The cerebellum was initially viewed as having a primary role in motor programming, largely in feedback and feedforward control evident in decerebrate animals walking on a treadmill and the clinical condition of spinocerebellar ataxia. Comparative input from the disynaptic parallel fibre pathway and the climbing fibre pathway on Purkinje cells (PCs) was postulated to serve as an ‘error-correction’ circuit (Fig. 1). From elegant lesion studies, it became clear early on that the cerebellum was also involved in motor learning using, for example, the associative eyelid conditioning task (McCormick et al. 1985). Hebbian modulation resulting from the parallel fibre and climbing fibre inputs onto the Purkinje cells had been postulated on the basis of their apparent anatomical interaction, and correspondingly, both long-term potentiation (LTP) and long-term depression (LTD) were later demonstrated to occur along these input pathways. This synaptic plasticity provides substrates for motor learning.
Figure 1.
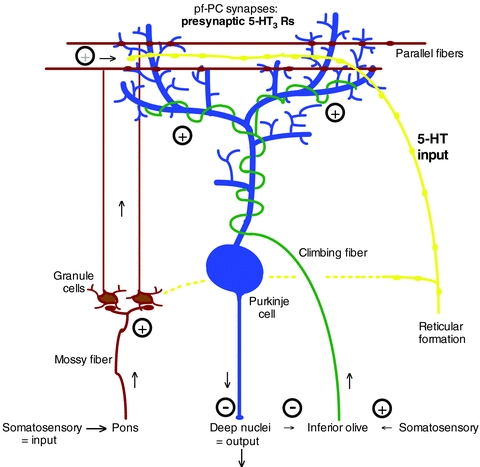
Serotonin (5-HT) input into the molecular layer of cerebellar cortex, where parallel fibers synapse onto Purkinje cell dendrites (pf-PC synapses)
Serotonergic fibres have been found to innervate virtually all sensorimotor areas, including the cerebellum (Bishop & Ho, 1985) where they arrive early in development. In addition, prominent serotonergic input into areas involved in memory, such as the hippocampus, has been noted. The role for serotonin in the cerebellum was thus first conjectured as a regulator of sensorimotor learning. Indeed, serotonin release into the cerebellum is strongly correlated with the level and type of motor activity, and serotonin strongly modulates Purkinje cell firing. However, the means by which serotonin regulates motor learning in the cerebellum, particularly in the developing animal, remain poorly understood.
In a recent article in The Journal of Physiology, Oostland and colleagues (2011), present evidence for strong serotonergic regulation of synaptic plasticity at parallel fibre presynaptic terminals on Purkinje cells during early postnatal development. Specifically, the authors demonstrate the essential role of presynaptic ligand-gated ion channel type 3 serotonin receptors (5-HT3 receptors) at this synapse. Indeed, the parallel fibre–Purkinje cell (PF–PC) synapse displayed no apparent synaptic plasticity in slices from 1-week-old mice, as measured using a paired-pulse protocol. By contrast, 5-HT3 receptor blockade converted the paired pulse ratio to depression. The authors interpret the results to mean that serotonin via presynaptic 5-HT3 maintains the immature PF–PC synapse at a normal level of transmission.
Earlier studies by several groups indicated little if any detectable 5-HT3 receptors in the cerebellum using various binding protocols. Later, discrete and highly localized immunoreactivity for 5-HT3 receptors was found on axonal-like fibres mainly in the molecular layer using immunocytochemistry, while functional 5-HT3 receptors were detected on isolated cerebellar presynaptic terminals using confocal imaging of Ca2+ signals. The work by the present authors now establishes an important role for serotonin impact through presynaptic 5-HT3 receptors in developing synaptic dynamics in the parallel fibre pathway (Fig. 1).
The work has important ramifications and raises a number of new, interesting questions. First, does serotonin influence PF–PC synapse maturation via activation of 5-HT3 receptors? Increased synaptic efficacy can enhance synaptic maturation (and competition). Second, it has been noted that parallel fibre input influences the development of climbing fibres on the Purkinje cells (Hashimoto et al. 2009). It would thus be of interest to know whether serotonin activation of presynaptic 5-HT3 receptors at PF–PC synapses has an impact on climbing fibre maturation. It would also be interesting to consider the functional impact of presynaptic regulation on postsynaptic GluRδ2s, which play a key role in synaptogenesis on the Purkinje cells (Mandolesi et al. 2009). Third, although the effect of receptor blockade was lost upon maturation (postnatal day 21), a facilitatory effect of presynaptic 5-HT3 receptor activation may remain. So far, serotonin's impact at mature PF–PC synapses (via presynaptic 5-HT3 receptors) has yet to be determined. In addition, as LTD at PF–PC synapses is considered to be key to motor learning (Linden, 2003), how do presynaptic 5-HT3 receptors fit into the equation? To date, no role for 5-HT3 receptors in cerebellar motor learning has been noted, but perhaps this could be re-examined using knock-outs. Lastly, is the effect of serotonin on the presynaptic terminals at PF–PC synapses (and the granule cells) mediated mainly by volume transmission? This would suggest a more tonic impact of serotonin. If so, the effect of sustained activation of presynaptic 5-HT3 receptors should be considered, as it might be more relevant.
It should also be noted that there are a host of other serotonin receptors at the level of the Purkinje cell bodies and the granule cells (Geurts et al. 2002), which also receive significant serotonergic input. While these other receptors may help shape Purkinje cell output, the work presented by Oostland and colleagues indicates direct involvement of 5-HT3 receptors at a crucial junction in the cerebellar motor learning circuit, namely the PF–PC synapse. Discovery of these receptors at presynaptic parallel fibre terminals thus opens up a new gateway for exploring serotonin's regulation of motor learning.
References
- Bishop GA, Ho RH. Brain Res. 1985;331:195–207. doi: 10.1016/0006-8993(85)91545-8. [DOI] [PubMed] [Google Scholar]
- Geurts FJ, De Schutter E, Timmermans JP. J Chem Neuroanat. 2002;24:65–74. doi: 10.1016/s0891-0618(02)00020-0. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Yoshida T, Sakimura K, Mishina M, Watanabe M, Kano M. Neuroscience. 2009;162:601–611. doi: 10.1016/j.neuroscience.2008.12.037. [DOI] [PubMed] [Google Scholar]
- Linden DJ. Science. 2003;301:1682–1684. doi: 10.1126/science.1090462. [DOI] [PubMed] [Google Scholar]
- Mandolesi G, Autuori E, Cesa R, Premoselli F, Cesare P, Strata P. PloS One. 2009;4:e5243. doi: 10.1371/journal.pone.0005243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Steinmetz JE, Thompson RF. Brain Res. 1985;359:120–130. doi: 10.1016/0006-8993(85)91419-2. [DOI] [PubMed] [Google Scholar]
- Oostland M, Sellmeijer J, van Hooft JA. J Physiol. 2011;589:4837–4846. doi: 10.1113/jphysiol.2011.217307. [DOI] [PMC free article] [PubMed] [Google Scholar]


