Summary
Dendritic cells (DCs) in tissues and lymphoid organs comprise distinct functional subsets that differentiate in situ from circulating progenitors. Tissue-specific signals that regulate DC subset differentiation are poorly understood. We report that DC-specific deletion of the Notch2 receptor caused a reduction of DC populations in the spleen. Within the splenic CD11b+ DC subset, Notch signaling blockade ablated a distinct population marked by high expression of the adhesion molecule Esam. The Notch-dependent Esamhi DC subset required lymphotoxin beta receptor signaling, proliferated in situ and facilitated CD4+ T cell priming. The Notch-independent Esamlo DCs expressed monocyte-related genes and showed superior cytokine responses. In addition, Notch2 deletion led to the loss of CD11b+ CD103+ DCs in the intestinal lamina propria and to a corresponding decrease of IL-17-producing CD4+ T cells in the intestine. Thus, Notch2 is a common differentiation signal for T cell-priming CD11b+ DC subsets in the spleen and intestine.
Introduction
Dendritic cells (DCs) represent the primary antigen (Ag)-presenting cell population in the immune system. They can detect pathogens through pattern recognition receptors such as Toll-like receptors (TLRs), migrate into the T cell areas of lymphoid organs, secrete immunostimulatory cytokines such as interleukin-12 (IL-12), and present pathogen-derived peptides to naïve T cells (Steinman and Idoyaga, 2010). To initiate appropriate immune responses to different pathogen types, DCs comprise distinct functional subsets including interferon-producing plasmacytoid DCs (pDCs) and two main subsets of classical DCs. The CD8α-expressing CD8+ CD11b− DCs in lymphoid organs and their CD103+ CD11b− counterparts in tissues mediate efficient cross-presentation to cytotoxic T cells (Shortman and Heath, 2010). The CD8α-negative CD8− CD11b+ subset is preferentially involved in MHC class II (MHC II)-restricted Ag presentation to CD4+ helper T cells (Dudziak et al., 2007). In the spleen, CD11b+ DCs are preferentially localized to the marginal zone (MZ), a unique structure that filters the incoming blood (Mebius and Kraal, 2005). In the intestinal lamina propria (LP), the CD11b+ DC population is comprised of two distinct subsets. The CD11b+ CD103+ subset is thought to mediate Ag capture and transport to mesenteric lymph node (LN). Recently, it was shown to be particularly efficient for the induction of interleukin 17 (IL-17)-secreting helper T cells (Th17) in vitro (Denning et al., 2011), although its role in T cell differentiation in vivo remains unclear. Conversely, the CD11b+ CD103− population does not migrate to LN, is capable of high-level cytokine secretion and appears closely related to macrophages (Bogunovic et al., 2009; Schulz et al., 2009; Varol et al., 2009).
Classical DCs along with pDCs, monocytes and macrophages originate from the common macrophage and DC progenitor (MDP) in the bone marrow (BM) (Fogg et al., 2006). Commitment to the DC lineage occurs in the BM at the level of common DC progenitors (CDP) (Naik et al., 2007; Onai et al., 2007), whereas the terminal differentiation of classical DC subsets occurs in the periphery. All DCs in the lymphoid organs and CD8+ or CD103+ DCs in tissues are thought to develop from pre-DC (Ginhoux et al., 2009; Liu et al., 2009), a blood-derived progenitor originally defined in the spleen (Naik et al., 2006). Similarly, the unique CD11b+ CD103+ subset in the intestinal LP is derived from pre-DCs. All pre-DC-derived subsets are low or negative for fractalkine receptor Cx3cr1 and preferentially depend on signaling by Flt3 ligand through its receptor Flt3. On the other hand, CD11b+ DCs in tissues arise from MDP-derived monocytes, express Cx3cr1 and depend on macrophage colony-stimulating factor receptor Csf1r rather than on Flt3 (Bogunovic et al., 2009; Ginhoux et al., 2009; Varol et al., 2009). Thus, the homogeneity and single pre-DC origin of DC subsets in lymphoid organs appears to contrast with the functional heterogeneity and dual origin of DC subsets in tissues such as the intestine. Furthermore, little is known about molecular signals that promote DC fate and impart subset and/or specificity on DC progenitors.
Notch is an evolutionarily conserved signaling pathway that allows cells to adopt cell fates dictated by their microenvironment (Bray, 2006). The interaction of Notch receptor with its ligand on a neighboring cell causes receptor cleavage that releases the intracellular domain of Notch (NICD), which translocates into the nucleus and binds the transcription factor CSL (called RBPJ in the mouse). The resulting NICD-RBPJ complex recruits coactivators of the Mastermind (MAML) family and activates Notch-dependent gene expression programs, including canonical targets such as Hes1 and Deltex (Dtx1). Notch signaling is regulated at multiple levels including ligand endocytosis, receptor glycosylation and composition of the transactivation complex. Mammals have five Notch ligands of the Delta-like and Jagged families and four Notch receptors (Notch1–4), of which only Notch1 and Notch2 contain transactivation domains. These two receptors appear to mediate the majority of Notch-dependent developmental processes and their deletion causes embryonic lethality.
Notch signaling plays essential roles in lymphocyte development by inducing progenitor differentiation in unique anatomic locations (Radtke et al., 2010; Yashiro-Ohtani et al., 2010; Yuan et al., 2010). Thus, Delta-like 4 (Dll4) on thymic epithelium drives T cell lineage development through Notch1 on thymic progenitors, whereas Delta-like 1 (Dll1) on the splenic MZ stroma specifies MZ B cell subset through Notch2. However, the role of Notch in the development of innate immune system remains poorly understood. Notch1 has been implicated in DC differentiation in vitro (Cheng et al., 2003; Zhou et al., 2009), although earlier studies on Notch1 deletion in vivo argue against this notion (Radtke et al., 2000). We have shown previously that DC-specific deletion of RBPJ caused partial reduction of the splenic CD11b+ DCs, which express Notch target Dtx1 in an RBPJ-dependent manner (Caton et al., 2007). However, major questions remain concerning the Notch receptor involved, the partial nature and functional consequences of the phenotype, and the role of Notch in DC differentiation in tissues.
We now report that the Notch2 receptor controls DC differentiation in the spleen. Among the CD11b+ DCs, Notch2-RBPJ signaling specifies a unique Cx3cr1lo Esamhi DC subset, which was required for efficient T cell priming in the spleen. Moreover, Notch2 was necessary for the development of lamina propria CD11b+ CD103+ DCs, which in turn maintain optimal numbers of Th17 cells. These results establish Notch2 signaling as an essential tissue-specific determinant of DC differentiation in the spleen and intestine. Furthermore, they demonstrate that CD11b+ DCs both in tissues and in lymphoid organs are heterogeneous and include a distinct Notch-dependent subset specialized in T cell priming.
Results
Notch2 controls splenic DC differentiation
To determine the role of individual Notch receptors in DC differentiation, we used Itgax-cre (CD11c-cre) deleter strain for DC-specific deletion of conditional Notch1 (Radtke et al., 1999) and Notch2 (McCright et al., 2006) alleles. As a complementary way to block canonical Notch signaling in DCs, we used Itgax-cre-induced expression of dominant-negative human MAML1 (DNMAML1) protein fused to green fluorescent protein (GFP) (Tu et al., 2005). The analysis of GFP fluorescence confirmed DC-specific activation of DNMAML1 expression in the resulting DC-DNMAML1 mice (Fig. S1). Indeed, Notch2-dependent MZ B cells showed minimal recombination and were present in normal numbers in mice with Notch2 deletion (DC-Notch2Δ) or with DNMAML1 activation (Fig. S1). As shown in Fig. 1A,B, mice with DC-specific deletion of Notch1 (DC-Notch1Δ) had normal splenic DC populations. This is consistent with normal DC development after Notch1 deletion in hematopoietic chimeras (Radtke et al., 2000). In contrast, DC-Notch2Δ and DC-DNMAML1 mice had reduced fraction and absolute numbers of splenic CD11chi MHC II+ classical DCs (Fig. 1A,B). The reduction was specific to cDCs, as pDC populations were normal despite efficient cre recombination (Fig. S1).
Figure 1. Notch2 signaling regulates splenic DC development.
Mice with Itgax-cre -mediated deletion of Notch1 or Notch2 or DC-specific overexpression of DNMAML1 were analyzed along with the respective cre-negative littermate controls. Statistically significant differences are indicated as follows: ***, P<0.001; **, P<0.01; *, P<0.05.
(A) Representative staining profiles of total splenocytes with the fraction of CD11chi MHC II+ DCs indicated.
(B) The fraction (top) and absolute number (bottom) of splenic DCs (mean ± S.D. of 3–6 animals per group).
(C) Staining profiles of gated CD11chi MHC II+ splenic DCs, with CD11b+ (blue), CD8+ (green), and double-negative (purple) subsets highlighted. The percentages represent mean ± S.D., n=3–6 for DC-Notch1Δ and DC-DNMAML1 and 11–12 for DC-Notch2Δ.
(D) The percentage among total splenocytes (top) and absolute number (bottom) of CD11b+ and CD8+ DC subsets (mean ± S.D. of 8–10 animals per group).
(E) The expression of SIRPα and CD24 in gated CD11chi MHC II+ CD11b− CD8− double-negative DCs from conditional Notch2 (DC-Notch2Δ) and littermate control (Ctrl) spleens. The SIRPα+ and CD24+ populations indicative of the differentiation towards CD11b+ and CD8+ DC subsets, respectively, are indicated (representative of 2 animals).
(F) Expression of surface markers in CD11b+ DCs from DC-Notch2Δ and control spleens.
As with DC-specific Rbpj deletion (DC-RbpjΔ, (Caton et al., 2007)), the CD8− CD11b+ subset was significantly reduced in the spleens of DC-Notch2Δ and DC-DNMAML1 mice (Fig. 1C,D). In addition, the CD8+ DC subset was decreased in DC-Notch2Δ spleens, whereas a distinct population of CD8− CD11b− double-negative cells became apparent within the DC population. A similar albeit less pronounced phenotype was observed in DNMAML1-overexpressing DCs, suggesting the involvement of canonical Notch signaling. The CD8− CD11b− double-negative DCs arising after Notch2 deletion included two distinct populations expressing either signal regulatory protein alpha (SIRPα) or CD24, the markers associated with CD11b+ and CD8+ DC lineages, respectively (Naik et al., 2006) (Fig. 1E). Similar populations could be detected among the rare CD8− CD11b− DCs in wild-type mice, suggesting that they represent natural immature stages of CD11b+ and CD8+ DC development. Earlier developmental stages such as pre-DCs were unaffected by Notch2 deletion (Fig. S1), consistent with a late differentiation defect.
In addition to the decrease in number, splenic CD11b+ DCs in DC-Notch2Δ mice exhibited an abnormal surface phenotype including higher CD11b and lower (albeit heterogeneous) SIRPα expression (Fig. 1F). Splenic CD11b+ DCs express the specific marker Dcir2 (33D1) (Dudziak et al., 2007) and a fraction of them express CD4 (Kamath et al., 2000). As shown in Fig. 1F, the fraction of Dcir2+ and CD4+ cells was profoundly reduced in Notch2-deficient CD11b+ DCs. Altogether, these data demonstrate that Notch2 deletion impairs the development of CD11b+ and CD8+ splenic DCs, and causes phenotypic abnormality of the residual CD11b+ DC population.
Notch2-RBPJ signaling specifies a distinct population of splenic CD8− DCs
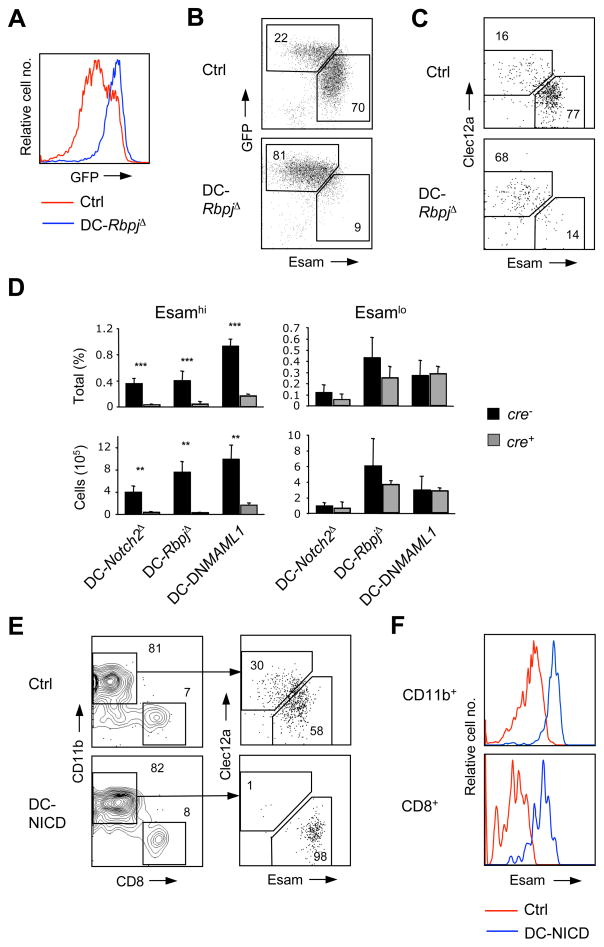
To explain the partial reduction and abnormal phenotype of CD11b+ DCs in DC-Notch2Δ, DC-DNMAML1 and DC-RbpjΔ mice, we hypothesized that CD11b+ DCs are heterogeneous and include a distinct population eliminated by Notch2-RBPJ blockade. We therefore crossed DC-RbpjΔ mice to a Cx3cr1-GFP reporter strain, which has been used to resolve DC subsets in the spleen and tissues (Bar-On et al., 2010; Varol et al., 2009). We found that CD11b+ DCs in wild-type spleens included GFPhi and GFPlo populations, whereas in DC-RbpjΔ spleens the GFPlo population was missing (Fig. 2A). To identify a positive marker of this Notch-dependent DC population, we performed microarray analysis of total CD11b+ DCs from DC-RbpjΔ spleens and focused on genes that encode cell surface markers (data not shown). One prominently reduced gene encoded Esam, an immunoglobulin superfamily adhesion molecule that is expressed on endothelium and regulates neutrophil extravasation (Wegmann et al., 2006). We found that among the splenocytes Esam was abundant in a subset of CD11b+ DCs, low in CD8+ DCs and absent from non-DCs (Fig. S2). The combination of Esam with Cx3cr1-GFP defined two distinct populations, Esamlo GFPhi and Esamhi GFPlo, the latter completely absent in DC-RbpjΔ splenocytes (Fig. 2B). The RBPJ-dependent Esamhi population was also defined by low expression of Clec12a, a C-type lectin expressed by CD8+ DCs, pDCs and myeloid cells (Lahoud et al., 2009). Staining with Esam, particularly in combination with Clec12a, resolved the two CD11b+ DC populations and allowed their analysis in the absence of Cx3cr1-GFP reporter (Fig. 2C). The Esamhi but not Esamlo CD11b+ DCs were nearly absent in all examined Notch mutants including DC-RbpjΔ, DC-Notch2Δ and DC-DNMAML1 (Fig. 2D). In contrast, the low Esam expression on CD8+ DCs was minimally affected by Notch2 loss (Fig. S2).
Figure 2. Notch2-RBPJ signaling specifies a distinct population of CD11b+ cDC.
(A) The expression of Cx3cr1-GFP reporter in CD11b+ splenic DCs from conditional RBPJ-deficient (DC-RbpjΔ) mice. Shown is the histogram of GFP expression in gated CD11chi MHC II+ CD11b+ DCs from Cx3cr1-GFP+ control (Ctrl) or DC-RbpjΔ mice (representative of 4 animals per group).
(B) The expression of Esam vs GFP in gated CD11b+ splenic DCs from Cx3cr1-GFP+ control or DC-RbpjΔ mice.
(C) The expression of Esam vs Clec12a in gated CD11b+ splenic DCs from DC-RbpjΔ or control mice.
(D) The percentage among total splenocytes (top) and absolute number (bottom) of the Esamhi and Esamlo CD11b+ DC subsets in mice with DC-specific targeting of the indicated genes (mean ± S.D. of 3–5 animals). Significance is indicated as in Fig. 1.
(E) Splenic DC populations in mice with DC-specific expression of Notch1 intracellular domain (DC-NICD). Shown are staining profiles of gated splenic CD11chi MHC II+ DCs and of their CD11b+ subset from DC-NICD mice or Cre-negative littermate controls (Ctrl). Representative of 8 animals per genotype.
(F) The expression of Esam in splenic CD11b+ and CD8+ DCs from DC-NICD and control mice.
To test the effect of Notch overactivation in DCs, we used the Itgax-cre strain in conjunction with a Cre-inducible overexpression cassette encoding Notch1 intracellular domain (NICD) (Buonamici et al., 2009). The NICDs of Notch1 and Notch2 are highly homologous (Radtke et al., 2010) and are likely interchangeable in gain-of-function experiments. In the resulting DC-NICD animals, splenic CD11b+ DCs consisted almost exclusively of Esamhi Clec12a− cells (Fig. 2E). Furthermore, the expression of Esam was increased in all DCs including the CD8+ subset (Fig. 2F), showing that Esam as a faithful readout of Notch activity in DCs. Altogether, these data suggest that splenic CD11b+ DCs contain a distinct Esamhi population that is directly regulated by Notch2-RBPJ signaling.
Genetic and phenotypic analysis of splenic Esamhi DCs
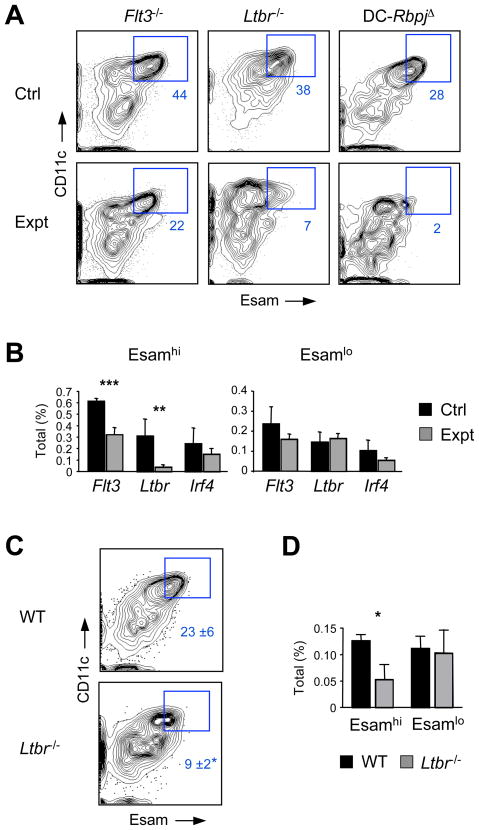
We have tested genetic requirements for the development of Notch-dependent Esamhi CD11b+ DC population. Splenocytes from Flt3-deficient mice showed a modest but significant reduction of Esamhi population (Fig. 3A,B), suggesting that Flt3 signaling contributes to its development or homeostasis. The blockade of lymphotoxin β receptor (LTβR) signaling partially reduces the number of splenic CD11b+ DCs and impairs their in situ proliferation in a cell-intrinsic manner (De Trez et al., 2008; Kabashima et al., 2005). We found that the fraction of Esamhi but not of Esamlo DCs was severely reduced in Ltbr−/− mice (Fig. 3A,B). To circumvent the pleiotropic defects of LTβR deficiency, we analyzed competitive chimeras reconstituted with a mixture of wild-type and LTβR-deficient BM. The fraction of LTβR-deficient donor cells was reduced in Esamhi DC population, suggesting that the phenotype was at least partially cell-intrinsic (Fig. 3C,D). In contrast, mice with DC-specific targeting of Irf4 (Klein et al., 2006), a transcription factor regulating CD11b+ DC development, showed marginal decrease of both Esamhi and Esamlo populations (Fig. 3B and data not shown). Thus, Esamhi CD11b+ DCs are genetically distinct from Esamlo DCs and specifically depend on Notch2-RBPJ and LTβR signaling, and to a lesser extent on Flt3 signaling.
Figure 3. The development of Esamhi CD11b+ cDCs requires LTβR signaling.
(A) Splenic CD11b+ Esamhi DC subset in experimental (Expt) Flt3−/− or Ltbr−/− animals or respective wild-type controls (Ctrl). The DC-RbpjΔ splenocytes that lack Esamhi DCs (Fig. 2D) are included for comparison. Shown are staining profiles of gated B220− CD11b+ CD8− splenocytes, with the CD11chi Esamhi DC population highlighted.
(B) The fraction of Esamhi and Esamlo DC subsets among total splenocytes from Flt3−/−, Ltbr−/− or DC-Irf4Δ experimental (Expt) animals or controls (Ctrl). Significance is indicated as in Fig. 1.
(C) Splenic CD11b+ Esamhi DC population among gated wild-type competitor (WT) or Ltbr−/− donor populations in competitively reconstituted BM chimeras, shown as in panel A (mean ± S.D. of 3 animals).
(D) The fraction of WT competitor- or Ltbr−/− donor-derived CD11b+ DC subsets among total splenocytes in the BM chimeras (mean ± S.D. of 3 animals).
Cell surface staining of CD11b+ DCs showed that the Esamhi Cx3cr1-GFPlo population was uniformly positive for CD4 and Dcir2 (Fig. 4A), in agreement with the preferential loss of CD4+ and Dcir2+ DCs after Notch2 deletion (Fig. 1F). Conversely, Esamlo Cx3cr1-GFPhi cells were heterogeneous for CD4 and Dcir2 and high for CD11b and Clec12a. Esamlo cells expressed high amounts of Flt3 and LTβR, despite their independence of these receptors (Fig 4A,B and Fig. S3). Cytospin preparations of sorted Esamhi DCs revealed irregular nuclei and thin cytoplasmic veils, whereas Esamlo DCs showed typical mononuclear morphology (Fig 4C). Notably, Esamlo DCs appeared distinct from splenic macrophages (Fig 4C) or intestinal CD11b+ CD103− DCs (Bogunovic et al., 2009), as they did not contain prominent cytoplasmic vesicles. After in vivo pulse with nucleoside analogue 5-bromo-2′-deoxyuridine (BrdU), Esamhi CD11b+ DC incorporated more BrdU than Esamlo DC (Fig. 4D), revealing a faster turnover. Although the contribution of progenitors cannot be ruled out, the short BrdU pulse revealed more proliferating cells among Esamhi DCs (Fig. 4E). Altogether, the Esamhi subset of CD11b+ DCs appears distinct in its higher turnover rate and dependence on Notch2 and LTβR signaling.
Figure 4. Phenotypic analysis of the splenic CD11b+ DC subsets.
Unless indicated otherwise, Cx3cr1-GFP reporter mice were used to separate the Esamhi GFPlo and Esamlo GFPhi subsets of CD11chi CD11b+ CD8− splenic DCs.
(A) Expression of the indicated surface markers in the gated Esamhi GFPlo and Esamlo GFPhi subsets. Dashed lines indicate positive staining threshold.
(B) Expression of LTβR on the indicated DC subsets from wild-type mice.
(C) Microphotographs of the sorted DC subsets stained by Giemsa stain on cytospin preparations. The CD11c− CD11b+ F4/80+ Cx3cr1-GFP+ macrophages (MΦ) are shown for comparison (magnification, 400x).
(D) Histograms of BrdU staining in the gated DC subsets after 2 days of in vivo BrdU pulse. The percentages of BrdU+ cells are indicated; representative of 4 animals.
(E) Cell cycle profiles of DC subsets after 2 hr of in vivo BrdU pulse. Shown are gated DC subsets stained for DNA content (DAPI) and BrdU incorporation; the fractions of cells in S and G2/M phases are indicated (representative of 2 animals).
Gene expression profiles of splenic CD11b+ DC populations
To confirm genetic differences between the two subsets of CD11b+ DCs, we compared genome-wide expression profiles of wild-type Esamhi and Esamlo CD11b+ DC populations, along with CD11b+ DCs from DC-RbpjΔ mice. No major differences in the expression of Notch2 or other pathway components were found by microarray or quantitative polymerase chain reaction (PCR) (qPCR, Fig. S3), suggesting control by other factors such as ligand availability. Clustering analysis confirmed that RBPJ-deficient DCs were very similar to the Esamlo subset (Fig 5A), as suggested by surface phenotype (Fig. 2). Pairwise comparison of Esamhi and Esamlo populations (Fig. 5B and Table S1) showed the expected differential expression of Esam and Clec12a. One of the most differentially expressed genes was Gpr4, which encodes a G protein-coupled receptor and is preferentially expressed in CD11chi cDCs as revealed by a Gpr4 locus-driven transgenic GFP reporter (Fig. S3). This reporter confirmed the expression of Gpr4 in Esamhi but not Esamlo DCs (Fig. S3). Finally, Esamhi DCs showed lower expression of cell cycle inhibitor Cdkn1a (p21), in accordance with their higher proliferation rate.
Figure 5. Gene expression profiles of splenic CD11b+ DC subsets.
(A) The comparison of control and RBPJ-deficient CD11b+ DCs. Splenic CD11b+ DCs from Cx3cr1-GFP+ DC-RbpjΔ mice were sorted along with the Esamhi GFPlo and Esamlo GFPhi subsets of CD11b+ splenic DCs from control Cx3cr1-GFP+ mice. Shown is unsupervised clustering analysis of the resulting microarray expression profiles.
(B) Pairwise comparison of the Esamhi and Esamlo subsets. The probes overexpressed >2 fold in the respective populations are shown in green and red, and their percentage among the total probes is indicated. Probes for select relevant genes are highlighted in blue.
(C) The expression of signature probe sets in the Esamhi and Esamlo subsets. The signature sets of total DCs, CD11b+ DCs and monocytes were derived from Immgen database and their distribution among the Esamhi and Esamlo subsets is depicted as in panel B.
(D) The expression of Ccr2-RFP reporter in DC subsets. Shown are histograms of RFP expression in the indicated splenic populations including CD11c− CD11b+ monocytes and macrophages (Mo-MΦ), and Clec12a− and Clec12a+ CD11b+ DCs corresponding to Esamhi and Esamlo subsets, respectively.
(E) Efficiency of Lyz2-cre recombination in DC subsets. Shown are histograms of YFP expression in the indicated splenic populations of Lyz2-cre+ mice with cre-inducible YFP reporter allele.
Next, the Immgen database (Heng and Painter, 2008) was used to derive expression signatures of splenic CD11b+ CD4+ and CD11b− CD8+ DCs and of blood Ly6C+ MHC II+ monocytes (Table S2). We then examined the distribution of these signature gene sets among Esamhi and Esamlo CD11b+ DC populations. As shown in Fig. 5C, genes characteristic of all DCs were equally expressed by both populations, confirming that they represent bona fide DCs. In contrast, CD11b+ DC-specific genes including the Notch target Dtx1 were preferentially expressed in the Esamhi subset. Conversely, monocyte-specific genes were preferentially expressed in Esamlo cells, including such canonical myeloid genes as Ly6c, cytokine (Csf1r, Csf3r) and chemokine (Ccr2) receptors and lysozyme (Lyz1, Lyz2). Notably, the expression of myeloid genes such as colony stimulating factor (CSF) receptors in Esamlo DCs appear significantly lower than in macrophages (Fig. S3). Thus, the Esamhi population features the unique expression profile of CD11b+ cDCs, whereas Esamlo population appears closer to but distinct from monocytes or macrophages.
Using red fluorescent protein (RFP) reporter driven by Ccr2 locus (Saederup et al., 2010), we found that Esamhi DCs were the only splenic DC population with low RFP expression, while Esamlo DCs and the majority of CD8+ DCs were Ccr2-RFPhi. To confirm the differential relationship of Esamhi and Esamlo DCs to myeloid cells, we used lineage tracing with a myeloid-specific Lyz2-cre (also known as LysM-cre) deleter strain (Jakubzick et al., 2008; Liu et al., 2009). In Lyz2-cre mice crossed to a cre-inducible yellow fluorescent protein (YFP) reporter, splenic Esamhi DCs as well as CD8+ DCs harbored only a small (5–7%) fraction of recombined YFP+ cells (Fig. 5E). In contrast, the Esamlo DCs showed substantial recombination (~30%) that was nevertheless lower than in monocytes and macrophages (~45%). These data show that the Esamhi DCs are uniquely low for Ccr2, have no developmental history of Lyz2 expression and therefore are not derived from the Esamlo population or from monocytes.
The differences in signaling requirements, gene expression profile and Lyz2 expression history suggest that the Esamhi and Esamlo population may arise from different precursors. Indeed, adoptively transferred Cx3cr1-GFP+ MDP gave rise to both GFPhi and GFPlo splenic CD11b+ DC populations, whereas CDP gave rise almost exclusively to GFPlo CD11b+ DCs corresponding to the Esamhi subset (Fig. S3). These data suggest that the GFPlo Esamhi subset represents the canonical CDP-derived splenic DC population; conversely, GFPhi DCs likely arise from different myeloid progenitors.
Functional analysis of splenic CD11b+ DC populations
The Esamlo DC subset showed preferential expression of multiple genes associated with TLR-mediated pathogen sensing, including several TLRs, MyD88, CD14 and CD36 (Table S1 and Fig. 6A). The expression of these genes in Esamlo DCs was nevertheless lower than in macrophages (Fig. 6B). We therefore tested the ability of CD11b+ DC subsets to produce cytokines in response to TLR ligands. Esamlo DCs efficiently produced tumor necrosis factor alpha (TNF-α) and IL-12 in response to the TLR9 agonist CpG DNA, in contrast to the weak production by Esamhi DCs (Fig. 6C). CD11c− CD11b+ monocytes and macrophages produced more TNF-α but less IL-12 than Esamlo DCs, underscoring the distinct functionality of the latter population. A similarly superior cytokine production by Esamlo over Esamhi DCs was observed in response to TLR2 agonist heat-killed L.monocytogenes (Fig. 6D) and TLR7 agonist gardiquimod (data not shown). No expression of interferon-β or interleukin-10 by either DC population could be detected under these conditions (data not shown). Furthermore, Esamlo DCs showed stronger TLR-mediated induction of costimulatory molecules than Esamhi DCs (Fig. 6E), suggesting a higher overall sensitivity to TLR ligands.
Figure 6. Functional properties of splenic CD11b+ DC subsets.
(A) The expression of indicated genes in GFPlo Esamhi and GFPhi Esamlo subsets of splenic CD11b+ DCs sorted from Cx3cr1-GFP mice. Data represent normalized expression values relative to the Esamhi sample as determined by qPCR (mean ± S.D. of triplicate reactions).
(B) The expression of indicated genes in the same DC subsets as well as in CD11c− CD11b+ F4/80+ GFP+ macrophages (MΦ), determined by qPCR as in (A) and shown on a log scale.
(C) Cytokine production by CD11b+ DCs in response to TLR9 ligand CpG. Lymphocyte-depleted splenocytes from Cx3cr1-GFP mice were incubated for 6 hr with CpG and stained for surface markers and intracellular TNF-α or IL-12. Shown are cytokine expression profiles in GFPlo Esamhi or GFPhi Esamlo subsets of CD11chi CD11b+ DCs, or in CD11c− CD11b+ GFPhi monocytes and macrophages (Mo-MΦ). Data are representative of three independent experiments.
(D) TNF-α production by CD11b+ DCs in response to TLR ligands. Splenocytes from Cx3cr1-GFP mice were activated with CpG or TLR2 ligand heat-killed L.monocytogenes (HKLM) and stained as above.
(E) Phenotypic maturation of CD11b+ DCs in vitro. Splenocytes from Cx3cr1-GFP mice were activated with CpG or HKLM and stained for DC markers as above. Shown are staining profiles of CD11b+ DC subsets for CD40, with mean fluorescence intensities indicated.
(F) In vivo CD4+ T cell priming in the absence of Esamhi DCs. DC-RbpjΔ or littermate control (Ctrl) mice were administered CFSE-labeled OVA-specific OT-II T cells and immunized with OVA. Shown are CFSE staining profiles of gated OT-II T cells from the recipient spleens 3 days after immunization. The fractions of T cells that did not divide or divided 1–3 and >3 times are indicated (mean of two recipients).
Both subsets of CD11b+ DCs showed efficient phagocytosis of in vivo injected polystyrene beads (Fig. S4). In an in vitro Ag presentation assay, both Esamlo and Esamhi DCs sorted from ovalbumin (OVA)-pulsed mice efficiently primed naïve OVA-specific CD4+ OT-II T cells (Fig. S4). Furthermore, splenic DCs from DC-RbpjΔ mice elicited strong OVA-specific OT-II proliferation in vitro, despite lack of the Esamhi DC subset. These data suggest that Esamhi and Esamlo DCs are equally capable of Ag-specific T cell priming in vitro on a per cell basis. To test the role of Esamhi DCs in T cell priming in vivo, we adoptively transferred carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled OT-II T cells into DC-RbpjΔ mice and immunized with OVA. Three days after immunization, OT-II T cells showed extensive proliferation (as measured by CFSE dye dilution) in the spleens of control mice, but only minor proliferation in the spleens of DC-RbpjΔ mice (Fig. 6F). Because DC-RbpjΔ spleens specifically lack Esamhi DCs, we conclude that this subset is required for optimal T cell priming in the spleen in vivo.
Notch2 is required for the development of intestinal CD11b+ CD103+ DCs
Given the tissue-specific nature of Notch signaling, we examined the role of Notch2 in DC differentiation outside of the spleen. We found that DC-Notch2Δ mice had normal numbers of CD11b+ and CD103+ DCs and Langerhans cells in the skin and/or skin-draining LN (Fig. S5). Similarly, CD11b+ and CD103+ DCs in the lung and liver were unaffected by Notch2 deletion (Fig. S5). However, analysis of DCs in the intestinal LP revealed selective depletion of the CD11b+ CD103+ subset (Fig. 7A, B). In contrast, the monocyte-derived CD11b+ CD103− population was unaffected, whereas the CD103+ population was moderately increased. Similarly, the fraction of CD11b+ CD103+ DCs migrating from the LP was selectively reduced in the mesenteric LN (Fig. 7C). The LP CD11b+ CD103+ did not express Esam or Dtx1 and were not reduced in DC-RBPJΔ or DC-DNMAML1 mice (data not shown), suggesting that the Notch2 signal is transient or qualitatively different in this subset. However, the expression of the Gpr4 reporter was detected in CD11b+ CD103+ but not in CD11b+ CD103− DCs (Fig. S3). This selective expression was similar to Gpr4 expression in Esamhi but not Esamlo splenic DCs, illustrating the similarity between Notch2-dependent DC subsets in the spleen and intestine.
Figure 7. Notch2 controls the development of intestinal CD11b+ CD103+ DCs.
(A) DC populations in the intestinal lamina propria from DC-Notch2Δ mice and littermate controls. Shown are staining profiles of CD45+ CD11chi MHC II+ DCs and of the gated CD11b+ DCs, highlighting the CD11b+ CD103+ DC subset. Fractions represent average ± S.D. of 4 animals per group. Statistical significance is indicated as in Fig. 1.
(B) Absolute numbers of the indicated DC subsets (mean ± S.D. of 4 animals).
(C) Staining profiles of CD11chi MHC II+ DCs from the mesenteric lymph nodes. Fractions represent average ± S.D. of 7 animals per group.
(D) Effector T cell populations in the intestine. Lymphocytes were isolated from the LP of small intestine and stained ex vivo for intracellular FoxP3 or activated in vitro with PMA plus ionomycin and stained for intracellular IL-17. Shown are profiles of CD11chi MHC II+ LP DCs and of gated TCRβ+ CD4+ T cells isolated ex vivo or activated in vitro (two pairs of littermates shown; representative of 4 DC-Notch2Δ animals).
To test the functional consequences of CD11b+ CD103+ DCs depletion, we examined intestinal T cell populations in DC-Notch2Δ mice. While the fraction of regulatory CD4+ T (Treg) cells expressing transcription factor FoxP3 was unchanged, the IL-17-producing Th17 cells were reduced ~50% (Fig. 7D). Similar results were observed in the small intestine (Fig. 7D) and colon (not shown). Thus, Notch2 provides a tissue-specific differentiation signal for the CD11b+ CD103+ DC population, which in turn support optimal effector T cell differentiation in the intestine.
Discussion
Although Notch signaling has been indirectly implicated in various aspects of DC development or function (Caton et al., 2007; Cheng et al., 2003; Feng et al., 2010; Zhou et al., 2009), the specific Notch receptor involved and DC populations affected were unclear. Here we have demonstrated that Notch2 receptor controls the development of specific DC subsets in the spleen and intestine. In contrast, Notch1 was dispensable for DC development, consistent with previous results of global inducible Notch1 deletion (Radtke et al., 2000). Similarly, Notch4 deficiency did not affect DC populations (data not shown), establishing Notch2 as a non-redundant Notch receptor regulating the DC lineage development.
Canonical Notch signaling occurs through the NICD-RBPJ-MAML transcriptional activation complex, and Rbpj deletion (Han et al., 2002) or DNMAML1-mediated complex disruption (Maillard et al., 2004) recapitulates Notch receptor deletion in lymphocytes. Indeed, CD11b+ splenic DCs were equally affected by the loss of Notch2 or RBPJ or by DNMAML expression. However, Notch2 deletion impaired the development of splenic CD8+ DCs and intestinal CD11b+ CD103+ DCs, whereas Rbpj deletion spared both subsets ((Caton et al., 2007) and data not shown). Although Notch2 signaling in these DC subsets may include a non-canonical RBPJ-independent pathway, this discrepancy likely reflects technical aspects of gene targeting in DCs. Because DCs have a rapid turnover and Itgax-cre recombination commences after DC commitment, delayed recombination kinetics and/or increased protein perdurance of RBPJ versus Notch2 would mitigate the phenotype. Indeed, continuous global deletion of RBPJ caused apparent loss of CD8+ DCs (Feng et al., 2010), suggesting the predominant role of canonical Notch2 signaling in DC development.
Notch signaling can provide the initial commitment signal as well as sustain further differentiation steps and/or homeostasis of a given cell type (Radtke et al., 2010; Yashiro-Ohtani et al., 2010). In splenic DCs, Notch2 deletion affected both DC subsets and caused accumulation of immature CD11b− CD8− cells. In addition, mature CD11b+ DCs demonstrate ongoing Notch signaling activity, as evidenced by RBPJ-dependent expression of Dtx1 and sensitivity to Notch2-RBPJ loss or DNMAML1 induction. In contrast, CD8+ DCs do not express Dtx1 and are less affected by Rbpj deletion or DNMAML1 induction. Thus, Notch2 signaling appears to initiate and maintain the differentiation of splenic CD11b+ DC subset, starting at the common pre-DC stage and thereby promoting CD8+ DC development as well. In tissues such as the intestine, Notch signaling in CD11b+ CD103+ DCs may commence after the pre-DC stage, and would not affect CD103+ DCs.
Our data illustrate a tissue-specific role of Notch signaling in DC differentiation in the spleen and intestinal LP, in contrast to its proposed general role in DC development (Cheng et al., 2003; Zhou et al., 2009). Indeed, both tissues are classical sites of Notch activity, which drives the respective differentiation of splenic MZ B cells and of the intestinal epithelium (Wilson and Radtke, 2006). MZ B cell development is mediated solely by Notch ligand Dll1 (Hozumi et al., 2004), whereas the latter involves both Dll1 and Dll4 (Pellegrinet et al., 2011). Because both Dll1 and Dll4 are also expressed in the splenic red pulp and MZ (Tan et al., 2009), these two ligands may redundantly regulate Notch2-mediated DC development both in the spleen and intestine. A notable molecular feature of the splenic MZ is active LTβR signaling, which is required both for MZ formation and MZ B cell maintenance, likely in a cell-extrinsic manner (Mebius and Kraal, 2005). We found that both Notch2 and LTβR signals control the development of CD11b+ splenic DCs. The contribution of LTβR was at least in part intrinsic to the hematopoietic compartment, consistent with previous studies on LTβR in DC development (Kabashima et al., 2005) and function (Summers deLuca et al., 2011). Our results establish these two pathways as common regulators of the splenic MZ and of its two major immune constituents, the MZ B cells and CD11b+ DCs.
The CD11b+ and CD8+ DC populations in the lymphoid organs of naive animals were thought to be genetically and functionally homogeneous. However, it has been demonstrated recently that CD8+ DCs are comprised of two distinct populations, the Cx3cr1-GFP− classical CD8+ DCs and Cx3cr1-GFPhi cells affiliated with the pDC lineage (Bar-On et al., 2010). Here we combined genetic analysis with the Cx3cr1-GFP reporter and surface marker Esam to similarly sub-divide the splenic CD11b+ DCs. In particular, we found that Notch or LTβR signaling blockade causes a complete ablation of the Esamhi DC subset, rather than a partial decrease of all splenic CD11b+ DCs. The Notch2-dependent Esamhi population exhibited properties of classical DCs, including cytoplasmic “veils” and rapid turnover. Although the CD4+ subset of CD11b+ DCs was reported to incorporate BrdU at a slower rate (Kamath et al., 2000), Esam is expressed only in a fraction of CD4+ DCs and thus provides a more precise subset definition. Furthermore, the higher turnover of RBPJ-deficient CD11b+ DCs (Caton et al., 2007) was based on prolonged BrdU pulse that aggregated proliferation with cell death and migration. Most importantly, the higher proliferation rate of Esamhi DCs is consistent with their dependence on Flt3 and LTβR signaling, both of which promote DC proliferation in situ (Kabashima et al., 2005; Waskow et al., 2008).
All blood-derived DCs in lymphoid organs are thought to originate from CDPs in the BM via the committed pre-DCs entering from the blood and differentiating in situ. Indeed, the Esamhi Cx3cr1lo CD11b+ splenic DCs apparently arise in this pathway as they can be derived from CDPs and lack developmental history of myeloid-specific Lyz2 gene expression. In contrast, the Esamlo Cx3cr1hi subset could not be derived from transferred CDPs and preferentially expressed myeloid genes, although at much lower level than monocytes or macrophages. Because splenic DCs cannot be derived from monocytes (Varol et al., 2007), the Esamlo DCs likely arise from earlier progenitors such as MDPs. Splenic DC development is not dependent on myeloid cytokine receptors Csf1r (Ginhoux et al., 2009) or Csf2r (our unpublished data), consistent with the lower expression of these genes in Esamlo DCs relative to macrophages (Fig. S3). In any case, our results suggest that the spleen is similar to non-lymphoid organs in the dual origin of its resident DCs from the CDPs and from a parallel myelomonocytic pathway.
In the spleen, the absence of Esamhi DCs after DC-specific Rbpj deletion impaired CD4+ T cell priming. This was not due to higher Ag uptake or presentation capacity of Esamhi DCs on a per cell basis; therefore, Esamhi DCs may be uniquely suited for T cell priming because of their specific localization in the MZ and/or migration properties. In the intestinal LP, the CD11b+ CD103+ cells represent a unique DC population that migrates to the mesenteric LN in the steady state and upon inflammation, suggesting its likely role in T cell priming (Bogunovic et al., 2009). We found that the loss of this population after DC-specific Notch2 targeting was associated with a reduced fraction of Th17 T cells, a major effector CD4+ T cell population in the intestine. Although this finding remains to be confirmed in mice with pure genetic background and more defined microflora, it is consistent with the recently reported capacity of CD11b+ CD103+ DCs to induce Th17 cell differentiation in vitro (Denning et al., 2011). Thus, DC-specific Notch2 targeting provided the first insight into the specific function of CD11b+ CD103+ DC subset in vivo, opening the way for future studies of immune responses and tolerance in the intestine. Collectively, our results establish Notch2 as a common regulator of the two key T cell-priming DC populations in the spleen and the intestine.
Methods
Animals
The Itgax-cre hemizygous transgene was used to generate the following experimental animals on C57BL/6 (B6) background unless indicated otherwise: DC-RbpjΔ (Rbpjflox/flox), DC-Notch1Δ (Notch1flox/flox), DC-Notch2Δ (Notch2flox/flox, mixed B6/129), DC-Irf4Δ (Irf4flox/flox), DC-DNMAML1 (Rosa26-StopFlox-DNMAML1, B6 or B6129F1), DC-NICD (Eef1a1-StopFlox-NICD1, mixed B6/129). Where indicated, DC-RbpjΔ mice carried heterozygous Cx3cr1-GFP reporter allele. Because the Itgax-cre transgene does not affect DC numbers or phenotype (Caton et al., 2007), Cre-negative littermates were used as controls for the respective Itgax-cre+ animals. For the analysis of intestinal T cells, co-housed sex-matched littermates were used. Germline null mutants of Flt3 and LTβR on B6 background, as well as competitive chimeras established from LTβR-null BM have been described (Summers deLuca et al., 2011; Waskow et al., 2008). Chimeras were analyzed 11 weeks after reconstitution and the donor- or competitor-derived cells were distinguished by differential CD45 isoform expression. Homo- or heterozygous Cx3cr1-GFP and heterozygous Ccr2-RFP reporter mice were on the B6 background. The Lyz2-cre lineage tracing was performed as described (Jakubzick et al., 2008), and utilized mice hemizygous for Lyz2-cre and heterozygous for Rosa26-StopFlox-EYFP. All animal studies were performed according to the investigator's protocol approved by the Institutional Animal Care and Use Committee of Columbia University.
Cell analysis
Cells were isolated ex vivo from lymphoid organs and stained for cell surface markers as described (Caton et al., 2007; Liu et al., 2009). The antibodies used for staining are listed in Table S3. The isolation and analysis of DCs from the intestinal LP and other tissues was done as in (Bogunovic et al., 2009; Ginhoux et al., 2009). The isolation and analysis of intestinal T cells was done as described (Ivanov et al., 2006). Fluorochrome- or biotin-conjugated antibodies were from eBiosciences or BD Biosciences. Biotinylated anti-Clec12a was kindly provided by Dr. M. Lahoud, at the Walter and Eliza Hall Institute. The samples were acquired on a LSR II flow cytometer or sorted on FACSAria flow sorter (BD Immunocytometry Systems), and analyzed using FlowJo software (Treestar Inc.). Cytospin preparations were done on Cytospin 3 centrifuge (Thermo Shandon) and stained by Giemsa stain.
For BrdU incorporation, Cx3cr1-GFP reporter mice were either injected i.p. with 1.5 mg BrdU and analyzed 2 hr later, or injected with 1.5 mg BrdU for two consecutive days and analyzed 48 hr after the first injection. BrdU staining was performed using APC BrdU Flow Kit (BD Biosciences), and combined with DAPI staining for 2 hr pulse. To measure cytokine production, splenocytes from Cx3cr1-GFP mice were depleted of lymphoid and erythroid cells by negative selection on MACS columns (Miltenyi). The DC-enriched flow-through fraction was incubated in serum-containing medium at 37°C with 100 nM CpG-B oligonucleotides (ODN1826) or 108 heat-killed L.monocytogenes (Invivogen). After 2 hours, Brefeldin A (10 μg/ml) was added and the cells were incubated for an additional 4 hrs. Cells were stained for surface markers, fixed, permeabilized and stained with APC-conjugated antibodies to IL-12 or TNF-α (BD Biosciences) according to the manufacturer’s instructions.
Gene expression analysis
For microarray analysis, spleens from Cx3cr1-GFP+ DC-RbpjΔ mice or Cre-negative littermate controls were isolated and depleted of lymphoid and erythroid cells by negative selection on MACS columns. Total CD11chi CD11b+ DCs from DC-RbpjΔ spleens (uniformly Esamlo GFPhi, see Fig. 2) or Esamlo GFPhi and Esamhi GFPlo subsets thereof from control mice were sorted and total RNA was isolated using RNeasy columns (Qiagen). Precipitated RNA was reverse-transcribed, labeled, and hybridized to GeneChip Mouse Gene 1.0 ST Array (Affymetrix) according to the manufacturer’s instructions. RMA-normalized gene expression values were analyzed using NIA Array software (Sharov et al., 2005). The signatures of normal DC populations were derived by clustering analysis of Immgen subsets DC.4+.Sp, DC.8+.Sp and Mo.6c+II+.Bl (Heng and Painter, 2008). Real-time qPCR was performed as described (Caton et al., 2007).
T cell proliferation assay
Splenic and LN CD4+ T cells from OVA-specific OT-II transgenic mice (Barnden et al., 1998) were enriched by negative selection using MACS columns, labeled with CFSE and injected intravenously (i.v.) (2×106 per animal) into DC-RbpjΔ or control mice. After 24 hr recipient mice received 10 mg OVA (Sigma) i.v. and were analyzed 72 hr later. Transferred T cells were identified as CD4+ TCR Vα2+ TCR Vβ5+.
Statistical analysis
Statistical significance was estimated using unpaired, two-tailed Student’s t-test.
Supplementary Material
Highlights.
Notch2 controls differentiation of splenic classical DCs
Notch2 dependence defines a distinct Esamhi population of splenic CD11b+ DCs
The Esamhi CD11b+ DCs are required for CD4+ T cell priming in the spleen
Notch2 controls differentiation of CD11b+ CD103+ DCs in the intestinal lamina propria
Acknowledgments
We thank U. Klein, A. Efstratiadis, F. Radtke, T. Gridley and W. Pear for mouse strains, A. Capobianco, D. Lin and A. Ferrante for animal donations, M. Lahoud for antibodies and J. Ericson for Immgen data. Supported by the American Asthma Foundation (B.R.), NIH grants AI072571 (B.R.) and DK085329 (I.I.I.), NIH training grants AI007161 (K.L.L.) and HD055165 (M.L.C.), and CIHR/IRSC award MOP #67157 (J.L.G.).
Footnotes
Accession numbers. The microarray data are available in the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/gds) under the accession number GSE31551.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bar-On L, Birnberg T, Lewis KL, Edelson BT, Bruder D, Hildner K, Buer J, Murphy KM, Reizis B, Jung S. CX3CR1+ CD8alpha+ dendritic cells are a steady-state population related to plasmacytoid dendritic cells. Proc Natl Acad Sci U S A. 2010;107:14745–14750. doi: 10.1073/pnas.1001562107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, Liu K, Jakubzick C, Ingersoll MA, Leboeuf M, et al. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- Buonamici S, Trimarchi T, Ruocco MG, Reavie L, Cathelin S, Mar BG, Klinakis A, Lukyanov Y, Tseng JC, Sen F, et al. CCR7 signalling as an essential regulator of CNS infiltration in T-cell leukaemia. Nature. 2009;459:1000–1004. doi: 10.1038/nature08020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J Exp Med. 2007;204:1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P, Nefedova Y, Miele L, Osborne BA, Gabrilovich D. Notch signaling is necessary but not sufficient for differentiation of dendritic cells. Blood. 2003;102:3980–3988. doi: 10.1182/blood-2003-04-1034. [DOI] [PubMed] [Google Scholar]
- De Trez C, Schneider K, Potter K, Droin N, Fulton J, Norris PS, Ha SW, Fu YX, Murphy T, Murphy KM, et al. The inhibitory HVEM-BTLA pathway counter regulates lymphotoxin receptor signaling to achieve homeostasis of dendritic cells. J Immunol. 2008;180:238–248. doi: 10.4049/jimmunol.180.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning TL, Norris BA, Medina-Contreras O, Manicassamy S, Geem D, Madan R, Karp CL, Pulendran B. Functional Specializations of Intestinal Dendritic Cell and Macrophage Subsets That Control Th17 and Regulatory T Cell Responses Are Dependent on the T Cell/APC Ratio, Source of Mouse Strain, and Regional Localization. J Immunol. 2011;187:733–747. doi: 10.4049/jimmunol.1002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- Feng F, Wang YC, Hu XB, Liu XW, Ji G, Chen YR, Wang L, He F, Dou GR, Liang L, et al. The transcription factor RBP-J-mediated signaling is essential for dendritic cells to evoke efficient anti-tumor immune responses in mice. Mol Cancer. 2010;9:90. doi: 10.1186/1476-4598-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Liu K, Helft J, Bogunovic M, Greter M, Hashimoto D, Price J, Yin N, Bromberg J, Lira SA, et al. The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med. 2009;206:3115–3130. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H, Tanigaki K, Yamamoto N, Kuroda K, Yoshimoto M, Nakahata T, Ikuta K, Honjo T. Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Int Immunol. 2002;14:637–645. doi: 10.1093/intimm/dxf030. [DOI] [PubMed] [Google Scholar]
- Heng TS, Painter MW. The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol. 2008;9:1091–1094. doi: 10.1038/ni1008-1091. [DOI] [PubMed] [Google Scholar]
- Hozumi K, Negishi N, Suzuki D, Abe N, Sotomaru Y, Tamaoki N, Mailhos C, Ish-Horowicz D, Habu S, Owen MJ. Delta-like 1 is necessary for the generation of marginal zone B cells but not T cells in vivo. Nat Immunol. 2004;5:638–644. doi: 10.1038/ni1075. [DOI] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Jakubzick C, Bogunovic M, Bonito AJ, Kuan EL, Merad M, Randolph GJ. Lymph-migrating, tissue-derived dendritic cells are minor constituents within steady-state lymph nodes. J Exp Med. 2008;205:2839–2850. doi: 10.1084/jem.20081430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabashima K, Banks TA, Ansel KM, Lu TT, Ware CF, Cyster JG. Intrinsic lymphotoxin-beta receptor requirement for homeostasis of lymphoid tissue dendritic cells. Immunity. 2005;22:439–450. doi: 10.1016/j.immuni.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Kamath AT, Pooley J, O'Keeffe MA, Vremec D, Zhan Y, Lew AM, D'Amico A, Wu L, Tough DF, Shortman K. The development, maturation, and turnover rate of mouse spleen dendritic cell populations. J Immunol. 2000;165:6762–6770. doi: 10.4049/jimmunol.165.12.6762. [DOI] [PubMed] [Google Scholar]
- Klein U, Casola S, Cattoretti G, Shen Q, Lia M, Mo T, Ludwig T, Rajewsky K, Dalla-Favera R. Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nat Immunol. 2006;7:773–782. doi: 10.1038/ni1357. [DOI] [PubMed] [Google Scholar]
- Lahoud MH, Proietto AI, Ahmet F, Kitsoulis S, Eidsmo L, Wu L, Sathe P, Pietersz S, Chang HW, Walker ID, et al. The C-type lectin Clec12A present on mouse and human dendritic cells can serve as a target for antigen delivery and enhancement of antibody responses. J Immunol. 2009;182:7587–7594. doi: 10.4049/jimmunol.0900464. [DOI] [PubMed] [Google Scholar]
- Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, Chu FF, Randolph GJ, Rudensky AY, Nussenzweig M. In Vivo Analysis of Dendritic Cell Development and Homeostasis. Science. 2009;324:392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard I, Weng AP, Carpenter AC, Rodriguez CG, Sai H, Xu L, Allman D, Aster JC, Pear WS. Mastermind critically regulates Notch-mediated lymphoid cell fate decisions. Blood. 2004;104:1696–1702. doi: 10.1182/blood-2004-02-0514. [DOI] [PubMed] [Google Scholar]
- McCright B, Lozier J, Gridley T. Generation of new Notch2 mutant alleles. Genesis. 2006;44:29–33. doi: 10.1002/gene.20181. [DOI] [PubMed] [Google Scholar]
- Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- Naik SH, Metcalf D, van Nieuwenhuijze A, Wicks I, Wu L, O'Keeffe M, Shortman K. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat Immunol. 2006;7:663–671. doi: 10.1038/ni1340. [DOI] [PubMed] [Google Scholar]
- Naik SH, Sathe P, Park HY, Metcalf D, Proietto AI, Dakic A, Carotta S, O'Keeffe M, Bahlo M, Papenfuss A, et al. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol. 2007;8:1217–1226. doi: 10.1038/ni1522. [DOI] [PubMed] [Google Scholar]
- Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat Immunol. 2007;8:1207–1216. doi: 10.1038/ni1518. [DOI] [PubMed] [Google Scholar]
- Pellegrinet L, Rodilla V, Liu Z, Chen S, Koch U, Espinosa L, Kaestner KH, Kopan R, Lewis J, Radtke F. Dll1- and dll4-mediated notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology. 2011;140:1230–1240. e1237. doi: 10.1053/j.gastro.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke F, Fasnacht N, Macdonald HR. Notch signaling in the immune system. Immunity. 2010;32:14–27. doi: 10.1016/j.immuni.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Radtke F, Ferrero I, Wilson A, Lees R, Aguet M, MacDonald HR. Notch1 deficiency dissociates the intrathymic development of dendritic cells and T cells. J Exp Med. 2000;191:1085–1094. doi: 10.1084/jem.191.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke F, Wilson A, Stark G, Bauer M, van Meerwijk J, MacDonald HR, Aguet M. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–558. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- Saederup N, Cardona AE, Croft K, Mizutani M, Cotleur AC, Tsou CL, Ransohoff RM, Charo IF. Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PLoS One. 2010;5:e13693. doi: 10.1371/journal.pone.0013693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, Pabst O. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206:3101–3114. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharov AA, Dudekula DB, Ko MS. A web-based tool for principal component and significance analysis of microarray data. Bioinformatics. 2005;21:2548–2549. doi: 10.1093/bioinformatics/bti343. [DOI] [PubMed] [Google Scholar]
- Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev. 2010;234:18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Idoyaga J. Features of the dendritic cell lineage. Immunol Rev. 2010;234:5–17. doi: 10.1111/j.0105-2896.2009.00888.x. [DOI] [PubMed] [Google Scholar]
- Summers deLuca L, Ng D, Gao Y, Wortzman ME, Watts TH, Gommerman JL. LTbetaR signaling in dendritic cells induces a type I IFN response that is required for optimal clonal expansion of CD8+ T cells. Proc Natl Acad Sci U S A. 2011;108:2046–2051. doi: 10.1073/pnas.1014188108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JB, Xu K, Cretegny K, Visan I, Yuan JS, Egan SE, Guidos CJ. Lunatic and manic fringe cooperatively enhance marginal zone B cell precursor competition for delta-like 1 in splenic endothelial niches. Immunity. 2009;30:254–263. doi: 10.1016/j.immuni.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Tu L, Fang TC, Artis D, Shestova O, Pross SE, Maillard I, Pear WS. Notch signaling is an important regulator of type 2 immunity. J Exp Med. 2005;202:1037–1042. doi: 10.1084/jem.20050923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R, Kalchenko V, Geissmann F, Jung S. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med. 2007;204:171–180. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varol C, Vallon-Eberhard A, Elinav E, Aychek T, Shapira Y, Luche H, Fehling HJ, Hardt WD, Shakhar G, Jung S. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Waskow C, Liu K, Darrasse-Jeze G, Guermonprez P, Ginhoux F, Merad M, Shengelia T, Yao K, Nussenzweig M. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat Immunol. 2008;9:676–683. doi: 10.1038/ni.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmann F, Petri B, Khandoga AG, Moser C, Khandoga A, Volkery S, Li H, Nasdala I, Brandau O, Fassler R, et al. ESAM supports neutrophil extravasation, activation of Rho, and VEGF-induced vascular permeability. J Exp Med. 2006;203:1671–1677. doi: 10.1084/jem.20060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Radtke F. Multiple functions of Notch signaling in self-renewing organs and cancer. FEBS Lett. 2006;580:2860–2868. doi: 10.1016/j.febslet.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Yashiro-Ohtani Y, Ohtani T, Pear WS. Notch regulation of early thymocyte development. Semin Immunol. 2010;22:261–269. doi: 10.1016/j.smim.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Yuan JS, Kousis PC, Suliman S, Visan I, Guidos CJ. Functions of notch signaling in the immune system: consensus and controversies. Annu Rev Immunol. 2010;28:343–365. doi: 10.1146/annurev.immunol.021908.132719. [DOI] [PubMed] [Google Scholar]
- Zhou J, Cheng P, Youn JI, Cotter MJ, Gabrilovich DI. Notch and wingless signaling cooperate in regulation of dendritic cell differentiation. Immunity. 2009;30:845–859. doi: 10.1016/j.immuni.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.