Abstract
During exposure to high strength static magnetic fields, humans report vestibular symptoms such as vertigo, apparent motion, and nausea. Rodents also show signs of vestibular perturbation after magnetic field exposure at 7 tesla (T) and above, such as locomotor circling, activation of vestibular nuclei, and acquisition of conditioned taste aversions. We hypothesized that the acute effects of the magnetic field might be seen as changes in head position during exposure within the magnet. Using a yoked restraint tube that allowed movement of the head and neck, we found that rats showed an immediate and persistent deviation of the head during exposure to a static 14.1 T magnetic field. The direction of the head tilt was dependent on the orientation of the rat in the magnetic field (B), such that rats oriented head-up (snout towards B+) showed a rightward tilt of the head, while rats oriented head–down (snout towards B−) showed a leftward tilt of the head. The tilt of the head during magnet exposure was opposite to the direction of locomotor circling immediately after exposure observed previously. Rats exposed in the yoked restraint tube showed significantly more locomotor circling compared to rats exposed with the head restrained. There was little difference in CTA magnitude or extinction rate, however. The deviation of the head was seen when the rats were motionless within the homogenous static field; movement through the field or exposure to the steep gradients of the field was not necessary to elicit the apparent vestibulo-collic reflex.
Keywords: Vestibulo-collic reflex, vestibular, conditioned taste aversion, locomotor circling
1. Introduction
The resolution of magnetic resonance imaging (MRI) machines can be increased with ultrahigh magnetic fields, e.g. > 7 tesla (T). There is increasing evidence, however, that exposure to high magnetic fields causes vestibular perturbations in humans and rodents. Humans report feelings of nausea, vertigo, and subjective rotation of the body during exposure to, or while moving through, high magnetic fields of 4 T and above [1–7]. Humans may also show deficits in visual-motor tasks after exposure to magnetic field in or around MRI machines that may be related to vestibular perturbation [8–10]. It has been proposed that the high magnetic fields cause vestibular sensations by interacting with the endolymph within the semicircular canals or the otoconia of the saccule and utricle, or by induction of current in sensory nerves [2, 11, 12].
The effects of high magnetic fields on rodents have been assessed from the behavior of rats and mice after exposure to the magnetic field [13]. In locomotor tests after exposure to 4 T, rats show decreased rearing; after exposure to 7 T and above, rats also walk in tight circles [14, 15]. The direction of circling is dependent on the orientation of the rat within the magnetic field: the rat walks counterclockwise if exposed with its head towards B+, and clockwise if exposed with its head towards B−. Similarly, rats and mice swim in circles after magnetic field exposure [16]. Exposure to magnetic fields at 7T or above can serve as the unconditioned stimulus for conditioned taste aversion or avoidance (CTA) learning, suggesting that exposure has an aversive component [14, 15, 17]. Consistent with stimulation of the vestibular system, magnetic field exposure induces c-Fos in vestibular and visceral relays of the rat brainstem [18, 19]. The effects of the high magnetic field on locomotion and CTA are maximal after head exposure [20]. Locomotor circling, CTA, and c-Fos responses are abolished by chemical labyrinthectomy [21].
In addition to these delayed effects observed after the magnetic field exposure, we hypothesize that the high magnetic field has an acute effect on rodents, such that they receive vestibular stimulation during magnetic field exposure comparable to the self-reports of humans. Vestibular perturbation is usually inferred from the posture or locomotion of the effected animal; the narrow inner diameter (6 cm) of our 14.1 T magnet, however, precludes observing the full range of postures in a freely-moving, unrestrained rat during the actual exposure to the magnetic field.
A prominent component of vestibular responses is the vestibulo-collic reflex (VCR), which stabilizes head position in space during involuntary head and body movement [22]. For example, rotation of the whole body causes compensatory head movements counter to the direction of rotation [23, 24]. The VCR can also be elicited artificially, e.g. by electrical stimulation of the afferents of the semicircular canals [25] or by transmastoid galvanic stimulation [26]. It is possible, therefore, that magnetic exposure might elicit a VCR response via its interactions with the vestibular apparatus of the inner ear.
While the rat cannot move freely within the magnet, the bore is wide enough to allow a range of head movements. We therefore exposed rats to 14.1 T in a restraint tube with a yoke that constrained body movements but allowed relatively free head movements.
2. Methods
2.1. Animals
Adult female Sprague-Dawley rats (8–10 weeks old, 175–200 g; Charles River) were housed individually in polycarbonate cages in the temperature-controlled (22 ± 2 °C, 30–40% humidity) animal facility at the National High Magnetic Field Laboratory at The Florida State University. The light/dark cycle was 12:12 with lights on at 0700 hours. All conditioning trials were conducted during the light cycle. The rats had free access to pelleted Purina Rat Chow 5001 and deionized-distilled water except as specified otherwise. All procedures were approved by the Institutional Animal Care and Use Committee of Florida State University (protocol #0912).
2.2. Magnet
A 600 MHz Magnex Cryo magnet with an 89 mm bore and a fixed central field (B0) strength of 14.1 T was used. The magnet was located approximately 50 m from the animal facility. The magnetic field was orientated vertically so that the positive pole was at the top of the magnet. The lower opening of the magnet’s bore was 73 cm above the floor, and the bore extended for 157 cm; the center of the bore at 14.1 T was 50 cm from the lower opening of the bore. Shim magnets extending along the magnet’s bore for approximately ± 15 cm from the magnet core stabilized the magnetic field to give a central core field of uniform strength. The magnet was operated without radiofrequency pulses, so rats were exposed to a static magnetic field only.
2.3. Exposure
Rats were placed in restraint tubes for sham- or magnet-exposure. The restraint tubes were 30 cm in length with an inside diameter of 5.6 cm and an outside diameter of 6.4 cm. Two types of restraints were used. The type of restraint tube which we have used in previous studies holds the head of the rat almost completely immobile within a plug at the rostral end of the tube and held in position by nylon screws. The inside of this rostral plug was fabricated in a cone shape to accommodate the head of the rat. A 1-cm hole was bored in this plug at the apex of this cone to allow fresh breathing air. A second type of restraint tube was constructed for this study that allowed relatively free head movements (“free-head restraint”). Hinged plates at the rostral end of the tube could be closed around the neck of the rat to form a yoke through which the head projected (see Figure 1). In both types of tube, a second plug was inserted into the caudal end of the tube and was adjusted to restrain the bodily movements of the rat. A hole in the center of this plug accommodated the rat’s tail.
Figure 1.
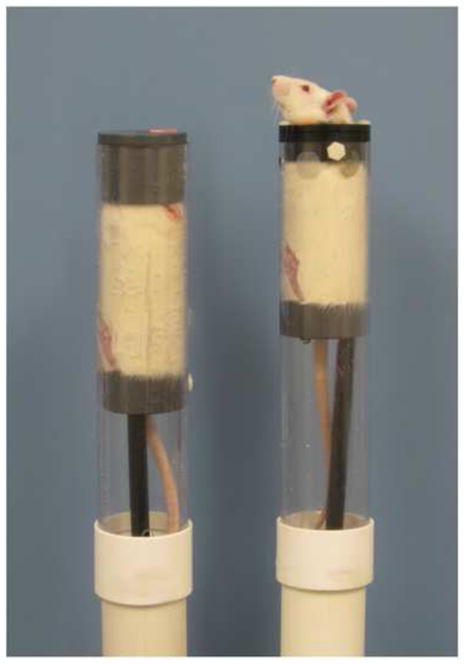
Examples of restraint tubes used in this study. On the left, a rat is shown in the restraint tube used in previous studies which holds the head almost completely immobile within a plug at the rostral end of the tube. On the right, a rat is shown in the restraint tube with a yoke that constrained body movements but allowed relatively free movement of the head and neck.
Restrained rats were transported from the animal facility to the 14.1 T magnet in approximately 30 seconds. Rats exposed to the magnetic field were inserted 60 cm into the bore of the magnet for 30 min at 14.1 T (“magnet exposure”). As controls for the effects of restraint, some rats were “sham-exposed” by placing them in the restraint tubes and inserting them into an opaque PVC pipe placed in the same room as the magnet but beyond the 500 μT line of the high magnetic field.
2.4. Experiment 1. Head Tilt during Magnet Exposure
Rats (n=18) were placed in free-head restraint and either exposed to 14.1 T magnet or sham-exposed. Heads-up rats (n=6) were also sham-exposed for 30 min either the day before or the day after magnet exposure. Separate groups of rats were exposed with their heads down in the 14.1T magnet (n=6) or sham-exposed with their heads down in the PVC “sham-magnet” (n=6).
An additional 4 rats were anesthetized with sodium pentobarbital (50 mg/kg) and exposed to 14.1 T in the heads-up position for 5 minutes to assess the effects of the magnetic field on unconscious rats.
During exposure, rats were videotaped to record any head movements. A fiber optic light source was used to illuminate the bore of the magnet. The videocamera was placed at the 500 μT line of the magnet (i.e. 3 m from the magnet to avoid interference from the high field), and the rats were imaged inside the magnet through a mirror above or below the bore of the magnet.
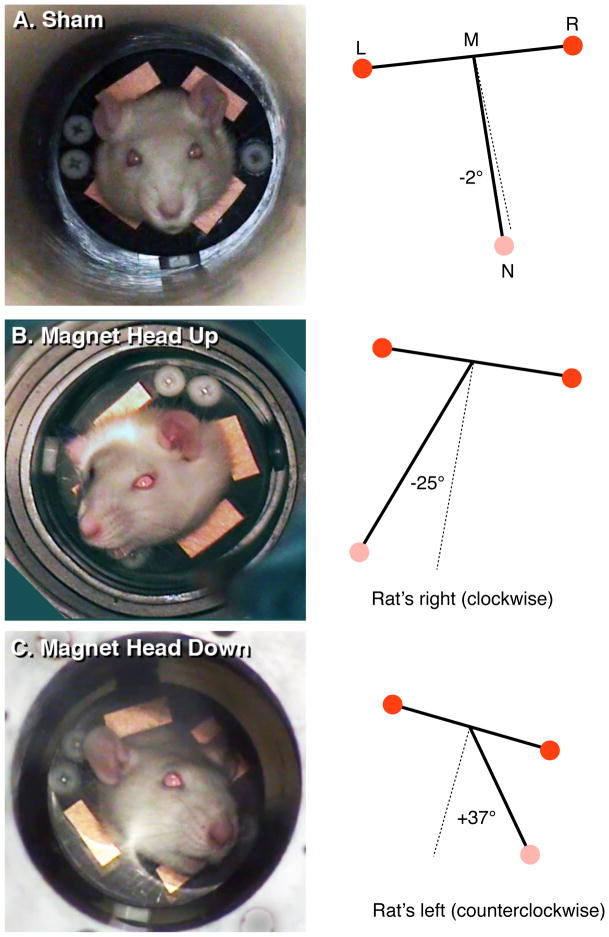
To quantify the position of the head, videotape was digitized onto a Macintosh computer and single frames were extracted at 1 min intervals across the 30-min exposures. A triangle was constructed from the position of the right eye (point R), left eye (point L), and nose (point N). Within this triangle, the median (line NM) of the nose vertex (angle RNL) with the line connecting the two eyes (line RL) was found. The degree of head tilt was quantified as the the angle (NMR) of median NM with the eye line RL; the more the rat’s head titled towards the side, the greater this angle deviated from the perpendicular (see Figure 2).
Figure 2.
Examples of rats during (A) sham exposure, (B) 14.1 T magnetic field with head up, and (C) 14.1 T magnetic field with head down. Panels on the left are frames from the video recording. Panels on the right demonstrate the quantification of head tilt calculated as the angle from the nose (N) to the midpoint (M) between the position of the left eye (L) and right eye (R). A deviation from the perpendicular towards the rat’s right was assigned a negative angle (A), while a deviation towards the rat’s left was assigned a positive angle (C).
2.5. Experiment 2. Locomotor Circling and Conditioned Taste Aversion
Eight days prior to conditioning day, rats (n = 16) were placed on a water restriction schedule under which they received daily water access in one drinking session, during which a water bottle was presented simultaneously with an empty bottle to accustom the rats to a 2-bottle choice. The first daily session was 3 h in length and the session times were diminished each day so that for two days before conditioning the rats received water access in a single 10-min session. On the conditioning day, rats were given access to 0.125% sodium saccharin solution (saccharin) for 10 min. Mean saccharin intake on conditioning day across all rats was 6.5 ± 0.6 g; there was no significant difference between groups.
Immediately following saccharin access, rats were placed in restraint tubes and exposed to 14.1 T for 30 min as described above. Half the rats were restrained in “free-head” tubes (n = 8), and half were placed in tubes with full head and body restraint (n =8).
After 30-min exposure within the bore of the magnet, each rat was released into an open polycarbonate cage (37 cm wide by 47 cm long by 20 cm high) with chip bedding. The locomotor behavior of each rat was recorded on videotape for 2 minutes after release into the cage. (Most rats exhibited locomotor effects of the magnetic field for less than 1 minute; thus, 2 minutes of recording captured most of the phenomena of interest.) The rat was then returned to its home cage and ad libitum water was returned. The videotapes were scored later by an observer blind to the rats’ treatment. Instances of tight-circling behavior were quantified. Rats were scored as “circling” if they moved continuously around a full circle with diameter less than the length of the rat’s body. Partial circles or circles interrupted by stationary pauses were not counted. Rearing behavior (both forepaws off the floor of the cage and one or both forepaws on the side of the cage) was also scored at this time. Rats were then returned to their home cage and given ad libitum access to water overnight.
The strength of the CTA induced by the magnet was measured with daily 24-h, 2-bottle preference tests that were initiated the day after conditioning. (Note that this measures conditioned taste avoidance, without explicitly measuring orofacial rejection responses to intraoral infusions typical of strong aversions [27]. However, provocative vestibular stimulation has been shown by others to condition intraoral rejection [28].) Two bottles were placed on the cages, one containing saccharin and the other distilled water. Fluid consumption was measured every 24 h and a preference score was calculated as the ratio of saccharin to total fluid consumption:
The preference tests were continued for 14 post-conditioning test days. The left/right position of saccharin and water bottles on the rats’ cages was reversed each day. Because saccharin access during the preference tests was not paired with any treatment, the preference tests constituted extinction trials.
2.6. Statistics
Comparisons between groups on single-day data were analyzed with appropriate ANOVAs or t-tests (Statistica). Results collected over multiple 2-bottle preference test days were analyzed by 2-way ANOVA, with groups as one factor and test days as the second factor, which consisted of repeated sampling of the same subjects across test days. Post-hoc comparisons were made with the Tukey’s HSD test. Data are presented as the mean ± standard error of the mean.
3. Results
3.1. Experiment 1
Sham-exposed rats showed only few and transient movement of the head, and maintained the head in a straight posture across the 30-min period (Figure 2A). Rats that were sham-exposed upside down appeared to display mild dorsoflexion, but otherwise kept the head straight. When quantified, the angle of the snout remained parallel to the body (Figures 2A, 3A, and 4).
Figure 3.
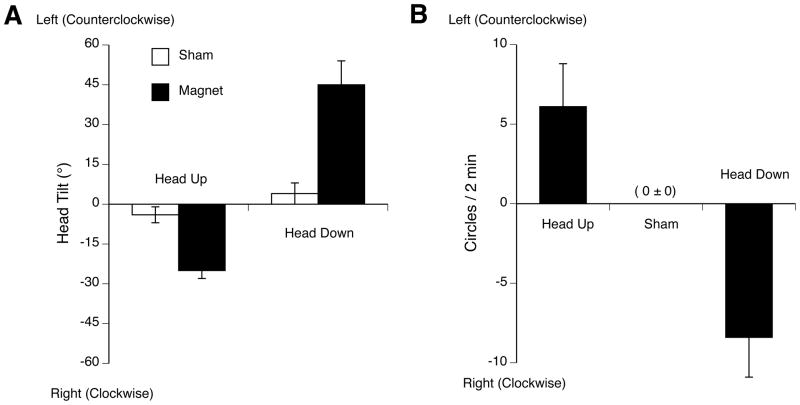
A. Mean ± s.e.m. head tilt during 30-min sham-exposure (white bars) or exposure to 14.1 T (black bars) in head-up orientation or head-down orientation. The data shown is the average across rats of the individual head tilt averaged at 1-min intervals across the 30 min exposure time for each rat. B. Data replotted from previous report [14] demonstrating locomotor circling immediately after 30-min exposure to 14.1 T while restrained in the head-up or head-down orientation. Note that rats walked in circles after exposure in the direction opposite to head tilt during exposure observed in the present study. * p < 0.05 vs. sham exposure.
Figure 4.
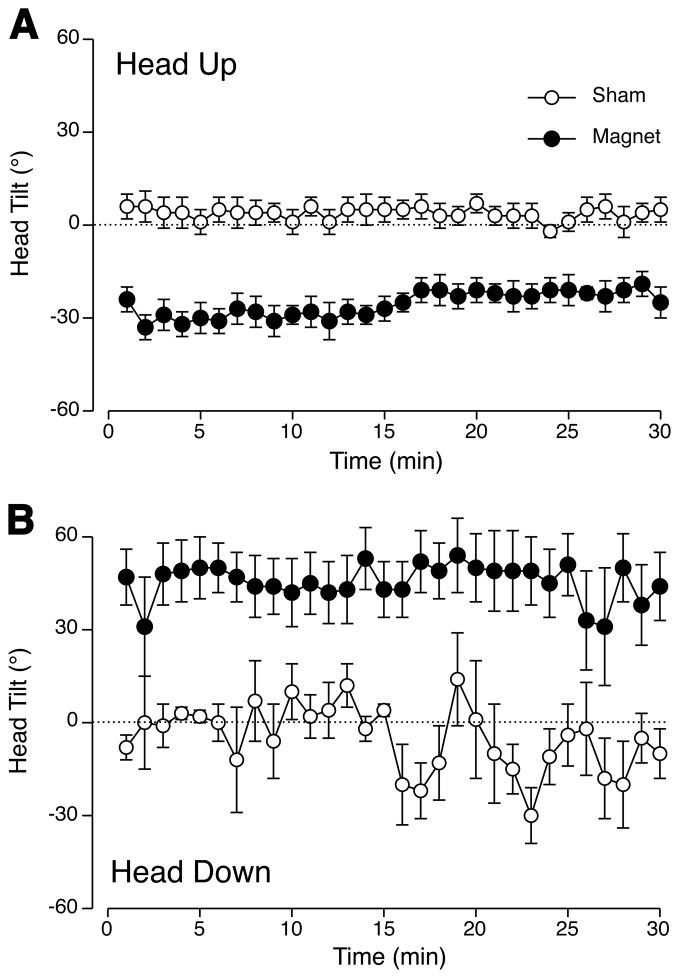
Mean ± s.e.m. across all rats of individual head tilts calculated at 1-min intervals across 30 min of sham-exposure (white circles) or exposure to 14.1 T (black circles) in the head-up orientation (A) or head-down orientation (B). Rats in the magnet maintained a stable degree of head tilt throughout the 30-min exposure.
Within seconds of being introduced into the bore of the 14.1 T magnet, rats tilted their heads to one side. (Example video can be seen at http://www.houptlab.org/freehead.mp4). Rats introduced head-up tilted the head to the right (Figures 2B). Rats introduced head-down (upside down) tilted the head to the left (Figures 2C). Rats also tended to tilt the snout slightly in the ventral direction, but this was not quantified. There was a significant interaction of exposure (sham vs. magnet) and orientation (head-up vs. head-down) on the mean angle of the snout across the 30-min exposure (F(1,20) = 50.7, p < 0.0001; Figure 3A). Rats exposed upside-down in the magnet showed a greater average deviation (44± 9 ° clockwise) than rats exposed head-up (26 ± 3° counterclockwise), but the difference in magnitude of snout deviation was not significant (p = 0.07). It is possible that hanging upside-down evoked additional postural reflexes that could have amplified the average snout deviation and increased the variability.
After the first few seconds, most rats in the magnet showed only occasional movements and maintained the head in a tilted position for the remainder of the 30-min period (Figure 4). There was a significant effect of group for both head-up and head-down exposures (F(1,290) = 19.28 and 24.6, p < 0.001 respectively).
Across all 1-min observations and rats, the range of head-tilt in the head-up position was from 4° clockwise to 54 ° counterclockwise while in the magnet, and from from 15° clockwise to 25 ° counterclockwise during sham-exposure. While upside down, the range of head-tilt was more variable: from 84° clockwise to 6° counterclockwise while in the magnet, and from 90° clockwise to 90° counterclockwise during sham-exposure.
The first full minute of video recordings from the “head-up” group were examined frame-by-frame to determine if rats showed small nystagmus of the head during exposure. Aside from a 2–3Hz motion due to breathing, no periodic turning of the head or rapid re-orientation of the head, characteristic of nystagmus, was observed.
The rats were briefly observed while still restrained after removal from the magnet. No pronounced head-tilt or head movement in any direction was seen.
Rats anesthetized with sodium pentobarbital showed no head movements in the bore of the magnet (data not shown). Thus the tilt of the head within the magnet was an active response by the rat, and not a passive physical effect of the magnetic field.
We have previously measured the amount and direction of circling induced by 30-min exposure of restrained rats within the same 14.1 T magnet [14]. In order to compare magnet-induced head tilt with magnet-induced circling, these data are replotted in Figure 3B. Note that the while rats exposed head-up showed a rightward (clockwise) head tilt, head-up exposure induced leftward (counterclockwise) locomotor circling immediately after circling (and vice-versa for head-down exposure.) Thus the direction of head tilt during magnetic field exposure is opposite to the direction of locomotor circling after exposure.
3.2. Experiment 2
Although most rats (12 of 16) showed some locomotor circling, rats that were exposed with their heads free showed significantly more locomotor circling compared to immobilized rats (6.0 ± 2.4 vs. 1.3 ± 0.6 circles/2 min, p < 0.05). Neither group of rats showed much rearing (head-free 0.5±0.3 vs. immobilized 0.3±0.2 rears/2 min, n.s.)
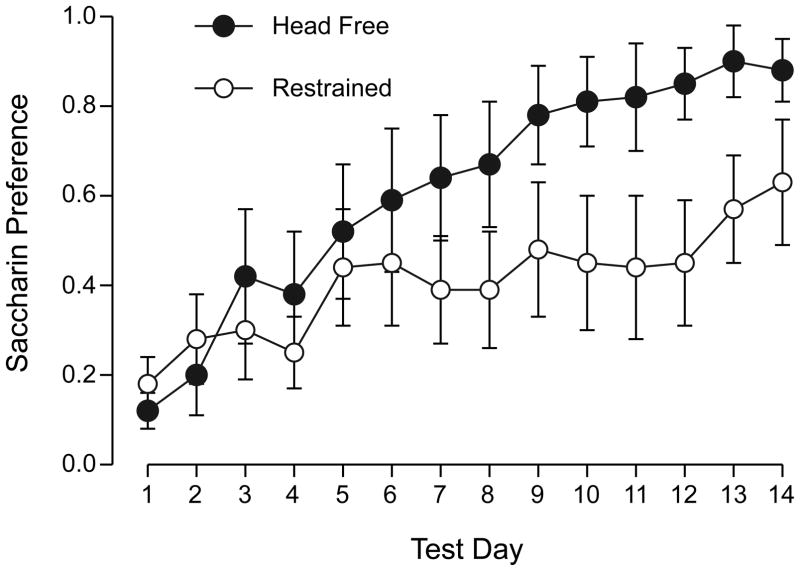
Both groups showed a low preference for saccharin on the first day of 2-bottle testing (head-free 0.12± 0.04 vs. immobilized 0.18 ± 0.06, n.s.), indicating acquisition of a strong CTA. Across 14 days of extinction testing, the was a significant effect of days (F (13, 15) = 7.89, p < 0.001) but no effect of restraint and no interaction (Figure 5.)
Figure 5.
Mean ± s.e.m saccharin preference across 14 days of 2-bottle extinction testing for rats conditioned by saccharin paired with “head-free” exposure (black circles) or immobilized exposure (white circles) to 14.1 T. There was no significant difference between groups on any single day.
4. Discussion
Using a yoked restraint tube, we found that rats show an immediate and persistent deviation of the head during exposure to a static 14.1 T magnetic field. The direction of the head tilt was dependent on the orientation of the rat in the magnetic field, such that rats oriented head-up (snout towards B+) showed a rightward tilt of the head, while rats oriented head-down (snout towards B−) showed a leftward tilt of the head. The tilt of the head during magnet exposure was opposite to the direction of locomotor circling immediately after exposure observed previously. Allowing head tilt using the yoked restraint tube during magnet exposure resulted in significantly more locomotor circling compared to rats exposed with the head restrained. There was little difference in CTA magnitude or extinction rate, however. (The divergence of extinction rates between groups suggests that a significant effect on extinction might be detected with a larger sample size.)
4.1. Vestibulo-collic reflex
The deviation of the head during magnet exposure is reminiscent of the VCR response to, e.g., whole body rotation [22]. VCR with compensatory head movements opposing the direction of rotation in any axis can be demonstrated using electromyography of head and neck muscles [23] or electromagnetic search coils attached to the head [24]. The VCR receives contributions from both semicircular canal stimulation (e.g. with sinusoidally varying rotation) and otolith organs (e.g. under constant rotation) [24]. We did not observe head nystagmus during magnet exposure, which suggests that rats were experiencing the equivalent of a static tilt, and not the equivalent of continuous rotation.
The VCR can also be elicited with artificial stimulation or manipulation of the vestibular apparatus of the inner ear. In humans, transmastoid galvanic stimulation that induces the subjective experience of the head tilting towards the cathode also induces deviation of the head in the opposite direction towards the stimulating anode [29]. In cats, unilateral electrical stimulation of the afferents of individual semicircular canals induced head movements which were in plane with the stimulated canals, but opposite in direction to the movements that would normally excite the canals [25]. A VCR can also be elicited by irrigation of the inner ear, with the head turning towards the side receiving cold water [30]. An exaggerated VCR is also seen in guinea pigs after unilateral labyrinthectomy, in which head deviations towards the lesioned side were seen for 72 h after surgery [31].
Thus, the deviation of the rat’s head while within the magnet could reflect a stimulation of the contralateral inner ear (as with electrical stimulation) or inhibiting the ipsilateral inner ear (as with cold water irrigation or after unilateral labyrinthectomy) resulting in a VCR response. The mechanisms of stimulation or inhibition are not known, however, although the inner ear is critical for other interactions with the magnetic field [21, 32].
The reasons for a consistent deviation to the right (in head-up rats) or to the left (in head-down rats) are also unknown. The magnetic field is extremely homogenous and symmetrical within the bore of the 14.1 T magnet. The asymmetry of response therefore is likely to reflect an asymmetry of interaction between the vestibular apparatus and the magnetic field, perhaps related to the structure of the inner ear itself.
4.2. Locomotor circling as vestibular after effect
Our results also suggest that the circular locomotion seen in rats and mice after magnet exposure is not a direct effect of the magnetic field. Rather, it may be an after effect of magnet exposure such that they walk in the direction opposite to the direction of vestibular perturbation experienced during magnet exposure. In other words, after a 30-min magnet exposure with a rightward head tilt (i.e. compensatory to a tonic leftward stimulation), upon release from the stimulation rats demonstrate a transient leftward locomotion. Similar locomotor after effects are seen after other forms of vestibular stimulation. For example, after whole-body rotation, rodents swim in the direction opposite to rotation [33]. After rotating optokinetic stimulation, human subjects walk in the direction opposite to the direction of rotation [34]. Podokinetic afterrotation has also been well-characterized; after walking on a circular treadmill, humans show a transient tendency to walk in the opposite direction [35]. Locomotor circling and postural changes after magnet exposure are also transient (2 – 10 min) [14, 16, 36], and may represent a similar post-stimulation vestibular response.
4.3. Comparison to Human Studies
In the psychophysical study of Glover et al., [2], humans being moved into the bore of a 7T magnet reported a sensation of falling forward or backward (depending on orientation), but no left-right displacements. Standing outside the magnet, some subjects showed a significant forward displacement of the head and reported the sensation of falling forward. Although humans report vestibular perturbations during exposure to ultra-high magnetic fields, there have been no reports of humans showing a reflexive head movement in response to the fields. However, the presence of such a reflex has not been explicitly tested, e.g., by observing head movements in the absence of visual input or other cues for orientation.
Furthermore, humans have not reported any left/right deviations of the head (or clockwise/counterclockwise rotations) similar to the apparent effect of high magnetic fields on rodents. One difference between human and rodent studies may be the different orientation of the vestibular apparatus relative to the magnetic fields. In rats restrained within the 14.1 T magnet, the head and, e.g., the horizontal canal lies parallel to the magnetic field. In humans lying in an MRI parallel to the magnetic field, the head is turned 90° compared to a rat, and, e.g., the horizontal canal is perpendicular to the magnetic field. We have previously found that the effects of magnetic field exposure are minimal in rats exposed perpendicular to the field [15], so it may be that the vestibular system in the supine human is aligned in such a way as to minimize interactions with the high magnetic field.
Humans report greatest vestibular effects during whole-body movement into a high magnetic field, or while moving the head within the static field. Humans can also show vestibular responses to much smaller magnetic fields (e.g. 0.2 to 2.8 mT) if the fields are pulsed or time-varying. Application to the head of specific pulsed magnetic fields of +/− 200 μT improved standing balance in human subjects as measured on a 3-D forceplate [37]. This effect can be modulated by ambient light intensity (with eyes open), further suggesting a visual-vestibular reflex modulation [38]. A decline in visual motor skills has also been observed after movement in the gradient field of MRI machines [8] or during head exposure to sinusoidal magnetic fields [39].
In rats, we have found that movement into and out of the 14.1 magnet cannot account for the entire response of the animal [40]. Rather, rats must be exposed to the static field at the center of the magnet for several minutes to observe maximal locomotor circling or CTA [14, 40]. The present results are consistent with these parameters. The head tilt in the rats was an effect of the static magnetic field and independent of gross movement through the field.
Models have been proposed of the interaction of high magnetic fields with the semicircular canals, otoconia, or vestibular nerves [2, 11, 12]. However, these models relay largely on motion through a static magnetic field (or equivalently, exposure to a time-varying magnetic field). Future models should explain the persistent reflexive response during static field exposure observed here.
Highlights.
High strength magnetic fields cause vestibular pertubations in humans and rodents.
During 30 min exposure within a 14 T magnet, rats tilt their heads to one side.
The direction of head tilt is dependant on orientation within the magnet.
The direction of head tilt is opposite to the direction of circling after exposure.
The high magnetic field appears to elicit a vestibulo-collic reflex.
Acknowledgments
Supported by National Institute on Deafness and Other Communication Disorders Grants RO1DC4607. We thank Drs. Timothy Cross and Zhehong Gan of the United States National Magnetic Field Laboratory for providing access to the magnet.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schenck JF, Dumoulin CL, Redington RW, Kressel HY, Elliot RT, McDougall IL. Human exposure to 4.0-Tesla magnetic fields in a whole body scanner. Med Phys. 1992;19:1089–1098. doi: 10.1118/1.596827. [DOI] [PubMed] [Google Scholar]
- 2.Glover PM, Cavin I, Qian W, Bowtell R, Gowland PA. Magnetic-field-induced vertigo: a theoretical and experimental investigation. Bioelectromagnetics. 2007:28. doi: 10.1002/bem.20316. [DOI] [PubMed] [Google Scholar]
- 3.Theysohn JM, Maderwald S, Kraff O, Moenninghoff C, Ladd ME, Ladd SC. Subjective acceptance of 7 Tesla MRI for human imaging. MAGMA. 2008;21:63–72. doi: 10.1007/s10334-007-0095-x. [DOI] [PubMed] [Google Scholar]
- 4.Kangarlu A, Burgess RE, Zhu H, Nakayama T, Hamlin RL, Abduljalil AM, Robitaille PML. Cognitive, cardiac, and physiological safety studies in ultra high field magnetic resonance imaging. Magn Reson Imag. 1999;17:1407–1416. doi: 10.1016/s0730-725x(99)00086-7. [DOI] [PubMed] [Google Scholar]
- 5.Chakeres DW, Kangarlu A, Boudoulas H, Young DC. Effect of static magnetic field exposure of up to 8 Tesla on sequential human vital sign measurements. J Magn Reson Imaging. 2003:18. doi: 10.1002/jmri.10367. [DOI] [PubMed] [Google Scholar]
- 6.Yang M, Christoforidis G, Abduljali A, Beversdorf D. Vital signs investigation in subjects undergoing MR imaging at 8T. AJNR Am J Neuroradiol. 2006;27:922–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Patel M, Williamsom RA, Dorevitch S, Buchanan S. Pilot study investigating the effect of the static magnetic field from a 9.4-T MRI on the vestibular system. J Occup Environ Med. 2008;50:576–583. doi: 10.1097/JOM.0b013e318162f5d6. [DOI] [PubMed] [Google Scholar]
- 8.de Vocht F, van Wendel de Joode B, Engels H, Kromhout H. Neurobehavioral effects among subjects exposed to high static and gradient magnetic fields from a 1.5 Tesla magnetic resonance imaging system--a case-crossover pilot study. Magn Reson Med. 2003;50:670–4. doi: 10.1002/mrm.10604. [DOI] [PubMed] [Google Scholar]
- 9.de Vocht F, Stevens T, van Wendel de Joode B, Engels H, Kromhout H. Acute neurobehavioral effects of exposure to static magnetic fields: analyses of exposure-response relations. J Magn Reson Imaging. 2006;23:291–7. doi: 10.1002/jmri.20510. [DOI] [PubMed] [Google Scholar]
- 10.de Vocht F, Glover P, Engels H, Kromhout H. Pooled analyses of effects on visual and visuomotor performance from exposure to magnetic stray fields from MRI scanners: application of the Bayesian framework. J Magn Reson Imaging. 2007;26:1255–60. doi: 10.1002/jmri.21142. [DOI] [PubMed] [Google Scholar]
- 11.Schenck JF. Health and physiological effects of human exposure to whole-body four-tesla magnetic fields during MRI. Ann NY Acad Sci. 1992;649:285–301. doi: 10.1111/j.1749-6632.1992.tb49617.x. [DOI] [PubMed] [Google Scholar]
- 12.Schenck JF. Physical interactions of static magnetic fields with living tissues. Prog Biophys Molec Biol. 2005;87:185–204. doi: 10.1016/j.pbiomolbio.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Houpt TA, Smith JC. Conditioned taste aversion induced by exposure to high-strength static magnetic fields. In: Reilly S, Schachtman TR, editors. Conditioned Taste Aversion: Behavioral and Neural Processes. Oxford University Press; NY: 2009. pp. 422–441. [Google Scholar]
- 14.Houpt TA, Pittman DM, Barranco JM, Brooks EH, Smith JC. Behavioral effects of high strength magnetic fields on rats. J Neurosci. 2003;23:1498–505. doi: 10.1523/JNEUROSCI.23-04-01498.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houpt TA, Pittman DW, Riccardi C, Cassell JA, Lockwood DR, Barranco JM, Kwon BS, Smith JC. Behavioral effects on rats of high strength magnetic fields generated by a resistive electromagnet. Physiol Behav. 2005;86:379–89. doi: 10.1016/j.physbeh.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Houpt TA, Houpt CE. Circular swimming in mice after exposure to a high magnetic field. Physiol Behav. 2010;100:284–90. doi: 10.1016/j.physbeh.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nolte CM, Pittman DW, Kalevitch B, Henderson R, Smith JC. Magnetic field conditioned taste aversion in rats. Physiol Behav. 1998;63:683–688. doi: 10.1016/s0031-9384(97)00526-x. [DOI] [PubMed] [Google Scholar]
- 18.Snyder D, Jahng JW, Smith JC, Houpt TA. c-Fos induction in visceral and vestibular nuclei of the rat brainstem by a 9.4 T magnetic field. NeuroReport. 2000;11:1681–5. doi: 10.1097/00001756-200008210-00015. [DOI] [PubMed] [Google Scholar]
- 19.Cason A, Kwon BS, Smith JC, Houpt TA. c-Fos induction by a 14T magnetic field in visceral and vestibular relays of the female rat brainstem is modulated by estradiol. Brain Res. 2010;1347:48–57. doi: 10.1016/j.brainres.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houpt TA, Cassell JA, Cason AM, Reidell A, Golden GJ, Riccardi C, Smith JC. Evidence for a cephalic site of action of high magnetic fields on the behavioral responses of rats. Physiol Behav. 2007;92:665–74. doi: 10.1016/j.physbeh.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cason AM, Kwon BS, Smith JC, Houpt TA. Labyrinthectomy abolishes the behavioral and neural response of rats to a high-strength static magnetic field. Physiol Behav. 2009;97:36–43. doi: 10.1016/j.physbeh.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 22.Goldberg JM, Cullen KE. Vestibular control of the head: possible functions of the vestibulocollic reflex. Exp Brain Res. 2011;210:331–45. doi: 10.1007/s00221-011-2611-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker J, Goldberg J, Peterson B. Spatial and temporal response properties of the vestibulocollic reflex in decerebrate cats. J Neurophysiol. 1985;54:735–56. doi: 10.1152/jn.1985.54.3.735. [DOI] [PubMed] [Google Scholar]
- 24.Baker JF. Dynamics and directionality of the vestibulo-collic reflex (VCR) in mice. Exp Brain Res. 2005;167:108–13. doi: 10.1007/s00221-005-0031-0. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki JI, Cohen B. Head, eye, body and limb movements from semicircular canal nerves. Exp Neurol. 1964;10:393–405. doi: 10.1016/0014-4886(64)90031-7. [DOI] [PubMed] [Google Scholar]
- 26.Watson SR, Colebatch JG. Vestibulocollic reflexes evoked by short-duration galvanic stimulation in man. J Physiol. 1998;513 ( Pt 2):587–97. doi: 10.1111/j.1469-7793.1998.587bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parker LA. Taste avoidance and taste aversion: evidence for two different processes. Learn Behav. 2003;31:165–172. doi: 10.3758/bf03195979. [DOI] [PubMed] [Google Scholar]
- 28.Cordick N, Parker LA, Ossenkopp KP. Rotation-induced conditioned rejection in the taste reactivity test. NeuroReport. 1999;10:1557–1559. doi: 10.1097/00001756-199905140-00030. [DOI] [PubMed] [Google Scholar]
- 29.Mars F, Vercher JL, Popov K. Dissociation between subjective vertical and subjective body orientation elicited by galvanic vestibular stimulation. Brain Res Bull. 2005;65:77–86. doi: 10.1016/j.brainresbull.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 30.Osanai R, Hayashida T, Yamane M, Futaki T, Murofushi T. The vestibulo-collic reflex induced by external ear canal irrigation. Acta Otolaryngol Suppl. 1991;481:337–8. doi: 10.3109/00016489109131416. [DOI] [PubMed] [Google Scholar]
- 31.Ris L, Capron B, de Waele C, Vidal PP, Godaux E. Dissociations between behavioural recovery and restoration of vestibular activity in the unilabyrinthectomized guinea-pig. J Physiol. 1997;500 ( Pt 2):509–22. doi: 10.1113/jphysiol.1997.sp022037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Houpt TA, Cassell JA, Riccardi C, DenBleyker MD, Hood A, Smith JC. Rats avoid high magnetic fields: dependence on an intact vestibular system. Physiol Behav. 2007;92:741–7. doi: 10.1016/j.physbeh.2007.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Semenov LV, Bures J. Vestibular stimulation disrupts acquisition of place navigation in the Morris water tank task. Behav Neural Biol. 1989;51:346–363. doi: 10.1016/s0163-1047(89)90987-4. [DOI] [PubMed] [Google Scholar]
- 34.Gordon CR, Tal D, Gadoth N, Shupak A. Prolonged optokinetic stimulation generates podokinetic after rotation. Ann N Y Acad Sci. 2003;1004:297–302. doi: 10.1196/annals.1303.027. [DOI] [PubMed] [Google Scholar]
- 35.Weber KD, Fletcher WA, Gordon CR, Melvill Jones G, Block EW. Motor learning in the “podokinetic” system and its role in spatial orientation during locomotion. Exp Brain Res. 1998;120:377–85. doi: 10.1007/s002210050411. [DOI] [PubMed] [Google Scholar]
- 36.Houpt TA, Cassell JA, Riccardi C, Kwon BS, Smith JC. Suppression of drinking by exposure to a high-strength static magnetic field. Physiol Behav. 2007;90:59–65. doi: 10.1016/j.physbeh.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 37.Thomas AW, Drost DJ, Prato FS. Human subjects exposed to a specific pulsed (200 microT) magnetic field: effects on normal standing balance. Neurosci Lett. 2001;297:121–4. doi: 10.1016/s0304-3940(00)01688-8. [DOI] [PubMed] [Google Scholar]
- 38.Prato FS, Thomas AW, Cook CM. Human standing balance is affected by exposure to pulsed ELF magnetic fields: light intensity-dependent effects. NeuroReport. 2001;12:1501–5. doi: 10.1097/00001756-200105250-00040. [DOI] [PubMed] [Google Scholar]
- 39.de Vocht F, Liket L, De Vocht A, Mistry T, Glover P, Gowland P, Kromhout H. Exposure to alternating electromagnetic fields and effects on the visual and visuomotor systems. Br J Radiol. 2007;80:822–8. doi: 10.1259/bjr/22263979. [DOI] [PubMed] [Google Scholar]
- 40.Houpt TA, Carella L, Gonzalez D, Janowitz J, Mueller A, Mueller K, Neth B, Smith JC. Behavioral effects on rats of motion within a high static magnetic field. Physiol Behav. 2011;102:338–346. doi: 10.1016/j.physbeh.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]