Abstract
Evidence suggests an association between brain ischemia and Alzheimer’s disease (AD) development. Amyloid plaques consisted of β-amyloid peptide (Aβ) in the brain are a pathological hallmark of AD. Little is known about how brain ischemia induces AD-like neuropathology. Strategy effective to block such brain changes has not been reported. Here, adult male Sprague-Dawley rats were subjected to a 90-min right middle cerebral artery occlusion (MCAO). Pyrrolidine dithiocarbamate (PDTC) at various doses was given daily via gastric gavage with the first dose given at 10 min after the onset of reperfusion. The MCAO increased Aβ1-42 concentrations in the ischemic brain tissues. PDTC attenuated this increase. PDTC also decreased the ischemia-reduced expression of neprilysin, an Aβ degrading enzyme. Aβ1-42 levels were negatively correlated with neprilysin protein abundance. Brain ischemia decreased the expression of β-amyloid converting enzyme 1, a key enzyme to produce Aβ, and increased the expression of insulin-degrading enzyme, another Aβ degrading enzyme. Animals had impaired learning and memory at 2 months after the MCAO. PDTC attenuated this impairment. PDTC also improved long-term neurological outcomes. Our findings suggest that PDTC improves long-term neurological outcome of rats after transient focal brain ischemia. PDTC reduces ischemia-induced Aβ accumulation, possibly via preserving neprilysin expression.
Keywords: β-amyloid peptide, cognition, focal brain ischemia, pyrrolidine dithiocarbamate
Introduction
Ischemic brain injury is the underlying pathophysiology for many common human diseases, such as stroke and brain trauma (Martin et al., 1999). A long-term effect of brain ischemia is cognitive impairments. It has been shown that 29% of patients develop new onset dementia in 3 years after a stroke (Henon et al., 2001).
Alzheimer’s disease (AD) is the most common form of dementia in the elderly. Brain ischemia significantly increases the risk of AD (Tatemichi et al., 1992; Vermeer et al., 2003). One pathological hallmark in the brain of a patient with AD is extracellular amyloid plaques formed by accumulated β-amyloid peptide (Aβ) (Selkoe, 2001), an enzymatic product of amyloid precursor protein (APP) by β-secretase/β-amyloid converting enzyme 1 (BACE1) and γ-secretase (Vassar and Citron, 2000). The popular amyloid cascade hypothesis states that generation and accumulation of Aβ are the central event for developing AD (Selkoe, 2000). It has been suggested that Aβ induces local neurotoxicity that ultimately results in AD.
Although clinical evidence has suggested a strong association between brain ischemia and AD and animal studies have shown that brain ischemia induces learning and memory impairment (Heim et al., 2000; Takeo et al., 2003), it is just beginning to reveal the mechanisms for brain ischemia to lead to AD neuropathology. Earlier studies have shown that ischemia increases APP accumulation in the astrocytic processes and neuronal axons of ischemic tissues (Kalaria et al., 1993; Stephenson et al., 1992). Recent evidence has suggested that brain ischemia in animals increases the expression of BACE1 (Tesco et al., 2007; Wen et al., 2004). BACE1 is the rate-limited enzyme for the proteolytic cleavage of APP in the amyloidogenic pathway. A recent study has shown that brain ischemia increases the Aβ accumulation in human brain (Qi et al., 2007). However, strategy that is effective to block brain ischemia-induced AD-like brain pathology has not been reported.
Pyrrolidine dithiocarbamate (PDTC) is an anti-oxidant and anti-inflammatory agent (Liu et al., 1999). PDTC reduces focal brain ischemic injury (Nurmi et al., 2004b). PDTC also improves learning and memory functions of transgenic mice modeling for AD (Malm et al., 2007). Thus, we hypothesize that PDTC attenuates AD-like brain changes after focal brain ischemia.
Materials and Methods
The animal protocol was approved by the institutional Animal Care and Use Committee of the University of Virginia (Charlottesville, VA). All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publications number 80–23) revised in 1996.
Transient middle cerebral arterial occlusion (MCAO)
The transient MCAO was performed as we described before (Li and Zuo, 2009; Zheng and Zuo, 2004). Briefly, male Sprague-Dawley rats weighing 280 to 300 g were intubated and mechanically ventilated with 35% O2-63% N2-2% isoflurane. A temperature probe was placed in the temporalis muscle. A servo-controlled warming blanket was used to maintain the temporalis muscle temperature at 37°C. The MCAO was achieved by advancing a 3–0 monofilament nylon suture (Beijing Sunbio Biotech Co. Ltd., Beijing, China) with a rounded tip to the right internal carotid artery via the external carotid artery until slight resistance was felt. Isoflurane anesthesia was stopped and the endotracheal tube was removed once the suture was in place. Rats were reanesthetized by isoflurane at 90 min after the onset of MCAO to remove the suture. This re-anesthesia lasted for about 2 min. All animals with surgery received infiltration of the surgical wound with 0.25% bupivacaine before general anesthesia was stopped. They also received 0.1 mg/kg buprenorphine subcutaneously every 12 h for three days after the surgery.
Sham-operated rats were subjected to the same surgical procedure but without inserting the nylon suture to cause occlusion of the MCA.
Pyrrolidine dithiocarbamate (PDTC) treatment
PDTC solution (30 mg/ml) was prepared freshly each day by dissolving it in normal saline. PDTC at 20, 50 and 100 mg/kg/d or 0.5 ml saline was given to the rats daily via gastric gavage (Fine Science Tools, CA, USA). The first dose was given at 10 min after the onset of reperfusion. The treatment was continued till the animal was sacrificed for brain harvest at 3 days, 1 week, 2 weeks or 2 months after the MCAO. Animals were weighed every 2 weeks for adjusting PDTC dosage.
Infarct size measurement
This measurement was performed as we described before (Li and Zuo, 2009; Zheng and Zuo, 2004). Briefly, animals were euthanized by isoflurane and transcardiacally perfused by saline. Brains were removed and sliced into 2-mm thick slices. They were incubated in 2% 2,3,5-triphenyltetrazolium chloride for 30 min. The infarct areas in the rostral and caudal sides of each brain slice were quantified using the NIH Image 1.60. The sum of the infarct areas in the rostral and caudal sides of each brain slice was divided by 2 to get the average infarct area of the brain slice. The infarct volume of the brain slice was calculated by multiplying the average infarct area of the slice by the thickness of the slice (2 mm). The total infarct volume in the brain was the sum of infarct volume of each brain slice. The percentage of infarct volume in the ipsilateral hemisphere volume was calculated to account for cerebral edema and differential shrinkage from brain ischemia and tissue processing and to correct for individual differences in brain volumes.
Barnes maze
Rats were tested in a Barnes maze equipped with ANY-Maze video tracking system (San Diego Instruments, San Diego, CA). The test was performed as described before (Reiserer et al., 2007) with minor modifications. The test was administered and evaluated by a person blinded to the group assignment of rats. Each animal was subjected to two test protocols: cued-target and hidden-target. In cued-target protocol, the location of the target hole varied on each trial and was marked by a conspicuous polystyrene cone attached to the maze perimeter. In hidden-target protocol, the location of the target hole was fixed and was not marked by any intra-maze references. Both protocols involved training sessions on 5 consecutive days that consisted of four training sessions on each day with a 15-min inter-session interval. Each session ended when the rat entered the target hole or after 3 min had elapsed. The probe trial was performed at 2 h after the last training session on the fifth day. The hidden-target protocol was started at 2 days after the completion of the cued-target protocol. Each trial was recorded and analyzed by using the ANY-Maze tracking system to calculate the latency for the rat to enter the target hole and the time spent in the zone of each hole. The zone for each hole was defined to include the area that was within 2 cm to the hole.
Fear conditioning
The fear conditioning was performed as described previously (Stratmann et al., 2009) and administered and evaluated by a person blinded to the group assignment of rats. Briefly, a rat was placed in a Plexiglas conditioning training chamber. After a 3-min baseline exploratory period in the chamber, rats received 3 tone (2000 Hz, 90 db)–shock (1 mA, 2 s) pairings separated by 1 min between each pairing. Twenty-four hours after the training session, each rat was placed again in the training chamber for a period of 8 min in the absence of tone and foot shock to test its contextual fear conditioning. Each animal’s freezing behavior was scored every 8 s during the 8 min observation period. One hour later, the rat was placed into a new chamber. After a 3-min exploratory period in this new chamber, a 30-s tone (2000 Hz, 90 db) was applied. Freezing behavior also was scored during this tone test period.
Evaluation of motor coordination and neurological deficit scores
Motor coordination was evaluated as we described before (Zhao et al., 2007). Rats were placed on an accelerating rotarod. The speed of the rotarod was increased from 4 rpm to 40 rpm in 5 min. All rats were trained for two consecutive days, three times per day, before the formal tests. Each rat was tested for three times in the formal test. The latency and speed of rat’s falling off the rotarod were recorded. The speed–latency index (latency in seconds x speed in rpm) of each of the three tests was calculated and averaged for reporting.
Neurological deficit scores were evaluated by a person blinded to the group assignment based on an eight-point scale as described before (Li and Zuo, 2009; Zheng and Zuo, 2004): 0, no apparent deficits; 1, failure to extend left forepaw fully; 2, decreased grip of the left forelimb; 3, spontaneous movement in all directions, contralateral circling only if pulled by the tail; 4, circling or walking to the left; 5, walking only if stimulated; 6, unresponsiveness to stimulation and with depressed level of consciousness; 7, dead (Rogers et al., 1997).
Aβ1-42 assay
Aβ was extracted from brain tissues as described before (Gravina et al., 1995). Briefly, striatum and frontal cortex area 1 (Fr1) were homogenized in 8 x volume of 70% glass distilled formic acid. The formic acid extract layer was used for Aβ1-42 quantification by an Aβ1-42 ELISA kit (catalog number KMB3441; Invitrogen, Carlsbad, CA, USA). The level of the Aβ1-42 in each brain sample was standardized to the brain tissue weight.
Western blotting
Standard Western blotting procedure was followed. Primary antibodies used were rabbit polyclonal anti-neprilysin antibody (Millipore, Billerica, MA; 1:500 dilution), rabbit polyclonal anti-BACE1 antibody (Calbiochem, Darmstadt, Germany; 1:1000 dilution); rabbit polyclonal anti-insulin-degrading enzyme (IDE) antibody (Abcam, Cambridge, MA, USA; 1:600 dilution); mouse monoclonal anti-NeuN antibody (Millipore; 1:1000 dilution); rabbit polyclonal anti-actin antibody (Sigma chemical, St Louis, MO, USA; 1:4000 dilution ); rabbit monoclonal anti-APP antibody (Abcam; 1:5000 dilution) and rabbit polyclonal anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (Sigma chemical; 1:1000 dilution). Protein bands were visualized and quantified using G:Box equipped with Gene tools analysis software (Syngene, Frederick, MD, USA). All protein band density was normalized by that of actin or GAPDH in the same sample.
Immunofluorescent staining
Rats under deep isoflurane anesthesia were transcardially perfused with cold normal saline. The brain was removed and fixed with 4% paraformaldehyde in 0.1 M phosphate-buffered saline (pH 7.4) for 24 h at 4°C followed by being sequentially immersed in 10, 20, and 30% sucrose-phosphate buffered solution. Coronal 14-μm-thick cryostat brain sections were cut.
Antigen retrieval was performed by heating the section in the microwave for 15 min in 0.01 M tri-sodium citrate buffer (pH 6.0) containing 0.05% tween-20. The primary antibody was rabbit polyclonal anti-Aβ1-42 antibody (Abcam; 1:400 dilution) that does not cross-react with Aβ1-40 or APP per company. After being incubated with the primary antibody at 4°C overnight, the Cy3-conjugated goat polyclonal anti-rabbit IgG antibody (Abcam; 1:100 dilution) was applied. The sections were viewed under an Olympus BX51 high magnification microscope equipped with an Olympus DP70 12 megapixel digital camera (Olympus, Center Valley, PA, USA).
Statistical analysis
Parametric results are presented as means ± S.D. (n ≥ 5) and were analyzed by Student’s t test or one way analysis of variance followed by the Tukey test after confirmation of normal distribution of the data. The results were analyzed by Kruskal-Wallis analysis of variance on ranks followed by the Dunn’s test when the data are not normally distributed. Neurological deficit scores are presented as medians with 95% interval and were analyzed by Mann-Whitney rank sum test. Linear correlation analysis was performed to assess the relationship between the Aβ1-42 concentration ratio (ipsilateral/contralateral) and neprilysin protein abundance ratio (ipsilateral/contralateral) in the striatum from animals whose brains were harvested at 1 week after the MCAO. These included Sham-operated animals and animals used for PDTC dose-response study. A P ≤ 0.05 was accepted as significant.
Results
Total 253 rats were used in this study. Overall mortality rate in the animals subjected to MCAO during 1-week and 2-month observation periods is 20% and 25.7%, respectively. The mortality rates for animals received and not received 50 mg/kg/d PDTC during the 1-week observation period were 11.8% and 28.6%, respectively (P = 0.388). These rates for the 2-month observation period were 23.5% and 27.8%, respectively (P = 0.923).
Because physiological parameters, such as temperature, blood pressures, glucose level and arterial blood gases, can affect neurological outcome after ischemia, they were closely controlled during the period of surgery to induce the MCAO. There was no difference in these physiological parameters before and after the MCAO (Table 1). The systolic and diastolic blood pressures of these animals when awake were 127 ± 11 and 92 ± 7 mmHg, respectively, with a calculated mean arterial blood pressure 103 ± 8 mmHg, which was not significantly different from those pressures during the peri-MCAO period as shown in Table 1.
Table 1.
Physiological parameters during surgery
| Before MCAO | During MCAO | |
|---|---|---|
| MABP (mmHg) | 115 ± 6 | 116 ± 2 |
| Arterial blood pH | 7.34 ± 0.12 | 7.34 ± 0.08 |
| PaCO2 (mmHg) | 41 ± 10 | 41 ± 9 |
| PaO2 (mmHg) | 229 ± 62 | 211 ± 49 |
| Glucose (mg/dl) | 199 ± 27 | 222 ± 32 |
Arterial blood samples were taken 10 min before and 5 min after the onset of MCAO. Data are means ± SD (n = 5 – 11). MABP: mean arterial blood pressure; PaCO2: arterial CO2 partial pressure; PaO2: arterial O2 partial pressure.
PDTC treatment attenuated brain ischemia-increased expression of Aβ1-42 in the brain
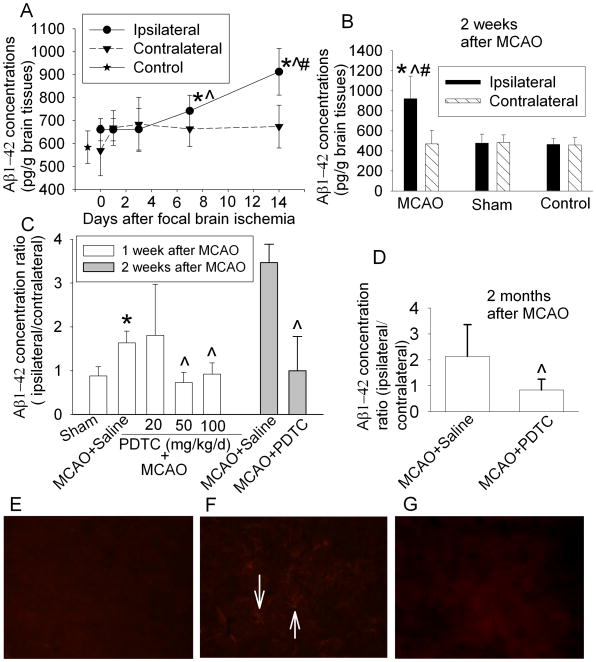
In this study, we determined Aβ1-42 expression because Aβ1-42 is generally considered as the key and more neurotoxic amyloidogenic Aβ species than Aβ1-40 (Finder et al., 2010). Similar to the previous studies using ELISA kit to measure Aβ concentrations in the brains of wild type rats (Iwata et al., 2000; Zou et al., 2006), measurable Aβ concentrations existed in the brains of our Sprague-Dawley rats under physiological conditions. The time-course experiments showed that Aβ1-42 concentrations in the ischemic striatum were increased with the time after the MCAO and that a significant increase occurred at 7 days after the MCAO. The Aβ1-42 concentrations in the striatum contralateral to the MCAO side did not change significantly over time and were not different from those in the striatum of control or sham-operated rats (Figs. 1A and 1B). These results suggest that surgery, anesthesia and postoperative pain do not increase Aβ1-42 in the brain tissues. The results also indicate that ischemia increased the expression of Aβ1-42 in the ischemia core, findings that are consistent with previous studies using immunohistochemistry to estimate Aβ expression (van Groen et al., 2005; Zhang et al., 2010).
Fig. 1.
Aβ1-42 expression in rat brain. A: The striatum ipsilateral or contralateral to the MCAO side was harvested at various time points after a 90-min MCAO. Results are means ± SD (n = 6). * P < 0.05 compared with control animals; ^ P < 0.05 compared with the ipsilateral striatum harvested immediately after the MCAO; # P < 0.05 compared with the corresponding contralateral stratum in the same animal. B: Both sides of striatum were harvested at 14 days after the MCAO or sham surgery. Results are means ± SD (n = 7). * P < 0.05 compared with control animals; ^ P < 0.05 compared with sham-operated animals; # P < 0.05 compared with the corresponding contralateral striatum in the same animal. C: Both sides of striatum from animals treated with saline or various doses of PDTC were harvested at 7 days after the MCAO or from animals treated with saline or 50 mg/kg/d PDTC were harvested at 14 days after the MCAO. Results are means ± SD (n = 5 – 7). * P < 0.05 compared with sham-operated animals. ^ P < 0.05 compared with animals treated with saline after the MCAO. D: Right and left frontal cortex area 1 of animals treated with saline or 50 mg/kg/day PDTC was harvested at 2 months after the MCAO. Results are means ± SD (n = 11 – 12). ^ P < 0.05 compared with animals treated with saline after the MCAO. E–G: Immunofluorecent staining of Aβ1-42 (red) in the non-ischemic striatum (E), ischemic striatum (F) and ischemic striatum from a rat treated with 50 mg/kg/d PDTC. These tissues were harvested at 7 days after the MCAO. Arrows in panel F indicate positive staining for Aβ1-42.
Interestingly, the increase of Aβ1-42 in the ischemic striatum at 1 week after the MCAO was not affected by 20 mg/kg/d PDTC but was abolished by 50 mg/kg/d or 100 mg/kg/d PDTC (Fig. 1C). Therefore, the dose of 50 mg/kg/d was used in our further experiments. This dose was effective to reduce Aβ1-42 concentrations in the ischemic striatum at 2 weeks after the MCAO (Fig. 1C). Since ischemic core tissue at 2 months after the MCAO was almost lost due to cell death, Fr1 was dissected for measuring Aβ1-42 at this time point. Fr1 ipsilateral to the MCAO side has a decreased blood flow that is not low enough to cause rapid and irreversible membrane failure and cell death. Thus, it has been classified as an ischemic penumbral area (Memezawa et al., 1992; Nagasawa and Kogure, 1989). PDTC at 50 mg/kg/d also reduced Aβ accumulation in the ischemic Fr1 at 2 months after the MCAO (Fig. 1D). However, the Aβ concentrations in the Fr1 contralateral to the MCAO side were not different among the animals in the control, MCAO plus saline and MCAO plus PDTC groups (225 ± 131, 229 ± 116 and 283 ± 77 pg/g brain tissues, respectively, n = 5, P = 0.66), suggesting that PDTC does not reduce Aβ concentrations under physiological conditions.
Consistent with the quantitative Aβ1-42 results described above, Aβ1-42 expression detected by immunohistochemical method was higher in the ischemic striatum than that in the non-ischemic striatum at 7 days after the MCAO. Streaks that were positively stained by the anti-Aβ1-42 antibody existed in the ischemic striatum but did not appear in the non-ischemic striatum and ischemic striatum from rats treated with PDTC (Figs. 1E, 1F and 1G).
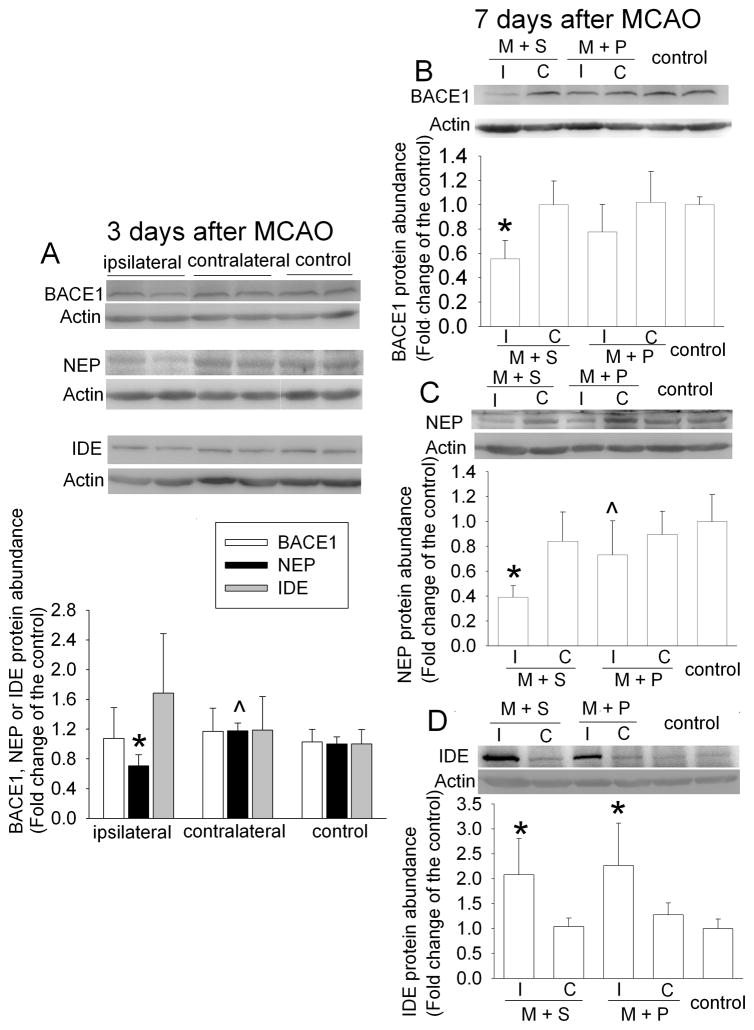
PDTC attenuated brain ischemia-induced decrease of neprilysin expression
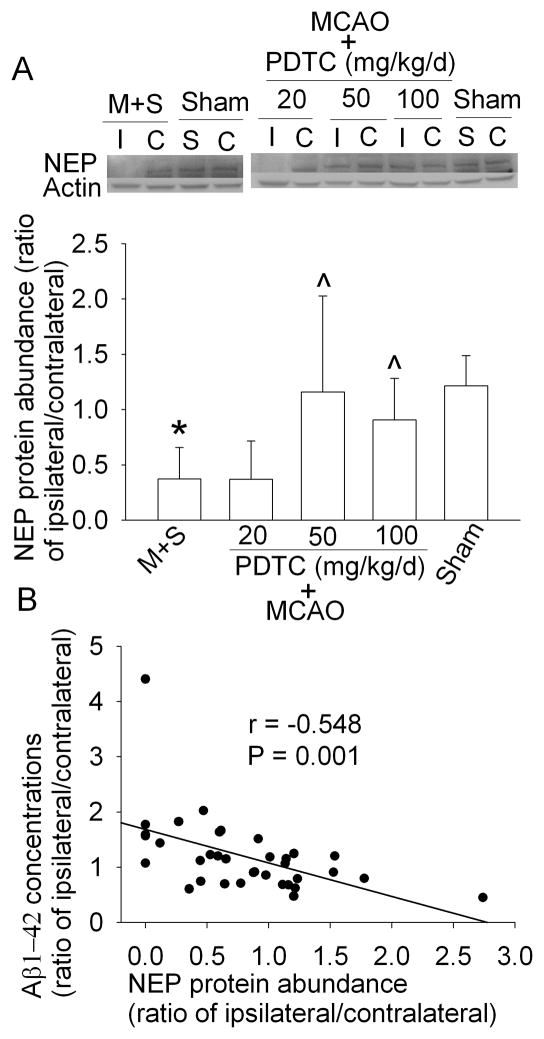
The increased expression of Aβ can be due to increased production and/or decreased clearance. BACE1 is a key enzyme for Aβ production (Vassar and Citron, 2000). Neprilysin and IDE are the major enzymes to degrade Aβ (Miners et al., 2008). As shown in figure 2, neprilysin expression was significantly decreased in the ischemic striatum at 3 days after the MCAO; whereas BACE1 and IDE expression in the ischemic striatum was not changed. The reduction of neprilysin expression in the ischemic striatum was more pronounced at 7 days after MCAO. IDE expression was increased and BACE1 level was decreased in the ischemic striatum at 7 days after brain ischemia (Fig. 2). PDTC treatment attenuated the decrease of neprilysin and BACE1 in the ischemic striatum at this time point (Fig. 2). Similar to the dose-response of PDTC on Aβ1-42 expression, PDTC also dose-dependently attenuated the ischemia-induced reduction of neprilysin. Importantly, there was a very significant negative correlation between the expression of neprilysin and Aβ1-42 (Fig. 3).
Fig. 2.
Expression of BACE1, neprilysin (NEP) and IDE in rat brain. The striatum ipsilateral (I) or contralateral (C) to the MCAO side or striatum from control animals was harvested at 3 days or 7 days after the MCAO. The graphic presentation of the BACE1, neprilysin and IDE protein abundance quantified by integrating the volume of autoradiograms from 5 – 6 rats for each experimental condition is shown as fold change over the control rats. Results are means ± SD. * P < 0.05 compared with control. ^ P < 0.05 compared with the corresponding ipsilateral striatum in animals treated with saline after the MCAO. M + S: animals with MCAO were treated with saline; M + P: animals with MCAO were treated with 50 mg/kg/d PDTC.
Fig. 3.
Effects of PDTC on the expression of neprilysin (NEP) and Aβ1-42 in the brain. The striatum ipsilateral (I) or contralateral (C) to the MCAO side or striatum from sham-operated animals (S: surgical side, C: contralateral side) was harvested at 7 days after the MCAO. A: The graphic presentation of the neprilysin protein abundance quantified by integrating the volume of autoradiograms from 7 rats for each experimental condition is shown as expression ratio in the ipsilateral and contralateral striatum. Results are means ± SD. * P < 0.05 compared with sham-operated animals. ^ P < 0.05 compared with the corresponding ipsilateral striatum in animals treated with saline after the MCAO. M + S: animals with MCAO were treated with saline. B: Linear correlation analysis to assess the relationship between NEP and Aβ1-42 expression.
Since Aβ is generated from APP (Selkoe, 2001), increased APP expression can be a potential mechanism for the increased Aβ in the ischemic striatum. Our results showed that the APP expression in the ischemic striatum was not changed (supplemental Fig. 1).
Similar to the results in the ischemic striatum, the BACE1 expression in the ischemic Fr1 was reduced compared with that in control animals (supplemental Fig. 2).
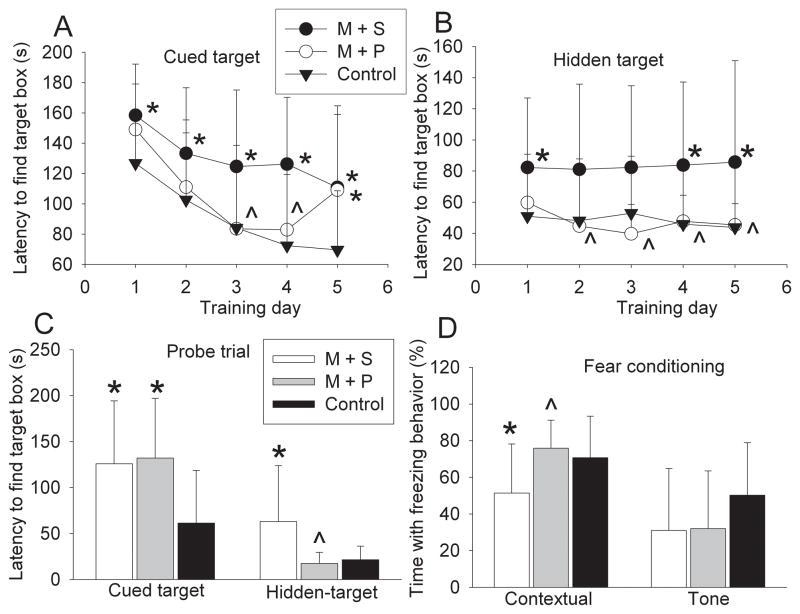
PDTC treatment attenuated cognitive impairment in rats after the MCAO
Barnes maze and fear conditioning tests were started at 45 days and 2 months after the MCAO, respectively. The performance of both control rats and rats after the MCAO during the cued-target training sessions generally improved over the sessions, suggesting that rats with or without focal brain ischemia can develop spatial learning. However, rats after the MCAO took significantly longer than control rats to find the target hole (Fig. 4A), indicating significant spatial learning impairment in rats with brain ischemia. This impairment was partially restored by PDTC treatment (Fig. 4A). Rats after brain ischemia also took longer than control rats to find the target hole in the probe trial (Fig. 4), indicating that rats with brain ischemia had impaired spatial reference memory.
Fig. 4.
Performance on Barnes maze and fear conditioning. Animals were tested with Barnes maze or fear conditioning tasks 2 months after the MCAO. The time for animals to find the target hole during the training sessions of Barnes maze is shown in panel A and panel B. The time to find the target box during the probe trial of Barnes maze test is shown in panel C. The percentage of the time with freezing behavior in the total observation time during the fear conditioning tests is shown in panel D. Results are means ± SD (n = 13). * P < 0.05 compared with control rats; ^ P < 0.05 compared with animals subjected to the MCAO and then treated with saline. M + S: animals with MCAO were treated with saline; M + P: animals with MCAO were treated with 50 mg/kg/d PDTC.
Similar to the results in the cued-target test, rats after focal brain ischemia took longer than control rats to find the target hole in both the training sessions and the probe trial in the hidden target test (Figs. 4B and 4C), suggesting that those rats had significant impairment in spatial leaning and memory. This impairment was attenuated by PDTC treatment (Fig. 4).
The contextual fear conditioning, which tests hippocampus-dependent learning and memory functions (Kim and Fanselow, 1992), was significantly impaired in rats at 2 months after MCAO (Fig. 4). This impairment was abolished by PDTC. No difference was observed in the tone-related fear conditioning whose development may not be heavily affected by hippocampal functions (Kim and Fanselow, 1992).
PDTC treatment improved short-term and long-term neurological outcome
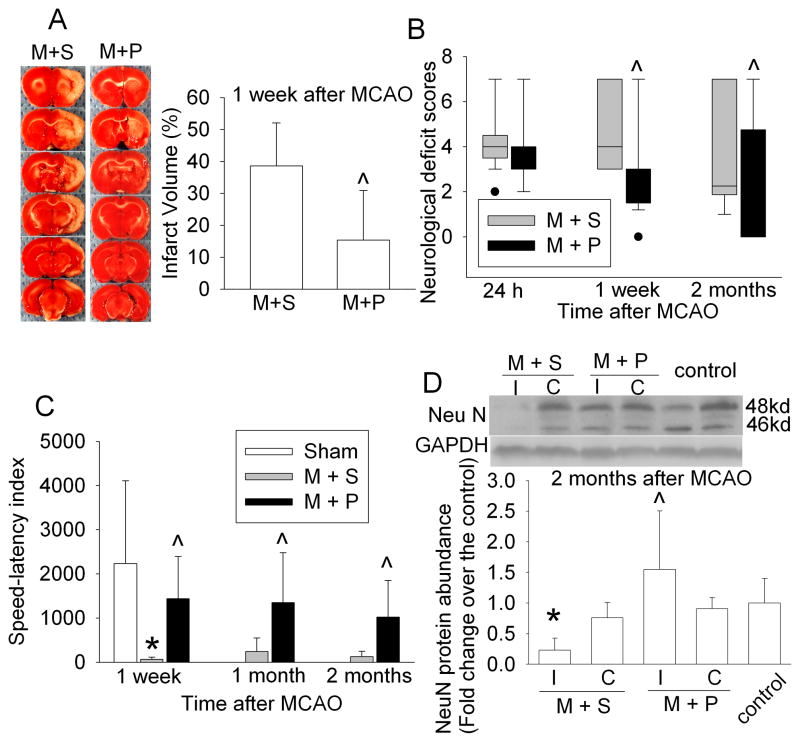
PDTC has been shown to reduce brain infarct volume at 1 and 3 days after MCAO (Nurmi et al., 2004b). Our results showed that PDTC reduced the brain infarct volume assessed at 1 week after the MCAO (Fig. 5A). In addition, animals treated with PDTC had better neurological functions as indicated by the performance on rotarod test and neurological deficit scores at 1 week, 1 month and 2 months after the brain ischemia (Figs. 5B and C). Similarly, NeuN, a neuronal specific protein, in the ischemic Fr1 was significantly reduced at 2 months after the MCAO and this reduction was abolished by PDTC treatment (Fig. 5C), suggesting that PDTC also helps to preserve neural tissues at a long time after brain ischemia.
Fig. 5.
Neurological outcome. A: Brain slices stained with 2,3,5-triphenyltetrazolium chloride from representative rats are shown in the left panel and the percentage of infarct volume in ipsilateral hemisphere volume is shown in the right panel. Results are means ± S.D. (n = 8). ^ P < 0.05 compared with animals subjected to the MCAO and then treated with saline. B: Neurological deficit scores evaluated at 24 h, 1 week and 2 months after the MCAO. The neurological deficit scores of all sham-operated rats were 0 and are not plotted in the figure. Results are presented in a box plot format (n = 17 – 21). ●: lowest or highest score (the score will not show up if it falls in the 95% interval). ^ P < 0.05 compared with animals subjected to the MCAO and then treated with saline. C: Performance on rotarod was assessed at 1 week, 1 month or 2 months after the MCAO. Results are means ± S.D. (n = 5 – 7). ^ P <0.05 compared with animals subjected to the MCAO and then treated with saline. D: NeuN protein expression. The frontal cortex area 1 (Fr1) ipsilateral (I) or contralateral (C) to the MCAO side or the Fr1 from control animals was harvested at 2 months after the MCAO. The Fr1 ipsilateral to the MCAO side has been considered to be ischemic penumbral region. The graphic presentation of the NeuN protein abundance quantified by integrating the volume of autoradiograms from 5 – 6 rats for each experimental condition is shown as fold change over the control rats. Results are means ± SD. * P < 0.05 compared with control. ^ P < 0.05 compared with the corresponding ipsilateral Fr1 in animals treated with saline after the MCAO. M + S: animals with MCAO were treated with saline; M + P: animals with MCAO were treated with 50 mg/kg/d PDTC.
Discussion
Brain ischemia increases the risk for AD (Tatemichi et al., 1992; Vermeer et al., 2003). A recent study showed that brain ischemia increased Aβ expression in human brain (Qi et al., 2007). Since Aβ accumulation in the brain is known to play an important role in AD neuropathology (Selkoe, 2001), it has been a focus of laboratory studies to identify the mechanisms for brain ischemia to increase Aβ. However, quantitative and dynamic changes of Aβ expression in animal brains after ischemia have not been reported. Our results showed that transient focal brain ischemia induced a time-dependent increase of Aβ1-42 in the ischemic brain tissues. Brain tissues contralateral to the ischemia side did not have an increased Aβ1-42 expression. Importantly, the increased Aβ1-42 expression in the ischemic striatum was dose-dependently attenuated by PDTC. Similarly, PDTC also attenuated the Aβ1-42 expression in the Fr1, an ischemic penumbral region, at 2 months after brain ischemia.
BACE1 is the rate-limiting enzyme for amyloidogenic cleavage of APP (Vassar and Citron, 2000). It has been suggested that BACE1 increase after brain ischemia contributes to Aβ increase in the brain (Tesco et al., 2007; Wen et al., 2004). We evaluated BACE1 expression in the striatum, an ischemic core region, and the Fr1, an ischemic penumbral region, at various times. We did not see an increase of BACE1 expression in either of these two brain regions. Instead, BACE1 expression in the ischemic striatum was significantly decreased at 7 days after the MCAO. These results suggest that BACE1 may not contribute to the increased Aβ1-42 expression in these ischemic tissues. The reasons for the discrepancy in BACE1 expression between our study and previous studies are not known. Our method of using specific brain regions for measuring BACE1 expression should have increased the chances of detecting an increase than that of using the whole brain hemisphere ipsilateral to the MCAO side in a previous study (Tesco et al., 2007). This situation should be especially true because we detected an increased Aβ expression in the ischemic striatum and Fr1 that were used to measure BACE1 expression. However, we subjected adult male rats to a 90-min MCAO and the previous studies applied a 60-min MCAO to young female rats (Tesco et al., 2007; Wen et al., 2004). These experimental differences may have contributed to the different findings on BACE1 expression between our study and previous studies.
In addition to BACE1, Aβ production may be changed by an altered expression of APP, the substrate for Aβ production. A previous study has shown that APP mRNA is increased in the ischemic core and penumbral tissues at 4 and 7 days after permanent MCAO (Shi et al., 2000). However, the study did not measure APP protein level. Our results showed that APP protein expression in the ischemic striatum was not changed at 3 or 7 days after the MCAO, indicating that alteration of APP expression may not be a mechanism for the ischemia-increased Aβ1-42 expression.
Since increased Aβ1-42 expression can be due to increased production or decreased degradation, we determined the expression of neprilysin and IDE, two major Aβ degrading enzymes in the brain (Miners et al., 2008). The levels of neprilysin and IDE are decreased in AD brains (Miners et al., 2008; Russo et al., 2005). Our study showed that the expression of neprilysin in the ischemic striatum was significantly reduced at 3 and 7 days after the MCAO. While the expression of IDE was not changed at 3 days after the MCAO; its expression in the ischemic striatum was increased at 7 days after the MCAO. These results, along with the time-course of Aβ1-42 expression in the ischemic striatum, suggest that decreased neprilysin expression may be a primary cause for the increased Aβ1-42 in the ischemic brain tissues. This suggestion is strongly supported by our findings that PDTC dose-dependently blocked brain ischemia-induced neprilysin reduction and Aβ1-42 increase in the ischemic striatum and that the Aβ1-42 increase was significantly correlated with the reduction of neprilysin expression. In addition, many studies have shown convincingly that neprilysin plays a critical role in Aβ metabolism in the brain: while over-expression of neprilysin reduces Aβ concentrations and amyloid plaque load in the brains of APP transgenic mice; silencing the expression of neprilysin or inhibiting its activity significantly increases Aβ concentrations in the brains of wild-type rats or mice (Hemming et al., 2007; Iwata et al., 2000; Marr et al., 2004; Zou et al., 2006).
Since BACE1 and IDE were not changed at 3 days after MCAO when Aβ1-42 expression was not significantly increased and was altered at 7 days after MCAO when Aβ1-42 expression was increased, the altered expression of BACE1 and IDE at 7 days after MCAO may be secondary to the increase of Aβ1-42 expression in the ischemic striatum. Consistent with this interpretation, PDTC also blocked the brain ischemia-induced decrease of BACE1 in the ischemic striatum.
Our results clearly showed that PDTC attenuated ischemia-induced reduction of neprilysin. PDTC is an inhibitor of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB). This inhibition leads to reduced proinflammatory cytokine production (Liu et al., 1999). A very recent study has shown that increased NF-κB activity can lead to decreased neprilysin expression (Thompson et al., 2011). It is well known that brain ischemia activates NF-κB (Nurmi et al., 2004a; Nurmi et al., 2004b). Thus, the attenuation of ischemia-induced reduction of neprilysin by PDTC may be mediated by its inhibition of NF-κB. In addition to the anti-inflammatory effect, PDTC also is an anti-oxidant. Ischemic brain tissues have increased oxidative stress (Margaill et al., 2005). However, the effects of oxidative stress on neprilysin expression are controversial: oxidative stress increases neprilysin in human endothelial cells (Muangman et al., 2005) and decreases neprilysin in human blastoma cells (Fisk et al., 2007). The effects of oxidative stress on neprilysin in normal neurons or glia are not known yet.
Brain ischemia and hypoperfusion induce cognitive impairments (Heim et al., 2000; Takeo et al., 2003). However, previous studies determined cognitive functions within 2 weeks after brain ischemia. Our results showed that rats had cognitive impairments at 2 months after the MCAO. These animals had impairments in spatial reference learning as evidenced by a prolonged latency of finding the target hole during the training sessions of the hidden-target and cued-target test. These animals also had significant impairments in spatial memory as presented by a prolonged latency of finding the target hole during the probe trial of the hidden-target and cued target tests. However, these animals formed memory as they presented with freezing behavior in the fear conditioning tests. Animals subjected to MCAO and then received saline had significant impairments in the hippocampus-dependent learning and memory as they presented with less freezing behavior in the contextual memory tests. Our results also showed that the brain ischemia-induced learning and memory impairments were attenuated by PDTC.
Animals after brain ischemia can have significant sensorimotor function impairments, especially during the acute phase of brain ischemia. This function impairment can influence the performance of animals in learning and memory tasks. We performed Barnes maze and fear conditioning tests at 2 months after the MCAO when animals have very mild focal neurological deficits. Also, the performance of fear conditioning does not require animals to move around. Our animals after the MCAO had sensory functions that were good enough for performing the fear conditioning tests because they all had freezing behavior. In addition, they presented with similar degree of freezing behavior to that of control animals in tone-related learning and memory.
PDTC has been shown to provide neuroprotection against brain ischemia (Nurmi et al., 2004b). Our results showed that PDTC reduced the infarct volumes at 1 week after transient focal brain ischemia. However, whether PDTC-induced neuroprotection translates into a better long-term neurological outcome has not been examined. We showed here that animals subjected to the MCAO and then treated with PDTC had better neurological functions evaluated at 2 months after the MCAO than animals subjected to the MCAO only. The PDTC-treated animals also had higher NeuN expression in the ischemic penumbral brain tissues, suggesting that PDTC reduces neural tissue injury after brain ischemia. In addition, PDTC may reduce the mortality rate of rats during the one-week observation period after the MCAO (11.8% versus 28.6%). However, this difference did not reach statistic significance. Power analysis suggests that 101 rats are needed in each group for a desired power of 0.8 at an α level of 0.05 by z-test.
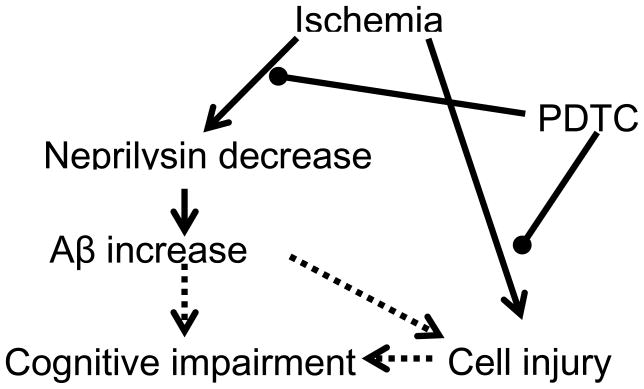
Our results showed that PDTC treatment attenuated brain ischemia-induced Aβ increase and impairments in learning, memory and neurological functions. However, the relationship among these beneficial effects is not clear. PDTC has been shown to reduce Aβ-induced neuroinflammation in wild-type animals and improve the learning and memory functions but without affecting the Aβ load in animals modeling for AD (Malm et al., 2007). The attenuation of brain ischemia-induced Aβ increase may be a mechanism for PDTC to improve the learning and memory functions in our animals (Fig. 6). Since Aβ-induced neuroinflammation can cause damage to the brain (Craft et al., 2006), the attenuation of Aβ expression may contribute to the improvement of neurological outcome. On the other hand, preservation of neurostructure by PDTC after brain ischemia may contribute to the attenuation of brain ischemia-induced Aβ expression and cognitive impairments (Fig. 6).
Fig. 6.
Diagram of the major findings. Arrow heads imply that one effect leads to another effect. Circle heads indicate inhibition of the process. Solid lines after arrow or circle heads indicate that our study provides evidence for this effect. Dotted lines after arrow heads indicate that evidence in the literature suggests this effect.
Our data of fear conditioning suggests impairment in hippocampal functions after the MCAO. Although hippocampus is not considered as a ischemic core or penumbral region after MCAO, evidence of hippocampal damage is frequently seen after MCAO (Zheng and Zuo, 2004). Hippocampus is very close to the ischemic brain tissues. Neurotoxic chemicals, such as Aβ, may be transported or diffused from those tissues to hippocampus.
We focused on analysis of Aβ and protein expression in the ischemic core and penumbral tissues because the change of Aβ and protein expression in these tissues should be bigger than that in non-ischemic brain tissues after MCAO. Also, it is conceivable that various neuroprotective strategies manifest their beneficial effects mainly by protecting the ischemic penumbral tissues. However, penumbral tissues may be more heterogeneous in the degrees of cell injury than ischemic core or non-ischemic tissues, which may increase variability of the results generated from using ischemic penumbral tissues. In this study, we did not harvest hippocampus for analysis because hippocampus is not an ischemic core or penumbral region after the MCAO. In addition, harvesting multiple brain regions from the same brains will increase the time and difficulty of the procedure. Future studies are needed to investigate the biochemical changes in the hippocampus to understand their role in PDTC-induced improvement of cognitive impairment after the MCAO.
PDTC was applied chronically in this study. Our results showed that BACE1 expression was decreased in the ischemic brain tissues and that PDTC blocked this decrease. Conceivably, it should be beneficial if PDTC therapy can attenuate the reduction of neprilysin but do not affect the decrease of BACE1 after brain ischemia. This status may be difficult to achieve because our results suggest that the changed BACE1 expression is secondary to the change of neprilysin and Aβ. PDTC therapy normalizes all of these changes. However, this inference needs to be tested by detailed time-course experiments. These experiments may identify an optimal time window to use PDTC for neuroprotection.
We started PDTC therapy after the onset of reperfusion. This method of application should make it easy to apply in the clinical situations because most patients are under intensive care only after brain ischemia has already occurred. Future studies are needed to determine whether our findings can be translated to humans.
Supplementary Material
Research highlights.
Focal brain ischemia time-dependently increase Aβ in ischemic brain tissues
PDTC attenuates brain ischemia-increased Aβ via preservation of neprilysin expression
PDTC also improves long-term neurological outcome after focal brain ischemia
Acknowledgments
This study was supported by a grant (R01 GM065211 to Z Zuo) from the National Institute of Health, Bethesda, Maryland, by a grant from the International Anesthesia Research Society (2007 Frontiers in Anesthesia Research Award to Z Zuo), by a Grant-in-Aid from the American Heart Association Mid-Atlantic Affiliate (10GRNT3900019 to Z Zuo), Baltimore, Maryland, by the Robert M. Epstein Professorship endowment, University of Virginia, and a grant (81070912 to W Sheng) from National Natural Science Foundation of China, Beijing, China.
Abbreviations
- Aβ
β-amyloid peptide
- AD
Alzheimer’s disease
- APP
amyloid precursor protein
- BACE1
β-secretase/β-amyloid converting enzyme 1
- Fr1
frontal cortex area 1
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- IDE
insulin-degrading enzyme
- MCAO
middle cerebral artery occlusion
- NEP
neprilysin
- PDTC
pyrrolidine dithiocarbamate
Footnotes
The research work was performed in and should be attributed to the Department of Anesthesiology, University of Virginia, Charlottesville, VA 22908, USA.
Disclosure/conflict of interest: None from the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Craft JM, Watterson DM, Van Eldik LJ. Human amyloid beta-induced neuroinflammation is an early event in neurodegeneration. Glia. 2006;53:484–490. doi: 10.1002/glia.20306. [DOI] [PubMed] [Google Scholar]
- Finder VH, Vodopivec I, Nitsch RM, Glockshuber R. The recombinant amyloid-beta peptide Abeta1-42 aggregates faster and is more neurotoxic than synthetic Abeta1-42. J Mol Biol. 2010;396:9–18. doi: 10.1016/j.jmb.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Fisk L, Nalivaeva NN, Boyle JP, Peers CS, Turner AJ. Effects of hypoxia and oxidative stress on expression of neprilysin in human neuroblastoma cells and rat cortical neurones and astrocytes. Neurochem Res. 2007;32:1741–1748. doi: 10.1007/s11064-007-9349-2. [DOI] [PubMed] [Google Scholar]
- Gravina S, Ho L, Eckman C, Long K, Otvos L, Jr, Younkin L, Suzuki N, Younkin S. Amyloid beta protein in Alzheimer’s disease brain. J Biol Chem. 1995;270:7013, 7017–7016. doi: 10.1074/jbc.270.13.7013. [DOI] [PubMed] [Google Scholar]
- Heim C, Zhang J, Lan J, Sieklucka M, Kurz T, Riederer P, Gerlach M, Sontag KH. Cerebral oligaemia episode triggers free radical formation and late cognitive deficiencies. Eur J Neurosci. 2000;12:715–725. doi: 10.1046/j.1460-9568.2000.00916.x. [DOI] [PubMed] [Google Scholar]
- Hemming ML, Patterson M, Reske-Nielsen C, Lin L, Isacson O, Selkoe DJ. Reducing amyloid plaque burden via ex vivo gene delivery of an Abeta-degrading protease: a novel therapeutic approach to Alzheimer disease. PLoS Med. 2007;4:e262. doi: 10.1371/journal.pmed.0040262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henon H, Durieu I, Guerouaou D, Lebert F, Pasquier F, Leys D. Poststroke dementia: incidence and relationship to prestroke cognitive decline. Neurology. 2001;57:1216–1222. doi: 10.1212/wnl.57.7.1216. [DOI] [PubMed] [Google Scholar]
- Iwata N, Tsubuki S, Takaki Y, Watanabe K, Sekiguchi M, Hosoki E, Kawashima-Morishima M, Lee HJ, Hama E, Sekine-Aizawa Y, Saido TC. Identification of the major Abeta1-42-degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition. Nat Med. 2000;6:143–150. doi: 10.1038/72237. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Bhatti SU, Palatinsky EA, Pennington DH, Shelton ER, Chan HW, Perry G, Lust WD. Accumulation of the beta amyloid precursor protein at sites of ischemic injury in rat brain. Neuroreport. 1993;4:211–214. doi: 10.1097/00001756-199302000-00025. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Li L, Zuo Z. Isoflurane preconditioning improves short-term and long-term neurological outcome after focal brain ischemia in adult rats. Neuroscience. 2009;164:497–506. doi: 10.1016/j.neuroscience.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SF, Ye X, Malik AB. Inhibition of NF-kappaB activation by pyrrolidine dithiocarbamate prevents In vivo expression of proinflammatory genes. Circulation. 1999;100:1330–1337. doi: 10.1161/01.cir.100.12.1330. [DOI] [PubMed] [Google Scholar]
- Malm TM, Iivonen H, Goldsteins G, Keksa-Goldsteine V, Ahtoniemi T, Kanninen K, Salminen A, Auriola S, Van Groen T, Tanila H, Koistinaho J. Pyrrolidine dithiocarbamate activates Akt and improves spatial learning in APP/PS1 mice without affecting beta-amyloid burden. J Neurosci. 2007;27:3712–3721. doi: 10.1523/JNEUROSCI.0059-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margaill I, Plotkine M, Lerouet D. Antioxidant strategies in the treatment of stroke. Free Radic Biol Med. 2005;39:429–443. doi: 10.1016/j.freeradbiomed.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Marr RA, Guan H, Rockenstein E, Kindy M, Gage FH, Verma I, Masliah E, Hersh LB. Neprilysin regulates amyloid Beta peptide levels. J Mol Neurosci. 2004;22:5–11. doi: 10.1385/JMN:22:1-2:5. [DOI] [PubMed] [Google Scholar]
- Martin JA, Smith BL, Matthews TJ, Ventura SJ. Births and Deaths: Preliminary Data for 1998. Natl Vital Stat Rep. 1999;47:1–45. [PubMed] [Google Scholar]
- Memezawa H, Minamisawa H, Smith M-L, Siesjo BK. Ischemic penumbra in a model of reversible middle cerebral artery occlusion in the rat. Exp Brain Res. 1992;89:67–78. doi: 10.1007/BF00229002. [DOI] [PubMed] [Google Scholar]
- Miners JS, Baig S, Palmer J, Palmer LE, Kehoe PG, Love S. Abeta-degrading enzymes in Alzheimer’s disease. Brain Pathol. 2008;18:240–252. doi: 10.1111/j.1750-3639.2008.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muangman P, Tamura RN, Gibran NS. Antioxidants inhibit fatty acid and glucose-mediated induction of neutral endopeptidase gene expression in human microvascular endothelial cells. J Am Coll Surg. 2005;200:208–215. doi: 10.1016/j.jamcollsurg.2004.09.043. [DOI] [PubMed] [Google Scholar]
- Nagasawa H, Kogure K. Correlation between cerebral blood flow and histologic changes in a new rat model of middle cerebral artery occlusion. Stroke. 1989;20:1037–1043. doi: 10.1161/01.str.20.8.1037. [DOI] [PubMed] [Google Scholar]
- Nurmi A, Lindsberg PJ, Koistinaho M, Zhang W, Juettler E, Karjalainen-Lindsberg ML, Weih F, Frank N, Schwaninger M, Koistinaho J. Nuclear factor-kappaB contributes to infarction after permanent focal ischemia. Stroke. 2004a;35:987–991. doi: 10.1161/01.STR.0000120732.45951.26. [DOI] [PubMed] [Google Scholar]
- Nurmi A, Vartiainen N, Pihlaja R, Goldsteins G, Yrjanheikki J, Koistinaho J. Pyrrolidine dithiocarbamate inhibits translocation of nuclear factor kappa-B in neurons and protects against brain ischaemia with a wide therapeutic time window. J Neurochem. 2004b;91:755–765. doi: 10.1111/j.1471-4159.2004.02756.x. [DOI] [PubMed] [Google Scholar]
- Qi JP, Wu H, Yang Y, Wang DD, Chen YX, Gu YH, Liu T. Cerebral ischemia and Alzheimer’s disease: the expression of amyloid-beta and apolipoprotein E in human hippocampus. J Alzheimers Dis. 2007;12:335–341. doi: 10.3233/jad-2007-12406. [DOI] [PubMed] [Google Scholar]
- Reiserer RS, Harrison FE, Syverud DC, McDonald MP. Impaired spatial learning in the APPSwe + PSEN1DeltaE9 bigenic mouse model of Alzheimer’s disease. Genes Brain Behav. 2007;6:54–65. doi: 10.1111/j.1601-183X.2006.00221.x. [DOI] [PubMed] [Google Scholar]
- Rogers DC, Campbell CA, Stretton JL, Mackay KB. Correlation between motor impairment and infarct volume after permanent and transient middle cerebral artery occlusion in the rat. Stroke. 1997;28:2060–2065. doi: 10.1161/01.str.28.10.2060. [DOI] [PubMed] [Google Scholar]
- Russo R, Borghi R, Markesbery W, Tabaton M, Piccini A. Neprylisin decreases uniformly in Alzheimer’s disease and in normal aging. FEBS Lett. 2005;579:6027–6030. doi: 10.1016/j.febslet.2005.09.054. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Toward a comprehensive theory for Alzheimer’s disease. Hypothesis: Alzheimer’s disease is caused by the cerebral accumulation and cytotoxicity of amyloid beta-protein. Ann N Y Acad Sci. 2000;924:17–25. doi: 10.1111/j.1749-6632.2000.tb05554.x. [DOI] [PubMed] [Google Scholar]
- Shi J, Yang SH, Stubley L, Day AL, Simpkins JW. Hypoperfusion induces overexpression of beta-amyloid precursor protein mRNA in a focal ischemic rodent model. Brain Res. 2000;853:1–4. doi: 10.1016/s0006-8993(99)02113-7. [DOI] [PubMed] [Google Scholar]
- Stephenson DT, Rash K, Clemens JA. Amyloid precursor protein accumulates in regions of neurodegeneration following focal cerebral ischemia in the rat. Brain Res. 1992;593:128–135. doi: 10.1016/0006-8993(92)91274-i. [DOI] [PubMed] [Google Scholar]
- Stratmann G, Sall JW, May LD, Bell JS, Magnusson KR, Rau V, Visrodia KH, Alvi RS, Ku B, Lee MT, Dai R. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology. 2009;110:834–848. doi: 10.1097/ALN.0b013e31819c463d. [DOI] [PubMed] [Google Scholar]
- Takeo S, Niimura M, Miyake-Takagi K, Nagakura A, Fukatsu T, Ando T, Takagi N, Tanonaka K, Hara J. A possible mechanism for improvement by a cognition-enhancer nefiracetam of spatial memory function and cAMP-mediated signal transduction system in sustained cerebral ischaemia in rats. Br J Pharmacol. 2003;138:642–654. doi: 10.1038/sj.bjp.0705096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatemichi TK, Desmond DW, Mayeux R, Paik M, Stern Y, Sano M, Remien RH, Williams JB, Mohr JP, Hauser WA, Figueroa M. Dementia after stroke: baseline frequency, risks, and clinical features in a hospitalized cohort. Neurology. 1992;42:1185–1193. doi: 10.1212/wnl.42.6.1185. [DOI] [PubMed] [Google Scholar]
- Tesco G, Koh YH, Kang EL, Cameron AN, Das S, Sena-Esteves M, Hiltunen M, Yang SH, Zhong Z, Shen Y, Simpkins JW, Tanzi RE. Depletion of GGA3 stabilizes BACE and enhances beta-secretase activity. Neuron. 2007;54:721–737. doi: 10.1016/j.neuron.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RC, Herscovitch M, Zhao I, Ford TJ, Gilmore TD. NF-kappaB down-regulates expression of the B-lymphoma marker CD10 through a miR-155/PU.1 pathway. J Biol Chem. 2011;286:1675–1682. doi: 10.1074/jbc.M110.177063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Groen T, Puurunen K, Maki HM, Sivenius J, Jolkkonen J. Transformation of diffuse beta-amyloid precursor protein and beta-amyloid deposits to plaques in the thalamus after transient occlusion of the middle cerebral artery in rats. Stroke. 2005;36:1551–1556. doi: 10.1161/01.STR.0000169933.88903.cf. [DOI] [PubMed] [Google Scholar]
- Vassar R, Citron M. Abeta-generating enzymes: recent advances in beta- and gamma-secretase research. Neuron. 2000;27:419–422. doi: 10.1016/s0896-6273(00)00051-9. [DOI] [PubMed] [Google Scholar]
- Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- Wen Y, Onyewuchi O, Yang S, Liu R, Simpkins JW. Increased beta-secretase activity and expression in rats following transient cerebral ischemia. Brain Res. 2004;1009:1–8. doi: 10.1016/j.brainres.2003.09.086. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xiong M, Yan RQ, Sun FY. Mutant ubiquitin-mediated beta-secretase stability via activation of caspase-3 is related to beta-amyloid accumulation in ischemic striatum in rats. J Cereb Blood Flow Metab. 2010;30:566–575. doi: 10.1038/jcbfm.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Peng L, Li L, Xu X, Zuo Z. Isoflurane preconditioning improves long-term neurologic outcome after hypoxic-ischemic brain injury in neonatal rats. Anesthesiology. 2007;107:963–970. doi: 10.1097/01.anes.0000291447.21046.4d. [DOI] [PubMed] [Google Scholar]
- Zheng S, Zuo Z. Isoflurane preconditioning induces neuroprotection against ischemia via activation of p38 mitogen-activated protein kinase. Mol Pharmacol. 2004;65:1172–1180. doi: 10.1124/mol.65.5.1172. [DOI] [PubMed] [Google Scholar]
- Zou LB, Mouri A, Iwata N, Saido TC, Wang D, Wang MW, Mizoguchi H, Noda Y, Nabeshima T. Inhibition of neprilysin by infusion of thiorphan into the hippocampus causes an accumulation of amyloid Beta and impairment of learning and memory. J Pharmacol Exp Ther. 2006;317:334–340. doi: 10.1124/jpet.105.095687. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.