Abstract
To investigate the effects of bisphenol A (BPA) on embryo and uterine factors in embryo implantation, timed pregnant C57BL6 females were treated subcutaneously with 0, 0.025, 0.5, 10, 40, and 100 mg/kg/day BPA from gestation days 0.5 to 3.5. In 100 mg/kg/day BPA-treated females, no implantation sites were detected on day 4.5 but retention of embryos in the oviduct and delayed embryo development were detected on day 3.5. When untreated healthy embryos were transferred to pseudopregnant females treated with 100 mg/kg/day BPA, no implantation sites were detected on day 4.5. In 40 mg/kg/day BPA-treated females, delayed implantation and increased perinatal lethality of their offspring were observed. Implantation seemed normal in the rest BPA-treated groups or the female offspring from 40 mg/kg/day BPA-treated group. These data demonstrate the adverse effects of high doses of BPA on processes critical for embryo implantation: embryo transport, preimplantation embryo development, and establishment of uterine receptivity.
Keywords: Bisphenol A, embryo implantation, embryo transport, preimplantation embryo development, uterine receptivity, progesterone receptor
1. Introduction
Bisphenol A (BPA) is an organic compound with two phenol functional groups. It has been widely used as a monomer in manufacturing polycarbonate plastics and epoxy resins. BPA can leach from products made with these materials, such as food/liquid containers, medical devices, etc. The general human population can be exposed to BPA mainly via ingestion, inhalation and skin contact at micrograms per kilogram of body weight daily [1–3]. BPA is detectable in the urine (0.4–149 µg/L) and serum (2.84 µg/L) of the general human population [4–6], as well as amniotic fluid, placental tissue, and breast milk [7]. Studies on human populations have correlated higher BPA exposure with disorders such as cardiovascular diseases, diabetes, liver dysfunction, and male sexual dysfunction [1, 2, 8, 9]. Laboratory studies on animals have demonstrated multiple adverse effects of BPA, such as on development, behavior, reproduction, the immune system, and occurrence of cancer [7, 10–13]. However, it may also be argued that low doses of BPA could have adverse effects on human reproductive and developmental health [14, 15].
BPA is classified as an endocrine disruptor with weak estrogenicity [3]. Its estrogenic potency was estimated to be 10,000-fold less than that of 17β-estradiol (E2) [16, 17], which may reflect the affinity of BPA for the classical nuclear estrogen receptors (ERs) [18–20]. However, numerous studies demonstrate that BPA at concentrations that are too low to efficiently activate nuclear ERs also have cellular effects [15]. One mechanism postulated for the low-dose effects of BPA is a nongenomic response, e.g., BPA binding to membrane ERs other than nuclear ERs [3, 21]. Non-classical nuclear receptors such as estrogen-related receptor gamma (ERRγ) may also be involved in the estrogenic effects of BPA [22]. Epigenetic mechanisms, such as DNA methylation of ER target genes, have also been postulated [23].
The reproductive system is a main target of endocrine disruptors. Extensive laboratory studies have revealed multiple adverse effects of BPA on the reproductive system. In the male reproductive system, effects of BPA include decreased sperm motility, impaired spermatogenesis, and decreased fertility of male offspring [24–26]. In the female reproductive system, BPA may target the mammary gland, the ovary, the oviduct, the uterus, and the placenta [22, 27–36]. A recent study demonstrates that CD-1 mice exposed to environmentally relevant BPA levels (subcutaneously via osmotic pumps, 0.025, 0.25, and 25 µg/kg) during the perinatal period (gestation day 8 to postnatal day 16) show decreased reproductive capacity, although the causes of such a decrease have not been determined [37]. Various BPA-induced effects in the uterus have been reported, such as increased uterine wet weight and luminal epithelium height, uterine cell proliferation, and induced expression of genes such as lactoferrin and c-fos [38–41]. In utero BPA exposure (5 mg/kg intraperitoneal injection) can alter DNA methylation of the Hox10 gene [23], which has been implicated in uterine development and decidualization [42].
One important function of the uterus is to accept an embryo for implantation. Embryo implantation is a hormonally controlled process involving synchronized readiness of an embryo and a receptive uterus [42, 43]. It was reported that BPA exposure (10.125 mg/mouse/day, ~400 mg/kg/day) during gestation days 1.5~4.5 (it was expressed as day 1 to day 4 in this referred study when the day that a vaginal plug was detected was defined as day 0) led to fewer implantation sites [44]. However, it is not known whether the fewer number of implantation sites is due to any adverse effects of BPA on the embryos and/or the uterus. The objective of this study was to examine the effects of preimplantation BPA exposure on embryonic and uterine factors critical for embryo implantation in mice.
2. Materials and Methods
2.1. Animal Husbandry
C57BL6 mice were obtained from Jackson Laboratory (Bar Harbor, ME, USA). The mice were housed in polypropylene cages with free access to food (rodent diet 5053, Purina Mills LabDiet) and water on a 12h light/dark cycle (6:00 AM to 6:00 PM) at 23±1°C with 30–50 relative humidity. All methods used in this study were approved by the University of Georgia IACUC Committee (Institutional Animal Care and Use Committee) and conform to National Institutes of Health guidelines and public law.
2.2. Animal treatment and detection of implantation sites
Young virgin females (2–3 months old) were mated naturally with untreated young stud males. The animals were checked each morning and when a vaginal plug was seen, that day was designated as gestation day 0.5. The plugged females were randomly distributed into seven treatment groups with five to fourteen females in each group. A subcutaneous (s.c.) exposure was used in this study in order to do comparisons with two other studies on BPA in embryo implantation, which were either to test the estrogenicity of BPA using a delayed implantation model [17] or to determine the consequence of peri-implantation BPA exposure on embryo implantation [44]. The plugged females were s.c. injected daily (between 9:00 AM and 10:00 AM) with 0, 0.025, 0.5, 10, 40, and 100 mg/kg/day (~ 0, 0.000625, 0.0125, 0.25, 1, 2.5 mg/mouse/day, respectively) of BPA (Sigma-Aldrich, St. Louis, MO, USA); or with 0.01 mg/kg/day E2 (Sigma-Aldrich) in 100 µl sesame oil (Sigma-Aldrich) from gestation days 0.5 to 3.5. The estrogenicity of 0.01 mg/kg/day E2 was assumed to be equivalent to 100 mg/kg/day of BPA based on the estimation that the estrogenic potency of BPA was ~10,000-fold less than that of E2 [16, 17]. Implantation normally initiates at about gestation day 4.0 in mice when the mating night is defined as gestation day 0. At gestation day 4.5 or day 5.5, the mice were anesthetized with isoflurane (Webster Veterinary, Devens, MA, USA) by inhalation and intravenously (i.v.) injected with Evans blue dye (Alfa Aesar, Ward Hill, MA, USA) to visualize the implantation sites as previously described [45]. The number and position of implantation sites were recorded and analyzed. If no implantation sites were detected on day 4.5, the uterine horns were flushed with 1xPBS to determine the presence of embryos and thus the status of pregnancy. Uterine tissues were snap frozen and kept in −80°C for immunohistochemistry.
2.3. Embryo transport and development
Pregnant mice were treated with 0 and 100 mg/kg/day BPA from gestation day 0.5 to day 3.5 as described above. Uteri and oviducts were flushed with PBS to detect the presence of embryos and the stages of embryo development.
2.4. Embryo transfer
Young virgin females (2–3 months old) were superovulated with intraperitoneal (i.p.) injections of 5 IU equine chorionic gonadotropin (Sigma-Aldrich) and 48 hours later with 5 IU human chorionic gonadotropin (Sigma-Aldrich). They were subsequently mated with stud males. Meanwhile, pseudopregnant females were prepared by mating with vasectomized males. The following day was designated as gestation day 0.5 when a vaginal plug was identified. The pseudopregnant females were s.c. injected daily with 0 or 100 mg/kg/day of BPA in 100 µl sesame oil between 9:00 AM and 10:00 AM from gestation day 0.5 to day 3.5. At gestation day 3.5 between 12:00 PM and 1:00 PM, blastocysts were harvested from superovulated females and transferred to the uteri of day 3.5 pseudopregnant females. Resultant implantation sites were detected using blue dye injection at day 4.5. If no implantation sites were detected at day 4.5, the uterine horns were flushed with 1xPBS to determine the presence of transferred blastocysts. Since treatment with 100 mg/kg/day of BPA adversely affected preimplantation embryo development and embryo transport, the reverse embryo transfer (BPA-treated gestation day 3.5 embryos transferred to the uteri of untreated gestation day 3.5 pseudopregnant females) study was not performed.
2.5. Gestation period, litter size, postnatal survival rate, gender ratio, postnatal growth, and embryo implantation in the offspring females
To determine the consequences of delayed implantation in 40 mg/kg/day BPA-treated females, plugged females were treated with 0 or 40 mg/kg/day BPA as described above from gestation day 0.5 to day 3.5. The date of birth was recorded to determine gestation period. At birth (postnatal day 1), the number of pups from each female was counted to determine the litter size. The body weight of each pup was recorded each week until 9 weeks old. The gender ratios were determined on postnatal day 21 (weaning time). The offspring females (8–12 weeks old) were also mated and examined for embryo implantation as previously described [45].
2.6. Immunohistochemistry
To determine the presence and location of progesterone receptor (PR), frozen uterine sections (10 µm) were fixed in 4% paraformaldehyde (EMD Millipore, Darmstadt, Germany) in PBS for 10 minutes at room temperature; washed in PBS; and subjected to antigen retrieval in 0.01M sodium citrate buffer, pH 6.0, for 20 minutes. Endogenous peroxidase was inactivated with 3% H2O2 (Fisher Scientific Co., Fairlawn, NJ, USA). Non-specific staining was blocked using 10% goat serum. Sections were then incubated with primary rabbit-anti-progesterone receptor (PR) antibody (1:200, Dako, Denmark) at 4°C for overnight; washed in PBS and incubated with biotinylated goat anti-rabbit secondary antibody (1:200, Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 30 min at room temperature. PBS washed sections were incubated with ABComplex/HRP (Santa Cruz Biotechnology), washed in PBS, incubated with 3, 3’-diaminobenzidine tetrahydrochloride (DAB, Bio Basic Inc. Ontario, Canada) for 10 minutes, counterstained with hematoxylin (Sigma-Aldrich), and mounted for imaging. The negative control was processed exactly the same way except that the primary antibody was replaced with non-immune rabbit IgG (Santa Cruz Biotechnology).
2.7. Statistical analysis
One-way ANOVA with Dunnett’s-t test was used to compare the number of implantation sites among different groups. Two-tail unequal variance Student’s t- tests were used to compare the gestation periods and litter sizes. Pregnancy rate, implantation rate, rate of mice with embryo retention in the oviduct, rate of mice with delayed embryo development, rate of embryos in delayed developmental stages, and survival rate of pups were initially analyzed by the χ2 test and if a significant difference was observed, a Fisher’s exact test was performed. P≤0.05 was considered significant.
3. Results
3.1. Preimplantation 100 mg/kg/day BPA s.c. treatment inhibited embryo implantation
The BPA exposure regimen designed in this study was focused on the embryo implantation process but not the ovulation and fertilization processes. Since ovulation and fertilization happen during the dark cycle before 5:00 AM on gestation day 0.5 [46], the BPA exposure regimen in this study, which started 9:00 AM~10:00 AM of gestation day 0.5, should not affect the ovulation and fertilization processes.
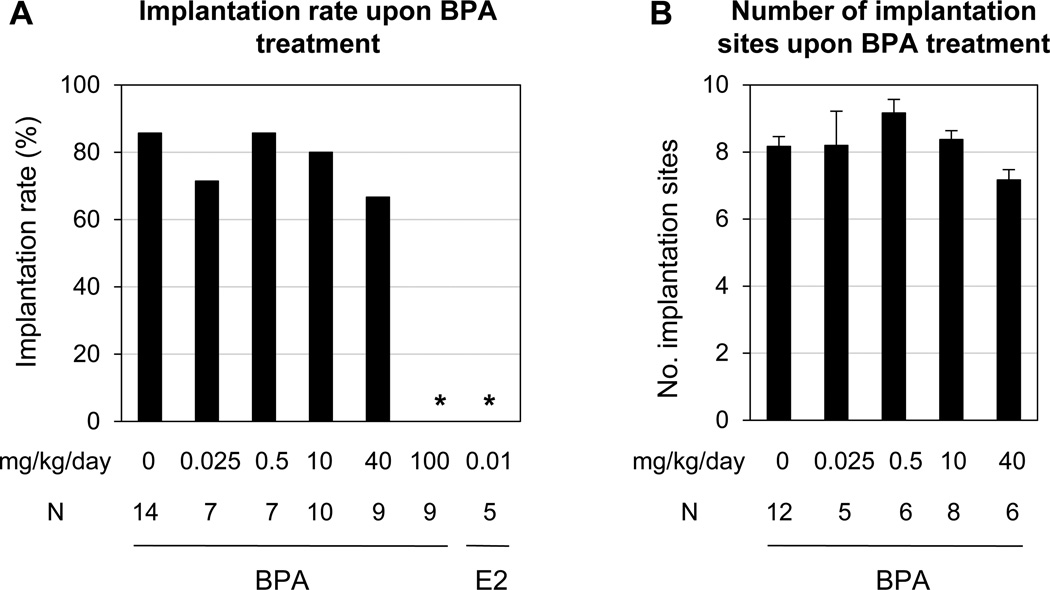
Comparable implantation rates were observed among 0, 0.025, 0.5, 10, and 40 mg/kg/day BPA-treated groups on gestation day 4.5 (Fig. 1A). There was also no significant difference in the numbers of implantation sites among these five groups (Fig. 1B). None of the nine females treated with 100 mg/kg/day BPA or the five females treated with 0.01 mg/kg/day E2 (as a positive control) showed any implantation sites. The implantation rates in these two groups were significantly lower than that in the control group (Fig. 1A).
Figure 1.
Effects of preimplantation bisphenol A (BPA) exposure on embryo implantation detected on gestation day 4.5. A. Implantation rates. They were determined by calculating the ratio of the total number of mice with implantation sites over the total number of plugged mice in each group × 100. N=5–14; * P<0.001 compared to control. E2, 17β-estradiol, was included as a positive control for estrogenicity. B. The average number of implantation sites. The implantation sites were detected as blue bands shown in Figure 3. The number of implantation sites was counted for each mouse and the average was determined for each dose group. Only the mice with implantation sites were counted. N=5–12. Error bars: standard error.
The pregnancy status of the females without implantation sites were determined by flushing their reproductive tract for the presence of embryos. None of these females in the 0, 0.025, 0.5, and 10 mg/kg/day BPA-treated groups had embryos in the uterus, indicating that the mice without implantation sites were not pregnant. One of the three females without implantation sites in the 40 mg/kg/day BPA-treated group had hatched blastocysts in the uterus and the other two had no embryos flushed from the uterus. In the 100 mg/kg/day BPA-treated group, the first five plugged females, whose oviducts were not examined at the time of dissection, had no embryos detected in the uterine flushing. Among the next four plugged females, one had hatched blastocysts in the uterus; two had embryos with delayed development in the oviducts only; and one had unfertilized eggs in the oviducts only, suggesting adverse effects of 100 mg/kg/day BPA on embryo transport and embryo development. All five females treated with 0.01 mg/kg/day E2 had embryos in the oviducts only. The embryos recovered from E2-treated females were all in the blastocyst stage on gestation day 4.5, 67% of them were hatched and the rest 33% were still associated with zona pellucida (data not shown).
3.2. Preimplantation 100 mg/kg/day s.c. BPA treatment delayed embryo transport in reproductive tract
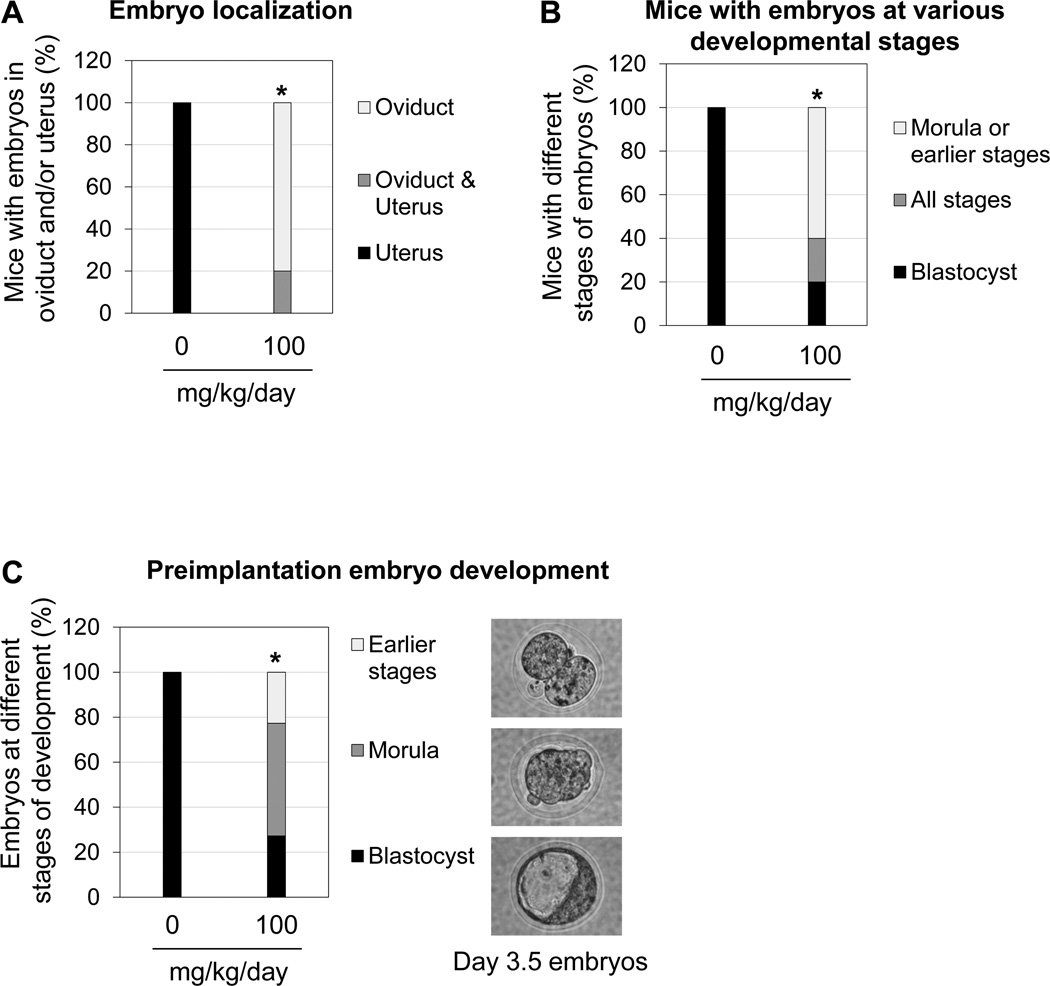
To determine the effect of 100 mg/kg/day BPA on embryo transport, gestation day 3.5 females were examined. There was no significant difference in the pregnancy rate (based on the presence of embryos) between control and 100 mg/kg/day BPA-treated groups. Among the five pregnant females treated with 100 mg/kg/day BPA, four of them had embryos in the oviducts only; one had embryos in both the uterus and the oviducts. However, all the embryos were detected in the uterus on gestation day 3.5 in the eight pregnant control females (Fig. 2A).
Figure 2.
Effects of preimplantation 100 mg/kg/day BPA treatment on embryo transport and embryo development detected on gestation day 3.5. A. Percentage of pregnant females with embryo localization in the uterus and oviduct. * P=0.00031 when the numbers of mice with oviduct retention were compared. B. Percentage of pregnant females with embryos in different developmental stages. * P=0.0023 when the numbers of mice with delayed embryo development (morula and/or earlier stages) were compared. A & B. N=8 in control and N=5 in 100 mg/kg/day BPA-treated group. C. Percentage of embryos in earlier stages than morula, and blastocyst stages. A representative image of embryos at each of these three stages from 100 mg/kg/day BPA-treated group is shown on the right. All the embryos from the control group were in the blastocyst stage. * P=4.01E-09. N= 34 in control and N=22 in 100 mg/kg/day BPA-treated group.
3.3. Preimplantation 100 mg/kg/day s.c. BPA treatment delayed early embryo development
The developmental stages of embryos flushed from gestation day 3.5 uteri and oviducts were determined. Among the five pregnant females treated with 100 mg/kg/day BPA, one had blastocysts only; one had embryos from the two-cell stage to the blastocyst stage; three had embryos in the morula and earlier stages (Fig. 2B). Among all the embryos recovered on gestation day 3.5 from the 100 mg/kg/day BPA-treated group, 27% were in blastocyst stage, 50% were in morula stage, and 23% were in earlier stages than morula (Fig. 2C). All the embryos in the control group were in blastocyst stage (Fig. 2B, 2C). The numbers of embryos recovered from the reproductive tract were comparable between these two groups (Control: 4.25±1.16 (SE); 100 mg/kg/day BPA-treated group: 4.33±0.40 (SE); P=0.95).
3.4. Preimplantation 100 mg/kg/day s.c. BPA treatment adversely affected uterine receptivity
Successful embryo implantation requires synchronized preparation of both an embryo and the uterus. Since 100 mg/kg/day BPA treatment adversely affected embryo transport and development (Fig. 2), an embryo transfer study was performed to differentiate the effect of BPA on the uterus, specifically uterine receptivity for embryo implantation. Four of the five females with successful embryo transfer in the control group had detectable implantation sites on gestation day 4.5. None of the four females with successful embryo transfer in the 100 mg/kg/day BPA-treated group had detectable implantation sites. However, all of them had hatched blastocysts flushed from the transferred uterine horns. The implantation rate in the 100 mg/kg/day BPA-treated group was significantly lower than that in the control group (P=0.04). It demonstrates that preimplantation 100 mg/kg/day BPA treatment causes defective uterine receptivity.
3.5. Preimplantation 40 mg/kg/day s.c. BPA treatment delayed embryo implantation
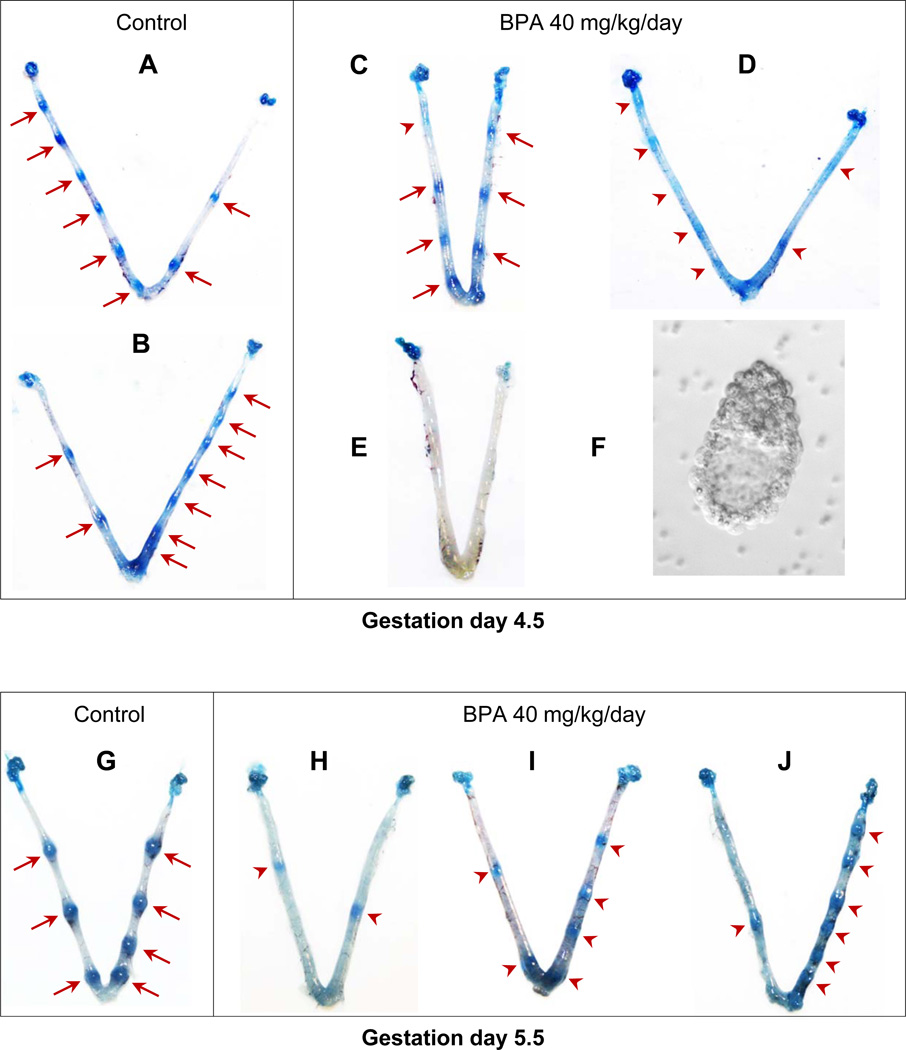
Although there was no significant difference in the number o f implantation sites in 40 mg/kg/day BPA-treated group detected on gestation day 4.5 compared to the control group (Fig. 1B), the appearance of implantation sites in the 40 mg/kg/day BPA-treated group was different from that of the control group. All the implantation sites in the control group were detected as distinct blue bands (Fig. 3A, 3B), but the appearance of implantation sites in the 40 mg/kg/day BPA-treated group varied. Of the seven pregnant mice in the 40 mg/kg/day BPA-treated group, most of the implantation sites from two of them were shown as defined blue bands with some not as defined (red arrow head in Fig. 3C); all the implantation sites from four of the seven pregnant mice were shown as faint with diffused blue bands (Fig. 3D); the 7th female had no detectable implantation sites (Fig. 3E) but hatched blastocysts were flushed from the uterus (Fig. 3F). Varied implantation sites that generally appeared smaller than those in the control group (Fig. 3G) were also observed on gestation day 5.5 in the 40 mg/kg/day BPA-treated group (Fig. 3H~J). These data indicate delayed embryo implantation in the 40 mg/kg/day BPA-treated group.
Figure 3.
Effects of preimplantation 40 mg/k g/day BPA treatment on embryo implantation detected on gestation day 4.5 (A~F) and day 5.5 (G~J). A & B. Two representative uteri from the control group showing defined implantation sites as distinctive blue bands (red arrows). C & D. Two representative uteri with implantation sites from 40 mg/kg/day BPA-treated group. Red arrows in C indicate defined implantation sites. Red arrow heads in C and D indicate the locations of faint blue bands (implantation sites) suggesting delayed implantation. E. A uterus from 40 mg/kg/day BPA-treated group showing no detectable implantation sites (E) but with hatched embryos flushed from the uterus. F. A representative embryo from the uterus in E. G. A representative uterus from gestation day 5.5 control uterus showing implantation sites (red arrows). H~J. Images of three gestation day 5.5 uteri from 40 mg/kg/day BPA-treated mice showing implantation sites (red arrow heads) with variable sizes but at relatively earlier stages (especially those in H and I) compared to those in the control (G).
3.6. Preimplantation 40 and 100 mg/kg/day s.c. BPA treatment altered uterine progesterone receptor (PR) expression
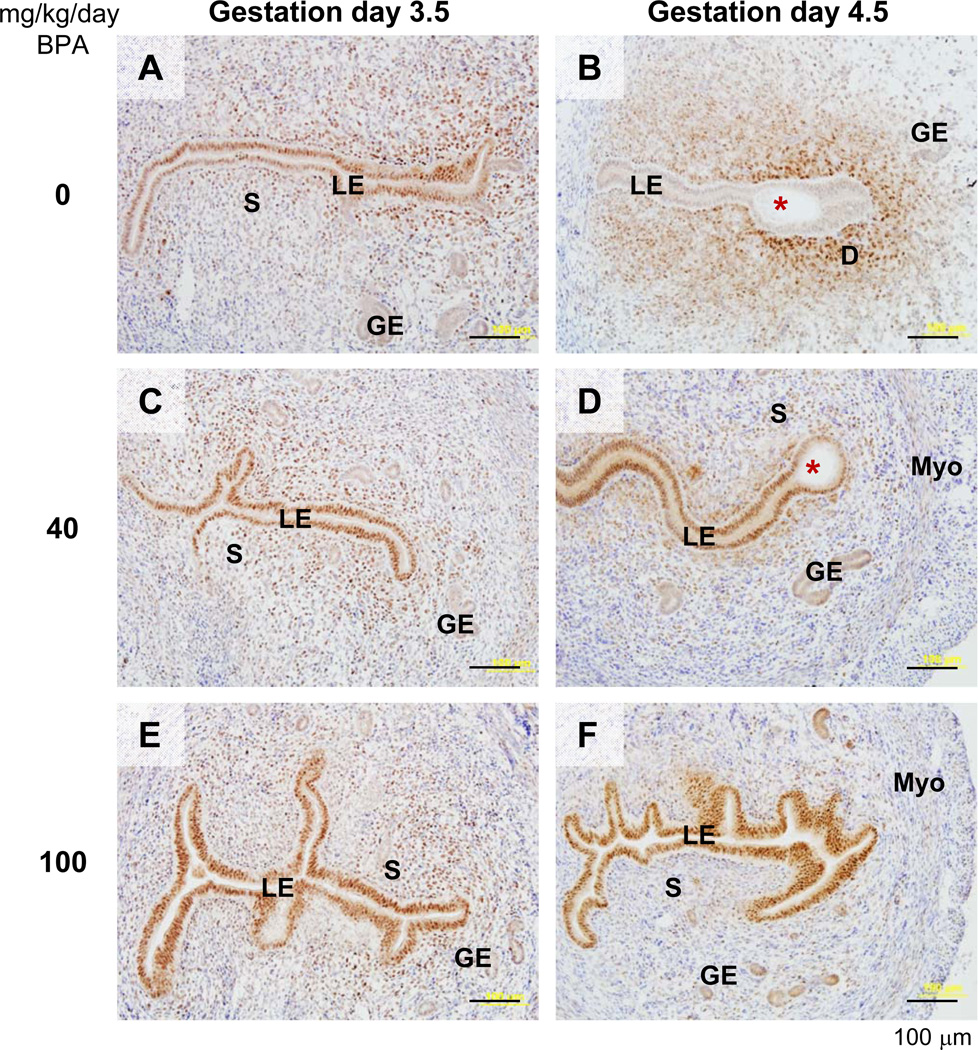
To confirm the implantation defects (delayed and failed implantation in 40 and 100 mg/kg/day BPA-treated groups, respectively) in the gestation day 4.5 uterus, immunohistochemistry was used to examine the expression of a molecular marker, progesterone receptor (PR). PR has dynamic spatiotemporal expression patterns in the peri-implantation uterus. It is highly expressed in the luminal epithelium in the preimplantation uterus and disappears from luminal epithelium after implantation has occurred, when PR is highly expressed in the primary decidual zone [47–49]. On gestation day 3.5, PR was highly expressed in the luminal epithelium in all the studied groups (Fig. 4A, 4C, 4E, and data not shown). On gestation day 4.5, PR had disappeared from the luminal epithelium and staining indicated that PR was in the primary decidual zone surrounding the implanting embryo in 0, 0.025, 0.5, and 10 mg/kg/day BPA-treated groups, in which on-time implantation had taken place (Fig. 4B and data not shown). However, PR remained highly expressed in the luminal epithelium of all examined uteri with faint blue bands and/or no implantation sites in 40 and 100 mg/kg/day BPA-treated groups (Fig. 4D, 4F). Fig. 4D showed that the luminal epithelium surrounding the embryo had become shorter compared to the luminal epithelium in the non-implantation site, but no obvious primary decidual zone had formed yet, indicating an early implantation process [49] that was delayed compared to the control (Fig. 4B).
Figure 4.
Immunohistochemical detection of progesterone receptor (PR) in gestation day 3.5 and day 4.5 uteri upon preimplantation BPA treatment. Uterine cross sections (10 µm) were processed for detecting PR localization (brown staining). A representative section was from each of the following groups: A. Gestation day 3.5, 0 mg/kg/day BPA (control). B. Gestation day 4.5, 0 mg/kg/day BPA (control). C. Gestation day 3.5, 40 mg/kg/day BPA. D. Gestation day 4.5, 40 mg/kg/day BPA. E. Gestation day 3.5, 100 mg/kg/day BPA. F. Gestation day 4.5, 100 mg/kg/day BPA. No specific staining was detected in the negative control (data not shown). Red star, embryo; LE, luminal epithelium; S, stroma; GE, glandular epithelium; D, decidual z one; Myo, myometrium. Scale bar: 100 µm.
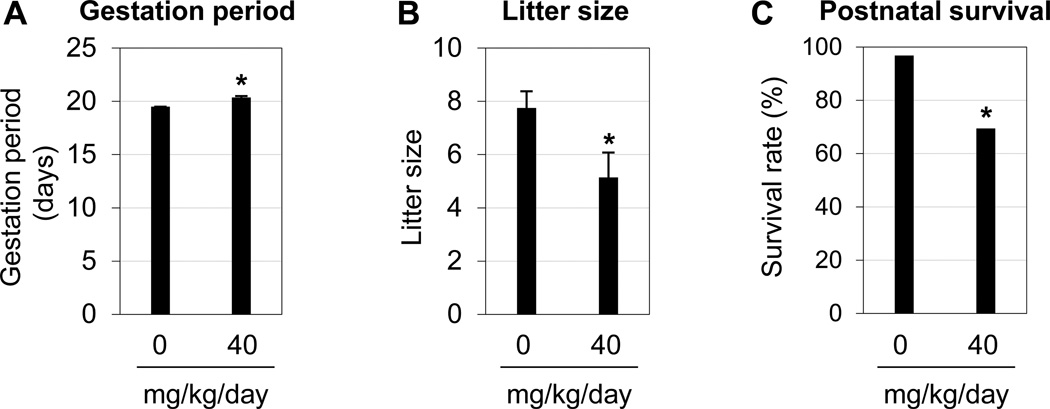
3.7. Consequences from preimplantation 40 mg/kg/day s.c. BPA treatment
To determine potential consequences from delayed implantation in the 40 mg/kg/day s.c. BPA treatment, the following parameters were examined: gestation period, litter size, offspring perinatal survival rate, gender ratio, postnatal growth, and embryo implantation in the offspring females. Significantly increased gestation period (Fig. 5A), reduced litter size (Fig. 5B), and reduced postnatal survival rate (Fig. 5C) were observed in the 40 mg/kg/day BPA-treated group. No significant difference in the gender ratios was observed at weaning time (three weeks old) (data not shown). By 9 weeks old, the offspring from BPA-treated females had bodyweights comparable to the control (data not shown). On-time implantation, normal embryo spacing, and comparable number of implantation sites were detected in the offspring females from control and 40 mg/kg/day BPA-treated groups (data not shown).
Figure 5.
Effects of preimplantation BPA (40 mg/kg/day) exposure on pregnancy outcome. A. Gestation period. *, P=0.00096. All the animals in the control group (0 mg/kg/day BPA) happened to have the same gestation period; the standard error for this group was zero. B. Litter size. *, P=0.046. A & B, N=4–7. Error bars: standard error. C. Postnatal survival rate. *, P=0.0036. N=31 in control and 36 in BPA-treated group.
4. Discussion
This study investigated the effects of preimplantation BPA exposure on the embryo and the uterus, two factors critical for embryo implantation in mice. A study by Berger et al demonstrated that BPA given s.c. (10.125 mg/mouse/day, ~400 mg/kg/day) to CF-1 mice from gestation day 1.5 to day 4.5 significantly reduced the percentage of females with visible implantation sites and the number of implantation sites detected on day 6.5 [44]. In our study, C57BL6 mice were treated with 100 mg/kg/day BPA s.c. from gestation day 0.5 to day 3.5 and no embryo implantation was detected on gestation day 4.5 (Fig. 1). These results indicate that the regimen used in our study was more sensitive than the one used by Berger et al [44]. Two main factors could be involved: different mouse strain sensitivities and/or the treatment regimens. C57BL6 is one of the most sensitive strains to endocrine disruption [50], although the relative sensitivity of CF-1 to endocrine disruptors is unknown. Treatment regimen (timing and duration) could be another important factor. Exposure to endocrine disruptors on gestation day 0.5 could be more sensitive than on gestation days 2.5 and 3.5 in disrupting embryo implantation based on the data from methoxychlor (MXC), another environmental estrogen [51]. In addition, all four injections (day 0.5~day 3.5) in our study were in the preimplantation period, whereas only three injections (day 1.5~day 4.5) in Berger et al [44] fell in the preimplantation period (implantation normally occurs ~gestation day 4.0 in mice).
An abrupt drop of implantation rates as the dose was raised from 40 to 100 mg/kg/day BPA in the treated groups suggests a threshold for toxicity of BPA on the events critical for successful embryo implantation, for example preimplantation embryo development, embryo transport, and uterine receptivity. These events were all adversely affected in the 100 mg/kg/day BPA-treated group (section 3.1~3.4), and each of these adverse effects could lead to failed embryo implantation.
BPA treatment affects preimplantation embryos both in vitro and in vivo. In vitro studies have demonstrated that exposure of two-cell embryos to 100 µM BPA in culture for 48 hours significantly increased the degeneration of preimplantation embryos, whereas more of those exposed to 1 nM BPA reached the blastocyst stage [52]. Our results indicate that preimplantation BPA exposure at 100 mg/kg/day adversely affected preimplantation embryo development and embryo transport (Fig. 2). The tube-locking effect of BPA on embryo transport may reflect the estrogenic effect of BPA [18, 19].
Although BPA at high doses can affect embryo implantation (Fig. 1) [44] and BPA can have various effects on the uterus [38–41], the effects of BPA on uterine receptivity, which is also indispensable for successful embryo implantation, was unknown. Preimplantation treatment of 100 mg/kg/day BPA is detrimental not only to the embryos (Sections 3.2 & 3.3) but also to the establishment of uterine receptivity, which was demonstrated by the embryo transfer study. Since the blastocysts were transferred to the BPA-treated pseudopregnant mice, which received the last dose only three hours prior to embryo transfer, the transferred blastocysts were exposed to residual BPA. It is possible that failed implantation in the embryo transfer study might be the result of blastocyst exposure to BPA. However, two lines of evidence would argue against this possibility. First, hatched blastocysts (data not shown) were flushed from the BPA-treated uteri; second, when BPA was tested for its estrogenicity in a delayed implantation rat model, up to 200 mg/kg BPA single s.c. injection on the day before induction of implantation (equivalent to gestation day 3.5 in this study) could induce embryo implantation [17], indicating that exposure to BPA (up to 200 mg/kg) right before implantation did not harm the ability of the blastocysts to implant in rats.
The progesterone receptor (PR) contributes to embryo implantation [53], and it has dynamic spatiotemporal expression patterns in the uterus during peri-implantation [47, 48] (Fig. 4). Loss of PR in uterine epithelium is associated with the establishment of uterine receptivity in all mammals examined [54, 55], while sustained PR expression in the uterine epithelium during the expected “implantation window” has been associated with defective uterine receptivity in both the human and the mouse [56–58]. Our hourly time-course study demonstrates that PR disappears from uterine luminal epithelium several hours after implantation sites become detectable by blue dye reaction and right before decidualization occurs [49]. Therefore, PR expression in the uterine luminal epithelium can be used as a sensitive temporal marker in the early implantation process. However, if embryo implantation has proceeded to the decidualization stage, PR is no longer a good molecular marker for detecting delayed implantation. Based on immunohistochemistry, the sustained expression of PR in the luminal epithelium of gestation day 4.5 uteri exposed to 40 and 100 mg/kg/day BPA confirmed delayed implantation (Fig. 4D) and failed implantation (Fig. 4F), respectively.
Preimplantation BPA exposure affects not only embryo implantation processes but also post-implantation processes, such as increased post-implantational death, which was suggested by reduced litter size (Fig. 5B), and increased postnatal death (Fig. 5C) from 40 mg/kg/day BPA-treated group. The female offspring had normal embryo implantation, indicating that the effect of preimplantation exposure to 40 mg/kg/day BPA on embryo implantation was not manifested in the next generation.
The focus of this study was to differentiate the effects of preimplantation BPA exposure on embryo and on uterine receptivity. Both of these two aspects are critical for successful embryo implantation, for which the molecular mechanism is still not fully understood. BPA at high doses was used as a pharmacologic agent to study its effects on embryo implantation. Based on the data from this study, many aspects, which are not the focus of the current study but future directions, can be potentially addressed. For example, what specific genes are differentially regulated in the uterus by BPA that could be associated with the adverse effect of BPA on uterine receptivity, thus embryo implantation, and how these genes are differentially regulated by BPA in the uterus. Such information will provide more insight into the molecular mechanism of the establishment of uterine receptivity. In addition, such information can also be used to assess any potential effects of long-term exposure to environmentally relevant BPA levels on genes critical for the establishment of uterine receptivity, thereby helping with risk assessment.
5. Conclusions
Preimplantation exposure to 100 mg/kg/day s.c. BPA adversely affects embryo transport, preimplantation embryo development, and uterine receptivity, leading to failed embryo implantation detected on gestation day 4.5. Preimplantation exposure to 40 mg/kg/day s.c. BPA delays embryo implantation, resulting in an increased gestation period, increased post-implantational and postnatal death, and reduced litter size. These BPA treatments alter progesterone receptor expression patterns in gestation day 4.5 uteri that correlate with the defective embryo implantation. Preimplantation exposure of BPA at environmentally relevant doses does not appear to have an adverse effect on embryo implantation.
Research highlights.
Preimplantation exposure to 100 mg/kg/day s.c. BPA adversely affects embryo transport, preimplantation embryo development, and uterine receptivity, leading to failed embryo implantation detected on gestation day 4.5.
Preimplantation exposure to 40 mg/kg/day s.c. BPA delays embryo implantation, resulting in an increased gestation period, increased post-implantational and postnatal death, and reduced litter size.
Preimplantation exposure to 40 and 100 mg/kg/day s.c. BPA alters progesterone receptor expression patterns in gestation day 4.5 uteri that correlate with the defective embryo implantation.
Preimplantation exposure of BPA at environmentally relevant doses does not have an adverse effect on embryo implantation.
Acknowledgments
The authors thank Dr. Zhen Fu at Department of Pathology, University of Georgia for the access to the imaging system; Dr. Michael K. Skinner at Washington State University and Dr. John J. Wagner at University of Georgia for insightful comments on the discussion; Ms. Kali King at Department of Physiology & Pharmacology, University of Georgia for English editing; the Office of the Vice President for Research, Interdisciplinary Toxicology Program, and Department of Physiology & Pharmacology at University of Georgia, and National Institute of Health (NIH R15HD066301 to X.Y.) for financial support.
Role of the funding sources: The experimental design, data collection, analysis and interpretation for this study and the writing of this report were supported by the Office of the Vice President for Research, Interdisciplinary Toxicology Program, and Department of Physiology & Pharmacology at University of Georgia; and NIH R15HD066301.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest statement: The authors declare that there are no conflicts of interest.
Contributor Information
Shuo Xiao, Email: shuoxiao@uga.edu.
Honglu Diao, Email: hldiao@uga.edu.
Mary Alice Smith, Email: masmith@uga.edu.
Xiao Song, Email: xsong@uga.edu.
Xiaoqin Ye, Email: ye@uga.edu.
References
- 1.Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, et al. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300:1303–1310. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- 2.Ranjit N, Siefert K, Padmanabhan V. Bisphenol-A and disparities in birth outcomes: a review and directions for future research. J Perinatol. 2010;30:2–9. doi: 10.1038/jp.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takeuchi T, Tsutsumi O. Serum bisphenol a concentrations showed gender differences, possibly linked to androgen levels. Biochem Biophys Res Commun. 2002;291:76–78. doi: 10.1006/bbrc.2002.6407. [DOI] [PubMed] [Google Scholar]
- 6.He Y, Miao M, Herrinton LJ, Wu C, Yuan W, Zhou Z, et al. Bisphenol A levels in blood and urine in a Chinese population and the personal factors affecting the levels. Environ Res. 2009;109:629–633. doi: 10.1016/j.envres.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Palanza P, Gioiosa L, vom Saal FS, Parmigiani S. Effects of developmental exposure to bisphenol A on brain and behavior in mice. Environ Res. 2008;108:150–157. doi: 10.1016/j.envres.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 8.Li D, Zhou Z, Qing D, He Y, Wu T, Miao M, et al. Occupational exposure to bisphenol-A (BPA) and the risk of self-reported male sexual dysfunction. Hum Reprod. 2010;25:519–527. doi: 10.1093/humrep/dep381. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Jonathan N, Hugo ER, Brandebourg TD. Effects of bisphenol A on adipokine release from human adipose tissue: Implications for the metabolic syndrome. Mol Cell Endocrinol. 2009;304:49–54. doi: 10.1016/j.mce.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubin BS, Soto AM. Bisphenol A: Perinatal exposure and body weight. Mol Cell Endocrinol. 2009;304:55–62. doi: 10.1016/j.mce.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prins GS, Tang WY, Belmonte J, Ho SM. Perinatal exposure to oestradiol and bisphenol A alters the prostate epigenome and increases susceptibility to carcinogenesis. Basic Clin Pharmacol Toxicol. 2008;102:134–138. doi: 10.1111/j.1742-7843.2007.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Susiarjo M, Hunt P. Bisphenol A exposure disrupts egg development in the mouse. Fertil Steril. 2008;89:e97. doi: 10.1016/j.fertnstert.2008.01.060. [DOI] [PubMed] [Google Scholar]
- 13.Holladay SD, Xiao S, Diao H, Barber J, Nagy T, Ye X, et al. Perinatal bisphenol A exposure in C57B6/129svj male mice: potential altered cytokine/chemokine production in adulthood. Int J Environ Res Public Health. 2010;7:2845–2852. doi: 10.3390/ijerph7072845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodman JE, Witorsch RJ, McConnell EE, Sipes IG, Slayton TM, Yu CJ, et al. Weight-of-evidence evaluation of reproductive and developmental effects of low doses of bisphenol A. Crit Rev Toxicol. 2009;39:1–75. doi: 10.3109/10408440903279946. [DOI] [PubMed] [Google Scholar]
- 15.Sekizawa J. Low-dose effects of bisphenol A: a serious threat to human health? Toxicol Sci. 2008;33:389–403. doi: 10.2131/jts.33.389. [DOI] [PubMed] [Google Scholar]
- 16.Milligan SR, Balasubramanian AV, Kalita JC. Relative potency of xenobiotic estrogens in an acute in vivo mammalian assay. Environ Health Perspect. 1998;106:23–26. doi: 10.1289/ehp.9810623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cummings AM, Laws SC. Assessment of estrogenicity by using the delayed implanting rat model and examples. Reprod Toxicol. 2000;14:111–117. doi: 10.1016/s0890-6238(00)00062-9. [DOI] [PubMed] [Google Scholar]
- 18.Fang H, Tong W, Perkins R, Soto AM, Prechtl NV, Sheehan DM. Quantitative comparisons of in vitro assays for estrogenic activities. Environ Health Perspect. 2000;108:723–729. doi: 10.1289/ehp.00108723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersen HR, Andersson AM, Arnold SF, Autrup H, Barfoed M, Beresford NA, et al. Comparison of short-term estrogenicity tests for identification of hormone-disrupting chemicals. Environ Health Perspect. 1999;107 Suppl 1:89–108. doi: 10.1289/ehp.99107s189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hewitt SC, Korach KS. Estrogenic activity of bisphenol A and 2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane (HPTE) demonstrated in mouse uterine gene profiles. Environ Health Perspect. 2011;119:63–70. doi: 10.1289/ehp.1002347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147:S56–S69. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- 22.Takeda Y, Liu X, Sumiyoshi M, Matsushima A, Shimohigashi M, Shimohigashi Y. Placenta expressing the greatest quantity of bisphenol A receptor ERR{gamma} among the human reproductive tissues: Predominant expression of type-1 ERRgamma isoform. J Biochem. 2009;146:113–122. doi: 10.1093/jb/mvp049. [DOI] [PubMed] [Google Scholar]
- 23.Bromer JG, Zhou Y, Taylor MB, Doherty L, Taylor HS. Bisphenol-A exposure in utero leads to epigenetic alterations in the developmental programming of uterine estrogen response. FASEB J. 2010;24:2273–2280. doi: 10.1096/fj.09-140533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salian S, Doshi T, Vanage G. Perinatal exposure of rats to Bisphenol A affects the fertility of male offspring. Life Sci. 2009;85:742–752. doi: 10.1016/j.lfs.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Aikawa H, Koyama S, Matsuda M, Nakahashi K, Akazome Y, Mori T. Relief effect of vitamin A on the decreased motility of sperm and the increased incidence of malformed sperm in mice exposed neonatally to bisphenol A. Cell Tissue Res. 2004;315:119–124. doi: 10.1007/s00441-003-0806-1. [DOI] [PubMed] [Google Scholar]
- 26.Toyama Y, Yuasa S. Effects of neonatal administration of 17beta-estradiol, beta-estradiol 3-benzoate, or bisphenol A on mouse and rat spermatogenesis. Reprod Toxicol. 2004;19:181–188. doi: 10.1016/j.reprotox.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Newbold RR, Jefferson WN, Padilla-Banks E. Prenatal exposure to bisphenol environmentally relevant doses adversely affects the murine female reproductive tract later in life. Environ Health Perspect. 2009;117:879–885. doi: 10.1289/ehp.0800045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adewale HB, Jefferson WN, Newbold RR, Patisaul HB. Neonatal bisphenol-a exposure alters rat reproductive development and ovarian morphology without impairing activation of gonadotropin-releasing hormone neurons. Biol Reprod. 2009;81:690–699. doi: 10.1095/biolreprod.109.078261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dominguez MA, Petre MA, Neal MS, Foster WG. Bisphenol A concentration-dependently increases human granulosa-lutein cell matrix metalloproteinase-9 (MMP-9) enzyme output. Reprod Toxicol. 2008;25:420–425. doi: 10.1016/j.reprotox.2008.05.059. [DOI] [PubMed] [Google Scholar]
- 30.Susiarjo M, Hassold TJ, Freeman E, Hunt PA. Bisphenol A exposure in utero disrupts early oogenesis in the mouse. PLoS Genet. 2007;3:e5. doi: 10.1371/journal.pgen.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikaido Y, Yoshizawa K, Danbara N, Tsujita-Kyutoku M, Yuri T, Uehara N, et al. Effects of maternal xenoestrogen exposure on development of the reproductive tract and mammary gland in female CD-1 mouse offspring. Reprod Toxicol. 2004;18:803–811. doi: 10.1016/j.reprotox.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Markey CM, Luque EH, Munoz De Toro M, Sonnenschein C, Soto AM. In utero exposure to bisphenol A alters the development and tissue organization of the mouse mammary gland. Biol Reprod. 2001;65:1215–1223. doi: 10.1093/biolreprod/65.4.1215. [DOI] [PubMed] [Google Scholar]
- 33.Colerangle JB, Roy D. Profound effects of the weak e nvironmental estrogen-like chemical bisphenol A on the growth of the mammary gland of Noble rats. J Steroid Biochem Mol Biol. 1997;60:153–160. doi: 10.1016/s0960-0760(96)00130-6. [DOI] [PubMed] [Google Scholar]
- 34.Benachour N, Aris A. Toxic effects of low doses of Bisphenol-A on human placental cells. Toxicol Appl Pharmacol. 2009;241:322–328. doi: 10.1016/j.taap.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Schonfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect. 2002;110:A703–A707. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imanishi S, Manabe N, Nishizawa H, Morita M, Sugimoto M, Iwahori M, et al. Effects of oral exposure of bisphenol A on mRNA expression of nuclear receptors in murine placentae assessed by DNA microarray. J Reprod Dev. 2003;49:329–336. doi: 10.1262/jrd.49.329. [DOI] [PubMed] [Google Scholar]
- 37.Cabaton NJ, Wadia PR, Rubin BS, Zalko D, Schaeberle CM, Askenase MH, et al. Perinatal Exposure to Environmentally Relevant Levels of Bisphenol A Decreases Fertility and Fecundity in CD-1 Mice. Environ Health Perspect. 2011;119:547–552. doi: 10.1289/ehp.1002559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Markey CM, Michaelson CL, Veson EC, Sonnenschein C, Soto AM. The mouse uterotrophic assay: a reevaluation of its validity in assessing the estrogenicity of bisphenol A. Environ Health Perspect. 2001;109:55–60. doi: 10.1289/ehp.0110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steinmetz R, Mitchner NA, Grant A, Allen DL, Bigsby RM, Ben-Jonathan N. The xenoestrogen bisphenol A induces growth, differentiation, and c-fos gene expression in the female reproductive tract. Endocrinology. 1998;139:2741–2747. doi: 10.1210/endo.139.6.6027. [DOI] [PubMed] [Google Scholar]
- 40.Ashby J, Tinwell H. Uterotrophic activity of bisphenol A in the immature rat. Environ Health Perspect. 1998;106:719–720. doi: 10.1289/ehp.98106719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamasaki K, Sawaki M, Takatsuki M. Immature rat uterotrophic assay of bisphenol A. Environ Health Perspect. 2000;108:1147–1150. doi: 10.1289/ehp.001081147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7:185–199. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
- 43.Paria BC, Reese J, Das SK, Dey SK. Deciphering the cross-talk of implantation: advances and challenges. Science. 2002;296:2185–2188. doi: 10.1126/science.1071601. [DOI] [PubMed] [Google Scholar]
- 44.Berger RG, Hancock T, deCatanzaro D. Influence of oral and subcutaneous bisphenol-A on intrauterine implantation of fertilized ova in inseminated female mice. Reprod Toxicol. 2007;23:138–144. doi: 10.1016/j.reprotox.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 45.Ye X, Hama K, Contos JJ, Anliker B, Inoue A, Skinner MK, et al. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature. 2005;435:104–108. doi: 10.1038/nature03505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagy A, Gertsenstein M, Vintersten K, Behringer R. Manipulating the Mouse Embryo: A Laboratory Manual. Third Edition. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2003. pp. 48–55. [Google Scholar]
- 47.Tan J, Paria BC, Dey SK, Das SK. Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology. 1999;140:5310–5321. doi: 10.1210/endo.140.11.7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheon YP, Li Q, Xu X, DeMayo FJ, Bagchi IC, Bagchi MK. A genomic approach to identify novel progesterone receptor regulated pathways in the uterus during implantation. Mol Endocrinol. 2002;16:2853–2871. doi: 10.1210/me.2002-0270. [DOI] [PubMed] [Google Scholar]
- 49.Diao H, Paria BC, Xiao S, Ye X. Temporal expression pattern of progesterone receptor in the uterine luminal epithelium suggests its requirement during early events of implantation. Fertil Steril. 2011;95:2087–2093. doi: 10.1016/j.fertnstert.2011.01.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spearow JL, Doemeny P, Sera R, Leffler R, Barkley M. Genetic variation in susceptibility to endocrine disruption by estrogen in mice. Science. 1999;285:1259–1261. doi: 10.1126/science.285.5431.1259. [DOI] [PubMed] [Google Scholar]
- 51.Hall DL, Payne LA, Putnam JM, Huet-Hudson YM. Effect of methoxychlor on implantation and embryo development in the mouse. Reprod Toxicol. 1997;11:703–708. doi: 10.1016/s0890-6238(97)00026-9. [DOI] [PubMed] [Google Scholar]
- 52.Takai Y, Tsutsumi O, Ikezuki Y, Kamei Y, Osuga Y, Yano T, et al. Preimplantation exposure to bisphenol A advances postnatal development. Reprod Toxicol. 2001;15:71–74. doi: 10.1016/s0890-6238(00)00119-2. [DOI] [PubMed] [Google Scholar]
- 53.Mulac-Jericevic B, Conneely OM. Reproductive tissue selective actions of progesterone receptors. Reproduction. 2004;128:139–146. doi: 10.1530/rep.1.00189. [DOI] [PubMed] [Google Scholar]
- 54.Bazer FW, Slayden OD. Progesterone-induced gene expression in uterine epithelia: a myth perpetuated by conventional wisdom. Biol Reprod. 2008;79:1008–1009. doi: 10.1095/biolreprod.108.072702. [DOI] [PubMed] [Google Scholar]
- 55.Bazer FW, Wu G, Spencer TE, Johnson GA, Burghardt RC, Bayless K. Novel pathways for implantation and establishment and maintenance of pregnancy in mammals. Mol Hum Reprod. 2010;16:135–152. doi: 10.1093/molehr/gap095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palomino WA, Fuentes A, Gonzalez RR, Gabler F, Boric MA, Vega M, et al. Differential expression of endometrial integrins and progesterone receptor during the window of implantation in normo-ovulatory women treated with clomiphene citrate. Fertil Steril. 2005;83:587–593. doi: 10.1016/j.fertnstert.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 57.Lessey BA, Killam AP, Metzger DA, Haney AF, Greene GL, McCarty KS., Jr Immunohistochemical analysis of human uterine estrogen and progesterone receptors throughout the menstrual cycle. J Clin Endocrinol Metab. 1988;67:334–340. doi: 10.1210/jcem-67-2-334. [DOI] [PubMed] [Google Scholar]
- 58.Wakitani S, Hondo E, Phichitraslip T, Stewart CL, Kiso Y. Upregulation of Indian hedgehog gene in the uterine epithelium by leukemia inhibitory factor during mouse implantation. J Reprod Dev. 2008;54:113–116. doi: 10.1262/jrd.19120. [DOI] [PubMed] [Google Scholar]