Non-technical summary
The transient and progressive decrease in skeletal muscle performance during contraction is known as fatigue. One of the phenomena associated with fatigue is an alteration in the excitation–contraction coupling mechanism. Using isolated muscle fibres loaded with the fast Ca2+ dye Magfluo-4, we have found that after a protocol of repetitive stimulation there are alterations in the ability of the fibres to release and reuptake Ca2+, which are more evident and rapidly established in fast fibres compared to slow ones. All alterations were reversed after several minutes of rest and were not related to a phenomenon of inactivation of Ca2+ release. These data increase our knowledge of the events present during muscle fatigue and will help understand the mechanisms responsible for them.
Abstract
Abstract
We used enzymatically dissociated flexor digitorum brevis (FDB) and soleus fibres loaded with the fast Ca2+ dye Magfluo-4 AM, and adhered to Laminin, to test whether repetitive stimulation induces progressive changes in the kinetics of Ca2+ release and reuptake in a fibre-type-dependent fashion. We applied a protocol of tetani of 350 ms, 100 Hz, every 4 s to reach a mean amplitude reduction of 25% of the first peak. Morphology type I (MT-I) and morphology type II (MT-II) fibres underwent a total of 96 and 52.8 tetani (P < 0.01 between groups), respectively. The MT-II fibres (n = 18) showed significant reductions of the amplitude (19%), an increase in rise time (8.5%) and a further reduction of the amplitude/rise time ratio (25.5%) of the first peak of the tetanic transient after 40 tetani, while MT-I fibres (n = 5) did not show any of these changes. However, both fibre types showed significant reductions in the maximum rate of rise of the first peak after 40 tetani. Two subpopulations among the MT-II fibres could be distinguished according to Ca2+ reuptake changes. Fast-fatigable MT-II fibres (fMT-II) showed an increase of 32.2% in the half-width value of the first peak, while for fatigue-resistant MT-II fibres (rMT-II), the increase amounted to 6.9%, both after 40 tetani. Significant and non-significant increases of 36.4% and 11.9% in the first time constant of decay (t1) values were seen after 40 tetani in fMT-II and rMT-II fibres, respectively. MT-I fibres did not show kinetic changes in any of the Ca2+ reuptake variables. All changes were reversed after an average recovery of 7.5 and 15.4 min for MT-I and MT-II fibres, respectively. Further experiments ruled out the possibility that the differences in the kinetic changes of the first peak of the Ca2+ transients between fibres MT-I and MT-II could be related to the inactivation of Ca2+ release mechanism. In conclusion, we established a model of enzymatically dissociated fibres, loaded with Magfluo-4 and adhered to Laminin, to study muscle fatigue and demonstrated fibre-type-dependent, fatigue-induced kinetic changes in both Ca2+ release and reuptake.
Introduction
Excitation–contraction coupling (ECC) refers to the entire sequence of events that, starting with an action potential, leads to muscle contraction (Sandow, 1952; Caputo, 1983). One of the key elements of this phenomenon is the Ca2+ ion (Berchtold et al. 2000). Many factors and conditions affect ECC, including different drugs, ions, development, ageing and fatigue (Caputo, 1983; Payne & Delbono, 2004; Capote et al. 2005; Allen et al. 2008; Calderón-Vélez & Figueroa-Gordon, 2009). Fatigue can be defined as the transient and progressive decrease in muscle performance during continuous stimulation, and is a complex process with central and peripheral components. In several preparations, fatigue has been shown to be mainly peripheral (Grabowski et al. 1972; Bigland-Ritchie et al. 1979; Bigland-Ritchie & Woods, 1984; Vøllestad et al. 1988; Moussavi et al. 1989; Kent-Braun, 1999), explained in part by alterations in the Ca2+ release mechanism (Grabowski et al. 1972; Allen et al. 1989; Westerblad & Allen, 1991). In the same way, alterations of the sarcoendoplasmic reticulum Ca2+-ATPase (SERCA) function and slowing of single-twitch and tetanic relaxation have been demonstrated as a result of fatigue (Fletcher, 1902; Gollnick et al. 1991; Westerblad & Lännergren, 1991; Tupling, 2004).
Metabolic factors, such as changes in Ca2+ itself, adenosine triphosphate (ATP), phosphocreatine (PCr), H+, Mg2+, phosphate (Pi) and reactive oxygen species (ROS) concentrations observed during fatigue may be involved in altering the ability of the sarcoplasmic reticulum (SR) to release and reuptake Ca2+ (Stephenson et al. 1998; Lamb, 2002; Tupling, 2004; Allen et al. 2008; Calderón-Vélez & Figueroa-Gordon, 2009). Other phenomena, such as a decrease in the intra-SR Ca2+ content, the inactivation of the Ca2+ release mechanism and muscle damage may also be involved (Caputo, 1983; Takehura et al. 2001; Tupling, 2004). However, the mechanisms involved in the development of muscle fatigue may not be the same for all fibre types. It has been shown that fibre types show differences in the kinetics of Ca2+ transients (Carroll et al. 1997; Calderón et al. 2009), being in general type I, the slowest, types IIX and IIB, the fastest, and type IIA, intermediate, all regarding Ca2+ release and reuptake (Calderón et al. 2010). There is biochemical and structural support for these findings (Reggiani & te Kronnie, 2006; Calderón et al. 2009, 2010). The different muscles and fibre types also show different fatigue resistance (Burke et al. 1971; Burke et al. 1973; Petrofsky & Lind, 1979; Stephenson et al. 1998; Bruton et al. 2003), which could be explained by the susceptibility or resistance to the fatigue of the different steps involved in ECC (Stephenson et al. 1998) and also by the intrinsic (qualitative and quantitative) differences in the ECC found in the fibre types (Carroll et al. 1997; Bottinelli & Reggiani, 2000; Reggiani & te Kronnie, 2006; Calderón et al. 2009, 2010). The amplitude of the whole tetanic Ca2+ transient, measured with slow dyes, changes differentially in fatigued slow and fast fibre types (Westerblad & Allen, 1991; Bruton et al. 2003); furthermore, parvalbumin (PV)-deficient fast muscles are more fatigue resistant than normal fast muscles (Chen et al. 2001), which links the machinery involved in the kinetics of Ca2+ transients with the fatigue resistance.
Unfortunately, with the slow, high-affinity Ca2+ dyes that have been used to demonstrate the reduction of the tetanic Ca2+ transient amplitude during fatigue, it is difficult to reliably demonstrate kinetic changes of the transients. A new generation of Ca2+ dyes has been available during the last decade and some of them can faithfully track the kinetics of Ca2+ signalling during muscle contraction (Katerinopoulos & Foukaraki, 2002; Hollingworth et al. 2009; Calderón et al. 2010). One of these dyes, Magfluo-4, has provided reliable measurement of kinetic parameters related to Ca2+ release and reuptake processes and has proven to be useful in recognizing Ca2+ transient differences among fibre types (Caputo et al. 2004; Hollingworth et al. 2009; Calderón et al. 2009, 2010). Actually, using Magfluo-4, it has been recently shown that fibre types I and IIA share the same Ca2+ transient morphology, type I (MT-I), while fibre types IIX/D and IIB share the morphology type II (MT-II). Ca2+ transient morphology itself is a useful tool for functionally identifying fibre types (Calderón et al. 2009, Calderón et al. 2010).
In this work, we hypothesize that before the final result of reduced tetanic Ca2+ transients is established, some alterations in the kinetics of: (i) Ca2+ release, such as a reduction of the amplitude, an increase in rise time and a decrease in the rate of rise of the first peak of the tetanic Ca2+ transients, and (ii) Ca2+ reuptake, such as a decrease in the rate of tetanic decay, may occur in a fibre-type-dependent fashion. These changes would be larger in MT-II compared to MT-I fibres, in agreement with the general profile of fatigability of the fibre types. Moreover, Ca2+ release from the SR is subject to an inactivation mechanism (Schneider & Simon, 1988), itself dependent on the myoplasmic Ca2+ levels (Jong et al. 1993). Since basal Ca2+ levels are differentially altered during fatigue in fast and slow fibres (Westerblad & Allen, 1991; Bruton et al. 2003), we tested the possibility that the phenomenon of inactivation of Ca2+ release may explain eventual fatigue-induced alterations in the kinetics of Ca2+ release if the fast fibres present a different recovery from inactivation after fatigue compared to resting conditions.
We used enzymatically dissociated murine fibres loaded with either the fast Ca2+ dye Magfluo-4 AM or Fluo-3 AM, and adhered to Laminin, to measure the changes in kinetics of the tetanic Ca2+ transients induced by repetitive stimulation. This preparation allowed us to record more than 100 tetanic responses in a fixed part of the fibre and provided reliable information on the kinetic alterations in Ca2+ release and reuptake induced by fatigue.
Methods
Ethical approval
All manipulations and procedures carried out in mice during the development of this work were approved by the local Bioethics Committee on Animal Research (COBIANIM) at the Venezuelan Institute for Scientific Research (IVIC).
Dyes
Fluo-3 AM and Magfluo-4 AM were from Molecular Probes (Eugene, OR, USA). Stocks of all compounds were prepared in dimethyl sulfoxide (Sigma; MO, USA) and aliquots were frozen. Aliquots were thawed and dissolved in Tyrode solution (in mm: 5.4 KCl, 1 MgCl2, 140 NaCl, 0.33 NaH2PO4, 10 glucose, 10 N-(2-hydroxyethyl) piperazine-N′-2-ethanesulfonic acid (Hepes, Sigma), 2 CaCl2, pH 7.3) to the final concentration just before starting the experiments.
Fibres preparation
The enzymatic dissociation method is a modification of previously published ones (Bekoff & Betz, 1977; Capote et al. 2005; Calderón et al. 2010). Briefly, 42- to 49-day-old male mice (NMRI-IVIC, Navy Medicine Research Institute-Venezuelan Institute for Scientific Research) were killed by rapid cervical dislocation. Flexor digitorum brevis (FDB) and soleus muscles were dissected out and incubated in a modified Ringer solution (in mm: 2.7 KCl, 1.2 KH2PO4, 0.5 MgCl2, 138 NaCl, 0.1 Na2HPO4, 1 CaCl2, pH 7.4) containing 3 or 2.5 mg ml−1 collagenase (Worthington CLS2; 250 units mg−1), respectively, for 52–64 min at 36.6°C. Soleus was divided longitudinally before collagenase incubation. After collagenase treatment the muscles were washed twice with Tyrode solution at room temperature and gently separated from tendons and remaining tissue with a set of fire-polished Pasteur pipettes. In the present work, about 80–85% of the fibres responded to electrical stimulation immediately after dissociation, and contracted briskly even after 36 h in Tyrode solution.
Fluorescence recordings and stimulation protocols
Dissociated fibres were transferred to the experimental chamber and incubated for 40–45 min at room temperature in a Tyrode solution containing 10 μm Fluo-3 AM or 8–10 μm Magfluo-4 AM. This procedure allowed loading the fibres while they were adhering to the chamber bottom consisting of a glass coverslide, previously coated with 2–4 μl of Laminin (1 mg ml−1 (Sigma). We have shown that this method is effective in reducing movement artifacts in the Ca2+ records (Calderón et al. 2009). Although a large number of fibres detached after the first 10–20 tetani, we succeeded in recording complete protocols of fatigue (of up to 120 tetani) and recovery in 25 fibres (2 fibres loaded with Fluo-3 AM and 23 fibres loaded with Magfluo-4 AM), without any detachment or movement artifact. Twenty-nine more fibres were evaluated for inactivation of Ca2+ release or used in different types of control experiments.
The chamber was mounted on the stage of an inverted Nikon Diaphot TMD (Nikon Co., Tokyo, Japan) microscope equipped for epifluorescence and the fibres were illuminated with a xenon lamp (100 W). Precautions were taken to diminish photodamage and photobleaching of the dye. The characteristic wavelengths (in nm) of the filter combination (excitation/dichroic/barrier) were 450–490/510/520. The light signals were collected with a photomultiplier connected to a Nikon P1 amplifier and the output was fed into a TL1 DMA interface (Axon Instruments, Foster City, CA, USA). The data were acquired and analysed using the pCLAMP 6 software (Axon Instruments). Acquisition frequency for tetanic Ca2+ transients was 10 kHz.
Intracellular Ca2+ transients were elicited by applying supra-threshold rectangular current pulses (0.6–1.4 ms) through two platinum plate electrodes placed on either side along the groove containing the fibre. Tetanic stimulation lasted 350 ms, at 100 Hz. To induce fatigue in Magfluo-4 AM-loaded fibres, tetanic stimulation was applied every 4 s until either the amplitude of the first peak of the transient diminished by 25% or a total amount of 120 tetani was reached. Recovery of the Ca2+ transients was assessed at minutes 1, 3, 5, 7, 10 and 15 after finishing the fatiguing protocol. Recovery from inactivation of Ca2+ release was studied by a double-stimuli protocol (Jong et al. 1993; Caputo et al. 2004) that was applied before the first tetanus and 4 s after the last tetanus of the fatiguing protocol. The amplitudes of both transients (F1 and F2) were calculated and the ratio F2/F1 was plotted for each of the 10 episodes. The points were fitted with a function of the form y = y0+A1*[1 – exp(−x/τ1)].
For tetanic Ca2+ transients recorded with Magfluo-4, we analysed the amplitude (ΔF/F), the rise time from 10 to 90% of the amplitude (ms), the amplitude/rise time ratio (ΔF/RT) and the maximum rate of rise (ΔF/F, ms−1), as calculated from the first time derivative, of the first peak. The decay phase of the whole MT-I tetanic transients was fitted by a single exponential function, while the decay of the MT-II transients was fitted by a biexponential function (Calderón et al. 2009). Only the changes in the values of τ1 were analysed. We also analysed the half-width of the first peak, the decay phase of the whole record and the changes in basal fluorescence measured during the 80 ms before the start of the tetanic transient. The first peak of the tetanus reflects the physiological phenomenon of Ca2+ release after the first stimulus. Our approach is somewhat different from the one found in the literature in which the mean value from the last part of a tetanic transient has been reported, since the previously used slow dyes did not resolve every peak of the tetani. We believe that in order to give an idea of the alterations of the Ca2+ release induced by fatigue, it is more useful to report the kinetics of the first peak of the record rather than the last part, which can be ‘contaminated’ with phenomena happening during the tetanic stimuli itself.
Due to uncertainties regarding the concentration and the dissociation constant (Kd) of the dye in the fibres, we present the Ca2+ transients as ΔF/F = ((Fmax–Frest)/Frest). The low affinity of Magfluo-4 for Ca2+ (Kd = 22 μm according to the manufacturer or 81 μm in the presence of 1 mm Mg2+ according to Hollingworth et al. 2009), and the fact that the peak Ca2+ concentration reached in mammalian muscles during a twitch has been reported to be around 20 μm, suggests that the dye is far from saturation under our experimental conditions (Caputo et al. 2004). Since Magfluo-4 is a fluorescent dye sensitive to both Ca2+ and Mg2+ ions, it is necessary to consider the possibility of artifacts arising from changes in the myoplasmic Mg2+ concentration due to its displacement from PV by Ca2+ ions. Available literature suggests that during a single twitch, one would expect the dye to reliably respond to changes in myoplasmic Ca2+ concentration with little contribution from changes in Mg2+ myoplasmic concentration (Konishi et al. 1991; Hollingworth et al. 2009; Calderón et al. 2010). Konishi et al (1991), using Magfura-2, a dye sensitive to both Ca2+ and Mg2+, found that during a twitch, interference by Mg2+ occurred very late (100 ms) after stimulation, opening the possibility that this ion might have an important effect for the case of tetanic transients, due to their prolonged decay phase. However, this possibility is dispproved by the fact that tetanic transients reported by different dyes insensitive to Mg2+ also show long decay phases (Baylor & Hollingworth, 1988; Caputo et al. 1994).
All experiments were done at room temperature (21–23°C).
Functional identification of fibre types
For identifying fibre types we used the morphology of both single and tetanic Ca2+ transients as previously proposed (Calderón et al. 2009, 2010). Briefly, the Ca2+ transients of fibre types I and IIA have the MT-I, and the fibre types IIX/D and IIB share the MT-II when recorded with Magfluo-4. Single transients classified as MT-I are wider and slower than those of MT-II. In both cases the time course of transient decay can be described by a double exponential function. Tetanic transients MT-I have a staircase shape and a decay phase that can be fitted by a single exponential function with a time constant of decay τ1, while those classified as MT-II have a first peak larger than the others which form a plateau and a double exponential decay phase with time constants τ1 and τ2. The specificity of this model was evaluated using anti-myosin antibodies and SDS-PAGE of isolated fibres in which Ca2+ transients were previously measured. Since we used FDB and soleus fibres from NMRI-IVIC mice (see Calderón et al. 2009), MT-I fibres include both fibre types I and IIA, which are known to be fatigue resistant (Burke et al. 1973), and MT-II fibres are exclusively fibres type IIX/D, which are more fatigue sensitive.
Statistics
For comparing mean values, Student's t test for paired or two independent populations or analysis of variance (ANOVA) were used when appropriate (using Origin 7.5 software, Microcal Software Inc., Northampton, MA, USA). Results are given as mean ± SEM. Differences were considered statistically significant at P < 0.05.
Results
In the experiment shown in Supplemental Fig. S1 we used a manually dissected, small bundle of FDB fibres loaded with the fast Ca2+ dye Magfluo-4 AM to simultaneously record tension (black trace) and Ca2+ (grey trace). The decay phase of the transient has a hump not seen in fibres isolated by enzymatic dissociation and either adhered to Laminin or exposed to N-benzyl-p-toluene sulphonamide (BTS) (Calderón et al. 2009). Therefore, this hump can be considered a movement artifact.
To avoid this problem and to evaluate the kinetics of the tetanic Ca2+ transients in fibres under repetitive stimulation, using a non-ratiometric, low-affinity Ca2+ indicator such as Magfluo-4, we preferred the use of enzymatically dissociated fibres. However, we first checked whether such fibres, subjected to repetitive stimulation, could reproduce the two main observations regarding Ca2+ and fatigue, namely, the decrease in the tetanic Ca2+ amplitude and the increase in the basal level of free Ca2+ (Westerblad & Allen, 1991).
Figure 1A shows the effects of repetitive tetanic stimulation on a FDB fibre loaded with the Ca2+ indicator Fluo-3 AM. Figure 1B illustrates some of the kinetic changes induced by the repetitive stimulation. The graph shows the first peak of Ca2+ release of each tetanic record during the fatiguing protocol and the recovery period, as well as its first derivative in the inset. The figure demonstrates the progressive reduction of the maximum rate of rise during repetitive stimulation, and the partial recovery during the first 20 min of rest after the end of the fatiguing stimulation. The amplitude of the fluorescence transients was reduced from 5.81 to 1.9 ΔF/F (Fig. 1C, upper panel), in part mediated by the increase in basal fluorescence level, which changed from 5.3 to 9.74 arbitrary units (AU), as it is shown in Fig. 1C, middle panel. After recovery, amplitude reached 4.52 ΔF/F and basal fluorescence decreased to 5.4. In two FDB fibres, a mean of 45 tetani reduced the amplitude of the signal to 25.2% and increased the basal fluorescence by 108.7%. After a resting period of 20 min the amplitude recovered to 76.9% of the initial value, while basal fluorescence recovered by 93.3% of the initial values (Fig. 1C). The bottom trace in Fig. 1C shows the decrease of the ΔF/RT ratio of the first peak, indicative of the mean rate of rise of the first peak of the record, as fatigue develops and its later recovery during the resting time. After the fatiguing protocol, the amplitude of the first peak and the relationship ΔF/RT were reduced in the two fibres to 18.5% and 18%, respectively. Both variables recovered to 82.2% of the initial values. A control experiment, not shown, was carried out in one of the fibres loaded with Fluo-3 AM, in which the stimulation was turned off from episodes 2 to 39, and the fluorescence was recorded from the episodes 1, 10th, 20th, 30th and 40th. In this case no changes were observed in either the basal fluorescence or the amplitude of the tetanic Ca2+ transient.
Figure 1. Fatigue-induced changes in tetanic Ca2+ transients recorded in an enzymatically dissociated FDB fibre loaded with Fluo-3 AM.
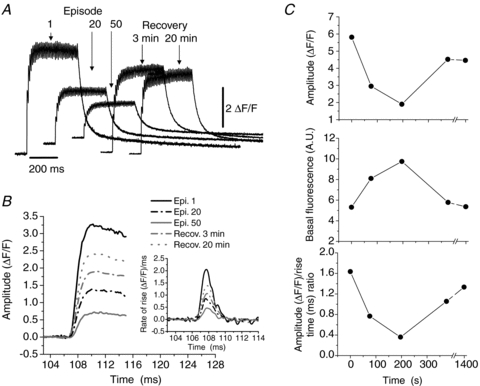
In A the 1st, 20th and 50th tetanic records during the fatiguing protocol and two tetani during the recovery period are shown. In B the rising phase of the first peak of each tetanic transient labelled in A is shown at a faster time scale. The inset illustrates the time derivative of these records. Note the progressive decrease in the maximum rate of rise. In C, the decrease in the amplitude of the tetanic transient, the increase in the basal fluorescence level and the change in the amplitude of the first peak/rise time ratio are shown, for the episodes illustrated in A. Note the fatigue-induced kinetic alterations. All changes partially recovered within 20 min.
A further control was carried out in which an isolated fibre was adhered to Laminin, loaded during 60 min with the intramitochondrial Ca2+ indicator Rhod-2 AM, at 7 μm, and stimulated with 75 tetani. The fibre was imaged with a confocal microscope (Supplemental Fig. S2). The result showed that the fibre was not displaced or damaged during the stimulation protocol. Experiments were also carried out loading the fibre with both Rhod-2 AM and Mitotracker Green-FM to verify that the increase in fluorescence occurs inside the mitochondria.
Two types of controls were also run using fibres loaded with Magfluo-4 AM. In the first one we used a protocol of tetanic stimulation of 350 ms at 100 Hz every 20 s between 2 and 4 min for 13 MT-II and 1 MT-I. This period is equivalent to between 30 and 60 tetani of the fatiguing protocol. There were no differences in the time constants of decay of the whole tetani, in the half-width of the first peak or in the basal fluorescence, between the first and the last episode (not shown). There was a reduction of 6% in the amplitude of the first peak comparing the first and the last tetani (not shown). In a second set of controls, three fibres were stimulated every 20 s for 2 min, then allowed to rest for 5–6 min and then stimulated again every 20 s for 2 min. The results were similar to those reported for the first group of controls (not shown). This ruled out the possibility of any kinetic changes induced by a non-fatiguing protocol or simply by the movement induced by the tetanic stimulation.
These results confirm that this model reproduces the reversible alterations described in Ca2+ kinetics during fatigue, using other preparations (Westerblad & Allen, 1991; Bruton et al. 2003).
Fatigue and tetanic Ca2+ transients measured with a fast Ca2+ dye
After running the above control experiments, we evaluated the effect of repetitive stimulation on the kinetics of the tetanic Ca2+ transients recorded with Magfluo-4. To reach a 25% decrease in the amplitude of the first peak of the tetanic Ca2+ transients, 96 ± 13 and 52.8 ± 5 (P < 0.01) tetani were applied to MT-I and MT-II fibres, respectively.
Maximal recovery was attained after a mean of 15.4 and 7.5 min of rest for MT-I and MT-II fibres (P = 0.06 between groups), respectively, and all variables followed approximately the same time course within a group of fibres.
Changes in the first peak of tetanic Ca2+ transients
Figure 2 shows the changes induced by the fatiguing stimulation on the amplitude and maximum rate of rise of the first peak of the tetanic Ca2+ transients in a MT-I (A) and a MT-II (B) fibre. In Fig. 2Aa, the episodes numbered 1, 60 and 120, as well as the recovery, are illustrated. In this fibre the amplitude of the first peak started to diminish after 80 tetanic stimuli (Ab, left) and diminished by 13% after 120 stimuli, with a reduction of 18% in the maximum rate of rise (Ab, right). For the MT-II fibre, the episodes 1 and 50, as well as the recovery (Ba), are shown. This fibre showed a reduction of 28% in the peak amplitude (Bb, left), as well as a decrease of 38% in the maximum rate of rise (Bb, right) after only 50 tetanic stimuli.
Figure 2. Fatigue-induced kinetic alterations in the first peak of the tetanic Ca2+ transients in a MT-I (A) and a MT-II (B) fibre loaded with Magfluo-4 AM.
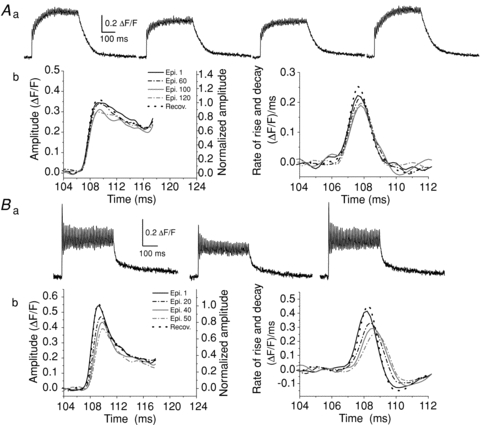
In Aa the episodes (epi) number 1, 60 and 120 and recovery (recov) are shown. The decrease in the amplitude of the first peak of the tetanic Ca2+ transients obtained in a MT-I fibre (A) started after 80 tetani and amounted to 13% after 120 tetani, as shown in Ab, on the left. This change was accompanied by a 18% decrease in the maximum rate of rise as shown by the first derivative of the respective records, as shown in Ab, on the right. In Ba, the Ca2+ transients number 1, 50 and the recovery in a MT-II fibre are shown. In this fibre the decrease in the amplitude of the first peak of the tetanic transients was of 28% after 50 tetani (Bb, left) and the maximum rate of rise diminished by 38%, as shown by the first derivative of the respective records (Bb, right). All changes fully recovered after 13 and 3 min for the MT-I and the MT-II fibres, respectively.
Figure 3 summarizes the stimulation-induced changes in the kinetics of the first peak of the tetanic Ca2+ transients, in MT-I and MT-II fibres. Figure 3A–C shows the fatigue-induced changes in the amplitude, rise time and ΔF/RT ratio of the first peak, in MT-I and MT-II fibres, in the first, 40th and last episode, as well as after recovery. The results show a faster reduction of the amplitude of the first peak of the tetanic records in the MT-II fibres compared to the MT-I fibres (A) and no change in the rise time for MT-I fibres while there was a significant increase for MT-II fibres (B). This means that the MT-II fibres are taking more time to reach an even lower amplitude, as is illustrated by the changes in the ΔF/RT ratio (C), in which after 40 tetani, the MT-I fibres show no change while the MT-II fibres show a significant reduction (P < 0.01). After a mean of 96 and 52 tetani, the reduction amounted to 26% and 31% for MT-I and MT-II fibres, respectively. Figure 3D shows the changes in the maximum rate of rise of the first peak (ΔF/F. ms−1), obtained from its first derivative. The reduction of this parameter after 40 tetani in both MT-I and MT-II fibres was statistically different from their respective controls, and was similar between groups of fibres (24% and 29%, respectively). After the last episode, the reduction amounted to 27 and 33%, showing no statistical difference between groups. The figure also shows that the alterations were reversed by more than 92% compared to the pre-fatiguing conditions, with the recovered values statistically different from those of the last episodes. Three MT-II fibres were not included in the calculations of recovery in Fig. 3D since they were evaluated for inactivation of the Ca2+ release mechanism immediately after the fatiguing protocol (see below).
Figure 3. Fatigue-induced kinetic alterations in the first peak of the tetanic Ca2+ transients in MT-I and MT-II fibres loaded with Magfluo-4 AM.
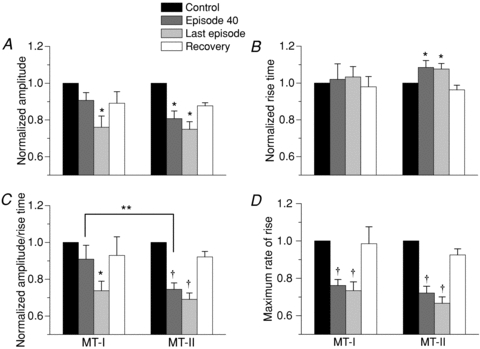
A, the decrease in the amplitude of the first peak of tetanic Ca2+ transients after 40 stimuli, compared to the first tetanus, was statistically significant only in MT-II fibres (19%). Both MT-I and MT-II fibres showed a significant reduction of the amplitude at the end of the protocol (last episode). The protocol finished after 96 and 52.8 tetani for MT-I and MT-II fibres (P < 0.01), respectively, which were the number of tetani needed to reach an amplitude decrease of about 25%. The recovery was over 88% for both fibre types. The change in A was accompanied by a significant increase of 8.5% in the rise time of MT-II fibres (B). The amplitude/rise time ratio showed a significant reduction of 25.5% after 40 tetani only for fibres MT-II (C), a significant decrease for both fibre types at the end of the protocol and a statistically significant recovery (over 92% for both fibre types). D, the decrease in the maximum rate of rise, as calculated from the first derivative of the first peak of every tetanic Ca2+ transient, after 40 stimuli was statistically significant in both MT-I (24%) and MT-II (28%) fibres. The decay continued from episode 40 to the last one, and reached 27% and 33% after 96 and 52.8 tetani for MT-I and MT-II fibres, respectively. The recovery was over 92% for both fibre types. All values were normalized to the first tetanus. *P < 0.05 compared to control, paired t test; **P < 0.05 between MT-I and MT-II, unpaired t test; †P < 0.01 compared to control, paired t test. MT-I, n = 5 for all graphs; MT-II, n = 18 for all graphs, except the white bar in D, which includes 15 fibres.
Changes in the decay phase of the tetanic Ca2+ transients
Figure 4 shows the changes induced by the repetitive stimulation on the decay phase of the tetanic records. Values of the τ1 of the decay of the first tetanic records ranged from 10.2 to 71.4 ms among all fibres and between 10.2 and 37.4 ms among MT-II fibres. Figure 4A shows the effects of repetitive stimulation on the normalized decay phase of the 1st, 20th, 40th and 50th records of a MT-II fibre and its recovery. In this fibre the stimulation induced an increase of τ1 from 23.4 to 27.3 ms (16.7%); full recovery was seen after 3 min. When analysing the changes in MT-II fibres, two subpopulations of fibres could be distinguished. One subgroup showed very rapid, noticeable changes (fast-fatigable MT-II, fMT-II) and the other group showed less severe changes that were established later (fatigue-resistant, rMT-II). Both groups differed regarding the amount of tetani applied (40.8 ± 2.3 and 76.7 ± 8.0 for fMT-II and rMT-II, respectively, P < 0.01) and the τ1 of the decay phase (15.2 ± 1.2 and 27.1 ± 3.7 ms for fMT-II and rMT-II, respectively, P < 0.01); these variables showed a significant correlation and allowed rMT-II and fMT-II fibres to be distinguished from each other (r = 0.74; P < 0.01, Supplemental Fig. S3), although some overlap exists between subgroups.
Figure 4. Effect of repetitive stimulation on the first time constant of decay (τ1) of the whole tetanic transients in MT-I and MT-II fibres loaded with Magfluo-4 AM.
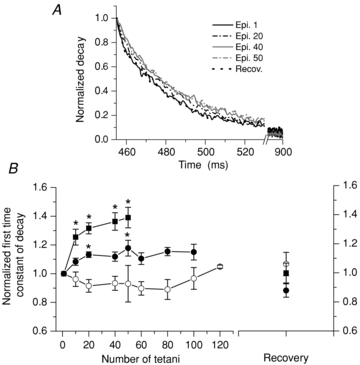
This figure shows only the decay phase of the tetanic records, corresponding to that of their last individual transient. The alteration of the decay phase in a MT-II fibre is illustrated in A. The maximum increase in the time constant of decay occurs after 50 tetani and after recovery the dotted trace superimposes on the first one. No such changes were seen in MT-I fibres. All traces were normalized. B summarizes the change of the τ1 of decay of fibres MT-I (open circles, n = 5), fMT-II (filled squares, n = 9) and rMT-II (filled circles, n = 6). The values were normalized to the first tetanus. *Significant differences with the first point demonstrated by a Tukey post hoc test, after a one-way ANOVA. The MT-II curves were different between them when compared with a two-way ANOVA.
Figure 4B summarizes the changes seen in all populations of fibres. For fMT-II fibres (filled squares) a significant increase was observed after 10 episodes and a maximum increase of 39% occurred after 50 episodes, while for rMT-II fibres (filled circles) a statistically significant increase of only 13.6% was seen after 20 tetani and a maximum of 17.8% was found after 50 tetani. When a two-way ANOVA was performed for the first 50 tetani, significant differences were found between the two subgroups of fibres. The mean τ1 of the MT-I fibres under control conditions was 63.5 ± 2.9 ms, and no change was observed during the protocol (open circles).
Figure 5 illustrates the effect of repetitive stimulation on the half-width of the first peak of the tetanic Ca2+ transients in MT-II fibres. Notice that when discussing this figure we refer to the half-width and decay phase of the first peak of the transient, and not to half-width or the decay phase of the whole tetani (which was described in Fig. 4). To focus on the decay phase (arrow) of the first tetanic peak, we normalized each episode and aligned them with regard to the time of the peak amplitude of the first episode. In this fibre (Fig. 5A), appreciable changes occurred only after 10 episodes of tetanic stimulation, and the half-width increased from 3.8 to 4.8 ms (26.3%) from the 1st to the 50th episode, and was fully reversed after 3 min of recovery. In fMT-II fibres (Fig. 5B, filled squares) the half-width values significantly increased (32.7%) after 40 tetani. The alterations were fully reversed, as seen in the right-most part of the graph in Fig. 5B. rMT-II fibres (filled circles) showed small, non-significant changes in the mean half-width values. In this figure, four fibres were not included since they have either control or fatigue values that did not allow us to measure a half-width value. The half-width value of the MT-I fibres could not be measured because the decay was so slow that it did not reach half of decay before arrival of the next stimuli. In these fibres, instead, we fitted the decay of the first peak of the tetani with a single exponential function and compared the τ1 before and after fatigue without seeing any change.
Figure 5. Effect of repetitive tetanic stimulation on the half-width of the first tetanic peak in MT-II fibres loaded with Magfluo-4 AM.
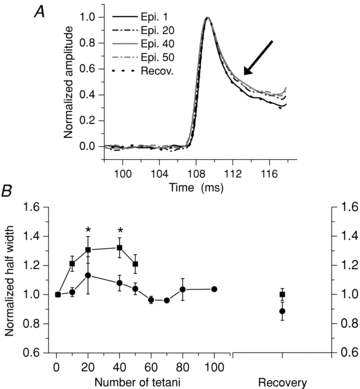
In A the increase in the time to half-relaxation is shown (arrow) for the selected episodes (epi). Note that the recovery (recov) trace superimposes on the first trace. All traces were normalized. B is a summary of the half-width values of the first peak in fMT-II (filled squares, n = 9) and rMT-II fibres (filled circles, n = 6). *Significant differences with the first point demonstrated by a Tukey post hoc test, after a one-way ANOVA. Differences between the two curves were also found by a two-way ANOVA.
Ca2+ release inactivation
To test whether differences in the susceptibility to fatigue between MT-I and MT-II fibres could be related to differences in the inactivation of the Ca2+ release mechanism, we evaluated the recovery from Ca2+ release inactivation in nine soleus fibres and compared the results with those previously obtained by Caputo et al. (2004) in FDB fibres (Fig. 6) and also compared the kinetics of recovery from inactivation of resting and fatigued fibres (Fig. 7).
Figure 6. Recovery from inactivation of Ca2+ release in soleus and FDB fibres.
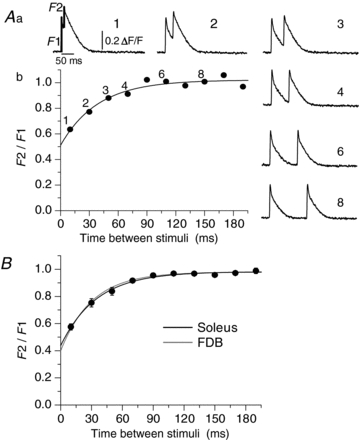
The procedure to study the phenomenon and to construct the curve is shown in A. Aa shows the transients obtained in a soleus fibre which underwent a double-stimulus protocol with 10 ms between them (record labelled 1), a time which is increased by 20 ms for each episode, as seen in the transients labelled 2 to 8. There are 20 s of rest between each episode. Ab, the ratio F2/F1 calculated as shown in Aa. The numbers above the points indicate the record where F2/F1 ratio is plotted. The points were fitted with a function of the form y = y0+A1*[1 – exp(−x/τ1)]. B shows a summary of the inactivation curves of 9 soleus fibres (black trace) to compare with the curve constructed (grey trace) with the values previously reported for 26 FDB fibres by Caputo et al. (2004). Note that the curves almost superimpose.
Figure 7. Recovery from inactivation of Ca2+ release in FDB (A) and soleus (B) fibres under control conditions and after a protocol of repetitive stimulation.
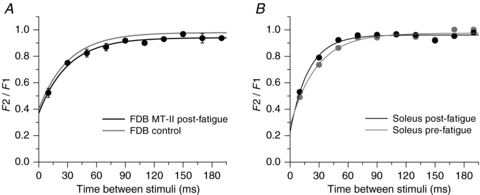
The procedure to construct the graphs is as in Fig. 6. In A the curve constructed with the values previously reported for 26 FDB fibres by Caputo et al. (2004), and used as control (grey trace), is compared to the curve obtained for 3 FDB fibres in which recovery from inactivation was evaluated immediately after a fatiguing protocol (black trace). In B, the curves of a soleus fibre are compared before (grey trace) and after (black trace) a fatiguing protocol. It is demonstrated that fatigue does not induce alterations in the recovery from inactivation in either FDB or soleus fibres.
The graph in Fig. 6A illustrates the procedure to obtain the curve of recovery from inactivation of Ca2+ release in a resting soleus fibre (see Methods and figure legend). In Fig. 6B we replotted the results obtained in 26 FDB fibres by Caputo et al. (2004), using the reported values: y0 = 0.4; A1 = 0.579 yτ1 = 30.4 (grey line) and compared them with the fit obtained for the nine soleus fibres evaluated in this work, whose values were: y0 = 0.44; A1 = 0.55 y; τ1 = 34.8 (black line). The great overlap of the curves show that resting MT-I and MT-II fibres have a similar recovery from inactivation of Ca2+ release.
Since MT-II fibres showed greater alterations of the first peak of the tetanic Ca2+ transients after the protocol of repetitive stimulation than MT-I fibres, we next evaluated if these fibres showed alterations of the recovery of the inactivation of Ca2+ release. The graph in Fig. 7A compares the curve obtained immediately after the fatiguing protocol in three FDB fibres MT-II (black line) with the curve previously obtained in resting FDB fibres (grey line) according to Caputo et al. (2004). Figure 7B compares the curve obtained after a fatiguing protocol in a soleus fibre MT-I (black line) with the curve previously obtained in the same fibre under resting conditions (grey line). No differences were found in either type of fibre in the recovery from inactivation of Ca2+ release after the protocol of repetitive stimulation.
Discussion
We aimed to test whether we could detect any fibre type-related difference in fatigue-induced kinetic changes of tetanic Ca2+ transients in enzymatically dissociated fibres loaded with the fast Ca2+ dye Magfluo-4, and if so, whether the phenomenon of inactivation of Ca2+ release might be involved.
Enzymatically dissociated fibres as a suitable model for the study of muscle fatigue
The morphological evaluation (Bekoff & Betz, 1977; Wang et al. 2007), and measurements of the levels of resting basal Ca2+ (Williams et al. 1990), electrical properties of the sarcolemma (Bekoff & Betz, 1977; Szentesi et al. 1997), charge movement (Szentesi et al. 1997), amplitude of the action potentials (Woods et al. 2004) and release of Ca2+ from the SR (Szentesi et al. 1997) have shown that the procedure of enzymatic dissociation yields functionally intact fibres suitable for physiological studies.
Our experiments using enzymatically dissociated fibres adhered to Laminin and loaded with Fluo-3 AM reproduced the two main changes described for the Ca2+ transients (Fig. 1) in fatigued fibres, namely a decrease in the amplitude of the tetanic Ca2+ transient and an increase in cytoplasmic basal Ca2+ (Westerblad & Allen, 1991). Besides that, in dissociated FDB fibres loaded with Rhod-2 under repetitive stimulation, we saw the increase in intramitochondrial Ca2+ previously reported in manually dissected, fatigued fibres (Supplemental Fig. S2 in this work; Bruton et al. 2003). This figure also demonstrates that the fibres remain very well attached to the Laminin layer at the bottom of the chamber. Laminin overcome the limitations of BTS, which was not used since: (i) it does not work for fibres of type I or hybrid fibres I/IIA (Cheung et al. 2002; Calderón et al. 2010), and (ii) it has been shown to modify the time course of the development of fatigue-induced changes in Ca2+ transients in mouse fibres (Bruton et al. 2006).
Fluo-3 has been used to study tetanic Ca2+ transients with no detectable effect on the mechanical response of the fibre (Caputo et al. 1994). Moreover, the signals show no Mg2+ interference, allow us to detect every individual peak of Ca2+ release in a tetani, and measure the changes in the basal level of fluorescence. Thus, it gives more information than dyes such as Fura-2 or Indo-1, whose smaller value of Koff limits its ability to track rapid changes in Ca2+ concentration during sustained activity (Baylor & Hollingworth, 1988). Fluo-3, however, it is still not as fast as Magfluo-4 and not suitable to study differences between fibre types. Magfluo-4 seems to be one of the best available dyes to reliably track Ca2+ movements in skeletal muscle fibres (Caputo et al. 2004; Hollingworth et al. 2009; Calderón et al. 2010). Enzymatically dissociated FDB and soleus fibres give us access to the fibre types I, IIA and IIX/D and we have previously shown that, when loaded with Magfluo-4 AM, fibre types I and IIA have tetanic Ca2+ transients with a MT-I, while fibres of type IIX/D have a tetanic Ca2+ transient MT-II (Calderón et al. 2009, 2010). Here, we used this methodology to compare the kinetics of tetanic Ca2+ transients obtained in the classical groups of fatigue-resistant (types I and IIA) and fast-fatigable (type IIX/D) fibres under fatiguing stimulation.
Potential pitfalls when Magfluo-4 is used include the possibility of Mg2+ interference and movement artifacts. The first one has been discussed elsewhere (Calderón et al. 2010) and shown not to affect our analysis. Supplemental Fig. S4 shows no significant mean changes in basal fluorescence in either MT-I or MT-II fibres. Regarding the issue of the movement artifacts, three lines of evidence suggest that they did not affect our experiments: (i) the use of Laminin strongly reduces movement artifacts (Calderón et al. 2009), (ii) we have measured, under confocal microscopy, the movement of the fibres after up to 75 tetani and found that the fibres (n = 5) displaced less than 4 μm, and (iii) all the stimulation-induced kinetic changes reversed during recovery, something unexpected if they were related to movement artifacts.
All these results indicate that the model of enzymatically dissociated muscle fibres is adequate to study ECC under fatiguing stimulation as well as to study physiological events in a fibre knowing its classification while still alive. It must be stressed that despite the use of Laminin, because of the elevated number of tetani applied to induce fatigue, some fibres moved and movement artifacts appeared; in these cases the experiments were terminated and the fibres were not included in the analysis.
Fatigue-induced kinetic changes in tetanic Ca2+ transients
The most important new findings of this work are the following: (i) the use of enzymatically dissociated mouse fibres loaded with Magfluo-4 AM and adhered to Laminin provides a suitable model for the study of muscle fatigue in functionally identified muscle cells; (ii) a fatiguing protocol induces a decrease in the amplitude as well as in the ΔF/RT ratio of the first peak of the tetanic Ca2+ transients, which appear earlier in MT-II than in MT-I fibres. An increase in the rise time was only seen in MT-II fibres (Fig. 3A–C); (iii) there is a proportional similar reduction in the maximum rate of rise of the first peak in both fibre types (Fig. 3D); (iv) there is a rapid decrease in the rate of the decay phase of the whole tetanic transients in MT-II but not in MT-I fibres (Fig. 4); (v) there is a rapid increase in the half-width of the first peak of the Ca2+ transients of MT-II fibres, which reflects a lengthening of its relaxation phase (Fig. 5); and (vi) fatigue does not induce alterations in the recovery from inactivation of Ca2+ release in any type of fibre (Fig. 7).
These findings were possible due to the properties of Magfluo-4, which resolves every peak in a tetanus and is the first dye used for recognizing fibre types for physiological experiments. Finding (iv) completes the panorama of the alteration of the decay phase of the whole tetanic transient, since our results refer to the first part of the decay, better seen by the use of the fast Ca2+ dye, while previously published results have focused on the last part of the decay, the ‘tail’, as better seen with slow Ca2+ dyes (Westerblad & Allen, 1993).
Metabolic factors have long been implicated in the development of fatigue (Fitts, 1994; Westerblad et al. 2002; Allen et al. 2008; Calderón-Vélez & Figueroa-Gordon, 2009).
The main changes in basal Ca2+, Mg2+, lactate and pH occur during the second half of the fatiguing protocols in different experimental models, although they depend on the intensity of the activity (Vøllestad et al. 1988; Westerblad & Allen, 1991, 1992b; Kent-Braun et al. 1993; Chin & Allen, 1998; Westerblad et al. 2002). Moreover, the protocol used in this work has been shown to induce only minor pH alterations (Westerblad & Allen, 1992a; Bruton et al. 1998; Chin & Allen, 1998) and it has not been possible to demonstrate an increase in the generation of ROS in isolated mouse fibres (Bruton et al. 2008).
Changes in Pi can start early during the fatigue development, although is more notorious during the second half of the stimulation periods (Bergström & Hultman, 1988; Moussavi et al. 1989; Kent-Braun et al. 1993; Kent-Braun, 1999) and several studies have shown that Pi is important for the early changes in the kinetics of Ca2+ signals (Dahlstedt et al. 2000, 2001; Westerblad et al. 2002). Since Pi can regulate both the release of Ca2+ and the function of SERCA (Duke & Steele, 2000; see also Dahlstedt et al. 2001; Westerblad et al. 2002), an increase of this by-product could explain our results (ii to v). The higher energy substrate content and utilization rates by the fast compared to slow fibres led to a higher concentrations of Pi in these fibres during fatigue (Kushmerick et al. 1992; Fitts, 1994; Potma et al. 1995; Bottinelli & Reggiani, 2000; Dahlstedt et al. 2000; He et al. 2000). Slow fibres appear to be as sensitive as the fast fibres to Pi increases regarding some, but not all, physiological variables (Potma et al. 1995; Bottinelli & Reggiani, 2000; He et al. 2000), and the effect of Pi on slow and fast fibre types depends on the interrelation with other factors such as pH (Potma et al. 1995). The management of and sensitivity to Pi alterations by the different fibre types in the model of isolated, intact muscle cells is an issue which deserves further research.
Since the first part of the decay of the first peak of the tetanic transient is probably due to the PV action and the second part to SERCA function (Caputo et al. 1999, 2005; Calderón et al. 2009; J. C. Calderón, unpublished results), the change (v) probably reflects the ‘fatigue’ of these mechanisms. However, we cannot fully explain this new finding, as no previous works have demonstrated fatigue-induced alterations of PV.
It is still possible that unknown factors may explain in part the time course of the Ca2+ kinetics alterations induced by fatigue and the differences among fibre types, such as rapid changes in the proteins that regulate Ca2+ release and SERCA function.
All fatigue-induced kinetic alterations reversed to more than 85% of the original values after rest. Small differences found among groups: (i) may reflect true differences among fibre types; (ii) may be related to more or less dye photobleaching or compartmentalization; (iii) or may be due to some degree of local damage in those fibres subjected to more prolonged stimulation. We have also seen a slight decrease of the amplitude of the Ca2+ transients as a function of the duration of different types of experiments not related to fatigue. This may reflect some loss of the dye from the cytoplasm and may explain why some fibres do not reach full recovery of the amplitude.
As indicated by many different biochemical and physiological parameters (Hintz et al. 1982; Franzini-Armstrong et al. 1988; Bottinelli & Reggiani, 2000; Calderón et al. 2010), the fatigability of the Ca2+ signals shows great variability. When analysing the results of the fMT-II, rMT-II and MT-I fibres, it appears a continuum in which the fMT-II, the fibres with the fastest Ca2+ kinetics, and the MT-I, the fibres with the slowest kinetics, are the extremes of the susceptibility to present kinetic changes under fatiguing stimulation, while the rMT-II, with intermediate kinetics, behave also intermediate regarding the fatigability. It is interesting that we can recognize subgroups of fast-fatigable and fast-fatigue resistant cells within MT-II fibres from mouse FDB, which are type IIX/D (Calderón et al. 2009). Although we did not evaluate any biochemical property, this variability may reflect the fact that the this type of fibre spans the spectrum from the more oxidative, fatigue-resistant IIA fibres to the more glycolytic, fast-fatigable IIB fibres (Gorza, 1990). For instance, the succinate dehydrogenase activity in fibre type IIX/D ranges from intermediate to high in mouse (Gorza, 1990; Füchtbauer et al. 1991). In agreement with this, Hintz et al. (1982) found that individual murine fibres varied enough to create a continuous spectrum of metabolite levels from one extreme to the other under both resting and stimulated conditions. Thus, it seems that there is a relationship between oxidative–glycolytic activity of a fibre, slow–fast Ca2+ kinetics, resistance–susceptibility to fatigue and resistance–susceptibility to present alterations in Ca2+ kinetics under fatiguing stimulation. All these variables show a continuum in which the extremes are the fibres of type I and IIB and the intermediates are the fibres of type IIA and IIX/D and some overlap exists between the nearest neighbours. At this point, we want to call attention to the fact that enzymatically dissociated muscles yield different types of fibres which seem not to behave in an homogeneous way; this is something important to take into account when performing physiological experiments, and seems to be a neglected issue, since many physiological papers never recognize subpopulations, or even when some heterogeneity in the results appears, the issue about which type of fibre is being used in the work is not discussed.
Besides the effect of metabolites, a process of inactivation of Ca2+ release may play a role in the alterations of Ca2+ release induced by fatigue (Caputo, 1983). It is interesting to note that the first and second points of our figures (which are the points taken immediately after fatigue) of the control and post-fatigue records superimpose (Fig. 7); i.e. the fact that the protocols were not different rule out the possibility of artifacts induced by the time between episodes. The remaining points are within 3 min of recovery, a time that is clearly lower than the reported values for total recovery of the Ca2+ alterations. These results indicate that the phenomenon of inactivation of Ca2+ release does not play a role in the alterations in the first peak of the tetanic Ca2+ transients during fatigue development.
The mechanisms to explain the different resistance to fatigue in different fibre types are not yet known. Many fatigue-induced changes in tetanic Ca2+ transients were ignored because of the lack of a model to evaluate fatigue using fast Ca2+ dyes with special reference to fibre types. We have broadened the spectrum of physiological alterations developed during fatigue in intact fibres and ruled out the effect of the mechanism of Ca2+ release inactivation in explaining these alterations. Our results will help to better dissect the mechanisms involved in fatigue of different muscle fibre types since it is now possible to study subtle changes in several kinetic parameters of the Ca2+ transients during fatigue development, before the final result of reduction in the amplitude is established.
In conclusion, we demonstrated fibre-type dependent, early fatigue-induced kinetic changes in both Ca2+ release and reuptake in enzymatically dissociated murine fibres loaded with the Ca2+ dyes Magfluo-4 AM and Fluo-3 AM and adhered to Laminin. The amount of these changes is related to the velocity of the Ca2+ kinetics of the fibres under unfatigued conditions and ruled out the possibility that the differences in the kinetic changes of Ca2+ release between fibres MT-I and MT-II could be related to the inactivation of the Ca2+ release mechanism.
Acknowledgments
Grant support was provided by FONACIT project G-2001000637. We want to thank Lic Alis Guillén at IVIC and Dr Jaime Pérez at University of Antioquia for support to complete the manuscript.
Glossary
Abbreviations
- BTS
N-benzyl-p-toluene sulphonamide
- ECC
excitation–contraction coupling
- MT-I
morphology type I
- MT-II
morphology type II
- rMT-II
fatigue-resistant morphology type II
- fMT-II
fast-fatigable morphology type II
- FDB
flexor digitorum brevis
- PCr
phosphocreatine
- Pi
inorganic phosphate
- ROS
reactive oxygen species
- PV
parvalbumin
- SR
sarcoplasmic reticulum
- SERCA
sarcoendoplasmic reticulum Ca2+-ATPase
Author contributions
J.C.C. and C.C. contributed to the conception and design, and J.C.C. conducted the experiments, analysed the data and drafted the article. C.C. and P.B. contributed to the interpretation of the data, critically reviewed the manuscript and made important intellectual contributions. All authors approved the final version to be published. All experiments were done at the Venezuelan Institute for Scientific Research (IVIC). J.C.C is now at University of Antioquia, Colombia, but held a position as visitor colaborator at IVIC to participate in this project.
Supplementary material
Supplemental Online Figure 1
Supplemental Online Figure 2
Supplemental Online Figure 3
Supplemental Online Figure 4
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Allen D, Lamb G, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- Allen D, Lee J, Westerblad H. Intracellular calcium and tension during fatigue in isolated single muscle fibres from Xenopus laevis. J Physiol. 1989;415:433–458. doi: 10.1113/jphysiol.1989.sp017730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S, Hollingworth S. Fura-2 calcium transients in frog skeletal muscle fibres. J Physiol. 1988;403:151–192. doi: 10.1113/jphysiol.1988.sp017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekoff A, Betz W. Physiological properties of dissociated muscle fibres obtained from innervated and denervated adult rat muscle. J Physiol. 1977;271:25–40. doi: 10.1113/jphysiol.1977.sp011988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchtold M, Brinkmeier H, Müntener M. Calcium ion in skeletal muscle: its crucial role for muscle function, plasticity, and disease. Physiol Rev. 2000;80:1215–1265. doi: 10.1152/physrev.2000.80.3.1215. [DOI] [PubMed] [Google Scholar]
- Bergström M, Hultman E. Energy cost and fatigue during intermittent electrical stimulation of human skeletal muscle. J Appl Physiol. 1988;65:1500–1505. doi: 10.1152/jappl.1988.65.4.1500. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Jones D, Woods J. Excitation frequency and muscle fatigue: electrical responses during human voluntary and stimulated contractions. Exp Neurol. 1979;64:414–427. doi: 10.1016/0014-4886(79)90280-2. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Woods J. Changes in muscle contractile properties and neural control during human muscular fatigue. Muscle Nerve. 1984;7:691–699. doi: 10.1002/mus.880070902. [DOI] [PubMed] [Google Scholar]
- Bottinelli R, Reggiani C. Human skeletal muscle fibres: molecular and functional diversity. Prog Biophys Mol Biol. 2000;73:195–262. doi: 10.1016/s0079-6107(00)00006-7. [DOI] [PubMed] [Google Scholar]
- Bruton J, Lännergren J, Westerblad H. Effects of CO2-induced acidification on the fatigue resistance of single mouse muscle fibers at 28°C. J Appl Physiol. 1998;85:478–483. doi: 10.1152/jappl.1998.85.2.478. [DOI] [PubMed] [Google Scholar]
- Bruton J, Pinniger G, Lännergren J, Westerblad H. The effects of the myosin-II inhibitor N-benzyl-p-toluene sulphonamide on fatigue in mouse single intact toe muscle fibres. Acta Physiol. 2006;186:59–66. doi: 10.1111/j.1748-1716.2005.01499.x. [DOI] [PubMed] [Google Scholar]
- Bruton J, Place N, Yamada T, Silva J, Andrade F, Dahlstedt A, et al. Reactive oxygen species and fatigue-induced prolonged low-frequency force depression in skeletal muscle fibres of rats, mice and SOD2 overexpressing mice. J Physiol. 2008;586:175–184. doi: 10.1113/jphysiol.2007.147470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton J, Tavi P, Aydin J, Wasterblad H, Lännergren J. Mitochondrial and myoplasmic [Ca2+] in single fibres from mouse limb muscles during repeated tetanic contraction. J Physiol. 2003;551:179–190. doi: 10.1113/jphysiol.2003.043927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R, Levine D, Tsairis P, Zajac F. Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973;234:723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R, Levine D, Zajac F. Mammalian motor units: physiological-histochemical correlation in three types in cat gastrocnemius. Science. 1971;174:709–712. doi: 10.1126/science.174.4010.709. [DOI] [PubMed] [Google Scholar]
- Calderón JC, Bolaños P, Caputo C. Myosin heavy chain isoform composition and Ca2+ transients in fibres from enzymatically dissociated murine soleus and extensor digitorum longus muscles. J Physiol. 2010;588:267–279. doi: 10.1113/jphysiol.2009.180893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón JC, Bolaños P, Torres SH, Rodríguez-Arroyo G, Caputo C. Different fibre populations distinguished by their calcium transient characteristics in enzymatically dissociated murine flexor digitorum brevis and soleus muscles. J Muscle Res Cell Motil. 2009;30:125–137. doi: 10.1007/s10974-009-9181-1. [DOI] [PubMed] [Google Scholar]
- Calderón-Vélez J, Figueroa-Gordon L. El acoplamiento excitación-contracción en el músculo esquelético: preguntas por responder a pesar de 50 años de estudio. Biomédica. 2009;29:140–160. [PubMed] [Google Scholar]
- Capote J, Bolaños P, Schuhmeier R, Melzer W, Caputo C. Calcium transients in developing mouse skeletal muscle fibres. J Physiol. 2005;564:451–464. doi: 10.1113/jphysiol.2004.081034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C. Pharmacological investigations of excitation-contraction coupling, Chap. 14. In: Peachey L, Adrian R, editors. Handbook of Physiology. Bethesda: American Physiological Society; 1983. [Google Scholar]
- Caputo C, Bolaños P, Escobar A. Fast calcium removal during single twitches in amphibian skeletal muscle fibres. J Muscle Res Cell Motil. 1999;20:555–567. doi: 10.1023/a:1005526202747. [DOI] [PubMed] [Google Scholar]
- Caputo C, Bolaños P, González A. Inactivation of Ca2+ transients in amphibian and mammalian muscle fibres. J Muscle Res Cell Motil. 2004;25:315–328. doi: 10.1007/s10974-004-4071-z. [DOI] [PubMed] [Google Scholar]
- Caputo C, Edman K, Lou F, Sun Y. Variations in myoplasmic Ca2+ concentration during contraction and relaxation studied by the indicator fluo-3 in frog muscle fibres. J Physiol. 1994;478:137–148. doi: 10.1113/jphysiol.1994.sp020237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S, Klein M, Schneider M. Decay of calcium transients after electrical stimulation in rat fast- and slow-twitch skeletal muscle fibres. J Physiol. 1997;501:573–588. doi: 10.1111/j.1469-7793.1997.573bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Carroll S, Racay P, Dick J, Pette D, Traub I, et al. Deficiency in parvalbumin increases fatigue resistance in fast-twitch muscle and upregulates mitochondria. Am J Physiol Cell Physiol. 2001;281:C114–C122. doi: 10.1152/ajpcell.2001.281.1.C114. [DOI] [PubMed] [Google Scholar]
- Cheung A, Dantzig J, Hollingworth S, Baylor S, Goldman Y, Mitchison T, et al. A small-molecule inhibitor of skeletal muscle myosin II. Nat Cell Biol. 2002;4:83–89. doi: 10.1038/ncb734. [DOI] [PubMed] [Google Scholar]
- Chin E, Allen D. The contribution of pH-dependent mechanisms to fatigue at different intensities in mammalian single muscle fibres. J Physiol. 1998;512:831–840. doi: 10.1111/j.1469-7793.1998.831bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlstedt A, Katz A, Westerblad H. Role of myoplasmic phosphate in contractile function of skeletal muscle: studies on creatine kinase-deficient mice. J Physiol. 2001;533:379–388. doi: 10.1111/j.1469-7793.2001.0379a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlstedt A, Katz A, Wieringa B, Westerblad H. Is creatine kinase responsible for fatigue? Studies of isolated skeletal muscle deficient in creatine kinase. FASEB J. 2000;14:982–990. doi: 10.1096/fasebj.14.7.982. [DOI] [PubMed] [Google Scholar]
- Duke A, Steele D. Characteristics of phosphate-induced Ca2+ efflux from the SR in mechanically skinned rat skeletal muscle fibers. Am J Physiol Cell Physiol. 2000;278:C126–C135. doi: 10.1152/ajpcell.2000.278.1.C126. [DOI] [PubMed] [Google Scholar]
- Fitts R. Cellular mechanisms of muscle fatigue. Physiol Rev. 1994;74:49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- Fletcher W. The relation of oxygen to the survival metabolism of muscle. J Physiol. 1902;28:474–498. doi: 10.1113/jphysiol.1902.sp000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong C, Ferguson D, Champ C. Discrimination between fast- and slow-twitch fibres of guinea pig skeletal muscle using the relative surface density of junctional transverse tubule membrane. J Muscle Res Cell Motil. 1988;9:403–414. doi: 10.1007/BF01774067. [DOI] [PubMed] [Google Scholar]
- Füchtbauer E, Rowlerson A, Gotz K, Friedrich G, Mabuchi K, Gergely J, et al. Direct correlation of parvalbumin levels with myosin isoforms and succinate dehydrogenase activity on frozen sections of rodent muscle. J Histochem Cytochem. 1991;39:355–361. doi: 10.1177/39.3.1825216. [DOI] [PubMed] [Google Scholar]
- Gollnick P, Korge P, Karpakka J, Saltin B. Elongation of skeletal muscle relaxation during exercise is linked to reduced calcium uptake by the sarcoplasmic reticulum in man. Acta Physiol Scand. 1991;142:135–136. doi: 10.1111/j.1748-1716.1991.tb09139.x. [DOI] [PubMed] [Google Scholar]
- Gorza L. Identification of a novel type 2 fiber population in mammalian skeletal muscle by combined use of histochemical myosin ATPase and anti-myosin monoclonal antibodies. J Histochem Cytochem. 1990;38:257–265. doi: 10.1177/38.2.2137154. [DOI] [PubMed] [Google Scholar]
- Grabowski W, Lobsiger E, Lüttgau H. The effect of repetitive stimulation at low frequencies upon the electrical and mechanical activity of single muscle fibres. Pflügers Arch. 1972;334:222–239. doi: 10.1007/BF00626225. [DOI] [PubMed] [Google Scholar]
- He Z, Bottinelli R, Pellegrino M, Ferenczi M, Reggiani C. ATP consumption and efficiency of human single muscle fibers with different myosin isoform composition. Biophys J. 2000;79:945–961. doi: 10.1016/S0006-3495(00)76349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintz C, Chi M, Fell R, Ivy J, Kaiser K, Lowry C, et al. Metabolite changes in individual rat muscle fibers during stimulation. Am J Physiol. 1982;242:C218–C228. doi: 10.1152/ajpcell.1982.242.3.C218. [DOI] [PubMed] [Google Scholar]
- Hollingworth S, Gee K, Baylor S. Low-affinity Ca2+ indicators compared in measurements of skeletal muscle Ca2+ transients. Biophys J. 2009;97:1864–1872. doi: 10.1016/j.bpj.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong DS, Pape P, Chandler W, Baylor S. Reduction of calcium inactivation of sarcoplasmic reticulum calcium release by Fura-2 in voltage-clamped cut twitch fibers from frog muscle. J Gen Physiol. 1993;102:333–370. doi: 10.1085/jgp.102.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katerinopoulos H, Foukaraki E. Polycarboxylate fluorescent indicators as ion concentration probes in biological systems. Curr Med Chem. 2002;9:275–306. doi: 10.2174/0929867023371193. [DOI] [PubMed] [Google Scholar]
- Kent-Braun J. Central and peripheral contributions to muscle fatigue in humans during sustained maximal effort. Eur J Appl Physiol. 1999;80:57–63. doi: 10.1007/s004210050558. [DOI] [PubMed] [Google Scholar]
- Kent-Braun J, Millar R, Weiner M. Phases of metabolism during progressive exercise to fatigue in human skeletal muscle. J Appl Physiol. 1993;75:573–580. doi: 10.1152/jappl.1993.75.2.573. [DOI] [PubMed] [Google Scholar]
- Konishi M, Hollingworth S, Harkins A, Baylor S. Myoplasmic calcium transients in intact frog skeletal muscle fibers monitored with the fluorescent indicator furaptra. J Gen Physiol. 1991;97:271–301. doi: 10.1085/jgp.97.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmerick M, Moerland T, Wiseman R. Mammalian skeletal muscle fibres distinguished by contents of phosphocreatine, ATP, and Pi. Proc Natl Acad Sci U S A. 1992;89:7521–7525. doi: 10.1073/pnas.89.16.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G. Excitation-contraction coupling and fatigue mechanisms in skeletal muscle: studies with mecanically skinned fibres. J Muscle Res Cell Motil. 2002;23:81–91. doi: 10.1023/a:1019932730457. [DOI] [PubMed] [Google Scholar]
- Moussavi R, Carson P, Boska M, Weiner M, Miller R. Nonmetabolic fatigue in exercising human muscle. Neurology. 1989;39:1222–1226. doi: 10.1212/wnl.39.9.1222. [DOI] [PubMed] [Google Scholar]
- Payne A, Delbono O. Neurogenesis of excitation-contraction uncoupling in aging skeletal muscle. Exerc Sport Sci Rev. 2004;32:36–40. doi: 10.1097/00003677-200401000-00008. [DOI] [PubMed] [Google Scholar]
- Petrofsky J, Lind A. Isometric endurance in fast and slow muscles in the cat. Am J Physiol Cell Physiol. 1979;236:C185–C191. doi: 10.1152/ajpcell.1979.236.5.C185. [DOI] [PubMed] [Google Scholar]
- Potma E, van Grass I, Stienen G. Influence of inorganic phosphate and pH on ATP utilization in fast and slow skeletal muscle fibers. Biophys J. 1995;69:2580–2589. doi: 10.1016/S0006-3495(95)80129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiani C, te Kronnie T. RyR isoforms and fibre-type specific expression of proteins controlling intracellular calcium concentration in skeletal muscles. J Muscle Res Cell Motil. 2006;27:327–335. doi: 10.1007/s10974-006-9076-3. [DOI] [PubMed] [Google Scholar]
- Sandow A. Excitation-contraction coupling in muscular response. Yale J Biol Med. 1952;XXV:176–201. [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Simon B. Inactivation of calcium release from the sarcoplasmic reticulum in frog skeletal muscle. J Physiol. 1988;405:727–745. doi: 10.1113/jphysiol.1988.sp017358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson D, Lamb G, Stephenson G. Events of the excitation-contraction-relaxation (E-C-R) cycle in fast- and slow-twitch mammalian muscle fibres relevant to muscle fatigue. Acta Physiol Scand. 1998;162:229–245. doi: 10.1046/j.1365-201X.1998.0304f.x. [DOI] [PubMed] [Google Scholar]
- Szentesi P, Jacquemond V, Kovács L, Csernoch L. Intramembrane charge movement and sarcoplasmic calcium release in enzymatically isolated mammalian skeletal muscle fibres. J Physiol. 1997;502:371–384. doi: 10.1111/j.1469-7793.1997.371bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehura H, Fujinami N, Nishizawa T, Ogasawara H, Kasuga N. Eccentric exercise-induced morphological changes in the membrane systems involved in excitation–contraction coupling in rat skeletal muscle. J Physiol. 2001;533:571–583. doi: 10.1111/j.1469-7793.2001.0571a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupling A. The sarcoplasmic reticulum in muscle fatigue and disease: Role of the sarco(endo)plasmic reticulum Ca2+-ATPase. Can J Appl Physiol. 2004;29:308–329. doi: 10.1139/h04-021. [DOI] [PubMed] [Google Scholar]
- Vøllestad N, Sejersted O, Bahr R, Woods J, Bigland-Ritchie B. Motor drive and metabolic responses during repeated submaximal contractions in humans. J Appl Physiol. 1988;64:1421–1427. doi: 10.1152/jappl.1988.64.4.1421. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zheng Z, Messi M, Delbono O. Muscle fibers from senescent mice retain excitation-contraction coupling properties in culture. In vitro Cell Dev Biol. 2007;43:222–234. doi: 10.1007/s11626-007-9047-z. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Allen D. Changes of myoplasmic calcium concentration during fatigue in single mouse muscle fibers. J Gen Physiol. 1991;98:615–635. doi: 10.1085/jgp.98.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen D. Changes of intracellular pH due to repetitive stimulation of single fibres from mouse skeletal muscle. J Physiol. 1992a;449:49–71. doi: 10.1113/jphysiol.1992.sp019074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen D. Myoplasmic free Mg2+ concentration during repetitive stimulation of single fibres from mouse skeletal muscle. J Physiol. 1992b;453:413–434. doi: 10.1113/jphysiol.1992.sp019236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen D. The contribution of [Ca2+]i to the slowing of relaxation in fatigued single fibres from mouse skeletal muscle. J Physiol. 1993;468:729–740. doi: 10.1113/jphysiol.1993.sp019797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen D, Lännergren J. Muscle fatigue: lactic acid or inorganic phosphate the major cause? News Physiol Sci. 2002;17:17–21. doi: 10.1152/physiologyonline.2002.17.1.17. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Lännergren J. Slowing of relaxation during fatigue in single mouse muscle fibres. J Physiol. 1991;434:323–336. doi: 10.1113/jphysiol.1991.sp018472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D, Head S, Bakker A, Stephenson G. Resting calcium concentrations in isolated skeletal muscle fibres of dystrophic mice. J Physiol. 1990;428:243–256. doi: 10.1113/jphysiol.1990.sp018210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods C, Novo D, DiFranco M, Vergara J. The action potential-evoked sarcoplasmic reticulum calcium release is impaired in mdx mouse muscle fibres. J Physiol. 2004;557:59–75. doi: 10.1113/jphysiol.2004.061291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


