Abstract
Children of parents with bipolar disorder (BD), especially those with attention deficit hyperactivity disorder (ADHD) and symptoms of depression or mania, are significantly at high-risk for developing BD. As we have previously shown amygdalar reductions in pediatric BD, the current study examined amygdalar volumes in offspring of parents with (BD offspring) who have not yet developed a full manic episode. Youth participating in the study included 22 BD offspring and 22 healthy controls of comparable age, gender, handedness, and IQ. Subjects had no history of a manic episode, but met criteria for ADHD and moderate mood symptoms. MRI was performed on a 3T GE scanner, using a 3D volumetric spoiled gradient echo series. Amygdalae were manually traced using BrainImage Java software on positionally normalized brain stacks. Bipolar offspring had similar amygdalar volumes compared to the control group. Exploratory analyses yielded no differences in hippocampal or thalamic volumes. Bipolar offspring do not show decreased amygdala volume, possibly because these abnormalities occur after more prolonged illness rather than as a preexisting risk factor. Longitudinal studies are needed to determine whether amygdalar volumes change during and after the development of BD.
Keywords: bipolar disorder, MRI, adolescents, amygdala, neuroimaging
1. INTRODUCTION
Children of parents with bipolar disorder (BD), referred to henceforth as “BD offspring”, have been shown to be at high-risk for developing BD (DelBello and Geller, 2001; Chang et al., 2003). While there have been proposed risk criteria in such children (Chang et al., 2006; Bechdolf et al., 2010; Duffy et al., 2010; Correll, et al., 2007), there has been no standardization of criteria or “staging” of BD development that has been studied systematically to inform the field. Nonetheless, it appears that BD offspring with symptoms of ADHD, anxiety, depression, or mania may be at the highest risk for BD (Chang et al., 2006).
BD offspring with ADHD and mood symptoms may be one group at particularly high risk for developing a full manic episode. While some studies have shown increased rates of ADHD among offspring of bipolar parents (for review, see DelBello et al., 2001; Chang et al., 2003), others have not (Hillegers et al., 2005; Duffy et al., 2010; Shaw et al., 2005; Birmaher 2010). Nevertheless, the clinical course of pediatric BD often begins in childhood with ADHD presentations (Tillman & Geller, 2006). Furthermore, parents with BD who also had ADHD as children may have younger age at onset of BD (Sachs et al., 2000) and may be more likely to already have a child with BD and ADHD (Chang et al., 2000). Thus, those BD offspring who do have ADHD may still be at high-risk for developing a subtype of early-onset BD that begins with an ADHD presentation (Carlson and Weintraub, 1993; Faraone et al., 1997). Studying the brain anatomy of these symptomatic BD offspring may shed light on neurobiological factors predicting development and progression of BD.
Magnetic resonance imaging (MRI) has been used in previous studies to examine various regional brain volumes in patients with BD, finding abnormalities in regions involved in mood regulation, including the amygdala, hippocampus, thalamus, caudate, and putamen (Frazier et al., 2005; Frazier et al., 2005). Recent meta-analyses have reported decreased whole-brain and prefrontal volumes, and increased globus pallidus and ventricular volumes in patients with BD compared to controls (Arnone et al., 2009). The meta-analysis by Kempton and colleagues (2008) also reported increased lateral ventricular enlargement, as well as increased rates of deep white matter hyperintensities. Both increased (Dewan et al., 1988; Dupont et al., 1995) and normal (Dolan et al., 1990; Strakowski et al., 1993; Caetano et al., 2001) thalamic volume has been reported in adults with BD. In adolescents with BD, there are reports of increased (Frazier et al., 2005), decreased (Dasari et al., 1999), and similar (Chang et al., 2005) thalamic volumes compared to controls. Hippocampal volumes in a group of adults and adolescents with BD were found to be smaller than those of healthy controls (Blumberg et al., 2003), while adults with BD have shown either unchanged (Altshuler et al., 1998; Hauser, Matochik et al., 2000) or decreased (Swayze et al., 1992) hippocampal volumes.
The amygdala has been one of the most prominent brain structures of interest in studies of BD, as it is highly involved in emotion processing and shows abnormal activation in functional imaging studies of adults with BD (Altshuler et al., 2005; Blumberg et al., 2005; Pavuluri et al., 2007). Morphometric abnormalities in the amygdala have also strengthened its likely role in the pathophysiology of BD (Dupont et al., 1995; Stoll et al., 2000; Strakowski et al., 2000; Blumberg et al., 2003; Chang et al., 2004; Blumberg et al., 2005; Chang et al., 2005; Strakowski et al., 2005; Garrett and Chang, 2008). Various research groups have reported increased (Strakowski et al., 1999; Altshuler et al., 2000), decreased (Pearlson et al., 1997), and similar (Swayze et al., 1992) amygdalar volumes in adults with BD. Findings in pediatric samples have been more consistent, as most, but not all, morphometric studies of children and adolescents with BD have found decreased amygdalar volumes compared with healthy controls (Blumberg et al., 2003; DelBello et al., 2004; Chang et al., 2005; Frazier et al., 2005; Pfeifer et al, 2008). Although the functional consequences of decreased volume are not known, a study of adults with unipolar depression found an inverse correlation between amydalar volume and activation during emotion processing (Siegle et al., 2003). Thus, decreased amygdalar volume in youth with BD might be associated with increased amygdala activation to an emotional stimulus. In fact, increased activation of the amygdala has been reported in pediatric BD patients (Rich et al., 2006). Taken together, these findings are consistent with theories of mood dysregulation in BD that posit limbic hyperactivity and prefrontal cortex hypoactivity to psychosocial stressors (Chang et al., 2004; Blumberg et al., 2005).
In the current study, we used structural MRI to examine subcortical structures in BD offspring who already have ADHD and prominent mood symptoms. These individuals may already have early forms of BD and are at high risk for progressing to develop a full manic episode. Other groups have studied brain morphometry in BD offspring who are either healthy (Ladouceur et al., 2008; Hajek et al., 2009a), with depression or BD already (Hajek et al., 2009a) or mixed groups of healthy offspring and offspring with depression (Singh et al., 2008). Such healthy offspring are distinct from our sample, as they are less ill, and may even represent a resilient, rather than high-risk, sample. As we sought to investigate whether amygdalar abnormalities are present in youth with BD before the onset of mania, we chose a more symptomatic group who were more likely closer to their onset of mania, but not yet meeting criteria for a bipolar I or II disorder. We hypothesized that our high-risk subjects would have decreased amygdalar volume when compared to healthy controls. Furthermore, because of the variability of past studies regarding thalamus and hippocampus in youth with BD, we conducted exploratory analyses of these structures.
2. METHODS
This protocol was approved by the Stanford University Panel of Medical Research in Human Subjects. Twenty-two patients and twenty healthy volunteers were recruited from an ongoing study of BD offspring and from the community. Patients were included consecutively if they met inclusion criteria. Inclusion criteria for high-risk subjects were age 9 – 18 years, a biological parent with bipolar I or II disorder, and a diagnosis of “high-risk” for BD, as defined below. Exclusion criteria were presence of a pervasive development disorder (such as Autism or Aspergers Disorder), a neurological condition (such as a seizure disorder), a substance use disorder, IQ less than 80, or presence of metallic implants or orthodontic braces, which would make the MRI scan not feasible.
Oral and written consent from the parents as well as oral and written assent from the youth were obtained, and both the parents and the offspring were interviewed. At least one parent had BD I or II diagnosed by the Structured Clinical Interview for DSM-IV Axis I disorders (SCID) (First MB 1995), administered by a trained master's degree level clinician and/or a psychiatrist board certified in general psychiatry. Both parents were interviewed, and on occasion, the non-bipolar parent had an Axis I diagnosis within the high-risk group. For inclusion in the high-risk BD group, in addition to parental diagnosis of BD, all children met criteria for ADHD and had at least moderate mood symptoms, as indicated by a score of >10 on the Young Mania Rating Scale (YMRS, (Fristad et al., 1995)) or a score of >30 on the Children's Depressive Rating Scale – Revised (CDRS-R, (Poznanski et al., 1985)). Subjects could have depression or dysthymia, but not cyclothymia, bipolar I, or bipolar II disorder. Subjects were allowed to meet bipolar disorder, not otherwise specified (BD-NOS) criteria, defined as having either one less criterion B symptom than necessary for a (hypo)-manic episode, or enough criterion B symptoms but only a 2–3 day duration of the episode. All subjects, patients and healthy volunteers, were evaluated by the affective disorders module of the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) (Geller et al., 1996; Geller et al., 2001) and the Schedule for Affective Disorders and Schizophrenia for School-Age Children, Present and Lifetime (K-SADS-PL) (Kaufman et al., 1997). Researchers with at least a Masters degree and two years of clinical experience administered the KSADS-PL and WASH-U-KSADS. Diagnostic decisions were ultimately made by a board-certified child psychiatrist who reviewed the interview, and also performed a clinical interview on the child to confirm the diagnoses. . Inter-rater reliability was established at the outset by rating videotaped interviews, observing trained rater interviews, and performing interviews with observation by a trained rater, as described by Geller et al. (1998) (four consecutive patients with 100% agreement on diagnoses). The inter-rater reliability for diagnoses was a kappa of > 0.9. Current and lifetime diagnoses were established according to DSM-IV criteria.
Bipolar offspring had psychostimulants discontinued for at least 24 hours before the scan, primarily due to a concurrent functional MRI study of attention. They were allowed to continue any other current medications such as mood stabilizers or antidepressants due to the risk of mood destabilization. Medication history was obtained from interviews with subjects and parents and review of medical records when available. Past exposure to lithium or valproate was recorded if the subject had at least 6 months treatment of either agent at standard doses or serum levels.
For inclusion in the control group, healthy volunteers could not have a current or lifetime DSM-IV psychiatric diagnosis, neither parent had a lifetime or current psychiatric diagnosis by SCID, and the participant did not have a first or second degree relative with BD as determined by the Family History Research Diagnostic Criteria (Andreasen et al., 1977). None of the healthy control group parents had any Axis I diagnoses.
All the study participants and healthy volunteers were scanned on a GE 3Tesla scanner. Coronal 3D volumetric spoiled gradient echo (SPGR) series were obtained with the following parameters: TR = 35, TE = 6, flip angle = 45, slice thickness = 1.5 or 1.6 mm, and matrix = 256×192 for 124 slices. The number of scans using a 1.6 mm rather than a 1.5 mm slice thickness was not different between the at-risk and control groups (at-risk=5 of 22 scans; control=3 of 22 scans; chi-square(1)=.611, p=.43.)
The volumetric analysis was performed using BrainImageJava software v. 0.13.4 ((Reiss, 2002), Center for Interdisciplinary Brain Sciences Research, CIBSR; http://cibsr.stanford.edu) for semi-automated image processing and quantification.
Image processing included removal of non-brain tissue, correction of non-uniformity, and positional normalization to anterior and posterior commissures in a stereotactic space (Talairach and Tournoux, 1988). Each brain was divided into lobes with a semi-automated stereotactic-based parcellation method (Kates et al., 1999), based on the raters' identification of the anterior commissure, the posterior commissure, and a midsagittal point above the axis created by the first two points. Raters who conducted morphometric analyses were blind to the diagnosis of each subject. Voxels comprising brain tissue were then segmented into gray matter, white matter, and cerebrospinal fluid (CSF) using a semi-automated fuzzy tissue segmentation algorithm (Reiss et al., 1998). The total brain volume (TBV) was calculated as the sum of all brain regions. Total cerebral volume was calculated by adding cerebral total tissue with cortical and ventricular CSF. Total brain tissue was calculated by adding cerebral total tissue, cerebellar tissue, and brainstem tissue.
Subcortical regions were outlined manually by a rater who had demonstrated reliability with gold standard tracings established with an independent rater and dataset (single measure intraclass correlation coefficient) > 0.9). Regions were drawn on positionally normalized brain image stacks in the coronal orientation. Hippocampi were traced starting at the slice where a clear distinction between amygdala and hippocampus was first visible and outlined proceeding posteriorly until the structure disappeared. The superior white matter tract extending from the temporal lobe was used as an inferior border of the hippocampus, medial border was defined by CSF and by the pons, where present, and the lateral border was marked by CSF or white matter tracts on the lateral edge of the hippocampus.
Thalami were traced starting on the slice where the structure was first visible and followed until thalamic gray matter disappeared; the border between the gray matter of the thalamus and the surrounding white matter was used to outline the thalamus.
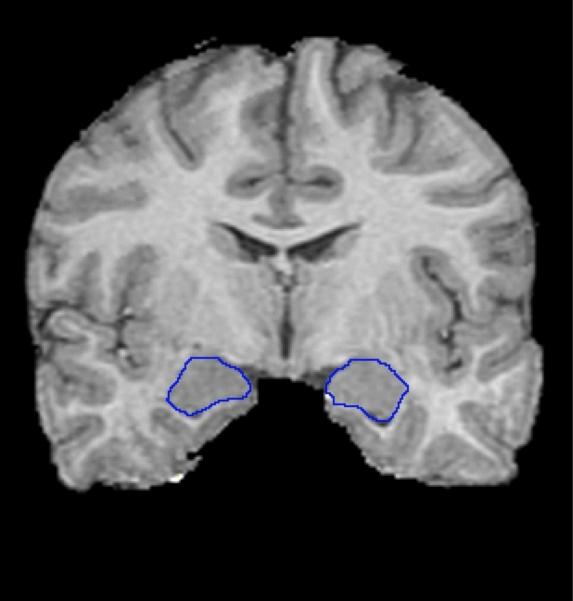
Amygdalae were traced starting on the slice demonstrating the thickest extent of the anterior commissure and following the structure towards the posterior end of the brain. The most superior white matter tract extending from the temporal lobe marked the inferior border, CSF marked the medial border, endorhinal sulcus marked the superior border, and a thick, central white matter tract of the temporal lobe was used as the lateral border of amygdala (Figure 1).
Figure 1.
Outline of the left and right amygdalae on the positionally normalized brain stack in coronal orientation. The most superior white matter tract extending from the temporal lobe marked the inferior border, CSF marked the medial border, endorhinal sulcus marked the superior border, and a thick, central white matter tract of the temporal lobe was used as the lateral border of amygdala.
2.1 Statistical Analysis
Independent t-tests were used to compare demographic measures and TBV in BD offspring and healthy controls. Brain volume data distributions were first examined for normality to confirm the assumptions of parametric statistics. One-way analyses of covariance (ANCOVAs) were used to compare brain structure volumes, using age and TBV as covariates. A p value of 0.05 (two-tailed) was chosen as the significance threshold. No corrections for multiple comparisons were made for these exploratory analyses. We calculated the effect size (Cohen's d) as the difference between the means (at-risk group mean volume minus control group mean volume for each region) divided by the pooled (average) standard deviation for that region.
3. RESULTS
6 out of the 28 bipolar offspring scans collected were excluded because of movement (21%). Therefore, 22 subjects at high-risk for BD, 15 males and 7 females, were included in this study. Eight of the subjects met our criteria for bipolar disorder, not otherwise specified (BD-NOS). Thirteen of the parents of the high-risk subjects had BD I and 9 had BD II. Fourteen of the parents also had a retrospective diagnosis of childhood ADHD. Mean age of the subjects was 12.3 ± 2.5 years (note: ± relates to standard deviation). The average duration of psychotropic medication exposure was 21 months. Twenty-two age, gender, handedness, and IQ matched healthy controls (13.1 ± 2.7 years old) comprised the comparison group (Table 1). The controls were individually chosen from the 29 usable control scans to group match the subjects in the study for age and gender. TBV was similar in the high-risk and control groups (1462.555 ± 150.254 cm3 versus 1493.255 ± 126.847 cm3, F (1, 41) = 0.69, p = 0.41) (see Table 2 for unadjusted means and SD). Nonetheless, due to a high correlation between total brain volume and total amygdala volume (r=0.49, p=.001), TBV was used as a covariate in the analysis of all the regions of interest. To be consistent with our previous manuscripts on the BD population, we included age as a covariate, although the group difference in mean age was not statistically significant. However, there was no correlation between age and brain volume in any region.
Table 1.
Demographics of Subjects
| Measure | Bipolar Offspring (n = 22) | Controls (n = 22) | P value |
|---|---|---|---|
| Mean age, yr (SD) | 12.3 (2.5) | 13.1 (2.7) | 0.31 |
| Gender | 15 males, 7 females | 15 males. 7 females | - |
| Socioeconomic status (SD) | 4.3 (.76) | 4.3 (.78) | 0.97 |
| Race Hispanic | 1 | 1 | 0.92 |
| Multiracial | 4 | 3 | |
| Caucasian | 17 | 18 | |
| IQ (SD) | 108 (13) | 114 (9) | 0.09 |
| Handedness | 20 R/2 L | 20 R/2 L | - |
| Diagnoses (%) | |||
| ADHD | 22 (100) | 0 (0) | - |
| Any Anxiety | 8 (36.4) | 0 (0) | - |
| MDD | 9 (40.9) | 0 (0) | |
| ODD | 9 (40.9) | 0 (0) | - |
| YMRS score | 13.8 (5.5) | - | - |
| CDRS-R score | 34 (7.6) | - | - |
| Dx of BD Parent | 13 BD I, 9 BD II 14 ADHD | - | - |
| Dx of non-BD parent | 2 SA, 2 MDD, 1 ADHD | ||
| Medication exposure (months) | |||
| Lithium | 3 (13.6) | 0 (0) | - |
| Stimulants | 7 (31.8) | 0 (0) | - |
| Valproate | 9 (40.9) | 0 (0) | - |
| Antipsychotics | 4 (18.2) | 0 (0) | - |
| Mean medication duration | 21.2 (26.3; range = 0−84 months) | 0(0) |
SD = standard deviation, R = right, L = left, ADHD = attention-deficit/hyperactivity disorder, MDD = major depressive disorder, ODD = oppositional defiant disorder, YMRS = Young Mania Rating Scale, CDRS-R = Children's Depression Rating Scale-Revised, Dx = diagnoses, BD = bipolar disorder, SA = substance abuse disorder, MDD = major depressive disorder
Table 2.
Brain Regional Volumes in Subsyndromal Bipolar Offspring and Controls (showing unadjusted means).
| Regions of Interest | Bipolar Offspring cm3 (SD) | Control cm3 (SD) | P value* |
|---|---|---|---|
| Total brain | 1462.555 | 1493.255 | 0.41 |
| volume | (150.254) | (126.847) | |
| Total cerebral | 686.277 | 699.668 | 0.85 |
| grey | (73.289) | (55.908) | |
| Total cerebral | 440.191 | 456.305 | 0.57 |
| white | (57.078) | (57.238) | |
| Total cerebral | 1126.455 | 1155.977 | 0.47 |
| tissue | (124.931) | (104.329) | |
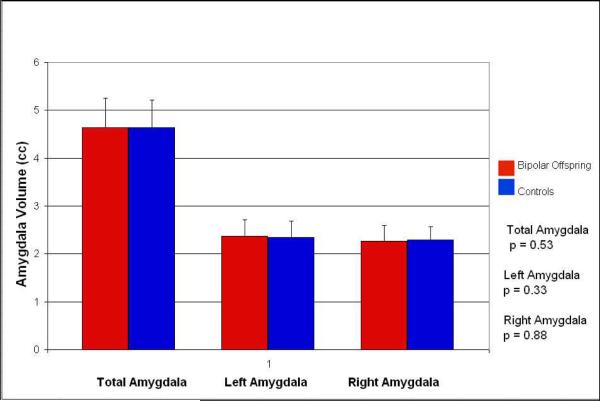
| Left amygdalar tissue | 2.368 (0.344) | 2.347 (0.341) | 0.33 |
| Right amygdalar tissue | 2.270 (0.328) | 2.293 (0.274) | 0.88 |
| Total amygdalar tissue | 4.640 (0.607) | 4.641 (0.567) | 0.53 |
| Left thalamus | 7.803 (0.669) | 8.007 (0.783) | 0.54 |
| Right thalamus | 7.741 (0.610) | 8.081 (0.816) | 0.20 |
| Total thalamus | 15.544 (1.264) | 16.088 (1.567) | 0.32 |
| Left hippocampus | 3.362 (0.348) | 3.599 (0.456) | 0.12 |
| Right hippocampus | 3.485 (0.407) | 3.657 (0.442) | 0.29 |
| Total hippocampus | 6.847 (0.721) | 7.256 (0.837) | 0.17 |
Total brain volume and age were used as covariates in the analysis in all of the regions of interest.
Covarying for age and TBV, total amygdalar volume in high-risk subjects was not significantly different from the amygdalar volume of the healthy controls (4.640 ± 0.607 cm3versus 4.641 ± 0.567 cm3, F (1, 40) = 0.409, p = 0.53), with neither the right amygdala (2.270 ± 0.328 cm3 versus 2.293 ± 0.274 cm3, F (1, 40) = 0.024, p = 0.88) nor the left amygdala significantly different in volume (2.368 ± 0.341 cm3 versus 2.347 ± 0.341 cm3, F (1, 40) = 0.982, p = 0.33), (see table 2 for unadjusted means and error bars). The effect size (Cohen's d) for the difference in left amygdala tissue volume was 0.062, for the right amygdala tissue 0.076, and for the total amygdala tissue 0.002. We also analyzed our data without the age covariate and these findings did not change. There was no significant effect of gender on any amygdala or hippocampus measure. The interaction between gender and diagnosis was also not significant.
In further exploratory analyses, there was no significant difference in thalamic volume (15.544 ± 1.264 cm3 versus 16.088 ± 1.568 cm3, F (1, 40) = 0.99, p = 0.32) or hippocampal volume in high-risk subjects compared with healthy controls (6.847 ± 0.721 cm3 versus 7.256 ± 0.837 cm3, F (1, 40) = 1.973, p = 0.17) (Table 2). The effect size (Cohen's d) for the difference in total thalamic volume was 0.382, and for the difference in total hippocampal volume was 0.524.
The TBV and amygdalar volumes of high-risk subjects exposed to lithium and/or valproate (12 subjects total) were not significantly different from those of subjects without these medication exposures (TBV: 1490.490 ± 147.155 cm3 versus 1439.275 ± 155.185 cm3, F (1, 19) = 0.53, p = 0.48; total amygdala tissue: 4.751 ± 0.784 cm3 versus 4.547 ± 0.423 cm3, F (1, 18) = 0.055, p = 0.82). No difference in total amygdalar volume was found between those subjects with an anxiety and/or depression diagnosis (9 subjects total) and those without (4.466 ± 0.601 versus 4.760 ± 0.604, F (1, 18) = 0.23, p = 0.64). There was no significant correlation between amygdala size and YMRS scores in the high-risk cohort (p = 0.909).
4. DISCUSSION
Decreased amygdalar volumes have been relatively consistently found in cohorts of children and adolescents with BD (Pfeifer et al., 2008). It is not known whether amygdalar volume abnormalities predate the onset of mania, possibly predisposing individuals to BD, or if they occur as a result of prolonged mood disorder episodes after the first manic episode. Early onset, or pediatric, bipolar samples have had somewhat distinct MRI findings so far, probably due to early onset status and relative lack of prolonged exposure to mood states, medications, and substances of abuse. The most consistent finding in pediatric age samples has been a decreased amygdalar volume. In a previous high risk sample that had already developed BD, our group did not find white matter hyperintensities or ventricular enlargement (Chang et al., 2004), but did find decreased amygdalar volume (Chang et al., 2005). Therefore, we focused on detecting amygdalar abnormalities in this study.
The current study aimed to address this question by examining children at high risk for BD development by virtue of their genetic susceptibility and symptoms of ADHD and mood dysregulation (Chang et al., 2004; Blumberg et al., 2005). We hypothesized that these children would have decreased amygdalar volumes compared with healthy controls, indicating a neuropathology that might be partially responsible for their mood difficulties and increased risk for full mania. As we found no significant difference between the amygdalar volumes of the BD offspring and healthy controls, our hypotheses were not supported. Thus, amygdalar volumes may be reduced compared with healthy controls only after a full manic episode and/or more prolonged illness. However, chronicity may produce different effects on amygdala volumes depending on age, as some studies have reported larger amygdala volumes in more heterogeneous samples of adults with BD (Strakowski et al., 1999; Altshuler et al., 2000), a finding supported by meta-analyses (Pfiefer et al., 2008; Hajek et al., 2009b). These results in adults with BD may also be due to external factors, such as substance abuse or, more likely, exposure to medications such as lithium (Chang et al., 2005; Foland et al., 2008).
Regardless, abnormalities of amygdalar structure may not predate a full bipolar diagnosis. To confirm this impression, longitudinal follow-up of these subjects to determine whether they do indeed develop fully characterized BD is necessary. Also, we found no significant thalamic or hippocampal volume differences between the BD offspring and the healthy controls. These findings are consistent with our earlier study of BD adolescents (Chang et al., 2005). However, hippocampal volume comparisons revealed a moderate effect size of 0.52, indicating that 93 subjects and controls would be needed at a power of 0.80 to reveal a statistically significant decrease in hippocampal volume in high-risk offspring. To detect decreased thalamic volume, 172 subjects would be needed. The effect size of total amygdalar volume difference (.002) was too low to even perform a meaningful power calculation to determine sample size needed to detect differences between groups. To our knowledge, ours is the first study to find no amygdalar, thalamus, or hippocampal volume differences in a pediatric cohort of symptomatic bipolar offspring who are high-risk for BD.
Both animal and human studies have established that the amygdala plays an important role in emotion-related processing (Garrett and Chang, 2008). The amygdala has been shown to be involved in the expression and acquisition of fear conditioning (Wilensky et al., 2006) and emotional responses to threatening stimuli (Izquierdo et al., 2005). Humans with bilateral amygdala damage have impaired judgment of emotional facial expressions (Adolphs and Tranel, 2004), and humans with unilateral or bilateral amygdala damage have impaired recognition of social emotions (Adolphs et al., 2002). Thus, abnormal amygdalar volume may indicate abnormal emotion perception and regulation.
BD offspring who are experiencing significant mood symptoms, and especially those already with ADHD, may have a prodromal form of BD, and, if untreated, may develop full BD. For example, in a large prospective naturalistic study of BD in children and adolescents, 25% of children with subsyndromal BD experienced a full manic or hypomanic episode within 24 months (Birmaher et al., 2006). Similarly, early childhood ADHD may represent a risk factor for BD development, as 28% of 81 children with ADHD developed BD over 6 years (Tillman and Geller, 2006). While some studies have not shown increased rates of ADHD among offspring of bipolar parents (Hillegers et al., 2005; Duffy et al., 2010; Shaw et al., 2005), others found that children with ADHD and a first degree relative with BD appear to be at high-risk for BD development, although the exact risk is unknown (Carlson and Weintraub, 1993; Faraone et al., 1997). Thus, while we cannot be certain that all of our high-risk subjects will develop DSM-IV bipolar I or II disorder, they do represent a population at fairly high risk for a full manic episode.
A possible explanation for our negative findings is that not all of our high-risk subjects will develop full mania. Thus, a subset of our cohort may develop mania, while the rest will not. Other studies of bipolar offspring have also reported normal amygdalar volumes (Ladouceur et al., 2008; Singh et al., 2008; Hajek et al., 2009a). However, these studies differed significantly from ours. First, Singh and colleagues (2008) studied 21 offspring who had various levels of psychopathology, ranging from healthy (24%), to ADHD (19%) to non-bipolar mood disorders such as dysthymia and depression (43%). Subjects were also younger than those in our study (mean age 9.7 years). Thus, this sample represented a younger, more heterogeneous and potentially less ill group than our sample. Ladouceur and colleagues studied 20 healthy offspring of parents with BD, none having a current DSM-IV diagnosis, with a mean age of 13 years. These researchers also used voxel-based morphometry and did not perform manual tracings on regions including the amygdala. Thus, this sample, being fairly old and free of psychopathology, might be considered a particularly low-risk “at-risk” group and may even be displaying neurobiological markers of resilience, such as the reported finding of increased volume of the left hippocampal gyrus (Ladouceur et al., 2008). The study by Hajek and colleagues (2009a) included a wider age range of at-risk subjects, from ages 15 to 30, included offspring of parents with MDD (but with a second-degree relative with BD), and their affected high-risk group included subjects with MDD as well as subjects with bipolar I or II disorder. Again, our sample represented a symptomatic, relatively homogenous group of bipolar offspring with ADHD and at least moderate symptoms of mania and depression, with 36% meeting BD NOS criteria. These children are arguably closer to a full manic episode (Birmaher et al., 2009), and thus one might expect to find decreased amygdalar volume in this cohort, which was not the case.
One significant limitation of our study is that all of the subjects in the high-risk group had ADHD, the presence of which might have an impact on our findings. While some studies indicate possible amygdalar and hippocampal abnormalities in the ADHD populations (Plessen et al., 2006; Brotman et al., 2010), others do not implicate the amygdala (Bush et al., 2005). A future study with a comparison group of non-bipolar offspring with ADHD would provide further insight into this issue. Nine of the subjects in the current study had anxiety and/or depression diagnoses. While there have been reports of amygdala abnormalities in populations with anxiety and/or depression, we did not find a significant difference in amygdala volume between these subjects and the other bipolar offspring, although it should be noted that these comparisons were underpowered and should be considered preliminary. Also, we studied only one subset of children likely to be prodromal for BD. Other studies should be conducted on BD offspring with unipolar depression and children with other forms of BD NOS.
The fact that some of our subjects were likely to be prepubertal while others are postpubertal is a possible confounding factor, as pubertal state may influence amygdalar volume (Kraemer et al., 2000). The subjects' medication exposure is another potentially confounding variable: 3 of our subjects had been exposed to lithium, 9 to valproate, and 4 to antipsychotics. While it has been postulated that such medications may increase cortical and subcortical gray matter volumes (Pfeifer et al., 2008), amygdalar volume was not statistically different between the participants with and without lithium or valproate exposure. However, psychotropic medications clearly affect brain function and structure, and may have had an effect on our results that was not detected because of the small sample size.
While it is challenging to study children at risk for psychiatric disorders, it may be the best method to determine “state vs. trait” effects and to identify characteristics that could indicate risk factors for illness. Our results are preliminary in some aspects and limited by the cross-sectional nature of our study, but these findings provide a rare initial view of the amygdala in high-risk individuals shortly before the possible onset of an initial manic episode. Examining this cohort over time to determine clinical outcome, and to observe the associated changes in brain structure would greatly improve our understanding of the susceptibilities, development and progression of BD in youth.
Figure 2.
Amygdala volumes in high-risk bipolar offspring (n=22) and healthy controls (n=22).
Acknowledgements
The authors gratefully acknowledge the assistance of Melody Chang for scan acquisition and Jessica Yee for data management. This work was supported in part by a grant from the Heinz C. Prechter Fund For Manic Depression, a NARSAD Young Investigators Award, a Klingenstein Third Generation Foundation Fellowship, a gift from the Hahn family, and NIH grant MH64460-01 (Dr. Chang). Dr. Chang receives research support from GlaxoSmithKline, NARSAD, and the NIMH. He is a consultant for Eli Lilly & Co., GlaxoSmithKline, Bristol Myers-Squibb and Merck, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of this manuscript were presented at the 53rd Annual Meeting of the American Academy of Child and Adolescent Psychiatry, San Diego, CA, October 25–30, 2006
The other authors have no conflicts of interest to disclose.
REFERENCES
- Adolphs R, Baron-Cohen S, Tranel D. Impaired recognition of social emotions following amygdala damage. Journal of Cognitive Neuroscience. 2002;14:1264–74. doi: 10.1162/089892902760807258. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D. Impaired judgments of sadness but not happiness following bilateral amygdala damage. Journal of Cognitive Neuroscience. 2004;16:453–462. doi: 10.1162/089892904322926782. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Bartzokis G, Grieder T, Curran J, Jimenez T, Leight K, Wilkins J, Gerner R, Mintz J. An MRI study of temporal lobe structures in men with bipolar disorder or schizophrenia. Biological Psychiatry. 2000;48:147–162. doi: 10.1016/s0006-3223(00)00836-2. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Bartzokis G, Grieder T, Curran J, Mintz J. Amygdala enlargement in bipolar disorder and hippocampal reduction in schizophrenia: an MRI study demonstrating neuroanatomic specificity. Archives of General Psychiatry. 1998;55:663–664. doi: 10.1001/archpsyc.55.7.663. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Bookheimer SY, Townsend J, Proenza MA, Eisenberger N, Sabb F, Mintz J, Cohen MS. Blunted activation in orbitofrontal cortex during mania: a functional magnetic resonance imaging study. Biological Psychiatry. 2005;58:763–769. doi: 10.1016/j.biopsych.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria. Reliability and validity. Archives of General Psychiatry. 1977;34:1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. The British Journal of Psychiatry. 2009;195:194–201. doi: 10.1192/bjp.bp.108.059717. [DOI] [PubMed] [Google Scholar]
- Bechdolf A, Nelson B, Cotton SM, Chanen A, Thompson A, Kettle J, Conus P, Amminger GP, Yung AR, Berk M, McGorry PD. A preliminary evaluation of the validity of at-risk criteria for bipolar disorders in help-seeking adolescents and young adults. Journal of Affective Disorders. 2010;127:316–320. doi: 10.1016/j.jad.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Monk K, Kalas C, Goldstein B, Hickey MB, Obreja M, Ehmann M, Iyengar S, Shamseddeen W, Kupfer D, Brent D. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring study. Archives of General Psychiatry. 2009;66:287–296. doi: 10.1001/archgenpsychiatry.2008.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Goldstein B, Monk K, Kalas C, Obreja M, Hickey MB, Iyengar S, Brent D, Shamseddeen W, Diler R, Kupfer D. Psychiatric disorders in preschool offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring Study (BIOS) American Journal of Psychiatry. 2010;167:321–330. doi: 10.1176/appi.ajp.2009.09070977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Strober M, Gill MK, Valeri S, Chiappetta L, Ryan N, Leonard H, Hunt J, Iyengar S, Keller M. Clinical course of children and adolescents with bipolar spectrum disorders. Archives of General Psychiatry. 2006;63:175–83. doi: 10.1001/archpsyc.63.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg HP, Fredericks C, Wang F, Kalmar JH, Spencer L, Papademetris X, Pittman B, Martin A, Peterson BS, Fulbright RK, Krystal JH. Preliminary evidence for persistent abnormalities in amygdala volumes in adolescents and young adults with bipolar disorder. Bipolar Disorders. 2005;7:570–576. doi: 10.1111/j.1399-5618.2005.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg HP, Kaufman J, J, Martin A, Whiteman R, Zhang JH, Gore JC, Charney DS, Krystal JH, Peterson BS. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Archives of General Psychiatry. 2003;60:1201–1208. doi: 10.1001/archpsyc.60.12.1201. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Martin A, Kaufman J, Leung HC, Skudlarski P, Lacadie C, Fulbright RK, Gore JC, Charney DS, Krystal JH, Peterson BS. Frontostriatal abnormalities in adolescents with bipolar disorder: preliminary observations from functional MRI. The American Journal of Psychiatry. 2003;160:1345–1347. doi: 10.1176/appi.ajp.160.7.1345. [DOI] [PubMed] [Google Scholar]
- Brotman MA, Rich BA, Guyer AE, Lunsford JR, Horsey SE, Reising MM, Thomas LA, Fromm SJ, Towbin K, Pine DS, Leibenluf E. Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. The American Journal of Psychiatry. 2010;167:61–69. doi: 10.1176/appi.ajp.2009.09010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Valera EM, Seidman LJ. Functional neuroimaging of attention-deficit/hyperactivity disorder: a review and suggested future directions. Biological Psychiatry. 2005;57:1273–1284. doi: 10.1016/j.biopsych.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Caetano SC, Sassi R, Brambilla P, Harenski K, Nicoletti M, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC. MRI study of thalamic volumes in bipolar and unipolar patients and healthy individuals. Psychiatry Research. 2001;108:161–168. doi: 10.1016/s0925-4927(01)00123-8. [DOI] [PubMed] [Google Scholar]
- Carlson GA, Weintraub S. Childhood behavior problems and bipolar disorder--relationship or coincidence? Journal of Affective Disorders. 1993;28:143–153. doi: 10.1016/0165-0327(93)90100-x. [DOI] [PubMed] [Google Scholar]
- Chang K, Adleman N, Dienes K, Simeonova DI, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder. Archives of General Psychiatry. 2004;61:781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- Chang K, Karchemskiy A, Barnea-Goraly N, Garrett A, Simeonova DI, Reiss A. Reduced amygdalar gray matter volume in familial pediatric bipolar disorder. Journal of American Academy of Child and Adolescent Psychiatry. 2005;44:565–573. doi: 10.1097/01.chi.0000159948.75136.0d. [DOI] [PubMed] [Google Scholar]
- Chang K, Steiner H, Dienes K, Adleman N, Ketter T. Bipolar offspring: a window into bipolar disorder evolution. Biological Psychiatry. 2003;53:945–951. doi: 10.1016/s0006-3223(03)00061-1. [DOI] [PubMed] [Google Scholar]
- Chang KD, Steiner H, Ketter TA. Psychiatric phenomenology of child and adolescent bipolar offspring. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:453–460. doi: 10.1097/00004583-200004000-00014. [DOI] [PubMed] [Google Scholar]
- Correll CU, Penzner JB, Lencz T, Auther A, Smith CW, Malhotra AK, Kane JM, Cornblatt BA. Early identification and high-risk strategies for bipolar disorder. Bipolar Disorders. 2007;9:324–338. doi: 10.1111/j.1399-5618.2007.00487.x. [DOI] [PubMed] [Google Scholar]
- Dasari M, Friedman L, Jesberger J, Stuve TA, Findling RL, Swales TP, Schulz SC. A magnetic resonance imaging study of thalamic area in adolescent patients with either schizophrenia or bipolar disorder as compared to healthy controls. Psychiatry Research. 1999;91:155–162. doi: 10.1016/s0925-4927(99)00028-1. [DOI] [PubMed] [Google Scholar]
- DelBello MP, Geller B. Review of studies of child and adolescent offspring of bipolar parents. Bipolar Disorders. 2001;3:325–334. doi: 10.1034/j.1399-5618.2001.30607.x. [DOI] [PubMed] [Google Scholar]
- DelBello MP, Strakowski SM, Zimmerman ME, Hawkins JM, Sax KW. MRI analysis of the cerebellum in bipolar disorder: a pilot study. Neuropsychopharmacology. 1999;21:63–68. doi: 10.1016/S0893-133X(99)00026-3. [DOI] [PubMed] [Google Scholar]
- DelBello MP, Zimmerman ME, Mills NP, Getz GE, Strakowski SM. Magnetic resonance imaging analysis of amygdala and other subcortical brain regions in adolescents with bipolar disorder. Bipolar Disorders. 2004;6:43–52. doi: 10.1046/j.1399-5618.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- Dewan MJ, Haldipur CV, Boucher MF, Ramachandran T, Major LF. Bipolar affective disorder. II. EEG, neuropsychological, and clinical correlates of CT abnormality. Acta Psychiatrica Scandinavica. 1988;77:677–682. doi: 10.1111/j.1600-0447.1988.tb05187.x. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Poynton AM, Bridges PK, Trimble MR. Altered magnetic resonance white-matter T1 values in patients with affective disorder. The British Journal Of Psychiatry: The Journal of Mental Science. 1990;157:107–110. doi: 10.1192/bjp.157.1.107. [DOI] [PubMed] [Google Scholar]
- Duffy A, Grof P, Hajek T, Alda M. Resolving the discrepancy in childhood bipolar high-risk study findings. American Journal of Psychiatry. 2010;167:716–717. doi: 10.1176/appi.ajp.2010.10020170. [DOI] [PubMed] [Google Scholar]
- Dupont RM, Jernigan TL, Heindel W, Butters N, Shafer K, Wilson T, Hesselink J, Gillin JC. Magnetic resonance imaging and mood disorders. Localization of white matter and other subcortical abnormalities. Archives of General Psychiatry. 1995;52:747–755. doi: 10.1001/archpsyc.1995.03950210041009. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Wozniak J, Mundy E, Mennin D, O'Donnell D. Is comorbidity with ADHD a marker for juvenile-onset mania? Journal of American Academy of Child and Adolescent Psychiatry. 1997;36:1046–1055. doi: 10.1097/00004583-199708000-00012. [DOI] [PubMed] [Google Scholar]
- First MB SR, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P, version 2.0) Biometric Research, New York State Psychiatric Institute; New York, NY: 1995. [Google Scholar]
- Foland LC, Altshuler LL, Sugar CA, Lee AD, Leow AD, Townsend J, Narr KL, Asuncion DM, Toga AW, Thompson PM. Increased volume of the amygdala and hippocampus in bipolar patients treated with lithium. Neuroreport. 2008;19(2):221–4. doi: 10.1097/WNR.0b013e3282f48108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier JA, Ahn MS, DeJong S, Bent EK, Breeze JL, Giuliano AJ. Magnetic resonance imaging studies in early-onset bipolar disorder: a critical review. Harvard Review of Psychiatry. 2005;13(3):125–140. doi: 10.1080/10673220591003597. [DOI] [PubMed] [Google Scholar]
- Frazier JA, Chiu S, Breeze JL, Makris N, Lange N, Kennedy DN, Herbert MR, Bent EK, Koneru VK, Dieterich ME, Hodge SM, Rauch SL, Grant PE, Cohen BM, Seidman LJ, Caviness VS, Biederman J. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. The American Journal of Psychiatry. 2005;162:1256–1265. doi: 10.1176/appi.ajp.162.7.1256. [DOI] [PubMed] [Google Scholar]
- Fristad MA, Weller RA, Weller EB. The Mania Rating Scale (MRS): further reliability and validity studies with children. Annals of Clinical Psychiatry. 1995;7:127–132. doi: 10.3109/10401239509149039. [DOI] [PubMed] [Google Scholar]
- Garrett A, Chang K. The role of the amygdala in bipolar disorder development. Development and Psychopathology. 2008;20:1285–1296. doi: 10.1017/S0954579408000618. [DOI] [PubMed] [Google Scholar]
- Geller B, Todd RD, Luby J, Botteron KN. Treatment-resistant depression in children and adolescents. The Psychiatric Clinics of North America. 1996;19:253–267. doi: 10.1016/s0193-953x(05)70287-2. [DOI] [PubMed] [Google Scholar]
- Geller B, Williams M, Zimerman B, Frazier J, Beringer L, Warner KL. Prepubertal and early adolescent bipolarity differentiate from ADHD by manic symptoms, grandiose delusions, ultra-rapid or ultradian cycling. Journal of Affective Disorders. 1998;51:81–91. doi: 10.1016/s0165-0327(98)00175-x. [DOI] [PubMed] [Google Scholar]
- Geller B, Zimerman B, Williams M, Bolhofner K, Craney JL, DelBello MP, Soutullo C. Reliability of the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) mania and rapid cycling sections. Journal of American Academy of Child and Adolescent Psychiatry. 2001;40:450–455. doi: 10.1097/00004583-200104000-00014. [DOI] [PubMed] [Google Scholar]
- Hajek T, Gunde E, Staney C, Propper L, MacQueen G, Duffy Al, Alda M. Amygdala and hippocampal volumes in relatives of patients with bipolar disorder: a high-risk study. Canadian Journal of psychiatry. 2009 Nov.54(11):726–33. doi: 10.1177/070674370905401102. [DOI] [PubMed] [Google Scholar]
- Hajek T, Kopecek M, Kozeny J, Gunde E, Alda M, Hoschl C. Amygdala volumes in mood disorders-meta-analysis of magnetic resonance volumetry studies. J Affective Disorders. 2009b;115(3):395–410. doi: 10.1016/j.jad.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Hauser P, Matochik J, Altshuler LL, Denicoff KD, Conrad A, Li X, Post RM. MRI-based measurements of temporal lobe and ventricular structures in patients with bipolar I and bipolar II disorders. Journal of Affective Disorders. 2000;60:25–32. doi: 10.1016/s0165-0327(99)00154-8. [DOI] [PubMed] [Google Scholar]
- Hillegers MH, Reichart CG, Wals M, Verhulst FC, Ormel J, Nolen WA. Five-year prospective outcome of psychopathology in the adolescent offspring of bipolar parents. Bipolar Disorders. 2005;7:344–505. doi: 10.1111/j.1399-5618.2005.00215.x. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. The Journal of Neuroscience. 2005;25:8534–8542. doi: 10.1523/JNEUROSCI.1232-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates WR, Warsofsky IS, Patwardhan A, Abrams MT, Liu AM, Naidu S, Kaufmann WE, Reiss AL. Automated Talairach atlas-based parcellation and measurement of cerebral lobes in children. Psychiatry Research. 1999;91:11–30. doi: 10.1016/s0925-4927(99)00011-6. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kempton MJ, Geddes JR, Ettinger U, Williams SC, Grasby PM. Meta-analysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder. Archives of General Psychiatry. 2008;65:1017–1032. doi: 10.1001/archpsyc.65.9.1017. [DOI] [PubMed] [Google Scholar]
- Kempton MJ, Haldane M, Jogia J, Grasby PM, Collier D, Frangou S. Dissociable brain structural changes associated with predisposition, resilience, and disease expression in bipolar disorder. The Journal of Neuroscience. 2009;29:10863–10868. doi: 10.1523/JNEUROSCI.2204-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Yesavage JA, Taylor JL, Kupfer D. How can we learn about developmental processes from cross-sectional studies, or can we? American Journal of Psychiatry. 2000;157:163–171. doi: 10.1176/appi.ajp.157.2.163. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Almeida JR, Birmaher B, Axelson DA, Nau S, Kalas C, Monk K, Kupfer DJ, Phillips ML. Subcortical gray matter volume abnormalities in healthy bipolar offspring: potential neuroanatomical risk marker for bipolar disorder? Journal of American Academy of Child and Adolescent Psychiatry. 2008;47:532–539. doi: 10.1097/CHI.0b013e318167656e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, O'Connor MM, Harral E, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biological Psychiatry. 2007;62:158–167. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Pearlson GD, Barta PE, Powers RE, Menon RR, Richards SS, Aylward EH, Federman EB, Chase GA, Petty RG, Tien AY. Ziskind-Somerfeld Research Award 1996. Medial and superior temporal gyral volumes and cerebral asymmetry in schizophrenia versus bipolar disorder. Biological Psychiatry. 1997;41:1–14. doi: 10.1016/s0006-3223(96)00373-3. [DOI] [PubMed] [Google Scholar]
- Pfeifer JC, Welge J, Strakowski SM, Adler CM, DelBello MP. Meta-analysis of amygdala volumes in children and adolescents with bipolar disorder. Journal of American Academy of Child and Adolescent Psychiatry. 2008;47:1289–1298. doi: 10.1097/CHI.0b013e318185d299. [DOI] [PubMed] [Google Scholar]
- Plessen KJ, Bansal R, Zhu H, Whiteman R, Amat J, Quackenbush GA, Martin L, Durkin K, Blair C, Royal J, Hugdahl K, Peterson BS. Hippocampus and amygdala morphology in attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2006;63:795–807. doi: 10.1001/archpsyc.63.7.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznanski E, Mokros HB, Grossman J, Freeman LN. Diagnostic criteria in childhood depression. The American Journal of Psychiatry. 1985;142:1168–1173. doi: 10.1176/ajp.142.10.1168. [DOI] [PubMed] [Google Scholar]
- Reiss A. BrainImage software. Stanford Psychiatry NeuroImaging Laboratory, Stanford University School of Medicine; Stanford, CA: 2002. [Google Scholar]
- Reiss AL, Hennessey JG, Rubin M, Beach L, Abrams MT, Warsofsky IS, Liu AM, Links JM. Reliability and validity of an algorithm for fuzzy tissue segmentation of MRI. Journal of Computer Assisted Tomography. 1998;22:471–479. doi: 10.1097/00004728-199805000-00021. [DOI] [PubMed] [Google Scholar]
- Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, Fromm SJ, Pine DS, Leibenluft E. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs GS, Baldassano CF, Truman CJ, Guille C. Comorbidity of attention deficit hyperactivity disorder with early- and late-onset bipolar disorder. American Journal of Psychiatry. 2000;157:466–468. doi: 10.1176/appi.ajp.157.3.466. [DOI] [PubMed] [Google Scholar]
- Shaw JA, Egeland JA, Endicott J, Allen CR, Hostetter AM. A 10-year prospective study of prodromal patterns for bipolar disorder among Amish youth. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:1104–1111. doi: 10.1097/01.chi.0000177052.26476.e5. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Konecky RO, Thase ME, Carter CS. Relationships between amygdala volume and activity during emotional information processing tasks in depressed and never-depressed individuals: an fMRI investigation. Annals of New York Academy of Sciences. 2003;985:481–484. doi: 10.1111/j.1749-6632.2003.tb07105.x. [DOI] [PubMed] [Google Scholar]
- Singh MK, Delbello MP, Adler CM, Stanford KE, Strakowski SM. Neuroanatomical characterization of child offspring of bipolar parents. Journal of American Academy of Child and Adolescent Psychiatry. 2008;47:526–531. doi: 10.1097/CHI.0b013e318167655a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll AL, Renshaw PF, Yurgelun-Todd DA, Cohen BM. Neuroimaging in bipolar disorder: what have we learned? Biological Psychiatry. 2000;48:505–517. doi: 10.1016/s0006-3223(00)00982-3. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Adler C, Cecil DM, Sax KW. Neuroimaging in bipolar disorder. Bipolar Disorders. 2000;2:148–164. doi: 10.1034/j.1399-5618.2000.020302.x. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Delbello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Molecular Psychiatry. 2005;10:105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, et al. Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Archives of General Psychiatry. 1999;56:254–260. doi: 10.1001/archpsyc.56.3.254. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Wilson DR, Sax KW, Zimmerman ME, Shear PK, Hawkins JM, Larson ER. Structural brain abnormalities in first-episode mania. Biological Psychiatry. 1993;33:602–609. doi: 10.1016/0006-3223(93)90098-x. [DOI] [PubMed] [Google Scholar]
- Swayze VW, 2nd, Andreasen NC, Alliger RJ, Yuh WT, Ehrhardt JC. Subcortical and temporal structures in affective disorder and schizophrenia: a magnetic resonance imaging study. Biological Psychiatry. 1992;31:221–240. doi: 10.1016/0006-3223(92)90046-3. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux . Co-planar stereotaxic atlas of the human brain. Thieme; New York: 1988. [Google Scholar]
- Tillman R, Geller B. Controlled study of switching from attention-deficit/hyperactivity disorder to a prepubertal and early adolescent bipolar I disorder phenotype during 6-year prospective follow-up: rate, risk, and predictors. Development and Psychopathology. 2006;18:1037–1053. doi: 10.1017/S0954579406060512. [DOI] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. The Journal of Neuroscience. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]