Non-technical summary
In young men, sympathetic nerve activity is directly related to the level of vasoconstrictor tone in the peripheral vasculature. However, in young women this relationship does not exist, suggesting that certain factors (potentially related to the female sex hormones) offset the transfer of sympathetic nerve activity into vasoconstrictor tone in this population. In the present study we show that, in young women, the β-adrenergic receptors (which cause vasodilatation in response to noradrenaline) blunt the vasoconstrictor effect of resting sympathetic nerve activity in young women. This mechanism does not occur in young men or postmenopausal women. It is possible that the β-adrenergic receptors may partially protect young women against the sometimes harmful effects of high sympathetic nerve activity. This may explain why the risk of developing hypertension is greater in young men and postmenopausal women (who have very high sympathetic nerve activity) compared to young women.
Abstract
Abstract
In men, muscle sympathetic nerve activity (MSNA) is positively related to total peripheral resistance (TPR) and inversely related to cardiac output (CO). However, this relationship was not observed in young women. We aimed to investigate whether simultaneous β-adrenergic stimulation offsets this balance in young women. Furthermore, we aimed to examine whether the ability of the β-adrenergic receptors to offset the transduction of MSNA into vasoconstrictor tone was lost in postmenopausal women. We measured MSNA (peroneal microneurography), arterial pressure (brachial line), CO (Modelflow), TPR and changes in forearm vascular conductance (FVC) to increasing doses of noradrenaline (NA; 2, 4 and 8 ng (100 ml)−1 min−1) before and after systemic β-blockade with propranolol in 17 young men, 17 young women and 15 postmenopausal (PM) women. The percentage and absolute change in FVC to the last two doses of NA were greater during β-blockade in young women (P < 0.05), whereas the change in FVC was similar before and during β-blockade in young men and PM women (P > 0.05). Before β-blockade there was no relationship of MSNA to TPR or mean arterial pressure (MAP) in young women. Following β-blockade, MSNA became positively related to TPR (r = 0.59, P < 0.05) and MAP (r = 0.58, P < 0.05). In the PM women and young men, MSNA was positively associated with TPR. β-Blockade had no effect on this relationship. Our data suggest that the β-adrenergic receptors offset α-adrenergic vasoconstriction in young women but not young men or PM women. These findings may explain in part the tendency for blood pressure to rise after menopause in women.
Introduction
It is well known that young women are at less risk of developing hypertension and other cardiovascular diseases compared to men of a similar age (Burt et al. 1995; Wiinberg et al. 1995). However, as women age the risk of developing hypertension increases (Burt et al. 1995; Wiinberg et al. 1995). This is particularly apparent around the onset of menopause, where the incidence of hypertension becomes similar to, or even exceeds, that observed in men of the same age (Burt et al. 1995). Therefore, the female sex hormones have long been associated with a protective effect on the cardiovascular system (Wuest et al. 1953; Miller & Duckles, 2008). Understanding how the female sex hormones exert their cardioprotective effect is becoming increasingly important, since the postmenopausal world population is projected to rise 2- to 5-fold by 2050 (US Census Bureau on World Population; Meyer et al. 2008).
Recently, we and others have demonstrated fundamental sex differences in arterial pressure regulation (Shoemaker et al. 2001; Charkoudian et al. 2005; Carter & Ray, 2009; Hart et al. 2009a). It appears that a reciprocal balance between cardiac output and sympathetic control of peripheral vascular resistance (TPR) is a key factor in normal arterial pressure regulation in young men (Charkoudian et al. 2005; Hart et al. 2009a). This balance minimizes the effect of high muscle sympathetic nerve activity (MSNA) on arterial pressure. However, data from our lab demonstrate that in complete contrast to men, there is no relationship of MSNA to TPR or cardiac output in women (Hart et al. 2009a). The lack of relationship between MSNA and TPR suggests that factors in addition to sympathetic neural activity modulate whole body ‘net’ vasoconstrictor tone in young women.
Previous work indicates that β-adrenergic receptor sensitivity is enhanced in young women compared to men of a similar age (Kneale et al. 2000). Consequently, forearm vasoconstrictor responses to noradrenaline are blunted in young women due to concurrent β-adrenergic-mediated vasodilatation. Thus, it is possible that β2-adrenergic receptor-mediated vasodilatation is enhanced in young women and offsets the vasoconstrictor effects of high levels of MSNA. Therefore, our first aim in the present study was to examine the role of β-adrenergic receptors in the balance among MSNA, TPR and cardiac output in young normotensive men and women.
As men and women age the relationship between MSNA and blood pressure becomes positive: older men and women with higher MSNA have higher blood pressure. In contrast, in younger people at rest, MSNA is unrelated to arterial pressure (Sundlof & Wallin, 1978a; Charkoudian et al. 2005; Narkiewicz et al. 2005). The MSNA–arterial pressure relationship is particularly robust in older women (Matsukawa et al. 1998; Narkiewicz et al. 2005). In the present study, our second aim was to examine whether the decrease in oestrogens and progesterone after menopause modulates the role of the β-adrenergic receptors in counterbalancing sympathetic vasoconstrictor activity. Changes in the ability of the β-adrenergic receptors to counterbalance the effects of MSNA on the peripheral vasculature might explain (in part) the steeper MSNA–blood pressure relationship in older women (Narkiewicz et al. 2005).
We hypothesized that (a) systemic non-selective β-blockade would augment the forearm vasoconstrictor response to noradrenaline in young women, but not in young men or postmenopausal women, (b) systemic β-blockade in young women would cause the relationship between MSNA and TPR to become positive (similar to that seen in men) and (c) systemic β-blockade would not affect any relationship observed among MSNA, TPR, cardiac output and MAP in postmenopausal women.
Methods
Participants
After the protocol was approved by the Institutional Review Board of the Mayo Clinic, 17 young men, 17 young women and 15 postmenopausal women gave written informed consent to participate in this study (Table 1 for demographics). The subjects were non-smokers with no history of cardiovascular or other chronic diseases. Participants were excluded if their body mass index was ≥28 kg m−2 or if they were taking any medications other than oral contraceptives. In the young women, 11 out of 17 subjects were taking oral contraceptives. Young women using an inter-uterine device were excluded. Postmenopausal women were excluded if they were taking hormone replacement therapy. In the older women, we accepted participants who were taking medication for hypothyroidism. We did this since a large percentage of the postmenopausal population is prescribed drugs for hypothyroidism. Thirteen out of fifteen were taking this type of medication. Women treated for hypothyroidism have thyroid hormones (e.g. thyroid stimulating hormone) that remain within a normal range. Postmenopause was defined as at least 1 year since last menstruation (Gracia et al. 2005). To minimize the effects of the reproductive hormones on autonomic control or cardiovascular function, all women were studied in the early follicular phase of the menstrual cycle or in the low hormone phase of oral contraceptive use (Minson et al. 2000). All women of child bearing age were asked to complete a pregnancy test at least 48 h before the study day. Subjects were asked not to consume anything within 2 h before the experiment, and not to consume caffeine or alcohol for 24 h before the experiment.
Table 1.
Demographic characteristics in young men, young women and postmenopausal (PM) women
| Demographics | Young men (n = 17) | Young women (n = 17) | PM women (n = 15) |
|---|---|---|---|
| Height (m) | 1.78 ± 0.01 | 1.68 ± 0.03† | 1.65 ± 0.01† |
| Weight (kg) | 79.1 ± 2.8 | 65.0 ± 3.1† | 66.5 ± 1.5† |
| BMI (kg m−2) | 24.9 ± 0.6 | 23.1 ± 0.7 | 24.4 ± 0.6 |
| Age (years) | 25 ± 1 | 25 ± 2 | 59 ± 2 |
P ≤ 0.05 vs. young men. Mean ± SEM.
Procedures
All of the studies were performed in a CTSA Clinical Research Unit laboratory at the Mayo Clinic, where ambient temperature was controlled between 22°C and 24°C. On arrival at the laboratory, subjects rested in the supine position during instrumentation. After local anaesthesia with 2% lidocaine, a 5 cm, 20-gauge arterial catheter was placed in the brachial artery of the non-dominant arm, using aseptic technique. The catheter was connected to a pressure transducer, which was positioned at the level of the heart and interfaced with a personal computer to monitor arterial pressure. A 3-lead ECG was used for continuous recordings of heart rate.
Multiunit MSNA was measured from the right peroneal nerve at the fibular head using insulated tungsten microelectrodes. A muscle sympathetic fascicle was identified when taps on the muscle belly or passive muscle stretch evoked mechanoreceptive impulses, and no afferent neural response was evoked by skin stimuli (Sundlof & Wallin, 1977, 1978b) The recorded signal was amplified 80,000-fold, band pass filtered (700 to 2000 Hz), rectified, and integrated (resistance-capacitance integrator circuit time constant 0.1 s) by a nerve traffic analyser.
Stroke volume was measured from the brachial artery using Modelflow analysis. Briefly, the brachial pressure wave was downloaded onto a personal computer and analysed offline using data acquisition software (WinDaq194; DATAQ instruments, Ohio, USA). Beat-to-beat stroke volume was then calculated using Modelflow which computes an aortic waveform based on non-linear pressure–volume, pressure–compliance and pressure–characteristic impedance equations, incorporating age, sex, height and body mass (Wesseling et al. 1993). Cardiac output was calculated as stroke volume × heart rate and TPR was calculated as MAP/cardiac output.
Forearm blood flow (FBF) was measured using mercury-in-silastic strain gauge plethysmography (Joyner et al. 2001). Briefly, a paediatric blood pressure cuff was placed around the wrist and inflated to suprasystolic levels (220 mmHg) to arrest the circulation of the hand, and a venous occlusion cuff was placed on the upper arm and rapidly inflated to 50 mmHg every 7.5 s, yielding one blood flow every 15.0 s. FBF was expressed as milliliters per 100 ml of tissue per minute.
Brachial artery infusion of noradrenaline
Noradrenaline infusion was adjusted for forearm volume and administered via the brachial artery catheter at total rates of 2 to 4 ml min−1. Noradrenaline (noradrenaline bitartrate, Bedford Laboratories) was administered at 2, 4 and 8 ng (100 ml)−1 min−1.
Systemic β-adrenergic blockade
Non-selective β-blockade was achieved using intravenous infusion of propranolol. A 0.25 mg kg−1 bolus of propranolol was followed by a continuous infusion of 0.004 mg kg−1 min−1 propranolol to maintain β-blockade. This dose of propranolol has been previously proven to cause total β-blockade in adult humans (Bell et al. 2001)
Protocol
After the placement of arterial catheters, ECG leads and instrumentation for plethysmography, subjects rested supine during instrumentation for microneurography. Once a good electrode site for measurement of MSNA was found, 5 min of baseline data (cardiac output, arterial pressures and MSNA) were recorded with the subject resting quietly. After this baseline period, forearm dose–response trials were performed for noradrenaline at the doses noted above. Each dose–response trial included 2 to 4 min of resting FBF measurement, followed by 2 min infusions of each dose. β-Blockade was then achieved via infusion of propranolol. Once a stable heart rate had been reached (∼5 min) MSNA, arterial pressures, heart rate and cardiac output were again recorded over 5 min. FBF responses to noradrenaline were then repeated.
Data analysis
Data were sampled at 250 Hz and stored on a personal computer for offline analysis. MSNA, heart rate, mean arterial pressure, systolic blood pressure (SBP) and diastolic blood pressure (DBP) were assessed as 5 min averages at the end of the initial baseline period. Sympathetic bursts in the integrated neurogram were identified by a custom-manufactured semiautomated analysis program (Kienbaum et al. 2001; Charkoudian et al. 2005); burst identification was controlled visually by a single investigator. The program then compensated for baroreflex latency and associated each sympathetic burst with the appropriate cardiac cycle.
FBF was determined from the slope of the plethysmographic recording during venous occlusion (Joyner et al. 2001). FBF data are presented as an average of four measurements during the last minute of each dose of drug infusion, when steady-state vasoconstriction was observed. Vasoconstrictor responses were analysed both as absolute change and as the percentages of change in FBF and forearm vascular conductance from baseline. Forearm vascular conductance was calculated as FBF/MAP × 100.
Statistics
Data were analysed statistically using commercially available software (Sigma Stat 2.03, SPSS Inc., Chicago, IL, USA). Group data are expressed as means ± SEM. A multifactorial ANOVA was used to examine whether neural and cardiovascular variables were different in response to β-blockade within and between each group, with a Bonferroni adjustment for pairwise comparisons. To measure whether there was a relationship of baseline MSNA to cardiovascular variables, linear regression analysis was performed and Pearson's correlation coefficients calculated. The α-level was set at 0.05.
Results
Average group differences in neural–haemodynamic variables
Average group haemodynamic and neural variables before and during β-blockade are outlined in Table 2. We did not gain a recording site for MSNA in one postmenopausal woman and we also lost the recording site for MSNA in three men and two young women during β-blockade. Therefore average haemodynamic and neural data reflect that from 14 young men, 15 young women and 14 postmenopausal women. Before β-blockade there was no difference in arterial blood pressures, heart rate and MSNA between young men and women. However, cardiac output and stroke volume were lower in young women vs. young men (P < 0.05). Conversely, TPR was higher in the young women compared to the men (P < 0.05). In the postmenopausal women, SBP, TPR and MSNA were higher compared to young men and women (P < 0.05), whereas stroke volume and cardiac output were lower (P < 0.05). Differences between young men and women were abolished after β-blockade, whereas differences between older women and the young groups persisted after β-blockade.
Table 2.
Average haemodynamic and neural variables at rest and during β-blockade (BB) in young men, young women and postmenopausal (PM) women
| Young men (n = 14) | Young women (n = 15) | PM women (n = 14) | ||||
|---|---|---|---|---|---|---|
| Haemodynamic and neural variables | Pre BB | During BB | Pre BB | During BB | Pre BB | During BB |
| SBP (mmHg) | 125 ± 2 | 126 ± 3 | 130 ± 3 | 130 ± 4 | 143 ± 5†$ | 145 ± 5†$ |
| DBP (mmHg) | 72 ± 2 | 74 ± 2* | 73 ± 2 | 75 ± 2* | 73 ± 2 | 74 ± 2 |
| MAP (mmHg) | 90 ± 2 | 93 ± 2 | 93 ± 2 | 96 ± 2* | 99 ± 3† | 98 ± 2 |
| HR (beats min−1) | 59 ± 2 | 51 ± 2* | 61 ± 3 | 54 ± 3* | 62 ± 2 | 51 ± 2* |
| SV (ml) | 99 ± 3 | 87 ± 6 | 85 ± 4† | 83 ± 4 | 66 ± 2†$ | 68 ± 3†$ |
| CO (l min−1) | 5.9 ± 0.2 | 4.9 ± 0.2* | 5.1 ± 0.2† | 4.4 ± 0.2* | 4.1 ± 0.2 †$ | 3.5 ± 0.2*†$ |
| TPR (mmHg l−1 min−1) | 15.5 ± 0.2 | 19.2 ± 0.8* | 18.8 ± 0.9† | 22.2 ± 1.1* | 25.1 ± 1.3†$ | 29.3 ± 1.8*†$ |
| FBF (ml/100 ml min−1) | 2.31 ± 0.16 | 2.05 ± 0.15* | 2.51 ± 0.23 | 2.00 ± 0.13 | 1.87 ± 0.18 | 1.70 ± 0.14†$ |
| FVC (ml min−1 mmHg−1) | 2.47 ± 0.16 | 2.14 ± 0.17* | 2.63 ± 0.21 | 2.14 ± 0.14 | 1.96 ± 0.19 | 1.69 ± 0.15†$ |
| MSNA (bursts (100 heart beats)−1) | 38 ± 4 | 46 ± 3 | 31 ± 4 | 35 ± 5 | 62 ± 3†$ | 74 ± 5*†$ |
| MSNA (bursts min−1) | 24 ± 3 | 23 ± 2 | 18 ± 2 | 19 ± 2 | 38 ± 2†$ | 37 ± 2†$ |
SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HR, heart rate; SV, stroke volume; CO, cardiac output; TPR, total peripheral resistance, MSNA, muscle sympathetic nerve activity.
P ≤ 0.05 vs. pre β-blockade (i.e. within group)
P ≤ 0.05 vs. young men
P ≤ 0.05 vs. young women.
For example, PM women had a significantly lower CO during β-blockade vs. pre condition. They also had a lower CO vs. young men and women during β-blockade. Mean ± SEM.
During β-blockade, heart rate and cardiac output where reduced, whereas TPR was increased in all groups (P < 0.05). The changes in TPR, HR and cardiac output were not different among groups. Arterial pressures were unchanged by β-blockade in all groups, with the exception of DBP, which increased in young men and women (P < 0.05). MSNA (burst incidence and burst frequency) was unchanged during propranolol infusion in young men and women, but increased in the postmenopausal women (bursts incidence only, P < 0.05).
Forearm blood flow and conductance responses to noradrenaline before and during propranolol
Baseline forearm blood flow and conductance before and during β-blockade
We recorded blood flow response to noradrenaline in all subjects (young men, n = 17; young women, n = 17; postmenopausal women, n = 15). There was no difference in baseline FBF before β-blockade between young men and women (Table 2, P < 0.05). There was also no difference in FVC between young men and women before β-blockade. Resting FBF and FVC were lower in postmenopausal women (P < 0.05) compared to the two younger groups. During β-blockade, baseline FBF was similar among groups (P > 0.05). However, there was a trend towards a lower FVC in older women during β-blockade compared to the two younger groups (P = 0.06).
Changes in forearm blood flow and conductance to noradrenaline before and during β-blockade
All young men had a dose-dependent vasoconstrictor response to noradrenaline before and during β-blockade. In 2 of the 17 young women, no dose-dependent vasoconstriction was observed before β-blockade, but became apparent during β-blockade. In addition, 2 of the 15 postmenopausal women demonstrated an increase in blood flow in response to noradrenaline at all doses of noradrenaline indicating vasodilatation. We analysed FBF and FVC responses in the postmenopausal group both with and without these two outliers, and we present both sets of data.
Figure 1 demonstrates percentage changes in FVC to noradrenaline before and during β-blockade in all groups. In the young men, the percentage reduction in FVC was similar before and during β-blockade at all doses of noradrenaline. As expected, in the young women the percentage reduction in FVC was greater during β-blockade at the last two doses of noradrenaline. Interestingly, the percentage reduction in FVC in postmenopausal women was similar before and during β-blockade at all doses of noradrenaline. This was true whether or not the two outliers were included in the postmenopausal averages. Results are similar when expressed as absolute changes in FVC (Fig. 2). Furthermore, percentage and absolute changes in FBF reflected the differences observed in FVC before and during β-blockade within groups (Table 3).
Figure 1. Percentage change in forearm vascular conductance (FVC) to increasing doses of noradrenaline (NA) in: A, young women (n = 17); B, young men (n = 17), and postmenopausal (PM) women with (C) outliers included (n = 15) and (D) excluded (n = 13).
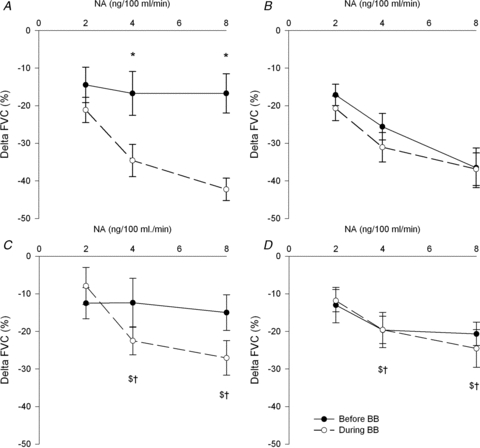
*P < 0.05 vs. before β-blockade, $P < 0.05 vs. young women during β-blockade, †P < 0.05 vs. young men during β-blockade, †P < 0.05 vs. young women before β-blockade and λP < 0.05 vs. before β-blockade young men. All comparisons between groups are vs. the same condition (for example during β-blockade in postmenopausal women vs. during β-blockade in young men). Removal of the PM outliers does not make any difference to this group's response to noradrenaline before and during β-blockade.
Figure 2. Delta forearm vascular conductance (FVC) responses to increasing doses of noradrenaline (NA) before and during β-blockade in: A, young women; B, young men; and postmenopausal (PM) women with outliers included (C, n = 15) and D, outliers excluded (n = 13).
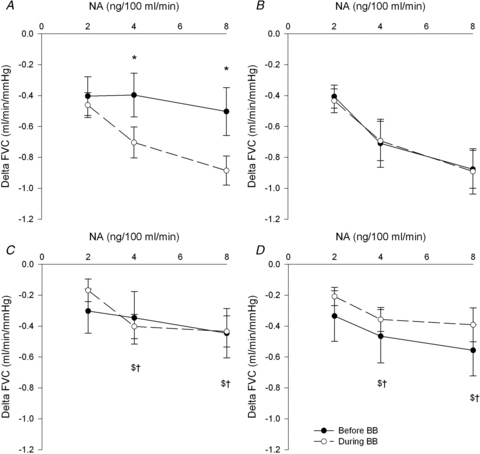
*P < 0.05 vs. before β-blockade, $P < 0.05 vs. young women during β-blockade, †P < 0.05 vs. young men during β-blockade, †P < 0.05 vs. young women before β-blockade. All comparisons between groups are vs. the same condition (for example during β-blockade in postmenopausal (PM) women vs. during β-blockade in young men). Removal of the PM outliers does not make any difference to this group's response to noradrenaline before and during β-blockade.
Table 3.
Absolute and percentage changes in forearm blood flow (FBF) in response to noradrenaline (NA) before and during β-blockade (BB) in young men, young women and postmenopausal (PM) women
| Young men (n = 17) | Young women (n = 17) | PM women (n = 15) | ||||
|---|---|---|---|---|---|---|
| FBF response to NA | Before BB | During BB | Before BB | During BB | Before BB | During BB |
| Absolute ΔFBF (ml min−1 (100 ml)−1) | ||||||
| 2 | −0.42 ± 0.06 | −0.38 ± 0.13 | −0.50 ± 0.12 | −0.43 ± 0.07 | −0.19 ± 0.06 | −0.20 ± 0.06†$ |
| 4 | −0.63 ± 0.12 | −0.62 ± 0.11 | −0.57 ± 0.14 | −0.67 ± 0.08 | −0.33 ± 0.04 | −0.35 ± 0.07†$ |
| 8 | −0.76 ± 0.16$ | −0.77 ± 0.16 | −0.63 ± 0.16 | −0.85 ± 0.08* | −0.41 ± 0.06† | −0.44 ± 0.1†$ |
| %ΔFBF | ||||||
| 2 | −17 ± 3 | −21 ± 3 | −20 ± 5 | −21 ± 3 | −9 ± 2 | −12 ± 3 |
| 4 | −25 ± 7 | −30 ± 4 | −21 ± 3 | −33 ± 3 | −20 ± 2 | −20 ± 5†$ |
| 8 | −37 ± 5$ | −36 ± 5 | −21 ± 3 | −43 ± 3* | −24 ± 3† | −22 ± 5†$ |
Group differences in the vasoconstrictor response to noradrenaline
The percentage reduction in FVC before β-blockade was similar between men and women at 2 and 4 ng (100 ml)−1 min−1 of noradrenaline, but was greater in young men at the final dose of noradrenaline. This difference was abolished during β-blockade. The percentage reduction in FVC to all doses of noradrenaline was lower in postmenopausal women vs. young men before and during β-blockade. The percentage reduction in FVC was greater in young women vs. postmenopausal women during β-blockade at the final two doses of noradrenaline. Again these differences were similar when expressed as absolute FVC (Fig. 2) or absolute and percentage change in FBF (Table 3).
Relationships between neural and haemodynamic variables before and during β-blockade
As previously demonstrated (Narkiewicz et al. 2005; Hart et al. 2009a) there was no relationship of MSNA to MAP before β-blockade among young men and women (Fig. 3), whereas there was a positive relationship between MSNA and MAP in the postmenopausal women (r = 0.55, P < 0.05). Notably, the relationship between MSNA and MAP among young women became positive during β-blockade (r = 0.58, P < 0.05, Fig. 3). Among young men the lack of relationship between MSNA and MAP persisted during β-blockade. During β-blockade, the relationship between MSNA and MAP was no longer significant in postmenopausal women.
Figure 3.
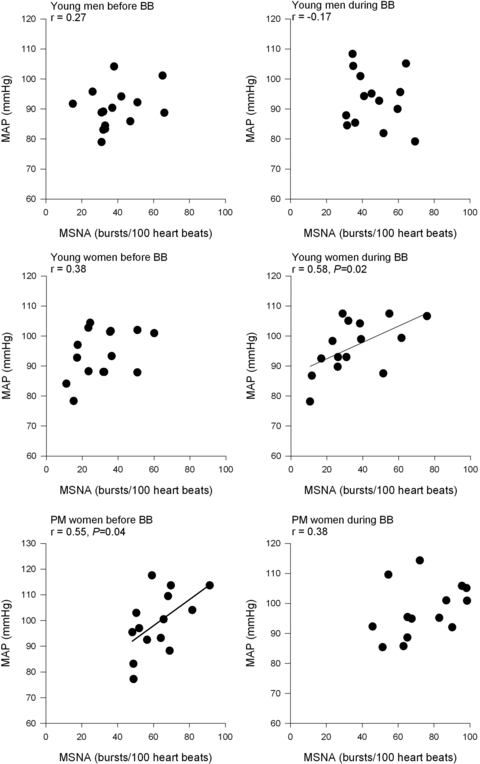
Linear regression analysis of the relationship between muscle sympathetic nerve activity (MSNA) and mean arterial pressure (MAP) in young men (n = 14), young women (n = 15) and postmenopausal (PM) women (n = 15) before and during β-blockade (BB).
As expected, before propranolol, the relationship between MSNA and TPR was positive in young men (r = 0.64, P < 0.05) but was not evident in young women (P > 0.05, Fig. 4). Interestingly, the relationship between MSNA and TPR among postmenopausal women was positive (r = 0.61, P < 0.05). During β-blockade, the relationship between MSNA and TPR became positive in young women (r = 0.59, P < 0.05). The relationship between MSNA and TPR in young men and postmenopausal women remained positive during β-blockade.
Figure 4.
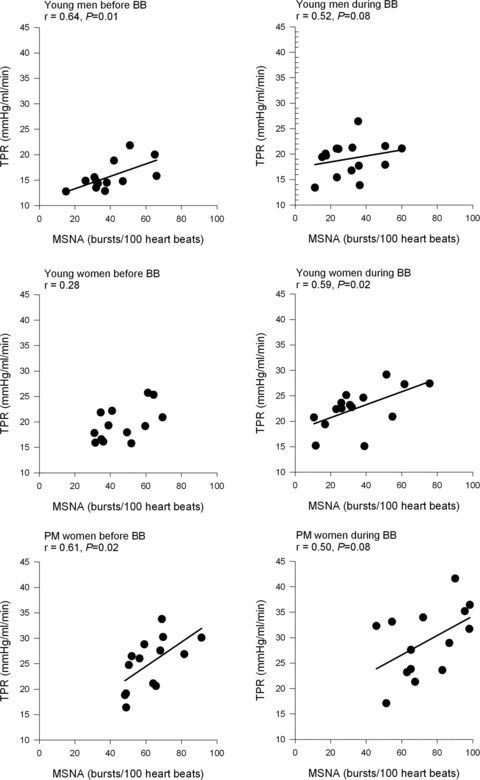
Linear regression analysis of the relationship between muscle sympathetic nerve activity (MSNA) and total peripheral resistance (TPR) in young men, young women and postmenopausal (PM) women before and during β-blockade (BB).
Before β-blockade, there was an inverse relationship between MSNA and cardiac output among young men (r = −0.55, P = 0.04) whereas no such relationship observed in the young women (r = −0.14, P = 0.2). Furthermore, there was no relationship between MSNA and cardiac output among the postmenopausal women (r = −0.39, P = 0.15). Propranolol infusion had no effect on relationships (or lack thereof) between MSNA and cardiac output observed in any group (young men; r = −0.77; P = 0.001, young women; r = −0.23; P = 0.15 and postmenopausal women; −0.43; P = 0.13).
Discussion
The major new findings of this study are: (a) there is a positive relationship of MSNA to TPR and MAP among postmenopausal women, (b) β-adrenergic blockade causes the relationship of MSNA to MAP and TPR to become positive in young women, which is similar to the relationship in postmenopausal women, and (c) the forearm vasoconstrictor responsiveness to noradrenaline is augmented in young women during β-blockade, but not in young men or postmenopausal women. These data suggest that enhanced β-adrenergic vasodilator responsiveness in young women is important in balancing the vasoconstrictor effects of MSNA on arterial pressure. This mechanism appears to be absent in postmenopausal women and young men.
Resting arterial pressure regulation and MSNA in postmenopausal women
In the present and previous studies we have found that MSNA is positively related to TPR but inversely related to cardiac output in young men. Thus, men with high MSNA and high TPR have a lower cardiac output (Charkoudian et al. 2005; Hart et al. 2009a). Consequently, the net effect of resting MSNA on the level of resting arterial pressure is minimal in young men. This is demonstrated by the lack of relationship between MSNA and arterial pressure in young men. Among young women, there is no relationship between MSNA and TPR, thus high (or low) levels of MSNA do not need to be balanced by cardiac output in young women (Hart et al. 2009a). This led us to hypothesize that other key factors are also important in determining vasoconstrictor tone in young women, including important influences of female sex hormones.
In the present study, we report that in postmenopausal women the relationship of MSNA to TPR is positive, but the lack of relationship between MSNA and cardiac output persists. Therefore, there is a positive relationship between MSNA and MAP in postmenopausal women, which has also been reported previously (Matsukawa et al. 1998; Narkiewicz et al. 2005). Consequently, postmenopausal women with high MSNA have a high TPR. In addition, because the vasoconstrictor effects of MSNA are not balanced by cardiac output in postmenopausal women, arterial pressure becomes directly related to MSNA. This is different to what has been observed in older men, where the relationship of MSNA to TPR and cardiac output does not exist (Hart et al. 2009b). Therefore, the effect of ageing on the interaction between the sympathetic nerves and the vasculature appears to differ between men and women. It is possible that other non-sympathetic factors may become more important in maintaining vasoconstrictor tone in older men, such as endothelin 1 and vasopressin. However, there is no scientific evidence to support this as yet. In contrast, in postmenopausal women, the sympathetic nerves may play a more dominant role in maintaining peripheral vasoconstrictor tone. Along these lines, we hypothesised that the female sex hormones (directly or indirectly) modify the transduction of MSNA into vasoconstrictor tone.
Sex differences in forearm vasoconstrictor responses: effect of the β-adrenergic receptors
Earlier studies investigating sex differences in adrenergic vasoconstrictor responsiveness demonstrated that young women demonstrated less forearm vasoconstriction to noradrenaline when compared to young men (Freedman et al. 1987). Kneale et al. (2000) demonstrated that this was due to greater β-adrenergic vasodilatation in young women, which balanced the α-adrenergic vasoconstrictor effect of noradrenaline. Thus, forearm vasoconstrictor responses to noradrenaline were enhanced in young women following β-blockade so their responses became similar to young men.
Our present findings are consistent with those of Kneale et al., and we extend those findings to include implications for changes in β-adrenergic responsiveness with ageing and menopause. In our group of young women, the reduction in FBF during noradrenaline infusion was greater after β-blockade with propranolol. In young men there was no difference in FBF responses to noradrenaline before and after β-blockade. In addition, we report a key new finding: in postmenopausal women, the vasoconstrictor response to noradrenaline was similar before and during β-blockade. This suggests that in postmenopausal women, the ability of the β-adrenergic receptors to offset α-mediated vasoconstriction caused by noradrenaline is minimized or abolished.
Resting blood pressure regulation and the β-adrenergic receptors
Since the reduction in FBF and FVC to noradrenaline was greater after β-blockade in young women, we hypothesized that the β-adrenergic receptors are a key mechanism by which the effect of MSNA on the vasculature is modified. In the present study, we demonstrate for the first time that following β-adrenergic blockade, the relationship between MSNA and TPR became positive in the young women, so that it looked similar to the MSNA–TPR relationship in postmenopausal women (Fig. 4). In addition, MAP became positively related to MSNA during β-blockade in the young women (Fig. 3). Therefore, following β-adrenergic blockade, young women with higher MSNA had higher TPR and MAP. In the young men and postmenopausal women, the relationship between MSNA and TPR was positive, and did not change with β-blockade.
Our data therefore suggest that vascular β-adrenergic receptors (most probably vasodilating β2-receptors) are fundamental to resting arterial pressure regulation in young women. Moreover, as women age the ability of the β-adrenergic receptors to offset the vasoconstrictor effects of MSNA disappears. Thus, TPR becomes related to the level of MSNA (Fig. 4). The loss of ability of the β-receptors to offset the vasoconstrictor effects of resting sympathetic traffic directed to the vasculature, combined with an increase in MSNA in postmenopausal women (Narkiewicz et al. 2005), probably contributes to the marked increase in risk of hypertension that occurs in women after menopause.
Sex hormones and the β-adrenergic receptors
Sherwood et al. (2010) recently demonstrated that cardiac β-adrenergic receptor responsiveness to noradrenaline was reduced in postmenopausal compared to pre-menopausal women. Our data in the peripheral vasculature suggest that β-adrenergic receptor sensitivity to noradrenaline is reduced in postmenopausal women, although we did not assess this directly. There are several possible mechanisms which may lead to modulations in β-adrenergic receptor sensitivity in postmenopausal women. First, the increase in sympathetic nerve activity which occurs with age in women (Narkiewicz et al. 2005) may lead to decreased β-adrenergic sensitivity via agonist-mediated receptor downregulation (or desensitization). However, it is not possible to evaluate from our present data whether increases in sympathetic nerve activity are a result rather than the cause of receptor desensitization.
Second, it is possible that the female sex hormones have a direct effect on the β-adrenergic receptors. In this context, Ferrer et al. (1996) demonstrated that oestrogen supplementation in ovariectomised rats increased isoproterenol (isoprenaline)-mediated relaxation in the rat mesenteric arteries. This response was abolished when propranolol was infused. Furthermore, increased vasodilatation to isoproterenol in the treated rats was not altered when the endothelium was removed, suggesting that oestrogen may enhance β-adrenergic receptor sensitivity through an endothelium-independent mechanism. Whether oestrogen (or other female sex hormones) has a similar direct effect on the vascular β-adrenergic receptors in humans is unknown. In addition to studies focusing on oestrogen, other animal-based experiments indicate that progesterone may increase β2-adrenergic receptor expression in the myometrium (Vivat et al. 1992). Whether progesterone has a similar effect on the expression of β-adrenergic receptors in the vasculature is unknown. Finally, the female sex hormones may enhance β-adrenergic receptor sensitivity in women via an indirect mechanism. It is well known that the female sex hormones increase nitric oxide availability (Sudhir et al. 1996; Miller & Duckles, 2008). Since the β-adrenergic receptors cause vasodilatation partially through a nitric oxide mechanism (Cardillo et al. 1997; Ferro et al. 1999; Jordan et al. 2001; Eisenach et al. 2002), increased nitric oxide availability may increase β-adrenergic-mediated vasodilatation in the peripheral vasculature.
Other observations
We also noted that in postmenopausal women, there was reduced α-adrenergic receptor responsiveness to noradrenaline during β-blockade compared to young men and young women. This suggests that α-adrenergic receptor sensitivity in the forearm is reduced in postmenopausal women. This is similar to that previously reported in older men (Dinenno et al. 2002) where α-adrenergic receptor sensitivity in the forearm was reduced compared to young men. However, this appeared to be specific to the forearm, as Dinenno et al. (2001) indicated that α-adrenergic receptor sensitivity in leg vasculature was preserved in healthy older men. Whether this occurs in postmenopausal women is unknown. It is important to determine whether there is a global decrease in α-adrenergic receptor sensitivity in postmenopausal women since these receptors are fundamental in blood pressure regulation. A decrease in sensitivity to noradrenaline in older women may explain why this group suffers from increased rates of orthostatic intolerance.
Possible mechanisms which may underlie the reduction in α-adrenergic receptor sensitivity include chronic over-stimulation of these receptors. Along these lines MSNA increases as women age, thus the receptors are chronically exposed to high levels of noradrenaline, which typically leads to desensitization, downregulation or changes in receptor subtype expression. However, it should be noted that from these data we cannot make inferences according to whether all or one α-subtype is desensitized (or downregulated) in our group of postmenopausal women.
Limitations
We used a systemic infusion of the non-selective β-antagonist, propranolol, to block the β-adrenergic receptors. Consequently, all β-adrenergic receptors were blocked, including those on the myocardium. Thus, cardiac output was reduced due to the effect of propranolol on heart rate. However, we do not feel this change in cardiac output limited our ability to interpret our data relative to the relationships among MSNA, TPR and arterial pressure in our groups of subjects.
Additionally, we focused on adrenergic vasoconstrictor responsiveness in the forearm before and during β-blockade. Consequently, these findings are only specific to this vascular bed. Whether α-adrenergic receptor responsiveness to noradrenaline is altered during β-blockade in other vascular beds in young and older women needs to be elucidated.
In addition, we used Modelflow analysis to determine stroke volume (and thus cardiac output) from the brachial arterial wave form. It is possible that this analysis may underestimate cardiac output compared to the more accepted thermodilution method in certain experimental conditions (Shibasaki et al. 2011). In our investigation individuals were resting in a normothermic environment. Under these conditions Modelflow-derived cardiac output has been shown to be similar to cardiac output derived from thermodilution. In our previous experiments focusing on resting neural–haemodynamic balance in men and women, we used the open-circuit acetylene approach to measure cardiac output. In the current study we have found similar relationships between MSNA and cardiac output derived from the Modelflow in men and women compared to our previous study where we used the open circuit acetylene method. We did not use the open circuit method to measure cardiac output because the Modelflow technique simplified the logistical elements of this complex experiment.
Perspectives
According to the data presented in this study, the β-adrenergic receptors offset resting sympathetic nerve activity directed towards the peripheral vasculature in young women, and therefore may prevent increases in resistance when resting MSNA increases. However, in older women this phenomenom does not occur. In older women, MSNA is very high vs. young men and women. In addition MSNA becomes related to the resting level of arterial pressure. Thus, the lack of ability for the β-adrenergic receptors to offset vasoconstrictor tone may explain, in part, why the incidence of hypertension increases in this population (Burt et al. 1995). Furthermore, it is possible that since the β-adrenergic receptors do not balance high levels of MSNA, further increases in MSNA due to mental stress, for example, could explain why the risk of cerebrovascular and other cardiovascular-related events increase in ageing women in the UK and USA (Rothwell et al. 2005; Roger et al. 2011).
Conclusions
In summary, we report that the β-adrenergic receptors appear to offset the vasoconstrictor effects of sympathetic nerve activity in young women at rest. This mechanism appears to be minimal or non-existent in young men and in postmenopausal women. Our data therefore suggest that the β-adrenergic receptors play a fundamental role in resting arterial pressure regulation in young women. Furthermore, these changes in the contributions of β-adrenergic receptors with ageing, combined with an increase in MSNA, probably contribute to the marked increase in risk of hypertension that occurs in women after menopause.
Acknowledgments
We are grateful to Christopher Johnson for his technical assistance and to Shelly Roberts, Jean Knutson, Karen Krucker, Shirley Kingsley-Berg, Jessica Sawyer, Casey Hines, Pam Engrav and Nancy Meyer for their assistance in the conduct of the studies. Finally, we thank the subjects for their participation. This study was supported by NIH HL083947 (M.J.J., B.G.W., N.C.) and AHA 070036Z (E.C.H.). This projected was also supported by grant 1 UL1 RR024150 from the National Center for Research Resources (NCRR) and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Additional support came from the Mayo Foundation including a philanthropic gift from the Caywood family and the Mayo Clinic Department of Anaesthesia. The authors have no interests to disclose.
Glossary
Abbreviations
- CO
cardiac output
- FVC
forearm vascular conductance
- MSNA
muscle sympathetic nerve activity
- NA
noradrenaline
- PM
postmenopausal
- TPR
total peripheral resistance
Author's present address
Emma C. Hart, PhD, Department of Physiology and Pharmacology, Medical Sciences Building, University of Bristol, Bristol, BS8 1TD, UK.
Author contributions
E.C.H.; conception, design and completion of study. Data analysis and manuscript write-up. N.C.; conception, design and completion of study. Data collection and mentoring throughout study. B.G.W.; conception and design of study. Manuscript revision. T.B.C.; completion of study and manuscript revision. J.E.; completion of study. M.J.J.; conception, design and completion of study. Manuscript revision and mentoring throughout study.
References
- Bell C, Seals DR, Monroe MB, Day DS, Shapiro LF, Johnson DG, Jones PP. Tonic sympathetic support of metabolic rate is attenuated with age, sedentary lifestyle, and female sex in healthy adults. J Clin Endocrinol Metab. 2001;86:4440–4444. doi: 10.1210/jcem.86.9.7855. [DOI] [PubMed] [Google Scholar]
- Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- Cardillo C, Kilcoyne CM, Quyyumi AA, Cannon RO, 3rd, Panza JA. Decreased vasodilator response to isoproterenol during nitric oxide inhibition in humans. Hypertension. 1997;30:918–921. doi: 10.1161/01.hyp.30.4.918. [DOI] [PubMed] [Google Scholar]
- Carter JR, Ray CA. Sympathetic neural responses to mental stress: responders, nonresponders and sex differences. Am J Physiol Heart Circ Physiol. 2009;296:H847–H853. doi: 10.1152/ajpheart.01234.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charkoudian N, Joyner MJ, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Balance between cardiac output and sympathetic nerve activity in resting humans: role in arterial pressure regulation. J Physiol. 2005;568:315–321. doi: 10.1113/jphysiol.2005.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm postjunctional α-adrenergic vasoconstriction in healthy men. Circulation. 2002;106:1349–1354. doi: 10.1161/01.cir.0000028819.64790.be. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Tanaka H, Stauffer BL, Seals DR. Reductions in basal limb blood flow and vascular conductance with human ageing: role for augmented α-adrenergic vasoconstriction. J Physiol. 2001;536:977–983. doi: 10.1111/j.1469-7793.2001.00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach JH, Clark ES, Charkoudian N, Dinenno FA, Atkinson JL, Fealey RD, Dietz NM, Joyner MJ. Effects of chronic sympathectomy on vascular function in the human forearm. J Appl Physiol. 2002;92:2019–2025. doi: 10.1152/japplphysiol.01025.2001. [DOI] [PubMed] [Google Scholar]
- Ferrer M, Meyer M, Osol G. Estrogen replacement increases β-adrenoceptor-mediated relaxation of rat mesenteric arteries. J Vasc Res. 1996;33:124–131. doi: 10.1159/000159140. [DOI] [PubMed] [Google Scholar]
- Ferro A, Queen LR, Priest RM, Xu B, Ritter JM, Poston L, Ward JP. Activation of nitric oxide synthase by β2-adrenoceptors in human umbilical vein endothelium in vitro. Br J Pharmacol. 1999;126:1872–1880. doi: 10.1038/sj.bjp.0702512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman RR, Sabharwal SC, Desai N. Sex differences in peripheral vascular adrenergic receptors. Circ Res. 1987;61:581–585. doi: 10.1161/01.res.61.4.581. [DOI] [PubMed] [Google Scholar]
- Gracia CR, Sammel MD, Freeman EW, Lin H, Langan E, Kapoor S, Nelson DB. Defining menopause status: creation of a new definition to identify the early changes of the menopausal transition. Menopause. 2005;12:128–135. doi: 10.1097/00042192-200512020-00005. [DOI] [PubMed] [Google Scholar]
- Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex differences in sympathetic neural-hemodynamic balance: implications for human blood pressure regulation. Hypertension. 2009a;53:571–576. doi: 10.1161/HYPERTENSIONAHA.108.126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart EC, Joyner MJ, Wallin BG, Johnson CP, Curry TB, Eisenach JH, Charkoudian N. Age-related differences in the sympathetic-hemodynamic balance in men. Hypertension. 2009b;54:127–133. doi: 10.1161/HYPERTENSIONAHA.109.131417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan J, Tank J, Stoffels M, Franke G, Christensen NJ, Luft FC, Boschmann M. Interaction between β-adrenergic receptor stimulation and nitric oxide release on tissue perfusion and metabolism. J Clin Endocrinol Metab. 2001;86:2803–2810. doi: 10.1210/jcem.86.6.7567. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Dietz NM, Shepherd JT. From Belfast to Mayo and beyond: the use and future of plethysmography to study blood flow in human limbs. J Appl Physiol. 2001;91:2431–2441. doi: 10.1152/jappl.2001.91.6.2431. [DOI] [PubMed] [Google Scholar]
- Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol. 2001;531:861–869. doi: 10.1111/j.1469-7793.2001.0861h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol. 2000;36:1233–1238. doi: 10.1016/s0735-1097(00)00849-4. [DOI] [PubMed] [Google Scholar]
- Matsukawa T, Sugiyama Y, Watanabe T, Kobayashi F, Mano T. Gender difference in age-related changes in muscle sympathetic nerve activity in healthy subjects. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1600–R1604. doi: 10.1152/ajpregu.1998.275.5.R1600. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Haas E, Barton M. Need for research on estrogen receptor function: importance for postmenopausal hormone therapy and atherosclerosis. Gend Med. 2008;5:S19–S33. doi: 10.1016/j.genm.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev. 2008;60:210–241. doi: 10.1124/pr.107.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000;101:862–868. doi: 10.1161/01.cir.101.8.862. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension. 2005;45:522–525. doi: 10.1161/01.HYP.0000160318.46725.46. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics – 2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CE, et al. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study) Lancet. 2005;366:1773–1783. doi: 10.1016/S0140-6736(05)67702-1. [DOI] [PubMed] [Google Scholar]
- Sherwood A, Park SB, Hughes JW, Blumenthal JA, Hinderliter A, Trivedi R, McFetridge-Durdle J. Cardiovascular hemodynamics during stress in premenopausal versus postmenopausal women. Menopause. 2010;17:403–409. doi: 10.1097/gme.0b013e3181b9b061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki M, Wilson TE, Bundgaard-Nielsen M, Seifert T, Secher NH, Crandall CG. Modelflow underestimates cardiac output in heat-stressed individuals. Am J Physiol Regul Integr Comp Physiol. 2011;300:R486–R491. doi: 10.1152/ajpregu.00505.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker JK, Hogeman CS, Khan M, Kimmerly DS, Sinoway LI. Gender affects sympathetic and hemodynamic response to postural stress. Am J Physiol Heart Circ Physiol. 2001;281:H2028–H2035. doi: 10.1152/ajpheart.2001.281.5.H2028. [DOI] [PubMed] [Google Scholar]
- Sudhir K, Jennings GL, Funder JW, Komesaroff PA. Estrogen enhances basal nitric oxide release in the forearm vasculature in perimenopausal women. Hypertension. 1996;28:330–334. doi: 10.1161/01.hyp.28.3.330. [DOI] [PubMed] [Google Scholar]
- Sundlof G, Wallin BG. The variability of muscle nerve sympathetic activity in resting recumbent man. J Physiol. 1977;272:383–397. doi: 10.1113/jphysiol.1977.sp012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundlof G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol. 1978a;274:621–637. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundlof G, Wallin BG. Muscle-nerve sympathetic activity in man. Relationship to blood pressure in resting normo- and hyper-tensive subjects. Clin Sci Mol Med Suppl. 1978b;4:387s–389s. doi: 10.1042/cs055387s. [DOI] [PubMed] [Google Scholar]
- Vivat V, Cohen-Tannoudji J, Revelli JP, Muzzin P, Giacobino JP, Maltier JP, Legrand C. Progesterone transcriptionally regulates the beta 2-adrenergic receptor gene in pregnant rat myometrium. J Biol Chem. 1992;267:7975–7978. [PubMed] [Google Scholar]
- Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol. 1993;74:2566–2573. doi: 10.1152/jappl.1993.74.5.2566. [DOI] [PubMed] [Google Scholar]
- Wiinberg N, Hoegholm A, Christensen HR, Bang LE, Mikkelsen KL, Nielsen PE, et al. 24-h ambulatory blood pressure in 352 normal Danish subjects, related to age and gender. Am J Hypertens. 1995;8:978–986. doi: 10.1016/0895-7061(95)00216-2. [DOI] [PubMed] [Google Scholar]
- Wuest JH, Jr, Dry TJ, Edwards JE. The degree of coronary atherosclerosis in bilaterally oophorectomized women. Circulation. 1953;7:801–809. doi: 10.1161/01.cir.7.6.801. [DOI] [PubMed] [Google Scholar]


