Abstract
Initially identified as an RNA modification in the anticodon loop of tRNAs from animal, plant and eubacterial origin, the deamination of adenosine-to-inosine by RNA editing has become increasingly recognized as an important RNA processing event to generate diversity in both the transcriptome and proteome and is essential for modulating the activity of numerous proteins critical for nervous system function. Here, we focus on the editing of transcripts encoding the 2C-subtype of serotonin receptor (5HT2C) to generate multiple receptor isoforms that differ in G-protein coupling efficacy and constitutive activity. 5HT2C receptors have been implicated in the regulation of anxiety, components of the stress response, and are thought to play a role in compulsive behavioral disorders, depression and drug addiction. A number of studies have been conducted to assess whether 5HT2C editing is altered in individuals suffering from psychiatric disorders, yet the results from these studies have been inconsistent, and thus inconclusive. This review provides a discussion of the challenges involved with characterizing 5HT2C editing patterns in human postmortem tissue samples and how differences in quantitative methodology have contributed to the observed inconsistencies between multiple laboratories. Additionally, we discuss new high-throughput sequencing tools, which provide an opportunity to overcome previous methodological challenges, and permit reliable systematic analyses of RNA editing in control and pathologic disease states.
Introduction
A major goal of current neurobiology research is to define and characterize the cellular and molecular pathophysiology underlying nervous system dysfunction including neurodegenerative disorders and psychiatric illness. Over the past two decades, a fundamental component of this effort has involved human postmortem brain studies where gene expression profiles of matched tissue samples from healthy individuals and patients diagnosed with specific nervous system disorders have been compared (Horvath et al., 2011; Iwamoto and Kato, 2006; Mehta et al., 2010; Sequeira and Turecki, 2006). While this traditional approach can be confounded by a number of variables such as postmortem interval, medication history, secondary effects of illness, cause of death and the small number of brain samples available for analysis (Bahn et al., 2001; Mirnics et al., 2004; Mirnics and Pevsner, 2004), technical artifacts of gene expression analysis may also contribute to inconsistencies between published datasets among multiple laboratories. The majority of transcriptome-wide gene expression studies have taken advantage of microarray strategies to simultaneously compare the relative expression of thousands of RNAs across sets of tissue samples. A limitation to this probe-based approach results from the inherent requirement to design probes based upon known (or predicted) sequences for genes of interest. The observation that a majority of human genes give rise to multiple mRNA isoforms by alternative splicing (Pan et al., 2008; Wang et al., 2008) or RNA editing (Gott and Emeson, 2000; Hogg et al., 2011; Zinshteyn and Nishikura, 2009) has further complicated these analyses as early microarrays typically contained probes consisting of full-length cDNAs or oligonucleotide probes located towards the 3′ end of transcripts which were unable to distinguish alternatively spliced or closely-related mRNA species. More recent microarray platforms have been developed to distinguish between splice variants by using either a) tiling arrays, consisting of overlapping probes across a known genomic region (Kwan et al., 2008); b) exon arrays, consisting of probe sets corresponding to annotated and predicted exons (Clark et al., 2007; Gardina et al., 2006); c) splice-junction arrays, consisting of probes crossing splice junctions (Castle et al., 2003; Johnson et al., 2003); or d) exon-junction arrays, consisting of probes within exons as well as across exon junctions (Fagnani et al., 2007; Pan et al., 2004). Despite these advances in expression profiling for alternatively spliced variants, no probe-based strategies have been developed to quantify RNA editing events where modifications may result in as little as a single nucleotide alteration between RNA isoforms. More recently, non-probe based approaches such as serial analysis of gene expression (SAGE) (Scott and Chrast, 2001; Velculescu et al., 2000; Yamamoto et al., 2001) and massively parallel high-throughput sequencing (deep-sequencing) platforms (Marioni et al., 2008; Mortazavi et al., 2008; Wang et al., 2009) have allowed de novo analysis of transcript composition within RNA samples, providing a more unbiased and quantitative analysis of gene expression. These advances in sequencing technology have led to the development of whole-transcriptome profiling strategies, often referred to as RNA-Seq (Haas and Zody, 2010; Liu et al., 2011; Lovci et al., 2011; Mortazavi et al., 2008; Nagalakshmi et al., 2010; Wilhelm et al., 2010), where junctions between exons can be assayed without prior knowledge of the gene structure, subtle modifications resulting from RNA editing events can be readily detected and knowledge of polymorphisms can provide direct measurements of allele-specific expression. These approaches have led to an increased appreciation for the extensive sequence diversity generated by RNA processing events such as splicing and editing (Li et al., 2011) and provide powerful tools for more detailed analyses of changes in gene expression associated with human disease.
Post-transcriptional modification of transcripts by RNA editing
The conversion of adenosine-to-inosine (A-to-I) by RNA editing is a post-transcriptional processing event in which genomically-encoded sequences are altered in precursor and mature mRNAs to generate functional diversity in the proteome (Gott and Emeson, 2000). A-to-I editing is generally identified as an adenosine-to-guanosine (A-to-G) discrepancy during comparisons of genomic and cDNA sequences that result from the base-pairing of cytosine to inosine during reverse transcriptase-mediated first-strand cDNA synthesis. Because inosine is recognized as guanosine during translation (Basilio et al., 1962), adenosine deamination has been shown to “recode” amino acid sequences for key regulators of nervous system function including ligand- and voltage-gated ion channels, G-protein coupled receptors and components of the synaptic release machinery (Burns et al., 1997; Hoopengardner et al., 2003; Li et al., 2009; Schaub and Keller, 2002; Seeburg and Hartner, 2003; Zinshteyn and Nishikura, 2009). Indeed, A-to-I editing generally alters residues that are both highly conserved and critical for modulating the function of the encoded protein products (Jepson and Reenan, 2008; Rula and Emeson, 2007). Such alterations in the primary nucleotide sequence of mRNA transcripts can affect not only coding potential, but also can occur in untranslated regions and introns to alter the structure, stability, translation efficiency and splicing patterns of the modified transcripts, thereby affecting numerous aspects of RNA function in the cell (Gott and Emeson, 2000).
The first example of A-to-I editing in mammalian mRNAs was identified in transcripts encoding the GluR-2 subunit of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) subtype of ionotropic glutamate receptor in which a genomically-encoded glutamine codon (CAG) was altered to an arginine codon (CIG) (Melcher et al., 1995; Rueter et al., 1995; Sommer et al., 1991; Yang et al., 1995). This single amino acid alteration (Q/R site) regulates both the electrophysiologic and ion-permeation properties of heteromeric AMPA receptors. AMPA channels containing the edited GluR-2(R) subunit are impermeant to calcium ions, whereas those that lack or contain a non-edited GluR-2(Q) subunit, demonstrate a dramatic increase in their relative divalent cation permeability (Dingledine et al., 1992; Hollmann et al., 1991; Sommer et al., 1991; Verdoorn et al., 1991). Mice that are heterozygous for a mutant GluR-2 allele that cannot be edited appear healthy until postnatal day 14 (P14), when they begin to develop seizures that lead to death by P20. This phenotype results from dramatically increased AMPA receptor permeability to Ca2+, concomitant with neuronal degeneration, and emphasizes the importance of RNA editing for nervous system function (Brusa et al., 1995).
Several lines of evidence have suggested that site-specific A-to-I editing is regulated spatio-temporally (Hackler et al., 2006; Jacobs et al., 2009; Niswender et al., 1998; Wahlstedt et al., 2009) and can be influenced by both genetic (Du et al., 2006) and environmental factors (Englander et al., 2005). The profound effect that A-to-I editing can have on protein function makes it essential to understand how the editing of specific RNA substrates is regulated in both normal and disease states. Efforts to gain a more complete picture of editing dynamics require methods to systematically and accurately determine the profile of edited RNA isoforms within experimental samples. This review provides a critical survey of approaches that have been used to compare editing profiles for transcripts encoding the 2C-subtype of serotonin receptor in postmortem brain tissue from healthy individuals and patients diagnosed with psychiatric disorders. Furthermore, it will discuss current and future approaches that will provide an opportunity to understand the biological significance of RNA editing in normal and pathological brain function.
Modulation of serotonin 2C receptor function by RNA editing
Serotonin (5-hydroxytryptamine; 5HT) is a monoaminergic neurotransmitter that modulates numerous sensory and motor processes as well as a wide variety of behaviors including sleep, appetite, pain perception, locomotion, thermoregulation, hallucinations, and sexual behavior (Werry et al., 2008). The multiple actions of 5HT are mediated by specific interaction with multiple receptor subtypes. Pharmacological, physiologic and molecular cloning studies have provided evidence for fifteen distinct 5HT receptor subtypes which have been subdivided into seven families (5HT1–5HT7) based on relative ligand binding affinities, genomic structure, amino acid sequence similarities and coupling to specific signal transduction pathways (Barnes and Sharp, 1999; Bockaert et al., 2006; Hoyer et al., 1994; Hoyer et al., 2002). The 5HT2 family of receptors includes three receptor subtypes: 5HT2A, 5HT2B and 5HT2C, which belong to the G-protein-coupled receptor (GPCR) superfamily. The G-protein:5HT2C receptor interactions occur in highly conserved regions of the second and third intracellular loops to potentiate subsequent signal transduction pathways via Gαq/11, Gα12/13 and Gαi to modulate effector molecules such as phospholipases C, D and A2, as well as the extracellular signal-regulated kinases 1 and 2 (Berg et al., 1994; Berg et al., 1998; Werry et al., 2005; Werry et al., 2008). 5HT2C mRNA expression has been shown to be widely distributed in neocortical areas, hippocampus, nucleus accumbens, amygdala, choroid plexus, dorsal striatum and substantia nigra (Pasqualetti et al., 1999; Pompeiano et al., 1994), suggestive of physiologic roles in reward behavior, locomotion, energy balance, and also when dysregulated, in the development of certain disease states such as obesity, epilepsy, anxiety, sleep disorders and motor dysfunction (Giorgetti and Tecott, 2004). Many of these anatomical predictions for 5HT2C function have been supported by analyses of 5HT2C-null mice that exhibit adult-onset obesity, seizures and enhanced cocaine-mediated locomotor activity and reward behavior (Abdallah et al., 2009; Brennan et al., 1997; Giorgetti and Tecott, 2004; Rocha et al., 2002; Tecott et al., 1995). Pharmacologic data have provided further support for these functional roles as selective 5HT2C agonists promote appetite suppression (Halford et al., 2007; Somerville et al., 2007), facilitate anxiety-like behaviors (Harada et al., 2006; Kennett et al., 1997), reduce cocaine-mediated locomotion and drug self-administration (Fletcher et al., 2004; Harada et al., 2006) and have been implicated in the pharmacological actions of both hallucinogens and atypical antipsychotics (Giorgetti and Tecott, 2004; Marquis et al., 2007; Siuciak et al., 2007).
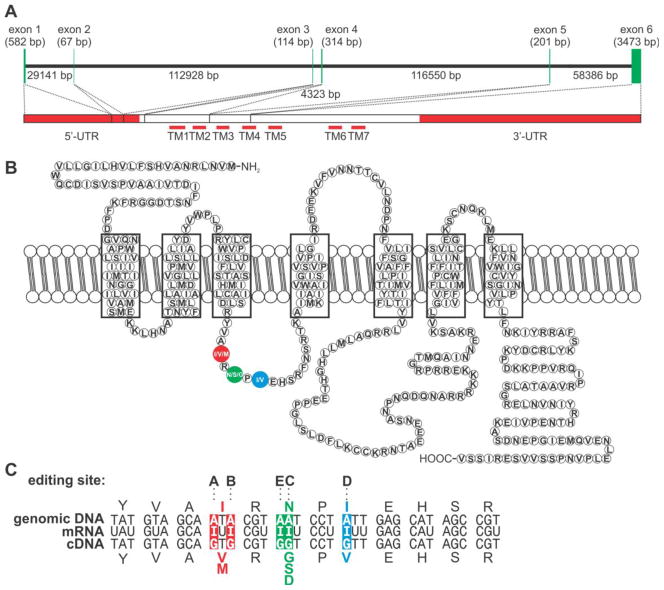
RNA transcripts encoding the 5HT2C receptor undergo up to five A-to-I editing events that predict alterations in the identity of three amino acids within the second intracellular loop of the receptor to generate as many as twenty-four receptor isoforms from thirty-two edited mRNA species (Fig. 1) (Burns et al., 1997; Wang et al., 2000). Sequence analysis of cDNAs isolated from dissected rat, mouse and human brains predicted the region-specific expression of seven major 5HT2C isoforms encoded by eleven distinct mRNA (Abbas et al., 2010; Burns et al., 1997; Morabito et al., 2010b; Niswender et al., 1998), suggesting that differentially edited 5HT2C receptors may serve distinct biological functions in those regions in which they are expressed. Sequencing studies have further revealed that edited mRNAs encoding isoforms with valine, serine and valine (VSV) or valine, asparagine and valine (VNV) at amino acids 156, 158 and 160 are the most highly expressed in a majority of dissected brain regions isolated from human and rat/mouse brains, respectively (Burns et al., 1997; Fitzgerald et al., 1999), whereas the major 5HT2C transcripts in the choroid plexus encode the less edited (INV) and non-edited (INI) receptor isoforms (Burns et al., 1997; Morabito et al., 2010b). Functional comparisons in heterologous expression systems, between the non-edited (INI) and the fully-edited (VGV) 5HT2C isoforms have revealed a 40-fold decrease in serotonergic potency to stimulate phospho-inositide hydrolysis for the VGV isoform due to reduced Gq/11-protein coupling efficiency and decreased coupling to other signaling pathways (Burns et al., 1997; Niswender et al., 1999). In addition, cells expressing more highly edited 5HT2C receptors (e.g. VSV and VGV) demonstrate considerably reduced (or absent) constitutive activation in the absence of ligand compared to cells expressing the non-edited isoform (Niswender et al., 1999). This reduction in coupling efficiency and constitutive activity derives from a difference in the ability of edited 5HT2C isoforms to spontaneously isomerize to the active R* conformation, a form of the receptor that interacts efficiently with G-proteins in the absence of agonist (Burns et al., 1997; Niswender et al., 1999). Mutant mice solely expressing the fully-edited (VGV) isoform of the receptor pheno-copy numerous aspects of Prader-Willi Syndrome (PWS) (Kawahara et al., 2008; Morabito et al., 2010a), a maternally-imprinted human disorder resulting from a loss of paternal gene expression on chromosome 15q11-13 which is characterized by a complex phenotype including cognitive deficits, infantile hypotonia and failure-to-thrive, short stature, hypogonadism and hyperphagia which can lead to morbid obesity (Goldstone, 2004; Nicholls and Knepper, 2001). The dramatic phenotypic alterations observed in these mutant animals clearly demonstrate the importance of normal patterns of 5HT2C RNA editing in vivo and suggest a potential role for altered 5HT2C function in the etiology of this imprinted disorder (Kawahara et al., 2008; Morabito et al., 2010a).
Figure 1. Structures of the human 5HT2C gene, mRNA, receptor protein and RNA editing events.
A) A schematic diagram of the human 5HT2C receptor gene (Htr2C) is mapped onto the mature mRNA transcript; the positions of the mRNA encoding the transmembrane domains (TM), the untranslated regions (UTR) and the sizes of exon and introns (bp, base pairs) are indicated. B) Schematic representation of the predicted topology and primary amino acid sequence for the human 5HT2C receptor is presented along with the positions of amino acid alterations within the second intracellular loop resulting from RNA editing events (colored circles). C) Nucleotide and predicted amino acid sequence alignments between 5HT2C genomic, mRNA and cDNA sequences; the positions of the five editing sites (A-E) are indicated and nucleotide discrepancies and predicted alterations in amino acid sequence are shown with colors corresponding to each codon in which they reside.
5HT2C pre-mRNA editing in human psychiatric disease
Serotonin signaling has been implicated widely in the etiology of behavioral and psychiatric disorders and several 5HT receptors, including the 5HT2C receptor, are thought to be important targets for pharmacologic intervention. In humans, antipsychotic drugs used in the treatment of schizophrenia interact with 5HT2C receptors (Meltzer, 1999; Siuciak et al., 2007) and the 5HT2C receptor has been hypothesized to play a role in psychiatric impairment associated with depression, anxiety and schizophrenia (Giorgetti and Tecott, 2004). For these reasons, several research groups have examined potential changes in 5HT2C editing patterns to determine whether alterations in this RNA processing event play a role in the neuronal processes leading to psychiatric dysfunction. While multiple reports have provided evidence for changes in editing within human disease cohorts, and in rodents exposed to various pharmacologic and behavioral paradigms, such reports have been inconsistent and no reproducible alterations in editing have been effectively associated with any particular human disease cohort (Dracheva et al., 2008; Gurevich et al., 2002a; Gurevich et al., 2002b; Iwamoto and Kato, 2002; Iwamoto and Kato, 2003; Niswender et al., 2001; Sodhi et al., 2001). This is likely due to several factors including inconsistency in the precise brain regions analyzed, inherent diversity in human populations, and technical limitations that have made it difficult to efficiently and accurately determine the profile of edited 5HT2C transcript isoforms in postmortem tissue samples.
The most common methodology to quantify site-specific editing has involved reverse-transcription polymerase chain reaction (RT-PCR) amplification of specific RNAs followed by a modified primer-extension analysis (Burns et al., 1997; Rueter et al., 1995); however, this analytical paradigm is less useful for analyses of editing patterns in transcripts with multiple editing sites, such as 5HT2C mRNAs. The two more commonly used methods to quantify editing of 5HT2C RNA isoforms involve either direct sequencing or pyrosequencing analysis of individual cDNA clones generated by RT-PCR and subcloned into plasmids (Iwamoto et al., 2005; Sodhi et al., 2005; Werry et al., 2008). While these methods yield unambiguous results, the accuracy of such methods is directly related to the number of clones sequenced, which can vary widely between studies and has been reported to be as few as 10 clones per sample (Sodhi et al., 2001). The limited number of clones analyzed can produce significant sampling errors that may either obscure or overestimate differences between experimental groups. Recent studies have analyzed 5HT2C editing using 454 deep sequencing technology, increasing the number of 5HT2C cDNAs sequenced to nearly 800, while simultaneously avoiding the laborious subcloning, bacterial transformation and preparation of individual cDNA clones (Wahlstedt et al., 2009).
Most studies attempting to determine the editing profile for 5HT2C transcripts in postmortem brain samples from patients diagnosed with psychiatric disorders have focused primarily on RNA isolated from regions of the dorsolateral prefrontal cortex (DLPFC), specifically Brodmann areas 8, 9, 10, and 46. The first of these studies (Niswender et al., 2001) analyzed RNA in postmortem tissue (Brodmann areas 8 and 9) from individuals diagnosed with major depressive disorder, schizophrenia and healthy, age-matched controls. This study relied upon primer-extension-based approaches to quantify the extent of editing at the A, C, and D sites, yet revealed no significant differences in RNA editing among the three populations. However, subjects who had committed suicide (regardless of diagnosis) exhibited a statistically significant elevation of editing at the A-site. A contemporaneous examination of 5HT2C editing patterns in DLPFC samples (Brodmann area 46) from schizophrenic and control subjects obtained from the Stanley Foundation Brain Collection (Torrey et al., 2000) employed direct sequencing of individual cDNA isolates to quantify RNA editing patterns (Sodhi et al., 2001) In this approach, only 10 cDNA clones were sequenced from each individual to estimate changes among the thirty-two potential 5HT2C isoforms that can be generated by editing. Results from this study reported a significant reduction in editing at all five sites in the DLPFC from individuals diagnosed with schizophrenia compared to controls and the authors suggested that observed differences from the results reported by Niswender (Niswender et al., 2001) could be explained as a result of different cortical regions being used for these analyses. A third research group (Gurevich et al., 2002b) used the sequencing of individual cDNA clones to compare 5HT2C editing profiles in DLPFC (Brodmann area 10) of suicide victims with a history of major depression to that of non-depressed controls. These investigators used the same direct sequencing strategy employed by Sodhi and colleagues (Sodhi et al., 2001), but attempted to increase the accuracy of their estimates by analyzing 30–60 clones per tissue sample, rather than the 10 clones reported previously. These studies identified a significant increase in C-site editing and a decrease in D-site editing in depressed suicide victims compared to controls. Interestingly, they also quantified 5HT2C editing patterns in the forebrain neocortex of mice treated for 28 days with oral fluoxetine and reported that drug treatment lead to changes in editing at the C- and D-sites that were opposite to those observed in depressed suicide victims. The authors suggested that this result supports a model where C- and D-site editing could be important for the etiology of depression and that compensatory alterations in editing efficiency at these sites may play a role in the therapeutic efficacy of the antidepressant, fluoxetine (Gurevich et al., 2002b). A fourth study examined RNA editing efficiencies of the A- and D-sites in 5HT2C transcripts in prefrontal cortex samples of patients diagnosed with bipolar disorder, schizophrenia, and major depression using primer-extension combined with denaturing high performance liquid chromatography. Results from these analyses did not find significant alterations in RNA editing between patients and controls, although trends for increased RNA editing at the D-site in depressive patients (P=0.08) and the A-site in suicide victims (P=0.07) was noted. These results disagree with those described in the Gurevich study (Gurevich et al., 2002b), despite the fact that both studies quantified 5HT2C editing in RNA samples isolated from Brodmann area 10. Finally, more recent studies (Dracheva et al., 2008) analyzed editing in DLPFC (Brodmann area not specified) by sequencing 45 clones per individual with cohorts of 82 normal controls and 22 suicide victims to report significant increases in the efficiency of editing at the A-, C-, and D-sites in suicide victims compared to controls (Dracheva et al., 2008). The discordant results between these studies raise significant concerns regarding the well-known limitations of postmortem brain analysis (Bahn et al., 2001; Mirnics et al., 2004; Mirnics and Pevsner, 2004) as well as the use of inadequate methodologies for effective quantification of 5HT2C editing patterns.
New approaches for quantification of editing profiles
In 2006, Sodhi and colleagues described a pyrosequencing-based paradigm to quantify 5HT2C editing patterns that circumvented some of the laborious steps previously employed for direct sequence analysis of individual cDNA clones (Sodhi et al., 2005). These studies also provided a careful analysis of the number of clones that need to be sequenced per RNA sample to detect 5HT2C editing changes of different magnitudes, indicating that to detect “small” changes in editing between samples with adequate power (>80% confidence) several hundred clones would need to be analyzed from each sample. Recently, quantitative strategies have been developed to take advantage of deep-sequencing technologies that can provide simultaneous sequence identities for thousands to millions of PCR amplicons. These deep-sequencing approaches can be multiplexed for simultaneous analysis of editing from multiple RNA samples or from multiple RNA targets in several samples. Analyzing thousands of sequences from each sample provides sufficient statistical power to identify small changes in editing with high confidence (Abbas et al., 2010; Morabito et al., 2010b) while reducing sample preparation, overall cost and time to facilitate analysis of large numbers of samples. This approach allows for a more detailed and accurate analysis of potential editing alterations in human disease cohorts and experimental animal models leading to new insights concerning the biological significance RNA editing.
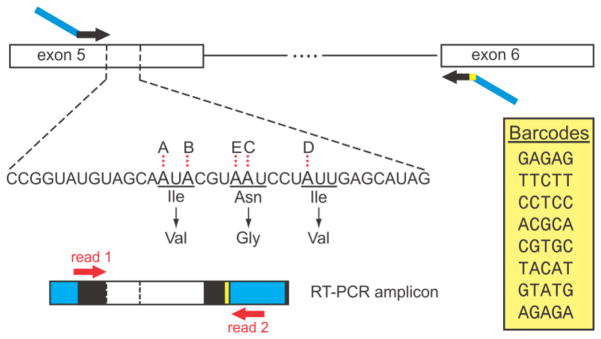
Deep-sequencing analysis of 5HT2C editing patterns takes advantage of the Illumina Genome Analyzer platform to directly sequence RT-PCR amplicons derived from 5HT2C transcripts. This is achieved by designing sense and antisense oligonucleotide primers containing a region of complementarity to the target RNA and 5′-extensions that correspond to the adapter sequences required for bridge amplification and direct paired-end sequencing on the Illumina platform (Fig. 2) (Bentley et al., 2008). This technique can be multiplexed by including unique “barcode” sequences into the antisense PCR primer which provides maintenance of sample identity. This multiplexing strategy allows for the combination of many samples into one sequencing run, taking full advantage of the vast number (>10 million) of sequencing reads which are simultaneously generated on the Illumina platform. This large number of sequencing reads provides investigators with the opportunity to make more accurate estimates of the overall 5HT2C isoform profile in a specific RNA sample and allows for enhanced detection of rare sequence variants (Abbas et al., 2010; Morabito et al., 2010b). Comparisons of 5HT2C editing profiles in RNA samples isolated from whole C57BL/6J mouse brain using pyrosequencing and deep-sequencing approaches revealed significantly decreased experimental variability using the high-throughput approach (Morabito et al., 2010b). Quantification of 5HT2C editing patterns using 100 cDNA clones from each of five brain samples by pyrosequencing suggested significant intra-animal variability, whereas the same five RNA samples analyzed using the Illumina-based strategy (>100,000 sequences per sample) decreased inter-animal variability by more than 10-fold (<3 % of mean). These results demonstrate that there is very little inter-animal variability in whole brain 5HT2C isoforms when controlled for age, gender, and background strain in matched mouse cohorts (Morabito et al., 2010b) and suggests that similar analyses of human brain samples may be systematically applied, if appropriately controlled for other confounding variables. Furthermore, the overestimation of inter-sample differences observed when analyzing 100 clones illustrates that a degree of caution must be taken when interpreting estimates of 5HT2C editing patterns that are based on tens or even hundreds of clones.
Figure 2. Summary of the high-throughput sequencing strategy for analysis of 5HT2C editing profiles.
The structure of 5HT2C pre-mRNA in the exon 5–6 region is presented (top). The location of RT-PCR primers are indicated (black arrows). Adapter sequences for paired-end sequencing on the Illumina Genome Analyzer (dark blue) were incorporated into each primer and one of eight distinct barcode sequences (yellow) was incorporated into the antisense primer. The sequence of the non-edited RNA, beginning immediately after the primer sequence, is specified below the pre-mRNA RT-PCR diagram. The positions of the five edited adenosines are indicated in bold (sites A, B, E, C and D) and the amino acids encoded by the non-edited (upper) and fully edited transcripts (lower) are indicated below their respective codons. The position of the 36nt sequence obtained from read 1 is indicated between the dashed lines on both the RT-PCR and pre-mRNA diagrams. Two sequencing primers (read 1 and read 2, red arrows) were used for paired-end sequence analysis of RT-PCR amplicons generated from 5HT2C mRNA (bottom). Each of eight unique barcode sequences incorporated into the RT-PCR amplicon for sample identification is provided in the yellow inset.
Conclusion
Previous studies characterizing 5HT2C editing in the human brain have relied upon crude dissections of human cortex corresponding to either specific Brodmann areas or even less well defined samples of cortical tissue. Given the discordant results obtained by many laboratories investigating potential alterations in 5HT2C editing, it is increasingly unclear whether dynamic changes in editing are associated with 5HT2C genotype, expression level, medication history, or psychiatric diagnosis. While analyses of human postmortem brain samples have a number of limitations, the development of accurate and sensitive methodologies for quantifying 5HT2C editing patterns represents a critical first-step to minimize technical artifacts associated with inadequate sample size or extensive variability within and between isolated RNA samples. The advent of high-throughput sequencing approaches and high-precision tissue sampling techniques like laser-capture microdissection, offers opportunities to obtain precise site-specific editing profiles for individual neurons allowing comparisons of normal and pathological states.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbas AI, et al. Assessing serotonin receptor mRNA editing frequency by a novel ultra high-throughput sequencing method. Nucleic Acids Res. 2010 doi: 10.1093/nar/gkq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah L, et al. Impact of serotonin 2C receptor null mutation on physiology and behavior associated with nigrostriatal dopamine pathway function. J Neurosci. 2009;29:8156–65. doi: 10.1523/JNEUROSCI.3905-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn S, et al. Gene expression profiling in the post-mortem human brain--no cause for dismay. J Chem Neuroanat. 2001;22:79–94. doi: 10.1016/s0891-0618(01)00099-0. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Basilio C, et al. Synthetic polynucleotides and the amino acid code. V Proc Natl Acad Sci U S A. 1962;48:613–6. doi: 10.1073/pnas.48.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley DR, et al. Accurate whole human genome sequencing using reversible terminator chemistry. Nature. 2008;456:53–9. doi: 10.1038/nature07517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KA, et al. 5-Hydroxytryptamine type 2A receptors regulate cyclic AMP accumulation in a neuronal cell line by protein kinase C-dependent and calcium/calmodulin-dependent mechanisms. Mol Pharmacol. 1994;45:826–36. [PubMed] [Google Scholar]
- Berg KA, et al. Interactions between effectors linked to serotonin receptors. Ann N Y Acad Sci. 1998;861:111–20. doi: 10.1111/j.1749-6632.1998.tb10181.x. [DOI] [PubMed] [Google Scholar]
- Bockaert J, et al. Neuronal 5-HT metabotropic receptors: fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res. 2006;326:553–72. doi: 10.1007/s00441-006-0286-1. [DOI] [PubMed] [Google Scholar]
- Brennan TJ, et al. Sound-induced seizures in serotonin 5-HT2c receptor mutant mice. Nat Genet. 1997;16:387–90. doi: 10.1038/ng0897-387. [DOI] [PubMed] [Google Scholar]
- Brusa R, et al. Early-onset epilepsy and postnatal lethality associated with an editing-deficient GluR-B allele in mice. Science. 1995;270:1677–80. doi: 10.1126/science.270.5242.1677. [DOI] [PubMed] [Google Scholar]
- Burns CM, et al. Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature. 1997;387:303–8. doi: 10.1038/387303a0. [DOI] [PubMed] [Google Scholar]
- Castle J, et al. Optimization of oligonucleotide arrays and RNA amplification protocols for analysis of transcript structure and alternative splicing. Genome Biol. 2003;4:R66. doi: 10.1186/gb-2003-4-10-r66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark TA, et al. Discovery of tissue-specific exons using comprehensive human exon microarrays. Genome Biol. 2007;8:R64. doi: 10.1186/gb-2007-8-4-r64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, et al. Structural determinants of barium permeation and rectification in non-NMDA glutamate receptor channels. J Neurosci. 1992;12:4080–7. doi: 10.1523/JNEUROSCI.12-10-04080.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dracheva S, et al. Increased serotonin 2C receptor mRNA editing: a possible risk factor for suicide. Mol Psychiatry. 2008;13:1001–10. doi: 10.1038/sj.mp.4002081. [DOI] [PubMed] [Google Scholar]
- Du Y, et al. A-to-I pre-mRNA editing of the serotonin 2C receptor: comparisons among inbred mouse strains. Gene. 2006;382:39–46. doi: 10.1016/j.gene.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Englander MT, et al. How stress and fluoxetine modulate serotonin 2C receptor pre-mRNA editing. J Neurosci. 2005;25:648–51. doi: 10.1523/JNEUROSCI.3895-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagnani M, et al. Functional coordination of alternative splicing in the mammalian central nervous system. Genome Biol. 2007;8:R108. doi: 10.1186/gb-2007-8-6-r108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald LW, et al. Messenger RNA editing of the human serotonin 5-HT2C receptor. Neuropsychopharmacology. 1999;21:82S–90S. doi: 10.1016/S0893-133X(99)00004-4. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, et al. Injection of the 5-HT2C receptor agonist Ro60-0175 into the ventral tegmental area reduces cocaine-induced locomotor activity and cocaine self-administration. Neuropsychopharmacology. 2004;29:308–18. doi: 10.1038/sj.npp.1300319. [DOI] [PubMed] [Google Scholar]
- Gardina PJ, et al. Alternative splicing and differential gene expression in colon cancer detected by a whole genome exon array. BMC Genomics. 2006;7:325. doi: 10.1186/1471-2164-7-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgetti M, Tecott LH. Contributions of 5-HT(2C) receptors to multiple actions of central serotonin systems. Eur J Pharmacol. 2004;488:1–9. doi: 10.1016/j.ejphar.2004.01.036. [DOI] [PubMed] [Google Scholar]
- Goldstone AP. Prader-Willi syndrome: advances in genetics, pathophysiology and treatment. Trends Endocrinol Metab. 2004;15:12–20. doi: 10.1016/j.tem.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Gott JM, Emeson RB. Functions and mechanisms of RNA editing. Annu Rev Genet. 2000;34:499–531. doi: 10.1146/annurev.genet.34.1.499. [DOI] [PubMed] [Google Scholar]
- Gurevich I, et al. Modulation of serotonin 2C receptor editing by sustained changes in serotonergic neurotransmission. J Neurosci. 2002a;22:10529–32. doi: 10.1523/JNEUROSCI.22-24-10529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich I, et al. Altered editing of serotonin 2C receptor pre-mRNA in the prefrontal cortex of depressed suicide victims. Neuron. 2002b;34:349–56. doi: 10.1016/s0896-6273(02)00660-8. [DOI] [PubMed] [Google Scholar]
- Haas BJ, Zody MC. Advancing RNA-Seq analysis. Nat Biotechnol. 2010;28:421–3. doi: 10.1038/nbt0510-421. [DOI] [PubMed] [Google Scholar]
- Hackler EA, et al. 5-HT(2C) receptor RNA editing in the amygdala of C57BL/6J, DBA/2J, and BALB/cJ mice. Neurosci Res. 2006;55:96–104. doi: 10.1016/j.neures.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Halford JC, et al. Serotonergic drugs: effects on appetite expression and use for the treatment of obesity. Drugs. 2007;67:27–55. doi: 10.2165/00003495-200767010-00004. [DOI] [PubMed] [Google Scholar]
- Harada K, et al. Anxiolytic activity of a novel potent serotonin 5-HT2C receptor antagonist FR260010: a comparison with diazepam and buspirone. Eur J Pharmacol. 2006;553:171–84. doi: 10.1016/j.ejphar.2006.09.042. [DOI] [PubMed] [Google Scholar]
- Hogg M, et al. RNA editing by mammalian ADARs. Adv Genet. 2011;73:87–120. doi: 10.1016/B978-0-12-380860-8.00003-3. [DOI] [PubMed] [Google Scholar]
- Hollmann M, et al. Ca2+ permeability of KA-AMPA--gated glutamate receptor channels depends on subunit composition. Science. 1991;252:851–3. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- Hoopengardner B, et al. Nervous system targets of RNA editing identified by comparative genomics. Science. 2003;301:832–6. doi: 10.1126/science.1086763. [DOI] [PubMed] [Google Scholar]
- Horvath S, et al. Analyzing schizophrenia by DNA microarrays. Biol Psychiatry. 2011;69:157–62. doi: 10.1016/j.biopsych.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D, et al. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- Hoyer D, et al. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–54. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Iwamoto K, et al. Estimating RNA editing efficiency of five editing sites in the serotonin 2C receptor by pyrosequencing. RNA. 2005;11:1596–603. doi: 10.1261/rna.2114505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto K, Kato T. Effects of cocaine and reserpine administration on RNA editing of rat 5-HT2C receptor estimated by primer extension combined with denaturing high-performance liquid chromatography. Pharmacogenomics J. 2002;2:335–40. doi: 10.1038/sj.tpj.6500130. [DOI] [PubMed] [Google Scholar]
- Iwamoto K, Kato T. RNA editing of serotonin 2C receptor in human postmortem brains of major mental disorders. Neurosci Lett. 2003;346:169–72. doi: 10.1016/s0304-3940(03)00608-6. [DOI] [PubMed] [Google Scholar]
- Iwamoto K, Kato T. Gene expression profiling in schizophrenia and related mental disorders. Neuroscientist. 2006;12:349–61. doi: 10.1177/1073858406287536. [DOI] [PubMed] [Google Scholar]
- Jacobs MM, et al. ADAR1 and ADAR2 expression and editing activity during forebrain development. Dev Neurosci. 2009;31:223–37. doi: 10.1159/000210185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepson JE, Reenan RA. RNA editing in regulating gene expression in the brain. Biochim Biophys Acta. 2008;1779:459–70. doi: 10.1016/j.bbagrm.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Johnson JM, et al. Genome-wide survey of human alternative pre-mRNA splicing with exon junction microarrays. Science. 2003;302:2141–4. doi: 10.1126/science.1090100. [DOI] [PubMed] [Google Scholar]
- Kawahara Y, et al. Dysregulated editing of serotonin 2C receptor mRNAs results in energy dissipation and loss of fat mass. J Neurosci. 2008;28:12834–44. doi: 10.1523/JNEUROSCI.3896-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennett GA, et al. SB 242084, a selective and brain penetrant 5-HT2C receptor antagonist. Neuropharmacology. 1997;36:609–20. doi: 10.1016/s0028-3908(97)00038-5. [DOI] [PubMed] [Google Scholar]
- Kwan T, et al. Genome-wide analysis of transcript isoform variation in humans. Nat Genet. 2008;40:225–31. doi: 10.1038/ng.2007.57. [DOI] [PubMed] [Google Scholar]
- Li JB, et al. Genome-wide identification of human RNA editing sites by parallel DNA capturing and sequencing. Science. 2009;324:1210–3. doi: 10.1126/science.1170995. [DOI] [PubMed] [Google Scholar]
- Li M, et al. Widespread RNA and DNA sequence differences in the human transcriptome. Science. 2011;333:53–8. doi: 10.1126/science.1207018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, et al. A comparison of RNA-Seq and high-density exon array for detecting differential gene expression between closely related species. Nucleic Acids Res. 2011;39:578–88. doi: 10.1093/nar/gkq817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovci MT, et al. RNA-seq analysis of gene expression and alternative splicing by double-random priming strategy. Methods Mol Biol. 2011;729:247–55. doi: 10.1007/978-1-61779-065-2_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marioni JC, et al. RNA-seq: an assessment of technical reproducibility and comparison with gene expression arrays. Genome Res. 2008;18:1509–17. doi: 10.1101/gr.079558.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis KL, et al. WAY-163909 [(7bR,10aR)-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta-[b][1,4]diazepino[6,7,1hi]indole]: A novel 5-hydroxytryptamine 2C receptor-selective agonist with preclinical antipsychotic-like activity. J Pharmacol Exp Ther. 2007;320:486–96. doi: 10.1124/jpet.106.106989. [DOI] [PubMed] [Google Scholar]
- Mehta D, et al. Gene expression studies in major depression. Curr Psychiatry Rep. 2010;12:135–44. doi: 10.1007/s11920-010-0100-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher T, et al. Editing of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR-B pre-mRNA in vitro reveals site-selective adenosine to inosine conversion. J Biol Chem. 1995;270:8566–70. doi: 10.1074/jbc.270.15.8566. [DOI] [PubMed] [Google Scholar]
- Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology. 1999;21:106S–115S. doi: 10.1016/S0893-133X(99)00046-9. [DOI] [PubMed] [Google Scholar]
- Mirnics K, et al. DNA microarray analysis of postmortem brain tissue. Int Rev Neurobiol. 2004;60:153–81. doi: 10.1016/S0074-7742(04)60006-7. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Pevsner J. Progress in the use of microarray technology to study the neurobiology of disease. Nat Neurosci. 2004;7:434–9. doi: 10.1038/nn1230. [DOI] [PubMed] [Google Scholar]
- Morabito MV, et al. Mice with altered serotonin 2C receptor RNA editing display characteristics of Prader-Willi syndrome. Neurobiol Dis. 2010a doi: 10.1016/j.nbd.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morabito MV, et al. High-throughput multiplexed transcript analysis yields enhanced resolution of 5-hydroxytryptamine 2C receptor mRNA editing profiles. Mol Pharmacol. 2010b;77:895–902. doi: 10.1124/mol.109.061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortazavi A, et al. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008;5:621–8. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- Nagalakshmi U, et al. Curr Protoc Mol Biol. Unit 4. Chapter 4. 2010. RNA-Seq: a method for comprehensive transcriptome analysis; pp. 11pp. 1–13. [DOI] [PubMed] [Google Scholar]
- Nicholls RD, Knepper JL. Genome organization, function, and imprinting in Prader-Willi and Angelman syndromes. Annu Rev Genomics Hum Genet. 2001;2:153–75. doi: 10.1146/annurev.genom.2.1.153. [DOI] [PubMed] [Google Scholar]
- Niswender CM, et al. RNA editing of the human serotonin 5-hydroxytryptamine 2C receptor silences constitutive activity. J Biol Chem. 1999;274:9472–8. doi: 10.1074/jbc.274.14.9472. [DOI] [PubMed] [Google Scholar]
- Niswender CM, et al. RNA editing of the human serotonin 5-HT2C receptor. alterations in suicide and implications for serotonergic pharmacotherapy. Neuropsychopharmacology. 2001;24:478–91. doi: 10.1016/S0893-133X(00)00223-2. [DOI] [PubMed] [Google Scholar]
- Niswender CM, et al. Identification and characterization of RNA editing events within the 5-HT2C receptor. Ann N Y Acad Sci. 1998;861:38–48. doi: 10.1111/j.1749-6632.1998.tb10171.x. [DOI] [PubMed] [Google Scholar]
- Pan Q, et al. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet. 2008;40:1413–5. doi: 10.1038/ng.259. [DOI] [PubMed] [Google Scholar]
- Pan Q, et al. Revealing global regulatory features of mammalian alternative splicing using a quantitative microarray platform. Mol Cell. 2004;16:929–41. doi: 10.1016/j.molcel.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Pasqualetti M, et al. Distribution and cellular localization of the serotonin type 2C receptor messenger RNA in human brain. Neuroscience. 1999;92:601–11. doi: 10.1016/s0306-4522(99)00011-1. [DOI] [PubMed] [Google Scholar]
- Pompeiano M, et al. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Brain Res Mol Brain Res. 1994;23:163–78. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Rocha BA, et al. Enhanced locomotor, reinforcing, and neurochemical effects of cocaine in serotonin 5-hydroxytryptamine 2C receptor mutant mice. J Neurosci. 2002;22:10039–45. doi: 10.1523/JNEUROSCI.22-22-10039.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueter SM, et al. Glutamate receptor RNA editing in vitro by enzymatic conversion of adenosine to inosine. Science. 1995;267:1491–4. doi: 10.1126/science.7878468. [DOI] [PubMed] [Google Scholar]
- Rula EY, Emeson RB. Mouse models to elucidate the functional roles of adenosine-to-inosine editing. Methods Enzymol. 2007;424:333–67. doi: 10.1016/S0076-6879(07)24016-9. [DOI] [PubMed] [Google Scholar]
- Schaub M, Keller W. RNA editing by adenosine deaminases generates RNA and protein diversity. Biochimie. 2002;84:791–803. doi: 10.1016/s0300-9084(02)01446-3. [DOI] [PubMed] [Google Scholar]
- Scott HS, Chrast R. Global transcript expression profiling by Serial Analysis of Gene Expression (SAGE) Genet Eng (N Y) 2001;23:201–19. doi: 10.1007/0-306-47572-3_11. [DOI] [PubMed] [Google Scholar]
- Seeburg PH, Hartner J. Regulation of ion channel/neurotransmitter receptor function by RNA editing. Curr Opin Neurobiol. 2003;13:279–83. doi: 10.1016/s0959-4388(03)00062-x. [DOI] [PubMed] [Google Scholar]
- Sequeira A, Turecki G. Genome wide gene expression studies in mood disorders. OMICS. 2006;10:444–54. doi: 10.1089/omi.2006.10.444. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, et al. CP-809,101, a selective 5-HT2C agonist, shows activity in animal models of antipsychotic activity. Neuropharmacology. 2007;52:279–90. doi: 10.1016/j.neuropharm.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Sodhi MS, et al. A rapid new assay to detect RNA editing reveals antipsychotic-induced changes in serotonin-2C transcripts. Mol Pharmacol. 2005;68:711–9. doi: 10.1124/mol.105.014134. [DOI] [PubMed] [Google Scholar]
- Sodhi MS, et al. RNA editing of the 5-HT(2C) receptor is reduced in schizophrenia. Mol Psychiatry. 2001;6:373–9. doi: 10.1038/sj.mp.4000920. [DOI] [PubMed] [Google Scholar]
- Somerville EM, et al. 5-HT(2C) receptor activation inhibits appetitive and consummatory components of feeding and increases brain c-fos immunoreactivity in mice. Eur J Neurosci. 2007;25:3115–24. doi: 10.1111/j.1460-9568.2007.05567.x. [DOI] [PubMed] [Google Scholar]
- Sommer B, et al. RNA editing in brain controls a determinant of ion flow in glutamate- gated channels. Cell. 1991;67:11–9. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- Tecott LH, et al. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995;374:542–6. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- Torrey EF, et al. The stanley foundation brain collection and neuropathology consortium. Schizophr Res. 2000;44:151–5. doi: 10.1016/S0920-9964(99)00192-9. [DOI] [PubMed] [Google Scholar]
- Velculescu VE, et al. Analysing uncharted transcriptomes with SAGE. Trends Genet. 2000;16:423–5. doi: 10.1016/s0168-9525(00)02114-4. [DOI] [PubMed] [Google Scholar]
- Verdoorn TA, et al. Structural determinants of ion flow through recombinant glutamate receptor channels. Science. 1991;252:1715–8. doi: 10.1126/science.1710829. [DOI] [PubMed] [Google Scholar]
- Wahlstedt H, et al. Large-scale mRNA sequencing determines global regulation of RNA editing during brain development. Genome Res. 2009;19:978–86. doi: 10.1101/gr.089409.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ET, et al. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456:470–6. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, et al. Altered G protein-coupling functions of RNA editing isoform and splicing variant serotonin2C receptors. J Neurochem. 2000;74:1290–300. doi: 10.1046/j.1471-4159.2000.741290.x. [DOI] [PubMed] [Google Scholar]
- Wang Z, et al. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werry TD, et al. Characterization of serotonin 5-HT2C receptor signaling to extracellular signal-regulated kinases 1 and 2. J Neurochem. 2005;93:1603–15. doi: 10.1111/j.1471-4159.2005.03161.x. [DOI] [PubMed] [Google Scholar]
- Werry TD, et al. RNA editing of the serotonin 5HT2C receptor and its effects on cell signalling, pharmacology and brain function. Pharmacol Ther. 2008;119:7–23. doi: 10.1016/j.pharmthera.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Wilhelm BT, et al. Defining transcribed regions using RNA-seq. Nat Protoc. 2010;5:255–66. doi: 10.1038/nprot.2009.229. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, et al. Use of serial analysis of gene expression (SAGE) technology. J Immunol Methods. 2001;250:45–66. doi: 10.1016/s0022-1759(01)00305-2. [DOI] [PubMed] [Google Scholar]
- Yang JH, et al. Editing of glutamate receptor subunit B pre-mRNA in vitro by site- specific deamination of adenosine. Nature. 1995;374:77–81. doi: 10.1038/374077a0. [DOI] [PubMed] [Google Scholar]
- Zinshteyn B, Nishikura K. Adenosine-to-inosine RNA editing. Wiley Interdiscip Rev Syst Biol Med. 2009;1:202–9. doi: 10.1002/wsbm.10. [DOI] [PMC free article] [PubMed] [Google Scholar]