SUMMARY
1. The endogenous production of and/or the bioavailability of nitric oxide (NO) is decreased in pulmonary hypertensive diseases. L-arginine (L-arg) is the substrate for NO synthase (NOS). L-arg is transported into cells via the cationic amino acid transporters (CAT), of which there are two isoforms in endothelial cells, CAT-1 and CAT-2.
2. To test the hypothesis that hypoxia will decrease CAT expression and L-arg uptake resulting in decreased NO production in human pulmonary microvascular endothelial cells (hPMVEC), cells were incubated in either normoxia (21% O2, 5% CO2, balance N2) or hypoxia (1% O2, 5% CO2, balance N2).
3. The hPMVEC incubated in hypoxia had 80% less NO production than cells incubated in normoxia (p<0.01). The hPMVEC incubated in hypoxia had significantly lower CAT-2 mRNA levels than normoxic hPMVEC (p<0.005), and the transport of L-arg was 40% lower in hypoxic than in normoxic hPMVEC (p<0.01). In hypoxic cells, overexpression of CAT-1 resulted in significantly greater L-arg transport and NO production (p<0.05).
4. These results demonstrate that in hPMVEC, hypoxia decreased CAT-2 expression, L-arg uptake and NO production. Furthermore, the hypoxia-induced decrease in NO production in hPMVEC can be attenuated by overexpressing CAT in these cells. We speculate that the CAT may represent a novel therapeutic target for treating pulmonary hypertensive disorders.
Keywords: Nitric Oxide Synthase, Hypoxia, L-arginine, Pulmonary Hypertension
INTRODUCTION
Nitric oxide (NO) is a potent pulmonary vasodilator. NO is produced from L-arginine (L-arg) by a family of enzymes called the NO synthases (NOS), with L-citrulline as a co-product. The majority of NO made in non-inflamed endothelial cells is from endothelial NOS (eNOS). In pulmonary hypertensive diseases it has been found that endogenous NO production in the lung is decreased (1,2,3). Persistent pulmonary hypertension of the newborn (PPHN) is a common cause of need for neonatal intensive care. PPHN is usually a self-limited disease that is often associated with acute hypoxia (2), furthermore pulmonary hypertension associated with acute hypoxia can be seen in some adult lung diseases such as high-altitude pulmonary edema (HAPE) (4). In patients with PPHN it has been shown that NO production is decreased during the acute phase of the disease and then normalizes once the disease has run its course, usually in 1–2 weeks (5,6). The exact mechanisms resulting in lower NO production are unclear and probably very complex, given that there are a myriad of control mechanisms for NO production by eNOS, including transcriptional regulation, post-translational modifications, protein-protein interactions, eNOS regulatory phosphorylations, etc. (7).
In the lung NO production can be augmented in some circumstances by increasing the extracellular concentration of L-arg (8,9), despite the fact that the Km for L-arg for NOS is relatively low compared to the usual intracellular concentrations of L-arg, a finding that has been termed the “L-arginine paradox” (10). Thus, lower uptake of extracellular L-arg may be another mechanism that accounts for the decrease in NO production during hypoxia. Extracellular L-arg is predominantly taken up by pulmonary endothelial cells via a family of proteins called the cationic amino acid transporters (CAT). CAT-1 and CAT-2 are expressed in endothelial cells and are encoded by genes of the solute carrier family, SLC7A1 and SLC7A2, respectively (11). We hypothesized that hypoxia would decrease CAT expression and L-arg uptake contributing to the hypoxia-induced decrease in NO production. We further hypothesized that over-expressing CAT-1 using an adenoviral vector would augment NO production in pulmonary endothelial cells by increasing L-arg uptake. We utilized cultured human pulmonary microvascular endothelial cells (hPMVEC) to investigate the effects of hypoxia on CAT expression and function, and the role of CAT in NO production. A hypoxia model consisting of exposing “normal” human pulmonary endothelial cells was utilized to mimic the hypoxic exposure seen in some lung diseases such as PPHN and HAPE. We also utilized adenoviral vectors containing either the human CAT-1 gene or the human iNOS gene to further manipulate L-arginine uptake and/or NO production in hPMVEC.
METHODS
Cell Culture
Human pulmonary microvascular endothelial cells (hPMVEC) were cultured as previously described (12). Briefly, hPMVEC were purchased from Lonza (Allendale, NJ) and cultured in endothelial cell basal medium (EBM2; Lonza) supplemented with an EGM-2 bullet kit (Lonza) in T75 flasks. The hPMVEC were incubated at 37°C in 5% CO2, balance air and used between passages 3 – 8. On the day of study, hPMVEC were washed three times with 4 ml of HEPES Balanced Salt Solution (HBSS; Lonza). Five ml of EGM was placed on the hPMVEC and the plate was returned to an incubator at 37°C in either the usual CO2 incubator, i.e. 5% CO2, balance air (for purposes of this study referred to as normoxia), or into a three-gas incubator, i.e. 5% CO2, 1% O2, balance N2 (for purposes of this study referred to as hypoxia) for 24 hours. At the end of the experimental period the media was harvested and frozen in 1 ml aliquots at −80°C. For the transfection experiments, hPMVEC were cultured on 6 well plates.
Protein Isolation
Protein was isolated from the hPMVEC as previously described (12,13). Briefly, hPMVEC were washed twice with PBS and lysed in ice-cold lysis buffer (0.2M NaOH, 0.2% SDS with the following added to each ml 30 minutes before use: 2 μg aprotinin, 5 μg leupeptin, 0.7 μg pepstatin A, and 174 μg phenylmethylsulfonyl fluoride). The lysates were clarified by centrifugation at 12,000 g for 10 minutes at 4°C. The supernatants were collected and total protein concentration was determined by the Bradford method (BioRad, Hercules, CA).
RNA isolation
RNA was isolated from hPMVEC as previously described (13,14). Briefly, Trizol (Life Technologies, Carlsbad, CA) was added to the cells and incubated for 5 minutes at room temperature. Chloroform(0.2 ml) was added, and the tubes were shaken for 15 seconds and then incubated at room temperature for 3 minutes. The mixture was centrifuged at 12,000 g for 15 min at 4°C. The supernatant was transferred to a fresh tube. Isopropyl alcohol (0.5 ml) was added, and the mixture was incubated at room temperature for 10 min and then centrifuged at 12,000 g for 15 minutes at 4°C. The supernatant was discarded, and the pellet was washed with 75% ethanol and centrifuged at 7,500 g for 5 minutes at 4°C. The supernatant was discarded, and the pellet was partially dried, dissolved in RNase-free water, and stored at −80°C.
Real-time PCR
The measurement of CAT-1 and CAT-2 mRNA levels were performed using real-time PCR and SYBR Green as a detector (SA Biosciences, Frederick, MD) as previously described (13). All of the assays were performed on a Bio-Rad DNA Engine Peltier Thermal Cycler under standard thermal cycling parameters: 95°C for 10 minutes, 40 cycles of 95°C for 15 seconds and 60°C for 60 seconds. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the control gene for normalization and mRNA expression level was quantified using the threshold cycle method.
Western blots
Cell lysates from hPMVEC were assayed for eNOS protein as previously described (13,15,16). Samples (15–20 μg of protein) were denatured with Laemmli buffer, heated to 95°C for 5 minutes, and electrophoresed on 4–12% polyacrylamide gel in the presence of SDS. Separated proteins were electrotransferred to PVDF membranes, incubated with 5% nonfat milk (Bio-Rad) for 1 hour, and then incubated overnight with anti-eNOS (BD Biosciences, San Leandro, CA) antibodies. The membranes were then washed with of 0.1% Tween 20, 20 mM Tris · HCl, pH 7.5, and 150 mM NaCl (TBST) three times for 10 minutes. Secondary goat anti-mouse IgG conjugated to alkaline phosphatase (1:10,000; Bio-Rad) was diluted in TBST plus 5% nonfat milk and incubated with the membranes at room temperature for 1 hour. The bands of interest were visualized with enhanced chemiluminescence (Amersham, Piscataway, NJ) and quantified by densitometry (Sigma Gel, Jandel Scientific, San Rafael, CA). To control for protein loading, the blots were stripped using a stripping buffer and reprobed for β-actin (1:10,000; Abcam, Cambridge, MA).
Adenoviral transfection
Adenoviral transfections were done as previously described (16). Adenovirus, serotype 5, containing either the cDNA for Escherichia coli β-galactosidase and a CMV promoter (Adβ-gal), the cDNA for human iNOS and a CMV promoter (AdiNOS) or the cDNA for human CAT-1 and a CMV promoter (AdCAT1) were derived and prepared as previously described (17). The cDNA in the AdCAT1 vectors also contained the gene for eGFP. Five groups of hPMVEC were prepared in the following manner: hPMVEC were transfected with 1) AdCAT1, 2) AdiNOS, 3) AdCAT1+AdiNOS, 4) Adβ-gal as a control for nonspecific gene transfer effects, and/or 5) vehicle (media containing no virus). The amount of virus placed on the hPMVEC was determined by multiplying the number of cells per six well plate by the multiplicity of infection (MOI), divided by the number of plaque-forming units per milliliter of virus. For each six-well plate, the required amount of viral stock was added to the media and the hPMVEC were incubated for 24 hours. The medium was then removed, fresh media added, and the hPMVEC were then exposed to hypoxia or normoxia for an additional 24 or 48 hours. The medium was removed and frozen at −80°C for nitrite analysis. The viable hPMVEC were counted in each well using trypan blue exclusion as previously described (13,16).
L-[3H] arginine uptake
Specific L-[3H]arg (l-[2,3,4-[3H]arginine monohydrochloride; PerkinElmer, Waltham, MA) uptake by normoxia and hypoxia cells was measured as previously described (15,16). After the 24-hour exposure to normoxia or hypoxia, hPMVEC in six-well plates were washed with warm HEPES, and then incubated at room temperature for 2 minutes. Either 1 ml of HEPES containing 1 μCi/ml L-[3H]arg was placed on the cells to measure total L-[3H]arg uptake, or 1 ml of HEPES buffer with 1 μCi/ml L-[3H]arg and 10 mM nonlabeled L-arg was added to measure nonspecific L-[3H]arg uptake. Reactions were stopped by washing the cells four times with ice–cold HEPES buffer. The cells were lysed with 200 μl of lysis buffer containing 0.2M NaOH and 0.2% SDS in each well, and the radioactivity in the cell lysates was assayed by liquid scintillation spectrometry (Packard Instrument Co. Downers Grove, IL). The specific uptake of L-[3H]arg was determined by subtracting the nonspecific L-[3H]arg uptake from the total L-[3H]arg uptake. The kinetics of L-arg uptake were defined by measuring uptake of L-arg over a range of L-arg concentrations. The hPMVEC were incubated with 1 μCi/ml L-[3H]arg and 10 – 3,000 μM unlabeled (cold) L-arg for 2 minutes. The apparent Michealis constant (Km) and maximal transport velocity (Vmax) were determined under normoxic and hypoxic conditions by fitting the Michealis-Menton equation to the data (SigmaPlot, Jandel Scientific, Carlsbad, CA).
Immunocytochemistry
For immunocytochemistry, cells were grown on sterile glass coverslips, and fixed at −20°C for 10 minutes. The coverslips were blocked with 10% goat serum for 1 hour at room temperature. Mouse anti-eNOS antibodies (BD, San Leandro, CA) were applied to the cells for 1 hour at room temperature, and then incubated with goat anti-mouse IgG for 45 minutes at room temperature. The coverslips were mounted and stored.
Nitrite assay
The medium was assayed in duplicate for nitrite (NO−2) using a chemiluminescence NO analyzer (model 280i; Sievers Instruments, Boulder, CO) as previously described (12,14,15,16). Briefly, 50 μl of sample was placed in a reaction chamber containing a mixture of NaI in glacial acetic acid to reduce NO−2 to NO. The NO gas was carried into the NO analyzer by a constant flow of helium gas. The analyzer was calibrated using a NaNO2 standard curve.
Statistical analysis
Values are expressed as the mean ± SE. When only comparing two groups a t-test was used. When comparing multiple groups, a one-way analysis of variance (ANOVA) was used, and significant differences were identified using a Tukey post-hoc test. All statistical analysis was done using SigmaStat (Jandel Scientific, Carlsbad, CA). Differences were considered significant when p<0.05.
RESULTS
Hypoxia decreased NO production in hPMVEC
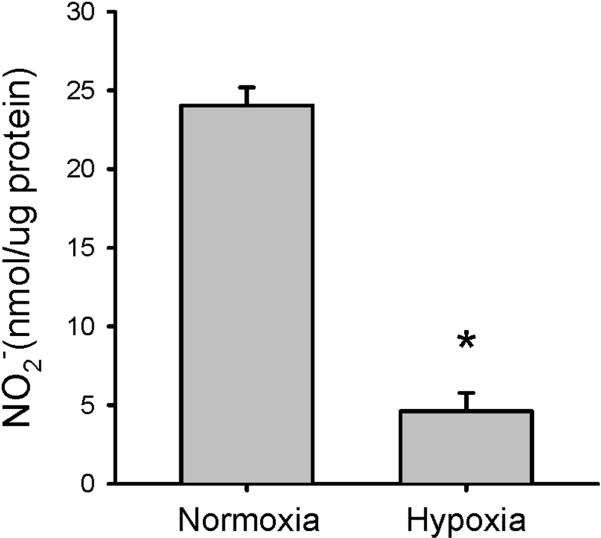
To determine the effect of hypoxia on NO production by hPMVEC, the cells were incubated in either normoxia or hypoxia for 24 hours. The medium was harvested for determination of nitrite levels, and the protein was harvested. Hypoxic hPMVEC had ~80% less NO production than did hPMVEC incubated in normoxia (Figure 1).
Figure 1.
Hypoxia results in lower NO production in hPMVEC. hPMVEC in T75 flasks were incubated in either normoxia or hypoxia for 24 hours. The media was harvested for determination of nitrites using chemiluminescence, and the hPMVEC were harvested for protein determination. Nitrite production is presented in nmol per mg protein. * hypoxia different from normoxia, p<0.01.
Hypoxia decreased CAT-2 mRNA expression and L-arg uptake
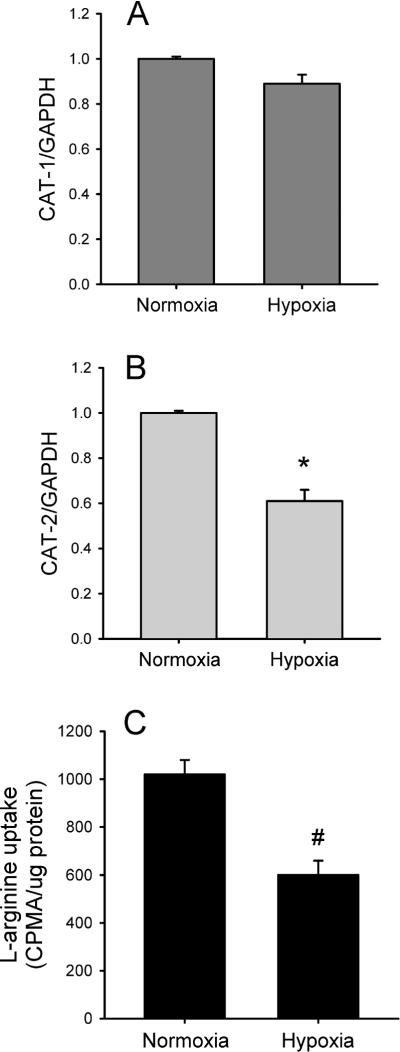
To determine if hypoxia affected the expression of either CAT-1 or CAT-2, hPMVEC were incubated in either normoxia or hypoxia for 24 hours. Total mRNA was then isolated from the cells. CAT-1 and CAT-2 mRNA levels were determined by real-time PCR. The hPMVEC incubated in normoxia expressed both CAT-1 and CAT-2. Hypoxia had little effect on CAT-1 mRNA levels (Figure 2A), while hypoxic hPMVEC had significantly less CAT-2 mRNA expression than did cells incubated for 24 hours in normoxia (Figure 2B). To determine if the hypoxia-induced decrease in CAT-2 expression was associated with decreased uptake of L-arg by hPMVEC, specific L-[3H]arg uptake was measured. Specific L-arg uptake was ~40% lower (p<0.01) in hypoxic hPMVEC than in normoxic hPMVEC (Figure 2C).
Figure 2.
Hypoxia decreased CAT-2 expression and L-arg uptake in hPMVEC. A) Expression of CAT-1 was not affected by hypoxic exposure. hPMVEC were incubated in normoxia or hypoxia for 24 hours and the cells were harvested for RNA. CAT-1 expression was determined by real-time PCR and normalized to GAPDH expression. B) Expression of CAT-2 was decreased by hypoxic exposure. CAT-2 expression was determined by real-time PCR in hPMVEC exposed to either 24 hours of normoxia or hypoxia and normalized to GAPDH expression. * Hypoxia different from normoxia, p<0.05. C) Hypoxia decreased specific L-arginine uptake by hPMVEC. Cells were incubated in either normoxia or hypoxia for 24 hours and then uptake of [3H]L-arg was determined in the presence and absence of 10 mM unlabelled L-arg. Specific [3H]L-arg uptake was normalized to μg of protein. * hypoxia different from normoxia, p<0.05.
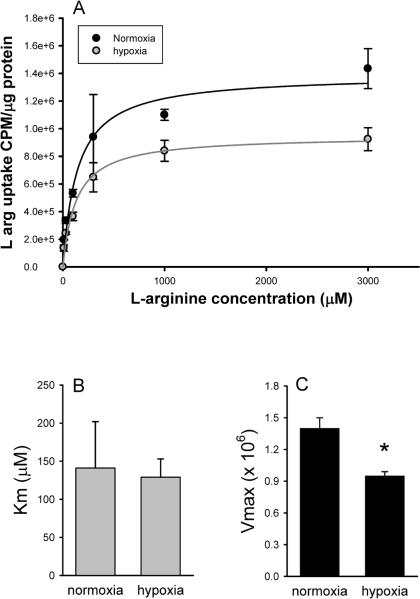
We then examined the kinetics of L-arg uptake in hPMVEC. Figure 3A shows that L-arg uptake by hPMVEC is a saturable process which follows Michaelis-Menton kinetics. There was no significant difference on the Km of L-arg uptake between normoxic and hypoxic hPMVEC (Figure 3B). The hPMVEC incubated in hypoxia had a significantly lower Vmax (1.4 ± 0.1 ×106, normoxia versus 0.95 ± 0.04 ×106, hypoxia, p<0.05) than did normoxic hPMVEC (Figure 3C).
Figure 3.
Hypoxia decreased Vmax for L-arg uptake. A) L-arg uptake determined in hPMVEC incubated with varying amounts of L-arg during normoxia (black circles) and hypoxia (grey circles). L-arg uptake by hPMVEC is a saturable process which follows Michaelis-Menton kinetics, and hypoxia decreased L-arg uptake. B) Hypoxia had no effect on the Km of L-arg uptake by hPMVEC. C) Hypoxia decreased the Vmax of L-arg uptake by hPMVEC. * hypoxia different from normoxia, p<0.05.
Overexpression of CAT-1 increased L-arg uptake in hPMVEC
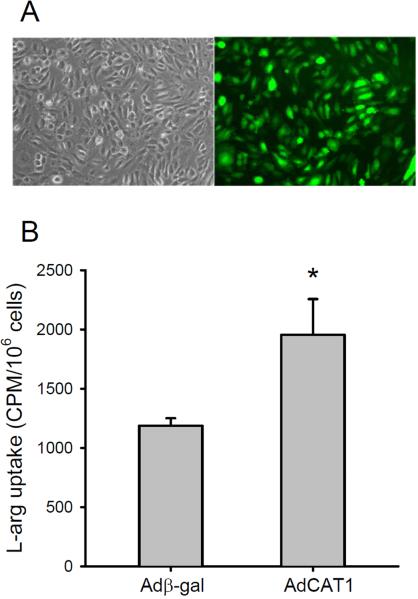
To establish that hPMVEC could be efficiently transfected with AdCAT1, we measured the expression of the eGFP reporter gene in the AdCAT1 vector (Figure 4A). The percentage of fluorescent cells was quantified using flow cytometry, which demonstrated a transfection efficiency of ~98%. To verify that transfection of hPMVEC with AdCAT1 increased L-arg uptake, the specific uptake of L-arg was determined in Adβ-gal- and AdCAT1-transfected hPMVEC. As shown in Figure 4B there was nearly a doubling of specific L-arg uptake in AdCAT1-transfected hPMVEC compared to Adβ-gal-transfected hPMVEC.
Figure 4.
Transfection of hPMVEC with an adenoviral vector containing the gene for human CAT-1 and GFP could be accomplished with high efficiency and resulted in increased L-arg uptake. A) Representative phase contrast and fluorescent images of hPMVEC transfected with AdCAT1 containing GFP. B) L-arg uptake was increased by AdCAT1 transfection. Specific [3H]L-arg uptake in hPMVEC transfected with either Adβ-gal or AdCAT1. * AdCAT1 different from Adβ-gal, p<0.05.
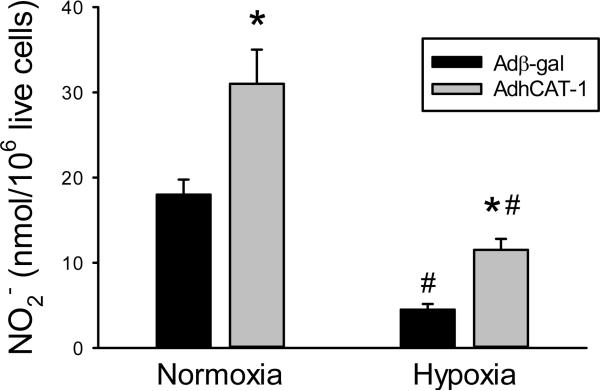
To determine the effect of overexpression of CAT-1 on NO production in hPMVEC, the cells were transfected with either Adβ-gal or AdCAT-1. After 24 hours the cells were washed and incubated with fresh media and placed in either normoxia or hypoxia for an additional 24 hours. The media was then harvested for determination of nitrites and the number of viable cells in each well was determined by trypan blue exclusion. In normoxia, the AdCAT1-transfected hPMVEC produced approximately two times more NO than did the Adβ-gal-transfected hPMVEC (Figure 5). Although, incubation in hypoxia resulted in significantly lower NO production in both the Adβ-gal and AdCAT1 transfected hPMVEC, AdCAT1 transfection resulted in significantly more hypoxic NO production than in cells transfected with Adβ-gal (Figure 5).
Figure 5.
Transfection with AdCAT1 increased NO production by hPMVEC both in normoxia and hypoxia. hPMVEC in 6 well plates were transfected with either Adβ-gal (black bars) or AdCAT1 (grey bars) for 24 hours, the cells were then washed and fresh media placed on the cells and the cells incubated in either normoxia or hypoxia for 24 hours. The media was then collected and assayed for nitrite (NO2−) concentration. Viable cell number in each well was determined using trypan blue exclusion. * AdCAT1 different from Adβ-gal same condition, p<0.05. # hypoxia different from normoxia same transfection group, p<0.05.
The effect of hypoxia on eNOS expression in hPMVEC
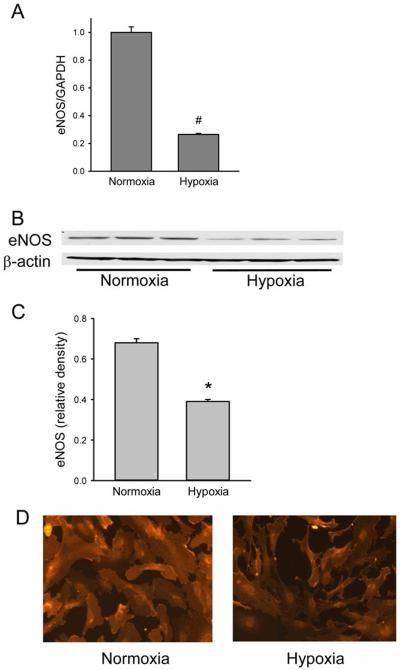
Given that in hypoxia, AdCAT1-transfected cells had lower NO production than did normoxic AdCAT1-transfected cells, we examined eNOS expression in hypoxic hPMVEC. The cells were exposed to either normoxia or hypoxia for 24 hours. The cells were harvested for mRNA and real-time PCR was done for eNOS and GAPDH expression. Hypoxia resulted in a significant decrease in eNOS mRNA expression (Figure 6A). In a second set of experiments, cells were exposed to normoxia or hypoxia for 24 hours and harvested for protein. Western blotting was performed to determine eNOS protein expression. eNOS protein expression was significantly lower in hypoxic hPMVEC than in normoxic cells (Figure 6 B&C). In a third set of studies, hPMVEC were again incubated in either normoxia or hypoxia for 24 hours and immunocytochemistry was performed as another measure of eNOS protein expression. Figure 6D demonstrates that eNOS protein expression was lower in hPMVEC incubated in hypoxia for 24 hours than in hPMVEC incubated in normoxia for 24 hours.
Figure 6.
Hypoxia decreased eNOS expression in hPMVEC. A) Hypoxia resulted in lower eNOS mRNA expression as demonstrated by real-time PCR. The graph shows eNOS mRNA levels normalized to GAPDH mRNA levels, which are plotted relative to normoxic levels. # hypoxia different from normoxia, p<0.001. B) Representative western blots for eNOS from hPMVEC incubated in normoxia or hypoxia for 24 hours. The blots were stripped and reprobed for β-actin. C) Relative density of eNOS bands normalized to β-actin bands. * hypoxia different from normoxia, p<0.01. D) eNOS immunoreactivity was decreased by 24 hours in hypoxia. Representative immunocytochemistry for eNOS from hPMVEC incubated in normoxia (right hand panel) or hypoxia (left hand panel). The representative pictures are from a total of 3 experiments.
Overexpression of iNOS and/or CAT-1 increased NO production in hPMVEC
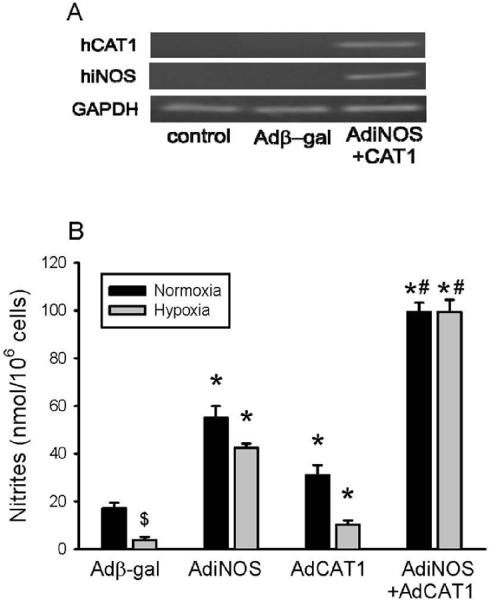
To further examine the role of arginine uptake in NO production we over-expressed iNOS using AdiNOS in hPMVEC. We have previously shown that transfection of endothelial cells with AdiNOS resulted in increased NO production (16). Thus, to continue to investigate the effect of CAT-1 overexpression we studied a condition wherein NO production is substantially increased, i.e. hPMVEC transfected with AdiNOS. In the first experiment, hPMVEC were transfected with either Adβ-gal, AdiNOS, AdCAT1 or both AdiNOS+AdCAT1. To determine that transfection with AdiNOS+AdCAT1 resulted in expression of both genes, hPMVEC were either not transfected, transfected with Adβ-gal, or transfected with AdiNOS+AdCAT1 for 24 hours and the cells were harvested and mRNA expression was measured. Figure 7A demonstrates that transfection with AdiNOS+AdCAT1 resulted in expression of both iNOS and CAT-1 mRNA in hPMVEC. In a second set of experiments, NO production after transfection with Adβ-gal, AdiNOS, AdCAT1, or AdiNOS+AdCAT1 was determined. The hPMVEC were transfected with the various viral vectors for 24 hours, the media was then changed and the cells were incubated for an additional 48 hours in either normoxia or hypoxia. In normoxia, transfection with either AdiNOS or AdCAT1 resulted in greater NO production than in cells transfected with Adβ-gal, however, transfection with both AdiNOS+AdCAT1 resulted in the greatest NO production in normoxic cells (Figure 7B). Adβ-gal-transfected hPMVEC incubated in hypoxia for 48 hours had significantly less NO production than did Adβ-gal-transfected cells incubated in normoxia (Figure 7B). AdiNOS-transfected or AdCAT1-transfected hPMVEC incubated in hypoxia had greater NO production than did hypoxic Adβ-gal-transfected cells (Figure 7B). AdiNOS+AdCAT1-transfected hPMVEC incubated in hypoxia had NO production levels similar to AdiNOS+AdCAT1-transfected cells incubated in normoxia (Figure 7B).
Figure 7.
Transfection with AdiNOS or AdCAT1 increased NO production in both normoxic and hypoxic hPMVEC, and the effects of AdiNOS+AdCAT1 were additive. A) Representative RT-PCR blots demonstrating that transfection with both AdiNOS+AdCAT1 results in expression of iNOS and CAT-1 in hPMVEC. B) Nitrite production in hPMVEC transfected for 24 hours with either Adβ-gal, AdiNOS, AdCAT1 or both AdiNOS+AdCAT1. T he cells were then washed and fresh media placed on the cells and they were incubated in either normoxia or hypoxia for an additional 48 hours. The media was then harvested for determination of nitrites, which was normalized to viable cell number. Transfection with either AdiNOS or AdCAT1 increased nitrite production in normoxia and hypoxia. The hypoxic NO production was lower than the normoxic NO production, except when both AdiNOS and AdCAT1 were transfected together. $ hypoxic Adβ-gal different from normoxic Adβ-gal, p<0.05. * different from Adβ-gal, same exposure (normoxia or hypoxia), p<0.01. # AdiNOS+AdCAT1 different from all other conditions, p<0.01.
NO production in AdiNOS+AdCAT1 transfected hPMVEC was dependent on L-arg uptake
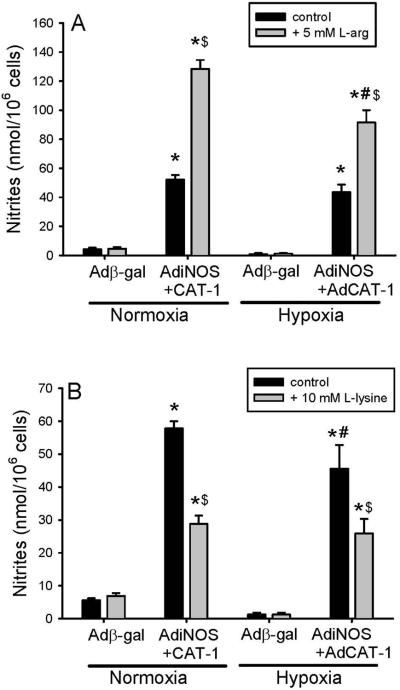
To determine the role of L-arg uptake in the vastly increased NO production seen in hPMVEC transfected with both AdiNOS+AdCAT1, NO production was determined in transfected cells either in the presence of excess L-arg, or in the presence of L-lysine, a competitive inhibitor of L-arg uptake. In the first set of experiments, hPMVEC were either transfected with Adβ-gal or both AdiNOS+AdCAT1. After 24 hours the cells were washed and media was placed on the cells that contained either no additional L-arg or 5 mM L-arg and the cells were incubated for 48 hours in either hypoxia or normoxia. The media was harvested for determination of nitrite production and the number of viable cells was counted using trypan blue exclusion. Again, AdiNOS+AdCAT1-transfected hPMVEC had markedly greater NO production then did Adβ-gal-transfected cells (Figure 8A). The addition of 5 mM L-arg to the media resulted in a significantly greater NO production in AdiNOS+AdCAT-transfected hPMVEC than in AdiNOS+AdCAT1-transfected cells without L-arg added to the media in both normoxia and hypoxia (Figure 8A). However, following the addition of 5 mM L-arg, the hypoxic AdiNOS+AdCAT1-transfected hPMVEC had significantly lower NO production than the normoxic AdiNOS+AdCAT1-transfected hPMVEC (Figure 8A).
Figure 8.
L-arg uptake affected the vastly increased NO production seen in hPMVEC transfected with both AdiNOS+AdCAT1. A) hPMVEC were transfected with either Adβ-gal or both AdiNOS+AdCAT1. After 24 hours the cells were washed and media was placed on the cells containing either no additional L-arg or 5 mM L-arg. The cells were incubated for 48 hours in either hypoxia or normoxia. The media was harvested for determination of nitrite production, which was normalized to viable cell number. AdiNOS+AdCAT1- transfected hPMVEC had markedly greater NO production than did Adβ-gal-transfected cells and the addition of 5 mM L-arg resulted in a significantly greater NO production in AdiNOS+AdCAT1-transfected hPMVEC in both hypoxia and normoxia. However, the hypoxic AdiNOS+AdCAT1-transfected hPMVEC with 5 mM L-arg had significantly lower NO production than the normoxic AdiNOS+AdCAT1-transfected hPMVEC with 5 mM L-arg. B) The experiments in A were repeated except that the hPMVEC were incubated in media containing either no additional L-lysine or 10 mM L-lysine. The addition of 10 mM L-lysine to the media resulted in significantly less NO production in AdiNOS+AdCAT-transfected hPMVEC than in AdiNOS+AdCAT1-transfected cells without added L-lysine in both hypoxia and normoxia. The hypoxic AdiNOS+AdCAT1-transfected hPMVEC with 10 mM L-lysine had NO production that was similar to normoxic AdiNOS+AdCAT1-transfected hPMVEC with 10 mM L-lysine. * AdiNOS+AdCAT1 different from Adβ-gal, same condition, p<0.05. $ Additive (L-arg or L-lysine) different from control, same transfection and condition, p<0.01. # hypoxia different from normoxia; same transfection, condition, and additive, p<0.05.
In a second set of experiments, hPMVEC were either transfected with Adβ-gal or both AdiNOS+AdCAT1. After 24 hours the cells were washed and media was placed on the cells that contained either no additional L-lysine or 10 mM L-lysine and the cells were incubated for 48 hours in either hypoxia or normoxia. Given that L-lysine is a competitive inhibitor of L-arg uptake and since the media contains ~400 μM L-arg, an L-lysine concentration of 10 mM was chosen to assure inhibition. The media was harvested for determination of nitrite production and the number of viable cells was counted using trypan blue exclusion. Again, AdiNOS+AdCAT1-transfected hPMVEC had markedly greater NO production than did Adβ-gal-transfected cells (Figure 8B). The addition of 10 mM L-lysine to the media resulted in significantly less NO production in AdiNOS+AdCAT1-transfected hPMVEC than in AdiNOS+AdCAT1-transfected cells without L-lysine added to the media in both normoxia and hypoxia (Figure 8B). The hypoxic AdiNOS+AdCAT1-transfected hPMVEC with 10 mM L-lysine had NO production that was similar to normoxic AdiNOS+AdCAT1-transfected hPMVEC with 10 mM L-lysine (Figure 8B).
DISCUSSION
The main findings of this study were that: 1) hypoxic hPMVEC had less NO production and lower eNOS protein expression than did normoxic hPMVEC, 2) hypoxia significantly decreased CAT-2 mRNA levels and L-arginine transport, 3) hypoxia decreased the Vmax without affecting the Km of L-arginine transport in hPMVEC, 4) transfection with AdCAT1 resulted in significantly greater L-arginine transport and NO production in hPMVEC in both normoxic and hypoxic cells, 5) transfection with both AdiNOS and AdCAT1 resulted in high levels of NO production and the difference in NO production between normoxic and hypoxic cells was abolished, 6) addition of 5mM L-arginine to normoxic and hypoxic cells transfected with both AdiNOS and AdCAT1 increased NO production even further, and 7) the competitive inhibitor o f C A T-mediated L-arg transport, L-lysine, decreased NO production by AdiNOS+AdCAT1 transfected hPMVEC in both normoxia and hypoxia. These findings suggest that basal NO production in hPMVEC depends, at least in part, on L-arg transport by the cationic amino acid transporters. Overexpressing CAT-1 in hPMVEC increased NO production even when eNOS expression was low such as seen in the hypoxic hPMVEC. Furthermore, co-transfection with both iNOS and CAT-1 eliminated the difference in NO production between normoxic and hypoxic cells. These results demonstrate that NO production both in hypoxia and normoxia can be influenced by L-arg transport, and that overexpressing CAT-1 leads to greater L-arg transport and greater NO production in both normoxic and hypoxic hPMVEC.
Depending on the underlying etiology of pulmonary hypertension a decrease in bioavailable NO may be associated with decreased, no change, or an increase in eNOS expression (1,2,3,18,19,20). However, even in pulmonary hypertensive diseases wherein eNOS expression is unchanged or increased the bioavailable NO, i.e. the NO which can activate the cGMP signaling cascade leading to vasodilation, is decreased. There are a variety of mechanisms associated with decreased bioavailable NO including increased superoxide scavenging of NO (18), increased hemoglobin scavenging of NO (20), increased nitration of downstream signaling proteins (19), etc. We found that in hPMVEC, hypoxia decreased both eNOS expression and NO production. Jelic et al. (21) found that eNOS expression was lower in forearm venous endothelial cells in patients with obstructive sleep apnea (OSA) than in endothelial cells from control subjects, and that the level of eNOS expression was directly correlated with the severity of the OSA and that adherence to CPAP treatment increased eNOS expression. Takemoto et al. (22) found in human pulmonary arterial endothelial cells that hypoxia caused a decrease in eNOS protein and mRNA expression that was dependent on Rhokinase signaling. Fish et al. (23) recently suggested that in human umbilical venous endothelial cells that hypoxia may decrease eNOS transcriptional activity due to histone eviction. Taken together these results suggest that eNOS expression is decreased in hypoxia in human endothelial cells, and this effect involves transcriptional regulation. Furthermore, the hypoxia-induced decrease in eNOS expression is associated with lower levels of NO production. Although the regulation of NO production is complex and the hypoxia-induced decrease in NO production may involve other mechanisms, such as chaperone protein binding, co-factor binding, etc., it is clear that decreased eNOS expression levels are involved in the decrease in NO production. It should be pointed out that one potential weakness of our study is the use of a hypoxia model. Although, this model may mimic pulmonary hypertension associated with acute lung diseases such as pneumonia, high-altitude pulmonary edema, neonatal respiratory distress syndrome; it may not completely mimic the changes seen in other types of pulmonary hypertensive diseases or more chronic diseases such as COPD.
We found that hypoxia also significantly decreased L-arg transport in hPMVEC. We found that the hypoxia-induced decrease in L-arg transport was associated with a decrease in CAT-2 mRNA expression, with no change in CAT-1 mRNA expression. Zharikov et al. (24) also reported a hypoxia-induced decrease in L-arg transport with no change in CAT-1 expression in porcine pulmonary arterial endothelial cells. Consistent with a decrease in CAT-2 expression we found a significant decrease in the Vmax for L-arg transport in hPMVEC. Taken together our results suggest that hypoxia reduces the total number of L-arg transporters expressed on hPMVEC. Thus, hypoxia results in a decrease in L-arg uptake by hPMVEC due, at least in part, to a decrease in CAT-2 expression.
Endothelial cell NO production can be influenced by extracellular L-arg concentrations, suggesting that L-arg uptake is vital for endothelial cell NO production in both normoxia and hypoxia. Indeed, the finding that the extracellular concentration of L-arg can influence NO production has been termed the “L-arginine paradox”. McDonald et al. (10) suggested that a caveolar complex between CAT-1 and eNOS resulted in transported L-arg being the substrate of choice for eNOS. We have previously found that cytokine stimulation of bovine pulmonary artery endothelial cells resulted in increased CAT-2 expression, greater L-arg uptake and NO production (15). We have also found that in the isolated perfused lung from chronically hypoxic neonatal pigs that L-arg increased NO production and decreased pulmonary vascular resistance (9). In the present study, we demonstrate that over-expression of CAT-1 using adenoviral vectors in both normoxic and hypoxic hPMVEC lead to increased L-arg uptake and NO production. This finding is consistent with a study in normoxic bovine aortic endothelial cells (bAEC) wherein overexpression of CAT-1 led to increased L-arg uptake and NO production (25). It should be pointed out that the NO production in hypoxic AdCAT1-transfected cells did not return to normoxic basal levels. This may be due to the decrease in eNOS protein abundance in the hPMVEC, which would be expected to lead to a lower maximal eNOS activity.
We further investigated the link between NO production and L-arg uptake by CAT in hypoxia by utilizing iNOS over-expression. Although both AdCAT-1 and AdiNOS transfection individually increased NO production in both hypoxia and normoxia, when hPMVEC were cotransfected with AdiNOS+AdCAT1 we found an additive effect on NO production. This suggests that intracellular L-arg stores were not fully available to support the iNOS-mediated NO production in cells overexpressing iNOS. Furthermore, and more importantly in terms of the role of CAT in NO production, these data clearly demonstrate that L-arg uptake is increased by CAT-1 overexpression in hPMVEC, and that the increased CAT-mediated L-arg uptake increases NO production. In fact when cells were transfected with both AdiNOS+AdCAT1, the difference between normoxic and hypoxic NO production disappeared. Thus, there is a clear increase in NO production, even in hypoxic conditions, when the uptake of extracellular L-arg is augmented in pulmonary endothelial cells. Indeed in both normoxic and hypoxic cells wherein both CAT-1 and iNOS were overexpressed, increasing the extracellular L-arg concentration resulted in enhanced NO production, while inhibiting L-arg uptake using L-lysine resulted in decreased NO production. These results clearly demonstrate that a measurable portion of endothelial cell NO production depends on the uptake of extracellular L-arg in hPMVEC in both normoxia and hypoxia. Our results support the notion that CAT-mediated L-arg uptake may represent a novel therapeutic target in pulmonary diseases, and that augmenting CAT-mediated L-arg uptake may be a viable option for augmenting NO production in pulmonary hypertensive disorders.
ACKNOWLEDGEMENTS
This study was supported by Grant Number R01HL075261 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
REFERENCES
- 1.Cua CL, Rogers LK, Chicoine LG, et al. Down syndrome patients with pulmonary hypertension have elevated plasma levels of asymmetric dimethylarginine. Eur. J. Pediatr. 2011;170:859–863. doi: 10.1007/s00431-010-1361-x. [DOI] [PubMed] [Google Scholar]
- 2.Pearson DL, Dawling S, Walsh WF, et al. Neonatal pulmonary hypertension--urea-cycle intermediates, nitric oxide production, and carbamoyl-phosphate synthetase function. N. Engl. J. Med. 2001;344:1832–8. doi: 10.1056/NEJM200106143442404. [DOI] [PubMed] [Google Scholar]
- 3.Xu W, Kaneko FT, Zheng S, et al. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J. 2004;18:1746–1748. doi: 10.1096/fj.04-2317fje. [DOI] [PubMed] [Google Scholar]
- 4.Dehnert C, Berger MM, Mairbäurl H, Bärtsch P. High altitude pulmonary edema: a pressure-induced leak. Respir. Physiol. Neurobiol. 2007;158:266–273. doi: 10.1016/j.resp.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Castillo L, DeRojas-Walker T, Yu YM, et al. Whole body arginine metabolism and nitric oxide synthesis in newborns with persistent pulmonary hypertension. Pediatr. Res. 1995;38:17–24. doi: 10.1203/00006450-199507000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Endo A, Ayusawa M, Minato M, Takada M, Takahashi S, Harada K. Endogenous nitric oxide and endothelin-1 in persistent pulmonary hypertension of the newborn. Eur. J. Pediatr. 2001;160:217–222. doi: 10.1007/pl00008431. [DOI] [PubMed] [Google Scholar]
- 7.Balligand JL, Feron O, Dessy C. eNOS activation by physical forces: from short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol. Rev. 2009;89:481–534. doi: 10.1152/physrev.00042.2007. [DOI] [PubMed] [Google Scholar]
- 8.Carter BW, Chicoine LG, Nelin LD. L-lysine decreases nitric oxide production and increases vascular resistance in lungs isolated from lipopolysaccharide treated neonatal pigs. Pediatr. Res. 2004;55:979–987. doi: 10.1203/01.pdr.0000127722.55965.b3. [DOI] [PubMed] [Google Scholar]
- 9.Fike CD, Kaplowitz MR, Thomas CJ, Nelin LD. L-arginine increases nitric oxide production in isolated lungs of chronically hypoxic newborn pigs. J. Appl. Physiol. 2000;88:1797–1803. doi: 10.1152/jappl.2000.88.5.1797. 2000. [DOI] [PubMed] [Google Scholar]
- 10.McDonald KK, Zharikov S, Block ER, Kilberg MS. A caveolar complex between the cationic amino acid transporter 1 and endothelial nitric-oxide synthase may explain the “arginine paradox”. J. Biol. Chem. 1997;272:31213–6. doi: 10.1074/jbc.272.50.31213. [DOI] [PubMed] [Google Scholar]
- 11.Mann GE, DL Yudilevich DK, Sobrevia L. Regulation of amino acid and glucose transporters in endothelial and smooth muscle cells. Physiol. Rev. 2003;83:183–252. doi: 10.1152/physrev.00022.2002. [DOI] [PubMed] [Google Scholar]
- 12.Chang R, Chicoine LG, Cui H, et al. Cytokine-induced arginase activity in pulmonary endothelial cells is dependent on Src family tyrosine kinase activity. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008;295:L688–697. doi: 10.1152/ajplung.00504.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toby I, Chicoine LG, Cui H, Chen B, Nelin LD. Hypoxia-induced proliferation of human pulmonary microvascular endothelial cells depends on epidermal growth factor receptor tyrosine kinase activation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010;298:L600–L606. doi: 10.1152/ajplung.00122.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelin LD, Chicoine LG, Reber KM, English BK, Young TL, Liu Y. Cytokine-induced endothelial arginase expression is dependent on epidermal growth factor receptor. Am. J. Respir. Cell Mol. Biol. 2005;33:394–401. doi: 10.1165/rcmb.2005-0039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelin LD, Nash HE, Chicoine LG. Cytokine treatment increases arginine metabolism and uptake in bovine pulmonary arterial endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;281:L1232–L1239. doi: 10.1152/ajplung.2001.281.5.L1232. [DOI] [PubMed] [Google Scholar]
- 16.Stanley KP, Chicoine LG, Young TL, et al. Gene transfer with inducible nitric oxide synthase decreases production of urea by arginase in pulmonary arterial endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;290:L298–L306. doi: 10.1152/ajplung.00140.2005. [DOI] [PubMed] [Google Scholar]
- 17.Chicoine LG, Tzeng E, Bryan R, et al. Intratracheal adenoviral-mediated delivery of iNOS decreases pulmonary vasoconstrictor responses in rats. J. Appl. Physiol. 2004;97:1814–1822. doi: 10.1152/japplphysiol.00193.2004. [DOI] [PubMed] [Google Scholar]
- 18.Norton CE, Jernigan NL, Kanagy NL, Walker BR, Resta TC. Intermittent hypoxia augments pulmonary vascular smooth muscle reactivity to NO: regulation by reactive oxygen species. J. Appl. Physiol. 2011 Jul 14; doi: 10.1152/japplphysiol.01286.2010. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao YY, Zhao YD, Mirza MK, et al. Persistent eNOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration. J/Clin. Invest. 229. 119:2009–2018. doi: 10.1172/JCI33338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris CR, Vichinsky EP. Pulmonary hypertension in thalassemia. Ann. N.Y. Acad. Sci. 2010;120:205–213. doi: 10.1111/j.1749-6632.2010.05580.x. [DOI] [PubMed] [Google Scholar]
- 21.Jelic S, Padeletti M, Kawut SM, et al. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation. 2008;117:2270–8. doi: 10.1161/CIRCULATIONAHA.107.741512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takemoto M, Sun J, Hiroki J, Shimokawa H, Liao JK. Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation. 2002;106:57–62. doi: 10.1161/01.cir.0000020682.73694.ab. [DOI] [PubMed] [Google Scholar]
- 23.Fish JE, Yan MS, Matouk CC, et al. Hypoxic repression of endothelial nitric-oxide synthase transcription is coupled with eviction of promoter histones. J. Biol. Chem. 2010;285:810–26. doi: 10.1074/jbc.M109.067868. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zharikov SI, Block ER. Association of L-arginine transporters with fodrin: implications for hypoxic inhibition of arginine uptake. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;278:L111–7. doi: 10.1152/ajplung.2000.278.1.L111. [DOI] [PubMed] [Google Scholar]
- 25.Li C, Huang W, Harris MB, JM Goolsby JM, Venema RC. Interaction of the endothelial nitric oxide synthase with the CAT-1 arginine transporter enhances NO release by a mechanism not involving arginine transport. Biochem. J. 2005;386:567–74. doi: 10.1042/BJ20041005. [DOI] [PMC free article] [PubMed] [Google Scholar]