Abstract
Mutations in the PARK6 gene coding for PTEN-induced kinase 1 (PINK1) cause recessive early-onset parkinsonism. Although PINK1 and Parkin promote the degradation of depolarized mitochondria in cultured cells, little is known about changes in signaling pathways that may additionally contribute to dopamine neuron loss in recessive parkinsonism. Accumulating evidence implicates impaired Akt cell survival signaling in sporadic and familial PD (PD). IGF-1/Akt signaling inhibits dopamine neuron loss in several animal models of PD and both IGF-1 and insulin are neuroprotective in various settings. Here, we tested whether PINK1 is required for insulin-like growth factor 1 (IGF-1) and insulin dependent phosphorylation of Akt and the regulation of downstream Akt target proteins. Our results show that embryonic fibroblasts from PINK1-deficient mice display significantly reduced Akt phosphorylation in response to both IGF-1 and insulin. Moreover, phosphorylation of glycogen synthase kinase-3β (GSK-3β) and nuclear exclusion of FoxO1 are decreased in IGF-1 treated PINK1-deficient cells. In addition, phosphorylation of ribosomal protein S6 is reduced indicating decreased activity of mitochondrial target of rapamycin (mTOR) in IGF-1 treated PINK1−/− cells. Importantly, the protection afforded by IGF-1 against staurosporine-induced metabolic dysfunction and apoptosis is abrogated in PINK1-deficient cells. Moreover, IGF-1-induced Akt phosphorylation is impaired in primary cortical neurons from PINK1-deficient mice. Inhibition of cellular Ser/Thr phosphatases did not increase the amount of phosphorylated Akt in PINK1−/− cells, suggesting that components upstream of Akt phosphorylation are compromised in PINK1-deficient cells. Our studies show that PINK1 is required for optimal IGF-1 and insulin dependent Akt signal transduction, and raise the possibility that impaired IGF-1/Akt signaling is involved in PINK1-related parkinsonism by increasing the vulnerability of dopaminergic neurons to stress-induced cell death.
Keywords: PINK1, Parkinson’s disease, IGF-1, Akt signaling, GSK-3β, apoptosis
INTRODUCTION
Recessive familial parkinsonism is caused by mutations in the genes encoding the proteins Parkin, PINK1, DJ-1 and ATP132A (Bonifati et al., 2003; Kitada et al., 1998; Ramirez et al., 2006; Valente et al., 2004). Many studies have shown that neurons and animals lacking Parkin, PINK1 or DJ-1 show increased sensitivity to oxidative stress and/or mitochondrial complex I inhibitors, and that all proteins harbor functions that directly or indirectly are critical for mitochondrial homoeostasis and, in Drosophila, the prevention of mitochondrial degeneration (Akundi et al., 2011; Canet-Aviles et al., 2004; Chen et al., 2010; Clark et al., 2006; Deng et al., 2008; Gautier et al., 2008; Gegg et al., 2009; Greene et al., 2005; Haque et al., 2008; Inden et al., 2006; Jiang et al., 2004; Kim et al., 2005b; Meulener et al., 2005; Park et al., 2005; Park et al., 2006; Paterna et al., 2007; Pesah et al., 2004; Pridgeon et al., 2007; Saini et al., 2010; Thomas et al., 2011; Wood-Kaczmar et al., 2008). In cell lines, PINK1 and Parkin cooperate to promote the degradation of depolarized mitochondria through autophagy (Dagda et al., 2009; Geisler et al., 2010; Narendra et al., 2010; Vives-Bauza et al., 2009). However, recent experiments have shown that PINK1/Parkin-mediated mitophagy of dysfunctional mitochondria is less effective in primary neurons due to different bioenergetic requirements (Van Laar et al., 2011). This does not rule out a critical involvement of impaired mitochondrial quality control in dopaminergic neuron loss. Rather, it suggests that impaired mitophagy as well as other defects jointly contribute to the demise of neurons in recessive parkinsonism. Mounting evidence points to a stimulatory role of recessive PD gene products in Akt cell survival signaling (Aleyasin et al., 2010; Kim et al., 2005a; Murata et al., 2011; Yang et al., 2005). Importantly, Akt signal transduction is also impaired in sporadic PD, where reduced levels of pSer473-Akt in the midbrain and tyrosine hydroxylase-positive neurons of the substantia nigra of PD patients compared to control subjects have been observed (Malagelada et al., 2008; Timmons et al., 2009). IGF-1 protects neurons against multiple stresses through induction of Akt signaling (Zheng et al., 2000). The importance of IGF-1 in the development and maintenance of dopaminergic neurons has been known for a long time (Beck, 1994) and is consistent with abundant expression of IGF-1 receptors in dopaminergic neurons of the substantia nigra (Quesada et al., 2007). Moreover, genetic defects in IGF-1 signaling increase neuroinflammation and neuron loss in a mouse model of sporadic PD (Nadjar et al., 2009). We have recently generated a new line of PINK1-deficient mice that develop age-dependent loss of striatal dopamine (DA) associated with increased DA turnover and display increased Ca2+-induced mitochondrial permeability transition (Akundi et al., 2011). In addition, basal and inflammatory cytokine-induced nuclear factor-κB (NF-κB) activity was reduced in PINK1−/− mouse embryonic fibroblasts (MEF) (Akundi et al., 2011). Because NF-κB inhibits apoptosis in neurons (Mattson and Camandola, 2001; Mattson et al., 2000), we reasoned that the lack of PINK1 might interfere with neuroprotective cell signaling. To further address this, we studied the effects of PINK1 deficiency on insulin-like growth factor 1 (IGF-1) and insulin-mediated Akt phosphorylation, because of the importance of this anti-apoptotic pathway for DA neuron physiology and survival in several animal models of PD (Clarkson et al., 2001; Ebert et al., 2008; Nadjar et al., 2009; Quesada et al., 2008). In addition, we studied whether PINK1 deficiency inhibits the phosphorylation of GSK-3β, because increased GSK-3β activity has been linked to Alzheimer’s disease (AD) (Avila et al., 2010; Giese, 2009; Sereno et al., 2009), PD (Nagao and Hayashi, 2009) and 6-OHDA induced neuron death (Chen et al., 2004). Given that forkhead box O (FoxO) transcription factors play an important role in regulating energy metabolism, oxidative stress response and cell death (Davila and Torres-Aleman, 2008; Gross et al., 2008; van der Horst and Burgering, 2007), we also investigated whether the lack of PINK1 affected the nuclear exclusion of the transcription factor FoxO1 downstream of activated Akt (Burgering and Medema, 2003). Finally, recent evidence points to abnormal regulation of translation in models of PD, possibly as a consequence of impaired Akt-mTOR signaling (Gehrke et al., 2010; Tain et al., 2009). We were thus interested to study whether phosphorylation of ribosomal protein S6 by mTOR was altered in response to IGF-1 in PINK1-deficient cells. Here we show that embryonic fibroblasts from PINK1−/− mice are severely impaired in the phosphorylation of Akt in response to IGF-1 and insulin. This defect is associated with abnormal phosphorylation of GSK-3β and reduced nuclear exclusion of FoxO1. Both proteins can promote apoptosis and have been linked to neurodegenerative diseases (Crews and Masliah, 2010; Giese, 2009; Nagao and Hayashi, 2009; Valencia et al., 2010). We also find that phosphorylation of ribosomal protein S6 by mTOR is impaired. Importantly, the protection afforded by IGF-1 against staurosporine-induced metabolic dysfunction and apoptosis is abrogated in PINK1-deficient cells, and impaired IGF-1 induced Akt phosphorylation is present in primary cortical neurons of PINK1−/− mice. These results reveal a novel function of PINK1 in promoting growth factor and hormone-induced Akt activation, and suggest that defects in IGF-1-mediated Akt survival signaling and abnormal regulation of pro-apoptotic Akt target proteins may contribute to DA neuron loss in PINK1-related parkinsonism.
MATERIALS AND METHODS
Preparation and culture of primary MEF
The generation of PINK1-deficient mice has recently been described (Akundi et al., 2011). All animal work has been conducted according to national guidelines and has been approved by the Animal Care and Use Committee of the University of Kentucky. Primary MEF were prepared from wildtype and PINK1−/− embryos at 15.5–16.5 dpc as described (Nagy et al., 2003: Manipulating the Mouse Embryo, 3rd ed., CSHL press). 5×106 cells were plated per 150-mm tissue culture dish. Cells were split 1:6 after growing confluent and frozen at passage 2. MEF were maintained in DMEM, 10% fetal bovine serum supplemented with 40 U/ml penicillin, 40 mg/ml streptomycin, 0.2 mM L-glutamine and 0.1 mM 2-mercaptoethanol (Invitrogen) in a 37°C incubator with 5% CO2.
Primary cortical neuron cultures
Primary cortical neurons were isolated from newborn (postnatal day 1) wildtype and PINK1−/− mice as previously described (Pang and Geddes, 1997). Briefly, cortices were dissected in Hank’s balanced salt solution (HBSS, Life Technologies) and digested in HBSS with 2 mg/ml trypsin for 15 min at RT. Cortices were washed four times in HBSS and trypsin inactivated with soybean trypsin inhibitor (1.5 mg/ml, 5 min incubation at RT). After four washes cortices were dissociated by repeated trituration in HBSS (4–5 cortices/ml) using a fire-polished pasteur pipette. Cells were seeded onto poly-D-lysine (50 µg/ml)-coated 35 mm culture dishes at a density of 25,000 cells per cm2 in Neurobasal-A medium supplemented with 2% B27, 0.5 mM L-glutamine and 25 µM L-glutamate (Life Technologies) and cultured at 37°C in a 5% CO2 humidified incubator. One and four days after plating we replaced half of the medium with medium lacking glutamate.
Cell treatments and Western blot analysis
5×105 MEF were serum-starved for 4 hours and incubated with 100 ng/ml IGF-1 (R&D Systems), 100 nM insulin (Tocris) or 0–1 mM H2O2 for the indicated times. In some experiments cells were incubated with 1 µM FK506 (Tocris) or 100 nM calyculin A (Enzo Biosciences) to inhibit phosphatases prior to and during stimulation with IGF-1, as indicated in the figure legends. On DIV7, primary cortical neurons were deprived of B27 supplement for 24 hours and subsequently incubated with 100 ng/ml IGF-1 for 30 min, or left untreated. For Western blot analysis, cells were washed in phosphate-buffered saline (PBS) and lysed in denaturing buffer (42 mM Tris-HCl, 1.3% SDS, 6.5% glycerol, 0.1 mM sodium orthovanadate, pH 6.8) containing protease and phosphatase inhibitor cocktails (Sigma). To isolate cytosolic proteins, the cytoplasmic extraction reagents CER-I and CER-II (Pierce) were used according to the manufacturer’s instructions. Purity of the cytosolic factions was confirmed by absence of the nuclear protein NP-62 (Santa Cruz, antibody H-122) and presence of GAPDH. Western blot analysis was carried out by standard methods, with 50µg total protein per sample. Primary antibodies were 1:1000 diluted rabbit anti-Akt, phospho-Akt (Ser473), phospho-Akt (Thr308) GSK-3β, phospho-GSK-3β (Ser 9), phospho-S6 ribosomal protein (Ser235/236), FoxO1 (Cell Signaling Technology) and 1:5000 diluted mouse monoclonal anti-GAPDH (Novus). Secondary antibodies were 1:5000 diluted IR-Dye 800CW-conjugated anti-rabbit IgG and Alexa Fluor 680-conjugated anti-mouse IgG. Membranes were scanned in the 700 nm (GAPDH) and 800 nm (primary antigens) channels of the Odyssey infrared imaging system (LI-COR Biosciences). Band quantification was done using the Odyssey software (LI-COR Biosciences) by selecting the background and subtracting it from the fluorescence intensity measured for each band. Bands for total protein and phosphoproteins were first normalized to GAPDH signals. Subsequently the ratio of normalized phosphoproteins to normalized total protein was calculated and is shown in the figures.
FoxO1 immunocytochemistry
3×104 MEF were plated into individual chambers of 8-well chambered coverglass (Nunc). After serum starvation cells were incubated with 100 ng/ml IGF-1 for 0, 30 or 60 minutes, followed by fixation in 4% paraformaldehyde. Cells were washed in PBS and blocked for 1 hour in PBS, 4% horse serum, 1% bovine serum albumin and 0.4% Triton X-100. FoxO1 was detected by incubation with 1:200 diluted rabbit anti-FoxO1 antibody (Cell Signaling) and 1:300 diluted Alexa Fluor 488-conjugated goat anti-rabbit IgG (Invitrogen). After washing in PBS cells were incubated for 5 min with propidium iodide (0.33 mg/ml in PBS) and subjected to five final washes in PBS. Cells were mounted with anti-fade media (Invitrogen). Cells incubated with secondary antibody alone showed that the background signal was negligible and thus FoxO1 signals were specific. Images were taken for both FoxO1 signal (FITC filter) and propidium iodide signal (Cy3 filter) from at least ten random areas of the slide. Images were captured using an Axiovert 40 fluorescence microscope equipped with an Axiocam MRc5 digital camera and AxioVision software 4.8 (Carl Zeiss). The total number of cells analyzed per condition is indicated in the figure legend. To determine the percentage of FoxO1-positive nuclei, images of FoxO1 and propidium signals were merged using Photoshop software (Adobe).
Quantification of cell viability and cell death
Cell viability was measured using the CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega G3580) according to the manufacturer’s instructions. In this assay, dehydrogenases of metabolically active cells produce NADH or NADPH that convert [3-(4,5-dimethylthiazol-2-yl)-5-(3-acrboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) through reduction into a colored formazan product. Briefly, 104 cells/well were plated in 96-well plates and grown for 24 hours in presence or absence of 100 ng/ml IGF-1 (± IGF-1), followed by incubation for 24 hours with 1 µM staurosporine in serum-free medium ± IGF-1. Twenty µl of MTS reagent was added to each well (100 µl medium) and cells were incubated for 3 hours at 37°C. The amount of formazan produced was measured by absorbance at 490 nm in a Biotek EL800 microplate reader. To quantify LDH released into the medium from dead cells we used the CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega G1780). Twenty-five µl supernatants from cells cultured and treated as above were incubated for 30 min with the assay buffer according to the manufacturer’s instructions and the amount of formazan produced measured by absorbance at 490 nm.
Phospho-tau Western blots
Mouse striatum was dissected on ice and homogenized in denaturing buffer (42 mM Tris-HCl, 1.3% SDS, 6.5% glycerol, 0.1 mM sodium orthovanadate, pH 6.8) containing protease and phosphatase inhibitor cocktails (Sigma). Cultured cells were lysed in the same buffer. Hippocampal tissue was homogenized in 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 0.1 mM EDTA containing Sigma protease inhibitors. Homogenates/lysates were spun at 13,000 × g for 10 minutes and the supernatants were used for Western blot analysis, which was carried out by standard methods using 50 µg total protein per sample. Mouse monoclonal PHF-1 antibody (Greenberg et al., 1992; Otvos et al., 1994) was used at a 1:250 dilution to detect Ser396/404 phosphorylated tau. Secondary Alexa Fluor 680-conjugated anti-mouse IgG was used at 1:5000. Membranes were scanned in the Odyssey infrared imaging system (LI-COR Biosciences).
Statistical analysis
Data were analyzed by ANOVA and two-tailed t-test. Data are presented as mean ± SD (unless stated otherwise) and values of p<0.05 were considered statistically significant.
RESULTS
PINK1 ablation impairs IGF-1-induced Akt activation and phosphorylation of GSK-3β
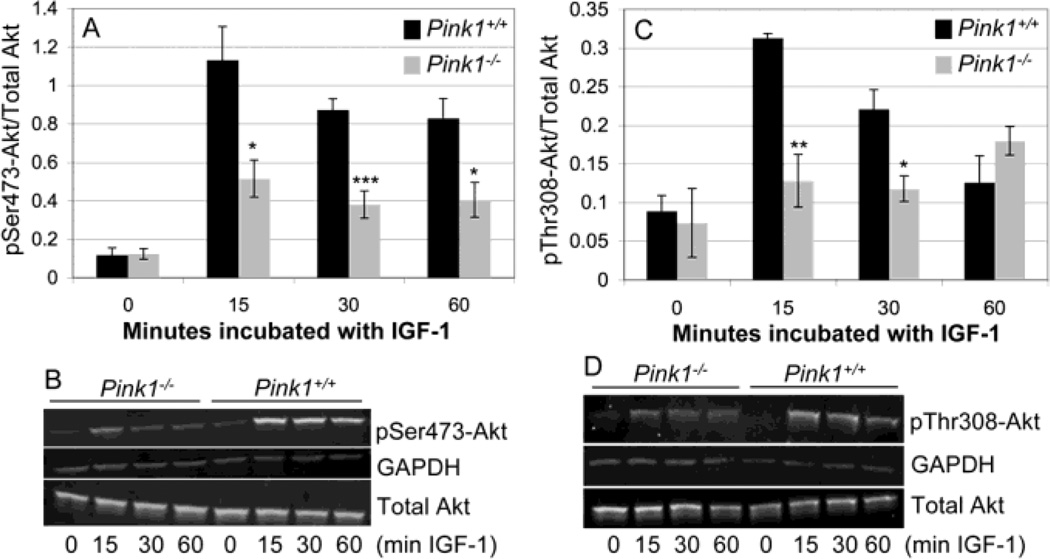
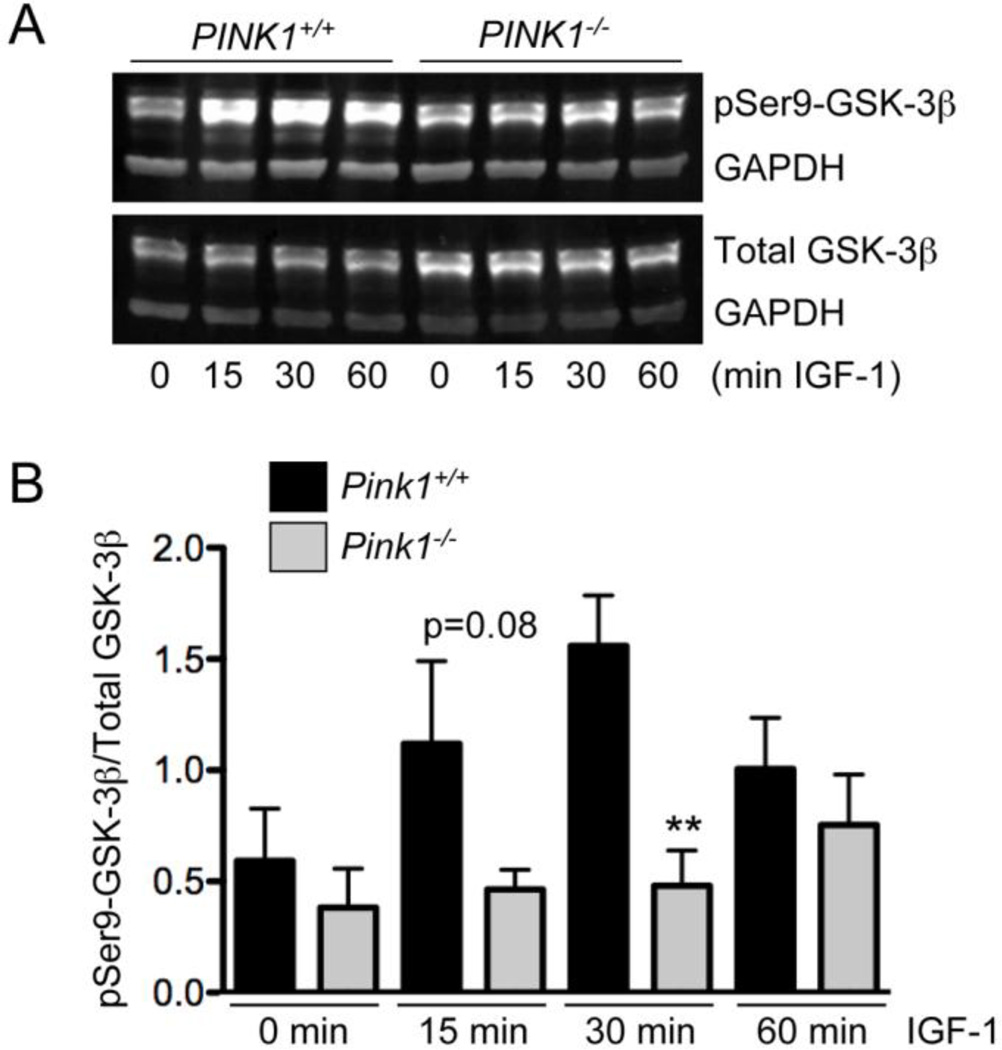
Akt is activated by phosphorylation on Ser473 and Thr308 (Alessi et al., 1997; Bozulic and Hemmings, 2009; Stephens et al., 1998). To test whether ablation of PINK1 affects IGF-1-mediated Akt activation, we studied phosphorylation of Akt on Ser473 and Thr308 in embryonic fibroblasts from wildtype and PINK1−/− mice. Basal levels of phosphorylated Akt were low and similar in wildtype and PINK1−/− MEF (Fig. 1A and C). IGF-1 led to a rapid and robust increase in both pSer473-Akt and pThr308-Akt in wildtype MEF (Fig. 1A–D). In contrast, IGF-1 induced Akt phosphorylation was drastically reduced in PINK1−/− MEF, such that the amounts of both pSer473-Akt and pThr308-Akt were significantly lower in PINK1−/− MEF compared to wildtype MEF after treatment with IGF-1, except for pThr308-Akt at 60 min (Fig. 1A–D). These results show that lack of PINK1 impairs IGF-1 dependent Akt phosphorylation. GSK-3β is a constitutively active kinase that is inhibited by Akt through phosphorylation on Ser9 (Cross et al., 1995). To study whether impaired IGF-1/Akt signaling in PINK1−/− fibroblasts leads to abnormal phosphorylation of GSK-3β, we quantified the amount of GSK-3β phosphorylated on Ser9 (inactive GSK-3β) relative to total GSK-3β in normal and IGF-1 treated cells. As shown in Figure 2A, IGF-1 strongly increased the levels of pSer9-GSK3 in wildtype MEF but not in PINK1−/− fibroblasts. Quantification of results from three independent experiments showed that the difference in GSK-3β phosphorylation was significant at 30 min, and nearly significant at 15 min (p=0.08) after IGF-1 treatment (Fig. 2B). These results demonstrate that PINK1 deficiency impairs IGF-1-induced phosphorylation and inactivation of GSK-3β.
Figure 1. IGF-1-induced phosphorylation of Akt is reduced in cells lacking PINK1.
Serum-starved wildtype (PINK1+/+) and PINK1−/− MEF were stimulated with 100 ng/ml IGF-1 for the indicated times and the levels of pSer473-Akt and pSer308-Akt were quantified by Western blots and normalized to total Akt. In addition, GAPDH served as an internal loading control. Normalized amounts of pSer473-Akt (A) and pThr308-Akt (C), n=4 experiments per genotype and incubation time. Representative Western blots for pSer473-Akt and pThr308-Akt are shown in (B) and (D).
Figure 2. Lack of PINK1 reduces IGF-1-induced phosphorylation of GSK-3β.
Serum-starved wildtype (PINK1+/+) and PINK1−/− MEF were stimulated with 100ng/ml IGF-1 for the indicated times. (A) Total GSK-3β and pSer9-GSK-3β were detected by Western blot. GAPDH served as an internal loading control. (B) Quantification of the results from three independent experiments (n=3), showing a significant reduction of GSK-3β phosphorylation in PINK1-deficient cells at 30 min and nearly significant decline at 15 min of IGF-1 incubation. Mean ± SEM, ** p<0.01.
Lack of PINK1 interferes with IGF-1-induced nuclear export of FoxO1
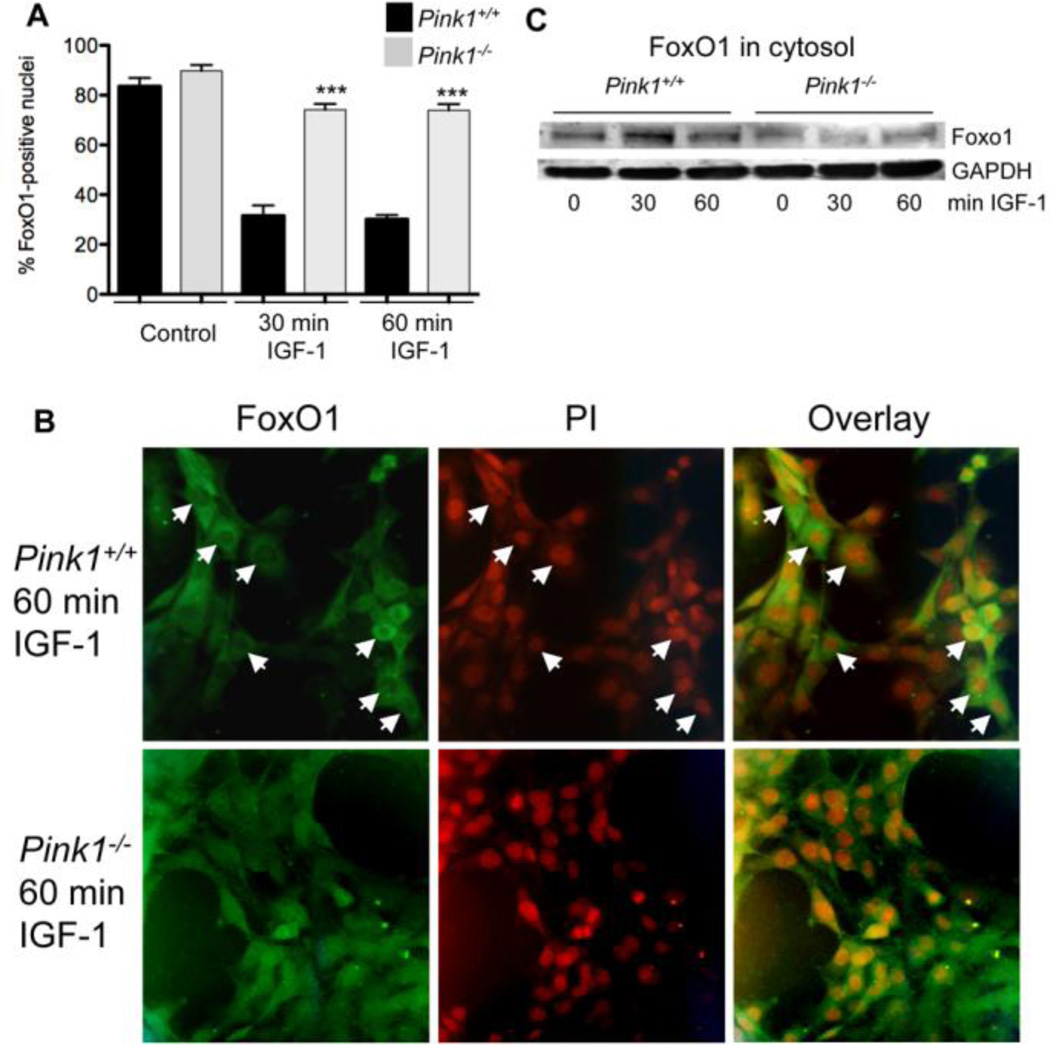
To study whether impaired activation of Akt after treatment with IGF-1 interferes with nuclear exclusion of FoxO1 in PINK1−/− MEF, we carried out immunocytochemistry with an antibody to FoxO1 and quantified the number of cells in which FoxO1 colocalized with the nuclear marker propidium iodide. In the absence of IGF-1, FoxO1 localized to the nucleus of most cells in both wildtype and PINK1−/− MEF (Fig. 3A). As expected, IGF-1 treatment of wildtype MEF led to a significant reduction in the number of cells with nuclear FoxO1 (Fig. 3A and B). In contrast, IGF-1 only weakly promoted nuclear exclusion of FoxO1 in PINK1−/− MEF, and both 30 minutes and 60 minutes after IGF-1 treatment significantly more PINK1−/− than wildtype MEF contained FoxO1 in the nucleus (Fig. 3A and B). In agreement with this finding, the cytosolic levels of FoxO1 were higher in wildtype MEF compared to PINK1−/− MEF after treatment with IGF-1 (Fig. 3C). Taken together, these results show that the lack of PINK1 inhibits IGF-1 mediated nuclear exclusion of FoxO1.
Figure 3. PINK1 deficiency interferes with IGF-1-induced nuclear exclusion of FoxO1.
Serum-starved wildtype (PINK1+/+) and PINK1−/− MEF were stimulated with 100 ng/ml IGF-1 for 0 (control), 30 or 60 min. (A and B) Cells were fixed, FoxO1 was detected with a specific antibody and nuclei were stained with PI. (A) Percent of cells with FoxO1-positive nuclei. (B) Representative pictures of cells from wildtype and PINK1−/− MEF 60 min after incubation with IGF-1, showing nuclei in wildtype cells that lack FoxO1 signals as a result of IGF-1-induced nuclear exclusion (arrows). Such nuclei were rarely detected in PINK1−/− MEF. In contrast, wildtype and PINK1−/− control cells (absence of IGF-1) demonstrated comparable expression of FoxO1 in the nucleus (panel A). Cells analyzed: n=214, 169, 272, 283, 220, and 347 for WT control, PINK1−/− control, WT 30min IGF-1, PINK1−/− 30min IGF-1, WT 60min IGF-1 and PINK1−/− 60min IGF-1, respectively. *** P<0.001, compared to wildtype cells. (C) Cytosolic fractions of cell lysates were analyzed by Western blot for FoxO1, showing that wildtype cells have higher amounts of FoxO1 in the cytosol after treatment with IGF-1, compared to PINK1−/− cells.
IGF-1-induced phosphorylation of ribosomal protein S6 is reduced in PINK1−/− cells
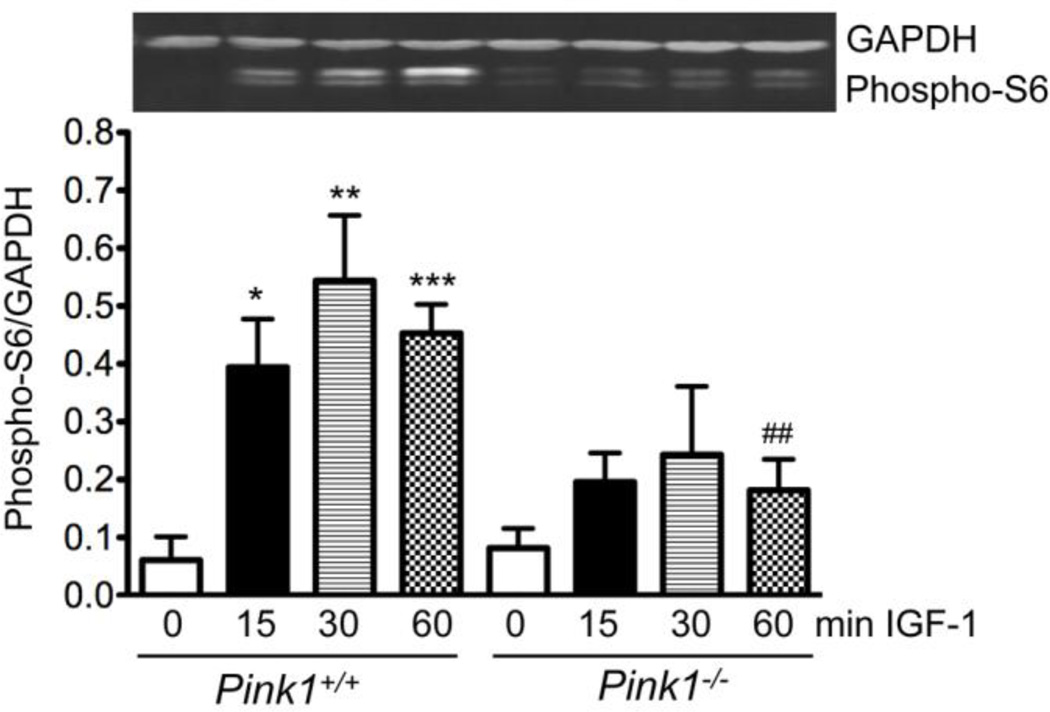
Akt increases the activity of the mitochondrial target of rapamycin (mTOR), which in turn activates p70 S6 kinase (p70S6K) to phosphorylate the ribosomal protein S6 (Bibollet-Bahena and Almazan, 2009; Huang and Manning, 2008). Through this pathway IGF-1 stimulates protein synthesis. As shown in Figure 4, IGF-1 significantly increased the phosphorylation of S6 in wildtype but not PINK1−/− MEF, suggesting that PINK1 ablation interferes with IGF-1 induced protein synthesis.
Figure 4. PINK1 deficiency interferes with IGF-1-induced phosphorylation of ribosomal protein S6.
Serum-starved wildtype (PINK1+/+) and PINK1−/− MEF were stimulated with 100 ng/ml IGF-1 for the indicated times and the levels of phospho-S6 (Ser235/236) were quantified by Western blots and normalized to GAPDH. The graph shows mean ± SEM from four independent experiments (n=4). A Western blot is shown above the graph (lanes correspond to x-axis labels of the graph). *p<0.05, **p<0.01, ***p<0.001, compared to PINK1+/+ without IGF-1. ##p<0.01, compared to PINK1+/+, 60 min IGF-1.
PINK1 is required for insulin-dependent Akt signaling and PINK1-deficient mice show increased body weight
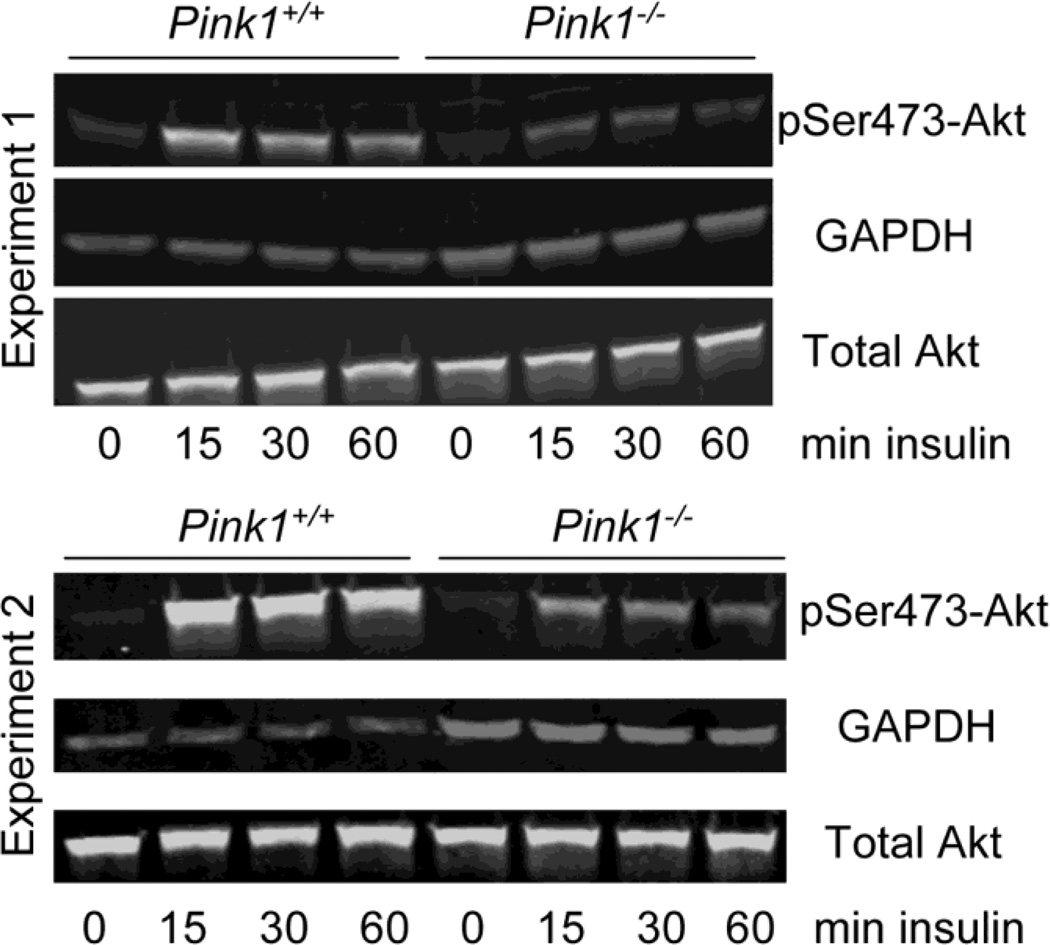
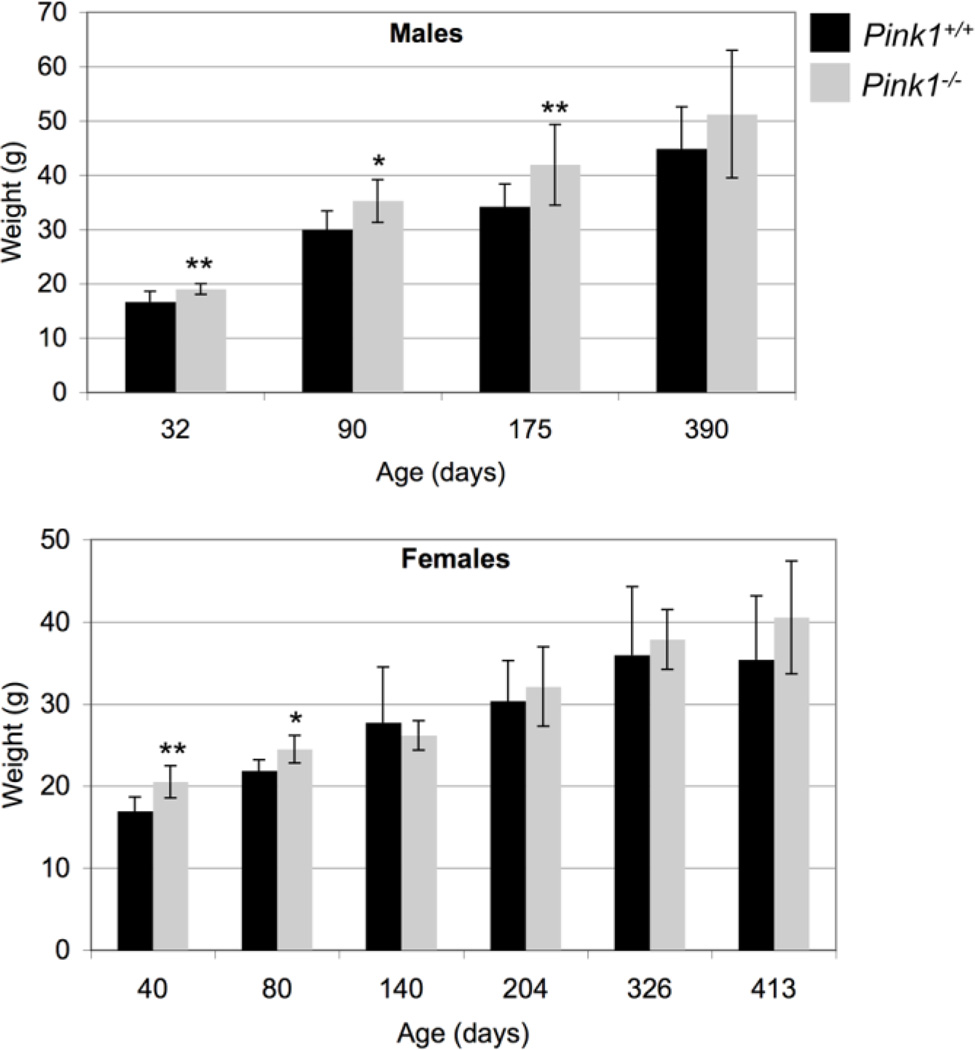
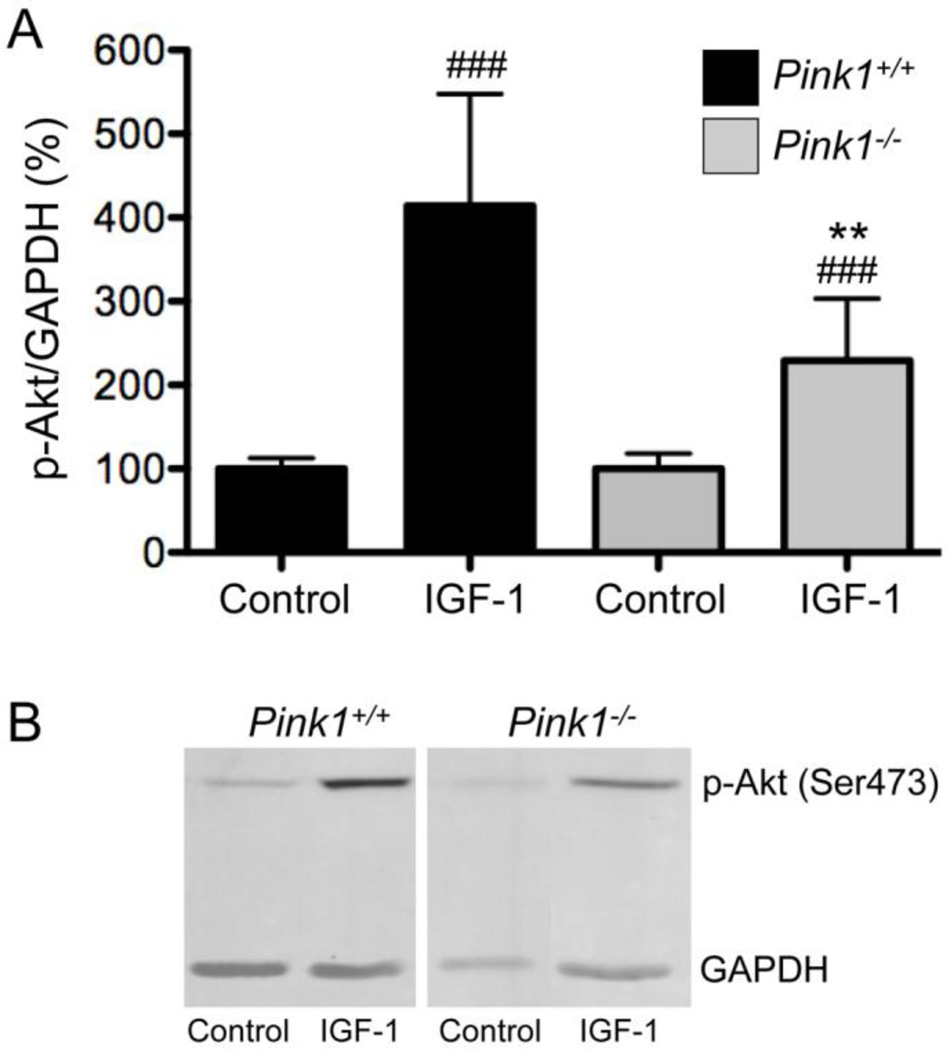
Akt plays a key role in regulating insulin-dependent metabolic responses including β-cell mass and pancreas plasticity, and impaired Akt signaling is involved in the development of insulin resistance and type-2 diabetes (Elghazi and Bernal-Mizrachi, 2009; Vasudevan and Garraway, 2010; Zoncu et al., 2011). To study insulin-dependent activation of Akt, wildtype and PINK1−/− MEF were treated with insulin for various times and the levels of pSer473-Akt relative to total Akt were analyzed by Western blot. As shown in Figure 5, phosphorylation of Akt on Ser473 in response to insulin was significantly reduced in PINK1−/− MEF in two independent experiments. Interestingly, PINK1−/− male mice were significantly heavier than their wildtype controls up to 175 days of age, but no longer at 390 days (Fig. 6A). Females lacking PINK1 showed increased weight up to 80 days of age (Fig. 6B). Although these data do not establish that the increased weight of PINK1−/− mice is related to insulin resistance, decreased insulin-dependent Akt signaling in PINK1−/− MEF warrants further investigation of this pathway in vivo.
Figure 5. Insulin-dependent phosphorylation of Akt is impaired in PINK1-deficient cells.
Serum-starved wildtype (PINK1+/+) and PINK1−/− MEF were stimulated with 100 nM insulin for the indicated times. Levels of total Akt and pSer473-Akt were analyzed by Western blots in two independent experiments, with GAPDH as an internal loading control. In both experiments the lack of PINK1 significantly reduced the insulin-dependent phosphorylation of Akt whereas basal Akt phosphorylation was unaffected. Total Akt levels were comparable between genotypes and not altered by insulin treatment.
Figure 6. PINK1−/− mice show increased body weight.
Mice were weighed at the indicated ages. Weight differences persisted to higher ages in males than females. Number of male mice: 32 days (7 WT and 8 PINK1−/−), 90 days (8 WT and 8 PINK1−/−), 175 days (14 WT and 18 PINK1−/−), 390 days (12 WT and 12 PINK1−/−). Number of female mice: 40 days (10 WT and 7 PINK1−/−), 80 days (6 WT and 3 PINK1−/−), 140 days (4 WT and 3 PINK1−/−), 204 days (9 WT and 8 PINK1−/−), 326 days (5 WT and 4 PINK1−/−), 413 days (4 WT and 5 PINK1−/−). * p<0.05, ** p<0.01.
IGF-1 ameliorates staurosporine-induced metabolic dysfunction and cell death in wildtype but not PINK1-deficient cells
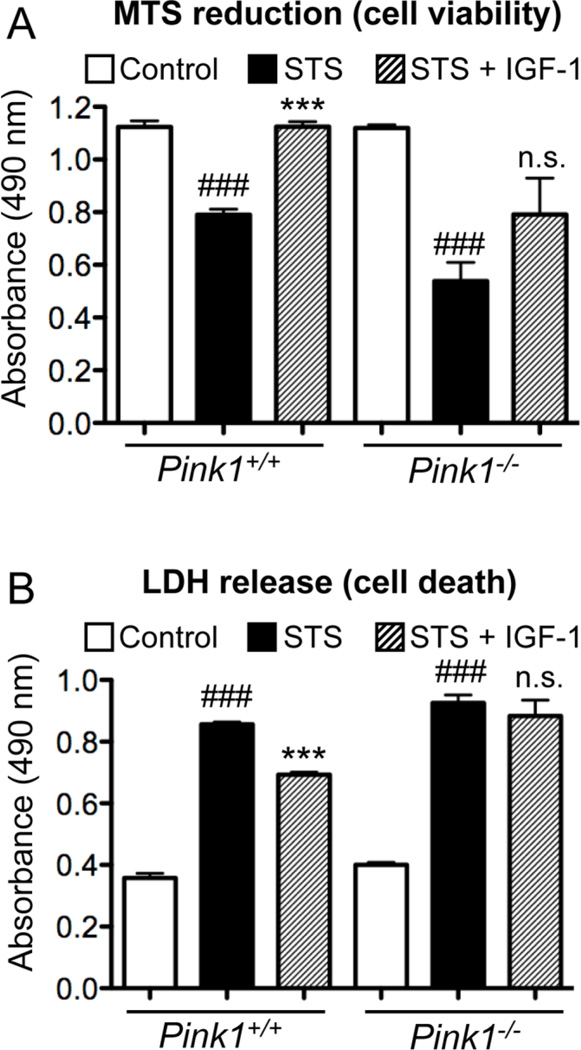
To study whether impaired IGF-1 induced Akt phosphorylation and signaling renders PINK1−/− cells more vulnerable to apoptosis, we treated cells with staurosporine (STS) in the absence and presence of IGF-1. It has been shown previously that IGF-1 inhibits STS-induced mitochondrial dysfunction and apoptosis (Allen et al., 2005; Chandrasekher and Sailaja, 2004; Hilmi et al., 2008; Pucci et al., 2008). We measured cell viability/metabolic activity by MTS reduction and cell death by quantification of lactate dehydrogenase (LDH) released into the medium. Control (untreated) wildtype and PINK1−/− cells showed comparable MTS reduction and LDH release. Treatment with STS decreased MTS reduction (Figure 7A) and increased cell death (Figure 7B) in both genotypes of cells (p<0.001). In addition, PINK1-deficient cells showed lower MTS reduction (p<0.01) and higher cell death (p<0.05) after STS treatment compared to wildtype cells. In STS-treated wildtype cells, IGF-1 restored MTS reduction to that of control cells (p<0.0001). In contrast, STS-treated and STS/IGF-1-treated PINK1−/− cells displayed similar MTS reduction (not significantly different). IGF-1 also reduced LDH release of STS-treated wildtype cells (p<0.0001) but not STS-treated PINK1−/− cells (Figure 7B). Finally, both MTS reduction (p<0.05) and cell death (p<0.01) were significantly different between wildtype and PINK1−/− cells after treatment with STS+IGF-1. In summary, PINK1−/− MEF are more sensitive to STS-induced metabolic dysfunction and apoptosis. Moreover, IGF-1 inhibited STS-induced metabolic dysfunction and cell death in WT but not PINK1−/− MEF. These data suggest that PINK1 ablation interferes with IGF-1-mediated protection against apoptosis.
Figure 7. Pink1 ablation interferes with IGF-1-mediated protection against staurosporine-induced metabolic dysfunction and cell death.
Cells were grown in 96-well plates in presence or absence of 100 ng/ml IGF-1 (± IGF-1) for 24 hours and then treated with 1 µM staurosporine for 24 hours ± IGF-1. (A) Cell viability/metabolism was measured by MTS reduction and (B) cell death was quantified by LDH release as described in Materials and Methods. ### p<0.0001 compared to control, *** p<0.0001 compared to staurosporine-treated cells, n.s. indicates not significantly different from staurosporine-treated cells (n=8 wells/condition). Two replicate experiments with similar results were done.
Impaired Akt signaling is not due to increased dephosphorylation of Akt in PINK1−/− cells
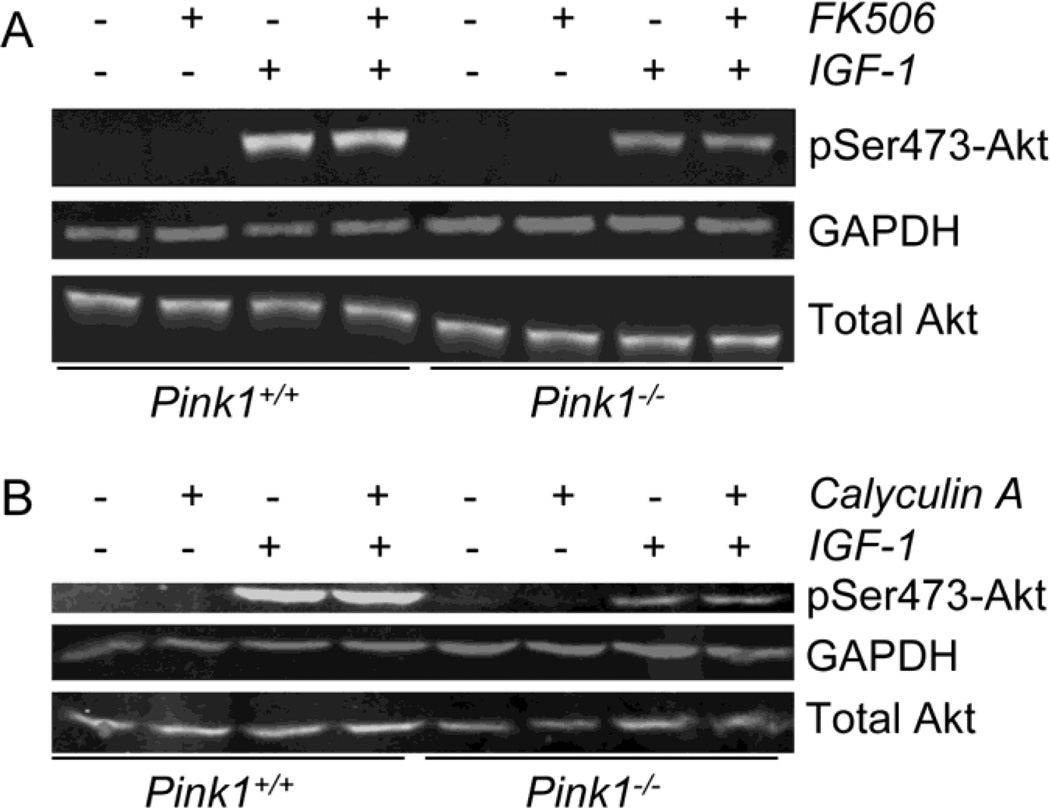
Because it has been shown that the phosphatase calcineurin is activated in human dopaminergic cells with a stable knockdown of PINK1 (Sandebring et al., 2009), we examined whether calcineurin may be responsible for the reduced levels of phosphorylated Akt in IGF-1 stimulated PINK1−/− MEF. Incubation of cells with the calcineurin inhibitor FK506 for one hour (Fig. 8A) and twenty-four hours (not shown) before and during the addition of IGF-1 did not increase Akt phosphorylation in PINK1−/− (and wildtype) cells. The same results were obtained when cyclosporine A was used to inhibit calcineurin (data not shown). We also inhibited all Ser/Thr phosphatases with calyculin A (Ni et al., 2007). Even under these conditions, the IGF-1 stimulated Akt phosphorylation was unchanged and lower in PINK1−/− cells than in wildtype cells (Figure 8B). These experiments show that the reduced Akt phosphorylation in PINK1−/− MEF is not due to enhanced Akt dephosphorylation.
Figure 8. Impaired Akt signaling in PINK1−/− cells is not due to enhanced Akt dephosphorylation.
Serum-starved wildtype (PINK1+/+) and PINK1−/− MEF were incubated with 1 mM FK506 for 60 min to inhibit calcineurin (A) or 100 nM calyculin A for 15 min to inhibit all Ser/Thr phosphatases (Ni et al., 2007). Subsequently cells were stimulated with 100 ng/ml IGF-1 for 15 min and the levels of pSer473-Akt, total Akt and GAPDH in whole cell lysates were analyzed by Western blot analysis. No difference in the amounts of pSer473-Akt and total Akt were seen without and with phosphatase inhibitor treatment (neither in PINK1+/+ nor PINK1−/− cells), indicating that dephosphorylation of Akt played no role in the lower amounts of pSer473 Akt observed in IGF-1 treated PINK1−/− cells. Identical results were obtained when the cells were treated for 24 hours with 1 mM FK506, or for one or 24 hours with 10 mM cyclosporine A (data not shown). Three replicate experiments with similar results were done.
Oxidative stress-induced Akt signaling is normal in PINK1-deficient MEF
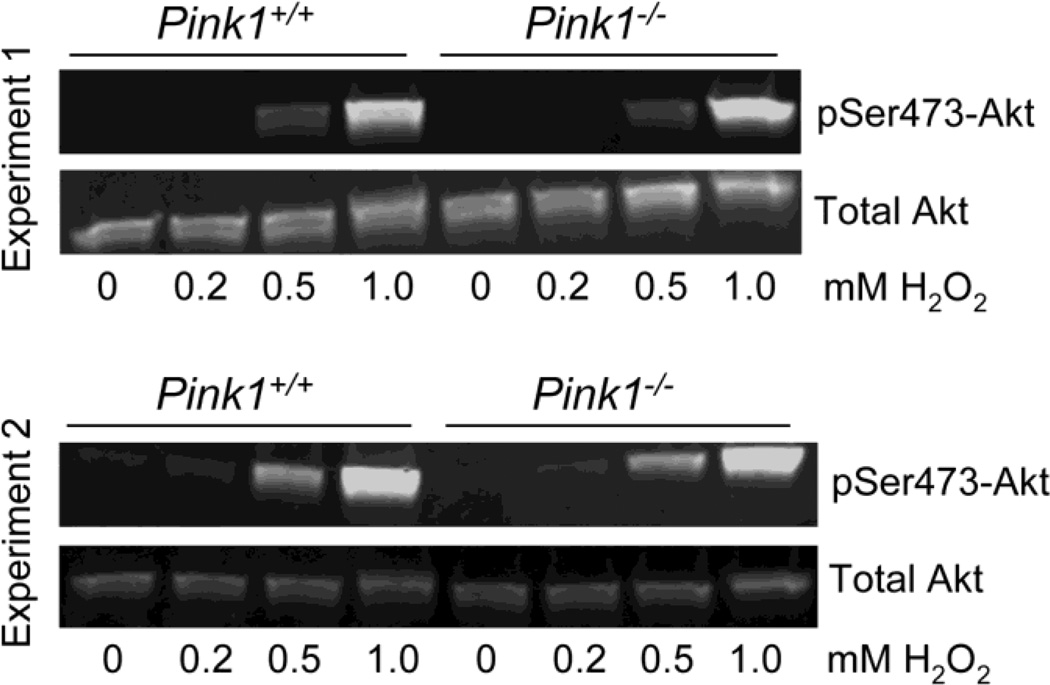
To study whether PINK1 is required for oxidative stress-induced activation of Akt, we treated wildtype and PINK1−/− MEF with increasing concentrations of hydrogen peroxide (H2O2). We found a dose-dependent phosphorylation of Akt with significant Akt activation occurring at 0.5 mM and 1 mM H2O2. However, no difference in the normalized pSer473-Akt levels was observed between wildtype and PINK1-deficient MEF (Figure 9). These results show that PINK1 is dispensible for Akt phosphorylation in response to oxidative stress, at least under the conditions used here.
Figure 9. Oxidative stress-induced phosphorylation of Akt is normal in the absence of PINK1.
Serum-starved wildtype (PINK1+/+) and PINK1−/− MEF were incubated for 30 min with the indicated concentrations of H2O2. Levels of total Akt and pSer473-Akt were analyzed by Western blots in two independent experiments and were comparable between genotypes. Even after 60 min, H2O2 concentrations up to 0.2 mM induced little or no Akt phosphorylation (data not shown). Two replicate experiments with similar results were done.
Impaired IGF-1-induced Akt phosphorylation in primary cortical neurons of PINK1−/− mice
To study whether the lack of PINK1 affected Akt phosphorylation in response to IGF-1 in neurons, we isolated primary cortical neurons from wildtype and PINK1−/− mice. Levels of pSer473-Akt were determined in neurons deprived of growth factor supplement for 24 hours and in growth factor-starved neurons after stimulation for 30 minutes with IGF-1. The levels of pSer473-Akt were not statistically different in wildtype and PINK1 mutant neurons after starvation (Figure 10A, control). In contrast, induction of Akt phosphorylation by IGF-1 was significantly larger in wildtype than in PINK1−/− cortical neurons (Fig. 10A and B), similar to what we found in MEF. These results show that the defect in IGF-1-induced Akt phosphorylation is also present in primary neurons of PINK1-deficient mice.
Figure 10. Induction of Akt phosphorylation in response to IGF-1 is reduced in primary cortical neurons of PINK1−/− mice.
B27-starved PINK1+/+ and PINK1−/− primary cortical neurons were stimulated with 100 ng/ml IGF-1 for 30 minutes and the levels of pSer473-Akt and GAPDH were quantified by Western blots. Basal levels of pSer473-Akt were not different between PINK1+/+ and PINK1−/− neurons and set to 100% for both genotypes. Data represent mean ± SD from n=4 experiments. ### p<0.001 compared to same genotype without IGF-1 (control), ** p<0.01 compared to PINK1+/+ with IGF-1.
Phosphorylation of tau on serine residues 396 and 404 is unaltered in the striatum and in cortical neurons of PINK1−/− mice
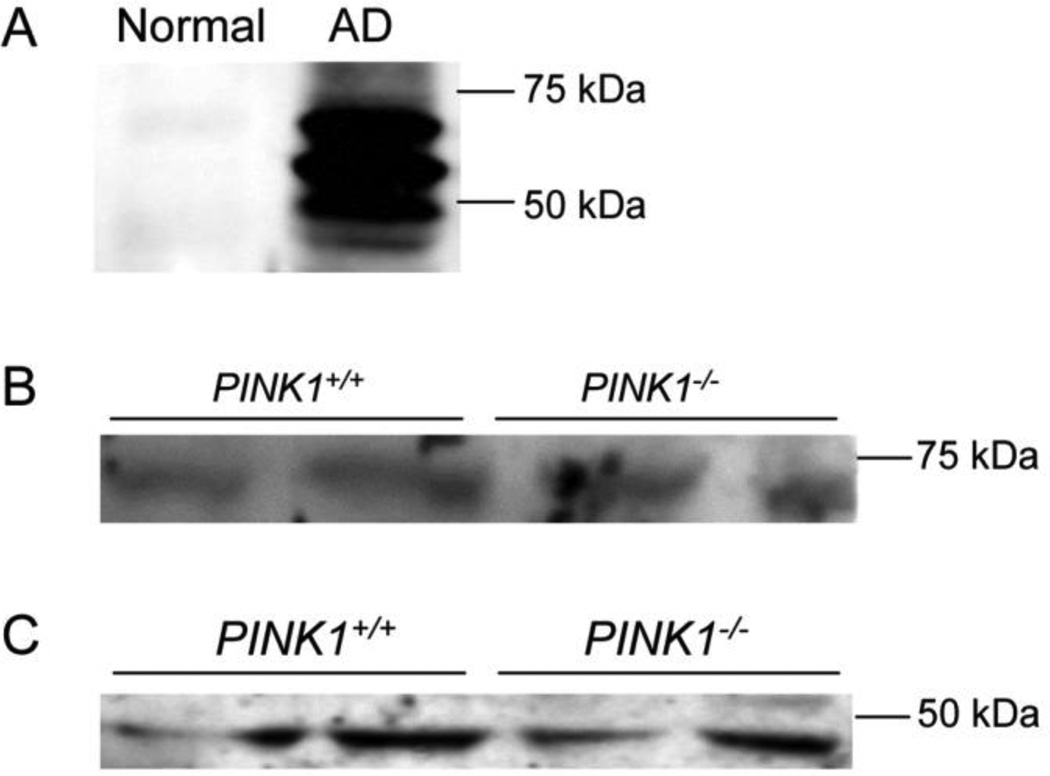
Tau is hyper-phosphorylated in several neurodegenerative diseases, notably AD, and contains multiple sites that can be phosphorylated by GSK-3β in vitro and in cells (Hanger and Noble, 2011). Among these sites are the serine residues 396/404, which are recognized when phosphorylated by the antibody PHF-1 (Greenberg et al., 1992; Otvos et al., 1994). PHF-1 also recognizes phosphorylated tau from mice, which is increased in a sporadic model of PD in a α-synuclein-dependent manner (Duka et al., 2006). To study whether tau phosphorylation on Ser396/404 may be altered in PINK1-deficient mice, we analyzed total extracts of mouse striatum and primary cortical neurons from wildtype and PINK1-deficient mice by Western blot with the PHF-1 antibody. In addition, we used extracts from MEF before and after treatment with IGF-1. As a control to verify antibody functionality, we used hippocampal extracts from normal individuals and late-stage AD patients. Normal human hippocampus contained no detectable phosphoSer396/404-tau. However, as expected, the hippocampus from an AD patient showed high levels of phospho-tau (Fig. 11A). In contrast, extracts from mouse striatum (Fig. 11B) and primary cortical neurons (Fig. 11C) showed very low levels of phospho-tau, which only became detectable upon over-exposure of the Western blots. However, no differences in phospho-tau levels between wildtype and PINK1−/− striatum and cortical neurons were observed in these studies (Fig. 11B and C). No phospho-tau was detected in MEF, neither before nor after IGF-1 treatment (data not shown).
Figure 11. Phosphorylation of tau at Ser396/404 is unaltered in PINK1−/− striatum and cortical neurons.
Phospho-tau (Ser396/404) was detected with the PHF-1 antibody (see Materials and Methods). (A) Human hippocampal tissue from control and AD patients was used to confirm functionality of the antibody. (B) Phospho-tau in the striatum and (C) phospho-tau in primary cortical neurons of wildtype and PINK1-deficient mice.
DISCUSSION
Mutations in PINK1 are linked to recessive familial PD. In cultured cells PINK1 and Parkin promote the degradation of depolarized mitochondria through autophagy (Narendra et al., 2010; Vives-Bauza et al., 2009). However, it remains to be seen whether impaired mitophagy of dysfunctional mitochondria is the major cause for DA neuron loss (Van Laar et al., 2011). It is likely that other defects in the absence of PINK1 also contribute to the increased DA neuron vulnerability in PD. Mounting evidence points to a stimulatory role of recessive PD gene products in Akt cell survival signaling (Aleyasin et al., 2010; Kim et al., 2005a; Murata et al., 2011; Yang et al., 2005). DJ-1 promotes the membrane localization and phosphorylation of Akt and is required for the neuroprotective effects of Akt in a mouse model of sporadic PD in which DA neuron loss occurs due to mitochondrial complex I inhibition and oxidative stress (Aleyasin et al., 2010). Importantly, Akt signal transduction is also impaired in sporadic PD (Malagelada et al., 2008; Timmons et al., 2009). At least a proportion of PINK1 has been reported to be cytosolic (Beilina et al., 2005; Haque et al., 2008; Takatori et al., 2008) and overexpression of cytosol-restricted PINK1 (by deletion of its mitochondrial targeting motif) protected neurons from the dopaminergic toxin MPTP, suggesting that PINK1 can exert neuroprotection through cytosolic mechanisms (Haque et al., 2008). Here we show for the first time that PINK1 is required for the full activation of Akt in response to IGF-1 and insulin. Calcineurin dephosphorylates Akt (Ni et al., 2007; Park et al., 2008; Wu et al., 2006), and it has been shown that calcineurin is activated in human dopaminergic cells with a stable knockdown of PINK1 (Sandebring et al., 2009). However, reduced Akt phosphorylation in PINK1-deficient MEF is not due to elevated phosphatase activity, as several calcineurin and pan-phosphatase inhibitors failed to increase Akt phosphorylation in PINK1-deficient cells stimulated with IGF-1. Therefore, we conclude that IGF-1 pathway signaling components upstream of Akt phosphorylation are compromised in PINK1−/− cells.
Consistent with impaired Akt signaling, we demonstrate that phosphorylation of GSK-3β and nuclear exclusion of FoxO1 are reduced in IGF-1 stimulated PINK1−/− cells. Besides Akt, protein kinase C and p90Rsk, a downstream kinase of Erk, can phosphorylate GSK-3β at the Ser9 residue (Eldar-Finkelman, 2002; Stambolic and Woodgett, 1994). IGF-1 can also activate Erk (Feldman et al., 1997) and our experiments have not addressed whether IGF-1 induced Erk signaling is impaired in PINK1-deficient cells. If PINK1 deficiency preferentially affects Akt activation it may explain why the differences in GSK-3β phosphorylation were significant at 30 min after IGF-1 but only nearly significant at 15 minutes after IGF-1 (p=0.08). GSK-3β is implicated in the pathogenesis of PD (Nagao and Hayashi, 2009) and PD risk is associated with polymorphisms of the GSK-3β gene (Kwok et al., 2005). In addition, GSK-3β mediates DA neuron death in 6-OHDA treated rats (Chen et al., 2004). It has been shown recently that GSK-3β directly contributes to the shrinkage and degeneration of neuronal dendrites in a Drosophila model of dominant PD that expresses G2019S LRRK2 (Lin et al., 2010). Furthermore, it has been reported that LRRK2 phosphorylates FoxO1 and enhances its transcriptional activity towards apoptotic target genes in both Drosophila and mammalian cells, leading to DA neuron loss and neurotoxicity (Kanao et al., 2010). Thus, our results show that two proteins implicated in the pathogenesis of dominant PD are also deregulated in PINK1-deficient cells and suggest that increased GSK-3β and FoxO1 activity may be involved in the pathogenesis of familial PD. Finally we show that the lack of PINK1 interferes with IGF-1-induced phosphorylation of the ribosomal protein S6, which is necessary for protein synthesis. In Drosophila, it has recently been shown that inactivating pink1 and parkin mutations as well as pathogenic LRRK2-G2019S result in deregulation of translation via abnormal phosphorylation of 4E-BP1 (Gehrke et al., 2010; Tain et al., 2009). Collectively, these results suggest that abnormalities in protein synthesis are common to various form of PD and may be related to impaired Akt-mTOR signaling. Importantly, we demonstrate that IGF-1 reversed/inhibited staurosporine-induced metabolic dysfunction and cell death in wildtype cells, but not in PINK1−/− cells. This shows that PINK1 is required for the protective effects of IGF-1 on staurosporine-induced cellular dysfunction and apoptosis and suggests that impaired IGF-1/Akt signaling may render PINK1-deficient cells more susceptible to apoptotic stimuli. In agreement with our results, it has recently been shown that adenoviral overexpression of PINK1 increases the phosphorylation of Akt on Ser473 in SH-SY5Y neuroblastoma cells via mTORC2 (Murata et al., 2011). PINK1-induced Akt phosphorylation was required for heightened resistance to rotenone-induced cell death and other apoptotic stimuli (Murata et al., 2011). Our results extend these findings by showing that physiological levels of PINK1 increase Akt phosphorylation in response to IGF-1, thereby protecting cells against apoptosis. Moreover we find that, when combined with IGF-1, PINK1 not only increases the phosphorylation of Akt on Ser472 (Murata et al., 2011) but also on Thr308. The only time point where Thr308 phosphorylation was not different was 60 min after IGF-1 treatment. Mitochondrial target of rapamycin complex 2 (mTORC2) is responsible for Ser473 phosphorylation while PDK1 phosphorylates Akt on Thr308 (Bozulic and Hemmings, 2009). It is possible that the lack of PINK1 affects IGF-1-induced activation of mTORC2 and PDK1 differentially, both in magnitude and time. This idea is consistent with the finding that overexpression of PINK1 (in the absence of IGF-1) enhanced phosphorylation of Akt on Ser473 but not Thr308 (Murata et al., 2011).
The finding of impaired IGF-1 induced Akt signaling and protection against apoptosis in PINK1-deficient cells is likely relevant for PD pathogenesis for several reasons. First, IGF-1 receptors are abundantly expressed in dopaminergic neurons of the substantia nigra (Quesada et al., 2007), and mice with a heterozygous deletion of the IGF-1 receptor gene (Igfr+/− mice) developed increased neuroinflammation and DA neuron loss after injection with the Parkinsonian mitochondrial complex I inhibitor MPTP (Nadjar et al., 2009). Second, IGF-1 improved the survival of grafted human DA neurons (Clarkson et al., 2001), antagonized DA toxicity (Offen et al., 2001) and inhibited DA neuron death in 6-hydroxydopamine (6-OHDA)-treated rats through activation of the PI3K-Akt pathway (Ebert et al., 2008; Quesada et al., 2008; Quesada and Micevych, 2004). Third, a constitutively active form of Akt suppressed both retrograde axon degeneration and DA neuron loss in toxin and axotomy-induced models of PD (Cheng et al., 2011), and promoted re-growth of dopaminergic axons after chemical lesion (Kim et al., 2011). Finally, Akt is involved in regulating signal transduction in DA-responsive postsynaptic striatal neurons (Beaulieu et al., 2007). Collectively, these data show that the IGF-1/Akt pathway protects DA neurons in vivo against mitochondrial dysfunction, oxidative stress and axonal pathologies induced by Parkinsonian toxins. Consequently, reduced IGF-1/Akt signaling in the absence of PINK1 may lower the threshold for stress-induced apoptosis and/or reduce regenerative axonal responses after insult, ultimately causing neuron death. This hypothesis is further supported by the observation that IGF-1-dependent induction of Akt phosphorylation is also impaired in primary cortical neurons from PINK1-deficient mice. We have recently shown that mitochondria from the brain of PINK1-deficient mice display increased calcium-induced permeability transition (Akundi et al., 2011). Calcium plays an important role in the normal physiology as well as cell death of DA neurons (Chan et al., 2007). Reduced IGF-1/Akt survival signaling in PINK1 deficiency may further increase the vulnerability of DA neurons to calcium-induced mitochondrial dysfunction and apoptosis. For example, IGF-1 has been shown to prevent Ca2+ overload-mediated death of motor neurons (Vincent et al., 2004) and photoreceptors (Arroba et al., 2009), and to block glutamate-induced death of oligodendrocytes progenitors (Ness et al., 2004; Ness and Wood, 2002; Wood et al., 2007) through the activation of the PI3K/Akt pathway. Decreased IGF-1 and/or insulin-dependent Akt signaling may also render neurons more vulnerable to oxidative stress-induced impairments in glucose metabolism (Duarte et al., 2006), glutamate (Digicaylioglu et al., 2004; Vincent et al., 2004) as well as hypoxia-ischemia and axonal injury (Kermer et al., 2000; Lin et al., 2009). Surprisingly, we found that oxidative stress (H2O2)-induced phosphorylation of Akt was normal in the absence of PINK1, although we cannot exclude that the relatively high concentrations of H2O2 required to activate Akt in MEF may have masked a differential activation of Akt in PINK1−/− cells. It has been shown that DJ-1 is required for the full activation of Akt in response to oxidative stress in Drosophila (Yang et al., 2005), suggesting that different recessive PD genes may enhance Akt signaling in response to different stimuli. Consistent with this idea, Parkin has been reported to enhance Akt signaling in response to epidermal growth factor (EGF) in an E3 ligase-dependent manner (Fallon et al., 2006). Because PINK1 acts upstream of Parkin (Clark et al., 2006; Park et al., 2006) and increases the Parkin E3 ligase activity (Sha et al., 2010), PINK1 and Parkin may jointly promote growth factor-induced Akt signal transduction. Parkin and PINK1 cooperate to promote nuclear factor kappa-B (NF-κB) signaling (Akundi et al., 2011; Sha et al., 2010), another pathway that inhibits apoptosis in neurons (Mattson and Camandola, 2001; Mattson et al., 2000). However, further studies are required to substantiate that PINK1 and Parkin jointly enhance Akt survival signaling and to elucidate the underlying mechanisms of impaired IGF-1/Akt signaling in PINK1-deficient cells. In summary, we demonstrate for the first time that endogenous PINK1 significantly enhances IGF-1-induced Akt signaling, a pathway that is neuroprotective in several models of PD. Our results suggest that defects in IGF-1-mediated Akt signal transduction and abnormal regulation of pro-apoptotic Akt target proteins may contribute to neuron loss in PINK1-related parkinsonism.
Highlights.
Pink1 enhances insulin-like growth factor 1 (IGF-1) and insulin-dependent Akt signaling.
Apoptotic Akt substrates GSK-3β and FoxO1 are deregulated in IGF-treated Pink1-mutant cells.
IGF-1-induced phosphorylation of ribosomal protein S6 is reduced in Pink1−/− cells.
Pink1 deficiency inhibits IGF-1-mediated protection against apoptosis
ACKNOWLEDGMENTS
We thank Vimala Bondada and Dr. Jim Geddes for practical advice and help with primary cortical neuron cultures. The authors gratefully acknowledge Dr. Peter Davies for providing PHF-1 antibody and Dr. George Smith for the phospho-S6 antibody. Frozen hippocampal tissue from normal and AD subjects were kindly provided in 2008 by Dr. William Markesbery from the Sanders Brown Center on Aging. This work was supported in part by a COBRE grant (P20 RR15592) from the National Center for Research Resources (NCRR), as well as by grants from Parkinson Schweiz and the American Parkinson’s Disease Association (APDA). The funding organizations had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akundi RS, et al. Increased mitochondrial calcium sensitivity and abnormal expression of innate immunity genes precede dopaminergic defects in pink1-deficient mice. PLoS One. 2011;6:e16038. doi: 10.1371/journal.pone.0016038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi DR, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- Aleyasin H, et al. DJ-1 protects the nigrostriatal axis from the neurotoxin MPTP by modulation of the AKT pathway. Proc Natl Acad Sci U S A. 2010;107:3186–3191. doi: 10.1073/pnas.0914876107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RT, et al. Sustained Akt/PKB activation and transient attenuation of c-jun N-terminal kinase in the inhibition of apoptosis by IGF-1 in vascular smooth muscle cells. Apoptosis. 2005;10:525–535. doi: 10.1007/s10495-005-1882-3. [DOI] [PubMed] [Google Scholar]
- Arroba AI, et al. IGF-I maintains calpastatin expression and attenuates apoptosis in several models of photoreceptor cell death. Eur J Neurosci. 2009;30:975–986. doi: 10.1111/j.1460-9568.2009.06902.x. [DOI] [PubMed] [Google Scholar]
- Avila J, et al. Role of glycogen synthase kinase-3 in Alzheimer's disease pathogenesis and glycogen synthase kinase-3 inhibitors. Expert Rev Neurother. 2010;10:703–710. doi: 10.1586/ern.10.40. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, et al. The Akt-GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol Sci. 2007;28:166–172. doi: 10.1016/j.tips.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Beck KD. Functions of brain-derived neurotrophic factor, insulin-like growth factor-I and basic fibroblast growth factor in the development and maintenance of dopaminergic neurons. Prog Neurobiol. 1994;44:497–516. doi: 10.1016/0301-0082(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Beilina A, et al. Mutations in PTEN-induced putative kinase 1 associated with recessive parkinsonism have differential effects on protein stability. Proc Natl Acad Sci U S A. 2005;102:5703–5708. doi: 10.1073/pnas.0500617102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibollet-Bahena O, Almazan G. IGF-1-stimulated protein synthesis in oligodendrocyte progenitors requires PI3K/mTOR/Akt and MEK/ERK pathways. J Neurochem. 2009;109:1440–1451. doi: 10.1111/j.1471-4159.2009.06071.x. [DOI] [PubMed] [Google Scholar]
- Bonifati V, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- Bozulic L, Hemmings BA. PIKKing on PKB: regulation of PKB activity by phosphorylation. Curr Opin Cell Biol. 2009;21:256–261. doi: 10.1016/j.ceb.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Burgering BM, Medema RH. Decisions on life and death: FOXO Forkhead transcription factors are in command when PKB/Akt is off duty. J Leukoc Biol. 2003;73:689–701. doi: 10.1189/jlb.1202629. [DOI] [PubMed] [Google Scholar]
- Canet-Aviles RM, et al. The Parkinson's disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci U S A. 2004;101:9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CS, et al. 'Rejuvenation' protects neurons in mouse models of Parkinson's disease. Nature. 2007;447:1081–1086. doi: 10.1038/nature05865. [DOI] [PubMed] [Google Scholar]
- Chandrasekher G, Sailaja D. Phosphatidylinositol 3-kinase (PI-3K)/Akt but not PI-3K/p70 S6 kinase signaling mediates IGF-1-promoted lens epithelial cell survival. Invest Ophthalmol Vis Sci. 2004;45:3577–3588. doi: 10.1167/iovs.04-0279. [DOI] [PubMed] [Google Scholar]
- Chen G, et al. Glycogen synthase kinase 3beta (GSK3beta) mediates 6-hydroxydopamine-induced neuronal death. FASEB J. 2004;18:1162–1164. doi: 10.1096/fj.04-1551fje. [DOI] [PubMed] [Google Scholar]
- Chen J, et al. Parkinson disease protein DJ-1 converts from a zymogen to a protease by carboxyl-terminal cleavage. Hum Mol Genet. 2010;19:2395–2408. doi: 10.1093/hmg/ddq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HC, et al. Akt suppresses retrograde degeneration of dopaminergic axons by inhibition of macroautophagy. J Neurosci. 2011;31:2125–2135. doi: 10.1523/JNEUROSCI.5519-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark IE, et al. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- Clarkson ED, et al. IGF-I and bFGF improve dopamine neuron survival and behavioral outcome in parkinsonian rats receiving cultured human fetal tissue strands. Exp Neurol. 2001;168:183–191. doi: 10.1006/exnr.2000.7593. [DOI] [PubMed] [Google Scholar]
- Crews L, Masliah E. Molecular mechanisms of neurodegeneration in Alzheimer's disease. Hum Mol Genet. 2010;19:R12–R20. doi: 10.1093/hmg/ddq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross DA, et al. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Dagda RK, et al. Loss of PINK1 Function Promotes Mitophagy through Effects on Oxidative Stress and Mitochondrial Fission. J Biol Chem. 2009;284:13843–13855. doi: 10.1074/jbc.M808515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila D, Torres-Aleman I. Neuronal death by oxidative stress involves activation of FOXO3 through a two-arm pathway that activates stress kinases and attenuates insulin-like growth factor I signaling. Mol Biol Cell. 2008;19:2014–2025. doi: 10.1091/mbc.E07-08-0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, et al. The Parkinson's disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci U S A. 2008;105:14503–14508. doi: 10.1073/pnas.0803998105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digicaylioglu M, et al. Acute neuroprotective synergy of erythropoietin and insulin-like growth factor I. Proc Natl Acad Sci U S A. 2004;101:9855–9860. doi: 10.1073/pnas.0403172101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte AI, et al. Insulin restores metabolic function in cultured cortical neurons subjected to oxidative stress. Diabetes. 2006;55:2863–2870. doi: 10.2337/db06-0030. [DOI] [PubMed] [Google Scholar]
- Duka T, et al. Alpha-synuclein induces hyperphosphorylation of Tau in the MPTP model of parkinsonism. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20:2302–2312. doi: 10.1096/fj.06-6092com. [DOI] [PubMed] [Google Scholar]
- Ebert AD, et al. Human neural progenitor cells over-expressing IGF-1 protect dopamine neurons and restore function in a rat model of Parkinson's disease. Exp Neurol. 2008;209:213–223. doi: 10.1016/j.expneurol.2007.09.022. [DOI] [PubMed] [Google Scholar]
- Eldar-Finkelman H. Glycogen synthase kinase 3: an emerging therapeutic target. Trends in molecular medicine. 2002;8:126–132. doi: 10.1016/s1471-4914(01)02266-3. [DOI] [PubMed] [Google Scholar]
- Elghazi L, Bernal-Mizrachi E. Akt and PTEN: beta-cell mass and pancreas plasticity. Trends Endocrinol Metab. 2009;20:243–251. doi: 10.1016/j.tem.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon L, et al. A regulated interaction with the UIM protein Eps15 implicates parkin in EGF receptor trafficking and PI(3)K-Akt signalling. Nat Cell Biol. 2006;8:834–842. doi: 10.1038/ncb1441. [DOI] [PubMed] [Google Scholar]
- Feldman EL, et al. Insulin-like growth factors regulate neuronal differentiation and survival. Neurobiology of disease. 1997;4:201–214. doi: 10.1006/nbdi.1997.0156. [DOI] [PubMed] [Google Scholar]
- Gautier CA, et al. Loss of PINK1 causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proc Natl Acad Sci U S A. 2008;105:11364–11369. doi: 10.1073/pnas.0802076105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegg ME, et al. Silencing of PINK1 expression affects mitochondrial DNA and oxidative phosphorylation in dopaminergic cells. PLoS ONE. 2009;4:e4756. doi: 10.1371/journal.pone.0004756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrke S, et al. Pathogenic LRRK2 negatively regulates microRNA-mediated translational repression. Nature. 2010;466:637–641. doi: 10.1038/nature09191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- Giese KP. GSK-3: a key player in neurodegeneration and memory. IUBMB Life. 2009;61:516–521. doi: 10.1002/iub.187. [DOI] [PubMed] [Google Scholar]
- Greenberg SG, et al. Hydrofluoric acid-treated tau PHF proteins display the same biochemical properties as normal tau. The Journal of biological chemistry. 1992;267:564–569. [PubMed] [Google Scholar]
- Greene JC, et al. Genetic and genomic studies of Drosophila parkin mutants implicate oxidative stress and innate immune responses in pathogenesis. Hum Mol Genet. 2005;14:799–811. doi: 10.1093/hmg/ddi074. [DOI] [PubMed] [Google Scholar]
- Gross DN, et al. The role of FoxO in the regulation of metabolism. Oncogene. 2008;27:2320–2336. doi: 10.1038/onc.2008.25. [DOI] [PubMed] [Google Scholar]
- Hanger DP, Noble W. Functional implications of glycogen synthase kinase-3-mediated tau phosphorylation. International journal of Alzheimer's disease. 2011;2011:352805. doi: 10.4061/2011/352805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque ME, et al. Cytoplasmic Pink1 activity protects neurons from dopaminergic neurotoxin MPTP. Proc Natl Acad Sci U S A. 2008;105:1716–1721. doi: 10.1073/pnas.0705363105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilmi C, et al. IGF1 promotes resistance to apoptosis in melanoma cells through an increased expression of BCL2, BCL-X(L), and survivin. J Invest Dermatol. 2008;128:1499–1505. doi: 10.1038/sj.jid.5701185. [DOI] [PubMed] [Google Scholar]
- Huang J, Manning BD. The TSC1–TSC2 complex: a molecular switchboard controlling cell growth. Biochem J. 2008;412:179–190. doi: 10.1042/BJ20080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inden M, et al. PARK7 DJ-1 protects against degeneration of nigral dopaminergic neurons in Parkinson's disease rat model. Neurobiol Dis. 2006;24:144–158. doi: 10.1016/j.nbd.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Jiang H, et al. Parkin protects human dopaminergic neuroblastoma cells against dopamine-induced apoptosis. Hum Mol Genet. 2004;13:1745–1754. doi: 10.1093/hmg/ddh180. [DOI] [PubMed] [Google Scholar]
- Kanao T, et al. Activation of FoxO by LRRK2 induces expression of proapoptotic proteins and alters survival of postmitotic dopaminergic neuron in Drosophila. Hum Mol Genet. 2010;19:3747–3758. doi: 10.1093/hmg/ddq289. [DOI] [PubMed] [Google Scholar]
- Kermer P, et al. Insulin-like growth factor-I protects axotomized rat retinal ganglion cells from secondary death via PI3-K-dependent Akt phosphorylation and inhibition of caspase-3 In vivo. J Neurosci. 2000;20:2–8. [PubMed] [Google Scholar]
- Kim RH, et al. DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell. 2005a;7:263–273. doi: 10.1016/j.ccr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Kim RH, et al. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc Natl Acad Sci U S A. 2005b;102:5215–5220. doi: 10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SR, et al. Dopaminergic pathway reconstruction by Akt/Rheb-induced axon regeneration. Ann Neurol. 2011;70:110–120. doi: 10.1002/ana.22383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- Kwok JB, et al. GSK3B polymorphisms alter transcription and splicing in Parkinson's disease. Ann Neurol. 2005;58:829–839. doi: 10.1002/ana.20691. [DOI] [PubMed] [Google Scholar]
- Lin CH, et al. LRRK2 G2019S mutation induces dendrite degeneration through mislocalization and phosphorylation of tau by recruiting autoactivated GSK3ss. J Neurosci. 2010;30:13138–13149. doi: 10.1523/JNEUROSCI.1737-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, et al. Intranasal administration of IGF-1 attenuates hypoxic-ischemic brain injury in neonatal rats. Exp Neurol. 2009;217:361–370. doi: 10.1016/j.expneurol.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malagelada C, et al. RTP801 is induced in Parkinson's disease and mediates neuron death by inhibiting Akt phosphorylation/activation. J Neurosci. 2008;28:14363–14371. doi: 10.1523/JNEUROSCI.3928-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Camandola S. NF-kappaB in neuronal plasticity and neurodegenerative disorders. J Clin Invest. 2001;107:247–254. doi: 10.1172/JCI11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, et al. Roles of nuclear factor kappaB in neuronal survival and plasticity. J Neurochem. 2000;74:443–456. doi: 10.1046/j.1471-4159.2000.740443.x. [DOI] [PubMed] [Google Scholar]
- Meulener M, et al. Drosophila DJ-1 mutants are selectively sensitive to environmental toxins associated with Parkinson's disease. Curr Biol. 2005;15:1572–1577. doi: 10.1016/j.cub.2005.07.064. [DOI] [PubMed] [Google Scholar]
- Murata H, et al. A New Cytosolic Pathway from a Parkinson Disease-associated Kinase, BRPK/PINK1: ACTIVATION OF AKT VIA MTORC2. J Biol Chem. 2011;286:7182–7189. doi: 10.1074/jbc.M110.179390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadjar A, et al. IGF-1 signaling reduces neuro-inflammatory response and sensitivity of neurons to MPTP. Neurobiol Aging. 2009;30:2021–2030. doi: 10.1016/j.neurobiolaging.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Nagao M, Hayashi H. Glycogen synthase kinase-3beta is associated with Parkinson's disease. Neurosci Lett. 2009;449:103–107. doi: 10.1016/j.neulet.2008.10.104. [DOI] [PubMed] [Google Scholar]
- Narendra DP, et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000298. e1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness JK, et al. IGF-I prevents glutamate-mediated bax translocation and cytochrome C release in O4+ oligodendrocyte progenitors. Glia. 2004;46:183–194. doi: 10.1002/glia.10360. [DOI] [PubMed] [Google Scholar]
- Ness JK, Wood TL. Insulin-like growth factor I, but not neurotrophin-3, sustains Akt activation and provides long-term protection of immature oligodendrocytes from glutamate-mediated apoptosis. Mol Cell Neurosci. 2002;20:476–488. doi: 10.1006/mcne.2002.1149. [DOI] [PubMed] [Google Scholar]
- Ni YG, et al. FoxO transcription factors activate Akt and attenuate insulin signaling in heart by inhibiting protein phosphatases. Proc Natl Acad Sci U S A. 2007;104:20517–20522. doi: 10.1073/pnas.0610290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offen D, et al. Protective effect of insulin-like-growth-factor-1 against dopamine-induced neurotoxicity in human and rodent neuronal cultures: possible implications for Parkinson's disease. Neurosci Lett. 2001;316:129–132. doi: 10.1016/s0304-3940(01)02344-8. [DOI] [PubMed] [Google Scholar]
- Otvos L, Jr, et al. Monoclonal antibody PHF-1 recognizes tau protein phosphorylated at serine residues 396 and 404. Journal of Neuroscience Research. 1994;39:669–673. doi: 10.1002/jnr.490390607. [DOI] [PubMed] [Google Scholar]
- Pang Z, Geddes JW. Mechanisms of cell death induced by the mitochondrial toxin 3-nitropropionic acid: acute excitotoxic necrosis and delayed apoptosis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:3064–3073. doi: 10.1523/JNEUROSCI.17-09-03064.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CH, et al. Calcineurin mediates AKT dephosphorylation in the ischemic rat retina. Brain Res. 2008;1234:148–157. doi: 10.1016/j.brainres.2008.07.082. [DOI] [PubMed] [Google Scholar]
- Park J, et al. Drosophila DJ-1 mutants show oxidative stress-sensitive locomotive dysfunction. Gene. 2005;361:133–139. doi: 10.1016/j.gene.2005.06.040. [DOI] [PubMed] [Google Scholar]
- Park J, et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- Paterna JC, et al. DJ-1 and Parkin modulate dopamine-dependent behavior and inhibit MPTP-induced nigral dopamine neuron loss in mice. Mol Ther. 2007;15:698–704. doi: 10.1038/sj.mt.6300067. [DOI] [PubMed] [Google Scholar]
- Pesah Y, et al. Drosophila parkin mutants have decreased mass and cell size and increased sensitivity to oxygen radical stress. Development. 2004;131:2183–2194. doi: 10.1242/dev.01095. [DOI] [PubMed] [Google Scholar]
- Pridgeon JW, et al. PINK1 protects against oxidative stress by phosphorylating mitochondrial chaperone TRAP1. PLoS Biol. 2007;5:e172. doi: 10.1371/journal.pbio.0050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucci B, et al. Insulin-like growth factor-1 inhibits STS-induced cell death and increases functional recovery of in vitro differentiated neurons. Cell Cycle. 2008;7:3869–3877. doi: 10.4161/cc.7.24.7261. [DOI] [PubMed] [Google Scholar]
- Quesada A, et al. PI3 kinase/Akt activation mediates estrogen and IGF-1 nigral DA neuronal neuroprotection against a unilateral rat model of Parkinson's disease. Dev Neurobiol. 2008;68:632–644. doi: 10.1002/dneu.20609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada A, Micevych PE. Estrogen interacts with the IGF-1 system to protect nigrostriatal dopamine and maintain motoric behavior after 6-hydroxdopamine lesions. J Neurosci Res. 2004;75:107–116. doi: 10.1002/jnr.10833. [DOI] [PubMed] [Google Scholar]
- Quesada A, et al. Distribution and localization patterns of estrogen receptor-beta and insulin-like growth factor-1 receptors in neurons and glial cells of the female rat substantia nigra: localization of ERbeta and IGF-1R in substantia nigra. J Comp Neurol. 2007;503:198–208. doi: 10.1002/cne.21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez A, et al. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nature genetics. 2006;38:1184–1191. doi: 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- Saini N, et al. Extended lifespan of Drosophila parkin mutants through sequestration of redox-active metals and enhancement of anti-oxidative pathways. Neurobiol Dis. 2010;40:82–92. doi: 10.1016/j.nbd.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Sandebring A, et al. Mitochondrial alterations in PINK1 deficient cells are influenced by calcineurin-dependent dephosphorylation of dynamin-related protein 1. PLoS One. 2009;4:e5701. doi: 10.1371/journal.pone.0005701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno L, et al. A novel GSK-3beta inhibitor reduces Alzheimer's pathology and rescues neuronal loss in vivo. Neurobiol Dis. 2009;35:359–367. doi: 10.1016/j.nbd.2009.05.025. [DOI] [PubMed] [Google Scholar]
- Sha D, et al. Phosphorylation of parkin by Parkinson disease-linked kinase PINK1 activates parkin E3 ligase function and NF-kappaB signaling. Hum Mol Genet. 2010;19:352–363. doi: 10.1093/hmg/ddp501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stambolic V, Woodgett JR. Mitogen inactivation of glycogen synthase kinase-3 beta in intact cells via serine 9 phosphorylation. The Biochemical journal. 1994;303(Pt 3):701–704. doi: 10.1042/bj3030701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L, et al. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science. 1998;279:710–714. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- Tain LS, et al. Rapamycin activation of 4E-BP prevents parkinsonian dopaminergic neuron loss. Nat Neurosci. 2009;12:1129–1135. doi: 10.1038/nn.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatori S, et al. Cytoplasmic localization and proteasomal degradation of N-terminally cleaved form of PINK1. Neurosci Lett. 2008;430:13–17. doi: 10.1016/j.neulet.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Thomas KJ, et al. DJ-1 acts in parallel to the PINK1/parkin pathway to control mitochondrial function and autophagy. Hum Mol Genet. 2011;20:40–50. doi: 10.1093/hmg/ddq430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons S, et al. Akt signal transduction dysfunction in Parkinson's disease. Neurosci Lett. 2009;467:30–35. doi: 10.1016/j.neulet.2009.09.055. [DOI] [PubMed] [Google Scholar]
- Valencia A, et al. Mutant huntingtin and glycogen synthase kinase 3-beta accumulate in neuronal lipid rafts of a presymptomatic knock-in mouse model of Huntington's disease. J Neurosci Res. 2010;88:179–190. doi: 10.1002/jnr.22184. [DOI] [PubMed] [Google Scholar]
- Valente EM, et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- van der Horst A, Burgering BM. Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol. 2007;8:440–450. doi: 10.1038/nrm2190. [DOI] [PubMed] [Google Scholar]
- Van Laar VS, et al. Bioenergetics of neurons inhibit the translocation response of Parkin following rapid mitochondrial depolarization. Hum Mol Genet. 2011;20:927–940. doi: 10.1093/hmg/ddq531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan KM, Garraway LA. AKT signaling in physiology and disease. Curr Top Microbiol Immunol. 2010;347:105–133. doi: 10.1007/82_2010_66. [DOI] [PubMed] [Google Scholar]
- Vincent AM, et al. IGF-I prevents glutamate-induced motor neuron programmed cell death. Neurobiol Dis. 2004;16:407–416. doi: 10.1016/j.nbd.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Vives-Bauza C, et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci U S A. 2009;107:378–383. doi: 10.1073/pnas.0911187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood TL, et al. Delayed IGF-1 administration rescues oligodendrocyte progenitors from glutamate-induced cell death and hypoxic-ischemic brain damage. Dev Neurosci. 2007;29:302–310. doi: 10.1159/000105471. [DOI] [PubMed] [Google Scholar]
- Wood-Kaczmar A, et al. PINK1 is necessary for long term survival and mitochondrial function in human dopaminergic neurons. PLoS One. 2008;3:e2455. doi: 10.1371/journal.pone.0002455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, et al. Delineating the mechanism by which selenium deactivates Akt in prostate cancer cells. Mol Cancer Ther. 2006;5:246–252. doi: 10.1158/1535-7163.MCT-05-0376. [DOI] [PubMed] [Google Scholar]
- Yang Y, et al. Inactivation of Drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/Akt signaling. Proc Natl Acad Sci U S A. 2005;102:13670–13675. doi: 10.1073/pnas.0504610102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng WH, et al. Insulin-like growth factor-1 (IGF-1): a neuroprotective trophic factor acting via the Akt kinase pathway. J Neural Transm Suppl. 2000:261–272. doi: 10.1007/978-3-7091-6301-6_17. [DOI] [PubMed] [Google Scholar]
- Zoncu R, et al. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]