Abstract
The incidence of obesity in the developed world is increasing at an alarming rate. Concurrent with the increase in the incidence of obesity is an increase in the incidence of type 2 diabetes. Cyclic AMP (cAMP) and cGMP are key second messengers in all cells; for example, when it comes to processes of relevance for the regulation of energy metabolism, cAMP is a key mediator in the regulation of lipolysis, glycogenolysis, gluconeogenesis and pancreatic β cell insulin secretion. PDE3B, one of several enzymes which hydrolyze cAMP and cGMP, is expressed in cells of importance for the regulation of energy homeostasis, including adipocytes, hepatocytes, hypothalamic cells, and β cells. It has been shown, using PDE3 inhibitors and gene targeting approaches in cells and animals, that altered levels of PDE3B result in a number of changes in the regulation of glucose and lipid metabolism and in overall energy homeostasis. This article highlights the complexity involved in the regulation of PDE3B by hormones, and in the regulation of downstream metabolic effects by PDE3B in several interacting tissues.
Introduction
The incidence of obesity in the developed world is increasing at an alarming rate. Concurrent with the increase in the incidence of obesity is an increase in the incidence of type 2 diabetes (T2D) [e.g. 1]. It has been reported that over 80% of adults diagnosed with T2D are obese. The connection between obesity and the development of T2D has been the focus of intense research in recent years. It has been demonstrated that low-grade, systemic inflammation originating from adipose tissue is a factor associated with systemic insulin resistance [e.g. 2]. Adipose tissue secretes numerous adipokines which affect whole body insulin sensitivity and dysregulation of production and secretion of these factors could contribute to the development of insulin resistance in obesity [e.g. 2, 3]. Also, excess fatty acids released from the adipocytes of obese persons contribute to ectopic fat storage in non-adipose tissues like liver and muscle, thereby exacerbating their insulin resistance [e.g. 4].
The composition of cAMP-signalling networks, which play key roles in target tissues of relevance for energy homeostasis, are growing in complexity [5, 6]. Cyclic nucleotide PDEs (phosphodiesterases) are important actors in this context. The PDE superfamily contains eleven structurally related, but functionally distinct, gene families (PDE1–11), which differ in primary structures, affinities for cAMP and cGMP, responses to specific effectors, sensitivities to specific inhibitors, and mechanisms of regulation [e.g. 7]. By virtue of their distinct intrinsic characteristics and their intracellular targeting to different subcellular locations, different PDEs integrate multiple cellular inputs and modulate the amplitude, duration, termination, and specificity of cyclic nucleotide signals and actions [6]. The PDE3 family contains two subfamilies, PDE3A and PDE3B [8, 9], which are encoded by distinct but related genes and exhibit distinct, but overlapping, patterns of expression. For example, PDE3A is more highly expressed in the cardiovascular system and PDE3B is more highly expressed in cells of importance for the regulation of glucose and lipid metabolism. This article highlights some key aspects of the role of PDE3B in normal and dysfunctional regulation of energy homeostasis.
Mechanisms for regulation of PDE3B and related signalling networks
When it comes to the overall regulation of PDE3B functions and the intracellular signalling networks involved in PDE3B regulation and action, the intracellular localization of the enzyme, phosphorylation events, as well as the formation of unique protein complexes containing PDE3B have critical roles.
Some molecular characteristics of relevance for the regulation and action of PDE3B
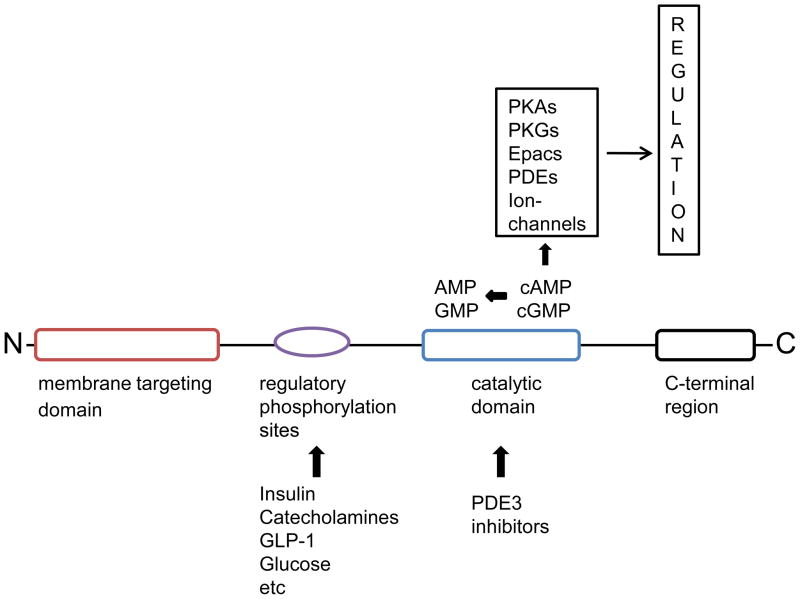
The structural organization of PDE3B (Fig. 1), which is identical to that of PDE3A, includes a catalytic domain conserved among all PDEs in the C-terminal portion of the enzymes, followed by a hydrophilic C-terminal region [8, 9]. The kinetic properties of PDE3 catalytic domains show high affinities for both cAMP and cGMP with Km values in the range of 0.1 to 0.8 μM, and velocity for cAMP hydrolysis being 4–10 fold higher than that for cGMP [8, 9]. Also, the catalytic domains of PDEs are the target for family and subfamily selective inhibitors [9, 10]. Such inhibitors have been very useful in dissecting specific functions for selected PDEs, including PDE3s, and are also used in the clinic or are being developed for the treatment of various diseases. The regulatory N-terminal portions of PDE3s contain large hydrophobic regions that are implicated in membrane targeting of these enzymes [8, 9, 11, 12]. Downstream of the hydrophobic regions lies serine residues that are subjected to reversible phosphorylation in intact cells. The importance of intracellular localization as well as reversible protein phosphorylation events for PDE3B function will be discussed below.
Figure 1. Structural organization of PDE3B.
The structural organization of PDE3B involves the catalytic domain conserved among all PDEs in the C-terminal portion of the enzymes followed by a hydrophilic C-terminal region. The catalytic domain hydrolyzes both cAMP and cGMP and is the target for PDE3 inhibitors. The regulatory N-terminal portion contains large hydrophobic regions with predicted transmembrane helical segments. Downstream of the hydrophobic regions lies regulatory serine residues that are phosphorylated in intact cells. Signalling induced by cAMP and cGMP primarily involves their activation of cAMP- and cGMP-activated protein kinases (PKA and PKG), with subsequent phosphorylation of critical effectors. However, direct interactions of cyclic nucleotides with binding proteins are now recognized as alternative mechanisms for transduction of their signals. These binding proteins include cAMP-activated guanine nucleotide exchange factors (GEFs) also called Epacs (exchange proteins activated by cAMP or cAMP GEF) which regulate Rap1, cyclic nucleotide-gated channels and several PDEs, which contain allosteric, non-catalytic cyclic nucleotide-binding sites located in GAF domains (the GAF domain is named after some of the proteins it is found in: cGMP-specific phosphodiesterases, adenylyl cyclases and FhlA).
Intracellular localization
PDE3B is predominantly associated with membranes in adipocytes, hepatocytes, and pancreatic β cells. In adipocytes and hepatocytes, PDE3B is localized to detergent resistant parts of the plasma membrane, including lipid rafts and caveolae, and to the endoplasmic reticulum [8, 9, 13, 14]. Caveolae are special forms of lipid rafts observed as small flask-shaped 50–100 nm invaginations of the plasma membrane [15]. Although caveolae are observed in many cell types they are particularly abundant in adipocytes. Caveolae have a high content of cholesterol and sphingolipids and are stabilized by one or more isoforms of caveolin. They are believed to be important in the organization of intracellular signalling events. Thus, caveolae localization of PDE3B and a potential interaction between caveolin and PDE3B is interesting in the context of signalling. In pancreatic β cells, PDE3B appears to be localized to the insulin granules and the plasma membrane, a location believed to be critical for a role of PDE3B in the regulation of exocytosis [16].
Hormone-induced phosphorylation, activation and complex formation
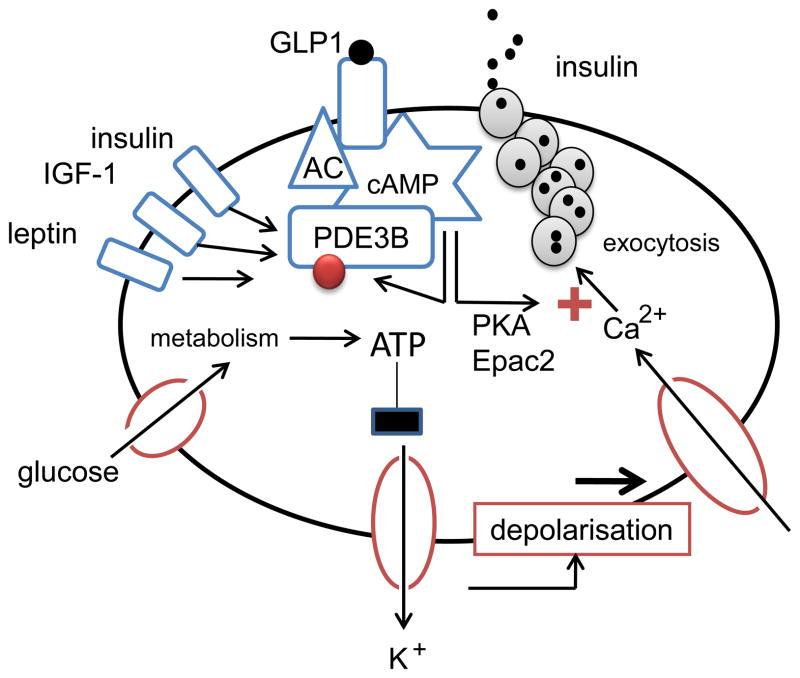
PDE3B enzymes are phosphorylated and activated in hepatocytes and adipocytes in response to stimulation by insulin and/or agents that increase cAMP, and are implicated in cAMP-mediated metabolic effects in those cells [8, 9]. In β cells, PDE3B regulates glucose-stimulated insulin secretion as well as cAMP-potentiation of glucose-stimulated insulin secretion, and is involved in regulation of both the acute first phase and the second sustained phase of insulin secretion [17–21] (Fig. 2). PDE3B is activated in cell models in response to a number of stimuli relevant to β cell function such as glucose, insulin, IGF-1, leptin, high K+, and cAMP-elevating agents [21–23]. Activation of PDE3B in response to glucose is associated with reduced phosphorylation of PDE3B whereas activation induced by insulin, forskolin and the phosphatase inhibitor okadaic acid is associated with increased phosphorylation [21]. In β cells the precise physiological role of PDE3B activation, identity of the phosphorylation sites and the particular kinases and phosphatases that are involved in its regulation remain to be elucidated.
Figure 2. Role of PDE3B in the regulation of insulin secretion.
The main stimulator of insulin secretion is glucose, which is metabolized inside the β cell. The subsequent increase in ATP/ADP ratio causes closure of KATP-dependent ion channels, resulting in depolarization of the plasma membrane. In consequence, L-type Ca2+ channels are opened, leading to influx of Ca2+. The increased intracellular concentration of Ca2+ stimulates exocytosis of insulin. cAMP initiates processes to enhance insulin secretion, including activation of PKA and binding to Epac2. Exocytosis of insulin is then stimulated through multiple pathways, only a few of which have been established so far. Glucagon-like peptide (GLP)-1 is an insulinoptropic gut hormone, which acts through a G-protein-coupled receptor to activate adenylate cyclase (AC) and thereby trigger an increase in cAMP. PDE3B in turn has been shown to negatively regulate insulin secretion through its cAMP-hydrolyzing activity. Other hormones known to activate β cell PDE3B are insulin, IGF-1 and leptin. The red ball indicates phosphorylation-sites.
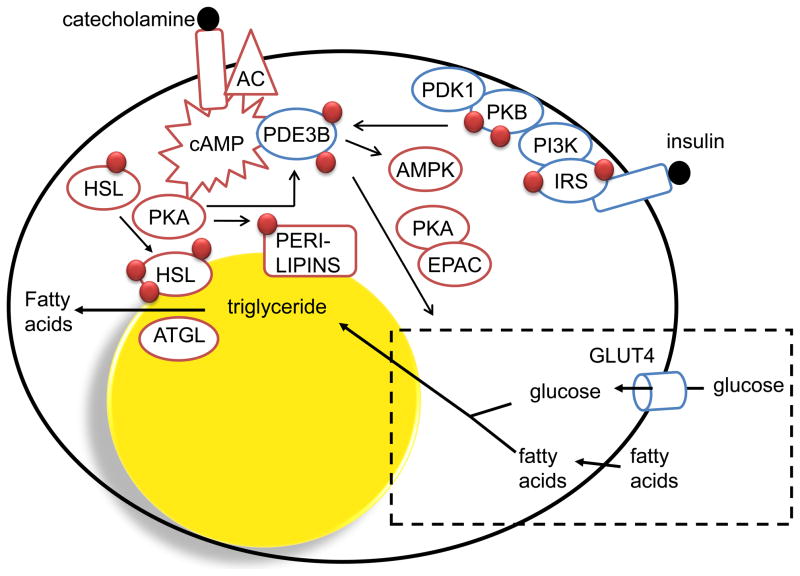
Most studies on mechanisms of regulation of PDE3B and PDE3B-associated signalling networks have utilized the adipocyte as a model. Insulin-induced phosphorylation and activation of PDE3B, partially mediated via PKB (protein kinase B), is a key event in the anti-lipolytic effect of insulin [8, 9] (Fig. 3). PDE3B is also involved in the regulation of insulin-induced glucose uptake and lipogenesis [24, 25]. cAMP activation of PKA (cAMP-dependent protein kinase) induces phosphorylation and activation of PDE3B, an effect that is important in negative feedback regulation of cAMP. The interplay between insulin and cAMP-mediated regulation of PDE3B also appears to involve cAMP/PKA-mediated activation of PKB [26] and PDE3B appears to have a role in the regulation of AMPK (AMP-activated protein kinase) [27, 28]. A number of serine residues have been identified as targets for insulin and cAMP-increasing hormones in adipocytes as well as in hepatocytes [e.g. 8, 9, 29–31]. It is likely that kinases other than PKB and PKA also contribute to the regulation of PDE3B. An additional level of complexity regarding regulatory mechanisms of PDE3B relates to the fact that insulin and cAMP increasing agents induce phosphorylation and activation of PDE3B at different intracellular locations, involving unique protein complexes. Thus, insulin preferentially phosphorylates and activates endoplasmic reticulum-associated PDE3B, whereas increases in cAMP preferentially lead to phosphorylation and activation of plasma membrane-associated PDE3B [30, 31]. The protein complexes present in insulin-stimulated cells contain tyrosine-phosphorylated insulin receptor substrate 1 and its downstream signalling proteins, whereas cAMP-induced complexes contain β3-adrenergic receptor, the PKA regulatory subunit II and hormone sensitive lipase. PDE3A and PDE4 are other examples of PDEs as components of signalling complexes, signalosomes, in other cells and contexts [32, 33].
Figure 3. Role of PDE3B in hormone-mediated regulation of adipocyte functions.
Activation of PDE3B leads to increased hydrolysis of cAMP and thereby inhibition of catecholamine-induced lipolysis, a process which involves PKA-dependent phosphorylation of hormone-sensitive lipase (HSL) and perilipin. Insulin-mediated phosphorylation and activation of PDE3B involves tyrosine phosphorylation of insulin receptor substrates (IRS) catalyzed by the activated insulin receptor tyrosine kinase (IRTK), activation of PI3K and increased production of phosphatidyl inositol 3,4/3,4,5 phosphates. This is followed by the activation of PKB which is believed to be one important kinase that phosphorylates and activates PDE3B. Phosphorylation and activation of PDE3B by cAMP-increasing hormones are thought to be important in feedback-regulation of cAMP and cAMP-mediated responses. PDE3B is also important in insulin-induced regulation of glucose uptake and lipogenesis which involves PKA as well as Epac proteins. Finally, PDE3B as well as PDE4 seem to regulate cAMP pools that affect the activation/phosphorylation state of AMPK. ATGL (adipose triglyceride lipase).
Implications for PDE3B in normal physiology and development of obesity and type 2 diabetes
PDE3B plays a key role in the regulation of insulin secretion
In agreement with results from studies using insulin-secreting cell lines and isolated pancreatic islets as models to modulate PDE3B expression and activity [17, 18] (Fig. 2), mice that specifically over-express PDE3B in β cells show a PDE3B protein dose-dependent decrease in glucose-induced insulin secretion as well as a decrease in the ability of GLP-1 to potentiate glucose-mediated insulin secretion [19, 20]. Furthermore, the seemingly moderate dysregulation of cAMP in pancreatic β cells negatively influences insulin secretion to the extent that it affects glucose homeostasis. Targeted PDE3B over-expression also sensitizes mice to high-fat feeding so as to precipitate the diabetes-like symptoms, which suggests that cAMP is important in preventing or delaying the development of fatty diet-induced insulin resistance. These results are in agreement with findings in PDE3B KO mice [34] and with findings using PDE3 inhibitors in vivo [e.g. 35, for more references see 8, 9]
Reduced PDE3B levels in vivo result in multiple alterations in the regulation of energy homeostasis
PDE3B knock-out (KO) mice [34] demonstrate a number of alterations in the regulation of energy homeostasis, both beneficial and non-beneficial, including signs of insulin resistance as well as reduced amounts of white adipose tissue and increased lean mass. Reduced ability of insulin to lower glucose output from the liver, shown using hyperinsulinemic-euglycemic clamps, as well as increased ability of catecholamines to induce lipolysis along with reduced ability of insulin to lower circulating fatty acid levels represent physiological alterations that contribute to the generation of systemic insulin resistance in PDE3B KO mice. Furthermore, liver triglycerides, cAMP content, expression of key gluconeogenic enzymes and several other insulin signalling-, inflammation-, and stress-related components are altered in PDE3B KO mice [14, 34]. Despite those non beneficial effects, these mice do not develop frank diabetes which could be explained by a potentiation of insulin secretion and some protective long term changes in adipose tissue. Islets from PDE3B KO mice show a potentiation of glucose-, as well as GLP-1- mediated insulin secretion, which is in agreement with other studies on cells and islets as discussed above.
With regard to the generation of a potential long term protective effect in adipose tissue which lacks PDE3B, PDE3B KO mice show reduced fat mass, smaller adipocytes, and reduced weight gain than control mice when maintained on a high fat diet. Unpublished studies indicate increased fatty acid oxidation and increased energy dissipation in isolated epididymal PDE3B KO adipocytes as well as increased oxygen consumption in intact PDE3B KO mice in response to intraperitoneal administration of a β3-adrenoreceptor agonist. In addition, mRNA levels of macrophage-related proteins were decreased in PDE3B KO adipose tissue, suggesting reduction in macrophage accumulation (unpublished results). Macrophage infiltration of adipose tissue is considered to be part of the chronic inflammation involved in development of insulin resistance and obesity [e.g. 2].
Consistent with our findings in PDE3B KO mice, administration of milrinone, a PDE3 inhibitor, to intact rats increases lipolysis and insulin secretionand blocks insulin-induced suppression of endogenous glucose production [e.g. 35, for more references see 8, 9].
PDE3B plays a role in the regulation of food intake and body weight
Leptin, a hormone primarily secreted from adipocytes, is required for normal food intake and body weight homeostasis via its actions in the hypothalamus [36, 37]. Leptin signalling activates PDE3B in the hypothalamus, and the PDE3 inhibitor, cilostamide, reverses anorexia and reduction in body weight produced by leptin [38–40]. Furthermore, the PDE3B pathway is responsible for the activation of proopiomelanocortin and neurotensin neurons, which play a critical role in energy homeostasis [39]. Thus, while central injection of leptin significantly increased both proopiomelanocortin and neurotensin mRNA levels in the medial basal hypothalamus, cilostamide completely reversed this effect of leptin. These results suggest that the PDE3B pathway plays an important role in mediating leptin signalling in the hypothalamus and thereby hypothalamic effects on energy homeostasis.
PDE3B expression in adipose tissue from patients with obesity and diabetes
Early studies on adipocytes from patients with diabetes demonstrated reduced PDE activity in adipose tissue [41]. Also, an important role of PDE3B in the regulation of lipolyis in humans has been demonstrated using a microdialysis approach [e.g. 42, for other references see 43]. Recently, PDE activities have been investigated with respect to differences in obesity and between different adipose tissue depots, considering the fact that the visceral depot is the metabolically toxic one as compared to the subcutaneous depot [44]. Results show that, in obese patients, total cAMP-hydrolyzing PDE, PDE3 and PDE4 activities were significantly reduced in both omental and subcutaneous adipose tissue depots compared to non-obese patients. Furthermore, there were inverse correlations between body mass index and total cAMP hydrolyzing PDE activity and PDE3 activities in adipocytes isolated from omental adipose tissue. Further studies are necessary to connect the altered activity profile of PDEs to different biological functions and to determine if and how they play a role in the development of adipose tissue insulin resistance. In obese subjects the antilipolytic effect of insulin is diminished [45], and PDE3B is the central enzyme controlling the antilipolytic effect of insulin. Thus, decreased PDE3B activity with increasing obesity may be a contributing factor to the diminished antilipolytic effect of insulin seen in obese patients. One possible mechanism whereby PDE3B is downregulated is via TNF-α, a cytokine known to be increased in obesity and known to be associated with insulin resistance in cell and animal models as well as in obese humans [46, 47]. Thus, in 3T3-L1 and human adipocytes, downregulation of PDE3B by TNF-α contributes to TNF-α - and ceramide-induced lipolysis, an effect that could be reversed by treating 3T3-L1 adipocytes with troglitazone [48–50]. Excess production of TNF-α has been shown to enhance the rate of adipose tissue lipolysis, hence increasing the concentration of circulating fatty acids and contributing to TNF-α-induced systemic insulin resistance.
Conclusion
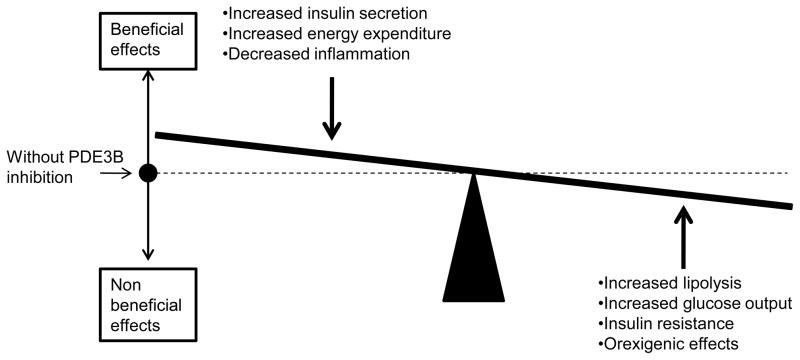
PDE3B is an important actor in the regulation of energy metabolism. However, with regard to PDE3B as a possible target for drugs in the context of treatment for obesity and T2D, one has to keep in mind the dynamic interplay among multiple tissues expressing PDE3B. Indeed, the final outcome of PDE3 inhibitors in the context of dyregulated energy homeostasis is difficult to predict (Fig. 4). Tissue-specific delivery systems appear to be necessary since the effects of PDE3B inhibition on hepatocytes, adipocytes and hypothalamus may result in worsening of glucose disposal and glucotoxicity, in the development of fatty acid-induced insulin resistance and even in weight gain. On the other hand, specific delivery to pancreatic β cells might indeed be beneficial by potentiating the effects of incretins such as GLP-1 on insulin secretion. Also, the induction of energy dissipation and reduced inflammation in adipose tissue would be beneficial. Thus, due to the different responses in different tissues, it is a challenge to target PDE3Bs to prevent and treat dysregulated metabolic states. To this end one must raise the question as to whether the PDE3B gene could function as a susceptibility or modifier gene for the development of specific types of diabetes.
Figure 4.
Multiple effects of PDE3 inhibitors. It is a challenge to target PDE3Bs to prevent and treat dysregulated metabolic states due to the different responses in different tissues.
Highlights.
Acute hormonal regulation of PDE3B involves reversible protein phosphorylation and protein complex formation at different subcellular locations
PDE3B plays a key role in the regulation of adipocyte lipolysis
PDE3B plays a key role in the regulation of insulin secretion
PDE3B plays an important role in overall energy homeostasis
PDE3B is down regulated in adipose tissue in human obesity
Acknowledgments
This work was supported, in part, by the NHLBI Intramural program and by the following grants to ED: Medical Research Council, Swedish Diabetes Association and Novo Nordisk Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
*of special interest
**of outstanding interest
- 1.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9(5):367–77. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curat CA, Wegner V, Sengenès C, Miranville A, Tonus C, Busse R, Bouloumié A. Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia. 2006;49(4):744–7. doi: 10.1007/s00125-006-0173-z. [DOI] [PubMed] [Google Scholar]
- 3.Antuna-Puente B, Feve B, Fellahi S, Bastard JP. Adipokines: the missing link between insulin resistance and obesity. Diabetes Metab. 2008;34(1):2–11. doi: 10.1016/j.diabet.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010;375(9733):2267–77. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarnaess E, Tasken K. Spatiotemporal control of cAMP signalling processes by anchored signalling processes. Biochem Soc Trans. 2007;35:931–37. doi: 10.1042/BST0350931. [DOI] [PubMed] [Google Scholar]
- 6.Houslay MD. Underpinning compartmentalised cAMP signalling through targeted cAMP breakdown. Trends Biochem Sci. 2010;35(2):91–100. doi: 10.1016/j.tibs.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Francis SH, Blount MA, Corbin JD. Mammalian cyclic nucleotide phosphodiesterases: molecular mechanisms and physiological functions. Physiol Rev. 2011;91(2):651–90. doi: 10.1152/physrev.00030.2010. [DOI] [PubMed] [Google Scholar]
- 8.Degerman E, Manganiello V. PDE3B: An important regulator of energy homeostasis. In: Beavo JA, Francis SH, Houslay MD, editors. Cyclic Nucleotide Phosphodiesterases in Health and Disease. CRC Press; Boca Raton: 2007. pp. 79–99. [Google Scholar]
- 9.Thompson PE, Manganiello V, Degerman E. Re-discovering PDE3 inhibitors--new opportunities for a long neglected target. Curr Top Med Chem. 2007;7(4):421–36. doi: 10.2174/156802607779941224. [DOI] [PubMed] [Google Scholar]
- 10.Schudt C, Hatzelmann A, Beume R, Tenor H. Phosphodiesterase inhibitors: history of pharmacology. Handb Exp Pharmacol. 2011;204:1–46. doi: 10.1007/978-3-642-17969-3_1. [DOI] [PubMed] [Google Scholar]
- 11.Kenan Y, Murata T, Shakur Y, Degerman E, Manganiello VC. Functions of the N-terminal region of cyclic nucleotide phosphodiesterase 3 (PDE 3) isoforms. J Biol Chem. 2000;275:12331–8. doi: 10.1074/jbc.275.16.12331. [DOI] [PubMed] [Google Scholar]
- 12.Sakur Y, Takeda K, Kenan Y, Yu ZX, Rena G, Brandt D, Houslay MD, Degerman E, Ferrans VJ, Manganiello VC. Membrane localization of cyclic nucleotide phosphodiesterase 3 (PDE3). Two N-terminal domains are required for the efficient targeting to, and association of, PDE3 with endoplasmic reticulum. J Biol Chem. 2000;275:38749–61. doi: 10.1074/jbc.M001734200. [DOI] [PubMed] [Google Scholar]
- 13.Nilsson R, Ahmad F, Swärd K, Andersson U, Weston M, Manganiello V, Degerman E. Plasma membrane cyclic nucleotide phosphodiesterase 3B (PDE3B) is associated with caveolae in primary adipocytes. Cell Signal. 2006;18(10):1713–21. doi: 10.1016/j.cellsig.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Berger K, Lindh R, Wierup N, Zmuda-Trzebiatowska E, Lindqvist A, Manganiello VC, Degerman E. Phosphodiesterase 3B is localized in caveolae and smooth ER in mouse hepatocytes and is important in the regulation of glucose and lipid metabolism. PLoS One. 2009;4(3):e4671. doi: 10.1371/journal.pone.0004671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pilch PF, Liu L. Fat caves: caveolae, lipid trafficking and lipid metabolism in adipocytes. Trends Endocrinol Metab. 2011;22(8):318–24. doi: 10.1016/j.tem.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walz HA, Wierup N, Vikman J, Manganiello VC, Degerman E, Eliasson L, Holst LS. Beta-cell PDE3B regulates Ca2+-stimulated exocytosis of insulin. Cell Signal. 2007;19(7):1505–13. doi: 10.1016/j.cellsig.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 17*.Furman B, Ong WK, Pyne NJ. Cyclic AMP signaling in pancreatic islets. Adv Exp Med Biol. 2010;654:281–304. doi: 10.1007/978-90-481-3271-3_13. An excellent review on cAMP signalling in pancreatic islets which includes a review of the role of PDE3B and other PDEs. [DOI] [PubMed] [Google Scholar]
- 18.Zhao AZ, Stenson Holst L. Regulation of cAMP levels by PDE3B-Physiological implications in energy balance and insulin secretion. In: Beavo JA, Francis SH, Houslay MD, editors. Cyclic Nucleotide Phosphodiesterases in Health and Disease. CRC Press; 2007. pp. 347–361. [Google Scholar]
- 19.Härndahl L, Wierup N, Enerbäck S, Mulder H, Manganiello VC, Sundler F, Degerman E, Ahrén B, Holst LS. Beta-cell-targeted overexpression of phosphodiesterase 3B in mice causes impaired insulin secretion, glucose intolerance, and deranged islet morphology. J Biol Chem. 2004;279(15):15214–22. doi: 10.1074/jbc.M308952200. [DOI] [PubMed] [Google Scholar]
- 20.Walz HA, Härndahl L, Wierup N, Zmuda-Trzebiatowska E, Svennelid F, Manganiello VC, Ploug T, Sundler F, Degerman E, Ahrén B, Holst LS. Early and rapid development of insulin resistance, islet dysfunction and glucose intolerance after high-fat feeding in mice overexpressing phosphodiesterase 3B. J Endocrinol. 2006;189(3):629–41. doi: 10.1677/joe.1.06522. [DOI] [PubMed] [Google Scholar]
- 21.Heimann E, Jones HA, Resjö S, Manganiello VC, Stenson L, Degerman E. Expression and regulation of cyclic nucleotide phosphodiesterases in human and rat pancreatic islets. PLoS One. 2010;5(12):e14191. doi: 10.1371/journal.pone.0014191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao AZ, Zhao H, Teague J, Fujimoto W, Beavo JA. Attenuation of insulin secretion by insulin-like growth factor 1 is mediated through activation of phosphodiesterase 3B. Proc Natl Acad Sci U S A. 1997;94:3223–8. doi: 10.1073/pnas.94.7.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao AZ, Bornfeldt KE, Beavo JA. Leptin inhibits insulin secretion by activation of phosphodiesterase 3B. J Clin Invest. 1998;102:869–73. doi: 10.1172/JCI3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zmuda-Trzebiatowska E, Oknianska A, Manganiello V, Degerman E. Role of PDE3B in insulin-induced glucose uptake, GLUT-4 translocation and lipogenesis in primary rat adipocytes. Cell Signal. 2006;18(3):382–90. doi: 10.1016/j.cellsig.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Eriksson JW, Wesslau C, Smith U. The cGMP-inhibitable phosphodiesterase modulates glucose transport activation by insulin. Biochim Biophys Acta. 1994;1189(2):163–7. doi: 10.1016/0005-2736(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 26.Zmuda-Trzebiatowska E, Manganiello V, Degerman E. Novel mechanisms of the regulation of protein kinase B in adipocytes; implications for protein kinase A, Epac, phosphodiesterases 3 and 4. Cell Signal. 2007;19(1):81–6. doi: 10.1016/j.cellsig.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 27.Omar B, Zmuda-Trzebiatowska E, Manganiello V, Göransson O, Degerman E. Regulation of AMP-activated protein kinase by cAMP in adipocytes: roles for phosphodiesterases, protein kinase B, protein kinase A, Epac and lipolysis. Cell Signal. 2009;21(5):760–6. doi: 10.1016/j.cellsig.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hardie DG. Sensing of energy and nutrients by AMP-activated protein kinase. Am J Clin Nutr. 2011;93(4):891S–6. doi: 10.3945/ajcn.110.001925. [DOI] [PubMed] [Google Scholar]
- 29.Lindh R, Ahmad F, Resjö S, James P, Yang JS, Fales HM, Manganiello V, Degerman E. Multisite phosphorylation of adipocyte and hepatocyte phosphodiesterase 3B. Biochim Biophys Acta. 2007;1773(4):584–92. doi: 10.1016/j.bbamcr.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 30.Ahmad F, Lindh R, Tang Y, Weston M, Degerman E, Manganiello VC. Insulin-induced formation of macromolecular complexes involved in activation of cyclic nucleotide phosphodiesterase 3B (PDE3B) and its interaction with PKB. Biochem J. 2007;404(2):257–68. doi: 10.1042/BJ20060960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31**.Ahmad F, Lindh R, Tang Y, Ruishalme I, Ost A, Sahachartsiri B, Strålfors P, Degerman E, Manganiello VC. Differential regulation of adipocyte PDE3B in distinct membrane compartments by insulin and the beta3-adrenergic receptor agonist CL316243: effects of caveolin-1 knockdown on formation/maintenance of macromolecular signalling complexes. Biochem J. 2009;424(3):399–410. doi: 10.1042/BJ20090842. The authors show that insulin and cAMP increasing agents induce phosphorylation and activation of PDE3B at different subcellular location in 3T3 L1 adipocytes, the endoplasmic reticulum and plasma membrane, respectively. Also they show that unique protein complexes containing PDE3B are formed in response to the different stimuli. Furthermore, the formation of macromolecular complexes was significantly attenuated in caveolin-1 knock down or atrovastatin-treated adipocytes suggesting a chaperon or scaffolding role of caveolin-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lynch MJ, Baillie GS, Houslay MD. cAMP-specific phosphodiesterase-4D5 (PDE4D5) provides a paradigm for understanding the unique non-redundant roles that PDE4 isoforms play in shaping compartmentalized cAMP cell signalling. Biochem Soc Trans. 2007;35(Pt 5):938–41. doi: 10.1042/BST0350938. [DOI] [PubMed] [Google Scholar]
- 33.Houslay MD, Baillie GS, Maurice DH. cAMP-Specific phosphodiesterase-4 enzymes in the cardiovascular system: a molecular toolbox for generating compartmentalized cAMP signaling. Circ Res. 2007;100(7):950–66. doi: 10.1161/01.RES.0000261934.56938.38. [DOI] [PubMed] [Google Scholar]
- 34.Choi YH, Park S, Hockman S, Zmuda-Trzebiatowska E, Svennelid F, Haluzik M, Gavrilova O, Ahmad F, Pepin L, Napolitano M, Taira M, Sundler F, Stenson Holst L, Degerman E, Manganiello VC. Alterations in regulation of energy homeostasis in cyclic nucleotide phosphodiesterase 3B-null mice. J Clin Invest. 2006;116(12):3240–51. doi: 10.1172/JCI24867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheung P, Yang G, Boden G. Milrinone, a selective phosphodiesterase 3 inhibitor, stimulates lipolysis, endogenous glucose production, and insulin secretion. Metabolism. 2003;52(11):1496–500. doi: 10.1016/s0026-0495(03)00271-3. [DOI] [PubMed] [Google Scholar]
- 36.Mantzoros CS, Magkos F, Brinkoetter M, Sienkiewicz E, Dardeno TA, Kim SY, Hamnvik OP. Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab. 2011 Jul 26; doi: 10.1152/ajpendo.00315.2011. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wauman J, Tavernier J. Leptin receptor signaling: pathways to leptin resistance. Front Biosci. 2011;17:2771–93. doi: 10.2741/3885. [DOI] [PubMed] [Google Scholar]
- 38.Sahu A, Metlakunta AS. Hypothalamic phosphatidylinositol 3-kinase-phosphodiesterase 3B-cyclic AMP pathway of leptin signalling is impaired following chronic central leptin infusion. J Neuroendocrinol. 2005;17(11):720–6. doi: 10.1111/j.1365-2826.2005.01362.x. [DOI] [PubMed] [Google Scholar]
- 39*.Sahu A. A role of phosphodiesterase-3B pathway in mediating leptin action on proopiomelanocortin and neurotensin neurons in the hypothalamus. Neurosci Lett. 2010;479(1):18–21. doi: 10.1016/j.neulet.2010.05.018. Proopiomelanocortin (POMC) and neurotensin (NT) neurons are known to play critical roles in energy homeostasis. The author shows that while central injection of leptin significantly increased both POMC and NT mRNA levels in the medial basal hypothalamus, cilostamide, a PDE3 inhibitor, completely reversed this effect of leptin suggesting a PDE3B-activation dependent induction of POMC and NT gene expression by leptin. This result suggests that the PDE3B pathway has an important role in mediating leptin action in the hypothalamus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sahu A. Intracellular leptin-signaling pathways in hypothalamic neurons: the emerging role of phosphatidylinositol-3 kinase-phosphodiesterase-3B-cAMP pathway. Neuroendocrinology. 2011;93(4):201–10. doi: 10.1159/000326785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Engfeldt P, Arner P, Bolinder J, Ostman J. Phosphodiesterase activity in human subcutaneous adipose tissue in insulin- and noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1982;55(5):983–8. doi: 10.1210/jcem-55-5-983. [DOI] [PubMed] [Google Scholar]
- 42.Hagström-Toft E, Bolinder J, Eriksson S, Arner P. Role of phosphodiesterase III in the antilipolytic effect of insulin in vivo. Diabetes. 1995;44(10):1170–5. doi: 10.2337/diab.44.10.1170. [DOI] [PubMed] [Google Scholar]
- 43.Snyder PB. The adipocyte cGMP inhibited cyclic nucleotide phosphodiesterase (PDE3B) as a target for lipolytic and thermogenic agents for the treatment of obesity. Expert opinion on therapeutic targets. 1999;3(4):587–599. [Google Scholar]
- 44*.Omar B, Banke E, Ekelund, Frederiksen, Degerman E. Alterations in cyclic nucleotide phosphodiesterase activities in omental and subcutaneous adipose tissues in humakn obesity. Nutrition and Diabetes. 2011 doi: 10.1038/nutd.2011.9. accepted. The authors demonstrate altered expression of PDEs, including PDE3B, in human obesity. In obese patients, total cAMP-hydrolyzing PDE, PDE3 and PDE4 activities were significantly reduced in both omental and subcutaneous adipose tissue depots compared to non-obese patients. Furthermore, there were inverse correlations between body mass index and total PDE and PDE3 activities in adipocytes isolated from omental adipose tissue. Thus, PDE activities including PDE3B are reduced in adipocytes from obese individuals. Further studies are necessary to connect the altered activity profile of phosphodiesterases to different biological functions and thereby determine if and how they play a role in the development of adipose tissue insulin resistance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson JA, Fried SK, Pi-Sunyer FX, Albu JB. Impaired insulin action in subcutaneous adipocytes from women with visceral obesity. Am J Physiol Endocrinol Metab. 2001;280(1):E40–9. doi: 10.1152/ajpendo.2001.280.1.E40. [DOI] [PubMed] [Google Scholar]
- 46.Tzanavari T, Giannogonas P, Karalis KP. TNF-alpha and obesity. Curr Dir Autoimmun. 2010;11:145–56. doi: 10.1159/000289203. [DOI] [PubMed] [Google Scholar]
- 47.Rydén M, Arner PJ. Tumour necrosis factor-alpha in human adipose tissue -- from signalling mechanisms to clinical implications. Intern Med. 2007;262(4):431–8. doi: 10.1111/j.1365-2796.2007.01854.x. [DOI] [PubMed] [Google Scholar]
- 48.Mei J, Holst LS, Landström TR, Holm C, Brindley D, Manganiello V, Degerman E. C(2)-ceramide influences the expression and insulin-mediated regulation of cyclic nucleotide phosphodiesterase 3B and lipolysis in 3T3-L1 adipocytes. Diabetes. 2002;51(3):631–7. doi: 10.2337/diabetes.51.3.631. [DOI] [PubMed] [Google Scholar]
- 49.Zhang HH, Halbleib M, Ahmad F, Manganiello VC, Greenberg AS. Tumor necrosis factor-alpha stimulates lipolysis in differentiated human adipocytes through activation of extracellular signal-related kinase and elevation of intracellular cAMP. Diabetes. 2002;51(10):2929–35. doi: 10.2337/diabetes.51.10.2929. [DOI] [PubMed] [Google Scholar]
- 50.Rahn Landström T, Mei J, Karlsson M, Manganiello V, Degerman E. Down-regulation of cyclic-nucleotide phosphodiesterase 3B in 3T3-L1 adipocytes induced by tumour necrosis factor alpha and cAMP. Biochem J. 2000;346(Pt 2):337–43. doi: 10.1042/bj3460337. [DOI] [PMC free article] [PubMed] [Google Scholar]