Abstract
Tuberous sclerosis complex (TSC) and cortical dysplasia Type IIB (CDIIB) share histopathologic features that suggest similar epileptogenic mechanisms. This study compared the morphological and electrophysiological properties of cortical cells in tissue from pediatric TSC (n=20) and CDIIB (n=20) patients using whole-cell patch clamp recordings and biocytin staining. Cell types were normal-appearing and dysmorphiccytomegalic pyramidal neurons, interneurons, and giant/balloon cells, including intermediate neuronal-glial cells. In the cortical mantle, giant/balloon cells occurred more frequently in TSC than in CDIIB cases, whereas cytomegalic pyramidal neurons were found more frequently in CDIIB. Cell morphology and membrane properties were similar in TSC and CDIIB cases. Except for giant/balloon and intermediate cells, all neuronal cell types fired action potentials and displayed spontaneous postsynaptic currents. However, the frequency of spontaneous glutamatergic postsynaptic currents in normal pyramidal neurons and interneurons was significantly lower in CDIIB compared with TSC cases and GABAergic activity was higher in all neuronal cell types in CDIIB. Further, acutely dissociated pyramidal neurons displayed higher sensitivity to exogenous application of GABA in CDIIB compared with TSC cases. These results indicate that, in spite of similar histopathologic features and basic cell membrane properties, TSC and CDIIB display differences in the topography of abnormal cells, excitatory and inhibitory synaptic network properties, and GABAA receptor sensitivity. These differences support the notion that the mechanisms of epileptogenesis could differ in patients with TSC and CDIIB. Consequently, pharmacologic therapies should take these findings into consideration.
Introduction
Tuberous sclerosis complex (TSC) is an autosomal dominant disease linked to TSC1 and TSC2 mutations. The proteins encoded by these genes, hamartin and tuberin respectively, are involved in cellular migration, proliferation, and differentiation in multiple organs via a negative regulation of the mTOR pathway (Crino et al., 2006). In the brain, TSC is associated with the presence of cortical and subcortical tubers, as well as subependymal giant cell astrocytomas and nodules. The histopathology of cortical tubers shows severe laminar disorganization and the presence of dysmorphic-cytomegalic neurons and giant cells, suggesting that tubers are areas of malformed cerebral cortex. Epilepsy occurs in up to 85% of TSC patients at some point in their lives (Curatolo et al., 2008; Sparagana and Roach, 2000). Seizures can begin as early as the first day of life, are often associated with infantile spasms, and can be frequently refractory to pharmacologic treatment (Chu-Shore et al 2010).
Focal cortical dysplasia (CD) of Taylor is another malformation of cortical development that is associated with refractory epilepsy (Taylor et al., 1971). It is the most common histopathologic substrate in infants and young children undergoing surgery for the treatment of epilepsy (Lerner et al., 2009; Mathern et al., 1999). According to the new consensus classification of CD (Blumcke et al., 2011), CD Type I (CDI) is characterized by mild cytoarchitectural abnormalities and CD Type II (CDII) by the additional presence of dysmorphic-cytomegalic neurons (CDIIA) and balloon (CDIIB) cells. Giant cells in TSC and balloon cells in CDIIB are morphologically indistinguishable (Blumcke et al., 2011). Hence, CDIIB and TSC share a number of histopathologic similarities (Gumbinger et al., 2009; Mackay et al., 2003; Vinters et al., 1993).
The morphology and electrophysiology of normal and abnormal cells in pediatric CD cases have been examined (Andre et al., 2007; Cepeda et al., 2006; Cepeda et al., 2003; Mathern et al., 2000). However, little is known about the electrophysiological properties of similar cortical cells in tissue from TSC patients (Cepeda et al., 2003; Wang et al., 2007). The aim of the present study was to compare the morphological and electrophysiological properties of normal and abnormal cells in the cerebral cortex of pediatric TSC and CDIIB patients. Based on our previous anatomical studies demonstrating differences in cortical neuronal densities between TSC and CD cases (Chandra et al., 2007) we hypothesized that, despite similar histopathology, there would be differences in network properties when comparing cells from TSC with CDIIB cases. A preliminary report on changes in excitatory synaptic activity based on a smaller cohort of TSC and CD patients and without separation of CDIIA and CDIIB types was published recently (Cepeda et al., 2010).
Methods
The research protocols were approved by the Institutional Review Board of the Human Protection Research Committee at the University of California Los Angeles (UCLA) and informed consent to use the surgically resected tissue for research was obtained through the parents or legal guardians.
Patient cohorts
Consecutive patients with TSC (n=20) who underwent surgery from 1997 to 2009 were included. All patients had cortical tubers as demonstrated by high resolution magnetic resonance imaging (MRI) and Fluoro-2-deoxyglucose positron emission tomography (FDG-PET) evaluations and verified by histopathologic examination. Genetic analysis of brain samples was obtained from 12 patients (Qin et al., 2010). Nine cases had TSC2 mutations, 1 case had a TSC1 mutation, and 2 had no recognizable TSC1 or TSC2 mutation. The cells from TSC cases were compared with those from CDIIB cases (n=20) displaying similar clinical features (Table 1).
Table I.
Clinical Characteristics of TSC and CDIIB Cohorts
| Clinical Variable | TSC (n=20) | CDIIB (n=20) | P value |
|---|---|---|---|
| Age Seizure Onset (Yrs) | 0.3±0.3 | 0.8±1.3 | P=0.080 |
| Age at Surgery (Yrs) | 4.0±2.8 | 2.8±2.5 | P=0.194 |
| Epilepsy Duration (Yrs) | 3.7±2.8 | 2.0±1.6 | P=0.026 |
| History of Infantile Spasms (%) | 75% | 35% | P=0.011 |
| Side of Surgery (% Left) | 45% | 60% | P=0.342 |
| Gender (% Female) | 60% | 55% | P=0.749 |
| Presurgery Seizure Frequency | P=0.072 | ||
| Daily or Greater | 85% | 100% | |
| Weekly | 15% | 0% | |
| Number of AEDs* Presurgery | 2.6±0.7 | 3.1±1.0 | P=0.108 |
| Type of Operation: % | P=0.009 | ||
| Hemispherectomy | 10% | 50% | |
| Multilobar | 30% | 5% | |
| Lobar/Focal | 60% | 45% | |
| Percent Seizure-Free Postsurgery | 75% | 80% | P=0.705 |
| Follow Up Duration (Years) | 2.1±1.1 | 2.1±1.3 | P=0.99 |
| Primary Sample Site: % | P=0.356 | ||
| Frontal | 45% | 65% | |
| Temporal | 30% | 20% | |
| Parietal | 25% | 15% | |
| Total Cells Sampled Per Case | 8.2±4.7 | 6.4±2.3 | P=0.133 |
| Cell Subtypes Per Case | |||
| Normal Pyramids | 3.9±3.1 | 3.4±1.9 | P=0.515 |
| Cytomegalic Neurons | 0.4±0.6 | 1.2±1.6 | P=0.032 |
| Immature Neurons | 0.7±1.0 | 0.3±0.6 | P=0.147 |
| Interneurons | 0.7±0.9 | 0.7±0.7 | P=0.891 |
| Balloon Cells | 2.5±2.3 | 0.7±1.8 | P=0.028 |
| Intermediate Neuronal-Glia | 0.1±0.4 | 0.05±0.2 | P=0.657 |
AEDs = antiepileptic drugs
Pre-surgical evaluation and clinical information
The clinical protocols used to evaluate patients have been described in detail elsewhere (Cepeda et al., 2005a; Mathern et al., 1999). The standardized evaluation included clinical history and neurological examinations, and intericatal and ictal scalp EEG video-recordings. Neuroimaging studies included MRI and FDG-PET (Salamon et al., 2008). Clinical data were abstracted from the medical records and included: age at seizure onset, age at surgery, history of infantile spasms, type of operation (hemispherectomy, multilobar, lobar/focal), side of operation, gender, seizure frequency presurgery (daily or weekly), number of antiepileptic drugs (AEDs) at surgery, seizure freedom and duration of follow-up (Hemb et al., 2010). Epilepsy duration was calculated as the interval in years from age at seizure onset to age at surgery. Also collected was the region of the brain sampled for in vitro cellular electrophysiological studies.
Sample selection and in vitro electrophysiology
Neocortical sample sites were excised for in vitro electrophysiological and histological evaluation based on abnormal neuroimaging and electrocorticography (ECoG) assessments. Tissue samples were classified as most (MA) and least abnormal (LA) according to published criteria (Cepeda et al., 2003). Sample sites (~2 cm3) were removed microsurgically and directly placed in ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM); NaCl 130, NaHCO3 26, KCl 3, MgCl2 5, NaH2PO4 1.25, CaCl2 1.0, glucose 10 (pH 7.2-7.4). Within 5-10 min, slices (350 μm) were cut (Microslicer, DSK Model 1500E or Leica VT1000S) and placed in ACSF for at least 1h (in this solution CaCl2 was increased to 2 mM and MgCl2 was decreased to 2mM). Slices were constantly oxygenated with 95% O2-5% CO2 (pH 7.2-7.4, osmolality 290-300 mOsm, at room temperature). Methodological details for cell visualization and identification have been published elsewhere (Cepeda et al., 2003). Patch electrodes (3-6 MΩ) were filled with (in mM) Cs-methanesulfonate 125, NaCl 4, KCl 3, MgCl2 1, MgATP 5, ethylene glycol-bis (β-aminoethyl ether)-N,N,N’,N’-tetraacetic acid (EGTA) 9, HEPES 8, GTP 1, phosphocreatine 10 and leupeptine 0.1 (pH 7.25-7.3, osmolality 280-290 mOsm) for voltage clamp recordings or K-gluconate 140, HEPES 10 MgCl2 2, CaCl2 0.1, EGTA 1.1, and 2 K2ATP (pH 7.25-7.3, osmolality 280-290 mOsm) for current clamp recordings. Electrodes also contained 0.2% biocytin (Sigma, St. Louis, MO) in the internal solution to label visualized cells. The access resistance ranged from 8-20 MΩ. Liquid junction potentials (~6 mV) were not corrected. After the experiment, the slice was fixed in 10% formaldehyde and processed according to published protocols (Horikawa and Armstrong, 1988).
Cells were initially held at -70 mV in voltage clamp mode. At this holding potential, passive membrane properties in slices were determined by applying a depolarizing step voltage command (10 mV) and using the membrane test function integrated in the pClamp (version 8) software (Axon Instruments, Foster City, CA). This function reports membrane capacitance (in pF), input resistance (in MΩ or GΩ) constant (in ms or μs). After characterizing the basic membrane properties of the cell, spontaneous excitatory (E) and inhibitory (I) postsynaptic currents (PSCs) were recorded for 3 min. The membrane current was filtered at 1 kHz and digitized at 200 μs using Clampex (Axon Instruments). Spontaneous EPSCs were isolated by holding the membrane at -70 mV, which closely corresponds to the resting membrane potential (RMP). At this potential glutamate currents mediated by α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptors are maximized and the γ-aminobutyric acid (GABA) receptor-mediated currents are minimized. In some cases, the GABAA receptor blocker bicuculline (BIC, 10 μM) was added to block residual GABA currents. IPSCs were isolated by holding the membrane at +10 mV. At this potential GABA currents are maximized and glutamate currents are minimized. In some cases, CNQX (10 μM) and AP5 (50 μM) were added to block possible residual glutamate currents. Miniature (m) IPSCs were isolated by addition of TTX (1 μM) to the external solution.
Spontaneous PSCs were analyzed off-line using the Mini Analysis Program (Jaejin Software, Leonia, NJ, USA). The threshold amplitude for the detection of an event was adjusted above root mean square noise level. This software was used to calculate EPSC/IPSC frequency and amplitude for synaptic events, and to construct amplitude-frequency and inter-event interval histograms. Frequencies were expressed as number of events per second (Hz). EPSC kinetic analysis was performed using the Mini Analysis Program.
Dissociated neurons
A detailed description of the methods used to obtain dissociated cells from human tissue samples has been published (Andre et al., 2004). Briefly, a slice was placed in an oxygenated cell-stir chamber (Wheaton, Millville, NJ) and enzymatically treated for 25-30 min with papain (0.625 mg/ml; Calbiochem, La Jolla, CA) at 35°C in a HEPES-buffered Hank's balanced salt solution (Sigma, St. Louis, MO). The tissue was rinsed with a low Ca2+ HEPES-buffered Na-isethionate solution and mechanically dissociated with a graded series of fire-polished Pasteur pipettes. The cell suspension was plated into a Petri dish containing a HEPES-buffered salt solution.Whole-cell voltage clamp recordings were obtained using an internal pipette solution that contained (in mM): 175 N-methyl-D-glucamine (NMDG), 40 HEPES, 2 MgCl2, 10 EGTA, 12 phosphocreatine, 2 Na2ATP, 0.2 Na2GTP, and 0.1 leupeptin. The Mg2+-free external solution consisted of (in mM): 127 NaCl, 20 CsCl, 2 CaCl2, 5 BaCl2, 10 glucose, 10 HEPES, and 0.0003 TTX. Signals were detected with an Axopatch 200A amplifier (Axon Instruments, Foster City, CA). Drugs were applied through capillaries positioned 400-500 μm from the cell using a pressure-driven fast perfusion system (SF-77B; Warner Instruments, Hamden, CT) synchronized by pClamp. GABA (Sigma, St. Louis, MO) was applied for 3 sec. An interval of 60 sec between each application was adequate to avoid GABA current run down.
Data analysis
Values are presented as mean ± standard deviation (SD) or standard error of the mean (SEM). Differences were considered statistically significant if p<0.05. Statistical differences between groups were examined using Student's t or Mann-Whitney Rank Sum tests, appropriate one-way or two-way ANOVAs followed by Bonferroni post hoc tests, and Kolmogorov-Smirnov (K-S) for distributions using the Sigmastat or Statview (SAS, Cary, NC) software. For dissociated neurons analyses were performed with Origin (Microcal Software, Northampton, MA) and pClamp software. Membrane properties and GABA current characteristics were compared by a one-way or two-way ANOVA followed by Fisher's PLSD tests. For EC50 measurements, currents induced by increasing concentrations of GABA were plotted semi-logarithmically, and fitted with the Hill equation. The comparison between clinical and experimental data was performed using ANOVA, t-tests, Chi-square, or linear correlations where appropriate (cf. Hemb et al., 2010).
Results
TSC and CDIIB cohorts and clinical findings
The TSC pediatric population comprised 20 cases (12 females) ranging in age from 1.1 to 10.1 yr. All TSC cases had one or more cortical tubers by MRI (Fig. 1A, left panel). Generalized and partial seizures occurred in all but one case that presented with complex partial seizures, and infantile spasms occurred in 15 cases (75%). The CDIIB group comprised 20 cases (11 females) ranging in age from 0.2 to 9.2 yr. Generalized and partial seizures were seen in all cases and a history of infantile spasms was documented in 7 patients (35%). All CDIIB patients had positive neuroimaging involving cortical malformations (Fig. 1A, right panel).
Fig. 1.
A. Representative axial MRI scans of children with refractory epilepsy from TSC (left) and CDIIB (right). Both children were 7 yr of age at the time of surgery. There are multiple cortical tubers (dashed circles) in the patient with TSC and one focal area of cortical dysplasia in the left temporal lobe in the child with CDIIB. In the TSC case the left frontal tuber was removed and both children are seizure free post-surgery. B. Left panels: Section of a tuber (originating from a 19 month-old male) showing abundant giant cells and disorganized collections of dysmorphic neurons. Arrows in left panel indicate giant cells in which the nucleus shows coarse chromatin, a pattern often seen in astrocytes. Right panels: Representative fields from a resection (originating from a 9 year-old female) showing features of CD type IIB. Note a balloon cell (arrow, left panel) with glassy eosinophilic cytoplasm, and dysmorphic neurons (arrows, right panel). The balloon cell shows ‘retraction’ of cytoplasm from the neuropil (images are from sections stained with H&E). Scale bar represents 50 μm and applies to all panels.
Most of the clinical characteristics were similar between TSC and CDIIB groups (Table I). There were no significant differences in age at seizure onset, age at surgery, gender, side of operation, seizure frequency before surgery, number of AEDs at the time of surgery, percent of patients seizure-free after surgery, and duration of follow-up (p>0.07). Epilepsy duration was longer (p=0.026) and the frequency of infantile spasms was higher (p=0.011) in the TSC compared with the CDIIB group. By neuroimaging, 4 patients had hemimegalencephaly, of which 1 was in the TSC and 3 were in the CDIIB group (p=0.373). This association, although rare, is not unprecedented (Guerra et al., 2007). More patients with CDIIB underwent hemispherectomy compared to the TSC group (p=0.009). Seizure freedom was obtained in 77.5% of this combined cohort without surgical mortality.
At the time of surgery all patients were taking AEDs, and a total of 13 AEDs were used in mono- (n=2) and poly-therapy (n=38). In descending order, the most common AEDs were; topiramate (n=16; 40%), levetiracetam (n=13; 32.5%), carbamazepine (n=13; 32.5%), lamotrigine (n=12; 30%), phenobarbital (n=9; 22.5%), zonisamide (n=8; 20%), vigabatrin (n=7; 17.5%), and clorazepate (n=6; 15%). Comparing patients with TSC and CDIIB, there were no differences in patients taking a particular AED (p>0.08, Chi-square) with one exception. At the time of surgery, more patients with TSC were taking vigabatrin (n=7) compared to those with CDIIB (n=0, p=0.004). However, it should be noted that only 35% of patients with TSC were taking vigabatrin at the time of surgery.
Tissue samples and principal pathologic findings
In the 20 TSC patients, a total of 30 cortical tissue samples were examined (16 from the right side). They were from the frontal (n=9), temporal (n=10), and parietal (n=11) regions. The majority of samples were classified as MA (n=25) and contained tissue from the tuber. Fewer samples (n=5) were classified as LA, and were at the edge of the tuber and part of the planned resection. All children had confirmed tubers upon histopathologic examination using Haematoxylin & Eosin, Nissl and Bielschowsky staining (Fig. 1B, left panels). The main findings included cortical dyslamination, pyramidal neuron misorientation, and the presence of dysmorphic-cytomegalic neurons. Cell clustering and heterotopic neurons were also a common finding. Giant cells with pale, glassy, eosinophilic cytoplasm were found in all cases, mostly in white matter but in many cases also extending to the cortical mantle. Astrogliosis and microcalcifications were observed in some TSC cases.
In the 20 CDIIB patients, 29 cortical tissue samples were examined (11 from the right side). The majority of samples were classified as MA (n=21) and a few samples (n=8) were classified as LA. They were from the frontal (n=16), temporal (n=7), and parietal (n=6) regions. There were no differences in sampled regions between the TSC and CDIIB groups (p=0.356, Chi-square, Table I). Similar to TSC cases, the histopathology of CDIIB cases showed severe architectural abnormalities, neuronal disarray and abnormal polarity, neuronal cytomegaly and balloon-type gemistocytic astrocytes restricted in three cases to the white matter (Fig. 1B, right panels).
Morphology of individual cells in TSC and CDIIB
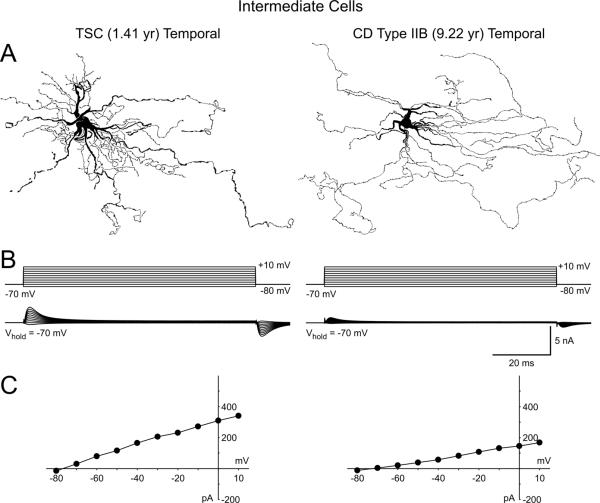
A total of 156 cells were recorded electrophysiologically from TSC tissue and 65% were successfully labeled with biocytin (Fig. 2). Based on previously published criteria (Cepeda et al., 2003) cells were classified, in descending order of frequency, as normal-appearing pyramidal neurons (n=87, including 13 immature-looking cells), giant cells (n=47), interneurons (n=14) and cytomegalic pyramidal neurons (n=8). As expected, the average area of cytomegalic pyramidal neurons and giant cells was larger than the somatic area of normal-appearing pyramidal neurons (p<0.05, Table II). The somata of most giant cells were round but others were elongated. They did not have axons and their processes (7-20) had a wavy, hair-like appearance (Supplementary Fig. 1). Overall these cells resembled enlarged astrocytes (Sosunov et al., 2008). However, three cells looked more neuronal-like with somata similar to those of pyramidal neurons but whose processes appeared like those of typical giant/balloon cells (Fig. 4A). We identified these as intermediate neuronal-glial cells (cf. Blumcke et al., 2011; Talos et al., 2008).
Fig. 2.
Morphology and electrophysiology of TSC cell types revealed by biocytin labeling and electrophysiology using whole-cell patch clamp recordings in current clamp mode. Left panels are examples of biocytin-filled normal pyramidal (A), cytomegalic (B), interneuron (C), and giant (D) cells. Calibration bar in C also applies to panels A and B. Middle panels illustrate hyperpolarizing and depolarizing voltage deflections induced by negative and positive current pulses. Note the large amplitude action potential after-hyperpolarization in the interneuron (arrow), and the strong rectification in the depolarizing direction in the giant cell (arrow). No action potentials could be evoked in giant cells despite high depolarizing current intensity. Right panels show the current-voltage relationships for each cell.
Table II.
| Cell Membrane Properties in Slices | |||||
|---|---|---|---|---|---|
| Cm (pF) | Ri (MΩ) | Tau (ms) | RMP (mV) | Somatic Area (μm2) | |
| Pyramidal | |||||
| TSC (n=87) | 132±7 | 284±48 | 2.3±0.2 | -65±2 | 176±10 |
| CDIIB (n=74) | 152±9 | 202±23 | 2.5±0.1 | -62±2 | 219±20 |
| Cytomegalic | |||||
| TSC (n=8) | 309±21 | 51±2 | 4.8±0.3 | -69±1 | 520±163 |
| CDIIB (n=25) | 353±14 | 58±6 | 4.5±0.2 | -65±2 | 380±33 |
| Interneuron | |||||
| TSC (n=14) | 73±14 | 329±106 | 0.9±0.1 | -63±1 | 187±24 |
| CDIIB (n=14) | 59±9 | 523±119 | 1.1±0.3 | -58±5 | 207±42 |
| Balloon | |||||
| TSC (n=47) | 244±16 | 210±46 | 3.2±0.3 | -74±3 | 445±48 |
| CDIIB (n=16) | 251±25 | 148±28 | 2.9±0.4 | NM | 339±45 |
| Membrane Properties of Dissociated Pyramidal Neurons | |||
|---|---|---|---|
| Cm (pF) | Ri (GΩ) | Tau (μs) | |
| Pyramidal | |||
| TSC (n=15) | 25.3±1.8 | 1.4±0.2 | 333±52 |
| CDIIB (n=13) | 28.5±2.5 | 1.1±0.2 | 294±30 |
NM. Not Measured.
Data are presented as mean ± SEM.
Fig. 4.
A. Camera lucida drawings from biocytin-filled cells with hybrid or neuronal-glial morphology from a TSC and a CDIIB case. Under IR-DIC the somata of these cells looked similar to that of pyramidal neurons. Only the lack of inward currents during electrophysiological recordings and the absence of spines and axons after biocytin processing allowed classification of these cells in the intermediate cell type category. B. Traces show that even highly depolarized voltage steps were unable to induce Na+ and Ca2+ inward currents. Due to the large size of these cells, the capacitive artifacts at the onset and offset of the step voltage commands could not be compensated completely. C. The graphs are current-voltage relationships indicating only an incipient outward current at depolarized potentials.
In the CDIIB cohort we recorded 129 cells and, as in TSC, the same types of cells were found (Fig. 3) but with different frequency. Sampled cells were normal-appearing pyramidal neurons (n=74, including 6 immature-looking pyramidal cells), cytomegalic pyramidal neurons (n=25), balloon cells (n=16, including one intermediate neuronal-glial cell), and interneurons (n=14). Thus, in our experimental conditions, we observed more giant cells in TSC than balloon cells in CDIIB samples (p=0.028), whereas more dysmorphic-cytomegalic neurons were observed in CDIIB than in TSC samples (p=0.032; Table 1). There were no differences in somatic areas and overall morphologic characteristics between cell types from TSC and CDIIB samples based on biocytin measurements.
Fig. 3.
Morphology and electrophysiology of CDIIB cell types revealed by biocytin labeling and whole-cell patch clamp recordings in current clamp mode. Left panels are examples of biocytin-filled normal pyramidal (A), cytomegalic (B), interneuron (C), and balloon (D) cells. Calibration in D applies to all panels. Middle panels illustrate hyperpolarizing and depolarizing voltage deflections induced by negative and positive current pulses. Note large amplitude action potential after-hyperpolarizations in the interneuron (arrow). No action potentials could be evoked in balloon cells despite high positive current intensity. Right panels show the current-voltage relationships for each cell. The properties by cell type are similar to those from TSC cases (Fig. 2).
Passive and active membrane properties of normal and abnormal cell types in TSC and CDIIB
Although there were differences in membrane capacitance, input resistance and time constant among cell types within TSC or CDIIB tissue (p<0.001, one-way ANOVA), these properties did not differ when comparing the same cell types between TSC and CDIIB tissue (Table II). In both TSC and CDIIB, interneurons had the smallest membrane capacitance and highest input resistance, whereas cytomegalic pyramidal neurons had the largest capacitance and lowest input resistance. Giant/balloon cells also had very large capacitance but their input resistance was variable, with some cells having very low (~20 MΩ) and others very high (~1 GΩ) values. This variability may reflect morphological differences and the predominance of a glial (low input resistance) over a neuronal-like (high input resistance) phenotype (Talos et al., 2008).
The RMP of normal-appearing pyramidal neurons and interneurons was similar, whereas cytomegalic pyramidal neurons and giant/balloon cells were more hyperpolarized (Table II). Action potentials could be evoked in all neurons but not in giant/balloon cells. The current-voltage (IV) relationships were fairly typical for normal-appearing and cytomegalic pyramidal neurons, with inward rectification in the hyperpolarizing direction. Giant/balloon cells displayed an almost linear relationship in the hyperpolarizing direction but in the depolarizing direction they showed strong rectification, probably caused by the opening of delayed rectifier K+ channels (Fig. 2, arrow).
In normal-appearing and cytomegalic pyramidal neurons repetitive action potentials were readily elicited once the threshold for action potential firing was reached. In interneurons the action potential duration was generally shorter than in normal-appearing pyramidal neurons and the amplitude of the action potential afterhyperpolarization was larger (Fig. 2, arrow). Action potentials could never be elicited in balloon cells, even at highly depolarized membrane potentials. There were no significant differences in resting membrane potentials, firing properties, or current-voltage relationships of normal pyramidal neurons from TSC and CDIIB cases (Figs. 2-3).
In voltage clamp recordings, normal-appearing and cytomegalic pyramidal neurons had prominent inward currents including persistent and fast Na+ currents, as well as transient and/or slowly inactivating Ca2+ currents. Most interneurons had negligible Ca2+ currents and fast-firing interneurons displayed repetitive Na+ currents (not shown). Giant/balloon and intermediate cells (Fig. 4B and C) did not display inward currents. In contrast, small outward currents were commonly observed in these cells.
Spontaneous glutamatergic currents in TSC and CDIIB
Except for the giant cells, all neurons from TSC cases displayed spontaneous EPSCs. Normal-appearing pyramidal neurons and interneurons displayed the highest frequencies of spontaneous EPSCs (average 2.95±0.5 and 4.6±0.9 Hz, respectively), whereas cytomegalic pyramidal neurons generally displayed low frequencies (average 1.8±0.5 Hz). The frequency of spontaneous EPSCs was significantly higher in normal-appearing pyramidal neurons from TSC compared with CDIIB cases (p=0.029, Mann-Whitney Rank Sum test) (Fig. 5A and 5B inset). Amplitude-frequency histograms showed that increased frequencies occurred at most amplitude bins, encompassing low-, medium- and large-amplitudes (Fig. 5B). Cumulative inter-event interval (the reciprocal of frequency) probability distributions between normal pyramidal neurons from TSC and CDIIB cases also were different (p<0.05, K-S, Fig. 5C). In contrast, cumulative amplitude probability histograms were similar, suggesting differences were probably due to presynaptic mechanisms.
Fig. 5.
A. Spontaneous EPSC activity recorded in voltage clamp mode at a holding potential of -70 mV (mostly glutamatergic) in different cell types from TSC and CDIIB tissue samples. Normal pyramidal neurons and interneurons from TSC cases displayed higher frequencies than cytomegalic pyramidal neurons. In comparison, synaptic activity was low in the same cell types from CDIIB cases. Giant/balloon cells from TSC and CDIIB had no spontaneous synaptic activity. B. Amplitude-frequency histograms of synaptic glutamate activity recorded in normal and cytomegalic pyramidal neurons and interneurons from TSC and CDIIB samples. Across amplitude bins, the frequency of spontaneous events was consistently higher in cells from TSC cases. The difference in the lower amplitude bins was significant. Insets show the average frequencies of the 3 types of cells in TSC and CDIIB. The frequencies were significantly higher in normal pyramidal neurons and interneurons from TSC samples. C. Cumulative inter-event interval histograms comparing the probability distributions in normal pyramidal, cytomegalic and interneurons from TSC and CDIIB samples. (In all panels asterisks indicate statistically significant differences of at least p<0.05).
The EPSC frequency of interneurons from TSC was significantly higher compared to interneurons from CDIIB (p=0.012, Student's t-test). Amplitude-frequency histograms demonstrated that differences occurred across most amplitude bins and cumulative inter-event interval distributions were significantly different (p<0.0001, K-S). Although the average frequency of spontaneous EPSCs in cytomegalic neurons from TSC and CDIIB was not different (p=0.995) due to the low number of cytomegalic pyramidal neurons sampled from TSC cases, the cumulative inter-event interval probability distributions were different (p=0.001, K-S). The kinetics of spontaneous synaptic events, including rise time, decay time and half-amplitude duration, of normal and cytomegalic pyramidal neurons and interneurons were similar between TSC and CDIIB groups (Table III).
Table III.
| Kinetics of Spontaneous EPSCs | ||||
|---|---|---|---|---|
| Rise (ms) | Decay (ms) | Half-width (ms) | Amplitude (pA) | |
| TSC | ||||
| Pyramidal | 1.7±0.1 | 7.4±0.3 | 8.2±0.3 | 10.1±0.4 |
| Cytomegalic | 2.9±0.7 | 9.4±1.9 | 9.3±1.9 | 11.8±1.1 |
| Interneuron | 2.2±0.8 | 5.8±1.3 | 7.7±2.2 | 11.6±3.4 |
| CDIIB | ||||
| Pyramidal | 1.9±0.2 | 7.7±0.4 | 9.3±0.5 | 10.6±0.4 |
| Cytomegalic | 2.5±0.3 | 10.8±1.3 | 12.5±1.3 | 12.3±1.2 |
| Interneuron | 1.5±0.5 | 7.6±2.7 | 8.3±2.7 | 12.6±1.5 |
| Kinetics of Spontaneous IPSCs | ||||
|---|---|---|---|---|
| Rise (ms) | Decay (ms) | Half-width (ms) | Amplitude (pA) | |
| TSC | ||||
| Pyramidal | 2.8±0.3 | 19.0±2.0 | 18.7±1.5 | 23.5±1.1 |
| Cytomegalic | 3.8±0.3 | 31.5±9.7 | 21.9±1.7 | 25.8±4.5 |
| Interneuron | 0.8±0.2 | 6.9±1.0 | 6.6±0.8 | 20.5±1.4 |
| CDIIB | ||||
| Pyramidal | 2.8±0.2 | 23.9±3.1 | 20.5±1.2 | 26.2±1.5 |
| Cytomegalic | 3.8±0.1 | 35.3±12.0 | 20.2±0.4 | 26.9±1.0 |
| Interneuron | 1.0±0.3 | 7.4±2.4 | 7.8±2.2 | 20.0±2.7 |
Data are presented as mean ± SEM.
Spontaneous GABAergic currents in TSC and CDIIB
GABAA receptor-mediated spontaneous currents were relatively abundant in all neuronal types from TSC cases (normal pyramidal 8.0±1.3 Hz, cytomegalic 10.8±3.6 Hz, and interneurons 6.7±0.6 Hz) (Fig. 6A). As expected, giant/balloon cells displayed no spontaneous GABA synaptic activity. The average frequency of spontaneous IPSCs in normal-appearing pyramidal neurons from CDIIB cases was higher than in cells from TSC cases (p=0.018, Mann-Whitney Rank Sum test). Amplitude-frequency histograms showed that increased frequencies occurred at most amplitude bins, but were more evident in the larger amplitude bins (>25 pA) and were significantly different for events >50 pA (Fig. 6B). Similarly, the average IPSC frequencies in cytomegalic pyramidal neurons and interneurons from CDIIB tended to be higher than in TSC samples, but because of the low numbers of cells sampled and high variability between cells the differences did not reach statistical significance. Reflecting increased frequencies, cumulative inter-event interval probability distributions demonstrated a significant leftward shift in pyramidal neurons (p<0.0001, K-S) and interneurons (p=0.0152, K-S), but only marginal in cytomegalic pyramidal neurons (p=0.0699), from CDIIB compared to TSC cases (Fig. 6C). In contrast, cumulative amplitude probability histograms were similar (not shown). Finally, the kinetic properties of spontaneous IPSCs were not different in cells from TSC and CDIIB cases although there was a trend for normal and cytomegalic pyramidal neurons from CDIIB cases to display slower decay times (Table III).
Fig. 6.
A. Spontaneous synaptic activity recorded in voltage clamp mode at a holding potential of +10 mV (mostly GABAergic) in different cell types from TSC and CDIIB tissue samples. In contrast to glutamatergic activity, the frequencies of spontaneous GABAergic currents were higher in CDIIB compared to TSC cases, particularly in normal pyramidal neurons and interneurons. B. Amplitude-frequency histograms of synaptic GABAergic activity recorded in normal and cytomegalic pyramidal neurons and interneurons from TSC and CDIIB samples. Across amplitude bins, the frequency of spontaneous events was consistently higher in cells from CDIIB cases. The difference in amplitude bins >50 was significant (p<0.01) in the pyramidal neuron group. Insets show the average frequencies of the 3 types of cells in TSC and CDIIB. The frequencies were higher in normal pyramidal neurons from CDIIB samples (p=0.018). Although a similar trend was seen for cytomegalic pyramidal neurons and interneurons, the low number of cells precluded reaching statistical significance. C. Cumulative inter-event interval histograms comparing the probability distributions in normal pyramidal, cytomegalic and interneurons from TSC and CDIIB samples.
In a few normal pyramidal cells from TSC (n=4) and CDIIB (n=4) cases mIPSCs were isolated by addition of TTX to the external solution (not shown). The difference in average frequency between both groups remained after TTX (TSC 1.5±0.3 Hz and CDIIB 2.6±0.4 Hz, p<0.05). Cumulative inter-event interval probability distributions were also different (p<0.0001, K-S, not shown). In contrast, the cumulative amplitude probability histogram and kinetics were similar, suggesting the involvement of presynaptic mechanisms (Redman, 1990).
GABA-glutamate synaptic ratio
In the subset of cases where the frequency of glutamate and GABA synaptic activities could be measured in the same cell, an index ratio was obtained by dividing the average GABA frequency by the average glutamate frequency for each patient (TSC, n=7 and CDIIB, n=9). This analysis included 56 cells from TSC (50 normal pyramidal, 2 cytomegalic, and 4 interneurons) and 72 cells from CDIIB (48 normal pyramidal, 13 cytomegalic, and 11 interneurons). The average GABA-glutamate ratio in TSC cases was lower (4.2±0.9) than in CDIIB cases (10.2±0.8, p<0.001) confirming that, relative to glutamate, GABA synaptic activity is higher in CDIIB compared with TSC cases.
GABAA receptor function in dissociated normal-appearing pyramidal neurons from TSC and CDIIB cases
To examine postsynaptic GABAA receptor sensitivity in TSC and CDIIB cases we used the isolated cell preparation. After recording the membrane properties, GABA was exogenously applied to normal-appearing pyramidal neurons (n=15, from 5 TSC cases and n=13, from 5 CDIIB cases). In all cells a clear apical dendrite and some basilar dendrites were present (Fig. 7A, left panels). No differences in capacitance, input resistance or time constant were found between cells from TSC and CDIIB cases (Table II). Application of increasing concentrations of GABA induced typical outward currents (Vhold=-40 mV) with a fast peak followed by a rapidly desensitizing current more evident at the higher concentrations (Fig. 7A, right panel). Peak current amplitudes and current densities at different GABA concentrations were larger in CDIIB compared with TSC pyramidal neurons at all concentrations tested (1, 10, 100 and 1000 μM) (Fig. 7B). Furthermore, the EC50 was significantly lower in cells from CDIIB (17.6±2.2 μM) compared with TSC cases (30.3±4.5 μM), indicating increased GABAA receptor sensitivity in CDIIB pyramidal neurons (Fig. 7C).
Fig. 7.
A. Responses to exogenous application of GABA in dissociated normal pyramidal neurons from TSC and CDIIB samples. Top left panels are examples of pyramidal neurons from a TSC and a CDIIB case. Traces on the right are outward currents induced by increasing concentrations of GABA at a holding potential of -40 mV. At the higher concentrations, the response consisted of a fast peak followed by a rapidly desensitizing current. B. Average peak currents and current densities at increasing concentrations of GABA. At all concentrations the response was larger in cells from CDIIB compared to TSC samples. C. In addition, the EC50 was significantly reduced in cells from CDIIB samples (right bar graph), indicating higher sensitivity of GABA receptors.
Correlation with Clinical Data
Clinical variables that differed between TSC and CDIIB cases, including epilepsy duration, history of infantile spasms and use of vigabatrin (Table 1), were compared with glutamate and GABA synaptic activities and GABAA receptor sensitivity. Results showed that none of these clinical variables was associated with differences in synaptic activity or GABAA receptor sensitivity (p>0.08). In other words, the observed differences were not confounded by identified clinical variables, including use of AEDs, between TSC and CDIIB cases.
Discussion
This study found that, despite similarities in cell type, morphology, and basic membrane properties, there are differences in cell distribution and network connectivity between TSC and CDIIB patients. Within the cortex, giant/balloon cells were more frequently sampled in TSC compared with CDIIB, whereas dysmorphic-cytomegalic neurons were more common in CDIIB. Glutamatergic synaptic activity was lower and GABAergic synaptic activity was higher in CDIIB compared with TSC cases and, in the dissociated cell preparation, normal pyramidal neurons had more robust responses to exogenous GABA application in CDIIB. We also characterized a small number of intermediate neuronal-glial cells in TSC and CDIIB cases and found that they were similar to giant/balloon cells as they lack the ability to generate action potentials. These results were not associated with different clinical variables, including use of AEDs, supporting the idea that the findings were intrinsic to cell and network properties of patients with TSC and CDIIB. Collectively, these results support that notion that, comparing TSC and CDIIB patients, there are unique cell and network properties that should be taken into consideration when contemplating mechanisms of epileptogenesis and possible pharmacologic treatment for these two epilepsy syndromes.
Cell morphology, distribution, and membrane properties
Similar histopathology and cell types have been described in cortical tissue from CDIIB and TSC cases (Andre et al., 2007; Cepeda et al., 2003; Sisodiya et al., 2009), but no electrophysiological studies had directly compared cells from these two etiologies. In both TSC and CDIIB normal-appearing pyramidal neurons were the predominant cell type and there were no differences in the frequency of occurrence of interneurons and immature cells. In contrast, balloon cells were more difficult to find in CDIIB, probably because they tend to cluster in the white matter, and dysmorphic-cytomegalic neurons were found more frequently. This observation suggests that the developmental migration patterns and final destination of abnormal dysmorphic-cytomegalic neurons and giant/balloon cells are different between TSC and CDIIB cases. This topographic variation could lead to differential synaptic arrangements and mechanisms of epileptogenesis. This observation is supported by our previous anatomical findings that neuronal density in superficial cortical layers and white matter is higher in CDIIB compared with TSC, whereas neuronal density in deep cortical layers is decreased in TSC (Chandra et al., 2007).
Balloon cells in CDIIB and giant cells in TSC have been difficult to classify due to their neuronal-glial morphology. Immunocytochemical studies have shown that giant/balloon cells express neuronal and glial markers alone or in combination (Englund et al., 2005; Farrell et al., 1992; Mathern et al., 2000), as well as a wide variety of embryological markers (Crino et al., 1996; Fukutani et al., 1992; Hirose et al., 1995; Thom et al., 2005; Ying et al., 2005). Anatomical studies first suggested that balloon cells were undifferentiated, mimicking features of neuroembryonic development (Huttenlocher and Heydemann, 1984; Machado-Salas, 1984; Probst and Ohnacker, 1977; Trombley and Mirra, 1981). Electrophysiological recordings demonstrated that giant/balloon and neuronal-glial cells from TSC and CDIIB are unable to generate action potentials, (Cepeda et al., 2005b; Cepeda et al., 2003), suggesting their role in epileptogenesis is probably minor (Boonyapisit et al., 2003). In contrast, dysmorphic-cytomegalic pyramidal neurons, both in TSC and CDIIB, have the potential to sustain epileptiform activity due to their hyperexcitability (Cepeda et al., 2005b). This differential contribution is supported by studies demonstrating cell-specific alterations in glutamate and GABA receptor subunit gene transcription and protein expression in dysmorphic neurons and giant cells in TSC (Crino et al., 2001; Talos et al., 2008; White et al., 2001).
Synaptic activity and GABAA receptor sensitivity
At the circuit level, this study demonstrated that glutamatergic tone was lower in CDIIB compared with TSC cases, while the GABAergic tone was higher in CDIIB. In slices from CDIIB tissue the frequency and sometimes the amplitude of spontaneous IPSCs were increased and, in dissociated normal pyramidal neurons, the sensitivity of GABAA receptors was higher. Thus, in pediatric CDIIB cases cortical cellular hyperexcitability cannot be due to decreased GABAergic inputs or GABAA receptor sensitivity alone. If there is reduced glutamate activity in CDIIB, one possibility is that, because of the relative immaturity of neuronal circuits, GABA may act as an excitatory neurotransmitter (Cepeda et al., 2007; Cherubini et al., 1991). The cause of cortical hyperexcitability in TSC cases is more difficult to grasp as we found no clear decreases in IPSC or increases in EPSC frequencies (cf. Cepeda et al., 2005a). A study in one patient with a TSC2 mutation but no recognizable tubers reported increased excitation after pharmacological blockade of GABAA receptors (Wang et al., 2007), supporting the hypothesis that inhibitory circuits are relatively intact in TSC. However, this manipulation is likely to increase cortical excitability even in non-pathologic tissue. Further, the frequency of spontaneous EPSCs was not reported, precluding a direct comparison with the present data.
What could be the etiology of increased GABAA receptor function in CDIIB compared with TSC? In terms of spontaneous synaptic activity, higher frequency of IPSCs and lower frequency of EPSCs are reminiscent of immature circuits and support the dysmature cerebral developmental hypothesis of severe CD (Cepeda et al., 2006). While on average the TSC cohort was slightly younger, the age difference was not significant between groups, and age at surgery did not correlate with IPSC or EPSC frequency. Higher GABA inputs could also be contributed by the recently characterized cytomegalic interneurons (Andre et al., 2007) and by supernumerary cells in superficial layers and white matter observed in CDIIB cases (Chandra et al., 2007), particularly if, as in rodents, layer I neurons are mostly GABAergic (Wozny and Williams, 2011). This differential network connectivity argues for diverse epileptogenic and propagation mechanisms in TSC and CDIIB. With regard to the possible mechanism of GABAA receptor up-regulation in CDIIB, although data from mIPSCs suggest increased probability of neurotransmitter release, our study can not rule out a concomitant increase in the number of release sites. At this point, we think that the most parsimonious explanation is that both presynaptic (increased probability of release and number of release sites), as well as postsynaptic (increased surface expression and different stoichiometry of postsynaptic GABAA receptors) mechanisms are probably involved. Additional studies are warranted to further characterize GABAergic receptor functions in CDIIB.
Up-regulation of GABA relative to glutamate activity in severe CD (CD Type IIA/B) compared to non-CD or mild CD (CD Type I) cases, was previously reported by our group (Andre et al., 2008; Cepeda et al., 2005a). The fact that the same difference occurred when comparing CDIIB versus TSC indicates that synaptic activity and GABAA receptor sensitivity in TSC more closely resembles non-CD and CD Type I cases and further underscores that the dysmature cerebral developmental hypothesis seems to be more relevant to CDIIB cases (Cepeda et al., 2006).
Clinical Relevance
It has been suggested that TSC and CDIIB can be considered similar pathological entities (Crino, 2007; Wong, 2008). If correct, then common treatments can be used for both etiologies. However, as shown in the present and previous studies (Baybis et al., 2004; Miyata et al., 2004), some distinctions exist between TSC and CDIIB tissue. Because of higher GABA tone in pediatric CDIIB, and the possibility that in this pathology GABA is excitatory (Cepeda et al., 2007), utilization of drugs that increase GABA may be less effective (Kahle and Staley, 2008). In contrast, in TSC drugs that increase GABA could be more useful, a possible reason why vigabatrin is fairly successful in controlling infantile spasms in TSC patients (Camposano et al., 2008). These findings at the cellular and network levels will need to be confirmed in clinical trials. However, our results should be taken into consideration when proposing clinical pharmacological studies using agents that alter the mTOR pathway and chloride-transporters in cases of TSC and CDIIB.
The reader should be aware of limitations of this human study when interpreting the results. For example, we compared findings from pediatric and not adult surgical cases. It is possible that different results might be obtained in tissue from older TSC and CDIIB patients (Calcagnotto et al., 2005; Wang et al., 2007). Furthermore, our study compared two abnormal etiologies in refractory patients undergoing epilepsy neurosurgery, and non-epileptic normal control tissue was not ethically available for this human study. While the lack of correlation between clinical variables and experimental findings in our cohort would indicate that these variables did not make a major contribution, with larger cohorts more subtle correlations could be discovered, including use of certain AEDs, longer seizure duration, and a history of infantile spasms, which might be confounding factors. Our results should be replicated in other clinical studies and appropriate experimental systems. In addition, the relative density and topography of abnormal cells in TSC and CDIIB cases need to be validated using non-biased cell counting techniques in histology specimens. Even with these potential problems, this study indicates that there are differences in network properties and GABAA receptor sensitivity in TSC and CDIIB cases that should be taken into consideration to further understand the mechanisms of epileptogenesis in these two refractory and surgically-treated epilepsy syndromes.
Supplementary Material
Legend for Supplementary Figure
Fig. 1
A wide variety of cells was observed in TSC tissue samples. Some cells had a neuronal appearance (e.g., A, C, D, and F), whereas others had a morphology more congruent with a glial cell type (B and E). The number of processes also varied considerably, with some cells having only a few thick processes (C) and others having abundant, hairy-like processes (B and E). Each cell in panel A was patched independently, whereas only one cell in panel B was patched, suggesting that in some cases astrocyte-like cells were coupled via gap junctions. Calibration in A also applies to panel B. Calibration in E also applies to panels C, D, and F.
Manuscript Highlights.
First characterization of normal and abnormal cells in Tuberous Sclerosis Complex
Differential distribution of abnormal cells in the cerebral cortex of TSC and CD Type IIB cases.
GABA synaptic activity significantly increased in CD Type IIB compared to TSC cases.
Electrophysiology of intermediate cells, demonstrating properties similar to balloon cells.
Acknowledgments
The authors would like to thank the patients and their parents for allowing use of resected specimen for experimentation. We also thank the UCLA Hospital Pediatric Neurology staff for their assistance. Donna Crandall helped with the illustrations and Dr. Yao-Ying Ma helped with the statistical analysis. This study was supported by NIH grant NS 38992. HVV was supported in part by the Daljit S. & Elaine Sarkaria Chair in Diagnostic Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andre VM, et al. Pyramidal cell responses to gamma-aminobutyric acid differ in type I and type II cortical dysplasia. J Neurosci Res. 2008;86:3151–62. doi: 10.1002/jnr.21752. [DOI] [PubMed] [Google Scholar]
- Andre VM, et al. NMDA receptor alterations in neurons from pediatric cortical dysplasia tissue. Cereb Cortex. 2004;14:634–46. doi: 10.1093/cercor/bhh024. [DOI] [PubMed] [Google Scholar]
- Andre VM, et al. Cytomegalic interneurons: a new abnormal cell type in severe pediatric cortical dysplasia. J Neuropathol Exp Neurol. 2007;66:491–504. doi: 10.1097/01.jnen.0000240473.50661.d8. [DOI] [PubMed] [Google Scholar]
- Baybis M, et al. mTOR cascade activation distinguishes tubers from focal cortical dysplasia. Ann Neurol. 2004;56:478–87. doi: 10.1002/ana.20211. [DOI] [PubMed] [Google Scholar]
- Blumcke I, et al. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia. 2011;52:158–74. doi: 10.1111/j.1528-1167.2010.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonyapisit K, et al. Epileptogenicity of focal malformations due to abnormal cortical development: direct electrocorticographic-histopathologic correlations. Epilepsia. 2003;44:69–76. doi: 10.1046/j.1528-1157.2003.08102.x. [DOI] [PubMed] [Google Scholar]
- Calcagnotto ME, et al. Dysfunction of synaptic inhibition in epilepsy associated with focal cortical dysplasia. J Neurosci. 2005;25:9649–57. doi: 10.1523/JNEUROSCI.2687-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camposano SE, et al. Vigabatrin in the treatment of childhood epilepsy: a retrospective chart review of efficacy and safety profile. Epilepsia. 2008;49:1186–91. doi: 10.1111/j.1528-1167.2008.01589.x. [DOI] [PubMed] [Google Scholar]
- Cepeda C, et al. Pediatric cortical dysplasia: correlations between neuroimaging, electrophysiology and location of cytomegalic neurons and balloon cells and glutamate/GABA synaptic circuits. Dev Neurosci. 2005a;27:59–76. doi: 10.1159/000084533. [DOI] [PubMed] [Google Scholar]
- Cepeda C, et al. Epileptogenesis in pediatric cortical dysplasia: the dysmature cerebral developmental hypothesis. Epilepsy Behav. 2006;9:219–35. doi: 10.1016/j.yebeh.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Cepeda C, et al. Are cytomegalic neurons and balloon cells generators of epileptic activity in pediatric cortical dysplasia? Epilepsia. 2005b;46(Suppl 5):82–8. doi: 10.1111/j.1528-1167.2005.01013.x. [DOI] [PubMed] [Google Scholar]
- Cepeda C, et al. Immature neurons and GABA networks may contribute to epileptogenesis in pediatric cortical dysplasia. Epilepsia. 2007;48(Suppl 5):79–85. doi: 10.1111/j.1528-1167.2007.01293.x. [DOI] [PubMed] [Google Scholar]
- Cepeda C, et al. Comparative study of cellular and synaptic abnormalities in brain tissue samples from pediatric tuberous sclerosis complex and cortical dysplasia type II. Epilepsia. 2010;51(Suppl 3):160–5. doi: 10.1111/j.1528-1167.2010.02633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, et al. Morphological and electrophysiological characterization of abnormal cell types in pediatric cortical dysplasia. J Neurosci Res. 2003;72:472–86. doi: 10.1002/jnr.10604. [DOI] [PubMed] [Google Scholar]
- Chandra PS, et al. Infantile spasm-associated microencephaly in tuberous sclerosis complex and cortical dysplasia. Neurology. 2007;68:438–45. doi: 10.1212/01.wnl.0000252952.62543.20. [DOI] [PubMed] [Google Scholar]
- Cherubini E, et al. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14:515–9. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Chu-Shore CJ, et al. The natural history of epilepsy in tuberous sclerosis complex. Epilepsia. 2010;51:1236–41. doi: 10.1111/j.1528-1167.2009.02474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crino PB. Focal brain malformations: a spectrum of disorders along the mTOR cascade. Novartis Found Symp. 2007;288:260–72. doi: 10.1002/9780470994030.ch18. discussion 272-81. [DOI] [PubMed] [Google Scholar]
- Crino PB, et al. Differential expression of glutamate and GABA-A receptor subunit mRNA in cortical dysplasia. Neurology. 2001;56:906–13. doi: 10.1212/wnl.56.7.906. [DOI] [PubMed] [Google Scholar]
- Crino PB, et al. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–56. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- Crino PB, et al. Embryonic neuronal markers in tuberous sclerosis: single-cell molecular pathology. Proc Natl Acad Sci U S A. 1996;93:14152–7. doi: 10.1073/pnas.93.24.14152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curatolo P, et al. Tuberous sclerosis. Lancet. 2008;372:657–68. doi: 10.1016/S0140-6736(08)61279-9. [DOI] [PubMed] [Google Scholar]
- Englund C, et al. Aberrant neuronal-glial differentiation in Taylor-type focal cortical dysplasia (type IIA/B). Acta Neuropathol. 2005;109:519–33. doi: 10.1007/s00401-005-1005-9. [DOI] [PubMed] [Google Scholar]
- Farrell MA, et al. Neuropathologic findings in cortical resections (including hemispherectomies) performed for the treatment of intractable childhood epilepsy. Acta Neuropathol. 1992;83:246–59. doi: 10.1007/BF00296786. [DOI] [PubMed] [Google Scholar]
- Fukutani Y, et al. An autopsy case of tuberous sclerosis. Histological and immunohistochemical study. Histol Histopathol. 1992;7:709–14. [PubMed] [Google Scholar]
- Guerra MP, et al. Intractable epilepsy in hemimegalencephaly and tuberous sclerosis complex. J Child Neurol. 2007;22:80–4. doi: 10.1177/0883073807299960. [DOI] [PubMed] [Google Scholar]
- Gumbinger C, et al. Focal cortical dysplasia: a genotype-phenotype analysis of polymorphisms and mutations in the TSC genes. Epilepsia. 2009;50:1396–408. doi: 10.1111/j.1528-1167.2008.01979.x. [DOI] [PubMed] [Google Scholar]
- Hemb M, et al. Improved outcomes in pediatric epilepsy surgery: the UCLA experience, 1986-2008. Neurology. 2010;74:1768–75. doi: 10.1212/WNL.0b013e3181e0f17a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose T, et al. Tuber and subependymal giant cell astrocytoma associated with tuberous sclerosis: an immunohistochemical, ultrastructural, and immunoelectron and microscopic study. Acta Neuropathol. 1995;90:387–99. doi: 10.1007/BF00315012. [DOI] [PubMed] [Google Scholar]
- Horikawa K, Armstrong WE. A versatile means of intracellular labeling: injection of biocytin and its detection with avidin conjugates. J Neurosci Methods. 1988;25:1–11. doi: 10.1016/0165-0270(88)90114-8. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Heydemann PT. Fine structure of cortical tubers in tuberous sclerosis: a Golgi study. Ann Neurol. 1984;16:595–602. doi: 10.1002/ana.410160511. [DOI] [PubMed] [Google Scholar]
- Kahle KT, Staley KJ. The bumetanide-sensitive Na-K-2Cl cotransporter NKCC1 as a potential target of a novel mechanism-based treatment strategy for neonatal seizures. Neurosurg Focus. 2008;25:E22. doi: 10.3171/FOC/2008/25/9/E22. [DOI] [PubMed] [Google Scholar]
- Lerner JT, et al. Assessment and surgical outcomes for mild type I and severe type II cortical dysplasia: a critical review and the UCLA experience. Epilepsia. 2009;50:1310–35. doi: 10.1111/j.1528-1167.2008.01998.x. [DOI] [PubMed] [Google Scholar]
- Machado-Salas JP. Abnormal dendritic patterns and aberrant spine development in Bourneville's disease--a Golgi survey. Clin Neuropathol. 1984;3:52–8. [PubMed] [Google Scholar]
- Mackay MT, et al. Malformations of cortical development with balloon cells: clinical and radiologic correlates. Neurology. 2003;60:580–7. doi: 10.1212/01.wnl.0000044053.09023.91. [DOI] [PubMed] [Google Scholar]
- Mathern GW, et al. Neurons recorded from pediatric epilepsy surgery patients with cortical dysplasia. Epilepsia. 2000;41(Suppl 6):S162–7. doi: 10.1111/j.1528-1157.2000.tb01575.x. [DOI] [PubMed] [Google Scholar]
- Mathern GW, et al. Postoperative seizure control and antiepileptic drug use in pediatric epilepsy surgery patients: the UCLA experience, 1986-1997. Epilepsia. 1999;40:1740–9. doi: 10.1111/j.1528-1157.1999.tb01592.x. [DOI] [PubMed] [Google Scholar]
- Miyata H, et al. Insulin signaling pathways in cortical dysplasia and TSC-tubers: tissue microarray analysis. Ann Neurol. 2004;56:510–9. doi: 10.1002/ana.20234. [DOI] [PubMed] [Google Scholar]
- Probst A, Ohnacker H. [Tuberous sclerosis in a premature infant (author's transl)]. Acta Neuropathol. 1977;40:157–61. doi: 10.1007/BF00688705. [DOI] [PubMed] [Google Scholar]
- Redman S. Quantal analysis of synaptic potentials in neurons of the central nervous system. Physiol Rev. 1990;70:165–98. doi: 10.1152/physrev.1990.70.1.165. [DOI] [PubMed] [Google Scholar]
- Qin W, et al. Analysis of TSC cortical tubers by deep sequencing of TSC1, TSC2 and KRAS demonstrates that small second-hit mutations in these genes are rare events. Brain Pathol. 2010;20:1096–105. doi: 10.1111/j.1750-3639.2010.00416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamon N, et al. FDG-PET/MRI coregistration improves detection of cortical dysplasia in patients with epilepsy. Neurology. 2008;71:1594–601. doi: 10.1212/01.wnl.0000334752.41807.2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisodiya SM, et al. Focal cortical dysplasia type II: biological features and clinical perspectives. Lancet Neurol. 2009;8:830–43. doi: 10.1016/S1474-4422(09)70201-7. [DOI] [PubMed] [Google Scholar]
- Sosunov AA, et al. Tuberous sclerosis: a primary pathology of astrocytes? Epilepsia. 2008;49(Suppl 2):53–62. doi: 10.1111/j.1528-1167.2008.01493.x. [DOI] [PubMed] [Google Scholar]
- Sparagana SP, Roach ES. Tuberous sclerosis complex. Curr Opin Neurol. 2000;13:115–9. doi: 10.1097/00019052-200004000-00001. [DOI] [PubMed] [Google Scholar]
- Talos DM, et al. Cell-specific alterations of glutamate receptor expression in tuberous sclerosis complex cortical tubers. Ann Neurol. 2008;63:454–65. doi: 10.1002/ana.21342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DC, et al. Focal dysplasia of the cerebral cortex in epilepsy. J Neurol Neurosurg Psychiatry. 1971;34:369–87. doi: 10.1136/jnnp.34.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom M, et al. Mcm2 labelling of balloon cells in focal cortical dysplasia. Neuropathol Appl Neurobiol. 2005;31:580–8. doi: 10.1111/j.1365-2990.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- Trombley IK, Mirra SS. Ultrastructure of tuberous sclerosis: cortical tuber and subependymal tumor. Ann Neurol. 1981;9:174–81. doi: 10.1002/ana.410090211. [DOI] [PubMed] [Google Scholar]
- Vinters HV, et al. Neuropathologic study of resected cerebral tissue from patients with infantile spasms. Epilepsia. 1993;34:772–9. doi: 10.1111/j.1528-1157.1993.tb00460.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, et al. Neocortical hyperexcitability in a human case of tuberous sclerosis complex and mice lacking neuronal expression of TSC1. Ann Neurol. 2007;61:139–52. doi: 10.1002/ana.21058. [DOI] [PubMed] [Google Scholar]
- White R, et al. Selective alterations in glutamate and GABA receptor subunit mRNA expression in dysplastic neurons and giant cells of cortical tubers. Ann Neurol. 2001;49:67–78. doi: 10.1002/1531-8249(200101)49:1<67::aid-ana10>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Wong M. Mechanisms of epileptogenesis in tuberous sclerosis complex and related malformations of cortical development with abnormal glioneuronal proliferation. Epilepsia. 2008;49:8–21. doi: 10.1111/j.1528-1167.2007.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozny C, Williams SR. Specificity of Synaptic Connectivity between Layer 1 Inhibitory Interneurons and Layer 2/3 Pyramidal Neurons in the Rat Neocortex. Cereb Cortex. 2011 doi: 10.1093/cercor/bhq257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Z, et al. Expression of neural stem cell surface marker CD133 in balloon cells of human focal cortical dysplasia. Epilepsia. 2005;4 6:1716–23. doi: 10.1111/j.1528-1167.2005.00276.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Legend for Supplementary Figure
Fig. 1
A wide variety of cells was observed in TSC tissue samples. Some cells had a neuronal appearance (e.g., A, C, D, and F), whereas others had a morphology more congruent with a glial cell type (B and E). The number of processes also varied considerably, with some cells having only a few thick processes (C) and others having abundant, hairy-like processes (B and E). Each cell in panel A was patched independently, whereas only one cell in panel B was patched, suggesting that in some cases astrocyte-like cells were coupled via gap junctions. Calibration in A also applies to panel B. Calibration in E also applies to panels C, D, and F.