Abstract
Previous studies have indicated that 2,2′-dipyridyl, a lipid-soluble ferrous iron chelator, can reduce brain injury after cerebral ischemia and reduce cerebral vasospasm after subarachnoid hemorrhage. In this study, we examined the efficacy of 2,2′-dipyridyl after intracerebral hemorrhage (ICH) in 12-month-old mice. ICH was modeled by intrastriatal injection of collagenase or autologous whole blood. 2,2′-Dipyridyl or vehicle was administered intraperitoneally 2 h before ICH (pretreatment) or 6 h after ICH (post-treatment) and then once daily for up to 3 days. Mice in the pretreatment group were sacrificed 1 or 3 days after ICH and examined for iron deposition, neuronal death, oxidative stress, microglial/astrocyte activation, neutrophil infiltration, and white matter damage. Mice in the post-treatment group were examined for brain lesion volume and edema on day 3 and for neurologic deficits on days 1, 3, and 28 after ICH. Pretreatment with 2,2′-dipyridyl decreased iron accumulation and neuronal death, attenuated production of reactive oxygen species, reduced microglial activation without affecting astrocytes or neutrophil infiltration, and attenuated white matter damage. Post-treatment reduced brain lesion volume and edema and improved neurologic function. These results indicate that the lipid-soluble ferrous iron chelator 2,2′-dipyridyl can reduce brain injury and improve functional outcome after ICH.
Keywords: 2,2′-dipyridyl; intracerebral hemorrhage; iron; neuronal death; white matter
Introduction
Intracerebral hemorrhage (ICH) is a common and often fatal stroke subtype. In the aftermath of ICH, iron, a heme degradation product, contributes significantly to the consequent brain injury (Wang, 2010). After erythrocyte lysis, iron concentrations in the brain can become high enough to stimulate free radical formation, which, in turn, leads to neuronal death and secondary brain injury (Wagner et al., 2003; Wang, 2010; Xi et al., 2006). When tested in preclinical studies, the iron chelator deferoxamine has inconsistently shown protection against hemorrhagic brain injury in rats (Okauchi et al., 2009; Warkentin et al., 2010). Deferoxamine was administered post-ICH in both studies, but in one it was delivered intramuscularly (Okauchi et al., 2009) and in the other intraperitoneally (i.p.) (Warkentin et al., 2010). In our previous study, we confirmed that iron toxicity contributes to collagenase-induced hemorrhagic brain injury in mice and that reducing iron accumulation by post-treatment with deferoxamine i.p. can improve neuronal survival; however, deferoxamine did not reduce brain lesion volume, edema, or swelling after ICH (Wu et al., 2011).
Lipid-soluble ferrous iron chelator 2,2′-dipyridyl (DP) has been reported to alleviate brain damage in different rodent models of cerebral ischemia (Demougeot et al., 2004; Methy et al., 2008; Millerot-Serrurot et al., 2008; Van Hoecke et al., 2005) and to reduce cerebral vasospasm in primate and rabbit models of subarachnoid hemorrhage (Horky et al., 1998; Yu et al., 2010). The lipid solubility of DP is evidenced by its ability to freely enter brain cells and chelate intracellular free iron, thereby reducing iron-induced cell death (Breuer et al., 1995; Kress et al., 2002; Methy et al., 2008). However, the efficacy of DP has not been tested in ICH models, and its effect on iron deposition, iron-mediated oxidative damage, neuroinflammation, and grey and white matter injury after ICH has not been examined. The objective of this study was, therefore, to investigate the efficacy of DP treatment on ICH outcomes and its effect on iron deposition, iron-mediated oxidative damage, neuroinflammation, and grey and white matter injury. Because the aged population is at greater risk for ICH than are young adults, we used 12-month-old mice to enhance the clinical relevance of the study.
Materials and methods
Animals
This study was conducted in accordance with the National Institutes of Health guidelines for the use of experimental animals. Animal protocols were approved by the Johns Hopkins University Animal Care and Use Committee. Twelve-month-old C57BL/6 male mice (25–35 g) were used in this study because cerebrovascular effects of aging in mice are well developed at this age (Park et al., 2007).
ICH models
As described previously (Grossetete and Rosenberg, 2008; Wang et al., 2003), the first model of ICH was produced by injecting mice in the left striatum with collagenase VII-S (0.075 U in 500 nL saline, Sigma, St. Louis, MO) at the following stereotactic coordinates: 0.8 mm anterior and 2.2 mm lateral of the bregma, 3.0 mm in depth. The second model of ICH entailed a double infusion of mice with a total of 10 μL autologous whole blood. Blood removed from the tail was infused into the left striatum in two time blocks, with a 7-min pause between infusions, according to our published protocol (Wang et al., 2008). Rectal temperature was maintained at 37.0 ± 0.5°C throughout the experimental and recovery periods. These two procedures resulted in reproducible lesions that were mostly restricted to the striatum.
Experimental groups
Mice were randomly assigned to receive either DP (Sigma; 20 mg/kg for pretreatment and 40 mg/kg for post-treatment) or vehicle (0.005% ethanol/saline). DP injections were administered i.p. at 2 h before collagenase injection (pretreatment group) or 6 h after collagenase or blood injection (post-treatment group) and then once daily for 1 or 3 days. The dosing regimens were based on previously published work that used a rodent focal ischemia model (Demougeot et al., 2004; Methy et al., 2008; Van Hoecke et al., 2005). Body weight was measured before collagenase or blood injection and at 1 and 3 days after ICH. Percent change in body weight was calculated according to the formula: change in body weight (%) = (post-hemorrhage weight at each time point – pre-hemorrhage weight)/pre-hemorrhage weight × 100.
Histology
Ferric iron deposition was evaluated with Perls staining of coronal brain sections (Gu et al., 2009; Wu et al., 2011). Fluoro-Jade B (FJB) was used to quantify neuronal death (Wang and Tsirka, 2005a; Xue et al., 2009). Luxol fast blue was used to stain intact myelin (Chen et al., 2011). To quantify iron- or FJB-positive cells or myelin in the perihematomal region along the rostral-caudal axis, we selected three sections each from mice with similarly sized hematomas (at the injection site and 360 μm to each side) to target similar regions of interest. Iron- or FJB-positive cells were quantified in the lateral edge of the hematoma in the caudate putamen, and myelin damage was quantified in the external and internal capsules. The numbers of iron- or FJB-positive cells or positive areas of Luxol fast blue-stained myelin from 12 locations per mouse (4 fields per section x 3 sections per mouse) were averaged and expressed as positive cells or areas per field (20x or 30x magnification; n=5 mice/group). Sections were analyzed by an observer blinded to the experimental cohort.
In situ detection of reactive oxygen species
Production of reactive oxygen species (ROS) after ICH was investigated by in situ detection of oxidized hydroethidine (Kamada et al., 2007; Park et al., 2007; Wang and Tsirka, 2005a). Hydroethidine, a cell-permeable oxidative fluorescent dye, is oxidized by superoxide to ethidium (Bindokas et al., 1996), which intercalates within the DNA and emits a red fluorescent signal. Fluorescence intensity was determined in predefined areas of the hemorrhagic striatum (at the injection site and at 360 μm on each side) after subtraction of the color density on the contralateral striatum (n=5 mice/group). For measurement of fluorescence intensity, all images were captured at the same intensity, contrast settings, and exposure times. Sections were analyzed by an observer blinded to the experimental cohort using ImageJ software (version 1.42q; NIH, Bethesda, MD).
Immunofluorescence
Immunofluorescence was carried out as described previously (Wang and Tsirka, 2005a). The primary antibodies used were: rabbit anti-myeloperoxidase (MPO, neutrophil marker; 1:500; Dako, Carpinteria, CA); rabbit anti-Iba 1 (microglia marker; 1:500; Wako Chemicals, Richmond, VA); rabbit anti-glial fibrillary acidic protein (GFAP, astrocyte marker; 1:500; Dako); rabbit anti-degraded myelin basic protein (dMBP; 1:2000; Millipore, Temecula, CA); rabbit anti-NG2 (oligodendrocyte progenitor cell marker; 1:200; Millipore); and rabbit anti-amyloid precursor protein (APP, 1:200, Sigma). Sections were then incubated with Alexa 488-conjugated goat anti-rabbit secondary antibody (1:1000; Molecular Probes, Eugene, OR). Stained sections were examined with a fluorescence microscope (ECLIPSE TE2000-E, Nikon, Japan). Immunoreactive cells were counted in the lateral edge of the hematoma along the rostral-caudal axis under a 40x objective for Iba1, GFAP, and MPO and under a 20x objective for NG2; staining intensities for dMBP and APP in the external and internal capsules were assessed under a 20x objective from 12 locations per mouse (4 fields per section x 3 sections per mouse). Images used for analysis were captured at the same intensity, contrast settings, and exposure times. Quantifications were averaged and expressed as positive cells or intensity/field (n=5 mice/group). To target similar regions of interest, sections with similar lesion size were selected and analyzed by an investigator blinded to the experimental cohort.
Spectrophotometric assay for hemoglobin
Drabkin’s reagent (Sigma) was used to quantify the hemoglobin content of brains subjected to collagenase-induced ICH, as described previously (MacLellan and Colbourne, 2005; Wang et al., 2003). Briefly, mice pretreated with DP (n = 6/group) were anesthetized 24 h after ICH with an overdose of isoflurane and transcardially perfused with 100 mL of normal saline. The tissue on the ipsilateral and contralateral sides was then trimmed to contain only the striatal region and was treated individually as follows. Each sample was homogenized for 5 min in 500 μL of distilled water and then centrifuged at 13,000g for 30 min. Drabkin’s reagent (80 μL) was added to a 20-μL aliquot of supernatant (which contains the hemoglobin) and allowed to stand for 15 min at room temperature. The concentration of cyanomethemoglobin produced was measured spectrophotometrically at 540 nm. Results from three samples per mouse were averaged.
Neurologic deficit
Neurologic deficits in the post-treatment group were assessed on days 1, 3, and 28 post-ICH for the collagenase model (n=8 mice/group) and on days 1 and 3 post-ICH for the blood model (n=6 mice/group). An investigator blinded to the experimental cohort scored all mice on six neurologic tests, including body symmetry, gait, climbing, circling behavior, front limb symmetry, and compulsory circling (Wang and Doré, 2007a). Each test was graded from 0 to 4, establishing a maximum deficit score of 24.
Brain lesion volume
Mice in the post-treatment group of the collagenase model (n=8/group) were euthanized after the neurologic examination on day 3 post-ICH. The entire brain of each mouse was cut into 50-μm sections with a cryostat. Sections through the entire striatum were stained with Luxol fast blue (for myelin) and Cresyl Violet (for neurons) before being quantified for grey- and white-matter injury with SigmaScan Pro software (version 5.0.0 for Windows; Systat, San Jose, CA). The lesion volume in cubic millimeters was calculated by multiplying the thickness by the damaged areas of each section, as determined by the lack of specific staining (Wang et al., 2003). Sections were analyzed by an investigator blinded to the experimental cohort.
Brain water content
Brain edema was determined in mice subjected to both ICH models on day 3 post-ICH by the wet/dry weight ratio method as described previously (Wang and Tsirka, 2005a; Wu et al., 2011). Brain water content was expressed as: (wet weight – dry weight) / wet weight of brain tissue × 100%.
Statistics
All data are expressed as means ± SD. Differences between two groups were analyzed with Student′s t-test. Statistical significance was set at p<0.05.
Results
DP pretreatment decreases iron deposits and neuronal death
Iron is released from the breakdown of hemoglobin during hematoma formation. Using Perls staining to examine iron clearance, we found that the number of Perls-positive amoeboid cells in the peri-ICH area was decreased on day 3 after ICH in mice given the DP regimen (n=5 mice/group, p<0.01; Fig. 1A). FJB staining was used to quantify neuronal cell death. As with the iron deposition, DP pretreatment reduced the number of FJB-positive cells in the peri-ICH area on day 3 after ICH (n=5 mice/group, p<0.01; Figs. 1B and C).
Fig. 1.
Dipyridyl (DP) pretreatment decreases iron deposition and neuronal death. (A) Compared with vehicle treatment, DP treatment significantly reduced the number of Perls-positive cells in the perihematomal region of mice 3 days after collagenase-induced ICH, indicating a reduction in ferric iron accumulation (n=5 mice/group, **p<0.01 versus vehicle-treated group). Values are means ± SD. (B) Fluoro-Jade B (FJB) histological staining of degenerating neurons in sections collected 3 days after collagenase injection shows intensely labeled neurons and processes in the perihematomal region. Arrows indicate FJB-positive neurons. Scale bar = 30 μm. (C) Quantification analysis showed that DP treatment reduced the number of degenerating neurons (n=5 mice/group, **p<0.01 versus vehicle-treated group). Values are means ± SD.
DP pretreatment attenuates ROS production
Iron-mediated oxidative stress in the brain can cause neuronal death (Wang, 2010). The fluorescent indicator hydroethidine was used to study the effect of DP pretreatment on hemorrhagic ROS production. In DP-treated mice, ROS signals (small red particles) were reduced in the peri-ICH area on days 1 and 3 after ICH (n=5 mice/group, p<0.01; Fig. 2).
Fig. 2.
Dipyridyl (DP) pretreatment attenuates ROS production. (A) The ROS signal was evident in the perihematomal region on days 1 and 3 after collagenase-induced ICH in vehicle-treated mice; DP treatment attenuated ROS production on both days. (B) Quantification analysis of fluorescence intensity indicated that DP treatment significantly reduced ROS production on days 1 and 3 after ICH (n=5 mice/group, **p<0.01 versus vehicle-treated group). Values are means ± SD.
DP pretreatment decreases microglial/macrophage activation, but not astrocytic activation or neutrophil infiltration
Microglial/macrophage activation contributes to ICH-induced early brain injury (Aronowski and Hall, 2005; Wang, 2010; Wang and Doré, 2007b). To examine the effect of DP pretreatment on microglial/macrophage activation after ICH, we used Iba1 immunofluorescence labeling (Shehadah et al., 2010; Wang and Doré, 2007a). After ICH, activated microglia/macrophages were characterized as cells with a spherical, amoeboid, or rod-like appearance and a cell body usually more than 7.5 μm in diameter, with short, thick processes and intense immunoreactivity. Resting microglia were characterized by small cell bodies (<7.5 μm in diameter), long processes, and weak immunoreactivity. By using a combination of morphological criteria and a cell body diameter cutoff of 7.5 μm, microglia/macrophages were classified as either resting or activated (Batchelor et al., 1999; Wang et al., 2008). DP pretreatment reduced the number of activated microglia/macrophages in the peri-ICH area on day 3 after ICH (n=5 mice/group, p<0.01; Figs. 3A and B).
Fig. 3.
Dipyridyl (DP) pretreatment decreases microglial/macrophage activation. (A) Activated microglia/macrophages (Iba1-immunoreactive cells), reactive astrocytes (GFAP-immunoreactive cells), and infiltrating neutrophils (MPO-immunoreactive cells) were evident in or around the injury site on day 3 after collagenase-induced ICH. DP treatment reduced the number of activated microglia/macrophages, but not activated astrocytes or infiltrating neutrophils. Arrows indicate Iba1-, GFAP-, and MPO-immunoreactive cells. Insets represent higher magnification of MPO-positive neutrophils. Scale bar = 30 μm. (B) Bar graph shows quantification analysis (n=5 mice/group, **p<0.01 versus vehicle-treated group). Values are means ± SD.
To examine the effect of DP pretreatment on astrocyte reactivity, we used GFAP immunofluorescence labeling. Compared with resting astrocytes, reactive astrocytes in the perihematomal region exhibit more intense GFAP immunoreactivity and a greater number, length, and thickness of GFAP-positive processes. We found that DP pretreatment did not change the number of reactive astrocytes in the perihematomal area on day 3 after ICH (n=5 mice/group, p>0.05; Figs. 3A and B).
In animal models of ICH, neutrophil infiltration occurs after activation of microglia/macrophages and also contributes to early hemorrhagic brain injury (Wang, 2010; Wang and Tsirka, 2005a). To examine the effect of DP on neutrophil infiltration, we used MPO immunofluorescence labeling. MPO-immunoreactive neutrophils were evident in the hemorrhagic striatum on day 3 after ICH. Insets in Fig. 3A represent higher magnification of MPO-positive neutrophils. DP pretreatment reduced their number, though the difference did not reach statistical significance (n=5 mice/group, p>0.05; Fig. 3B).
DP pretreatment decreases white matter damage
Mice with ICH exhibited marked white matter and axonal damage in the perihematomal region (Fig. 4). We used Luxol fast blue to label normal myelin, dMBP to label degraded myelin, and APP to label damaged axons (Chen et al., 2011). NG2 was used as a marker of oligodendrocyte progenitor cells. DP pretreatment reduced the loss of Luxol fast blue-stained myelin (Fig. 4A) and NG2-positive oligodendrocyte progenitor cells (Fig. 4D) and reduced immunostaining of dMBP (Fig. 4B) and APP (Fig. 4C) in the perihematomal region on day 3 after ICH (n=5 mice/group, all p<0.01). These data indicate that DP treatment reduced demyelination and axon loss. Because we performed all quantifications on brain sections with similar lesion sizes, the decrease in white matter damage in mice pretreated with DP is independent of lesion size.
Fig. 4.
Dipyridyl (DP) pretreatment decreases white matter damage. Immunostaining with Luxol fast blue (dark blue; A), dMBP (green; B), APP (green; C), and NG2 (green; D) and respective quantification data in the perihematomal region on day 3 after collagenase-induced ICH (n=5 mice/group, **p<0.01 versus vehicle-treated group). Arrows in A–D indicate normal myelin, damaged myelin, damaged axons, and normal oligodendrocyte progenitor cells, respectively. Values are means ± SD. Scale bar = 25 μm.
DP pretreatment has no effect on collagenase-induced bleeding
To ascertain whether neuroprotection conferred by DP pretreatment resulted from decreased collagenase-induced bleeding, we measured the initial levels of hemoglobin in the injured striatum as an indicator of the bleeding volume. No significant difference between DP- and vehicle-pretreated mice was observed 24 h after ICH (arbitrary units: 1.58±0.19 vs. 1.60±0.24; n=6 mice/group, p>0.05), indicating that DP pretreatment does not affect collagenase-induced bleeding.
DP post-treatment decreases brain lesion volume and edema and ameliorates neurobehavioral deficits
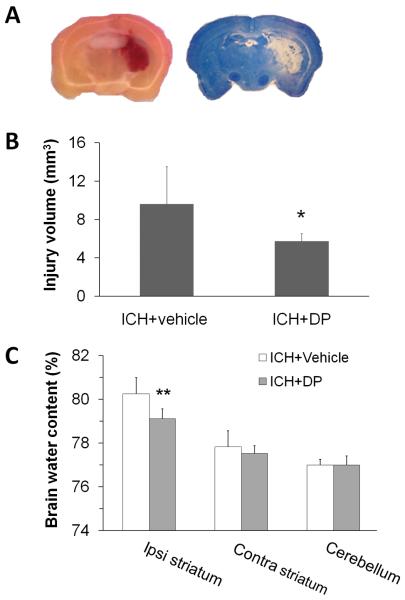
The collagenase-induced hematoma/lesion volume reaches a maximum by 3 days (Del Bigio et al., 1996; Michel-Monigadon et al., 2010). To examine the effect of DP post-treatment on ICH outcomes, we first assessed its effect on brain lesion volume in the collagenase model. Collagenase injection produced a hemorrhage that was largely restricted to the striatum, with blood only rarely observed in the cortex (Fig. 5A, left). After sections are stained with Luxol fast blue/Cresyl Violet, brain lesion can be identified easily by lack of color (Fig. 5A, right). The brain lesion volume comprised the hematoma, perihematomal edema, and surrounding damaged grey matter and white matter tracts. DP treatment begun 6 h after ICH produced significant reductions in brain lesion volume (p<0.05, n=8 mice/group; Fig. 5B) and perihematomal brain edema (p<0.01, n=6 mice/group; Fig. 5C) on day 3 after ICH.
Fig. 5.
Dipyridyl (DP) post-treatment decreases brain lesion volume and edema in the collagenase model. (A) Representative images of unstained (left) and Luxol fast blue/Cresyl Violet-stained (right) brain sections on day 3 after collagenase-induced ICH. The area of the lesion is indicated by the lack of staining (right). (B) Brain lesion volume was measured on Luxol fast blue/Cresyl Violet-stained brain sections. Quantification analysis revealed that brain lesion volume was smaller in the DP-treated group than in the vehicle-treated group 3 days after ICH (n=8 mice/group, *p<0.05). Values are means ± SD. (C) Percentage brain water content was measured 3 days after ICH. DP post-treatment reduced brain water content in the ipsilateral striatum after collagenase-induced ICH compared with that of the vehicle-treated group (n=6 mice/group, **p<0.01). Values are means ± SD. Ipsi, ipsilateral; Contra, contralateral.
To examine whether reduced brain injury results in improved neurologic function, we assessed the neurologic function at baseline and on days 1, 3, and 28 after ICH. Mice treated with DP beginning 6 h after collagenase injection had better neurologic function than did vehicle-treated mice on days 3 and 28 after ICH (n=8 mice/group, p<0.05; Fig. 6).
Fig.6.
Dipyridyl (DP) post-treatment ameliorates neurologic deficits in the collagenase model. (A) DP post-treatment improved the neurologic function of mice on days 3 and 28 after ICH compared with that of the vehicle-treated group. (B) Neurologic scores for each of the individual tests on days 1, 3, and 28 after ICH (n=8 mice/group, *p<0.05, **p<0.01). Values are means ± SD.
We further confirmed the efficacy of DP post-treatment in the blood model. We found that DP post-treatment begun 6 h after whole blood infusion significantly reduced brain water content in the ipsilateral striatum and improved neurologic function on day 3 after ICH (n=6 mice/group; both p<0.01; Figs. 7A and B). Neurologic scores for the six individual tests were reduced after DP post-treatment on day 3, though the difference did not reach statistical significance (all p>0.05). These results suggest that the efficacy of DP post-treatment is not animal model-dependent.
Fig. 7.
Dipyridyl (DP) post-treatment decreases brain edema formation and ameliorates neurologic deficits in the blood model. (A) Percentage brain water content was measured 3 days after ICH. DP post-treatment reduced brain water content in the ipsilateral striatum after ICH compared with that of the vehicle-treated group (n=6 mice/group, ***p<0.001). Values are means ± SD. (B) DP post-treatment improved neurologic function of mice on day 3 after ICH compared with that of the vehicle-treated group (n=6 mice/group, *p<0.05). Values are means ± SD. Ipsi, ipsilateral; Contra, contralateral.
DP post-treatment does not affect body weight loss or mortality rate
Mouse body weight was decreased compared to baseline on days 1 and 3 after ICH. Although deferoxamine was shown to exacerbate post-ICH weight loss in mice (Wu et al., 2011), DP post-treatment did not change body weight loss compared to vehicle treatment within 3 days after ICH in the collagenase model (6.4% ± 1.9% vs. 5.5% ± 3.5% on day 1, 6.2% ± 4.2% vs. 3.3% ± 5.4% on day 3, n=8 mice/group, both p>0.05). Similar results were obtained in the blood model (n=6 mice/group, both p>0.05). None of the mice post-treated with DP or vehicle died within 3 days after ICH.
Discussion
As most preclinical studies of ICH have been carried out in young animals, limiting the direct translation of preclinical studies into clinical trials, we investigated the efficacy of DP on ICH outcomes in 12-month-old mice. This work presents several novel findings. The results show for the first time that pretreatment with lipid-soluble ferrous iron chelator DP improves striatal neuronal survival and reduces white matter damage but does not affect collagenase-induced bleeding. This neuroprotection was manifest by decreases in iron accumulation and attenuation of ROS production and microglial activation. ICH-associated secondary brain injury has been suggested to be mediated by the generation of ferrous iron during heme degradation; the iron produced would potentiate oxidative damage and neuronal death (Wagner et al., 2003; Wang, 2010; Xi et al., 2006). Like the iron chelator deferoxamine, DP treatment begun 6 h after ICH improved functional outcome up to four weeks in the collagenase model. However, contrary to our previous findings with deferoxamine (Wu et al., 2011), DP post-treatment also significantly reduced brain lesion volume and edema in the collagenase model and brain edema in the blood model. DP post-treatment did not increase mortality rate or body weight loss.
As in other organs, the level of iron in the brain is tightly regulated. High concentrations of reactive iron are toxic to the brain tissue because iron catalyzes the Fenton reaction, which produces highly reactive hydroxyl radicals that lead to oxidative damage and neuronal death (Gaasch et al., 2007). Moreover, iron might induce neuronal death even after it has bound to ferritin because it can be locally released in its ferrous form under the acidic conditions that follow stroke (Bishop and Robinson, 2001). The major difference between iron chelators deferoxamine and DP is that DP is lipid-soluble, allowing it to freely enter brain cells and chelate both intra- and extracellular free iron after ICH, whereas deferoxamine does not alter intracellular iron levels in vitro, reflecting its insufficient cell permeability (Breuer et al., 1995; Kress et al., 2002). Moreover, the hydrophilicity and large size of deferoxamine would affect its blood-brain barrier penetration ability (Keberle, 1964; Lynch et al., 2000). In our study, DP significantly decreased iron accumulation and ROS production in the perihematomal region, demonstrating its blood-brain barrier permeability and efficacy for iron chelation in the hemorrhagic brain. As expected, the reduction in iron accumulation promoted striatal neuronal survival and reduced white matter damage after ICH. White matter damage in stroke patients is often associated with a higher risk of death and poor functional outcomes (Leys et al., 1999). The attenuation of white matter damage conferred by DP pretreatment might be attributable to the decreased iron deposition.
Free iron accumulates after ICH when it delocalizes from ferritin. The excess free iron then leads to the generation of ROS. Although ROS are produced during normal oxidative metabolism, high ROS levels can be lethal. Mounting evidence supports a critical role for ROS in oxidative brain damage after ICH (Aronowski and Hall, 2005; Wang and Doré, 2007b). It has been generally accepted that abnormal iron overload participates in the production of toxic ROS, which may be responsible for blood–brain barrier disruption, vasogenic edema formation, and subsequent aggravation of clinical outcomes. DP has been shown to be efficacious in mitigating ischemic brain damage (Demougeot et al., 2004; Methy et al., 2008; Millerot-Serrurot et al., 2008; Van Hoecke et al., 2005). The antioxidant property of DP is attributed to its ability to chelate reactive iron and thereby prevent the formation of hydroxyl radicals via the Fenton reaction. DP significantly attenuated ROS production, further supporting the idea that iron toxicity underlies the increase in oxidative damage after ICH.
Increasing evidence supports the premise that activated microglia/macrophages are major sources of proinflammatory mediators (Aronowski and Hall, 2005; Wang and Doré, 2007b). Consistent with this notion, we previously reported that inhibition of microglial activation before or early after ICH decreased neuronal death and brain injury and improved neurologic function (Wang et al., 2003; Wang and Tsirka, 2005b). Although the underlying mechanisms are not clear, microglia have a larger capacity to take up free iron than do neurons and astrocytes (Kress et al., 2002; Zecca et al., 2004). Iron accumulation in microglia might stimulate the activation of these cells in the hemorrhagic brain, and DP might inhibit microglial activation by reducing iron-mediated ROS production or blocking the signals that activate microglia. However, another possibility is that the decrease in ROS production might be caused by direct inhibition of microglial activation rather than decreased reactive iron levels.
Although our results indicate that DP is neuroprotective in the two mouse ICH models after a 3-day post-treatment regimen, the dose-response relationship remains to be assessed. Iron is an essential cofactor for many proteins and is involved in many important metabolic processes. Since tissue iron overload after ICH is highly localized, systemic long-term administration of iron chelators may be ill-advised due to potential toxicity of iron depletion.
Conclusions
In summary, systemic pretreatment with the lipid-soluble iron chelator DP decreased perihematomal iron accumulation, neuronal death, and white matter damage; attenuated ROS production; and reduced microglial activation. Post-treatment with DP significantly reduced the lesion volume and brain edema formation and improved neurologic function. These findings provide the first evidence that DP is neuroprotective and deserves further preclinical investigation for potential ICH therapy.
Highlights.
-
>
We examined the efficacy of 2, 2′-dipyridyl after ICH in 12-month-old mice.
-
>
Pretreatment decreased iron deposition and grey and white matter injury.
-
>
Post-treatment reduced brain lesion volume and edema, improved neurologic function.
-
>
Lipid-soluble iron chelator 2,2′-dipyridyl could protect brain against ICH injury.
Acknowledgements
This work was supported by AHA 09BGIA2080137 and NIH K01AG031926 (JW). We thank Sarah Busse and Jessica Wang for blind analysis of histology and immunofluorescence, Dr. Raymond C. Koehler for valuable suggestions and comments, and Claire Levine for assistance with manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aronowski J, Hall CE. New horizons for primary intracerebral hemorrhage treatment: experience from preclinical studies. Neurol Res. 2005;27:268–79. doi: 10.1179/016164105X25225. [DOI] [PubMed] [Google Scholar]
- Batchelor PE, et al. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. J Neurosci. 1999;19:1708–16. doi: 10.1523/JNEUROSCI.19-05-01708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindokas VP, et al. Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J Neurosci. 1996;16:1324–36. doi: 10.1523/JNEUROSCI.16-04-01324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop GM, Robinson SR. Quantitative analysis of cell death and ferritin expression in response to cortical iron: implications for hypoxia-ischemia and stroke. Brain Res. 2001;907:175–87. doi: 10.1016/s0006-8993(01)02303-4. [DOI] [PubMed] [Google Scholar]
- Breuer W, et al. Iron acquired from transferrin by K562 cells is delivered into a cytoplasmic pool of chelatable iron(II) J Biol Chem. 1995;270:24209–15. doi: 10.1074/jbc.270.41.24209. [DOI] [PubMed] [Google Scholar]
- Chen J, et al. White matter damage and the effect of matrix metalloproteinases in type 2 diabetic mice after stroke. Stroke. 2011;42:445–52. doi: 10.1161/STROKEAHA.110.596486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bigio MR, et al. Experimental intracerebral hemorrhage in rats. Magnetic resonance imaging and histopathological correlates. Stroke. 1996;27:2312–9. doi: 10.1161/01.str.27.12.2312. discussion 2319-20. [DOI] [PubMed] [Google Scholar]
- Demougeot C, et al. Cytoprotective efficacy and mechanisms of the liposoluble iron chelator 2,2′-dipyridyl in the rat photothrombotic ischemic stroke model. J Pharmacol Exp Ther. 2004;311:1080–7. doi: 10.1124/jpet.104.072744. [DOI] [PubMed] [Google Scholar]
- Gaasch JA, et al. Brain iron toxicity: differential responses of astrocytes, neurons, and endothelial cells. Neurochem Res. 2007;32:1196–208. doi: 10.1007/s11064-007-9290-4. [DOI] [PubMed] [Google Scholar]
- Grossetete M, Rosenberg GA. Matrix metalloproteinase inhibition facilitates cell death in intracerebral hemorrhage in mouse. J Cereb Blood Flow Metab. 2008;28:752–63. doi: 10.1038/sj.jcbfm.9600572. [DOI] [PubMed] [Google Scholar]
- Gu Y, et al. Deferoxamine reduces intracerebral hematoma-induced iron accumulation and neuronal death in piglets. Stroke. 2009;40:2241–3. doi: 10.1161/STROKEAHA.108.539536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horky LL, et al. Role of ferrous iron chelator 2,2′-dipyridyl in preventing delayed vasospasm in a primate model of subarachnoid hemorrhage. J Neurosurg. 1998;88:298–303. doi: 10.3171/jns.1998.88.2.0298. [DOI] [PubMed] [Google Scholar]
- Kamada H, et al. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: relation to blood-brain barrier dysfunction. Stroke. 2007;38:1044–9. doi: 10.1161/01.STR.0000258041.75739.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keberle H. The Biochemistry of Desferrioxamine and Its Relation to Iron Metabolism. Ann N Y Acad Sci. 1964;119:758–68. doi: 10.1111/j.1749-6632.1965.tb54077.x. [DOI] [PubMed] [Google Scholar]
- Kress GJ, et al. The relationship between intracellular free iron and cell injury in cultured neurons, astrocytes, and oligodendrocytes. J Neurosci. 2002;22:5848–55. doi: 10.1523/JNEUROSCI.22-14-05848.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leys D, et al. White matter changes in stroke patients. Relationship with stroke subtype and outcome. Eur Neurol. 1999;42:67–75. doi: 10.1159/000069414. [DOI] [PubMed] [Google Scholar]
- Lynch SG, et al. A multiple course trial of desferrioxamine in chronic progressive multiple sclerosis. Cell Mol Biol (Noisy-le-grand) 2000;46:865–9. [PubMed] [Google Scholar]
- MacLellan CL, Colbourne F. Mild to moderate hyperthermia does not worsen outcome after severe intracerebral hemorrhage in rats. J Cereb Blood Flow Metab. 2005;25:1020–9. doi: 10.1038/sj.jcbfm.9600099. [DOI] [PubMed] [Google Scholar]
- Methy D, et al. Beneficial effect of dipyridyl, a liposoluble iron chelator against focal cerebral ischemia: in vivo and in vitro evidence of protection of cerebral endothelial cells. Brain Res. 2008;1193:136–42. doi: 10.1016/j.brainres.2007.11.063. [DOI] [PubMed] [Google Scholar]
- Michel-Monigadon D, et al. c-Jun N-terminal kinase pathway inhibition in intracerebral hemorrhage. Cerebrovasc Dis. 2010;29:564–70. doi: 10.1159/000306643. [DOI] [PubMed] [Google Scholar]
- Millerot-Serrurot E, et al. Temporal changes in free iron levels after brain ischemia Relevance to the timing of iron chelation therapy in stroke. Neurochem Int. 2008;52:1442–8. doi: 10.1016/j.neuint.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Okauchi M, et al. Effects of deferoxamine on intracerebral hemorrhage-induced brain injury in aged rats. Stroke. 2009;40:1858–63. doi: 10.1161/STROKEAHA.108.535765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, et al. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J Cereb Blood Flow Metab. 2007;27:1908–18. doi: 10.1038/sj.jcbfm.9600491. [DOI] [PubMed] [Google Scholar]
- Shehadah A, et al. Combination treatment of experimental stroke with Niaspan and Simvastatin, reduces axonal damage and improves functional outcome. J Neurol Sci. 2010;294:107–11. doi: 10.1016/j.jns.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoecke M, et al. Apoptotic cell death progression after photothrombotic focal cerebral ischaemia: effects of the lipophilic iron chelator 2,2′-dipyridyl. Eur J Neurosci. 2005;22:1045–56. doi: 10.1111/j.1460-9568.2005.04297.x. [DOI] [PubMed] [Google Scholar]
- Wagner KR, et al. Heme and iron metabolism: role in cerebral hemorrhage. J Cereb Blood Flow Metab. 2003;23:629–52. doi: 10.1097/01.WCB.0000073905.87928.6D. [DOI] [PubMed] [Google Scholar]
- Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog Neurobiol. 2010;92:463–77. doi: 10.1016/j.pneurobio.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Doré S. Heme oxygenase-1 exacerbates early brain injury after intracerebral haemorrhage. Brain. 2007a;130:1643–52. doi: 10.1093/brain/awm095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Doré S. Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2007b;27:894–908. doi: 10.1038/sj.jcbfm.9600403. [DOI] [PubMed] [Google Scholar]
- Wang J, et al. The development of an improved preclinical mouse model of intracerebral hemorrhage using double infusion of autologous whole blood. Brain Res. 2008;1222:214–21. doi: 10.1016/j.brainres.2008.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, et al. Protective role of tuftsin fragment 1-3 in an animal model of intracerebral hemorrhage. Ann Neurol. 2003;54:655–64. doi: 10.1002/ana.10750. [DOI] [PubMed] [Google Scholar]
- Wang J, Tsirka SE. Neuroprotection by inhibition of matrix metalloproteinases in a mouse model of intracerebral haemorrhage. Brain. 2005a;128:1622–33. doi: 10.1093/brain/awh489. [DOI] [PubMed] [Google Scholar]
- Wang J, Tsirka SE. Tuftsin fragment 1-3 is beneficial when delivered after the induction of intracerebral hemorrhage. Stroke. 2005b;36:613–8. doi: 10.1161/01.STR.0000155729.12931.8f. [DOI] [PubMed] [Google Scholar]
- Warkentin LM, et al. Failure of deferoxamine, an iron chelator, to improve outcome after collagenase-induced intracerebral hemorrhage in rats. Brain Res. 2010;1309:95–103. doi: 10.1016/j.brainres.2009.10.058. [DOI] [PubMed] [Google Scholar]
- Wu H, et al. Iron toxicity in mice with collagenase-induced intracerebral hemorrhage. J Cereb Blood Flow Metab. 2011;31:1243–1250. doi: 10.1038/jcbfm.2010.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi G, et al. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- Xue M, et al. Contributions of multiple proteases to neurotoxicity in a mouse model of intracerebral haemorrhage. Brain. 2009;132:26–36. doi: 10.1093/brain/awn215. [DOI] [PubMed] [Google Scholar]
- Yu YY, et al. Ferrous chelator 2,2′-dipyridyl attenuates cerebral vasospasm after experimental subarachnoid haemorrhage in rabbits. J Int Med Res. 2010;38:583–92. doi: 10.1177/147323001003800220. [DOI] [PubMed] [Google Scholar]
- Zecca L, et al. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5:863–73. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]