Abstract
Abstract
Skeletal muscle fibres are highly heterogeneous regarding size, metabolism and contractile function. They also show a large capacity for adaptations in response to alterations in the activation pattern. A major part of this activity-dependent plasticity relies on transcriptional alterations controlled by intracellular Ca2+ signals. In this review we discuss how intracellular Ca2+ fluctuations induced by activation patterns likely to occur in vivo control muscle properties via effects on Ca2+–calmodulin-dependent proteins. We focus on two such Ca2+ decoders: calcineurin and Ca2+–calmodulin-dependent protein kinase II. Inherent Ca2+ transients during contractions differ rather little between slow- and fast-twitch muscle fibres and this difference is unlikely to have any significant impact on the activity of Ca2+ decoders. The major exception to this is fatigue-induced changes in Ca2+ transients that occur in fast-twitch fibres exposed to high-intensity activation typical of slow-twitch motor units. In conclusion, the cascade from neural stimulation pattern to Ca2+-dependent transcription is likely to be central in maintaining the fibre phenotypes in both fast- and slow-twitch fibres. Moreover, changes in Ca2+ signalling (e.g. induced by endurance training) can result in altered muscle properties (e.g. increased mitochondrial biogenesis) and this plasticity involves other signalling pathways.
Håkan Westerblad (left) is professor in cellular muscle physiology at the Karolinska Institutet in Stockholm, Sweden. He has developed techniques to study contractile function in isolated, intact fibres from mammalian skeletal muscle. A major focus of his research has been on cellular mechanisms of skeletal muscle fatigue. Pasi Tavi (right) is Finnish Academy Research Fellow at the University of Eastern Finland, A.I. Virtanen Institute for molecular sciences, Kuopio, Finland. He has developed several mathematical muscle cell models, and recently his research has focused on understanding the transcriptional control of muscle cell E–C coupling.
 |
Introduction
Skeletal muscle fibres are heterogeneous with respect to size, metabolism and contractile function. They also have a striking capability for adaptation and plasticity. This was demonstrated in classical experiments in the early 1960s where it was shown that the activity of the motoneuron defined the properties of the innervated muscle fibres (Buller et al. 1960; Vrbova, 1963). Subsequently it was shown that the phenotype of denervated muscles could be controlled by direct electrical stimulation; a fast activation pattern (bursts of high-frequency stimulation at long intervals) moved muscles towards a fast phenotype and a slow activation pattern (prolonged low-frequency stimulation) had the opposite effect (Lømo et al. 1974). Ever since the mechanisms behind this activity-dependent plasticity of skeletal muscle have been under intensive investigation (for reviews see e.g. Pette, 2001; Schiaffino et al. 2007; Gundersen, 2011). It has become evident that the matching between phenotype and environmental demands utilizes defined programmes of gene expression (Schiaffino & Reggiani, 1996). These muscle specific genetic programmes are recruited by transcription factors, most of which are affected by Ca2+-dependent signalling cascades (Bassel-Duby & Olson, 2006). It should be noted that the activity of these transcription factors is also affected by other factors in the cellular environment and the final result therefore depends on a combination of Ca2+-dependent and Ca2+-independent signalling (Gundersen, 2011).
Surprisingly little attention has been paid to the properties of the versatile Ca2+ signals that control Ca2+-dependent signalling cascades. In fact, changes in myoplasmic free [Ca2+] ([Ca2+]i) is frequently induced by physiologically rather primitive manoeuvres, such as, increasing baseline [Ca2+]i by Ca2+ ionophores or usage of prolonged continuous tetanic stimulation. Differences between fast and slow muscle fibres are frequently attributed to major differences in cellular Ca2+ handling where, for instance, slow-twitch fibre properties are suggested to be driven by slow [Ca2+]i transients and increases in baseline [Ca2+]i (Olson & Williams, 2000). However, the popularity of this belief is not a good guide to its accuracy. In fact, [Ca2+]i transients during individual contractions show only modest differences between mammalian fast- and slow-twitch fibres (Carroll et al. 1997; Baylor & Hollingworth, 2003; Calderon et al. 2010), especially considering the slow kinetics of cellular Ca2+ decoders and their downstream targets. Moreover, baseline [Ca2+]i shows little or no increase in slow-twitch fibres during highly intense fatiguing stimulation (Bruton et al. 2003; Lunde et al. 2006). In fact, facing continuous stimulation, slow type fibres are able maintain their contraction and [Ca2+]i signals relatively stable for long periods of time, whereas fast type fibres fatigue in minutes becoming unable to maintain a normal [Ca2+]i balance, excitability and contraction (Allen et al. 2008). In this review we discuss how [Ca2+]i changes induced by stimulation patterns likely to prevail in vivo can affect cellular Ca2+ decoders and thereby control muscle properties.
Muscle plasticity
Skeletal muscle fibres are commonly characterized as being of two major categories on the basis of their fatiguability, energy metabolism and speed of contraction. The fast-twitch, type II fibres exert fast contractions, frequently use anaerobic metabolism and fatigue easily. Conversely, the slow-twitch, type I fibres use preferentially oxidative metabolism, contract slower and are more fatigue resistant. The plasticity of the muscle cells is manifested as a large potential to change properties in response to altered demands. Well-known adaptations in this context are, on the one hand, the increase in myofibrillar proteins, resulting in larger muscle cross-sectional area and increased strength, induced by resistance training and, on the other hand, the increase in oxidative capacity and fatigue resistance observed with endurance training. Plasticity may also involve shifts in the expression of a given protein from one isoform to another isoform with different properties but with the same basic function. For example, human training studies have shown shifts between the two fast-twitch myosin heavy chains, where the fastest type IIx isoform and the intermediate IIa isoform are favoured by inactivity and activity, respectively (Harridge, 2007). However, switching between type II and type I fibres requires more dramatic changes in the activation pattern (e.g. denervation or electrical stimulation; Pette, 2001; Schiaffino et al. 2007) and is generally not observed under normal physiological conditions (Harridge, 2007).
Ca2+ decoders
Ca2+ decoders are enzymes able to respond to different kinds of Ca2+ stimuli and then initiate signalling that leads to, for instance, altered gene transcription. Two central Ca2+ decoders are calcineurin (CaN) and Ca2+–calmodulin-dependent protein kinase II (CaMKII). Both these rely on the interaction between Ca2+ and calmodulin. CaN is the only serine/threonine phosphatase that is under the control of Ca2+–calmodulin (Klee et al. 1998; Sakuma & Yamaguchi, 2010). CaN is a heterodimer consisting of a calmodulin binding catalytic subunit A and a Ca2+ binding regulatory subunit B. Upon an increase in [Ca2+]i, Ca2+ binds to calmodulin forming Ca2+–calmodulin complexes, which subsequently activate CaN by binding to the regulatory subunit. The apparent Ca2+ dissociation constant (Kd) of CaN is strongly dependent on the calmodulin concentration, varying from 1.3 to 0.6 μm at calmodulin concentrations from 0.03 to 20 μm (Stemmer & Klee, 1994). The deactivation time constant of CaN is relatively fast (Stemmer & Klee, 1994) and therefore a sustained CaN activity seems to require a sustained elevation [Ca2+]i or [Ca2+]i transients coming at short intervals. However, CaN can also translate the intervals at which [Ca2+]i transients occur in muscle cells into graded levels of activity (Tavi et al. 2004; Saucerman & Bers, 2008).
Ca2+-calmodulin also activates the CaMK family of serine/threonine protein kinases, of which CaMKII has been given most attention in relation to skeletal muscle plasticity (Chin, 2005). The Ca2+ sensitivity of CaMKII is considered adequate for decoding [Ca2+]i fluctuations occurring in the living cell. As is the case with CaN, CaMKII Ca2+ sensitivity is strongly dependent on the calmodulin concentration with Kd values varying from 0.5 to 5 μm depending on the concentration of calmodulin (Chin, 2005; Saucerman & Bers, 2008). The multimeric CaMKII is initially activated by Ca2+-calmodulin binding and this is followed by autonomous inter-subunit autophosphorylation. This complexity results in both activation and deactivation of CaMKII being relatively slow and partly Ca2+ independent. As a result, CaMKII acts as a frequency decoder, activated by the increases in [Ca2+]i that occur during prolonged contractions or when brief contractions are performed at short intervals (Dolmetsch et al. 1997; Aydin et al. 2007; Koivumäki et al. 2009), although it can also be activated by prolonged increases in basal [Ca2+]i (Wright et al. 2007). Because the deactivation is slow, CaMKII retains the information from the activating signal for prolonged periods and thereby has the potential of acting as a memory molecule (Hudmon & Schulman, 2002). Interestingly, activation of CaMKII can affect sarcoplasmic reticulum (SR) Ca2+ release and inhibition of CaMKII has been shown to decrease tetanic [Ca2+]i during repeated tetanic stimulation of mouse fast-twitch fibres (Tavi et al. 2003; Aydin et al. 2007).
To a large extent, the molecular evidence supporting the role of CaN and CaMK in muscle plasticity, including shifts in fibre type composition, comes from genetic manipulations where these Ca2+ decoders have been uncoupled form their normal regulation by robust over- or under-expression (Bassel-Duby & Olson, 2006) or by expression of modified, autonomously active versions of the proteins (Naya et al. 2000). Although these elegant approaches are instrumental in showing that certain signalling pathways exist, they cannot clarify whether or not a particular pathway is utilized under physiological conditions with normal [Ca2+]i fluctuations.
Differences in Ca2+ signals between fibre types
When linking physiological [Ca2+]i signalling to muscle plasticity, one fundamental question is whether the same type of stimulation pattern triggers similar or markedly different [Ca2+]i signals in different muscle cells. This fundamental question has received relatively little attention and appears sometimes to be almost completely ignored.
Primary isolated or cultured myotubes are in many respects valuable cell models to study muscle development and plasticity, as they are robust and more easily accessible with molecular biology methods than terminally differentiated, adult muscle fibres. However, when it comes to [Ca2+]i-dependent signalling they are less useful, at least if they are used in order to study properties of such signalling that is relevant to adult muscle fibres. Prior to the development of the adult tight coupling between the action potential-induced activation of transverse tubular voltage sensors (dihydropyridine receptors) and the rapid Ca2+ release from the SR (via ryanodine receptors), developing muscle cells rely on Ca2+ influx through voltage-activated Ca2+ channels and relatively slow Ca2+ release from the SR (Cognard et al. 1993; Imbert et al. 2001). Therefore, developing myotubes display [Ca2+]i transients that are orders of magnitude slower than those in adult muscle fibres (Fig. 1). As a result, Ca2+–calmodulin pathways can be activated in myotubes at much lower stimulation frequencies than in adult fibres; for instance, 1 Hz electrical stimulation activates CaN signalling in myotubes (Kubis et al. 2002), but not in adult fibres (Liu et al. 2001).
Figure 1. [Ca2+]i transients are much slower in developing than in mature skeletal muscle cells.
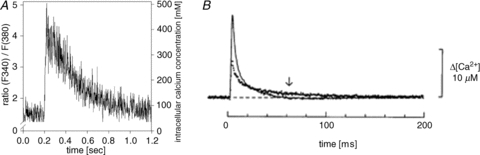
The response to a single electrical stimulation pulse in cultured rabbit myotubes (A) and adult EDL (continuous line) and soleus (dotted line) muscle fibres (B). Note the markedly different time scales in A and B. A is adapted from Kubis et al. (2003), with permission of the American Physiological Society; B is from Baylor & Hollingworth (2003).
Intuitively one would think that fast-twitch and slow-twitch muscle fibres display distinctly different [Ca2+]i signals upon activation, because of their marked difference in contractile properties. However, when measured with high-affinity, relatively slow Ca2+ indicators, fast-twitch fibres show [Ca2+]i transients in individual tetanic contractions that are only modestly faster than those in slow-twitch fibres (Fig. 2) (Carroll et al. 1997). Similarly, measurements with low-affinity, fast Ca2+ indicators show larger and faster tetanic [Ca2+]i transients in fast-twitch than in slow-twitch fibres (see Fig. 1B) (Baylor & Hollingworth, 2003; Calderon et al. 2010). However, the differences between fibre types are modest, especially in relation to the kinetics of CaN and CaMKII and their down-stream targets (discussed below). Thus, differences in [Ca2+]i signals between slow- and fast-twitch fibres during a single twitch or a brief tetanic contraction are unlikely to have markedly different effects on the Ca2+ decoders CaN and CaMKII.
Figure 2. Tetanic [Ca2+]i signals are similar in mouse fast-twitch FDB and slow-twitch soleus muscle fibres.
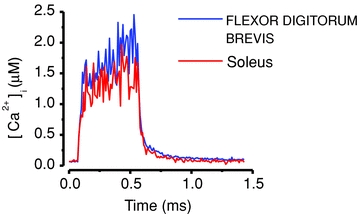
Fibres were in both cases stimulated with electrical pulses at 70 Hz for 500 ms and [Ca2+]i was measured with indo-1. Data from the FDB fibre included in Aydin et al. (2009) and the soleus fibre record adapted from Bruton et al. (2003).
The in vivo activation pattern differs markedly between motoneurons driving slow- and fast-twitch fibres, with prolonged activation at low frequencies (∼20 Hz) in the former and brief, infrequent bursts of high frequency (∼100–200 Hz) activation in the latter (Hennig & Lømo, 1985). Denervation experiments have shown that fibre type properties can be maintained with an electrical stimulation pattern similar to that occurring in vivo, whereas changes in properties towards the slow-twitch phenotype can be induced in fast-twitch fibres by a slow activation pattern and vice versa (Gundersen et al. 1988; Pette, 2001). Ca2+-dependent signalling is likely to be important in this context and it is then important to consider changes in [Ca2+]i that will occur with repeated activation, especially in experiments with electrical stimulation of a type that markedly differs from that in vivo. Stimulation with repeated tetani has markedly different effects on basal and tetanic [Ca2+]i in fast-twitch vs. slow-twitch fibres and this is illustrated in Fig. 3 (Allen et al. 2008). The typical fast-twitch flexor digitorum brevis (FDB) fibre in Fig. 3A displayed fatigue with tetanic force decreasing to 30% of the control in 88 tetani produced at a duty cycle of 0.14 and this was accompanied by an initial increase followed by a decrease in tetanic [Ca2+]i and a marked increase in basal [Ca2+]i. On the other hand, the typical slow-twitch soleus fibre in Fig. 3B was little affected by a more demanding stimulation protocol (1000 tetani at a duty cycle of 0.25) showing a decrease in tetanic force to ∼80% of the control, while basal and tetanic [Ca2+]i were little affected. Furthermore, experiments in our laboratory have shown that when mouse fast-twitch EDL fibres are exposed to continuous 20 Hz stimulation, fatigue is severe already within the first min of stimulation, and force and [Ca2+]i have basically returned to baseline values already after five min of stimulation (A. Hernández and H. Westerblad, unpublished). Thus, the marked differences in fatigue properties between fibre types have to be taken into account when studying the effects of different stimulation protocols on Ca2+-dependent signalling. This is especially important when fast-twitch muscles are exposed to the slow-type prolonged low-frequency stimulation (Simoneau & Pette, 1988; Tothova et al. 2006), which is likely to produce severe fatigue with markedly decreased, or even abolished, [Ca2+]i transients.
Figure 3. Fast-twitch FDB fibres, but not slow-twitch soleus fibres, show major changes in [Ca2+]i during fatiguing stimulation.
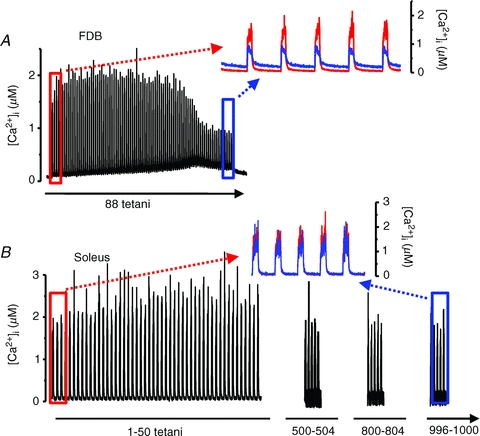
A, [Ca2+]i of a FDB fibre exposed to repeated 70 Hz, 350 ms tetani given every 2.5 s until force was decreased to 30% of the control. Inset shows a comparison between first four (red) and last four (blue) tetani; note the marked increase in basal [Ca2+]i and decrease in tetanic [Ca2+]i in fatigue. Data from this fibre included in Dahlstedt et al. (2000). B, [Ca2+]i of a soleus fibre during 1000 repeated 70 Hz, 500 ms tetani given every 2 s. Inset shows a comparison between the first five (red) and last five (blue) tetani; note that both basal and tetanic [Ca2+]i were little affected by fatiguing stimulation. Data from Bruton et al. (2003).
Activation of Ca2+ decoders by different stimulation patterns
From a physiological point of view, it is interesting to consider how CaN and CaMKII are activated by physiological [Ca2+]i signals. On the basis of simulations with generic CaN/CaMKII mathematical modelling, it can be shown that the [Ca2+]i transient induced by a single action potential is too fast to activate CaN or CaMKII (Tavi et al. 2003, 2004; Aydin et al. 2007). In support of these modelling results, 1 Hz continuous stimulation of adult muscle fibres did not activate CaN-induced nuclear translocation of the transcription factor NFAT (nuclear factor of activated T cells) (Liu et al. 2001). However, the modelling predicts that tetanic stimulation lasting 500 ms activates both CaN and CaMKII in a [Ca2+]i-dependent manner, which is followed by deactivation on a time scale ranging up to tens of seconds (Fig. 4). The rate of deactivation depends on the amplitude of the [Ca2+]i transient and is a crucial determinant of activation of the Ca2+ decoders, because cumulative activation occurs when the next [Ca2+]i transient arrives before full deactivation from the previous activation. Therefore, if 500 ms tetanic [Ca2+]i transients are repeated at short intervals, both CaN and CaMKII respond with cumulative activation (Fig. 4). Moreover, the modelling predicts that repeated activation with same sized tetanic [Ca2+]i transients results in slower speed of deactivation with time constants increasing ∼3-fold compared to after a single tetanus. Thus, these modelling experiments highlight important features of the Ca2+ decoders: they are not significantly activated by a fast event such as a single twitch [Ca2+]i transient and consequently their activity level depends on the pattern of repetitive activity; their deactivation is up to 100-fold slower than the decay of the activating [Ca2+]i and hence they have memory properties determined by the preceding activity.
Figure 4. Modelled activation of CaN and CaMKII in response to tetanic [Ca2+]i transients.
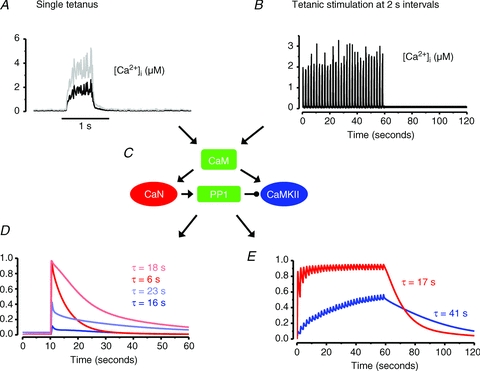
[Ca2+]i from a single 70 Hz, 500 ms tetanus (A) and from a series of 30 such tetani given at 2 s intervals (B; adapted from Fig. 3B). C, outline of the mathematical model into which the [Ca2+]i records were fed. CaM, calmodulin; PP1, protein phosphatase 1. Arrows indicate activation and punctuated arrow inhibition; for detailed description of the mathematical model see Tavi et al. (2003, 2004) and Aydin et al. (2007). D, modelled activation of CaN (red) and CaMKII (blue) induced by the single tetanus in A. τ values represent modelled deactivation time constants. In order to illustrate the effect of higher tetanic [Ca2+]i (e.g. induced by a higher stimulation frequency), A also shows tetanic [Ca2+]i of twice the amplitude (grey line) and the effect on CaN and CaMKII activation is shown in C (lighter colours). E, modelled activation of CaN and CaMKII by the repeated tetanic stimulation in B. Note that CaN is fully activated after a few tetani whereas CaMKII activity increases throughout the stimulation period. After the end of stimulation, the activity of both enzyme decays about three times slower than after a single 70 Hz tetanus.
In a variety of experimental settings, prolonged low-frequency stimulation vs. brief high-frequency bursts of stimulation have been used to mimic the activation pattern of slow-twitch and fast-twitch motor units, respectively (Pette & Vrbova, 1999; Gundersen, 2011). Figure 5 shows the activity of CaN and CaMKII predicted by mathematical modelling in response to different types of stimulation (Tavi et al. 2003, 2004; Aydin et al. 2007). Figure 5A shows the modelled response to continuous 20 Hz stimulation. With this type of stimulation CaN reaches close to maximum activity already after ∼1 s stimulation, whereas the increase in CaMKII activity occurs more slowly. In this modelling experiment the amplitude of [Ca2+]i transients was kept constant, which would be the situation in slow-twitch muscle fibres. On the other hand, in fast-twitch muscle fibres continuous 20 Hz stimulation would lead to severe fatigue with markedly decreased, or even abolished, [Ca2+]i transients, and hence the activity of CaN and CaMKII would return towards their basal state. Figure 5B and C shows the modelled response to the same, brief tetanic [Ca2+]i signal given at 1 and 32 s intervals. Due to faster kinetics, CaN responds more profoundly to an individual tetanus, rapidly reaching a saturated activity level at short (1 s) stimulation intervals, and it becomes fully deactivated during pauses with long (32 s) intervals. Conversely, CaMKII is activated and deactivated more slowly and thus shows a gradually developing increase in activity with short (1 s) intervals, whereas it is little affected at long (32 s) intervals.
Figure 5. The activation of CaN and CamKII critically depends on the rate at which contractions are produced.
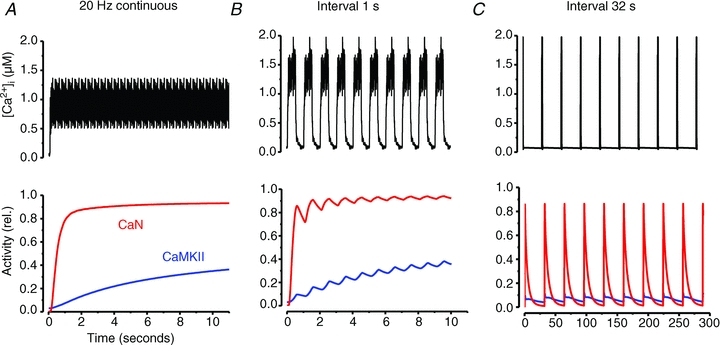
[Ca2+]i record from 20 Hz continuous stimulation (A) and the same representative 500 ms tetanic [Ca2+]i record given at 1 s (B) and 32 s (C) intervals were used as input to the mathematical model (Tavi et al. 2003, 2004; Aydin et al. 2007). The lower part shows the activities of CaN and CaMKII predicted by the model.
Ca2+ decoders and gene transcription
CaN and CaMKII mediate their effects on gene transcription by inducing nucleo-cytoplasmic shuttling of transcription factors (e.g. NFAT and myocyte enhancer factor-2, MEF2) (Chen et al. 2001; Liu et al. 2001) and transcription modulators (histone deacetylases, HDACs) (Liu et al. 2005; Shen et al. 2006). In principle, the activity of CaN and CaMKII is driven by the same changes in [Ca2+]i, but there are exemptions from this rule. For instance, translocation of HDAC4 from the nucleus reportedly involves activation of CaMKII localized in the nucleus and changes in nuclear [Ca2+] occur with substantially slower kinetics than changes in [Ca2+]i (Liu et al. 2005). This might explain why HDAC nuclear export can be activated by lower stimulus frequencies than NFAT nuclear import in adult muscle fibres (Liu et al. 2001, 2005).
The translocations of transcriptions factors and modulators are much slower processes than the Ca2+-induced activation of CaN and CaMKII. For instance, Ca2+-activated CaN promotes translocation of NFAT from the cytosol to the nucleus in a time frame of tens of minutes (Liu et al. 2001; Tothova et al. 2006). Similarly, CaMK-induced translocation of HDAC from the nucleus to the cytosol occurs with a time constant of tens of minutes (Liu et al. 2005). Consequently any [Ca2+]i signal able to activate Ca2+-dependent transcription has not only to be able to activate Ca2+ decoders, but also to maintain their activity for long enough periods to induce a significant translocation of the transcription factors and modulators. Thus, in general terms it can be stated that the activation and maintenance of a slow phenotype of transcription requires relatively constant muscle activity, whereas a fast phenotype is brought about by long lasting lack or infrequent, brief bursts of activity (Huey et al. 2001). However, while there are similarities in the response to complete lack of activity and activation with brief bursts of stimulation at long intervals, there are also marked differences. For instance, lack of activation results in marked muscle weakness and atrophy and this is not the situation with the fast pattern of activity (Gundersen, 2011). A striking example of this comes from experiments where 0.6 s high-frequency pulse trains delivered every 100 min resulted in ∼6-fold increase in tetanic force in denervated rat soleus muscle (Westgaard & Lømo, 1988). Moreover, the same study shows that stimulating rat soleus muscles with brief bursts of 100 Hz stimulation in combination with prolonged 10 Hz stimulation results in a switch towards faster contractile properties (Westgaard & Lømo, 1988). Thus, these latter findings cannot be explained on the basis of the modelled activity of CaN and CaMKII presented in Figs 4 and 5, which suggests the involvement of other important signalling pathways and/or that the activation of the Ca2+ decoders may be affected by other factors than global changes in [Ca2+]i, which will be discussed below.
In general, the prolonged slow-type stimulation of adult muscle fibres results in activation of both CaN and CaMKII pathways triggering translocation of both NFAT and HDAC and activation of MEF2 and peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) (Shen et al. 2006), thereby promoting gene expression towards a slow and oxidative phenotype (Chin et al. 1998). On the other hand, fast-type stimulation with high frequency bursts occurring at long intervals will only transiently activate the CaN and CaMKII, which is not sufficient to induce significant translocations and hence it promotes gene expressions towards a fast and anaerobic phenotype (Fig. 6). However, it again needs to be pointed out that the lack of translocations with a fast-type stimulation pattern does not result in the same phenotype as is observed with a total lack of activation (Gundersen, 2011).
Figure 6. Simplified scheme of the stimulus pattern-dependent transcription through Ca2+ activated pathways.

A, frequent activity mimicking that experienced by slow-twitch muscle fibres in vivo results in a Ca2+ binding to calmodulin (CaM) that is sufficient to induce a prolonged activation of CaN and CaMKII. Activated CaN controls transcription by inducing nuclear translocation of NFAT (Bassel-Duby & Olson, 2006) to promote transcription of slow type-specific genes, such as slow isoforms of myosin heavy chain and troponin I (Dunn et al. 1999; Serrano et al. 2001). Activated CaMKII stimulates transcription by removing repressive HDAC from the nucleus and by phosphorylating MEF2 (Bassel-Duby & Olson, 2006). MEF2 suppresses the myogenesis when it forms a complex with HDAC, but upon CaMKII-dependent disruption of MEF2-HDAC-complexes, MEF2 activates transcription (McKinsey et al. 2000) in co-operation with NFAT (Wu et al. 2000). When activated, Ca2+-dependent cascades also promote mitochondrial biogenesis via activation of PGC-1α (Wu et al. 2002). B, infrequent activation similar to that experienced by fast-twitch muscle fibres in vivo allows CaN and CaMKII deactivation between contractions. As a result, NFAT is not translocated into the nucleus, which suppresses the slow type of gene expression. In addition, HDAC remains in the nucleus and forms complexes with MEF2, which further suppresses slow type gene expression. Thus, this type of stimulation favours the expression of fast type-specific protein isoforms and it does not stimulate mitochondrial biogenesis; however, it does not promote the same phenotype as total lack of stimulation, which in addition involves muscle atrophy and weakness. Arrows indicate stimulatory actions, whereas dotted lines indicate lack of activation.
Physiological significance
There is overwhelming experimental support for CaN and CaMKII being capable of inducing major changes in muscle fibre properties, even including switches between fast-twitch type II and slow-twitch type I myosin heavy chain isoforms. However, major changes in muscle fibre properties directly attributed to CaN and CaMKII have generally been observed with rather drastic approaches, for example genetic manipulation of CaN or CaMKII (overexpression, knock-out, expression of constitutively active forms) or interference in the normal activation pattern of muscles (denervation, electrical stimulation). The importance of CaN and CaMKII in muscle plasticity under more physiological conditions is rather uncertain. Based on the properties of these two Ca2+ decoders and the prevailing [Ca2+]i signals in muscle fibres, it is clear that inactivity will result in limited activation of CaN and CaMKII and thus contribute to the inactivity-induced shift towards a fast phenotype with limited aerobic capacity. It is also evident that the prolonged activity in slow-twitch motoneurones during everyday activities will induce sufficient activation of CaN and CaMKII to maintain the slow, oxidative phenotype of slow-twitch type I fibres.
However, it is less clear whether increased physical activity can activate CaN and CaMKII to such an extent that this leads to a significant change in muscle fibre properties. For instance, are CaN and CaMKII involved in the beneficial muscle effects observed with relatively low-intensity endurance exercise performed for ∼30 min 3–5 days week-1 (American College of Sports Medicine Position Stand, 1998)? Intuitively, the rather modest and relatively short-lasting increase in [Ca2+]i directly induced by 30 min low-intensity exercise would have little impact on the overall activity of Ca2+ decoders. Even with the training performed by elite endurance athletes, the fraction of time that CaN and CaMKII are directly activated by exercise-induced increases in [Ca2+]i is limited; for example, with 4 h training per day, CaN and CaMKII would still be at their basal activity level for 20 h. Thus, for CaN and CaMKII to be important in this context, other modes of activation than the direct increase [Ca2+]i during contractile activity appear to be required. Accordingly, there are results supporting increased activity of the Ca2+ decoders also in the rest periods between training bouts (Rose et al. 2007). One mechanism behind this might be that exercise-induced changes in muscle fibre Ca2+ handling amplify the overall increase in [Ca2+]i. In support of this possibility, it was recently shown that endurance exercise can induce long-lasting changes in the RyR channel complex, which renders them more leaky (Bellinger et al. 2008). An increased SR Ca2+ leak due to modifications of the RyR channel complex has been associated with increased baseline [Ca2+]i and increases in mitochondrial biogenesis and fatigue resistance (Bruton et al. 2010). Furthermore, an increase in [Ca2+]i below the contraction threshold induced by exposing adult fast-twitch fibres to caffeine resulted in increased mitochondrial biogenesis and this was inhibited by pharmacological CaMKII inhibition (Wright et al. 2007). Thus, it appears that the Ca2+ decoders are involved in the adaptation of muscle fibres induced even by rather minor changes in global [Ca2+]i.
According to the established basic properties of CaN or CaMKII small baseline [Ca2+]i changes, such as those seen in cold acclimated muscles (60 to 90 mm) (Bruton et al. 2010), would most likely induce only minute activations of these enzymes. How can Ca2+ decoders still decode these [Ca2+]i elevations? Several mechanisms for this can be offered. For instance, when an increase in baseline [Ca2+]i is induced by increased RyR Ca2+ leak, inevitably the [Ca2+] close to the RYR channel complexes will be higher than in the cytoplasm as a whole. If Ca2+ decoders are located in these subcellular compartments, either through interactions with specific anchoring proteins, like αKAP (Bayer et al. 1998; O'Leary et al. 2006), or simply due to diffusion, they would be exposed to a high local [Ca2+]. In addition, if calmodulin is enriched in these microdomains, as reported to be the case in the vicinity of L-type calcium channels (Mori et al. 2004), local activation of Ca2+ decoders would be more extensive (Saucerman & Bers, 2008) and partly independent of the overall [Ca2+]i. However, the exact details and importance of spatial decoding of [Ca2+]i gradients for skeletal muscle plasticity are not known and will be an interesting field of future research.
The physiological changes in muscle fibre phenotype that occur with inactivity/endurance exercise involve not only the CaN and CaMKII but also several other signalling pathways, such as AMP-activated protein kinase, mitogen-activated protein kinases and hypoxia inducible factor 1α (Hawley et al. 2006; Lunde et al. 2011). In addition to [Ca2+]i, there are other changes in the cellular environment involved in muscle fibre plasticity and these include changes in energy metabolites and reactive oxygen/nitrogen species (ROS/RNS). There will also be interactions between these factors. For instance, the increased SR Ca2+ leak observed with endurance exercise involves ROS/RNS-induced modifications of the RyR channel complex (Bellinger et al. 2008), and oxidation of Ca2+–calmodulin-activated CaMKII leads to a sustained, Ca2+-independent increase in its activity (Anderson et al. 2008; Christensen et al. 2009). Thus, there is evidence for an intricate cross-talk between Ca2+ decoders, [Ca2+]i and ROS/RNS, and this constitutes another important area for future studies.
Conclusions
In the short term, the activity of the Ca2+ decoders CaN and CaMKII is mainly decided by the activity pattern imposed on muscle fibres by the nervous system, whereas intrinsic differences in [Ca2+]i transients between adult muscle fibre types is less important. However, fatigue will develop in fast-twitch fibres if these are exposed to prolonged, high-intensity stimulation and the [Ca2+]i signals are then not correlated with the stimulus pattern. The cascade from neural stimulation pattern to Ca2+-dependent transcription is likely to be central in maintaining the fibre phenotypes in both fast- and slow-twitch fibres. On the other hand, fibre type switching by altered activity of this cascade would require drastic changes in the neural activation pattern of the muscles and such drastic changes are unlikely to occur under normal physiological conditions. In the physiological context of muscle fibre plasticity, interactions and synergistic activations of Ca2+-activated cascades and other signalling pathways induced by muscle activity are likely to be important, as well as interactions between spatio-temporal changes in [Ca2+]i and other components of the cellular environment.
Acknowledgments
The authors thank T. Korhonen and J. Koivumäki for the help with the modelling. The authors acknowledge financial support from Academy of Finland, Sigrid Juselius Foundation, the Swedish Research Council, and the Swedish National Centre for Sports Research.
Glossary
Abbreviations
- CaM
calmodulin
- CaMKII
Ca2+–calmodulin-dependent protein kinase II
- CaN
calcineurin
- [Ca2+]i
myoplasmic free [Ca2+]
- FDB
flexor digitorum brevis
- HDAC
histone deacetylase
- MEF2
myocyte enhancer factor-2
- NFAT
nuclear factor of activated T-cells
- PGC-1α
peroxisome proliferator-activated receptor γ coactivator 1α
- RNS
nitrogen species
- ROS
reactive oxygen species
- RyR
ryanodine receptor
- SR
sarcoplasmic reticulum
References
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- American College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30:975–991. doi: 10.1097/00005768-199806000-00032. [DOI] [PubMed] [Google Scholar]
- Anderson ME, Erickson JR, Joiner MLA, Guan X, Kutschke W, Yang JY, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJL, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin J, Andersson DC, Hänninen SL, Wredenberg A, Tavi P, Park CB, Larsson NG, Bruton JD, Westerblad H. Increased mitochondrial Ca2+ and decreased sarcoplasmic reticulum Ca2+ in mitochondrial myopathy. Hum Mol Genet. 2009;18:278–288. doi: 10.1093/hmg/ddn355. [DOI] [PubMed] [Google Scholar]
- Aydin J, Korhonen T, Tavi P, Allen DG, Westerblad H, Bruton JD. Activation of Ca2+-dependent protein kinase II during repeated contractions in single muscle fibres from mouse is dependent on the frequency of sarcoplasmic reticulum Ca2+ release. Acta Physiol (Oxf) 2007;191:131–137. doi: 10.1111/j.1748-1716.2007.01725.x. [DOI] [PubMed] [Google Scholar]
- Bassel-Duby R, Olson EN. Signaling pathways in skeletal muscle remodeling. Annu Rev Biochem. 2006;75:19–37. doi: 10.1146/annurev.biochem.75.103004.142622. [DOI] [PubMed] [Google Scholar]
- Bayer KU, Harbers K, Schulman H. αKAP is an anchoring protein for a novel CaM kinase II isoform in skeletal muscle. EMBO J. 1998;17:5598–5605. doi: 10.1093/emboj/17.19.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor SM, Hollingworth S. Sarcoplasmic reticulum calcium release compared in slow-twitch and fast-twitch fibres of mouse muscle. J Physiol. 2003;551:125–138. doi: 10.1113/jphysiol.2003.041608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger AM, Reiken S, Dura M, Murphy PW, Deng SX, Landry DW, Nieman D, Lehnart SE, Samaru M, LaCampagne A, Marks AR. Remodeling of ryanodine receptor complex causes ‘leaky’ channels: a molecular mechanism for decreased exercise capacity. Proc Natl Acad Sci U S A. 2008;105:2198–2202. doi: 10.1073/pnas.0711074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton J, Tavi P, Aydin J, Westerblad H, Lännergren J. Mitochondrial and myoplasmic [Ca2+] in single fibres from mouse limb muscles during repeated tetanic contractions. J Physiol. 2003;551:179–190. doi: 10.1113/jphysiol.2003.043927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton JD, Aydin J, Yamada T, Shabalina IG, Ivarsson N, Zhang SJ, Wada M, Tavi P, Nedergaard J, Katz A, Westerblad H. Increased fatigue resistance linked to Ca2+-stimulated mitochondrial biogenesis in muscle fibres of cold-acclimated mice. J Physiol. 2010;588:4275–4288. doi: 10.1113/jphysiol.2010.198598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller AJ, Eccles JC, Eccles RM. Interactions between motoneurones and muscles in respect of the characteristic speeds of their responses. J Physiol. 1960;150:417–439. doi: 10.1113/jphysiol.1960.sp006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon JC, Bolanos P, Caputo C. Myosin heavy chain isoform composition and Ca2+ transients in fibres from enzymatically dissociated murine soleus and extensor digitorum longus muscles. J Physiol. 2010;588:267–279. doi: 10.1113/jphysiol.2009.180893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll SL, Klein MG, Schneider MF. Decay of calcium transients after electrical stimulation in rat fast- and slow-twitch skeletal muscle fibres. J Physiol. 1997;501:573–588. doi: 10.1111/j.1469-7793.1997.573bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SL, Wang SC, Hosking B, Muscat GE. Subcellular localization of the steroid receptor coactivators (SRCs) and MEF2 in muscle and rhabdomyosarcoma cells. Mol Endocrinol. 2001;15:783–796. doi: 10.1210/mend.15.5.0637. [DOI] [PubMed] [Google Scholar]
- Chin ER. Role of Ca2+/calmodulin-dependent kinases in skeletal muscle plasticity. J Appl Physiol. 2005;99:414–423. doi: 10.1152/japplphysiol.00015.2005. [DOI] [PubMed] [Google Scholar]
- Chin ER, Olson EN, Richardson JA, Yang Q, Humphries C, Shelton JM, Wu H, Zhu W, Bassel-Duby R, Williams RS. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev. 1998;12:2499–2509. doi: 10.1101/gad.12.16.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen MD, Dun W, Boyden PA, Anderson ME, Mohler PJ, Hund TJ. Oxidized calmodulin kinase II regulates conduction following myocardial infarction: A computational analysis. PLoS Comput Biol. 2009;5:e1000583.. doi: 10.1371/journal.pcbi.1000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cognard C, Constantin B, Rivet-Bastide M, Imbert N, Besse C, Raymond G. Appearance and evolution of calcium currents and contraction during the early post-fusional stages of rat skeletal muscle cells developing in primary culture. Development. 1993;117:1153–1161. doi: 10.1242/dev.117.3.1153. [DOI] [PubMed] [Google Scholar]
- Dahlstedt AJ, Katz A, Wieringa B, Westerblad H. Is creatine kinase responsible for fatigue? Studies of isolated skeletal muscle deficient in creatine kinase. FASEB J. 2000;14:982–990. doi: 10.1096/fasebj.14.7.982. [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- Dunn SE, Burns JL, Michel RN. Calcineurin is required for skeletal muscle hypertrophy. J Biol Chem. 1999;274:21908–21912. doi: 10.1074/jbc.274.31.21908. [DOI] [PubMed] [Google Scholar]
- Gundersen K. Excitation-transcription coupling in skeletal muscle: the molecular pathways of exercise. Biol Rev. 2011;86:564–600. doi: 10.1111/j.1469-185X.2010.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen K, Leberer E, Lømo T, Pette D, Staron RS. Fibre types, calcium-sequestering proteins and metabolic enzymes in denervated and chronically stimulated muscles of the rat. J Physiol. 1988;398:177–189. doi: 10.1113/jphysiol.1988.sp017037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harridge SD. Plasticity of human skeletal muscle: gene expression to in vivo function. Exp Physiol. 2007;92:783–797. doi: 10.1113/expphysiol.2006.036525. [DOI] [PubMed] [Google Scholar]
- Hawley JA, Hargreaves M, Zierath JR. Signalling mechanisms in skeletal muscle: role in substrate selection and muscle adaptation. Essays Biochem. 2006;42:1–12. doi: 10.1042/bse0420001. [DOI] [PubMed] [Google Scholar]
- Hennig R, Lømo T. Firing patterns of motor units in normal rats. Nature. 1985;314:164–166. doi: 10.1038/314164a0. [DOI] [PubMed] [Google Scholar]
- Hudmon A, Schulman H. Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem J. 2002;364:593–611. doi: 10.1042/BJ20020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey KA, Roy RR, Baldwin KM, Edgerton VR. Temporal effects of inactivty on myosin heavy chain gene expression in rat slow muscle. Muscle Nerve. 2001;24:517–526. doi: 10.1002/mus.1035. [DOI] [PubMed] [Google Scholar]
- Imbert N, Vandebrouck C, Duport G, Raymond G, Hassoni AA, Constantin B, Cullen MJ, Cognard C. Calcium currents and transients in co-cultured contracting normal and Duchenne muscular dystrophy human myotubes. J Physiol. 2001;534:343–355. doi: 10.1111/j.1469-7793.2001.00343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee CB, Ren H, Wang X. Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J Biol Chem. 1998;273:13367–13370. doi: 10.1074/jbc.273.22.13367. [DOI] [PubMed] [Google Scholar]
- Koivumäki JT, Korhonen T, Takalo J, Weckström M, Tavi P. Regulation of excitation-contraction coupling in mouse cardiac myocytes: integrative analysis with mathematical modelling. BMC Physiol. 2009;9:16. doi: 10.1186/1472-6793-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubis HP, Hanke N, Scheibe RJ, Meissner JD, Gros G. Ca2+ transients activate calcineurin/NFATc1 and initiate fast-to-slow transformation in a primary skeletal muscle culture. Am J Physiol Cell Physiol. 2003;285:C56–C63. doi: 10.1152/ajpcell.00377.2002. [DOI] [PubMed] [Google Scholar]
- Kubis HP, Scheibe RJ, Meissner JD, Hornung G, Gros G. Fast-to-slow transformation and nuclear import/export kinetics of the transcription factor NFATc1 during electrostimulation of rabbit muscle cells in culture. J Physiol. 2002;541:835–847. doi: 10.1113/jphysiol.2002.017574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Cseresnyes Z, Randall WR, Schneider MF. Activity-dependent nuclear translocation and intranuclear distribution of NFATc in adult skeletal muscle fibers. J Cell Biol. 2001;155:27–39. doi: 10.1083/jcb.200103020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Randall WR, Schneider MF. Activity-dependent and -independent nuclear fluxes of HDAC4 mediated by different kinases in adult skeletal muscle. J Cell Biol. 2005;168:887–897. doi: 10.1083/jcb.200408128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lømo T, Westgaard RH, Dahl HA. Contractile properties of muscle: control by pattern of muscle activity in the rat. Proc R Soc Lond B Biol Sci. 1974;187:99–103. doi: 10.1098/rspb.1974.0064. [DOI] [PubMed] [Google Scholar]
- Lunde IG, Anton SL, Bruusgaard JC, Rana ZA, Ellefsen S, Gundersen K. Hypoxia inducible factor 1α links fast-patterned muscle activity and fast muscle phenotype in rats. J Physiol. 2011;589:1443–1454. doi: 10.1113/jphysiol.2010.202762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunde PK, Sejersted OM, Thorud HM, Tonnessen T, Henriksen UL, Christensen G, Westerblad H, Bruton J. Effects of congestive heart failure on Ca2+ handling in skeletal muscle during fatigue. Circ Res. 2006;98:1514–1519. doi: 10.1161/01.RES.0000226529.66545.e5. [DOI] [PubMed] [Google Scholar]
- McKinsey TA, Zhang CL, Olson EN. Activation of the myocyte enhancer factor-2 transcription factor by calcium/calmodulin-dependent protein kinase-stimulated binding of 14-3-3 to histone deacetylase 5. Proc Natl Acad Sci U S A. 2000;97:14400–14405. doi: 10.1073/pnas.260501497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori MX, Erickson MG, Yue DT. Functional stoichiometry and local enrichment of calmodulin interacting with Ca2+ channels. Science. 2004;304:432–435. doi: 10.1126/science.1093490. [DOI] [PubMed] [Google Scholar]
- Naya FJ, Mercer B, Shelton J, Richardson JA, Williams RS, Olson EN. Stimulation of slow skeletal muscle fiber gene expression by calcineurin in vivo. J Biol Chem. 2000;275:4545–4548. doi: 10.1074/jbc.275.7.4545. [DOI] [PubMed] [Google Scholar]
- O'Leary H, Sui X, Lin PJ, Volpe P, Bayer KU. Nuclear targeting of the CaMKII anchoring protein αKAP is regulated by alternative splicing and protein kinases. Brain Res. 2006;1086:17–26. doi: 10.1016/j.brainres.2006.02.120. [DOI] [PubMed] [Google Scholar]
- Olson EN, Williams RS. Remodeling muscles with calcineurin. Bioessays. 2000;22:510–519. doi: 10.1002/1521-1878(200011)22:11<1049::AID-BIES14>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Pette D. Historical Perspectives: plasticity of mammalian skeletal muscle. J Appl Physiol. 2001;90:1119–1124. doi: 10.1152/jappl.2001.90.3.1119. [DOI] [PubMed] [Google Scholar]
- Pette D, Vrbova G. What does chronic electrical stimulation teach us about muscle plasticity? Muscle Nerve. 1999;22:666–677. doi: 10.1002/(sici)1097-4598(199906)22:6<666::aid-mus3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Rose AJ, Frosig C, Kiens B, Wojtaszewski JF, Richter EA. Effect of endurance exercise training on Ca2+ calmodulin-dependent protein kinase II expression and signalling in skeletal muscle of humans. J Physiol. 2007;583:785–795. doi: 10.1113/jphysiol.2007.138529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma K, Yamaguchi A. The functional role of calcineurin in hypertrophy, regeneration, and disorders of skeletal muscle. J Biomed Biotech. 2010;2010:721219. doi: 10.1155/2010/721219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucerman JJ, Bers DM. Calmodulin mediates differential sensitivity of CaMKII and calcineurin to local Ca2+ in cardiac myocytes. Biophys J. 2008;95:4597–4612. doi: 10.1529/biophysj.108.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev. 1996;76:371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Sandri M, Murgia M. Activity-dependent signaling pathways controlling muscle diversity and plasticity. Physiology (Bethesda) 2007;22:269–278. doi: 10.1152/physiol.00009.2007. [DOI] [PubMed] [Google Scholar]
- Serrano AL, Murgia M, Pallafacchina G, Calabria E, Coniglio P, Lømo T, Schiaffino S. Calcineurin controls nerve activity-dependent specification of slow skeletal muscle fibers but not muscle growth. Proc Natl Acad Sci U S A. 2001;98:13108–13113. doi: 10.1073/pnas.231148598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen T, Liu Y, Randall WR, Schneider MF. Parallel mechanisms for resting nucleo-cytoplasmic shuttling and activity dependent translocation provide dual control of transcriptional regulators HDAC and NFAT in skeletal muscle fiber type plasticity. J Muscle Res Cell Motil. 2006;27:405–411. doi: 10.1007/s10974-006-9080-7. [DOI] [PubMed] [Google Scholar]
- Simoneau JA, Pette D. Species-specific effects of chronic nerve stimulation upon tibialis anterior muscle in mouse, rat, guinea pig, and rabbit. Pflugers Arch. 1988;412:86–92. doi: 10.1007/BF00583735. [DOI] [PubMed] [Google Scholar]
- Stemmer PM, Klee CB. Dual calcium ion regulation of calcineurin by calmodulin and calcineurin B. Biochemistry. 1994;33:6859–6866. doi: 10.1021/bi00188a015. [DOI] [PubMed] [Google Scholar]
- Tavi P, Allen DG, Niemelä P, Vuolteenaho O, Weckström M, Westerblad H. Calmodulin kinase modulates Ca2+ release in mouse skeletal muscle. J Physiol. 2003;551:5–12. doi: 10.1113/jphysiol.2003.042002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavi P, Pikkarainen S, Ronkainen J, Niemelä P, Ilves M, Weckström M, Vuolteenaho O, Bruton J, Westerblad H, Ruskoaho H. Pacing-induced calcineurin activation controls cardiac Ca2+ signalling and gene expression. J Physiol. 2004;554:309–320. doi: 10.1113/jphysiol.2003.053579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tothova J, Blaauw B, Pallafacchina G, Rudolf R, Argentini C, Reggiani C, Schiaffino S. NFATc1 nucleocytoplasmic shuttling is controlled by nerve activity in skeletal muscle. J Cell Sci. 2006;119:1604–1611. doi: 10.1242/jcs.02875. [DOI] [PubMed] [Google Scholar]
- Westgaard RH, Lømo T. Control of contractile properties within adaptive ranges by patterns of impulse activity in the rat. J Neurosci. 1988;8:4415–4426. doi: 10.1523/JNEUROSCI.08-12-04415.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrbova G. The effect of motoneurone activity on the speed of contraction of striated muscle. J Physiol. 1963;169:513–526. doi: 10.1113/jphysiol.1963.sp007276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DC, Geiger PC, Han DH, Jones TE, Holloszy JO. Calcium induces increases in peroxisome proliferator-activated receptor γ coactivator-1α and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation. J Biol Chem. 2007;282:18793–18799. doi: 10.1074/jbc.M611252200. [DOI] [PubMed] [Google Scholar]
- Wu H, Kanatous SB, Thurmond FA, Gallardo T, Isotani E, Bassel-Duby R, Williams RS. Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science. 2002;296:349–352. doi: 10.1126/science.1071163. [DOI] [PubMed] [Google Scholar]
- Wu H, Naya FJ, McKinsey TA, Mercer B, Shelton JM, Chin ER, Simard AR, Michel RN, Bassel-Duby R, Olson EN, Williams RS. MEF2 responds to multiple calcium-regulated signals in the control of skeletal muscle fiber type. EMBO J. 2000;19:1963–1973. doi: 10.1093/emboj/19.9.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]


