Abstract
Damage to prefrontal cortex (PFC) impairs decision-making, but the underlying value computations that might cause such impairments remain unclear. Here we report that value computations are doubly dissociable within PFC neurons. While many PFC neurons encoded chosen value, they used opponent encoding schemes such that averaging the neuronal population eliminated value coding. However, a special population of neurons in anterior cingulate cortex (ACC) - but not orbitofrontal cortex (OFC) - multiplex chosen value across decision parameters using a unified encoding scheme, and encoded reward prediction errors. In contrast, neurons in OFC - but not ACC - encoded chosen value relative to the recent history of choice values. Together, these results suggest complementary valuation processes across PFC areas: OFC neurons dynamically evaluate current choices relative to recent choice values, while ACC neurons encode choice predictions and prediction errors using a common valuation currency reflecting the integration of multiple decision parameters.
Introduction
Prefrontal cortex (PFC) supports optimal and rational decision-making. Damage to areas such as orbitofrontal cortex (OFC), anterior cingulate cortex (ACC) and lateral prefrontal cortex (LPFC) is associated with severe decision-making impairments1–8; impairments not typically found with damage outside of PFC. This implies that PFC neurons must support value computations that are essential for decision-making. However, while anatomical sub-divisions within the PFC appear specialized in their function when examined with circumscribed lesions2,4,5 or fMRI9–12, such regional dissociations have rarely been described in the underlying neuronal activity. Similar determinants of an outcome's subjective value can be found in the firing rates of neurons in ACC, OFC and LPFC13–26.
While there is a technical reason for this apparent discrepancy – very few studies have simultaneously recorded single neurons from multiple PFC areas to identify regional specialization – there is also a more fundamental difficulty in uncovering such regional dissociations: the firing rates of PFC neurons exhibit substantial heterogeneity even within a PFC sub-region27. This heterogeneity is evident in two forms. First, neurons recorded within millimeters (or less) from each other may encode different features of the decision process28: neurons encode different parameters that influence the value of the choice (e.g., reward size or type; delay, risk, effort associated with obtaining reward)13,15,17,22–24, or neuronal activity may be modulated by different trial events (e.g., choice, movement, outcome)15,25. Second, and potentially more problematic, neurons which encode the same value parameter often do so with opponent encoding schemes; while one neuron may increase firing rate as a function of value, its neighbor’s activity may increase firing rate as value decreases15,17,19,21,23–25,29–31. This feature of PFC neurons is in stark contrast to activity measured, for example, in dopamine neurons, which code for reward and reward prediction error at predictable times, using a unified coding scheme32–34.
Such heterogeneity of prefrontal coding makes it particularly important to examine the activity of single neurons, but has rendered it difficult to dissociate regional patterns of PFC neuronal firing according to qualitative, rather than simply quantitative, differences. To address this issue, we simultaneously recorded the activity of single neurons in ACC, LPFC, and OFC while monkeys (Macaca mulatta) made choices that varied in both the cost and benefit of a decision. We report two clear functional dissociations between PFC areas. First, a subpopulation of ACC neurons exhibited two unique features that distinguish these neurons from other value coding neurons: i) at choice, these ACC neurons encoded all decision variables using a unified coding scheme of positive valence and ii) these same neurons also encoded reward prediction errors. Such activity was absent in OFC and LPFC. Second, we show that neurons in OFC - but not ACC - encoded the value of current choices relative to the recent history of choice values.
Results
We trained two subjects to choose between pairs of pictures associated with different probabilities or sizes of reward, or different physical effort (cost) to obtain reward (Fig. 1)15,25. In each trial the two stimuli varied along only one decision variable, hence there was always a correct choice. Trials from each decision variable were intermixed and randomly selected. Behavioral analyses revealed15 that both subjects performed at a high level, choosing the more valuable outcome on 98% of the trials. We report neuronal activity from 257, 140 and 213 neurons located in LPFC, OFC and ACC, respectively (see Methods, Supplementary Fig. 1 for recording locations). The current paper focuses on understanding the relationship between value encoding at both choice and outcome, and determining whether the encoding of choice value is sensitive to the previous history of choices and experienced outcomes.
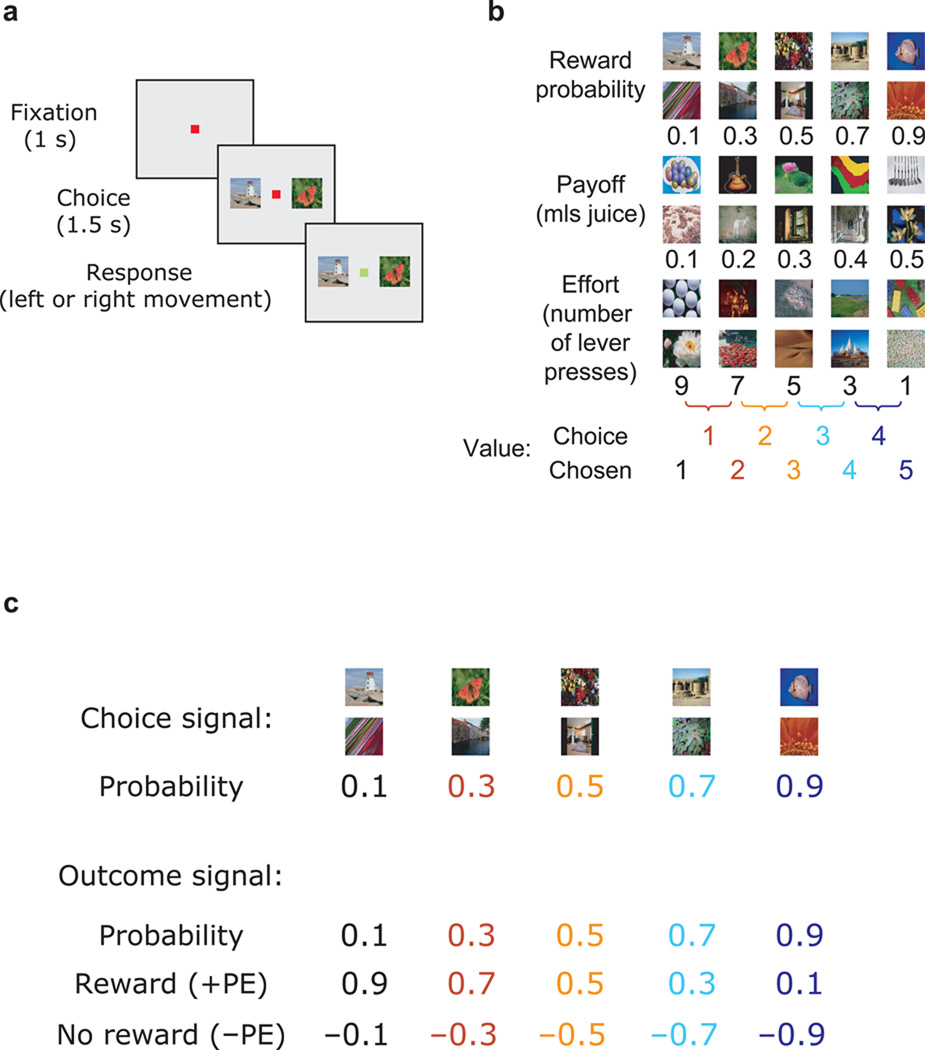
Figure 1.
The behavioral task and experimental contingencies. (a) Subjects made choices between pairs of presented pictures. (b) There were six sets of pictures, each associated with a specific outcome. We varied the value of the outcome by manipulating either the amount of reward the subject would receive (payoff), the likelihood of receiving a reward (probability) or the number of times the subject had to press a level to earn the reward (effort). We manipulated one parameter at time, holding the other two fixed. Presented pictures were always adjacent to one another in terms of value, i.e. choices were 1 vs. 2, 2 vs. 3, 3 vs. 4 or 4 vs. 5, hence there were four choice values (i.e., 1–4) per picture set and decision variable. All neuronal analyses were based on the chosen stimulus value (i.e., 1–5). (c) Relationship between the probability of receiving a reward and various value-related parameters. During the choice phase, neurons could encode the value of the choice (the probability of receiving a reward). During the outcome phase, neurons could encode a value signal reflecting the probability of reward delivery, or a prediction error, which is the difference between the subject’s expected value of the choice and the value of the outcome that was actually realized. On rewarded trials these values would always be positive (+PE) whereas on unrewarded trials they would always be negative (−PE).
Encoding of outcomes relative to choice expectancies
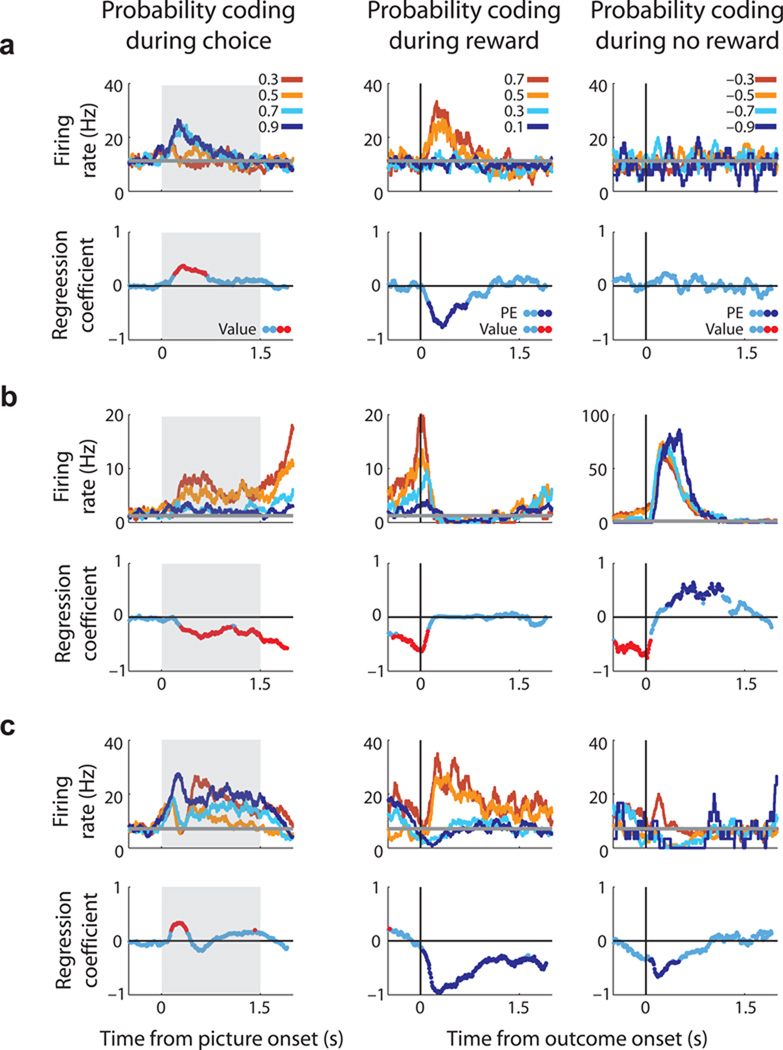
A hallmark feature of a prediction error neuron is that it encodes the discrepancy between experienced and expected outcomes. Thus, its firing rate should significantly correlate with probability at both choice and outcome, but should do so with an opposite relationship (Fig. 1c). For example, dopamine neurons show a positive relationship between firing rate and reward probability when the stimulus is evaluated, and a negative relationship during the experienced outcome32. In contrast, if a neuron was simply encoding reward probability at choice and outcome (i.e. a value rather than prediction error signal) then its firing rate should have the same relationship with probability during both epochs. Many ACC neurons exhibited increased activity when outcomes were better than expected (Fig. 2a), consistent with encoding a positive prediction error (+PE). Other neurons encoded a negative prediction error (−PE; Fig. 2b), indicating the outcome was worse than expected. In addition, some ACC neurons encoded probability at choice, and both a +PE and −PE at outcome (Fig. 2c), an activity pattern very similar to dopamine neurons32. Finally, some neurons encoded reward probability at both choice and outcome with the same pattern of activity, indicative of an outcome value (rather than prediction error) signal (Supplementary Figs. 2a, 2b).
Figure 2.
Three single neuron examples showing choice and outcome activity on probability trials during rewarded and unrewarded trials. For each neuron, the upper row of plots illustrates spike density histograms sorted according to the chosen stimulus value (choice epoch) or the size of the prediction error (outcome epochs). Data is not plotted for the least valuable choice since it was rarely chosen by the subjects. The horizontal grey line indicates the neuron’s baseline firing rate as determined from the 1-s fixation epoch immediately prior to the onset of the pictures. Grey box indicates choice epoch. The blue line in the lower row of plots illustrates how the magnitude of regression coefficients for reward probability coding (from GLMs 1–2) change across the course of the trial. Red data points indicate significant value encoding, while dark blue data points indicate significant prediction error encoding (i.e. encoding reward probability in the opposite direction in the outcome phase relative to the choice phase). (a) An ACC neuron that encoded +PE. It increased firing rate during choice as chosen probability increased and it increased firing rate during rewarded outcomes when the subject was least expecting to receive a reward (i.e., low probability trials). (b) An ACC neuron that encoded −PE. It increased firing rare during choice as chosen probability decreased and it increased firing rate during unrewarded outcomes when the subject was expecting to receive a reward (i.e., high probability trials). (c) An ACC neuron that encoded chosen probability, and at outcome encoded +PE and −PE.
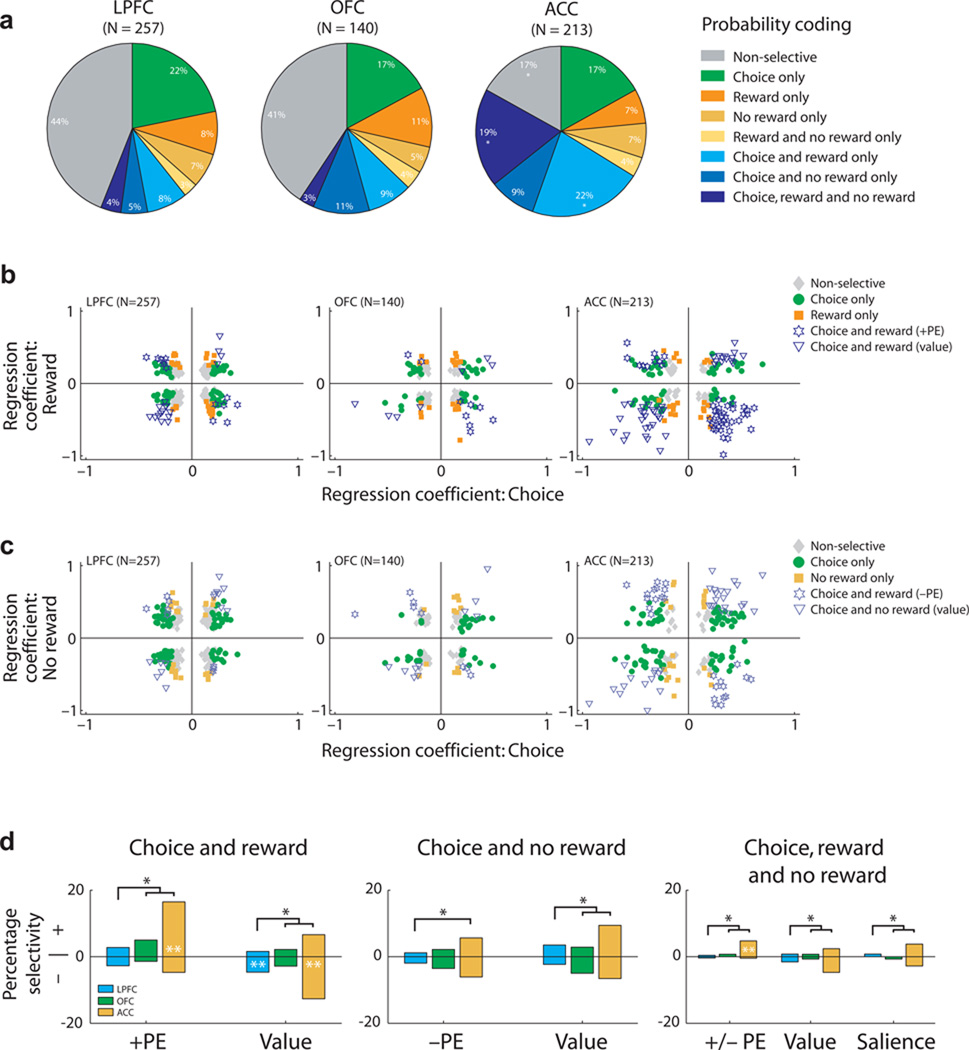
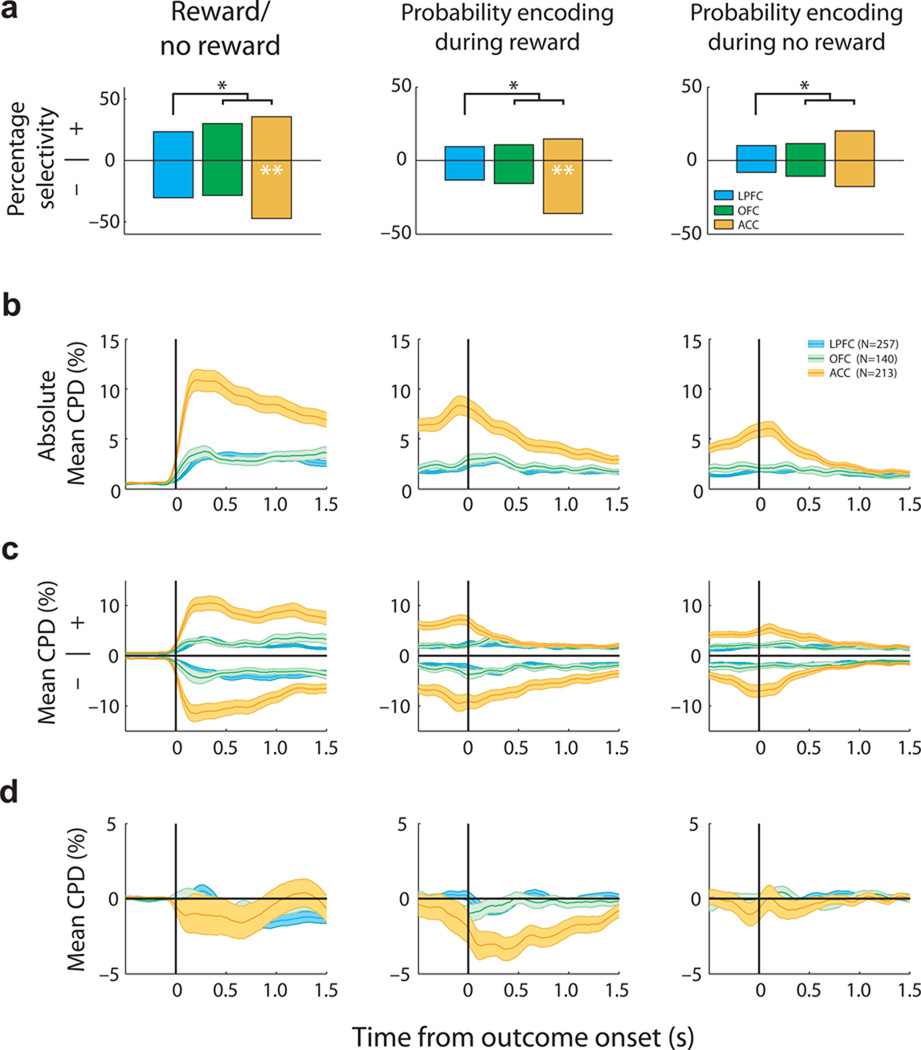
To distinguish whether neurons encoded a prediction error or a value signal at outcome, we compared the signs of the regression coefficients for encoding reward probability during the choice and outcome epochs (GLMs 1–2, see Methods). ACC neurons were significantly more likely than LPFC and OFC neurons to encode reward probability during both the choice and outcome epochs (Fig. 3a). Furthermore, many ACC neurons encoded reward probability information at both choice and outcome with opposite signed regression coefficients, indicative of a prediction error signal (Fig. 3b–c). Indeed, ACC neurons were significantly more likely to encode +PE (χ2=33, P<9×10−8), −PE (χ2=13, P=0.0016), or both +PE and −PE (χ2=10, P=0.0085) compared to both LPFC and OFC (Fig. 3d). ACC neurons were also significantly more likely to encode +PE than −PE (χ2=6.2, P=0.013). Finally, neurons that encoded reward probability in the same direction during the choice and outcome - indicative of an outcome value signal - were also most commonly found in ACC (Fig. 3d; χ2=24, P<7×10−6).
Figure 3.
Relationship between neuronal encoding during the choice and outcome epoch. (a) Prevalence of neurons encoding probability during different task events (choice, rewarded outcomes and/or unrewarded outcomes). Asterisks indicate that the proportion in ACC significantly differs from that in LPFC and OFC (chi-squared tests, P < 0.05). More ACC neurons encoded reward probability during both choice and outcome phases (blue shading). (b–c) Scatter plots of the regression coefficients for probability coding during both choice and (b) rewarded outcomes and (c) unrewarded outcomes for all neurons in each brain area. The point of maximum selectivity for each neuron and epoch is plotted, so data is biased away from zero. The different selectivity patterns are indicated by colored symbols. (b) ACC neurons that encoded probability positively during the choice encoded probability negatively during rewarded outcomes (star symbol), consistent with a +PE. Neurons that encoded probability positively during the choice also encoded probability positively during rewarded outcomes, consistent with encoding a value signal at outcome (triangle symbol). (c) There was no consistent relationship between the encoding of probability during the choice and unrewarded outcomes. (d) Bar plots summarizing the different probability encoding schemes, based on the sign of the choice regression coefficient (+ or −). Single black asterisks indicate significant differences between PFC areas (chi-squared tests, P < 0.05); double white asterisks indicate the proportion of neurons with positive or negative regression coefficients was significantly different from the chance 50/50 split (binomial test, P < 0.05). Position of white asterisks indicates the larger population.
A notable feature about ACC activity was that the sign of the regression coefficient at choice distinguished the type of signal that would be encoded at outcome – prediction error versus value representation (Fig. 3d). ACC neurons which encoded +PE (35/45; z-score=3.7, P<0.0002 binomial test) or both +PE and −PE (10/11; z-score=2.7, P=0.0059 binomial test) were significantly more likely to show a positive relationship between firing rate and probability during choice. In contrast, neurons which encoded a value signal on rewarded trials were significantly more likely to do so when the choice regression coefficient was negative (27/41; z-score=−2.2, P=0.03 binomial test). Thus, if an outcome selective neuron coded reward probability positively at choice then it flipped sign and encoded a prediction error at outcome, but if it coded probability negatively at choice then it encoded a value signal at outcome (Fig. 3b–d). Only 17 of 610 neurons encoded a saliency signal (see Methods, Supplementary Fig. 2c).
ACC multiplexes choice value with a positive valence
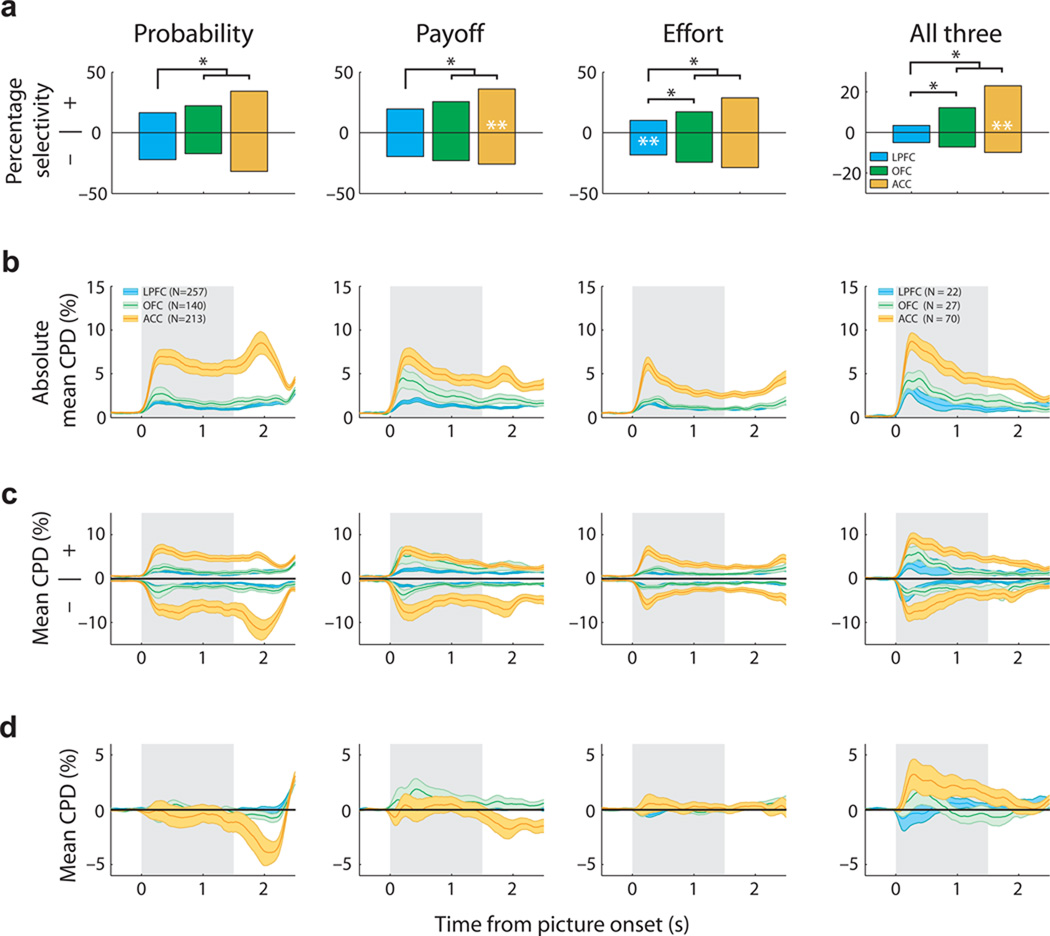
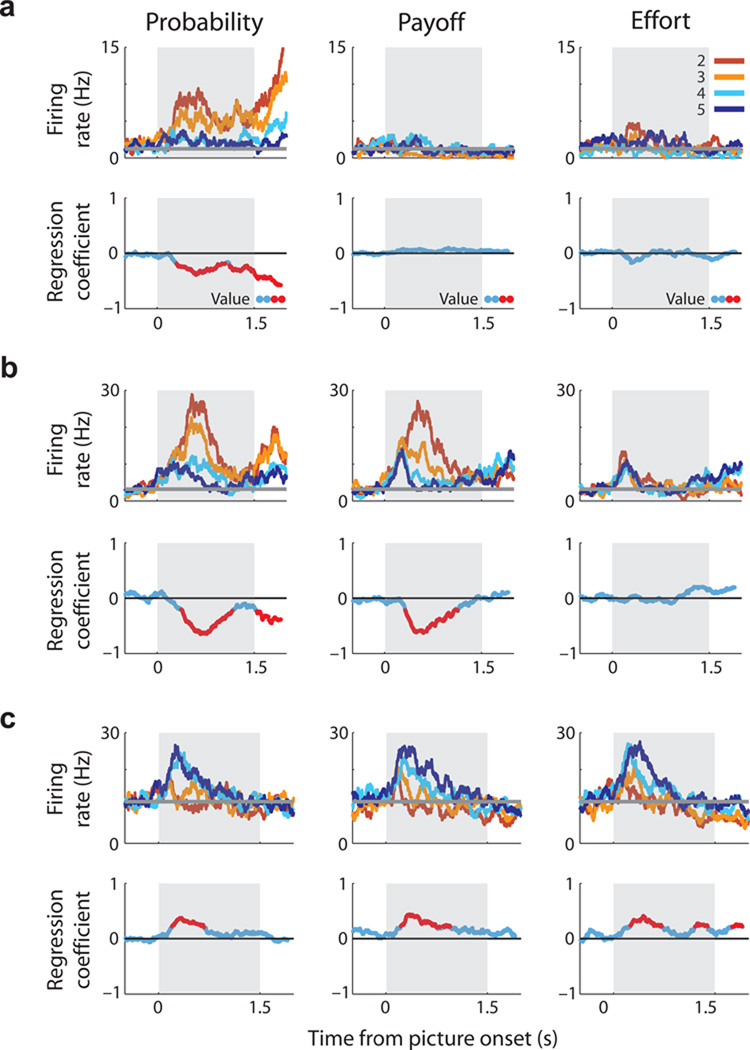
The previous analysis indicated that value coding during the choice phase predicted different value representations. Neurons that encoded chosen probability with a positive valence subsequently encoded a prediction error at outcome, while neurons that encoded chosen probability with a negative valence encoded a value signal at outcome. To determine whether the sign of the regression coefficient at choice could predict other types of value representations, we compared the relationship between firing rate and choice value for all three decision variables (GLM-1). Value coding for each decision variable was present in all PFC areas, but each was significantly more prevalent in ACC compared with both LPFC and OFC (Fig. 5a, χ2>23, P<2×10−6 in all cases). Neurons encoding all three decision variables (Fig. 4c) were also more common in ACC than LPFC and OFC (Fig. 5a, χ2=42, P<7×10−10). Notably, across all three decision variables and all three PFC areas, neurons were equally likely to increase firing rate as value either increased or decreased (Fig. 5a).
Figure 5.
Population encoding of value during the choice epoch. (a) Prevalence of neurons encoding choice value with a positive or negative regression coefficient. Conventions as in Figure 3d. The mean (solid line) and standard error (shading) of value selectivity as determined by (b) the absolute CPD for each value regressor, (c) the CPD shown separately for neurons encoding value either positively (+) or negatively (−) and (d) the population mean CPD. For the first three columns (probability, payoff, cost) the CPD is calculated from GLM-1 and averaged across all recorded neurons. The final column (all three) is restricted to neurons that encoded all three decision variables and the CPD is calculated from GLM-3. Value consistently explained more variance in ACC than OFC or LPFC (b), but because a similar amount of information was encoded by the populations with positive or negative regression coefficients (c), averaging the CPD across the two populations eliminated the encoding of value information at the population level (d). There were two exceptions: i) in ACC, neurons that encoded all three decision variables primarily did so with a positive regression coefficient and hence carried more information about choice value than the population of neurons with negative regression coefficients and ii) during the outcome phase of probability trials, ACC neurons tended to exhibit a negative coefficient for reward probability.
Figure 4.
Three neurons that encode the value of different choice variables. For each neuron, the upper row of plots illustrates spike density histograms sorted according to the value (2–5) of the expected outcome of the choice. Data is not plotted for the least valuable choice since it was rarely chosen by the subjects. The horizontal grey line indicates the neuron’s baseline firing rate as determined from the 1-s fixation epoch immediately prior to the onset of the pictures. Grey box indicates choice epoch. The blue line in the lower row of plots illustrates how the magnitude of regression coefficients for value coding (from GLM-1) changes across the course of the choice epoch. Red indicates time bins where significant value coding occurs. (a) An ACC neuron that encodes value solely on probability trials with an increase in firing rate as the value of the choice decreases. (b) An ACC neuron that encodes value on probability and payoff trials, increasing its firing rate as value decreases. (c) An ACC neuron that encodes value for all decision variables, increasing its firing rate as value increases.
The striking prevalence of opponent encoding of choice value may have important implications for interpreting brain activity in human studies, which typically use measures of average neuronal activity (e.g., EEG, fMRI) which may cancel out opposing value signals. To look at this issue directly, we calculated the coefficient of partial determination35 (CPD, see Methods), which measures how much variance in each neuron’s firing rate is explained by each GLM regressor. The mean CPD for all neurons in each PFC area is shown in Figure 5b. Chosen value consistently explained more of the variance in firing rate in ACC neurons relative to LPFC or OFC. We then sorted neurons by the sign of their regression coefficient and calculated the CPD for each population (Fig. 5c). As suggested by the single neuron analysis, choice value coding - when both the positive and negative neuronal populations are considered - averages to zero in all three PFC regions (Fig. 5d). This is particularly striking given the prevalence of value selectivity (Fig. 5a); the lack of a net value signal at the population level is the consequence of many highly selective neurons encoding the same value parameter but with opposite valence.
However, there was a clear exception to this averaging effect: at the time of choice, ACC neurons which encoded all three decision variables exhibited a strong bias to encode value positively at both the single neuron (Fig. 5a, right column; 49/70; z-score=3.3, P=0.0005 binomial test) and population level (Fig. 5d, right column). Thus, both in number, and in total explained variance, these ACC neurons are a specific subpopulation of neurons which multiplex value using a unified coding scheme of increasing firing rate as chosen value increased.
Outcome negativity in ACC explained by prediction errors
A second exception to the averaging effect occurred during the outcome of probability trials: ACC exhibited a negative net value bias (Fig. 5d, left column). There are two possible sources of this bias. First, the negative bias could arise from signaling errors, as has been previously reported in ACC activity36,37. If neurons signaled an error then their firing rate would increase as the chosen probability decreased, since the frequency of unrewarded trials (errors) increases. Second, the negative bias could arise from the many single ACC neurons that encoded reward prediction errors (Fig. 3b). If neurons encoded a +PE then firing rate would increase as chosen probability decreases, since a reward on a low probability trial is "better than expected".
To determine the source of this negative bias, we examined whether neurons were sensitive to reward/no reward, or showed a linear relationship between firing rate and chosen probability on rewarded or unrewarded trials (GLM-2, see Methods). Neurons that encode reward/no reward reflect a categorical outcome; a positive or negative regression coefficient indicates that neurons are more responsive to reward presence or absence, respectively (the latter is commonly considered an error signal). Although ACC neurons tended to encode categorical reward delivery with a negative regression coefficient (Fig. 6a, left), the amount of outcome information encoded by the populations with positive and negative regression coefficients was approximately equal (Fig. 6c, left) such that it averaged to zero and thus did not contribute to the negative bias at the population level (Fig. 6d, left). Similarly, although many ACC neurons were modulated by chosen probability on unrewarded outcomes (Fig. 6a, right), this information was absent at the population level (Fig. 6d, right). In contrast, ACC neurons exhibited a strong bias for a negative relationship with reward probability on rewarded trials (Fig. 6a, middle), leading to a large net negativity in the population response (Fig. 6d, middle). In other words, the ACC negativity at outcome (Fig. 5d, left column) is driven primarily by low probability trials being rewarded (i.e., +PE response) rather than unrewarded outcomes (i.e., error response).
Figure 6.
Population analyses of neuronal activity during the outcome epoch of probability trials. Conventions are the same as Figure 5. (a) The leftmost plot indicates the prevalence of neurons with positive or negative regression coefficients for encoding a categorical signal about reward presence or absence (e.g., neurons with a positive regression coefficient increase firing rate more on rewarded compared to unrewarded outcomes). The middle and right plots indicate the prevalence of neurons with positive or negative regression coefficients for encoding reward probability on rewarded (middle) or unrewarded (right) trials (e.g., neurons with a negative regression coefficient exhibit a linear increase in firing rate as reward probability decreases). (b) The absolute CPD for the three different regressors from GLM-2. ACC encoded more information than LPFC and OFC about the probability of receiving a reward as well as more information about whether or not a reward was actually received. (c) CPD plotted separately for those neurons which exhibited a positive or negative regression coefficient for the three different regressors. The two populations encoded approximately the same amount of outcome information. (d) The population mean CPD, averaged across neurons which exhibit both positive and negative regression coefficients for each of the three different regressors. The mirrored plots from panel (c) average so that approximately zero information about each regressor remains at the population level, with the exception that the ACC population retains information about rewarded outcomes with a negative bias (i.e., spiking on rewarded outcomes increases as probability decreases, akin to a +PE).
Prediction errors use an abstract value scale
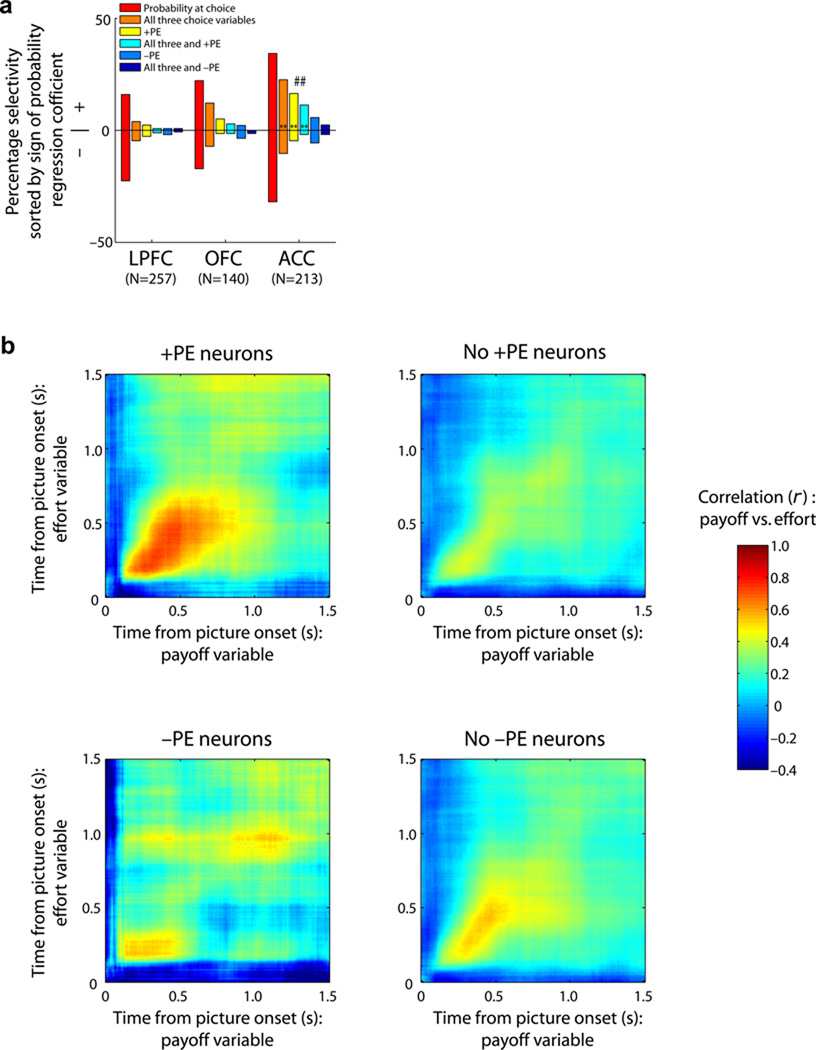
So far we identified two populations of ACC neurons that were biased towards encoding probability positively during choice: neurons that encoded value across all three decision variables (Fig. 5a, right column) and neurons that encoded +PEs (Fig. 3d). Our results suggest these two selectivity patterns are found in the same population of neurons, but only in ACC neurons (Fig. 7a). Neurons which encoded all three decision variables and +PE were significantly more likely to be found in ACC, but only when chosen probability was encoded with a positive regression coefficient. For example, the neuron in Figure 4c (choice activity) is the same neuron in Figure 2a (outcome activity).
Figure 7.
Relationship between encoding common value and +PE. (a) Proportion of ACC neurons encoding probability at choice with a positive (+) or negative (−) regression coefficient. ACC neurons that encoded all three decision variables during the choice and/or +PE at outcome were significantly more likely to encode probability during the choice phase with a positive regression coefficient. Double asterisks follow Figure 3d conventions but in black. ACC neurons that encoded +PE at outcome were the same subpopulation of neurons that also encoded all three decision variables at choice (## indicates that 69% of +PE neurons also encode all three decision variables, exceeding the expected frequency based on the odds of encoding payoff and effort information with a positive regression coefficient; P < 0.01 binomial test). (b) For those neurons that encoded probability during the choice, we plotted the correlation of their value encoding on payoff trials with the value encoding on effort trials (GLM-1) separated according to whether they did or did not code either +PE or −PE at outcome. This establishes whether the likelihood of encoding payoff and effort value information is dependent on encoding +PE or −PE. Only neurons that encoded probability during the choice phase and a +PE at outcome showed a significant correlation with value encoding on payoff and effort trials. This suggests that this subpopulation of ACC neurons uses a common value currency for computations related to representing expected values at choice, and discrepancies from those expectations at outcome (+PE).
To test whether +PE neurons were also likely to encode information about all three choice variables, we sorted neurons that encoded probability during choice into two groups, depending on whether these neurons did or did not encode a prediction error during outcome. There is no a priori reason why one group should be more likely to encode information about payoff and effort than the other group. However, we found a very strong correlation between the encoding of payoff and effort information for the group of neurons that encoded +PEs, but no correlation for the other groups (Fig. 7b). This correlation began as early as 200-ms after the start of the choice epoch, suggesting these neurons are multiplexing choice value early enough to reflect a role in the decision process. Thus, there appears to be a special population of neurons in ACC that multiplex value across decision variables, do so with a positive valence, and encode +PEs. This suggests that ACC encodes decision value and +PEs using a common value currency.
Relative value coding: the effect of value history
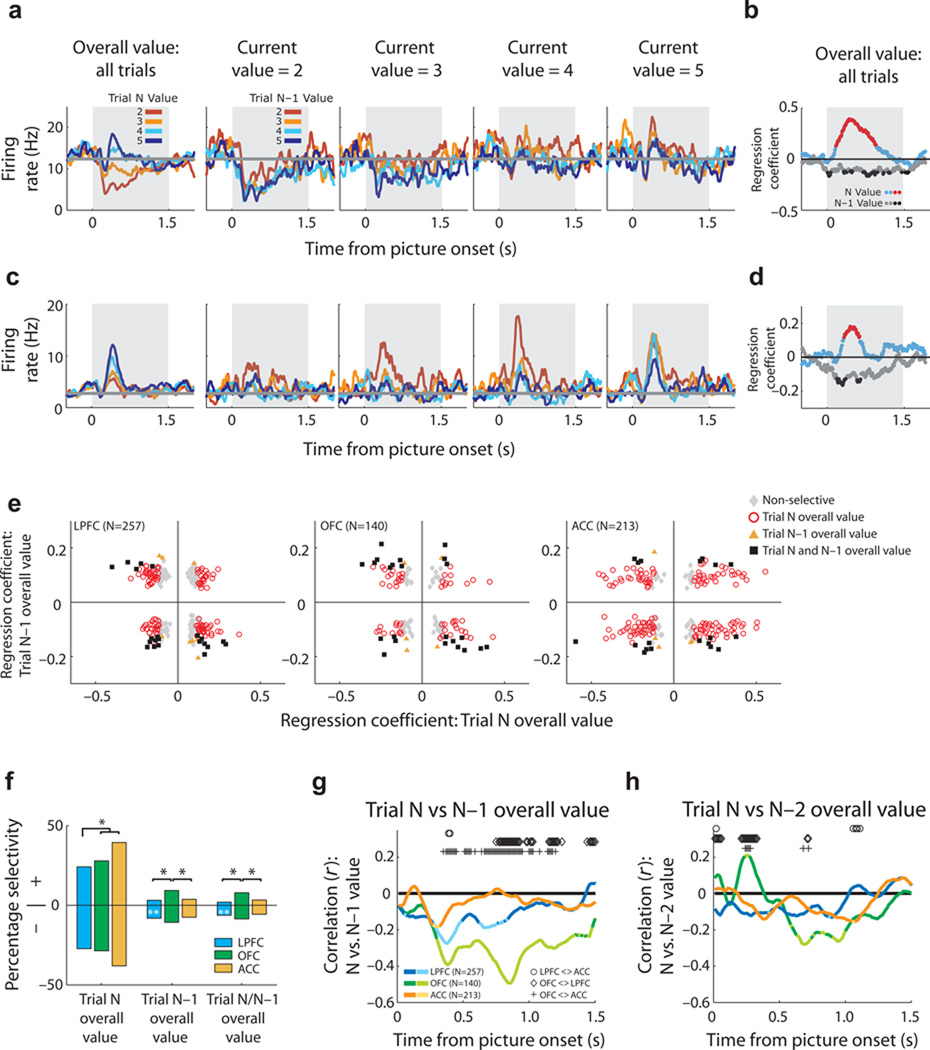
To facilitate optimal and adaptive choice, the brain must represent the reinforcement history of one’s choices. We examined whether PFC neurons encoded the overall value history of choices (i.e., value based on probability, payoff, and effort) while controlling for reward history (actual reward amount received) (GLM-3; see Methods). Many OFC neurons were sensitive to both the current and previous trial choice value, exhibiting increasing firing rate as the current trial value increased and as the past trial value decreased (Fig. 8a–b). In other words, this neuron encodes the current value relative to the value of the last choice. Some neurons in LPFC exhibited similar activity profiles (Figs. 8c–d).
Figure 8.
Neuronal encoding of value history. (a) An OFC neuron encoding current and past trial choice value. This neuron increases firing rate as current choice value increases (leftmost plot). Additionally, when the trials are sorted by the N–1 trial value, firing rate increases both as the current trial value increases and as the previous trial value decreases (four rightmost plots); this neuron is modulated by the difference in the current and past trial value. (b) Dynamics of the encoding shown in (a) as determined from the regression coefficients of GLM-3. Significant bins for current and N–1 value in red and black symbols, respectively. (c–d) An LPFC neuron that encodes current trial value relative to previous trial value. (e) Scatter plot of regression coefficients for current (N) and past trial (N–1) value for all neurons per brain area. Different colored symbols indicate different selectivity patterns. (f) Proportion of neurons encoding current trial N and/or N–1 trial chosen value at the time of the current trial choice, sorted by the sign of the regression coefficient (+ or −) for current trial (left plot) or N–1 trial (middle and right plot) value. Conventions as in Figure 3d. (g–h) Mean correlation (r) between regression coefficients for encoding value of the (g) current and previous trial or (h) current and two trials ago. Brighter colors signify the bins where the correlation was significant (P < 0.01). Symbols indicate significant differences in r values between areas (Fisher’s Z transformation, P<0.01).
We examined the distribution of regression coefficients for encoding present (N) and past (N–1) trial value for all neurons (Fig. 8e) to determine the number of neurons in each PFC area which encoded the current and/or past trial value at the time of the current choice (Fig. 8f). While 77% of ACC neurons encoded current choice value (significantly more than in LPFC and OFC, χ2=34, P<4×10−8) only 11% of ACC neurons encoded past trial value. In fact, OFC was significantly more likely to encode past trial value (χ2=6.2, P=0.04), or both current and past trial value (Fig. 8f; χ2=6.4, P=0.04) compared to both ACC and LPFC. Further, the value history representation within OFC was not simply persistent activity from the previous trial, but rather emerged at the time of the current choice as if it was used as a reference for the calculation of the current choice value (Supplementary Fig. 3a–c). Across several studies, this is the first time we have seen a value signal encoded more prevalently by OFC than ACC15,21,25. In contrast, at the time of the current choice, ACC neurons encoded the actual reward amount received from the previous trial (Supplementary Fig. 3; χ2=6.0, P=0.05).
If neurons encode current choice value relative to the past choice values, we might expect neurons to encode both value signals simultaneously with an negative correlation. Across the entire population, the correlation between current and past trial value was significantly stronger in the OFC population than in either LPFC or ACC, and it was negative (Fig. 8g; Fischer’s Z transformation, P<0.01). This correlation emerged 300-ms into the current choice, consistent with when the current choice would be evaluated. The current choice value was even modulated by the value of choices two trials into the past (Fig. 8h), but only in OFC. This effect was not driven by a subpopulation of very selective neurons, as this correlation remained significant across the OFC population even when the most selective neurons were excluded (see Supplementary Information; Supplementary Fig. 3d–e). Thus, the OFC population not only tracks the recent history of choice value, but uses this history to encode current choice value on a relative scale.
Discussion
Decision-making frameworks highlight the importance of multiple value computations necessary for decision-making, including representing decision variables that influence current choices and encoding choice outcomes to guide future choices28. This might explain why neuronal correlates of value have been identified across the entire brain, from PFC to sensorimotor areas16,27,38,39. To understand the relationship between value signals and decision-making we must identify what functions different value representations support. Recent comparative lesion studies suggest that despite the ubiquity of value representations across the brain, the computations supported by PFC areas make fundamental and specialized contributions to decision-making2,4–6. Here we describe our findings that different PFC neuronal populations encode different value computations.
Prefrontal encoding of prediction errors
Although previous studies have reported an outcome signal that correlates with prediction error in both ACC19,40,41 and OFC42 (but see26), our study is the first to report single PFC neuron activity time-locked to both stimulus presentation and outcome in a probabilistic context, analogous to the conditions used to test for these signals in dopamine neurons32,34. We identified three main findings. First, only ACC neurons encoded probability during the stimulus and outcome phases with an opposite relationship consistent with a prediction error signal. Second, ACC neurons more commonly encoded +PEs compared to −PEs, consistent with previous reports in both ACC40 and dopamine neurons43. We have also shown that focal ACC lesions causes reward-based, but not error-based, learning and decision-making impairments1, consistent with a functional link between dopamine and ACC for learning from +PEs. Third, just like dopamine neurons32, prediction error neurons in ACC encoded reward probability with a positive valence during stimulus presentation and a negative valence during reward delivery.
An important issue for future consideration is whether the value encoding scheme is of relevance. Many PFC neurons encode value with opposing encoding schemes, whereby neurons are equally likely to encode value positively or negatively15,17,19,21,23–25,29,30. While opponent encoding schemes might be a fundamental feature of a decision-making network31, such encoding schemes introduce potential interpretation difficulties for techniques which average across neuronal populations, such as in human neuroimaging. Averaging across opponent encoding schemes could average away value signals, as it did in many of our population analyses. However, despite an even distribution of ACC neurons which showed a positive or negative relationship between choice probability and firing rate, the former and latter group were predictive of a prediction error and value signal at outcome, respectively, suggesting that these two populations have functionally distinct roles.
ACC has been associated with error-related activity36,37. However, in many studies errors occur less frequently than rewards, suggesting ACC outcome activity might instead reflect violations in expectancy44,45, or how informative an outcome is for guiding adaptive behavior9,46,47. We counterbalanced the likelihood of a rewarded or unrewarded (error) outcome on probability trials to unconfound this issue. A comparison of ACC outcome activity suggested an approximately equal number of neurons were sensitive to reward and unrewarded (error) outcomes, but that error-related neurons were more likely to code a categorical signal while reward-related neurons were often modulated by prior reward expectancy, consistent with a +PE. Such results are consistent with our previous findings: ACC sulcus lesions did not impair error-related performance but impaired optimal choice after a new action was rewarded1. This suggests that when choices are rewarded in the context of a novel, volatile or low reward expectation environment, a critical function of ACC may be to signal a +PE to indicate the outcome is better than expected and hence promote future selection of that choice. In sum, rather than simply error detection, ACC encodes a rich outcome representation that incorporates prior expectancies and can inform future behavior25.
Choice value coded in a common neuronal currency
Formal decision models suggest that determining an action’s value requires the integration of the costs and benefits of a decision, thus generating a single value estimate for each decision alternative48. This net utility signal can be thought of as a type of neuronal currency which can facilitate comparison of very disparate choice outcomes48. However, such a valuation system operating in isolation would make learning about individual decision variables problematic, as it would not be possible to update the value estimate of individual variables (e.g., effort costs in isolation of reward benefits) if the only signal the valuation system received was in terms of overall utility. It may be optimal for the brain to encode two types of choice value signals; a variable-specific value signal that represents why an option is valuable, and a net utility signal that reflects the integrated net value of the option.
Our results suggest that variable-specific value signals were equally prevalent across all three PFC areas15, whereas neurons that encoded all three decision variables were primarily in ACC. Furthermore, ACC neurons that encoded all three decision variables during choice used a unified coding scheme of positive valence and encoded +PE on rewarded outcomes. This suggests that both choice value and prediction error coding – at least within many ACC neurons – is based on a common valuation scale reflecting the integration of individual decision variables.
Reinforcement history and fixed versus adaptive coding
Despite the abundance of value signals encoded by ACC neurons during both choice and outcome epochs, the encoding of current choice value within ACC was insensitive to the overall value of previous choices. Instead, ACC neurons encoded the actual reward history of choices, consistent with previous reports1,19,42,49. The encoding of reward history by ACC might reflect its role in tracking the reward rate, or volatility of possible outcomes, to inform when adaptive behavior is appropriate47.
Previous studies have shown that some ACC and OFC neurons adapt their firing rate when the scale of choice values is altered14,24,29,30, while other neurons encode a fixed value representation invariant to changes in the set of offers available30,50. Adaptive value coding is efficient because it provides flexibility to encode value across decision contexts that differ substantially in value, whereas fixed value coding is important because it provides value transitivity, a key characteristic of economic choice50.
Our results indicate that adaptive coding is specific to OFC and is extremely dynamic, operating on a trial-by-trial basis. OFC neurons exhibited a stronger response when the current trial value differed largely from the previous trial. Yet, OFC neurons did not encode a prediction error at outcome26, so this cannot be characterized as a general discrepancy signal. Adaptive coding in OFC also differed from ACC prediction error coding due to its distributed nature. The OFC neurons that had weak or non-significant value coding still exhibited a robust anti-correlation between current and past value, even for outcomes more than one trial into the past. Thus, adaptive value coding is a general feature of the OFC population, whereas prediction error computations are performed by a subpopulation of ACC neurons.
Interpretational issues
There are some caveats to our results. First, single-unit neurophysiology has sampling bias. We strived to reduce this bias (e.g., by not pre-screening neurons for response properties), but it is impossible to eliminate. The most easily detectable electrical signals are generated by the largest neurons and different areas may have subtly different sampling biases. Nevertheless, simultaneous recording eliminates many confounds associated with comparison of neurophysiological responses in different brain areas. Such comparisons frequently have to be made across different studies from different labs employing different behavioral and statistical methods. Second, the precise behavioral contingencies and training history may have influenced the direction of our effects. Indeed, neuroimaging studies suggest ACC activations can be stronger to errors or rewards depending on which outcome is more frequent as a result of task design44,45. Future research can test the generalizability of our findings to other behavioral contexts.
In conclusion, our results show complementary encoding of value between ACC and OFC. ACC encodes value on a fixed scale using a common value currency and uses this value signal to encode prediction errors: how choice outcomes relate to prior expectancies. In contrast, OFC encodes an adaptive value signal that is dynamically adjusted based on the recent value history of choice environments.
Methods
Subjects
Two male rhesus monkeys (Macaca mulatta), ages 5 and 6, were subjects. Our methods for neurophysiological recording are reported in detail elsewhere15,20. We recorded simultaneously from ACC, LPFC and OFC using arrays of 10–24 tungsten microelectrodes (Supplementary Fig. 1). We recorded 257 LPFC neurons from areas 9, 46, 45 and 47/12l (subject A: 113, subject B: 144), 140 OFC neurons from areas 11, 13 and 47/12o (A: 58, B: 82), and 213 ACC neurons from within area 24c in the dorsal bank of the cingulate sulcus (A: 70, B: 143). We randomly sampled neurons to enable a fair comparison of neuronal properties between different brain regions. There were no significant differences in the proportion of selective neurons between subjects, so we pooled neurons across subjects. All procedures complied with guidelines from the National Institute of Health guidelines and the U.C. Berkeley Animal Care and Use Committee.
Task
Task details have been described previously15. We used NIMH Cortex (http://www.cortex.salk.edu) to control the presentation of the stimuli and the task contingencies. We monitored eye position and pupil dilation using an infrared system sampling at 125 Hz (ISCAN, Burlington, MA). Briefly, subjects made choices between two pictures associated with either: i) a specific number of lever presses required to obtain a fixed magnitude of juice reward with the probability and magnitude of reward held constant (effort trials), ii) a specific amount of juice with probability and effort held constant (payoff trials), iii) a specific probability of obtaining a juice reward with effort and payoff held constant (probability trials, Fig. 1). All trials were randomly intermixed. We used five different picture values for each decision variable and the two presented pictures were always adjacent in value. Thus, there were four different choice values (1 vs. 2, 2 vs. 3, 3 vs. 4, 4 vs. 5) per decision variable. This ensured that, aside from pictures associated with the most or least valuable outcome, subjects chose or did not choose the pictures equally often. Moreover, by only presenting adjacent valued pictures we controlled for the difference in value for each of the choices and therefore the conflict or difficulty in making the choice. We used two sets of pictures for each decision variable to ensure that neurons were not encoding the visual properties of the pictures, but very few neurons encoded the picture set15 so for all analyses here we collapse across picture set. We defined correct choices as choosing the outcome associated with the larger reward probability, larger payoff, and least effort. We defined chosen value as the value of the chosen stimulus, even when the less valuable stimulus was chosen. Subjects rapidly learned to choose the more valuable outcomes consistently within a behavioral session. Once each subject had learned several picture sets for each decision variable, behavioral training was completed. Two picture sets for each decision variable were chosen for each subject and used during all recording sessions.
We tailored the precise reward amounts during juice delivery for each subject to ensure that they received their daily fluid aliquot over the course of the recording session. Consequently, the duration for which the reward pump was active (and hence the magnitude of delivered rewards) differed slightly in the two subjects. The reward durations in subject A were: probability=1400-ms; effort 1700-ms; payoff=150, 400, 750, 1150, 1600-ms. The reward durations in subject B were: probability=2200-ms; effort 2800-ms; payoff=400, 700, 1300, 1900, 2500-ms. We also individually adjusted the reward magnitudes so that the five levels of value for each decision variable were approximately subjectively equivalent to both subjects as confirmed by choice preference tests outside of the recording experiment. For example, subject A was indifferent in choices between the level 3 probability condition (0.5 probability of 1400-ms reward, no effort), the level 3 payoff condition (1.0 probability of 750-ms reward, no effort), and the level 3 effort condition (1.0 probability of 1600-ms reward, effort=5 presses) when faced with choice preference tests between these conditions, but consistently preferred any of the level 3 conditions compared to lower probability, lower payoff, or higher effort conditions. Although the task was not designed to pit the different decision variables against each other to determine subjective equivalence, the subjective indifference to each level of value for each decision variable suggested that each level of value of each decision variable had approximately equal value. We used this measure of value in GLM-3 below in order to assess the effects of choice value history on current trial neuronal activity.
Data analysis
Behavioral data has been reported elsewhere15. For all neuronal analyses, firing rate was regressed against the chosen stimulus value, even when the less valuable stimulus was chosen (<2% of all choices, see Supplementary Fig. 4). Analyses using average (instead of chosen) value produced nearly identical results. We constructed spike density histograms by averaging neuronal activity across appropriate conditions using a sliding window of 100-ms. The lowest value stimulus was rarely chosen, so histograms only show the four highest chosen values. To calculate neuronal selectivity, we fit three sliding general linear models (GLMs; see below) to each neuron’s standardized firing rate observed during a 200-ms time window, starting in the first 200-ms of the fixation epoch and then shifted in 10-ms steps until we had analyzed the entire trial. This enabled us to determine neuronal selectivity in a way that was independent of absolute neuronal firing rates, which is useful when comparing neuronal populations that can differ in the baseline and dynamic range of their firing rates. For each neuron, we also calculated the mean firing rate during the 1000-ms fixation epoch to serve as a baseline.
We used three different GLMs. In GLM-1, we modeled the relationship between the neuron's standardized firing rate in the choice epoch and the value of the stimulus that would subsequently be chosen as a function of each decision variable (i.e., probability, payoff, effort). Three regressors defined the relevant decision variable for the trial (probability, payoff or effort) to account for possible differences in mean firing rates across the different conditions. Three regressors defined the interaction of chosen value and decision variable (i.e. value in probability trials, value in payoff trials and value in effort trials).
GLM-2 focused solely on the probability trials and examined how each neuron's standardized firing rate in the outcome epoch was influenced by whether the trial was rewarded and by the probability of getting a reward. The outcome epoch was defined as the 1000-ms epoch between reward onset (or when reward would have been delivered on unrewarded trials) to 1000-ms after reward onset. We included 4 regressors in the design matrix. The first was a constant, to model the mean firing rate across trials. The second differentiated rewarded from unrewarded trials (i.e., the encoding of a categorical outcome). The third modeled a linear relationship between firing rate and reward probability on rewarded trials only. The fourth modeled a linear relationship between firing rate and reward probability on unrewarded trials only. The third and fourth regressors were orthogonalized with respect to the first and second to ensure that parameter estimates for the second regressor would reflect differences in mean firing rate between rewarded and unrewarded trials.
In GLM-3 we examined how each neuron's standardized firing rate in the choice epoch of all trials was influenced by either the choice value of the preceding trial or the actual reward history. The design matrix included 6 regressors. The first was a constant, to model the mean firing rate across trials. The second through fifth coded for the value of trials N (current value) through N–3 (value of the choice made 1, 2 or 3 trials into the past), and the sixth was the actual experienced amount of reward (zero for unrewarded trials) from trial N–1 to ensure that any satiety effect from a reward on the last trial did not influence encoding of value on the current trial.
All regressors in all GLMs were normalized to a scale of 1–5 (except for reward/no reward which was normalized to a scale of 0–1), so that regression coefficients would be of approximately equal weight across analyses. Although we used standardized firing rates for the GLMs, this becomes an issue when correlating regression coefficients since it tends to underweight neurons with large dynamic firing rate ranges (that tend to be selective) and overweight neurons with low dynamic ranges (that tend to be non-selective) potentially leading to type II errors. Therefore, for these correlation analyses (Figs. 7b, 8g–h, Supplementary Fig. 3d–e) we used actual neuronal firing rates
We defined a selective neuron as any neuron where a regressor from any of the time bins within the sliding GLMs had a t-statistic >= 3.1 for two consecutive time bins. We chose this criterion to produce acceptable type I error levels, quantified by examining how many neurons reached the criterion for encoding chosen value during the 1000-ms fixation epoch (before the choice stimuli appear, thus the number of neurons reaching criterion for this variable should not exceed chance levels). Our type I error for this analysis was acceptable: crossings of our criterion by chance occurred less than 3% of the time for a 1000-ms epoch. The point of “maximum selectivity” for each neuron was defined as the time bin in each sliding GLM which had the largest t-statistic for each regressor. The sign of the regression coefficient at this bin was used to define the relationship between firing rate and value and was also used in the scatter plots of Figs. 3b–c and 8e. For neurons which encoded all three decision variables at choice we determined the sign of the regression coefficient at peak selectivity for each decision variable. The majority of neurons (76%) which encoded all three decision variables at choice did so with the same signed RC across all variables. Excluding neurons which had opposing signed RCs across choice decision variables did not alter any of the results.
To determine the contribution of each regressor in explaining the variance in a neuron's firing rate, we calculated the coefficient of partial determination (CPD) and then averaged this value across all neurons in each brain area to obtain an estimate of the amount of variance explained for each regressor at the population level. The CPD for regressor Xi is defined as:
where SSE(X) refers to the sum of squared errors in a regression model that includes a set of regressors X, and X−i a set of all the regressors included in the full model except Xi35.
The signs of the regression coefficients become important when examining whether PFC neurons encode prediction errors. The relationship between encoding value and encoding the discrepancy from expected value (i.e. prediction error) at the time of outcome are opposite (Fig. 1c); the larger the probability at choice, the smaller the +PE if that trial is rewarded and the smaller (or more negative) the −PE if that trial is not rewarded. In other words, if a neuron has the same relationship between probability and firing rate during both the choice and the outcome then this neuron is not encoding a prediction error but rather encodes the value (or reward probability) of the trial. In contrast, if a neuron encodes probability at choice and outcome with an opposite relationship then this would reflect a prediction error signal. Thus, to be defined as a prediction error neuron, the neuron had to significantly encode probability at the time of choice (probability regressor from GLM-1) AND had to encode the discrepancy between actual and expected reward during the outcome (GLM-2) AND with an opposite relationship (i.e., opposite signed regression coefficients). We further examined whether neurons encoded a salience signal. To be defined as a salience signal, neurons had to have significant coding of probability during both rewarded and unrewarded outcomes but with opposite signed regression coefficients (Supplementary Fig. 2c).
Supplementary Material
Acknowledgments
The project was funded by NIDA grant R01DA19028 and NINDS grant P01NS040813 to J.W. and NIMH training grant F32MH081521 to S.K. T.B. and S.K. are supported by the Wellcome Trust. S.K. designed the experiment, collected and analyzed the data, and wrote the manuscript. T.B. analyzed the data and edited the manuscript. J.W. designed the experiment, supervised the project, and edited the manuscript. The authors have no competing interests.
References
- 1.Kennerley SW, Walton ME, Behrens TE, Buckley MJ, Rushworth MF. Optimal decision making and the anterior cingulate cortex. Nat Neurosci. 2006;9:940–947. doi: 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- 2.Rudebeck PH, et al. Frontal cortex subregions play distinct roles in choices between actions and stimuli. J Neurosci. 2008;28:13775–13785. doi: 10.1523/JNEUROSCI.3541-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walton ME, Behrens TE, Buckley MJ, Rudebeck PH, Rushworth MF. Separable learning systems in the macaque brain and the role of orbitofrontal cortex in contingent learning. Neuron. 2010;65:927–939. doi: 10.1016/j.neuron.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckley MJ, et al. Dissociable components of rule-guided behavior depend on distinct medial and prefrontal regions. Science. 2009;325:52–58. doi: 10.1126/science.1172377. [DOI] [PubMed] [Google Scholar]
- 5.Noonan MP, et al. Separate value comparison and learning mechanisms in macaque medial and lateral orbitofrontal cortex. Proc Natl Acad Sci U S A. 2010;107:20547–20552. doi: 10.1073/pnas.1012246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fellows LK. Deciding how to decide: ventromedial frontal lobe damage affects information acquisition in multi-attribute decision making. Brain. 2006;129:944–952. doi: 10.1093/brain/awl017. [DOI] [PubMed] [Google Scholar]
- 7.Williams ZM, Bush G, Rauch SL, Cosgrove GR, Eskandar EN. Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nat Neurosci. 2004;7:1370–1375. doi: 10.1038/nn1354. [DOI] [PubMed] [Google Scholar]
- 8.Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 9.Walton ME, Devlin JT, Rushworth MF. Interactions between decision making and performance monitoring within prefrontal cortex. Nat Neurosci. 2004;7:1259–1265. doi: 10.1038/nn1339. [DOI] [PubMed] [Google Scholar]
- 10.Daw ND, O'Doherty JP, Dayan P, Seymour B, Dolan RJ. Cortical substrates for exploratory decisions in humans. Nature. 2006;441:876–879. doi: 10.1038/nature04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prevost C, Pessiglione M, Metereau E, Clery-Melin ML, Dreher JC. Separate valuation subsystems for delay and effort decision costs. J Neurosci. 2010;30:14080–14090. doi: 10.1523/JNEUROSCI.2752-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rushworth MF, Behrens TE. Choice, uncertainty and value in prefrontal and cingulate cortex. Nat Neurosci. 2008;11:389–397. doi: 10.1038/nn2066. [DOI] [PubMed] [Google Scholar]
- 13.Padoa-Schioppa C, Assad JA. Neurons in the orbitofrontal cortex encode economic value. Nature. 2006;441:223–226. doi: 10.1038/nature04676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- 15.Kennerley SW, Dahmubed AF, Lara AH, Wallis JD. Neurons in the frontal lobe encode the value of multiple decision variables. J Cogn Neurosci. 2009;21:1162–1178. doi: 10.1162/jocn.2009.21100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roesch MR, Olson CR. Impact of expected reward on neuronal activity in prefrontal cortex, frontal and supplementary eye fields and premotor cortex. J Neurophysiol. 2003;90:1766–1789. doi: 10.1152/jn.00019.2003. [DOI] [PubMed] [Google Scholar]
- 17.O'Neill M, Schultz W. Coding of reward risk by orbitofrontal neurons is mostly distinct from coding of reward value. Neuron. 2010;68:789–800. doi: 10.1016/j.neuron.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 18.Seo H, Lee D. Behavioral and neural changes after gains and losses of conditioned reinforcers. J Neurosci. 2009;29:3627–3641. doi: 10.1523/JNEUROSCI.4726-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seo H, Lee D. Temporal filtering of reward signals in the dorsal anterior cingulate cortex during a mixed-strategy game. J Neurosci. 2007;27:8366–8377. doi: 10.1523/JNEUROSCI.2369-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallis JD, Miller EK. Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. Eur J Neurosci. 2003;18:2069–2081. doi: 10.1046/j.1460-9568.2003.02922.x. [DOI] [PubMed] [Google Scholar]
- 21.Kennerley SW, Wallis JD. Encoding of reward and space during a working memory task in the orbitofrontal cortex and anterior cingulate sulcus. J Neurophysiol. 2009;102:3352–3364. doi: 10.1152/jn.00273.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S, Hwang J, Lee D. Prefrontal coding of temporally discounted values during intertemporal choice. Neuron. 2008;59:161–172. doi: 10.1016/j.neuron.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrison SE, Salzman CD. The convergence of information about rewarding and aversive stimuli in single neurons. J Neurosci. 2009;29:11471–11483. doi: 10.1523/JNEUROSCI.1815-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sallet J, et al. Expectations, gains, and losses in the anterior cingulate cortex. Cogn Affect Behav Neurosci. 2007;7:327–336. doi: 10.3758/cabn.7.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kennerley SW, Wallis JD. Evaluating choices by single neurons in the frontal lobe: outcome value encoded across multiple decision variables. Eur J Neurosci. 2009;29:2061–2073. doi: 10.1111/j.1460-9568.2009.06743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi YK, et al. The orbitofrontal cortex and ventral tegmental area are necessary for learning from unexpected outcomes. Neuron. 2009;62:269–280. doi: 10.1016/j.neuron.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallis JD, Kennerley SW. Heterogeneous reward signals in prefrontal cortex. Curr Opin Neurobiol. 2010;20:191–198. doi: 10.1016/j.conb.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci. 2008;9:545–556. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Padoa-Schioppa C. Range-adapting representation of economic value in the orbitofrontal cortex. J Neurosci. 2009;29:14004–14014. doi: 10.1523/JNEUROSCI.3751-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi S, Pinto de Carvalho O, Schultz W. Adaptation of reward sensitivity in orbitofrontal neurons. J Neurosci. 2010;30:534–544. doi: 10.1523/JNEUROSCI.4009-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Machens CK, Romo R, Brody CD. Flexible control of mutual inhibition: a neural model of two-interval discrimination. Science. 2005;307:1121–1124. doi: 10.1126/science.1104171. [DOI] [PubMed] [Google Scholar]
- 32.Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005;307:1642–1645. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- 35.Cai X, Kim S, Lee D. Heterogeneous Coding of Temporally Discounted Values in the Dorsal and Ventral Striatum during Intertemporal Choice. Neuron. 2011;69:170–182. doi: 10.1016/j.neuron.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- 37.Debener S, et al. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J Neurosci. 2005;25:11730–11737. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Platt ML, Glimcher PW. Neural correlates of decision variables in parietal cortex. Nature. 1999;400:233–238. doi: 10.1038/22268. [DOI] [PubMed] [Google Scholar]
- 39.Shuler MG, Bear MF. Reward timing in the primary visual cortex. Science. 2006;311:1606–1609. doi: 10.1126/science.1123513. [DOI] [PubMed] [Google Scholar]
- 40.Matsumoto M, Matsumoto K, Abe H, Tanaka K. Medial prefrontal cell activity signaling prediction errors of action values. Nat Neurosci. 2007;10:647–656. doi: 10.1038/nn1890. [DOI] [PubMed] [Google Scholar]
- 41.Amiez C, Joseph JP, Procyk E. Anterior cingulate error-related activity is modulated by predicted reward. Eur J Neurosci. 2005;21:3447–3452. doi: 10.1111/j.1460-9568.2005.04170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sul JH, Kim H, Huh N, Lee D, Jung MW. Distinct roles of rodent orbitofrontal and medial prefrontal cortex in decision making. Neuron. 2010;66:449–460. doi: 10.1016/j.neuron.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bayer HM, Glimcher PW. Midbrain dopamine neurons encode a quantitative reward prediction error signal. Neuron. 2005;47:129–141. doi: 10.1016/j.neuron.2005.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oliveira FT, McDonald JJ, Goodman D. Performance monitoring in the anterior cingulate is not all error related: expectancy deviation and the representation of action-outcome associations. J Cogn Neurosci. 2007;19:1994–2004. doi: 10.1162/jocn.2007.19.12.1994. [DOI] [PubMed] [Google Scholar]
- 45.Jessup RK, Busemeyer JR, Brown JW. Error effects in anterior cingulate cortex reverse when error likelihood is high. J Neurosci. 2010;30:3467–3472. doi: 10.1523/JNEUROSCI.4130-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jocham G, Neumann J, Klein TA, Danielmeier C, Ullsperger M. Adaptive coding of action values in the human rostral cingulate zone. J Neurosci. 2009;29:7489–7496. doi: 10.1523/JNEUROSCI.0349-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Behrens TE, Woolrich MW, Walton ME, Rushworth MF. Learning the value of information in an uncertain world. Nat Neurosci. 2007;10:1214–1221. doi: 10.1038/nn1954. [DOI] [PubMed] [Google Scholar]
- 48.Montague PR, Berns GS. Neural economics and the biological substrates of valuation. Neuron. 2002;36:265–284. doi: 10.1016/s0896-6273(02)00974-1. [DOI] [PubMed] [Google Scholar]
- 49.Bernacchia A, Seo H, Lee D, Wang XJ. A reservoir of time constants for memory traces in cortical neurons. Nat Neurosci. 2011;14:366–372. doi: 10.1038/nn.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Padoa-Schioppa C, Assad JA. The representation of economic value in the orbitofrontal cortex is invariant for changes of menu. Nat Neurosci. 2008;11:95–102. doi: 10.1038/nn2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.