Non-technical summary
Relaxed skeletal muscles behave like springs that resist joint motion. There have been few in vivo studies of the spring-like properties of relaxed muscles. In this study, ultrasound was used to image human calf muscles while muscle length was changed by rotating the ankle of relaxed subjects. The muscles of some subjects buckled at short lengths. At short lengths most muscle fascicles (bundles of muscle cells) are slack. As the muscle is lengthened the slack is progressively taken up, first in some fascicles then in others. The increase in muscle length is due partly to increases in the length of muscle fascicles but most of the increase in muscle length occurs in the tendons.
Abstract
Abstract
Ultrasound imaging was used to measure the length of muscle fascicles in human gastrocnemius muscles while the muscle was passively lengthened and shortened by moving the ankle. In some subjects the muscle belly ‘buckled’ at short lengths. When the gastrocnemius muscle–tendon unit was passively lengthened from its shortest in vivo length by dorsiflexing the ankle, increases in muscle–tendon length were not initially accompanied by increases in muscle fascicle lengths (fascicle length remained constant), indicating muscle fascicles were slack at short muscle–tendon lengths. The muscle–tendon length at which slack is taken up differs among fascicles: some fascicles begin to lengthen at very short muscle–tendon lengths whereas other fascicles remain slack over a large range of muscle–tendon lengths. This suggests muscle fascicles are progressively ‘recruited’ and contribute sequentially to muscle–tendon stiffness during passive lengthening of the muscle–tendon unit. Even above their slack lengths muscle fascicles contribute only a small part (<∼30%) of the total change in muscle–tendon length. The contribution of muscle fascicles to muscle–tendon length increases with muscle length. The novelty of this work is that it reveals a previously unrecognised phenomenon (buckling at short lengths), posits a new mechanism of passive mechanical properties of muscle (recruitment of muscle fascicles), and confirms with high-resolution measurements that the passive compliance of human gastrocnemius muscle–tendon units is due largely to the tendon. It would be interesting to investigate if adaptations of passive properties of muscles are associated with changes in the distribution of muscle lengths at which fascicles fall slack.
Introduction
Relaxed skeletal muscle–tendon units generate passive tension that increases with muscle length. Passive tension is physiologically important because it constrains joint motion. In some movement disorders skeletal muscle–tendon units become abnormally short and stiff – they develop muscle contracture. Muscle contracture can prevent joint motion necessary for normal motor function.
Many muscles have long tendons which contribute to the passive mechanical properties of the muscle–tendon unit. It has been estimated that tendons contribute between about half and three-quarters of the total compliance of the relaxed rabbit soleus, human tibialis anterior and human gastrocnemius muscle–tendon units (Herbert & Crosbie, 1997; Herbert et al. 2002; Hoang et al. 2007). The surprisingly large contribution of tendon to muscle–tendon compliance occurs because the tendons of these muscles are many times longer than the muscle fascicles so even small tendon strains contribute substantially to muscle–tendon compliance.
At very short lengths, muscle–tendon units do not develop any passive tension – that is, they fall slack. Several observations suggest that some muscles fall slack within their physiological range of lengths (i.e. between the smallest and greatest lengths attained in vivo). For example the relaxed human brachialis muscle undergoes little change in pennation as it is lengthened by extending the elbow, and even very weak isometric contractions produce large increases in pennation (Herbert & Gandevia, 1995). Recent attempts to measure the length at which human gastrocnemius muscle–tendon units fall slack in vivo suggest the relaxed muscle is slack over about one-quarter of the physiological range (Hoang et al. 2005, 2007), although there is considerable variation between subjects. It is not known if all fascicles in a muscle fall slack at the same muscle–tendon length or if, for example, there is progressive ‘recruitment’ of muscle fascicles from one end of the muscle belly to the other during passive loading.
Here we report observations of the mechanical behaviour of the relaxed human gastrocnemius muscle in vivo. The observations were first made incidentally. While using ultrasound imaging to study mechanisms of contracture in people with spinal cord injury and stroke we observed that, even in control subjects (subjects who did not have contracture), (a) muscles sometimes ‘buckled’ at short lengths, (b) the muscle–tendon length at which muscle fascicles fell slack was widely distributed, and (c) above slack length, changes in muscle fascicle length were much smaller than changes in the length of the muscle–tendon unit. This paper presents a detailed analysis of the changes in the length and course of muscle fascicles and tendons observed in the gastrocnemius muscles of healthy control subjects in vivo.
Methods
Ethical approval
The procedures were approved by the University of Sydney's Human Research Ethics Committee. All subjects gave written, informed consent and the study was conducted according to the Declaration of Helsinki.
Subjects
Twenty-five healthy adults (15 men, 10 women) aged 21–86 (48 ± 21, mean ± SD) participated in the main study. All were currently free of musculoskeletal injury. None had a history of surgery for the ankle or knee.
Instrumentation and procedures
Subjects lay supine with the hip semi-flexed and the knee near-fully flexed (117 ± 13 deg). Knee angle was measured by taking the difference in the inclinations of the thigh and shank, measured with a digital inclinometer. The left foot was strapped to a footplate that could be manually rotated about a fixed axis. The axis was aligned with the presumed axis of the ankle (the inferior tip of the lateral malleolus; Lundberg et al. 1989; Breuning et al. 2008). EMG electrodes (diameter 30 mm, 50–60 mm centre-to-centre spacing) were attached to the skin over the belly of the lateral gastrocnemius muscle.
The subject was asked to relax as the ankle was passively plantarflexed and dorsiflexed at approximately 10 deg s−1 through the full range of ankle angles. Prior to data collection the ankle was preconditioned with five complete cycles. Data were collected from the subsequent three cycles. Ankle angle was measured with a rotary potentiometer built into the footplate axis. The torque applied to the foot about the same axis was measured with a load cell (XTran 250 N, Applied Measurement, Melbourne, Australia) attached to the base of the footplate. Ankle angle and torque were sampled at 50 Hz. EMG signals were band-pass filtered (20–1000 Hz) and sampled at 2 kHz using Spike2 software with a CED 1401 interface. EMG traces were inspected offline. Data obtained in periods of evident EMG activity were omitted from subsequent analysis.
Ultrasound was used to image the medial gastrocnemius muscle while the ankle was plantarflexed and dorsiflexed. The ultrasound system was designed to generate a wide field of view. This was necessary because it is often difficult to view whole muscle fascicles with conventional ultrasound systems, especially while muscle length is changing. Briefly, the complete system consisted of two portable ultrasound systems (Esaote MyLab25) each with a 46 mm linear array transducer (Esaote LA522E; 7.5–12 MHz). The two transducers were coupled together in a custom-made mold. The combined transducer was positioned over the distal end of the medial gastrocnemius muscle so that the muscle–tendon junction was visible in the distal part of the image when the ankle was in a plantarflexed position. The transducer was rotated slightly about its proximo-distal and antero-posterior axes to find the clearest image of the muscle fascicles (presumed to be the position in which the transducer was best aligned with the plane of the muscle fascicles). The position and orientation of the transducer was maintained manually while the ankle was plantarflexed and dorsiflexed through the full range of motion. Video sequences generated by the two ultrasound systems were captured with a dual-channel video capture card (ExtremeRGB, EMS Imaging, UK) at 15 Hz. The two video sequences were cropped and ‘stitched’ together offline to generate a composite video sequence with a field of view of 110 mm and a depth of 40 mm. The arrays of the two transducers did not extend to the edge of the transducer so the stitched videos contained a blank (‘gap’) region in the middle of the image (Fig. 1). Each frame of the video sequence was 1308 × 468 pixels and included a gap region of 214 × 468 pixels (18 mm, or 16% of the width of the image).
Figure 1. Composite images of the gastrocnemius muscle produced by joining images from two ultrasound transducers.
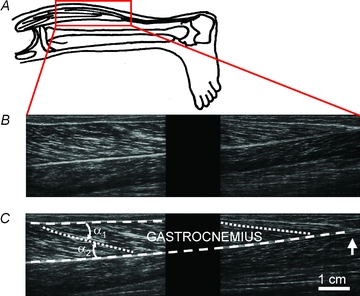
A, position of samples. B, sagittal image of the gastrocnemius. The proximal (knee) end of the muscle is to the left. The field of view of each transducer does not extend to the edge of the transducer so there is a blank ‘gap’ region between the left and right parts of the image. The muscle in these images is at an intermediate length (knee angle 125 deg, ankle angle 89 deg). C, same composite image as in B. Lines show the proximal (upper) and distal (lower) tendinous aponeuroses (dashed lines), and proximal and distal muscle fascicles (dotted lines). Pennation is the average of angles α1 and α2. The vertical arrow indicates the distal muscle–tendon junction. Note that the tendons (aponeuroses) and muscle fascicles follow a smooth course; that is, there is no evidence of buckling.
Measurement of muscle length, muscle fascicle length and pennation
The length of the gastrocnemius muscle–tendon unit was estimated from measurements of the (fixed) knee angle and the (variable) ankle angle using equations provided by Grieve et al. (1978). The length of muscle fascicles and pennation of the muscle were measured from ultrasound images using custom-written Matlab software. Briefly, the procedure was as follows. The investigator used a cursor to identify a series of control points along the proximal and distal tendinous aponeuroses of the gastrocnemius muscle in the first frame of a video sequence. A further series of control points was located along each of three muscle fascicles: a proximal fascicle, a distal fascicle and an intermediate fascicle. Additional control points were located on other distinctive features in the muscle belly. Each control point defined the centre of a region of interest of 31 × 31 pixels. Displacements of the regions of interest were tracked across video sequences using image cross-correlation routines in MATLAB. The search field for the location of the region of interest in any particular frame of the video sequence was a window of 61 × 61 pixels centred on the location of the control point in the preceding frame. The motion was smoothed with a 2-dimensional spatial filter to reduce random error (Garcia, 2010). This filter provided a full-field estimate of the motion which enabled estimation of the motion within the gap region. The tracking was inspected visually. If the tracked control points appeared to drift, the analysis was aborted, control points were repositioned, and the tracking was initiated once again. The procedure was repeated until the investigator was satisfied that the video sequence was tracked accurately. All tracking was carried out by a single investigator.
A regression line (first, second or third-order polynomial, selected by the user) was fitted to the control points lying along each fascicle in each frame of the video sequence. The intersections between the regression lines and spline curves fitted to control points along the aponeuroses defined the location of the proximal and distal insertions of muscle fascicles on the aponeuroses. Pennation was defined as the mean of the two acute angles (α1 and α2 in Fig. 1C) formed between the line passing from the proximal to distal fascicle insertions and the proximal and distal aponeuroses (Narici et al. 1996; Klimstra et al. 2007). Muscle fascicle length was calculated as the straight line distance between the muscle fascicle insertions, multiplied by the cosine of the pennation angle. This method of quantifying muscle fascicle length provides a measure of the effective length of the muscle fascicle (the absolute contribution of muscle fascicle length to muscle–tendon length) that accounts for fascicle curvature and pennation. For convenience we will refer to this measure as muscle fascicle length.
The length of the gastrocnemius muscle–tendon unit at which muscle fascicles fell slack was obtained by fitting a piecewise polynomial to the relationship between fascicle length (lf) and muscle–tendon length (lg):
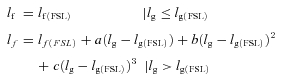 |
(1) |
where the subscript FSL means ‘at which the fascicle falls slack’. lf(FSL), lg(FSL), a, b and c are constants determined with a least-squares fitting procedure (Fig. 3A). Note that this fitting procedure generates two slack lengths: the length of the muscle fascicle when it falls slack (lf(FSL); the abscissa of the arrows in Fig. 3A) and the length of the muscle–tendon unit when the fascicle falls slack (lg(FSL); the ordinate of the arrows in Fig. 3A). In this report we focus on the latter.
Figure 3. Tracking of fascicle lengths.
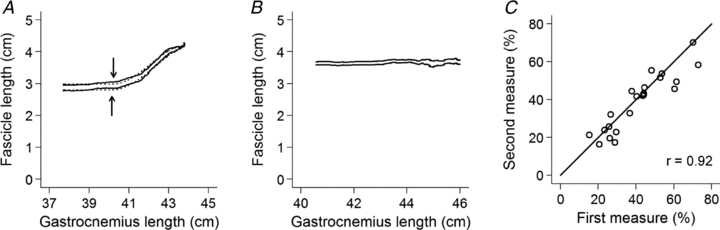
A, change in length of a single muscle fascicle as the muscle was passively lengthened. The two traces are from a single muscle fascicle tracked on two occasions. At short muscle–tendon lengths, increases in muscle–tendon length are not accompanied by changes in muscle fascicle length, indicating that the muscle fascicle is slack. The arrows show the slack lengths obtained by fitting eqn (1) to the data. The fitted equations are shown as dotted lines. B, same as in A, but data are from a fascicle which was slack at all observed lengths. C, relationship between repeated measurements of length of the gastrocnemius at which the fascicles fall slack, made by a single investigator on 22 subjects. The length of the gastrocnemius at which the fascicles fall slack is expressed as a percentage of the physiological range.
The errors involved in tracking of fascicle length and pennation were determined in the following way. Video sequences of one fascicle from each of 25 subjects were tracked by a single investigator on two occasions (Fig. 3A). To ensure that the resulting estimate of reliability included error associated with the initial placement of control points on the ultrasound images, the control points used in the first tracking were not used in the second tracking. Instead, the investigator generated a new set of control points for the aponeuroses and the marked fascicle when the muscle was re-tracked.
Estimates of within-investigator reliability of measures of the muscle-tendon length at which fascicles fell slack were obtained by comparing the slack lengths obtained from the two sets of tracked coordinates.
Results
Buckling
When the gastrocnemius muscle was observed at intermediate lengths or at the longest in vivo lengths (i.e. when the ankle was at an intermediate or dorsiflexed angle) the course of the aponeuroses and muscle fascicles followed a smooth curve (Fig. 1). However, when the muscle was taken to very short lengths (when the ankle was plantarflexed) the muscles of some subjects ‘buckled’ (Fig. 2). When buckling occurred it was always seen in the distal end of the distal aponeurosis (Fig. 2B) although it was sometimes also seen in the muscle fascicles (Fig. 2A). Buckling is more easily perceived in video sequences than in still images so videos which demonstrate this phenomenon have been supplied as Supplementary Videos S1–3. Buckling occurred when the muscle was electromyographically quiescent (Supplementary Fig. S1).
Figure 2. Examples of subtle, moderate and extreme buckling in the human gastrocnemius at very short lengths.
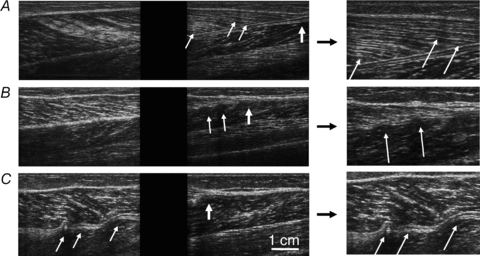
Thin, oblique arrows indicate buckles. Thicker, vertical arrows indicate distal muscle–tendon junction. The panels at the right show details of features in the left panels. A, subtle buckling in distal muscle fascicles. B, two buckles in the distal aponeurosis. C, gross buckling of the distal end of the muscle, most notably in the central part of the distal aponeurosis.
Overt buckling occurred in 6 (24%) of 25 subjects (1 man and 5 women; mean age 48 ± 17). Possible buckling was observed in a further 8 subjects (3 men and 5 women; mean age 47 ± 24). There was no evidence of buckling in the remaining 11 subjects (6 men and 5 women; mean age 49 ± 23). The chance of overt buckling was unrelated to age (odds ratio (OR) = 0.98; P = 0.99; exact logistic regression). The chance of buckling in men appeared to be lower than in women (OR = 0.23) but this difference was not statistically significant (P = 0.40; exact logistic regression). The following descriptions apply to the six subjects in whom there was clear buckling.
Buckling was only apparent at short muscle lengths. There was an abrupt onset of buckling when the muscle was slowly passively shortened. The buckling disappeared abruptly when the muscle was lengthened again. When the muscle was cyclically shortened and lengthened buckling was observed at the same location in the muscle and at the same muscle–tendon lengths.
The muscle–tendon length at which the gastrocnemius buckled was expressed as a proportion of the range between the shortest and longest in vivo lengths. In those people in whom buckling was evident, buckling was apparent at lengths below a mean of 33% of the range of in vivo lengths (SD 6%, range 25–39%).
All observations described above were obtained when subjects were relaxed. However, additional video sequences were obtained when subjects actively shortened the gastrocnemius by actively plantarflexing the ankle as far as possible. The footplate provided no resistance to rotation (although it provided a small weight torque that assisted plantarflexion), but at the extreme of plantarflexion the passive stiffness of the ankle provided resistance. The pattern and magnitude of buckling observed during active contraction to very short physiological lengths was similar to that during passive shortening.
Fascicle slack lengths
The tracking procedures yielded reproducible measures of fascicle length and pennation. The mean root mean square error (RMSE) between the tracked pairs of muscle fascicle lengths was 1.7 mm and the mean RMSE of pennation was 1.4 deg.
At very short muscle–tendon lengths, increases in muscle-tendon lengths (i.e. ankle dorsiflexion) were not accompanied by increases in muscle fascicle lengths (Fig. 3A; Supplementary Fig. S2). This implies that the fascicles were slack at those muscle–tendon lengths.
Further lengthening of the muscle–tendon unit resulted in an abrupt onset of lengthening of muscle fascicles (arrows in Fig. 3A). This was presumed to be the length at which the slack was taken up in the muscle. The relationship between muscle fascicle length and muscle–tendon length was fitted well with the piecewise polynomial regression. The regression provided estimates of lg(FSL), the muscle–tendon length at which fascicles fell slack.
As noted in the Methods section, the fascicles in three of the subjects included in the analysis of reliability did not exhibit any detectable increase in length (see Fig. 3B for an example). This may have been because, with the knee in a flexed position, those fascicles remained below their slack lengths even when the ankle was dorsiflexed. Data from the remaining 22 subjects showed that the within-investigator reliability of procedures for determining slack lengths was high (Fig. 3C). The correlation coefficient relating the two measures of muscle–tendon length at which the muscle fascicles fell slack was 1.00. When the muscle–tendon length at which the muscle fascicles fell slack was expressed as a percentage of the physiological range the correlation was 0.92 (Fig. 3C). The 95% limits of agreement (Bland & Altman, 1986) were −15% to 10% of the physiological range of gastrocnemius lengths.
The muscle–tendon lengths at which the onset of muscle fascicle lengthening occurred (or, equivalently, the muscle–tendon length at which fascicles fell slack during muscle–tendon shortening) were widely distributed (Fig. 4A and B) and varied from 3 to 79% of the physiological range (37 ± 20%).
Figure 4. Distributions of the gastrocnemius lengths at which muscle fascicles fall slack, expressed as a percentage of the physiological range of gastrocnemius lengths.
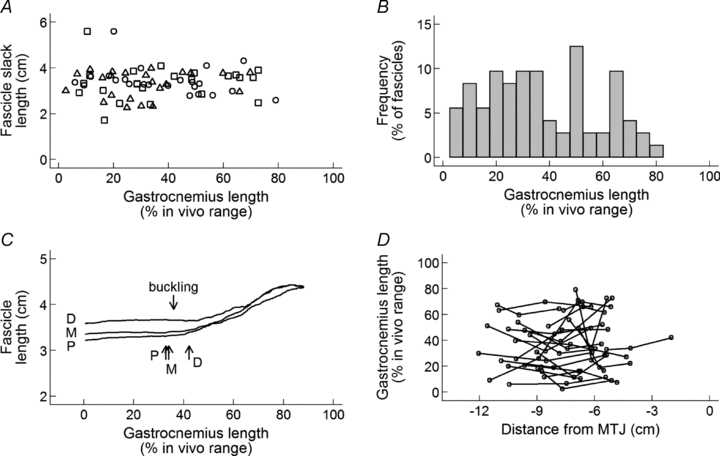
A, all three fascicles (open circles, proximal; triangles, middle; squares, distal) from one view of the gastrocnemius muscle in 22 subjects. B, frequency distribution of data in panel A. C, lengths of proximal, middle and distal fascicles from one view of the gastrocnemius muscle of a typical subject, as a function of muscle–tendon length. Upward-pointing arrows show the muscle–tendon lengths at which each of the three muscle fascicles fell slack. This subject displayed buckling in the distal aponeurosis. The downward-pointing arrow shows the length below which buckling was apparent. D, relationship between the location of muscle fascicles (distance of the proximal insertion of the fascicle from the muscle–tendon junction (MTJ)) and the gastrocnemius lengths at which muscle fascicles fall slack. Proximal, middle and distal fascicles from one muscle are joined with lines.
There was considerable variation in the muscle–tendon length at which fascicles from the three locations (proximal, middle and distal) fell slack (Fig. 4C and D). To determine if the between-location variance could be explained simply by tracking error, the geometric mean of the ratio of the location variance and the tracking error variance was obtained. A 95% confidence interval was obtained using a non-parametric bootstrap (1000 replications). This analysis showed that the between-location variance was greater than the tracking error by a factor of 10.2 (95% CI 5.9 to 65.7).
In subsequent analyses we investigated if the gastrocnemius length at which fascicles fell slack varied systematically with the proximo-distal location of the fascicle in the muscle. First, the distance from the distal muscle–tendon junction to the proximal end of each of the three fascicles (proximal, middle and distal) was measured. The relationship between the gastrocnemius length at which each fascicle fell slack and distance from the muscle–tendon junction to that fascicle was investigated using fractional polynomial regression with a clustered sandwich variance estimator to account for dependence of the three observations from each muscle. There was no evidence of a consistent relationship between the proximo-distal location of muscle fascicles and the gastrocnemius lengths at which they fell slack (P = 0.16; Fig. 4D).
Contribution of muscles fascicles to change in muscle–tendon length
Above the muscle–tendon length at which the fascicles were slack there was an abrupt increase in muscle fascicle length (Fig. 3A; Supplementary Fig. S2). The increase in fascicle length was always much less than the increase in muscle–tendon length. Figure 5 shows the contribution to the increase in muscle–tendon length above fascicle slack length of three mechanisms: muscle fascicle strain (by which we mean changes in the length of fascicles along their long axes; i.e. not corrected for pennation), decreases in pennation and tendon strain. At close to fascicle slack length almost all of the increase in length was due to tendon strain, but with increasing muscle–tendon length (and therefore with increasing passive tension) muscle fascicle strains contributed an increasing proportion of the total increase in muscle–tendon length. Changes in pennation contributed little (always <10%) to the total change in muscle–tendon length above fascicle slack length. At the longest muscle–tendon length at which the length of these fascicles was measured (mean 70% of maximum length, SD 13%) the mean contribution was 27% from muscle fascicle strain, 6% from decreases in pennation and 67% from tendon.
Figure 5.

Contribution of: A, muscle fascicle strain; B, changes in pennation; and C, tendon strain to total change in gastrocnemius muscle–tendon length above the muscle–tendon length at which fascicles fall slack. Curves in A–C are Lowess-smoothed curves from one fascicle (the middle of three) for each of 24 subjects. D, linear regressions (mixed models) through data in A–C, plotted over the range of gastrocnemius lengths between 37% and 80%.
Discussion
Buckling
In about one-quarter of the subjects in this study, the gastrocnemius muscle buckled at short lengths. Some subjects demonstrated small-amplitude localised buckling, but in two subjects (including the subject in Supplementary Video S3) large-amplitude buckling was apparent throughout the muscle belly. It has been reported that isolated intrafusal fibres ‘kink’ at short lengths (Gladden, 1976), and that single extrafusal muscle fibres embedded in compressible gelatine blocks buckle at short lengths (González-Serratos, 1971; Brown et al. 1984a,b;). Zuurbier and Huijing (1993) deduced, from observations of sarcomere lengths of rat gastrocnemius muscle at active slack length, that ‘the fibres may take on a wavelike pattern’ at short muscle lengths. However, to our knowledge there have not been previous descriptions of buckling in whole muscles in vivo. The salamander tongue is ‘folded’ when retracted, and achieves remarkable increases in length by unfolding as it is protracted (Lombard & Wake, 1976, 1977; Deban et al. 1997, 2007), but the highly regular folding of salamander tongues is quite different to the irregular buckling of the human gastrocnemius.
The presence of buckling implies the existence of a restoring force below slack length. Over a century ago Kaiser (1900) observed that an isolated frog sartorius muscle suspended in oil under no tension shortened when stimulated and lengthened on removal of the stimulus. The lengthening must have involved a restoring force (i.e. a force acting to lengthen the muscle), although it is not clear if the restoring force was apparent at physiological lengths. The magnitude of the restoring force is likely to depend on lateral constraints to buckling, so measures of the physiological restoring force would need to be made in situ, which could be difficult in skeletal muscle. In cardiac muscle, which is much stiffer than skeletal muscle, there is a large restoring force at short but physiological lengths. The restoring force in cardiac muscle has an important role in augmenting ventricular filling in early diastole (Gilbert & Glantz, 1989).
The origin of the restoring force is uncertain. Huxley and colleagues showed, in single muscle fibres embedded in compressible gelatine blocks, that myofibril buckling occurred with passive or active shortening at lengths about 5% below slack length, around the length at which the thin filaments of opposing half-sarcomeres came into opposition (González-Serratos, 1971; Brown et al. 1984b). They hypothesised that collision of opposing thin filaments resists longitudinal fibre compression. Myofibril buckling was not accompanied by buckling of muscle fibres in this preparation (González-Serratos, 1971). Helmes and colleagues showed that the restoring force observed in segments of rat cardiac fibres at very short lengths could be reduced by digestion of titin with trypsin (Helmes et al. 1996; Preetha et al. 2005) and that fibres which express different titin isoforms have different restoring forces (Helmes et al. 1996). These observations implicate titin in generation of the myofibrillar restoring force, at least in cardiac muscle.
Buckling occurs most readily when slender structures with low bending stiffness are exposed to axial loads (compression) without lateral constraints (Timoshenko, 1961). Thus, localised buckling strongly suggests that the buckled structures have been shortened to below their slack lengths. The instances of localised buckling may have occurred in structures that fell below their slack lengths while adjacent structures remained under tension, or in structures with particularly low bending stiffness or high axial load, or in structures that had relatively little lateral constraint from adjacent tissues. It is possible that stress concentrations occur due to a change in stiffness at the fascicle–aponeurosis junction where muscle fascicles insert obliquely onto the aponeurosis.
Fascicle slack lengths
Some fascicles began to lengthen early in the physiological range whereas others remained slack well beyond 50% of the physiological range of gastrocnemius lengths. This variability could not be attributed to tracking error because the reliability of tracking was high. Nor could the variability be due to misalignment of the transducer. The effect of aligning the transducer obliquely with respect to the plane of the fascicles would be to bias measures of muscle fascicle length (Benard et al. 2009; see further discussion of this issue below) but misalignment of the transducer should not bias estimates of the muscle–tendon length at which fascicles fall slack.
The variability in length of the gastrocnemius at which muscle fascicles fall slack could have arisen if there was little or no variability in the lengths at which fascicles within a muscle fell slack but a high degree of variability between muscles. Alternatively, there could be a high degree of variability within muscles but little between-muscle variability. A third possibility is that there is both substantial within- and between-muscle variability. It would appear unlikely that the variability in length of the gastrocnemius at which muscle fascicles fall slack is dominated by between-muscle variability because the implication that all of the fascicles in some muscles could fall slack at very long lengths seems implausible. The data indicate that there is significant within-muscle variability because the variance between the gastrocnemius lengths at which proximal, medial and distal fascicles fall slack is an order of magnitude greater than the variance due to tracking error. Within-muscle variability in passive behaviours of muscles would be consistent with other observations of within-muscle variability in muscle architecture and muscle fascicle strains during task performance (Lichtwark et al. 2007; Higham & Biewener, 2011).
We sought further evidence of within-muscle variability in the length of the gastrocnemius at which fascicles fell slack by obtaining images at different locations in the muscle. Figure 6 shows data obtained from three sites in a single subject: a site at the lateral margin of the medial gastrocnemius, a site on the medial aspect of the medial gastrocnemius, and a site between the other two. There were clear differences in the gastrocnemius lengths at which fascicles from the three sites fell slack. This provides further evidence of within-muscle variability in the length of the gastrocnemius at which fascicles from the medial gastrocnemius fall slack.
Figure 6. Comparison of gastrocnemius length at which fascicles at three sites (lateral, intermediate and medial) in a single muscle fall slack.
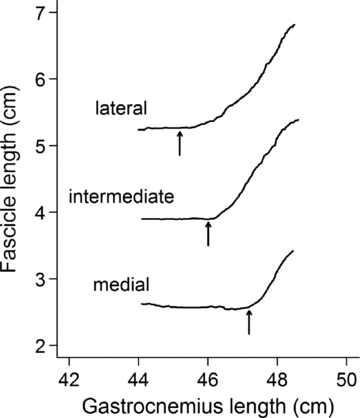
The arrows show the slack lengths obtained by fitting eqn (1) to the data.
Within-muscle variability implies that when a passive muscle is lengthened take-up of slack by muscle fascicles is not uniform: instead muscle fascicles are progressively ‘recruited’. That is, muscle fascicles contribute sequentially to the passive stiffness of the muscle. This is analogous to the sequential un-crimping of collagen fibrils that occurs during lengthening of ligaments and tendons (Franchi et al. 2007). A consequence is that individual muscle fascicles could remain slack at muscle–tendon lengths greater than the muscle–tendon slack length. The slack length of the muscle–tendon unit would presumably be the muscle–tendon length at which the first muscle fascicle was recruited. In practice it may be difficult to identify the first-recruited fascicles.
There was no evidence that fascicles were recruited in any particular proximo-distal order (proximal to distal, distal to proximal, middle first or middle last) that was consistent across muscles. However, because the primary study only sampled three fascicles in a single plane from each muscle, we cannot rule out the possibility of a more complex topographical pattern of recruitment. The data in Fig. 6, obtained from a single subject, suggest there could be a lateral-to-medial recruitment sequence. A further study in which there was broad sampling from many sites within a muscle might confirm or rule out the existence of topographical recruitment patterns.
The finding of variation in the gastrocnemius length at which muscle fascicles fall slack is consistent with and extends earlier observations of muscle morphology. Several groups have shown between-fibre variation of mean sarcomere lengths in architecturally complex mammalian skeletal muscles (Herring et al. 1979; Weijs & van der Wielen-Drent, 1983; van Eijden & Raadsheer, 1992; Zuurbier & Huijing, 1993). Of particular relevance is the study by Zuurbier and Huijing (1993) on rat gastrocnemius muscle, because these investigators obtained both morphological and physiological data. Their data show substantial between-fibre variation in mean sarcomere lengths at muscle active slack length (the shortest length at which the muscle could actively generate tension). It is not known if all fascicles in a muscle fall slack at the same sarcomere length, nor if the active and passive slack lengths of a muscle fascicle occur at the same muscle length. Our findings complement these morphological observations by showing variation in the muscle length at which fascicles in passive muscle fall slack.
Contribution of muscles fascicles to changes in muscle–tendon length
In 2002 we reported that changes in length of relaxed muscle fascicles in human gastrocnemius and tibialis anterior muscles, measured with ultrasound, were much smaller than changes in muscle–tendon length calculated from changes in ankle and knee angles (Herbert et al. 2002). The data were interpreted as indicating that, at the low forces experienced by passive muscle, tendon contributes a large part of the total compliance of the muscle–tendon unit. It was estimated that, when the gastrocnemius was lengthened over nearly the full range of physiological lengths, 27% of the total change in length was due to lengthening of muscle fascicles, 9% was due to decreases in pennation and 64% was due to lengthening of tendon. These estimates were broadly consistent with data reported by several other groups in studies not specifically designed to evaluate the contribution of muscle fascicles to passive changes in muscle–tendon length (Weijs & van der Wielen-Drent, 1983; Narici et al. 1996; Kawakami et al. 1998; Maganaris et al. 1998), and were subsequently confirmed by other investigators (Loram et al. 2007; Morse et al. 2008; but see Abellaneda et al. 2009). Unlike most previous studies, including previous studies from our laboratory, the current study estimated the contribution of muscle fascicles to changes in muscle–tendon length using semi-automated procedures to track changes in length of fascicles during passive lengthening with high resolution. (An exception was the study by Loram and colleagues (2007), which used automated tracking to measure displacements of muscle during small amplitude displacements in human gastrocnemius muscle.) Tendon contributes a large part of the total change in length, even though it is an intrinsically less compliant material than muscle (Herbert & Crosbie, 1997), because the tendon is about 10 times as long as the muscle fascicles (Herbert et al. 2002).
A methodological limitation of the earlier ultrasound studies, including our own, was that static measures of muscle fascicle length were made at each joint angle. The ultrasound transducer was repositioned at each joint angle so it was not possible to be certain that each measure was made on the same muscle fascicle. This meant there was considerable scatter in the relationship between fascicle length and gastrocnemius length. The scatter made it difficult to determine if the muscle fascicles were slack at short lengths. In the current study, high-resolution video sequences with a wide field of view were obtained. Consequently it was possible to track the changes in length of a single fascicle and reliably identify the slack length and changes in length of a single fascicle. Data from individual fascicles tracked up to 70% of the physiological range indicated that 27% of the total change in muscle–tendon length was due to lengthening of muscle fascicles, 6% was due to decreases in pennation and 67% was due to lengthening of tendon. These data are similar to the findings we previously reported based on static imaging of gastrocnemius muscles (Herbert et al. 2002).
A potential source of bias in estimates of the contribution of muscle fascicle strain to total change in muscle–tendon length is that the ultrasound transducer may not have been aligned with the plane of the muscle fascicles. It has generally been assumed that this would cause an overestimation of fascicle length and an underestimation of pennation (Scott et al. 1993; Kawakami et al. 1998) but, as Benard and colleagues have pointed out, this need not be the case – the fact that the aponeuroses are curved could mean that misalignment of the transducer could bias these measurements in either direction (Benard et al. 2009; see also Miyoshi et al. 2009). Moreover, if the alignment of the transducer changed with respect to the fascicles as the muscle was lengthened, the true change in length of muscle fascicles could have been overestimated or underestimated. Two observations suggest that this was not a major problem in the present study. First, Benard and colleagues (2009) compared ultrasound measures of fascicle length with measures of fascicle length made by anatomical dissection in the gastrocnemius muscles of four human cadavers. The concordance between the two measures was quite high (r = 0.92, mean absolute error 8.6% of fascicle length; calculated from data reported in their Table 1). In fact, this may underestimate the true accuracy because in Benard's study the transducer was aligned perpendicular to the skin whereas in most studies, including the current study, the transducer is rotated and tilted slightly from the perpendicular to obtain the best fascicle image. Presumably the best image is obtained when the transducer is in the plane of the fascicles. A second reason to suspect that our findings are not seriously biased is the consistency of the data. As Fig. 5A shows, the contribution of gastrocnemius muscle fascicles to change in gastrocnemius muscle–tendon length was less than 60% in all subjects and less than 50% in all but two subjects. It might be expected that changes in the alignment of the transducer with respect to the fascicles might cause the true change in length to be sometimes underestimated and sometimes overestimated, yet large changes in fascicle length were never observed.
In summary, observations of relaxed human gastrocnemius muscle fascicles in vivo show that (a) muscle fascicles may buckle at very short lengths, (b) the muscle–tendon length at which muscle fascicles fall slack varies greatly, and (c) most of the passive increase in muscle–tendon length occurs in the tendon. We propose the novel hypothesis that muscle fascicles are progressively recruited during passive lengthening of limb muscles. This phenomenon potentially provides insight into how muscle–tendon properties may change under many conditions. It would be interesting to investigate if changes in the passive properties of muscles caused by, for example, contracture, exercise-induced muscle damage, limb growth or ageing are associated with changes in the distribution of muscle lengths at which fascicles fall slack.
Acknowledgments
The projects were supported by the Australian Research Council and the Australian National Health and Medical Research Council. R.D.H., E.C.C., L.E.B. and S.C.G. hold NHMRC fellowships.
Author contributions
Conception and design of the experiments: R.D.H, L.E.B, S.C.G. Collection, analysis and interpretation of data: R.D.H., J.C., L.K.K, J.D., J.M., E.C.C, L.E.B., S.C.G. Drafting the article or revising it critically for important intellectual content: R.D.H., J.C., L.K.K, J.D., J.M., E.C.C, L.E.B., S.C.G. All authors approved the version to be published. Experiments were conducted at the George Institute for Global Health, Sydney.
Supplementary material
Supplementary Fig. S1
Supplementary Fig. S2
Supplementary Videos S1-3
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Abellaneda S, Guissard N, Duchateau J. The relative lengthening of the myotendinous structures in the medial gastrocnemius during passive stretching differs among individuals. J Appl Physiol. 2009;106:169–177. doi: 10.1152/japplphysiol.90577.2008. [DOI] [PubMed] [Google Scholar]
- Benard MR, Becher JG, Harlaar J, Huijing PA, Jaspers RT. Anatomical information is needed in ultrasound imaging of muscle to avoid potentially substantial errors in measurement of muscle geometry. Muscle Nerve. 2009;39:652–665. doi: 10.1002/mus.21287. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- Breuning DA, Crewe AN, Buczek FL. A simple, anatomically based correction to the conventional ankle joint centre. Clin Biomech (Bristol, Avon) 2008;23:1299–1302. doi: 10.1016/j.clinbiomech.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Brown LM, González-Serratos H, Huxley AF. Structural studies of the waves in striated muscle fibres shortened passively below their slack length. J Muscle Res Cell Motil. 1984a;5:273–292. doi: 10.1007/BF00713108. [DOI] [PubMed] [Google Scholar]
- Brown LM, González-Serratos H, Huxley AF. Sarcomere and filament lengths in passive muscle fibres with wavy myofibrils. J Muscle Res Cell Motil. 1984b;5:293–314. doi: 10.1007/BF00713109. [DOI] [PubMed] [Google Scholar]
- Deban SM, O'Reilly JC, Dicke U, van Leeuwen JL. Extremely high-power tongue projection in plethodontid salamanders. J Exp Biol. 2007;210:655–667. doi: 10.1242/jeb.02664. [DOI] [PubMed] [Google Scholar]
- Deban SM, Wake DB, Roth G. Salamander with a ballistic tongue. Nature. 1997;389:27–28. [Google Scholar]
- Franchi M, Fini M, Quaranta M, De Pasquale V, Raspanti M, Giavaresi G, Ottani V, Ruggeri A. Crimp morphology in relaxed and stretched rat Achilles tendon. J Anat. 2007;210:1–7. doi: 10.1111/j.1469-7580.2006.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia D. Robust smoothing of gridded data in one and higher dimensions with missing values. Comput Stat Data Anal. 2010;54:1167–1178. doi: 10.1016/j.csda.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert JC, Glantz SA. Determinants of left ventricular filling and of the diastolic pressure-volume relation. Circ Res. 1989;64:827–852. doi: 10.1161/01.res.64.5.827. [DOI] [PubMed] [Google Scholar]
- Gladden MH. Structural features relative to the function of intrafusal muscle fibres in the cat. Prog Brain Res. 1976;44:51–59. doi: 10.1016/S0079-6123(08)60722-0. [DOI] [PubMed] [Google Scholar]
- González-Serratos H. Inward spread of activation in vertebrate muscle fibres. J Physiol. 1971;212:777–799. doi: 10.1113/jphysiol.1971.sp009356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve D, Pheasant S, Cavanagh PR. Prediction of gastrocnemius length from knee and ankle posture. In: Asmussen E, Jorgensen K, editors. Biomechanics VI: Proceedings of the Sixth International Congress of Biomechanics, Copenhagen, Denmark. Baltimore: University Park Press; 1978. [Google Scholar]
- Helmes M, Trombitas K, Granzier H. Titin develops restoring force in rat cardiac myocytes. Circ Res. 1996;79:619–626. doi: 10.1161/01.res.79.3.619. [DOI] [PubMed] [Google Scholar]
- Herbert RD, Crosbie J. Rest length and compliance of non-immobilised and immobilised rabbit soleus muscle and tendon. Eur J Appl Physiol Occup Physiol. 1997;76:472–479. doi: 10.1007/s004210050277. [DOI] [PubMed] [Google Scholar]
- Herbert RD, Gandevia SC. Changes in pennation with joint angle and muscle torque: in vivo measurements in human brachialis muscle. J Physiol. 1995;484:523–532. doi: 10.1113/jphysiol.1995.sp020683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert RD, Moseley AM, Butler JE, Gandevia SC. Change in length of relaxed muscle fascicles and tendons with knee and ankle movement in humans. J Physiol. 2002;539:637–645. doi: 10.1113/jphysiol.2001.012756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring SW, Grimm AF, Grimm BR. Functional heterogeneity in a multipinnate muscle. Am J Anat. 1979;154:563–576. doi: 10.1002/aja.1001540410. [DOI] [PubMed] [Google Scholar]
- Higham TE, Biewener AA. Functional and architectural complexity within and between muscles: regional variation and intermuscular force transmission. Philos Trans R Soc Lond B Biol Sci. 2011;366:1477–1487. doi: 10.1098/rstb.2010.0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang PD, Gorman RB, Todd G, Gandevia SC, Herbert RD. A new method for measuring passive length-tension properties of human gastrocnemius muscle in vivo. J Biomech. 2005;38:1333–1341. doi: 10.1016/j.jbiomech.2004.05.046. [DOI] [PubMed] [Google Scholar]
- Hoang PD, Herbert RD, Todd G, Gorman RB, Gandevia SC. Passive mechanical properties of human gastrocnemius muscle–tendon unit, muscle fascicles and tendon in vivo. J Exp Biol. 2007;210:4159–4168. doi: 10.1242/jeb.002204. [DOI] [PubMed] [Google Scholar]
- Kaiser K. Ueber die Wiederausdehnung des contrahirten muskels. Zentralblatt für Physiologie. 1901;14:195–197. [Google Scholar]
- Kawakami Y, Ichinose Y, Fukunaga T. Architectural and functional features of human triceps surae muscles during contraction. J Appl Physiol. 1998;85:398–404. doi: 10.1152/jappl.1998.85.2.398. [DOI] [PubMed] [Google Scholar]
- Klimstra M, Dowling J, Durkin JL, MacDonald M. The effect of ultrasound probe orientation on muscle architecture measurement. J Electromyogr Kinesiol. 2007;17:504–514. doi: 10.1016/j.jelekin.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Lichtwark GA, Bougoulias K, Wilson AM. Muscle fascicle and series elastic element length changes along the length of the human gastrocnemius during walking and running. J Biomech. 2007;40:157–164. doi: 10.1016/j.jbiomech.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Lombard RE, Wake DB. Tongue evolution in the lungless salamanders, family plethodontidae. I. Introduction, theory and a general model of dynamics. J Morphol. 1976;148:265–286. doi: 10.1002/jmor.1051480302. [DOI] [PubMed] [Google Scholar]
- Lombard RE, Wake DB. Tongue evolution in the lungless salamanders, family Plethodontidae. II. Function and evolutionary diversity. J Morphol. 1977;153:39–79. doi: 10.1002/jmor.1051530104. [DOI] [PubMed] [Google Scholar]
- Loram ID, Maganaris CN, Lakie M. The passive, human calf muscles in relation to standing: the short range stiffness lies in the contractile component. The Journal of Physiology. 2007;584:677–692. doi: 10.1113/jphysiol.2007.140053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg A, Svensson OK, Nemeth G, Selvik G. The axis of rotation of the ankle joint. J Bone Joint Surg Br. 1989;71B:94–99. doi: 10.1302/0301-620X.71B1.2915016. [DOI] [PubMed] [Google Scholar]
- Maganaris CN, Baltzopoulos V, Sargeant AJ. In vivo measurements of the triceps surae complex architecture in man: implications for muscle function. J Physiol. 1998;512:603–614. doi: 10.1111/j.1469-7793.1998.603be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi T, Kihara T, Koyama H, Yamamoto S, Komeda T. Automatic detection method of muscle fiber movement as revealed by ultrasound images. Med Eng Phys. 2009;31:558–564. doi: 10.1016/j.medengphy.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Morse CI, Degens H, Seynnes OR, Maganaris CN, Jones DA. The acute effect of stretching on the passive stiffness of the human gastrocnemius muscle tendon unit. J Physiol. 2008;586:97–106. doi: 10.1113/jphysiol.2007.140434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narici MV, Binzoni T, Hiltbrand E, Fasel J, Terrier F, Cerretelli P. In vivo human gastrocnemius architecture with changing joint angle at rest and during graded isometric contraction. J Physiol. 1996;496:287–297. doi: 10.1113/jphysiol.1996.sp021685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preetha N, Yiming W, Helmes M, Norio F, Siegfried L, Granzier H. Restoring force development by titin/connectin and assessment of Ig domain unfolding. J Muscle Res Cell Motil. 2005;26:307–317. doi: 10.1007/s10974-005-9037-2. [DOI] [PubMed] [Google Scholar]
- Scott SH, Engstrom CM, Loeb GE. Morphometry of human thigh muscles. Determination of fascicle architecture by magnetic resonance imaging. J Anat. 1993;182:249–257. [PMC free article] [PubMed] [Google Scholar]
- Timoshenko S. Theory of Elastic Stability. New York: McGraw-Hill; 1961. [Google Scholar]
- van Eijden TM, Raadsheer MC. Heterogeneity of fiber and sarcomere length in the human masseter muscle. Anat Rec. 1992;232:78–84. doi: 10.1002/ar.1092320109. [DOI] [PubMed] [Google Scholar]
- Weijs WA, van der Wielen-Drent TK. The relationship between sarcomere length and activation pattern in the rabbit masseter muscle. Arch Oral Biol. 1983;28:307–315. doi: 10.1016/0003-9969(83)90073-0. [DOI] [PubMed] [Google Scholar]
- Zuurbier CJ, Huijing PA. Changes in geometry of actively shortening unipennate rat gastrocnemius muscle. J Morphol. 1993;218:167–180. doi: 10.1002/jmor.1052180206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


