SUMMARY
This review focuses upon the regulatory mechanisms of adiponectin (APN) gene expression during physiologic conditions, and both the clinical significance and underlying molecular mechanisms of hypoadiponectinemia during pathologic conditions.
APN is a versatile cardiovascular protective factor. It plays an important role in regulating insulin sensitivity and energy homeostasis, with anti-inflammatory and anti-atherosclerotic properties.
APN gene expression is down-regulated in both obesity and type 2 diabetes mellitus. Hypoadiponectinemia is an independent risk factor for coronary artery disease in type 2 diabetic patients.
Exogenous supplementation of recombinant APN attenuates insulin resistance, improving metabolic disorders. Therefore, APN-targeted pharmaceutical strategies increasing circulating APN levels may be therapeutic against type 2 diabetes.
There is great value in elucidating the regulatory mechanisms of APN gene expression during physiologic and pathologic conditions. APN biosynthesis regulation includes transcriptional expression and post-translational modification, oligomerisation, and secretion. Under pathological conditions, including obesity and type 2 diabetes mellitus, hypoxia, oxidative stress, and inflammation suppress APN mRNA levels and its secretion.
Keywords: Adiponectin, Gene Regulation, Hypoadiponectinemia, Type 2 diabetes
1. Introduction
APN is predominantly expressed in adipose tissue in vivo, and during in vitro adipocyte differentiation.1 We recently demonstrated that cardiomyocytes also locally produce biologically active APN, but of vastly significantly lower quantity.2 APN consists of a secretory signal sequence, a non-conserved N-terminal domain followed by 22 collagen repeats, and a C-terminal globular domain.3 APN monomers assemble into higher-order multimer structures via post-translational modification (e.g., hydroxylation and glycosylation) of the collagenous domain. Under native conditions, circulatory serum APN manifests as 3 major oligomeric isoforms: trimer (~90 kDa), low molecular weight hexamer (~180 kDa), and high-molecular weight 12- to 18-mers (>300 kDa).4
APN is a versatile regulator of energy homeostasis, insulin sensitisation, inflammation/atherosclerotic processes, and anti-ischemic cardioprotection. It is closely involved with the pathogenesis of diabetes and cardiovascular diseases.5 We have demonstrated that APN supplementation significantly attenuates hyperlipidemia-induced rat endothelial dysfunction via reduction of oxidative and nitrative stress through differential regulation of endothelial nitric oxide synthase (eNOS) and inducible nitric oxide synthase (iNOS) activity.6 Recently, we and others report the protective role APN plays against myocardial ischaemia-reperfusion (MI/R) induced injury. APN-knockout animals manifest exacerbated MI/R injury. APN administration diminishes infarct size and apoptosis index in both APN-knockout and wild-type mice.7,8 Studies further demonstrate APN mechanistically inhibits iNOS and NADPH-oxidase (nicotinamide adenine dinucleotide phosphate-oxidase) protein expression, ameliorating resultant oxidative/nitrative stress.7 APN dysregulation disrupts normal adipose physiology, and has deleterious effects upon endothelial and cardiac function. As mechanisms regulating APN production and signaling are increasingly elucidated, insight regarding diabetes etiology and efficacious treatment continually improves.
2. APN Gene and Gene Promoter
The human APN gene is located on chromosome 3q27. Its allelic variants are genetically linked to type 2 diabetes mellitus. The mouse APN gene is mapped to the telomere of mouse chromosome 16, syntenic to the human chromosomal locus.9 A proximal 1 kb fragment of the mouse APN gene has adipose-specific promoter activity, with cis-elements for several adipogenic transcriptional factors. Deletion analysis of the human APN promoter demonstrates the (−676 to +41) region is sufficient for basal transcriptional activity. Bindingsites for multiple transcription factors are present in the APN promoter, including CCAAT box, PPRE (PPAR-responsive element), and sterol regulatory element (SRE).10 In addition, it contains several E-boxes carrying the consensus sequence CANNTG (where N is any nucleotide), which are recognised as the binding site for class I basic helix-loop-helix proteins, also known as E-proteins.11
3. Transcriptional Regulation of Adiponectin
APN is up-regulated during preadipocyte differentiation into mature adipocytes. While the precise transcriptional mechanisms of the APN gene remain elusive, multiple upstream signals mediating the process are known. Some transcription factors, such as PPARγ (peroxisome proliferator activated receptor gamma), SREBP-1c (sterol-regulatory-element-binding protein-1c), C/EBP-α (CCAAT-enhancer binding protein-α) and Foxo1 (forkhead box O1), positively regulate APN gene transcription. Several transcription factors, such as CREB (cAMP-response-element-binding protein), NFAT (nuclear factor of activated T-cells), and IGFBP-3 (Insulin-like growth factor binding protein-3), are repressors contributing to obesity-induced down-regulation of APN transcription.
Positive Transcription Factors
Peroxisome proliferator activated receptor gamma (PPAR-γ)
PPARγ, abundantly expressed in adipose tissue, acts as a ligand-activated transcription factor, and plays a key role in adipocyte-differentiation induction. Both human and mouse APN gene promoters contain a PPRE site. PPARγ forms a heterodimer with retinoid X receptor-α (RXRα), and PPARγ-RXRα heterodimer bound to PPRE found in the APN promoter regions and stimulates its transcription.12 PPARγ agonists significantly increase adipose APN mRNA levels, while PPARγ deletion in adipose markedly decreases plasma adiponectin.13 PPRE site point mutations decrease, while PPARγ agonists augment APN promoter transactivation.11 Together, these results suggest PPARγ stimulates APN gene transcription.
Sterol-regulatory-element-binding protein-1c (SREBP-1c)
In addition to PPREs, a putative SRE is required for basal human APN promoter activity. SREBPs can recognise and bind to SREs sequence TCACNCCAC to stimulate APN promoter activity and up-regulate APN transcription. SREBP-1c overexpression increases both APN mRNA and protein levels in 3T3-L1 adipocytes. SRE motif mutations abolish APN promoter activation by SREBP-1c.14 Other studies have demonstrated that SREBPs can recognise E-boxes in the APN promoter. SREBP-1c also promotes APN gene transcription via association with E47, another bHLH factor, and subsequent binding to E-boxes within the APN promoter.15
CCAAT-enhancer binding protein-α (C/EBPα)
C/EBPα is a major transcription factor transactivating adipose-specific genes, critical in adipogenesis. Whereas over-expression of endogenous C/EBPα increases APN mRNA levels in differentiated human adipocytes, siRNA (short interfering RNA) mediated knockdown of C/EBPα results in the opposite effect.16 Moreover, whereas exogenous PPARγ can stimulate adipocyte differentiation in C/EBPα deficient adipocytes and induce APN expression to some extent, full C/EBPα restoration markedly augments APN expression in response to PPARγ. Taken together, these results suggest complete APN gene transcription requires both PPARγ and C/EBPα.
Forkhead box O1 (Foxo1)
Involved in adipocyte differentiation, Foxo1 is a member of the forkhead transcription factor class O family. Two adjacent Foxo1 binding sites exist within the mouse APN promoter. Foxo1 gene haploinsufficiency or knockdown leads to significant reduction of APN gene expression,17 whereas Foxo1 overexpression increases APN expression.18 Foxo1 interacts with C/EBPα to form a transcription complex accessing the mouse APN promoter, up-regulating APN transcription. Foxo1 protein levels are significantly decreased in epididymal fat tissues from db/db and high fat diet-induced obese mice compared to normal mice. Decreased Foxo1 expression impairs proper Foxo1-C/EBPα complex formation, potentially contributory to diminished APN expression observed in obesity and type 2 diabetes.19
Negative transcription factors
cAMP-response-element-binding protein (CREB)
Recent studies indicate that CREB acts as a transcriptional repressor of APN expression.20 During fasting, CREB prompts hepatic gluconeogenic gene expression. CREB is activated in adipocytes during obesity. Transgenic mice expressing a dominant-negative adipocyte CREB transgene manifest augmented total-body insulin sensitivity, enhanced APN mRNA levels, and increased circulating HMW APN protein compared to wild-type controls.20 In obesity and diabetes, enhanced hepatic CREB activity contributes to hyperglycemia and systemic insulin resistance via down-regulated APN expression.21 CREB does not directly bind the APN promoter, but instead inhibits APN transcription by up-regulating transcriptional repressor ATF3(activating transcription factor 3), which represses APN promoter activity via putative AP-1 (activator protein-1) site binding.22
Nuclear factor of activated T-cells (NFATc4)
NFAT negatively regulates APN expression. NFATc4 over-expression reduces APN promoter activity, whereas removal of the putative NFATc4 binding site increases promoter activity. The binding activities of NFATc4 and ATF3 increase significantly in white adipose tissues of ob/ob and db/db mice compared to control, which may play a critical role in down-regulating APN expression in obesity and type 2 diabetes.22
Insulin-like growth factor binding protein-3 (IGFBP-3)
IGFBP-3 is a binding partner for the retinoid X receptor-α (RXR-α), the PPAR-γ heterodimer partner. The binding of IGFBP-3 and RXR-α23 can inhibit PPARγ agonist (such as rosiglitazone) stimulated promoter activity, summarily resulting in decreased APN transcription, whereas IGFBP-3 mutants incapable of binding RXR-α do not.24 IGFBP-3 expression can be induced by hypoxia or proinflammatory cytokine TNF-α.
4. Post-Transcriptional Regulation of Adiponectin
The contribution of the various APN isoforms to specific physiological processes remains incompletely understood. However, considerable evidence suggests the multimerised HMW oligomeric forms of APN are more potent than its trimeric and hexameric forms.25. The ratio of HMW to total APN correlates better with insulin sensitivity than total serum APN quantity in diabetic humans.26 However, it must be indicated that proteolytic cleavage product of full APN, globular domain of APN, possesses the most potent biological activity, although its circulation level is extremely low.
Regulation of APN multimerisation and secretion occurs via post-translational modifications (PTMs), including hydroxylation, glycosylation and disulfide bond formation. Such processes are critical for proper formation and maintenance of HMW oligomeric complexes.27 The trimer is formed via hydrophobic interactions between three APN monomer globular heads, stabilised by non-covalent interactions of the collagen-like domains in a triple-helix stalk. Intertrimer disulfide bonds are crucial for hexameric and HMW multimeric APN isoform assemblage. Cys-39 mediated disulfide bonds located at the N-terminal variable domain are required for assembly.4 Four conserved lysine residues (positions 68, 71, 80, and 104) within the collagen domain are sequentially modified by hydroxylation and glycosylation.28 If these four conserved lysines within the consensus GXKG(E/D) motif are mutated, HMW oligomers is not formed and APN inhibitory action upon hepatic gluconeogenesis is lost. In addition, seven conserved proline residues (positions 44, 47, 53, 71, 76, 91, and 95) within the collagen domain are hydroxylated to enhance tri-helical structure stability.29
Mutations in human APN (Arg112Cys and Ile164Thr) preventing trimer assemblage cause impaired cellular secretion. Gly84Arg and Gly90Ser mutants can assemble into trimers and hexamers, but cannot form HMW multimers. These impaired PTMs influence APN multimer distribution, a characteristic significantly related to hypoadiponectinemia and the diabetic phenotype.30
Recently, studies have identified several molecular chaperones controlling the biosynthesis and secretion of APN oligomers in adipocytes, including ERp44 (ER protein of 44 kDa), Ero1-Lα (ER oxidoreductase 1-L alpha), and DsbA-L (disulfide-bond A oxidoreductase-like protein). All work together to ensure correct tertiary and quaternary APN structure.31 ERp44 retains APN intracellularly, inhibiting APN oligomer secretion via thiol-mediated retention. In contrast, Ero1-Lα releases HMW APN trapped by ERp44.27 TZD (thiazolidinediones) selectively enhance HMW APN secretion via Ero1-Lα up-regulation and ERp44 down-regulation.32,33
Recently identified, DsbA-L is a key APN biosynthesis regulator, serving as a molecular chaperone of APN assembly and secretion.34 Abundantly expressed in adipose tissue, its expression level is significantly reduced in obese mice and human adipose. In 3T3-L1 adipocytes, DsbA-L expression is stimulated by PPARγ agonist rosiglitazone, and is inhibited by the inflammatory cytokine TNF-α.35 DsbA-L overexpression facilitates APN folding and assembly, increases HMW form production, and protects against ER stress-mediated APN down-regulation in obesity. siRNA-knockdown of DsbA-L markedly reduces APN secretion in 3T3-L1 adipocytes. These findings reveal significant positive correlations between adipocyte DsbA-L levels and APN production.36
PPARγ has been demonstrated to promote APN transcription. However, some studies demonstrate PPARγ agonist increases HMW isoform secretion without modifying APN mRNA expression and protein synthesis. Therefore, PPARγ may promote APN assembly and secretion.37,38 Purported mechanisms of PPARγ include up-regulating cellular levels of critical ER chaperones, including Ero1-Lα and DsbA-L, potentially leading to assembly stimulation, and HMW isoform release.35 Therefore, PPARγ acts as a regulator promoting APN biosynthesis, assembly, and secretion at the transcriptional, translational, and/or post-translational levels.
Additionally, APN concentrations are significantly greater in women than men, a phenomenon attributed to the inhibitory effect of testosterone upon APN secretion.39 Sexual dimorphism exists regarding oligomeric distribution, as males harbor a predominance of LMW adiponectin, and females manifest more balanced distribution of both LMW and HMW isoforms.40
5. Hypoadiponectinemia and Type 2 Diabetes
Clinical significance of hypoadiponectinemia
Although APN is produced nearly exclusively from adipose tissue, plasma APN concentrations are negatively correlated with visceral fat accumulation.41 APN expression is significantly downregulated in genetically obese (ob/ob), diabetic (db/db) mice, and high-fat diet induced obese mice. Epidemiological studies encompassing diverse ethnic and geographic sources demonstrate a negative correlation between body mass index/body fat and plasma APN levels.42 Insulin resistance, type 2 diabetes, and cardiovascular disease may all share a common mechanism involving hypoadiponectinemia.
Hotta et al43 demonstrated a close relationship between hypoadiponectinemia and insulin resistance in high-fat diet induced obese monkeys. Furthermore, APN levels declined prior to insulin sensitivity decline, suggesting APN deficiency is one of the mechanisms accounting for the pathogenesis of insulin resistance in obesity. In contrast, serum APN augmentation was accompanied by weight loss and decreased plasma glucose, free fatty acids, and triglycerides, markedly enhancing insulin ability to suppress glucose production without stimulating insulin secretion.5
A strong association between hypoadiponectinemia and type 2 diabetes exists.44 Both adipose APN mRNA expression and circulating APN levels are significantly reduced in most rodent models of type 2 diabetes, as well as type 2 diabetes patients.45,46 The degree of glycosylated APN and HMW:total adiponectin ratio were significantly decreased in type 2 diabetic patients compared to healthy controls.28 Conversely, high APN levels are associated with reduced risk of developing diabetes.47
Hypoadiponectinemia is not only a predictor of type 2 diabetes, but also severity of macroangiopathy and coronary artery disease (CAD). Diabetic patients with CAD have decreased APN levels compared to those without CAD. Moreover, a number of cross-sectional and prospective studies have demonstrated the association of hypoadiponectinemia with CAD.42,48 Hypoadiponectinemia (<4.0 mg/mL) doubled CAD prevalence, independent of well-known CAD risk factors (such as smoking or elevated BMI).49 In contrast, high plasma APN levels are associated with lower CAD risk and acute myocardial infarction.50 Recent clinical observations have demonstrated that plasma APN levels correlated positively with post-MI myocardial salvage index and ejection fraction recovery.51 Additionally, hypoadiponectinemia is also associated with other cardiovascular diseases, such as hypertension, diabetic cardiomyopathy, and certain inflammatory diseases.52
Mechanisms of hypoadiponectinemia in type 2 diabetes
The underlying mechanism responsible for hypoadiponectinemia in the obese state is obscure. In obesity, increased fat mass results in adipose tissue hypoxia (ATH), increasing endoplasmic reticular (ER) stress. Obesity also induces macrophage filtration into adipocytes, resulting in a low-grade chronic inflammatory state accompanied by increased production of pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-18. These factors inducing hypoadiponectinemia may be involved with onset of type 2 diabetes.
Obesity, as well as diabetes, causes ER stress, a critical element mediating APN down-regulation.53,54 APN mRNA expression in adipose tissue of obese mice is negatively correlated with ER stress marker CHOP (C/EBP homologous protein) expression levels. CHOP expression attenuates APN promoter activity, and RNA interference of CHOP partly reverses suppression of APN mRNA expression in adipocytes.55
Serum concentrations of pro-inflammatory cytokines are elevated in obesity and type 2 diabetes, which can suppress APN mRNA levels, and its secretion in 3T3-L1 adipocytes. IL-18 selectively suppresses APN expression via ERK1/2-dependent NFATc4 activation. Pre-treatment of cells with pharmacological inhibitors of p44/42 MAPK (mitogen-activated protein kinase) partly reverses the inhibitory effect of IL-6 upon APN production. Most significantly, plasma APN levels are inversely correlated with TNF-α concentration.45 Increased TNF-α can significantly decrease APN expression and secretion in both dose and time-dependent manner; TNF-α also decreases rosiglitazone-stimulated APN promoter activity in adipocytes.24 Several mechanisms have been proposed to explain the association between TNFα and decreased APN expression. Firstly, TNFα decreases APN production by suppressing the expression levels of several factors promoting APN specifically, such as PPARγ, C/EBP, SREBP, DsbA-L, and retinoid X receptor-α. Secondly, TNFα induces production of inhibitory transcription factor IGFBP-3, which suppresses APN transcription and induces insulin resistance.56,57 Finally, TNF-α inhibits APN expression by activating JNK and nuclear factor κB systems.58,59
Finally, catecholamines, glucocorticoids, and β-adrenergic stimulators are hormones associated with insulin resistance and obesity; their activation down-regulates APN expression and secretion in adipocytes in vitro. Isoproterenol treatment reduces APN mRNA levels in dose-dependent fashion, and such inhibitory effect was almost completely reversed by β-adrenergic antagonist propranolol.60 Dexamethasone significantly inhibits APN release from obese subject adipocytes.61
Hypoadiponectinemia therapy
Clinical studies suggest that hypoadiponectinemia contributes to insulin resistance, and elevated serum APN levels are associated with increased insulin sensitivity and reduced cardiovascular injury. APN has therefore been an attractive development target for pharmaceutics treating of insulin resistance, type 2 diabetes, and other related cardiovascular diseases.
The insulin-sensitising thiazolidinediones (TZDs), specific agonist ligands for PPAR-γ, have been widely used to treat type 2 diabetes. PPARγ agonists, such as rosiglitazone and pioglitazone, significantly augment circulatory APN levels, particularly the HMW APN isoform,40 in diabetic patients.62 In cultured 3T3-L1 adipocytes, PPAR-γ agonists enhance APN mRNA expression and secretion in dose- and time-dependent manner,63 and reverse the inhibitory effect of TNFα upon APN secretion.64
PPAR-γ agonists achieve their insulin sensitisation and metabolic benefits via enhanced production and signal transduction of APN.63,65 In myocardial ischaemia/reperfusion processes, rosiglitazone directly protects ischemic cardiomyocytes, attenuates oxidative stress, and improves cardiac function.66 Rosiglitazone-induced APN overproduction and cardioprotection is completely abolished in APN knockout mice, suggesting rosiglitazone-associated APN production is causatively related to PPAR-γ agonist-mediated cardioprotection.67
6. Conclusion
APN is a protein hormone of nearly exclusively adipose origin. It plays an important role in regulating insulin sensitivity and energy homeostasis, and exerts anti-inflammatory and anti-atherosclerotic properties. Up-regulated during preadipocyte differentiation into mature adipocytes, APN transcription is regulated by various factors, such as PPARγ, C/EBP-α, SREBP-1c, Foxo1, CREB, NFATc4, and IGFBP-3. Regulation of APN multimerisation and secretion occurs via post-translational modifications (PTMs), including hydroxylation, glycosylation, and disulfide bond formation. Several molecular chaperones are involved in APN PTM, including ERp44, Ero1-Lα, and DsbA-L. In the obese and type 2 diabetic state, circulating APN level and expression are down-regulated. Clinical investigations have identified hypoadiponectinemia as an independent risk factor for type 2 diabetes and CAD. Furthermore, hypoadiponectinemia is involved with pathophysiological changes in adiposity, type 2 diabetes, and resultant cardiovascular abnormalities. Hypoxia, oxidative stress, and inflammation attenuate APN production. TNFα, IL-6, and IL-18 suppress APN mRNA levels and its secretion. TZDs, and other PPARγ agonists, increase circulating APN levels, providing potential therapeutic strategy against the metabolic syndrome and type 2 diabetes.
In this review, we have elucidated current information regarding molecular mechanisms regulating APN transcription, its post-translational modifications, oligomerisation, and secretion under physiological conditions. We have also dissected the pathological mechanisms responsible for APN down-regulation, and its clinical significance in type 2 diabetes.
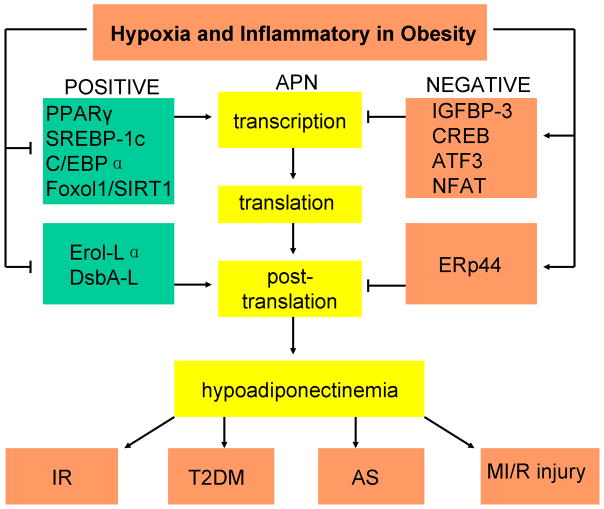
Figure 1. Relationships and mechanisms underlying obesity and hypoadiponectinemia, and the consequences of hypoadiponectinemia.
Many transcription factors positively and negatively regulate adiponectin (APN) gene expression. Positive transcription factors, such as PPARγ, SREBP-1c, C/EBPα, and Foxol1/SIRT1, increase APN gene transcription. Negative transcription factors, such as IGFBP-3, CREB, ATF3, and NFAT, suppress APN gene transcription. Several molecular chaperones regulate the post-translation of APN, including Ero1-Lα, DsbA-L and ERp44. ERp44 retains APN intracellularly, while Ero1-Lα and DsbA-L facilitate APN multimerisation and secretion. In obesity, increased fat mass results in adipose tissue hypoxia, increasing endoplasmic reticular stress. Obesity is a low-grade chronic inflammatory state. ER stress and increased pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-18 can inhibit the effect of positive transcription and post-translation factors, inducing production of inhibitory transcription factors, resulting in hypoadiponectinemia. Insulin resistance, type 2 diabetes, atherosclerosis formation, and increased myocardial ischaemia/reperfusion injury are all consequences of hypoadiponectinemia, and may all share a common etiological mechanism.
Acknowledgments
This research was supported by the following grants: NIH HL-63828, HL-96686 and American Diabetes Association 7-11-BS-93.
List of abbreviations
- APN
adiponectin
- MI/R
myocardial ischaemia/reperfusion
- C/EBP-α
CCAAT-enhancer binding protein-α
- CREB
cAMP-response-element-binding protein
- DsbA-L
disulfide-bond A oxidoreductase-like protein
- Ero1-Lα
ER oxidoreductase 1-L alpha
- ERp44
ER protein of 44 kDa
- Foxo1
forkhead boxhead protein O1
- IGFBP-3
insulin-like growth factor binding protein-3
- NFAT
nuclear factor of activated T-cells
- PPARγ
peroxisome proliferator-activated receptor γ
- PPRE
putative peroxisome proliferator response element
- SREBP-1c
sterol-regulatory-element-binding protein-1c
- TNFα
tumor necrosis factor alpha
- TZD
thiazolidinedione
References
- 1.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–9. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Lau WB, Gao E, et al. Cardiomyocyte-derived adiponectin is biologically active in protecting against myocardial ischemia-reperfusion injury. Am J Physiol Endocrinol Metab. 2009;298:E663–70. doi: 10.1152/ajpendo.00663.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shapiro L, Scherer PE. The crystal structure of a complement-1q family protein suggests an evolutionary link to tumor necrosis factor. Curr Biol. 1998;8:335–8. doi: 10.1016/s0960-9822(98)70133-2. [DOI] [PubMed] [Google Scholar]
- 4.Pajvani UB, Du X, Combs TP, et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–85. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 5.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–53. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 6.Li R, Wang WQ, Zhang H, et al. Adiponectin improves endothelial function in hyperlipidemic rats by reducing oxidative/nitrative stress and differential regulation of eNOS/iNOS activity. Am J Physiol Endocrinol Metab. 2007;293:E1703–8. doi: 10.1152/ajpendo.00462.2007. [DOI] [PubMed] [Google Scholar]
- 7.Tao L, Gao E, Jiao X, et al. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation. 2007;115:1408–16. doi: 10.1161/CIRCULATIONAHA.106.666941. [DOI] [PubMed] [Google Scholar]
- 8.Shibata R, Sato K, Pimentel DR, et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das K, Lin Y, Widen E, Zhang Y, Scherer PE. Chromosomal localization, expression pattern, and promoter analysis of the mouse gene encoding adipocyte-specific secretory protein Acrp30. Biochem Biophys Res Commun. 2001;280:1120–9. doi: 10.1006/bbrc.2001.4217. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi M, Arita Y, Yamagata K, et al. Genomic structure and mutations in adipose-specific gene, adiponectin. Int J Obes Relat Metab Disord. 2000;24:861–8. doi: 10.1038/sj.ijo.0801244. [DOI] [PubMed] [Google Scholar]
- 11.Iwaki M, Matsuda M, Maeda N, et al. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes. 2003;52:1655–63. doi: 10.2337/diabetes.52.7.1655. [DOI] [PubMed] [Google Scholar]
- 12.Yu JG, Javorschi S, Hevener AL, et al. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002;51:2968–74. doi: 10.2337/diabetes.51.10.2968. [DOI] [PubMed] [Google Scholar]
- 13.He W, Barak Y, Hevener A, et al. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci U S A. 2003;100:15712–7. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seo JB, Moon HM, Noh MJ, et al. Adipocyte determination- and differentiation-dependent factor 1/sterol regulatory element-binding protein 1c regulates mouse adiponectin expression. J Biol Chem. 2004;279:22108–17. doi: 10.1074/jbc.M400238200. [DOI] [PubMed] [Google Scholar]
- 15.Doran AC, Meller N, Cutchins A, et al. The helix-loop-helix factors Id3 and E47 are novel regulators of adiponectin. Circ Res. 2008;103:624–34. doi: 10.1161/CIRCRESAHA.108.175893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiao L, Maclean PS, Schaack J, et al. C/EBPalpha regulates human adiponectin gene transcription through an intronic enhancer. Diabetes. 2005;54:1744–54. doi: 10.2337/diabetes.54.6.1744. [DOI] [PubMed] [Google Scholar]
- 17.Nakae J, Kitamura T, Kitamura Y, Biggs WH, 3rd, Arden KC, Accili D. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev Cell. 2003;4:119–29. doi: 10.1016/s1534-5807(02)00401-x. [DOI] [PubMed] [Google Scholar]
- 18.Banks AS, Kon N, Knight C, et al. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–41. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiao L, Shao J. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex. J Biol Chem. 2006;281:39915–24. doi: 10.1074/jbc.M607215200. [DOI] [PubMed] [Google Scholar]
- 20.Qi L, Saberi M, Zmuda E, et al. Adipocyte CREB promotes insulin resistance in obesity. Cell Metab. 2009;9:277–86. doi: 10.1016/j.cmet.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang JW, Klemm DJ, Vinson C, Lane MD. Role of CREB in transcriptional regulation of CCAAT/enhancer-binding protein beta gene during adipogenesis. J Biol Chem. 2004;279:4471–8. doi: 10.1074/jbc.M311327200. [DOI] [PubMed] [Google Scholar]
- 22.Kim HB, Kong M, Kim TM, et al. NFATc4 and ATF3 negatively regulate adiponectin gene expression in 3T3-L1 adipocytes. Diabetes. 2006;55:1342–52. doi: 10.2337/db05-1507. [DOI] [PubMed] [Google Scholar]
- 23.Liu B, Lee HY, Weinzimer SA, et al. Direct functional interactions between insulin-like growth factor-binding protein-3 and retinoid X receptor-alpha regulate transcriptional signaling and apoptosis. J Biol Chem. 2000;275:33607–13. doi: 10.1074/jbc.M002547200. [DOI] [PubMed] [Google Scholar]
- 24.Zappala G, Rechler MM. IGFBP-3, hypoxia and TNF-alpha inhibit adiponectin transcription. Biochem Biophys Res Commun. 2009;382:785–9. doi: 10.1016/j.bbrc.2009.03.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aso Y, Yamamoto R, Wakabayashi S, et al. Comparison of serum high-molecular weight (HMW) adiponectin with total adiponectin concentrations in type 2 diabetic patients with coronary artery disease using a novel enzyme-linked immunosorbent assay to detect HMW adiponectin. Diabetes. 2006;55:1954–60. doi: 10.2337/db05-1525. [DOI] [PubMed] [Google Scholar]
- 26.Fisher FF, Trujillo ME, Hanif W, et al. Serum high molecular weight complex of adiponectin correlates better with glucose tolerance than total serum adiponectin in Indo-Asian males. Diabetologia. 2005;48:1084–7. doi: 10.1007/s00125-005-1758-7. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Lam KS, Yau MH, Xu A. Post-translational modifications of adiponectin: mechanisms and functional implications. Biochem J. 2008;409:623–33. doi: 10.1042/BJ20071492. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Lam KS, Chan L, et al. Post-translational modifications of the four conserved lysine residues within the collagenous domain of adiponectin are required for the formation of its high molecular weight oligomeric complex. J Biol Chem. 2006;281:16391–400. doi: 10.1074/jbc.M513907200. [DOI] [PubMed] [Google Scholar]
- 29.Richards AA, Stephens T, Charlton HK, et al. Adiponectin multimerization is dependent on conserved lysines in the collagenous domain: evidence for regulation of multimerization by alterations in posttranslational modifications. Mol Endocrinol. 2006;20:1673–87. doi: 10.1210/me.2005-0390. [DOI] [PubMed] [Google Scholar]
- 30.Waki H, Yamauchi T, Kamon J, et al. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278:40352–63. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 31.Wang ZV, Scherer PE. DsbA-L is a versatile player in adiponectin secretion. Proc Natl Acad Sci U S A. 2008;105:18077–8. doi: 10.1073/pnas.0810027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips SA, Kung J, Ciaraldi TP, et al. Selective regulation of cellular and secreted multimeric adiponectin by antidiabetic therapies in humans. Am J Physiol Endocrinol Metab. 2009;297:E767–73. doi: 10.1152/ajpendo.00378.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Long Q, Lei T, Feng B, et al. Peroxisome proliferator-activated receptor-gamma increases adiponectin secretion via transcriptional repression of endoplasmic reticulum chaperone protein ERp44. Endocrinology. 2010;151:3195–203. doi: 10.1210/en.2009-1501. [DOI] [PubMed] [Google Scholar]
- 34.Wang A, Liu M, Liu X, et al. Up-regulation of adiponectin by resveratrol: the essential roles of the Akt/FOXO1 and AMP-activated protein kinase signaling pathways and DsbA-L. J Biol Chem. 2010;286:60–6. doi: 10.1074/jbc.M110.188144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu M, Zhou L, Xu A, et al. A disulfide-bond A oxidoreductase-like protein (DsbA-L) regulates adiponectin multimerization. Proc Natl Acad Sci U S A. 2008;105:18302–7. doi: 10.1073/pnas.0806341105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou L, Liu M, Zhang J, Chen H, Dong LQ, Liu F. DsbA-L alleviates endoplasmic reticulum stress-induced adiponectin downregulation. Diabetes. 2010;59:2809–16. doi: 10.2337/db10-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banga A, Unal R, Tripathi P, et al. Adiponectin translation is increased by the PPARgamma agonists pioglitazone and omega-3 fatty acids. Am J Physiol Endocrinol Metab. 2009;296:E480–9. doi: 10.1152/ajpendo.90892.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qiang L, Wang H, Farmer SR. Adiponectin secretion is regulated by SIRT1 and the endoplasmic reticulum oxidoreductase Ero1-L alpha. Mol Cell Biol. 2007;27:4698–707. doi: 10.1128/MCB.02279-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cnop M, Havel PJ, Utzschneider KM, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46:459–69. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 40.Pajvani UB, Hawkins M, Combs TP, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152–62. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 41.Mazzali G, Di Francesco V, Zoico E, et al. Interrelations between fat distribution, muscle lipid content, adipocytokines, and insulin resistance: effect of moderate weight loss in older women. Am J Clin Nutr. 2006;84:1193–9. doi: 10.1093/ajcn/84.5.1193. [DOI] [PubMed] [Google Scholar]
- 42.Koenig W, Khuseyinova N, Baumert J, Meisinger C, Lowel H. Serum concentrations of adiponectin and risk of type 2 diabetes mellitus and coronary heart disease in apparently healthy middle-aged men: results from the 18-year follow-up of a large cohort from southern Germany. J Am Coll Cardiol. 2006;48:1369–77. doi: 10.1016/j.jacc.2006.06.053. [DOI] [PubMed] [Google Scholar]
- 43.Hotta K, Funahashi T, Bodkin NL, et al. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes. 2001;50:1126–33. doi: 10.2337/diabetes.50.5.1126. [DOI] [PubMed] [Google Scholar]
- 44.Lindsay RS, Funahashi T, Hanson RL, et al. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360:57–8. doi: 10.1016/S0140-6736(02)09335-2. [DOI] [PubMed] [Google Scholar]
- 45.Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003;52:1779–85. doi: 10.2337/diabetes.52.7.1779. [DOI] [PubMed] [Google Scholar]
- 46.Snehalatha C, Mukesh B, Simon M, Viswanathan V, Haffner SM, Ramachandran A. Plasma adiponectin is an independent predictor of type 2 diabetes in Asian indians. Diabetes Care. 2003;26:3226–9. doi: 10.2337/diacare.26.12.3226. [DOI] [PubMed] [Google Scholar]
- 47.Knobler H, Benderly M, Boyko V, et al. Adiponectin and the development of diabetes in patients with coronary artery disease and impaired fasting glucose. Eur J Endocrinol. 2006;154:87–92. doi: 10.1530/eje.1.02054. [DOI] [PubMed] [Google Scholar]
- 48.Schulze MB, Shai I, Rimm EB, Li T, Rifai N, Hu FB. Adiponectin and future coronary heart disease events among men with type 2 diabetes. Diabetes. 2005;54:534–9. doi: 10.2337/diabetes.54.2.534. [DOI] [PubMed] [Google Scholar]
- 49.Kumada M, Kihara S, Sumitsuji S, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–9. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 50.Frystyk J, Berne C, Berglund L, Jensevik K, Flyvbjerg A, Zethelius B. Serum adiponectin is a predictor of coronary heart disease: a population-based 10-year follow-up study in elderly men. J Clin Endocrinol Metab. 2007;92:571–6. doi: 10.1210/jc.2006-1067. [DOI] [PubMed] [Google Scholar]
- 51.Shibata R, Numaguchi Y, Matsushita K, et al. Usefulness of adiponectin to predict myocardial salvage following successful reperfusion in patients with acute myocardial infarction. Am J Cardiol. 2008;101:1712–5. doi: 10.1016/j.amjcard.2008.02.057. [DOI] [PubMed] [Google Scholar]
- 52.Iwashima Y, Katsuya T, Ishikawa K, et al. Hypoadiponectinemia is an independent risk factor for hypertension. Hypertension. 2004;43:1318–23. doi: 10.1161/01.HYP.0000129281.03801.4b. [DOI] [PubMed] [Google Scholar]
- 53.Gregor MF, Hotamisligil GS. Thematic review series: Adipocyte Biology. Adipocyte stress: the endoplasmic reticulum and metabolic disease. J Lipid Res. 2007;48:1905–14. doi: 10.1194/jlr.R700007-JLR200. [DOI] [PubMed] [Google Scholar]
- 54.Zhou L, Liu F. Autophagy: roles in obesity-induced ER stress and adiponectin downregulation in adipocytes. Autophagy. 2010;6:1196–7. doi: 10.4161/auto.6.8.13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hosogai N, Fukuhara A, Oshima K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–11. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 56.Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R. Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2002;290:1084–9. doi: 10.1006/bbrc.2001.6307. [DOI] [PubMed] [Google Scholar]
- 57.Kim HS, Ali O, Shim M, et al. Insulin-like growth factor binding protein-3 induces insulin resistance in adipocytes in vitro and in rats in vivo. Pediatr Res. 2007;61:159–64. doi: 10.1203/pdr.0b013e31802d8a30. [DOI] [PubMed] [Google Scholar]
- 58.Kim KY, Kim JK, Jeon JH, Yoon SR, Choi I, Yang Y. c-Jun N-terminal kinase is involved in the suppression of adiponectin expression by TNF-alpha in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2005;327:460–7. doi: 10.1016/j.bbrc.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 59.Kamon J, Yamauchi T, Muto S, et al. A novel IKKbeta inhibitor stimulates adiponectin levels and ameliorates obesity-linked insulin resistance. Biochem Biophys Res Commun. 2004;323:242–8. doi: 10.1016/j.bbrc.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 60.Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R. Adiponectin gene expression is inhibited by beta-adrenergic stimulation via protein kinase A in 3T3-L1 adipocytes. FEBS Lett. 2001;507:142–6. doi: 10.1016/s0014-5793(01)02960-x. [DOI] [PubMed] [Google Scholar]
- 61.Degawa-Yamauchi M, Moss KA, Bovenkerk JE, et al. Regulation of adiponectin expression in human adipocytes: effects of adiposity, glucocorticoids, and tumor necrosis factor alpha. Obes Res. 2005;13:662–9. doi: 10.1038/oby.2005.74. [DOI] [PubMed] [Google Scholar]
- 62.Miyazaki Y, Mahankali A, Wajcberg E, Bajaj M, Mandarino LJ, DeFronzo RA. Effect of pioglitazone on circulating adipocytokine levels and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2004;89:4312–9. doi: 10.1210/jc.2004-0190. [DOI] [PubMed] [Google Scholar]
- 63.Combs TP, Wagner JA, Berger J, et al. Induction of adipocyte complement-related protein of 30 kilodaltons by PPARgamma agonists: a potential mechanism of insulin sensitization. Endocrinology. 2002;143:998–1007. doi: 10.1210/endo.143.3.8662. [DOI] [PubMed] [Google Scholar]
- 64.Maeda N, Takahashi M, Funahashi T, et al. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50:2094–9. doi: 10.2337/diabetes.50.9.2094. [DOI] [PubMed] [Google Scholar]
- 65.Chinetti G, Zawadski C, Fruchart JC, Staels B. Expression of adiponectin receptors in human macrophages and regulation by agonists of the nuclear receptors PPARalpha, PPARgamma, and LXR. Biochem Biophys Res Commun. 2004;314:151–8. doi: 10.1016/j.bbrc.2003.12.058. [DOI] [PubMed] [Google Scholar]
- 66.Liu HR, Tao L, Gao E, et al. Anti-apoptotic effects of rosiglitazone in hypercholesterolemic rabbits subjected to myocardial ischemia and reperfusion. Cardiovasc Res. 2004;62:135–44. doi: 10.1016/j.cardiores.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 67.Tao L, Wang Y, Gao E, et al. Adiponectin: an indispensable molecule in rosiglitazone cardioprotection following myocardial infarction. Circ Res. 106:409–17. doi: 10.1161/CIRCRESAHA.109.211797. [DOI] [PMC free article] [PubMed] [Google Scholar]