick et al., 2010), and may impinge upon cognitive control. Nonetheless, one objective of this study was to evaluate brain function in MA-dependent subjects during early abstinence, simulating the time when they would approach a treatment episode. A previous study by our research group demonstrated that the duration of abstinence during the first 4–9 days did not correlate with any scores on a cognitive battery, nor did performance in the first 4–6 days differ from performance on the 7th through 9th days (Simon et al., 2010) potentially allaying concerns regarding withdrawal on cognitive functioning in the present study.
Cognitive impairments have previously been observed in individuals who abuse MA, with the persistence of some deficits during drug abstinence (Salo et al., 2009a; 2009b; Simon et al., 2000; 2010). The preliminary findings reported here suggest that MA-dependent subjects, during the first week of abstinence, demonstrate deficits in cognitive control, and reduced neural functioning within a network of regions that facilitate cognitive control during conflict adaptation.
Acknowledgments
Funding for this study was provided by National Institutes for Health (NIH) grants P20DA022539, R01 DA020726; RL1DA024853 (to EDL) and M01 RR00865 (UCLA GCRC). NIH had no further role in study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication. Additional support was provided by an endowment from the Thomas P. and Katherine K. Pike Chair in Addiction Studies and by a generous gift from the Marjorie M. Greene Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarts E, Roelofs A. Attentional control in anterior cingulate cortex based on probabilistic cueing. Journal of Cognitive Neuroscience. 2011;23:716–27. doi: 10.1162/jocn.2010.21435. [DOI] [PubMed] [Google Scholar]
- Albrecht B, Rothenberger A, Sergeant J, Tannock R, Uebel H, Banaschewski T. Interference control in attention-deficit/hyperactivity disorder: differential Stroop effects for colour-naming versus counting. Journal of Neurotransmission. 2008;115:241–7. doi: 10.1007/s00702-007-0818-1. [DOI] [PubMed] [Google Scholar]
- Aron A, Fletcher P, Bullmore E, Sahakian B, Robbins T. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience. 2003;6:115–6. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Baker SC, Rogers RD, Owen AM, Frith CD, Dolan RJ, Frackowiak RS, Robbins TW. Neural systems engaged by planning: a PET study of the Tower of London task. Neuropsychologia. 1996;34:515–26. doi: 10.1016/0028-3932(95)00133-6. [DOI] [PubMed] [Google Scholar]
- Banich MT, Crowley TJ, Thompson LL, Jacobson BL, Liu X, Raymond KM, Claus ED. Brain activation during the Stroop task in adolescents with severe substance and conduct problems: A pilot study. Drug and Alcohol Dependence. 2007;90:175–82. doi: 10.1016/j.drugalcdep.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20:1052–63. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Transactions on Medical Imaging. 2004;23:137–52. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Berman S, O’Neill J, Fears S, Bartzokis G, London ED. Abuse of Amphetamines and Structural Abnormalities in the Brain. Addiction Reviews 2008. 2008a;1141:195–220. doi: 10.1196/annals.1441.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman SM, Voytek B, Mandelkern MA, Hassid BD, Isaacson A, Monterosso J, Miotto K, Ling W, London ED. Changes in cerebral glucose metabolism during early abstinence from chronic methamphetamine abuse. Molecular Psychiatry. 2008b;13:897–908. doi: 10.1038/sj.mp.4002107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Cox RW, Bandettini PA. Detection versus estimation in event-related fMRI: choosing the optimal stimulus timing. Neuroimage. 2002;15:252–64. doi: 10.1006/nimg.2001.0964. [DOI] [PubMed] [Google Scholar]
- Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, Matochik J, Kurian V, Cadet J, Kimes A, Funderburk F, London E. Prefrontal cortical dysfunction in abstinent cocaine abusers. Journal of Neuropsychiatry and Clinical Neurosciences. 2004;16:456–64. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M, Nystrom L, Fissell K, Carter C, Cohen J. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–81. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Reviews. 2001;108:624–52. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences. 2004;8:539–46. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment brain activation during stroop task is associated with outcomes in cocaine-dependent patients. Biological Psychiatry. 2008;64:998–1004. doi: 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Campos M, Breznen B, Bernheim K, Andersen RA. Supplementary motor area encodes reward expectancy in eye-movement tasks. Journal of Neurophysiology. 2005;94:1325–35. doi: 10.1152/jn.00022.2005. [DOI] [PubMed] [Google Scholar]
- Carp J, Kim K, Taylor SF, Fitzgerald KD, Weissman DH. Conditional Differences in Mean Reaction Time Explain Effects of Response Congruency, but not Accuracy, on Posterior Medial Frontal Cortex Activity. Frontiers in Human Neuroscience. 2010:4. doi: 10.3389/fnhum.2010.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C, Braver T, Barch D, Botvinick M, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–9. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Speck O, Patel H, DeSilva M, Leonido-Yee M, Miller EN. Perfusion MRI and computerized cognitive test abnormalities in abstinent methamphetamine users. Psychiatry Research. 2002;114:65–79. doi: 10.1016/s0925-4927(02)00004-5. [DOI] [PubMed] [Google Scholar]
- Coles MG, Scheffers MK, Holroyd CB. Why is there an ERN/Ne on correct trials? Response representations, stimulus-related components, and the theory of error-processing. Biological Psychology. 2001;56:173–89. doi: 10.1016/s0301-0511(01)00076-x. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Dagher A, Owen AM, Boecker H, Brooks DJ. Mapping the network for planning: a correlational PET activation study with the Tower of London task. Brain. 1999;122 ( Pt 10):1973–87. doi: 10.1093/brain/122.10.1973. [DOI] [PubMed] [Google Scholar]
- Egner T. Multiple conflict-driven control mechanisms in the human brain. Trends in Cognitive Sciences. 2008;12:374–80. doi: 10.1016/j.tics.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nature Neuroscience. 2005;8:1784–90. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- Eldreth DA, Matochik JA, Cadet JL, Bolla KI. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. Neuroimage. 2004;23:914–20. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Bullmore ET, Craig KJ, Shabbir SS, Abbott S, Muller U, Ooi C, Suckling J, Barnes A, Sahakian BJ, Merlo-Pich EV, Robbins TW. Influence of compulsivity of drug abuse on dopaminergic modulation of attentional bias in stimulant dependence. Archives of General Psychiatry. 2010;67:632–44. doi: 10.1001/archgenpsychiatry.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philosophical Transactions of the Royal Society: Biological Sciences. 2008;363:3125–35. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Hutcheson DM, Ersche KD, Pelloux Y, Dalley JW, Robbins TW. The orbital prefrontal cortex and drug addiction in laboratory animals and humans. Annal of the New York Academy of Sciences. 2007;1121:576–97. doi: 10.1196/annals.1401.022. [DOI] [PubMed] [Google Scholar]
- Fassbender C, Murphy K, Foxe JJ, Wylie GR, Javitt DC, Robertson IH, Garavan H. A topography of executive functions and their interactions revealed by functional magnetic resonance imaging. Cognitive Brain Research. 2004;20:132–43. doi: 10.1016/j.cogbrainres.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Forman SD, Dougherty GG, Casey BJ, Siegle GJ, Braver TS, Barch DM, Stenger VA, Wick-Hull C, Pisarov LA, Lorensen E. Opiate addicts lack error-dependent activation of rostral anterior cingulate. Biological Psychiatry. 2004;55:531–7. doi: 10.1016/j.biopsych.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Garavan H, Kaufman JN, Hester R. Acute effects of cocaine on the neurobiology of cognitive control. Philosophical Transactions of the Royal Society: Biological Sciences. 2008;363:3267–76. doi: 10.1098/rstb.2008.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. American Journal of Psychiatry. 2000;157:1789–98. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross T, Murphy K, Roche R, Stein E. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection and correction. NeuroImage. 2002;17:1820–9. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Kaufman J, Stein EA. A midline dissociation between error-processing and response-conflict monitoring. Neuroimage. 2003;20:1132–9. doi: 10.1016/S1053-8119(03)00334-3. [DOI] [PubMed] [Google Scholar]
- Garavan H, Stout JC. Neurocognitive insights into substance abuse. Trends in Cognitive Sciences. 2005;9:195–201. doi: 10.1016/j.tics.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;159:1642–52. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MA, Critchley HD. Interoceptive basis to craving. Neuron. 2007;54:183–6. doi: 10.1016/j.neuron.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henik A, Salo R. Schizophrenia and the stroop effect. Behavioral and Cognitive Neuroscience Reviews. 2004;3:42–59. doi: 10.1177/1534582304263252. [DOI] [PubMed] [Google Scholar]
- Hester R, Fassbender C, Garavan H. Individual differences in error processing: a review and reanalysis of three event-related fMRI studies using the GO/NOGO task. Cerebral Cortex. 2004;14:986–94. doi: 10.1093/cercor/bhh059. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. Journal of Neuroscience. 2003;23:7839–43. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–6. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Klein TA, Endrass T, Kathmann N, Neumann J, von Cramon DY, Ullsperger M. Neural correlates of error awareness. Neuroimage. 2007;34:1774–81. doi: 10.1016/j.neuroimage.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Potenza MN, Skudlarski P, Wexler BE. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31:644–50. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Lau HC, Rogers RD, Ramnani N, Passingham RE. Willed action and attention to the selection of action. Neuroimage. 2004;21:1407–15. doi: 10.1016/j.neuroimage.2003.10.034. [DOI] [PubMed] [Google Scholar]
- Lee NK, Rawson RA. A systematic review of cognitive and behavioural therapies for methamphetamine dependence. Drug and Alcohol Review. 2008;27:309–17. doi: 10.1080/09595230801919494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Banich MT, Jacobson BL, Tanabe JL. Common and distinct neural substrates of attentional control in an integrated Simon and spatial Stroop task as assessed by event-related fMRI. Neuroimage. 2004;22:1097–106. doi: 10.1016/j.neuroimage.2004.02.033. [DOI] [PubMed] [Google Scholar]
- London ED, Berman SM, Voytek B, Simon SL, Mandelkern MA, Monterosso J, Thompson PM, Brody AL, Geaga JA, Hong MS, Hayashi KM, Rawson RA, Ling W. Cerebral metabolic dysfunction and impaired vigilance in recently abstinent methamphetamine abusers. Biological Psychiatry. 2005;58:770–8. doi: 10.1016/j.biopsych.2005.04.039. [DOI] [PubMed] [Google Scholar]
- London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, Bramen J, Shinn AK, Miotto K, Learn J, Dong Y, Matochik JA, Kurian V, Newton T, Woods R, Rawson R, Ling W. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Archives of General Psychiatry. 2004;61:73–84. doi: 10.1001/archpsyc.61.1.73. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Yucel M, Pantelis C. Addiction, a condition of compulsive behaviour? Neuroimaging and neuropsychological evidence of inhibitory dysregulation. Addiction. 2004;99:1491–502. doi: 10.1111/j.1360-0443.2004.00808.x. [DOI] [PubMed] [Google Scholar]
- Lyvers M. Loss of control in alcoholism and drug addiction: a neuroscientific interpretation. Experimental Clinical Psychopharmacology. 2000;8:225–49. doi: 10.1037//1064-1297.8.2.225. [DOI] [PubMed] [Google Scholar]
- MacDonald A, Cohen J, Stenger V, Carter C. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–8. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychological Bulleting. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- Maddock RJ. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends in Neurosciences. 1999;22:310–6. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- Magno E, Foxe JJ, Molholm S, Robertson IH, Garavan H. The anterior cingulate and error avoidance. Journal of Neuroscience. 2006;26:4769–73. doi: 10.1523/JNEUROSCI.0369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor C, Srisurapanont M, Jittiwutikarn J, Laobhripatr S, Wongtan T, White JM. The nature, time course and severity of methamphetamine withdrawal. Addiction. 2005;100:1320–9. doi: 10.1111/j.1360-0443.2005.01160.x. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The fifth edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGo response inhibition task. Human Brain Mapping. 2001;12:131–43. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RL. Linear increases in BOLD response associated with increasing proportion of incongruent trials across time in a colour Stroop task. Experimental Brain Research. 2010;203:193–204. doi: 10.1007/s00221-010-2225-3. [DOI] [PubMed] [Google Scholar]
- Mohanty A, Gitelman DR, Small DM, Mesulam MM. The spatial attention network interacts with limbic and monoaminergic systems to modulate motivation-induced attention shifts. Cerebral Cortex. 2008;18:2604–13. doi: 10.1093/cercor/bhn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug and Alcohol Dependence. 2005;79:273–7. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Murphy K, Garavan H. Artifactual fMRI group and condition differences driven by performance confounds. Neuroimage. 2004;21:219–28. doi: 10.1016/j.neuroimage.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends in Neurosciences. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–4. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton TF, Kalechstein AD, Duran S, Vansluis N, Ling W. Methamphetamine abstinence syndrome: preliminary findings. American Journal of Addiction. 2004;13:248–55. doi: 10.1080/10550490490459915. [DOI] [PubMed] [Google Scholar]
- Nordahl TE, Carter CS, Salo RE, Kraft L, Baldo J, Salamat S, Robertson L, Kusubov N. Anterior cingulate metabolism correlates with stroop errors in paranoid schizophrenia patients. Neuropsychopharmacology. 2001;25:139–48. doi: 10.1016/S0893-133X(00)00239-6. [DOI] [PubMed] [Google Scholar]
- Nordahl TE, Salo R, Leamon M. Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: a review. Journal of Neuropsychiatry and Clinical Neurosciences. 2003;15:317–25. doi: 10.1176/jnp.15.3.317. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Krueger DD, Tronson NC, Nairn AC, Taylor JR. Orbitofrontal cortex and cognitive-motivational impairments in psychostimulant addiction: evidence from experiments in the non-human primate. Annals of the New York Academy of Sciences. 2007;1121:610–38. doi: 10.1196/annals.1401.016. [DOI] [PubMed] [Google Scholar]
- Oldfield R. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paulus MP. Decision-making dysfunctions in psychiatry--altered homeostatic processing? Science. 2007;318:602–6. doi: 10.1126/science.1142997. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Frank LR. Anterior cingulate activity modulates nonlinear decision weight function of uncertain prospects. Neuroimage. 2006;30:668–77. doi: 10.1016/j.neuroimage.2005.09.061. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack N, Frank L, Brown GG, Schuckit MA. Decision making by methamphetamine-dependent subjects is associated with error-rate-independent decrease in prefrontal and parietal activation. Biological Psychiatry. 2003;53:65–74. doi: 10.1016/s0006-3223(02)01442-7. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack NE, Zauscher BE, Frank L, Brown GG, Braff DL, Schuckit MA. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002;26:53–63. doi: 10.1016/S0893-133X(01)00334-7. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Archives of General Psychiatry. 2005;62:761–8. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- Peoples LL. Will, anterior cingulate cortex, and addiction. Science. 2002;296:1623–4. doi: 10.1126/science.1072997. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Kane MJ, Alexander GM, Lacadie C, Skudlarski P, Leung HC, May J, Gore JC. An event-related functional MRI study comparing interference effects in the Simon and Stroop tasks. Cognitive Brain Research. 2002;13:427–40. doi: 10.1016/s0926-6410(02)00054-x. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Zahm DS. Specificity in the projections of prefrontal and insular cortex to ventral striatopallidum and the extended amygdala. Journal of Neuroscience. 2005;25:11757–67. doi: 10.1523/JNEUROSCI.3432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004a;306:443–7. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain and Cognition. 2004b;56:129–40. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Romo R, Hernandez A, Zainos A. Neuronal correlates of a perceptual decision in ventral premotor cortex. Neuron. 2004;41:165–73. doi: 10.1016/s0896-6273(03)00817-1. [DOI] [PubMed] [Google Scholar]
- Salo R, Henik A, Robertson LC. Interpreting Stroop interference: an analysis of differences between task versions. Neuropsychology. 2001;15:462–71. doi: 10.1037//0894-4105.15.4.462. [DOI] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Buonocore MH, Natsuaki Y, Waters C, Moore CD, Galloway GP, Leamon MH. Cognitive control and white matter callosal microstructure in methamphetamine-dependent subjects: a diffusion tensor imaging study. Biological Psychiatry. 2009a;65:122–8. doi: 10.1016/j.biopsych.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Galloway GP, Moore CD, Waters C, Leamon MH. Drug abstinence and cognitive control in methamphetamine-dependent individuals. Journal of Substance Abuse Treatment. 2009b;37:292–297. doi: 10.1016/j.jsat.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Possin K, Leamon M, Gibson DR, Galloway GP, Flynn NM, Henik A, Pfefferbaum A, Sullivan EV. Preliminary evidence of reduced cognitive inhibition in methamphetamine-dependent individuals. Psychiatry Research. 2002;111:65–74. doi: 10.1016/s0165-1781(02)00111-7. [DOI] [PubMed] [Google Scholar]
- Salo R, Ursu S, Buonocore MH, Leamon MH, Carter C. Impaired prefrontal cortical function and disrupted adaptive cognitive control in methamphetamine abusers: a functional magnetic resonance imaging study. Biological Psychiatry. 2009c;65:706–9. doi: 10.1016/j.biopsych.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffers MK, Coles MG, Bernstein P, Gehring WJ, Donchin E. Event-related brain potentials and error-related processing: an analysis of incorrect responses to go and no-go stimuli. Psychophysiology. 1996;33:42–53. doi: 10.1111/j.1469-8986.1996.tb02107.x. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychology Review. 2007;17:275–97. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Seo H, Barraclough DJ, Lee D. Dynamic signals related to choices and outcomes in the dorsolateral prefrontal cortex. Cerebral Cortex. 2007;17(Suppl 1):i110–7. doi: 10.1093/cercor/bhm064. [DOI] [PubMed] [Google Scholar]
- Simon SL, Dean AC, Cordova X, Monterosso JR, London ED. Methamphetamine dependence and neuropsychological functioning: evaluating change during early abstinence. Journal of Studies on Alcohol and Drugs. 2010;71:335–44. doi: 10.15288/jsad.2010.71.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SL, Domier C, Carnell J, Brethen P, Rawson R, Ling W. Cognitive impairment in individuals currently using methamphetamine. American Journal of Addiction. 2000;9:222–31. doi: 10.1080/10550490050148053. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smout MF, Longo M, Harrison S, Minniti R, Wickes W, White JM. Psychosocial treatment for methamphetamine use disorders: a preliminary randomized controlled trial of cognitive behavior therapy and Acceptance and Commitment Therapy. Substance Abuse. 2010;31:98–107. doi: 10.1080/08897071003641578. [DOI] [PubMed] [Google Scholar]
- Streeter CC, Terhune DB, Whitfield TH, Gruber S, Sarid-Segal O, Silveri MM, Tzilos G, Afshar M, Rouse ED, Tian H, Renshaw PF, Ciraulo DA, Yurgelun-Todd DA. Performance on the Stroop predicts treatment compliance in cocaine-dependent individuals. Neuropsychopharmacology. 2008;33:827–36. doi: 10.1038/sj.npp.1301465. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–62. [Google Scholar]
- Tedstone D, Coyle K. Cognitive impairments in sober alcoholics: performance on selective and divided attention tasks. Drug and Alcohol Dependence. 2004;75:277–86. doi: 10.1016/j.drugalcdep.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Thaler D, Chen YC, Nixon PD, Stern CE, Passingham RE. The functions of the medial premotor cortex. I. Simple learned movements. Experimental Brain Research. 1995;102:445–60. doi: 10.1007/BF00230649. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. Journal of Neuroscience. 2004;24:6028–36. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohka J, Foerde K, Aron AR, Tom SM, Toga AW, Poldrack RA. Automatic independent component labeling for artifact removal in fMRI. Neuroimage. 2008;39:1227–45. doi: 10.1016/j.neuroimage.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Perez-Garcia M. Profile of executive deficits in cocaine and heroin polysubstance users: common and differential effects on separate executive components. Psychopharmacology (Berlin) 2007;190:517–30. doi: 10.1007/s00213-006-0632-8. [DOI] [PubMed] [Google Scholar]
- Vocci FJ. Cognitive remediation in the treatment of stimulant abuse disorders: a research agenda. Experimental Clinical Psychopharmacology. 2008;16:484–97. doi: 10.1037/a0014101. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Wong C, Logan J. Higher cortical and lower subcortical metabolism in detoxified methamphetamine abusers. American Journal of Psychiatry. 2001a;158:383–9. doi: 10.1176/appi.ajp.158.3.383. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. American Journal of Psychiatry. 2001b;158:377–82. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiology of Learning and Memory. 2002;78:610–24. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Wagner G, Sinsel E, Sobanski T, Kohler S, Marinou V, Mentzel HJ, Sauer H, Schlosser RG. Cortical inefficiency in patients with unipolar depression: an event-related FMRI study with the Stroop task. Biological Psychiatry. 2006;59:958–65. doi: 10.1016/j.biopsych.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Chang L, Miller E, Sedler M, Hitzemann R, Zhu W, Logan J, Ma Y, Fowler JS. Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. American Journal of Psychiatry. 2004;161:242–8. doi: 10.1176/appi.ajp.161.2.242. [DOI] [PubMed] [Google Scholar]
- Wendelken C, Ditterich J, Bunge SA, Carter CS. Stimulus and response conflict processing during perceptual decision making. Cognitive, Affective, and Behavioral Neuroscience. 2009;9:434–47. doi: 10.3758/CABN.9.4.434. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–47. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J of Cerebral Blood Flow and Metabolism. 1992;12:900–18. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- Wunderlich K, Rangel A, O’Doherty JP. Neural computations underlying action-based decision making in the human brain. Proceedings of the National Academy of Sciences U S A. 2009;106:17199–204. doi: 10.1073/pnas.0901077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N, Nieuwenhuis S. Dissociating response conflict and error likelihood in anterior cingulate cortex. Journal of Neurosci. 2009;29:14506–10. doi: 10.1523/JNEUROSCI.3615-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorick T, Nestor L, Miotto K, Sugar C, Hellemann G, Scanlon G, Rawson R, London ED. Withdrawal symptoms in abstinent methamphetamine-dependent subjects. Addiction. 2010;105:1809–18. doi: 10.1111/j.1360-0443.2010.03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]