Abstract
The anorexia that results from extended periods of cellular dehydration is an important physiological adaptation that limits the intake of osmolytes from food and helps maintain the integrity of fluid compartments. The ability to experimentally control both the development and reversal of anorexia, together with the understanding of underlying hormonal and neuropeptidergic signals, make dehydration (DE)-anorexia a powerful model for exploring the interactions of neural networks that stimulate and inhibit food intake. However, it is not known which meal parameters are affected by cellular dehydration to generate anorexia. Here we use continuous and high temporal resolution recording of food and fluid intake, together with a drinking-explicit method of meal pattern analysis to explore which meal parameters are modified during DE-anorexia. We find that the most important factor responsible for DE-anorexia is the failure to maintain feeding behavior once a meal has started, rather than the ability to initiate a meal, which remains virtually intact. This outcome is consistent with increased sensitivity to satiation signals and post-prandial satiety mechanisms. We also find that DE-anorexia significantly disrupts the temporal distribution of meals across the day so that the number of nocturnal meals gradually decreases while diurnal meal number increases. Surprisingly, once DE-anorexia is reversed this temporal redistribution is maintained for at least 4 days after normal food intake has resumed, which may allow increased daily food intake even after normal satiety mechanisms are reinstated. Therefore, DE-anorexia apparently develops from a selective targeting of those neural networks that control meal termination, whereas meal initiation mechanisms remain viable.
Keywords: Feeding, Drinking, Meal pattern analysis, Anorexia, Circadian, Thirst
1 INTRODUCTION
Anorexia is the inhibition of feeding behavior despite an ongoing state of negative energy balance. We have extensively investigated the mechanisms and neural circuitry underlying anorexia in a rat model where anorexia develops as an adaptive response to cellular dehydration (DE). To induce DE, rats are given hypertonic saline (HS; 2.5% NaCl) to drink instead of water for up to five days. Although chow is freely available during this time, rats voluntarily and robustly limit their food intake (DE-anorexia) [1]. Nocturnal food intake progressively declines to approximately 20% of baseline intake and body weight is typically reduced by 15–20% [2, 3]. When water is returned rats rapidly exhibit a very reproducible sequence of behaviors that corrects the accrued energy and fluid deficits [2, 4].
Our knowledge of spontaneous eating and drinking patterns during DE-anorexia has been limited to two measures: total nocturnal and diurnal consumption, and the measurement of compensatory eating and drinking that follow the return of water [1, 2, 4]. However, these gross intake measures provide little insight into what aspect of feeding is compromised during DE-anorexia. Since the meal is considered the biological unit of feeding behavior [5, 6], any change in the amount of food consumed is the direct result of a change in meal size, meal number, or both [7, 8]. To determine which specific components of ingestive behavior are altered during the development and recovery from DE-anorexia we use the BioDAQ Intake Monitoring System to perform a detailed analysis of spontaneous meal patterns before, during, and after the onset of DE-anorexia.
A meal is traditionally defined as a cluster of smaller feeding bouts that are separated from other feeding clusters by an inter-meal interval (IMI) where feeding is absent. Furthermore, the relationship between eating and drinking has been extensively studied, and several reports have demonstrated that approximately 70–85% of water intake is temporally associated with meals [8–10]. Kissileff has emphasized the distinction between food-associated drinking, in which eating and drinking occur discretely but are temporally close, and prandial drinking, which occurs in rapid, alternating succession with feeding bouts, thus occurring within a meal [9]. More recently, Zorrilla and colleagues have extended this definition by proposing that drinking is an explicit component of the meal [11], and thus consider both feeding and drinking data in their analyses. This “drinking-explicit” analysis provides a method for validating an IMI that is used to define meals when both food and liquid intake are continuously monitored [11]. Given that DE-anorexia is provoked by drinking hypertonic saline, we now incorporate the notion of within-meal drinking into the framework for studying relationships between eating and drinking behavior before, during, and after the expression of DE-anorexia. We now use detailed meal pattern analyses to determine which aspects of feeding and drinking are modified as DE-anorexia develops and is reversed. In turn, changes in one or more meal components will provide powerful insights about the underlying mechanisms and neural networks that control ingestive behaviors during DE-anorexia.
2. MATERIALS AND METHODS
2.1 Animals
Adult male Sprague-Dawley rats (Harlan Laboratories; 250–275g) were individually housed in polysulfone home cages with sanitized wood chips. Cages were equipped with the BioDAQ® Food and Liquid Intake Monitors, a product of Research Diets Inc. (New Brunswick, NJ). Rats were maintained on a 12/12-h light-dark cycle (lights on at 06.30h) in a temperature-controlled environment (22–23°C), with ad libitum access to food (Teklad rodent chow 8604) and water, except where noted. Body weights were measured daily throughout the experiment (between 09.00h and 10.00h), and food and liquid intake were monitored as described below. All procedures have been approved by the Institutional Animal Care and Use Committee of the University of Southern California.
2.2 BioDAQ Food and Liquid Intake Monitoring System
The BioDAQ Food and Liquid Intake Monitoring System provides accurate and continuous collection of food and fluid intake data with minimal experimenter intervention. The system consists of multiple hoppers each coupled to a precision strain gauge-based load cell, or peripheral sensor controller (PSC), that is wired into a central controller. Each PSC outputs raw data to a laptop, as has been previously described in detail [12]. For our experiments, each cage was equipped with two PSCs; one coupled to the food hopper, and the second to an inverted fluid bottle with a ball-bearing spout. Each of a cage’s two PSCs was independently wired to the central controller to allow independent monitoring of food and fluid intake. The BioDAQ food hoppers have horizontal slots that allow rats to gnaw and paw at the chow but not remove entire pellets. The design of the hopper retains the crumbs that are not eaten due to gnawing, chewing, etc. Also, the design limits hoarding. The spillage beyond this retention is minimal, typically less than 0.5%. Cages were examined daily for food in the bedding, which was also minimal or not present. Recordings were halted for approximately 1h per day (between 09.00h and 10.00h) for animal maintenance, during which animals did not have access to food or water. This means that results reported for any 24h period consists of 23h of data collection and 1h of down time. Data were recorded using BioDAQ Monitoring Software 2.1.00, and analyzed using DataViewer 2.2.02 and Microsoft Excel 2004 and 2008 for Mac.
2.3 Euhydration, Dehydration, and Recovery
Rats were given at least five days of acclimation to the BioDAQ Monitoring system before any data were collected. After acclimation, ingestive behaviors were monitored for 5 days when chow and water were freely available. At 10.00h on experimental day 6, drinking water was replaced with hypertonic saline (HS; 2.5% NaCl (w/v) solution), which was then the only fluid available for the next five days (experimental days 6–10). At 12.00h on experimental day 11, water was returned and remained available for the remaining five days (experimental days 11–15). These three periods were designated as euhydration (EU), cellular dehydration invoked by drinking HS (DE), and recovery after the return of water (RE). A repeated measure design was implemented, by which each animal acted as its own control for the duration of the study to account for possible variations in between-animal body weights. Prior to replacing water with HS on day 6, rats were of similar body weight (297 ± 5g). Over the course of the DE period, rats lost on average 25 ± 0.7% of body weight, and on the last day of RE had reached 102 ± 0.9% of their EU body weight.
2.4 Drinking-explicit analysis of meal patterning during DE-anorexia
2.4.1 Meal Definition
Experiments used both the food and liquid intake monitors to determine interactions between eating and drinking behavior during the development and recovery from DE-anorexia. To capture this interaction we employed a drinking-explicit analytical method validated by Zorrilla and colleagues [11]. Here a meal is defined as any intake episode that contains at least 0.225 g of food (minimum meal size), and is separated from other burst clusters by an IMI of 300 s. In this study, the minimum meal size was defined as 0.23 g, as the BioDAQ software limits this value to two decimal places.
We also implemented a meal elimination criterion to separate and remove any large feeding cluster that resulted from mechanical errors, operator error, etc. Thus, any feeding cluster with an ingestion rate greater than 0.5 g/min was eliminated if two or more of the following rules were met: the feeding cluster consisted of less than 2 bouts; the feeding cluster contained a single bout that is greater than 1.0 g; the feeding cluster was independent of a drinking cluster.
2.4.2 Combining Ingestive Clusters into Composite Meals
The feeding and drinking data collected from each rat were manually combined into composite meals by first segmenting independent feeding and drinking files for each animal for each day into clusters using a 300 s IMI and a 0.23 g minimum meal size criterion (Microsoft Excel Mac2004/2008, Redmond, WA). Independent feeding and drinking clusters were then listed chronologically by cluster start time and scanned for feeding clusters that were separated from drinking clusters (and vice versa) by 300 s or less (Microsoft Excel). Clusters within this limit were assigned to a composite meal, the duration of which was taken as being between the start time of the first cluster and the end of the last response of the final cluster. Note that transition times between eating and drinking (inter-cluster interval) were included in the total meal duration. Drinking clusters not combined to a feeding cluster that did not fulfill the 0.23 g minimum food criteria were omitted from most meal-focused analysis. However, these drinking clusters were included in calculations of overall fluid consumption.
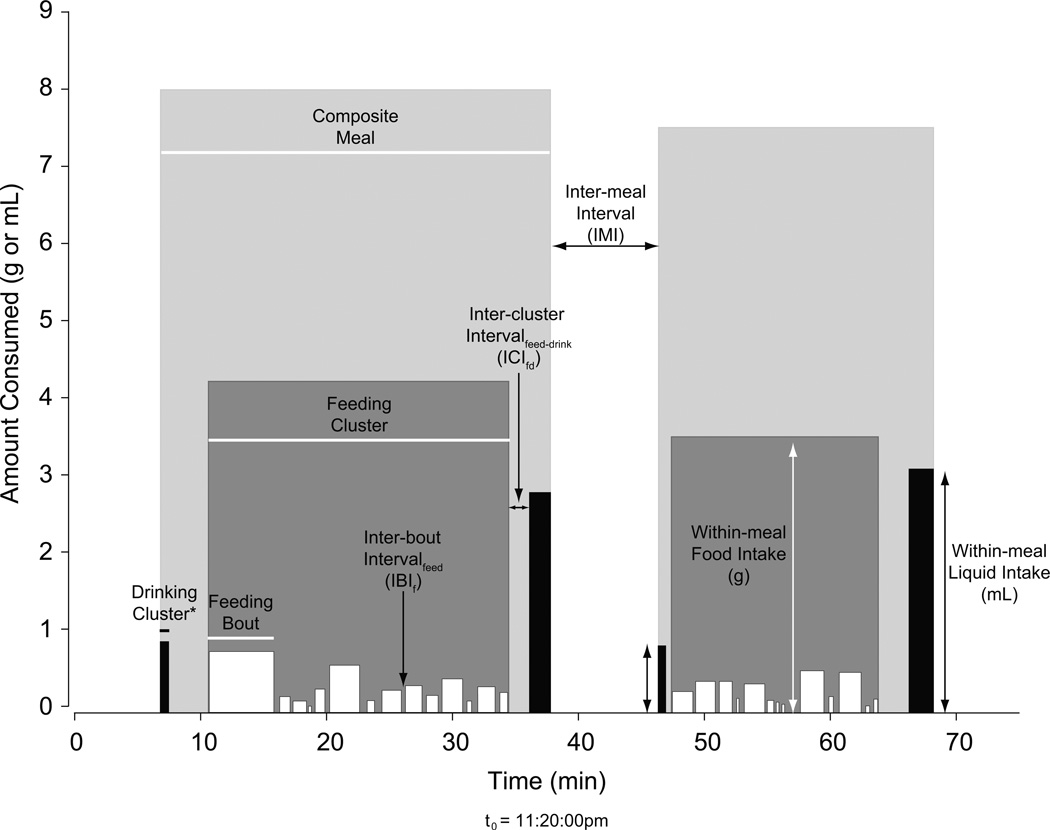
Figure 1 shows an example of two composite meals derived from 75 min of data collected from a EU rat. The figure also provides an illustration of various terms used in this paper. The terms and layout of this schema are derived from the microstructural analysis of licking established by Davis & Smith [13], together with the methods of Zorrilla and colleagues [11].
Figure 1. Anatomy of the Composite Meal.
Representative eating and drinking data from one EU rat that have been segmented into composite meals by the drinking-explicit method. Black vertical bars depict drinking clusters, white depict feeding bouts, dark gray depict feeding clusters, and light gray depict composite meals. Individual bar widths and horizontal lines (with and without arrows) represent duration (s). Individual bar heights and vertical lines represent within-meal food (white) or liquid (black) intake in g or mL; when a meal contains 2 or more clusters of feeding or drinking (as shown here in each meal for drinking), total within-meal intake or duration is equal to the sum of the individual clusters. Feeding bouts were assigned to clusters using meal criteria of 0.23 g minimum size, and a minimum IMI of 300 s. The same meal criteria were also used to combine feeding and drinking clusters.
*Although the resolution of the BioDAQ Liquid Intake Monitor does not capture drinking microstructure, drinking clusters also consist of smaller bouts, similar to those comprising feeding clusters.
2.5 Expt 1) Assessment of Feeding Patterns Before the Onset of Dehydration-Anorexia
Five days of EU feeding and drinking data were collected for each animal (n=10). Each 24h day (from lights on to lights on) was used to calculate descriptive statistics of average daily meal structure. Parameters measured (units) were number of composite meals; average composite meal duration (min); average IMI (min); percentage of total meals initiated during the nocturnal phase, average within-meal feeding and drinking intake (g or mL); average within-meal feeding and drinking duration (min); average food-to-liquid ratio for both intake (g/mL) and duration (min/min). Total daily intake of food (g), liquid (mL), and the ratio between the two (g/mL) were calculated from the difference in hopper weights over the 23h-recording period; these data were not subjected to the criteria used to define a meal. Composite meal duration was calculated as the time (min) between the start of the first cluster (feeding or drinking) and the end of the latest terminating feeding or drinking cluster, thus transition times between eating and drinking were included in the total composite meal duration. Durations of within-meal feeding and drinking cluster were calculated as consecutive time spent eating or drinking. IMI was defined as the time between the end and start times of two adjacent composite meals. Food-to-liquid ratios were calculated as the ratio between either within-meal quantities of food and liquid consumed, within-meal durations of food and liquid intake, or daily total quantities of food and liquid consumed. A feeding cluster not merged with a drinking cluster was treated as a composite meal and was thus included in calculations of composite meal duration, IMI, and within-meal food size and duration. However, these feeding clusters not merged with drinking were not included in either within-meal ratio calculation.
2.6 Expt 2) How do Composite Meal Structure Parameters Change as Dehydration-Anorexia Progresses?
Feeding and drinking data for five days (from lights on to lights on) of DE were used to calculate descriptive statistics of average daily meal structure. Parameters measured were identical to those described in Expt 1.
2.7 Expt 3) How Does Composite Meal Structure Change After Drinking Water Is Returned?
Feeding and drinking data for five days (from lights on to lights on) of RE were used to calculate descriptive statistics of average daily meal structure. Parameters measured were identical to those described in Expt 1.
2.8 Expt 4) Is the Diurnal Pattern of Composite Meals modified during and after Dehydration-Anorexia?
The 24h ingestive behavior data used in Experiments 1–3, were segmented by diurnal and nocturnal phase to investigate if DE or RE modulate the circadian organization of composite meals, and if changes observed in within-meal characteristics were phase-specific. Any composite meal that spanned a phase switch (i.e. portions of one composite meal were in contiguous light and dark phases) was analyzed within the phase in which the meal was initiated. Parameters measured were identical to those described for the single-phase analysis.
2.9 Statistical Analysis
Differences in parameter means (± SEM) between EU values and across the five days of DE or RE were analyzed using one-way ANOVA with repeated measures or, where missing values or unequal variances occurred, Welch’s test, a modified version of one-way ANOVA that does not assume equal variances [14]. When appropriate, Dunnett’s multiple comparison test was used to detect differences between the baseline EU data and each subsequent day of DE or RE. Data were statistically analyzed using a freely available software package R (http://www.r-project.org/), and Prism (Version 4, GraphPad Software, San Diego, CA). A P-value of <0.05 was considered to be statistically significant for all experiments.
3. RESULTS
3.1 Expt 1) Assessment of Feeding Patterns in Euhydrated Animals
There were no significant differences across the five-day control period for any of the parameters analyzed. Thus, five days of EU data from each rat were averaged for each parameter, and used as that individual’s baseline EU value for the repeated measures comparisons in Experiments 1–3. Table 1 shows descriptive statistics for each meal characteristic, representing average baseline EU values for each parameter during the 24h period, as well as during the light and dark periods.
TABLE 1.
The mean, range, and SEM for all composite meal variables expressed by euhydrated animals (EU). The values of all variables are presented for the entire 24h period, as well as their distribution within the light and dark periods. See text for further details.
| 24 h | Light | Dark | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Range | Range | Range | |||||||
| Measure | Mean | Min-Max | SE | Mean | Min-Max | SE | Mean | Min-Max | SE |
| Number of composite meals | 9.0 | 6.6–11.6 | 0.5 | 1.7 | 0.8–2.8 | 0.2 | 7.3 | 5.0–9.6 | 0.5 |
| Inter-meal Interval (min) | 87.7 | 62.6–111.1 | 5.7 | 139.3 | 61.1–286.7 | 23.3 | 77.7 | 61.5–105.2 | 5.5 |
| Composite meal duration (min) | 24.6 | 16.0–33.7 | 1.6 | 19.9 | 12.5–32.9 | 2.4 | 25.6 | 16.4–38.3 | 2.0 |
| Within-meal feeding (min) | 18.1 | 10.3–25.5 | 1.6 | 16.3 | 9.7–28.3 | 2.1 | 18.4 | 10.1–26.2 | 1.6 |
| Within-meal drinking (min) | 3.7 | 2.0–6.4 | 0.5 | 2.1 | 0.8–3.3 | 0.3 | 4.1 | 2.0–7.4 | 0.6 |
| Food:Liquid ratio (min) | 5.5 | 2.7–9.8 | 0.8 | 9 | 3.6–19.6 | 1.6 | 5.2 | 2.6–9.6 | 0.8 |
| Within-meal size | |||||||||
| Within-meal feeding (g) | 2.81 | 1.96–3.92 | 0.2 | 2.43 | 1.26–4.61 | 0.3 | 2.91 | 1.98–4.32 | 0.2 |
| Within-meal drinking (mL) | 3.13 | 2.5–5.13 | 0.2 | 2.28 | 0.80–3.45 | 0.3 | 3.29 | 2.55–5.64 | 0.2 |
| Food:Liquid ratio (g/mL) | 1.08 | 0.9–1.31 | 0.0 | 1.45 | 0.86–2.75 | 0.2 | 1.03 | 0.78–1.24 | 0.0 |
| Percentage of meals in dark phase | 83 | 76–91 | 2 | ||||||
| Total 24h food intake (g) | 24.00 | 21.53–25.98 | 0.5 | ||||||
| Total 24h liquid intake (mL) | 37.60 | 30.87–47.15 | 1.6 | ||||||
| Total 24h food:liquid ratio (g/mL) | 0.65 | 0.53–0.75 | 0.0 | ||||||
3.2 Expt 2) How do Composite Meal Structure Parameters Change as Dehydration-Anorexia Develops?
3.2.1 Composite Meal Duration and Temporal Distribution
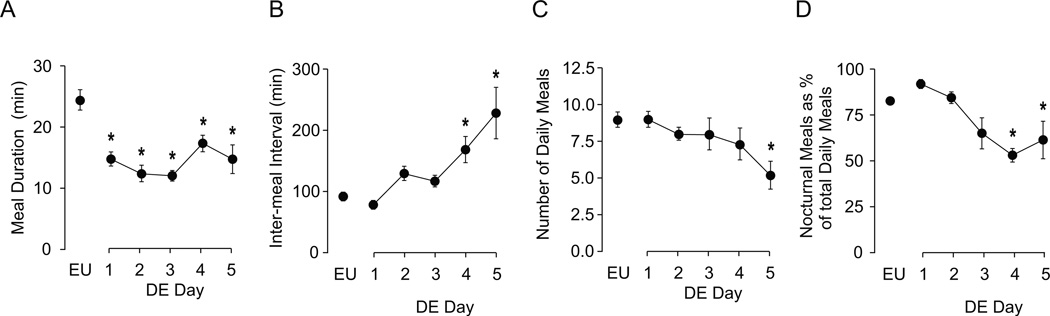
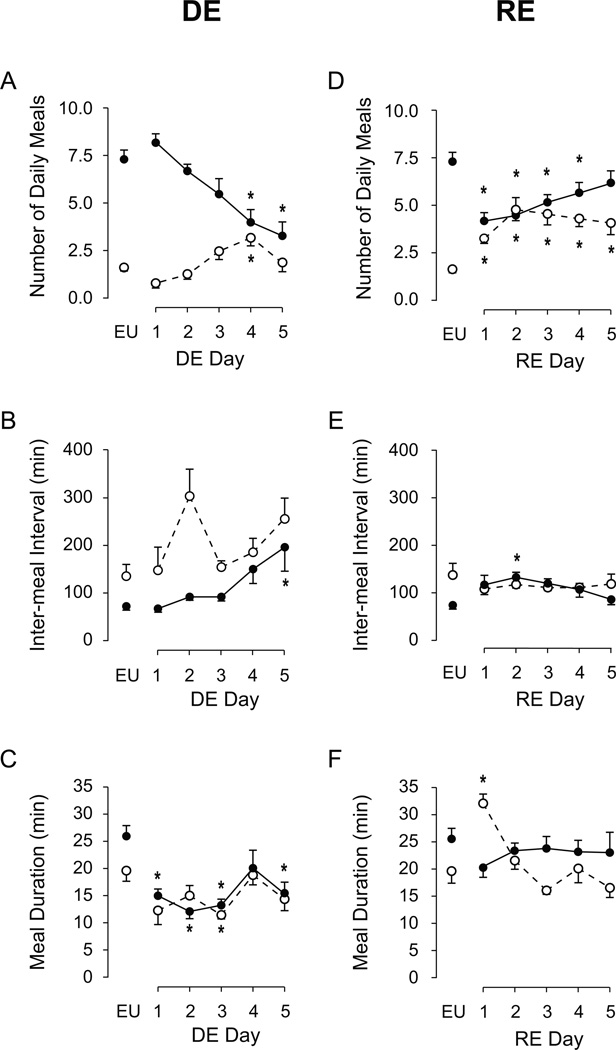
Drinking HS affected some but not all composite meal parameters. Figure 2A shows that composite meal duration was already significantly decreased from baseline on the first day, and continued to be shorter than controls throughout the remaining DE period (F [df 5, 9]=11.66, P<0.001). Not surprisingly, this was accompanied by a gradual increase in IMI (Fig. 2B), which became statistically significantly on days 4 and 5 (F [df 5, 23.539]=8.43, P<0.001). In contrast, there was no significant effect of drinking HS on meal number until the final day (Fig. 2C) when there was a small but significant suppression (F [df 5, 24.79]=2.80, P<0.05). Despite no significant change in the number of meals until Day 5, there was a decrease in the percentage of nocturnal meals by Day 3 (Fig. 2D), which reached significance on Days 4 and 5 (F [df 5, 9]=7.10, P<0.001).
Figure 2. Effects of DE progression on composite meal patterns.
Mean (± SEM) composite meal duration (A), IMI (B), total number of composite meals (C), and percentage of total daily meals initiated during the nocturnal phase (D), as measured daily over the course of five days of drinking hypertonic saline (DE). The values from euhydrated control animals (EU) are the baseline values shown in Table 1. Water was replaced with 2.5% saline at 1000 hours on Day 1, and remained the only fluid available for the next five days. Significant differences across days were determined using one-way ANOVA (see text for results). Symbols denote significant individual differences between EU and subsequent days of DE, where *P<0.05.
3.2.2 Within-Meal Characteristics
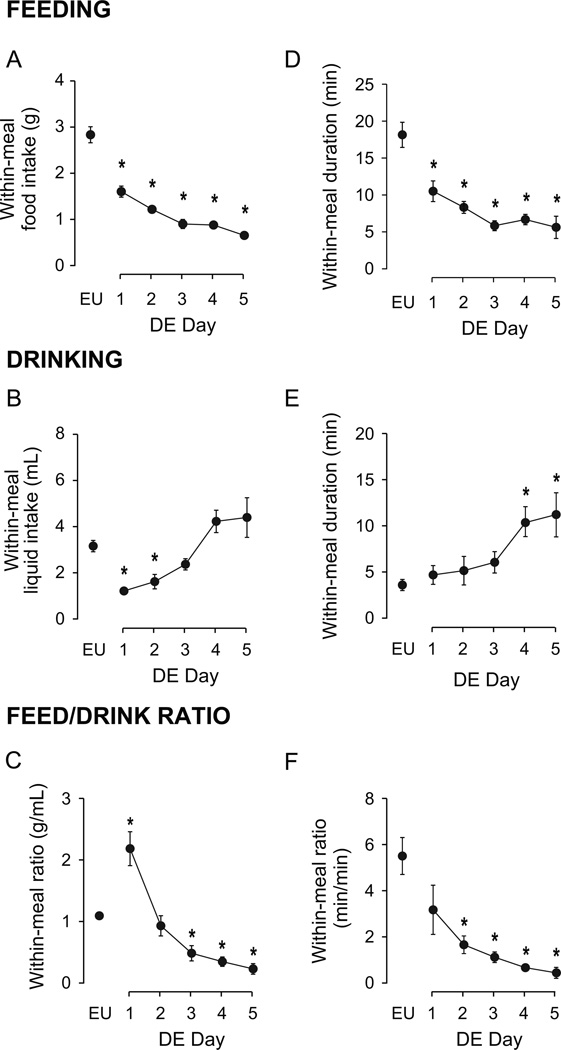
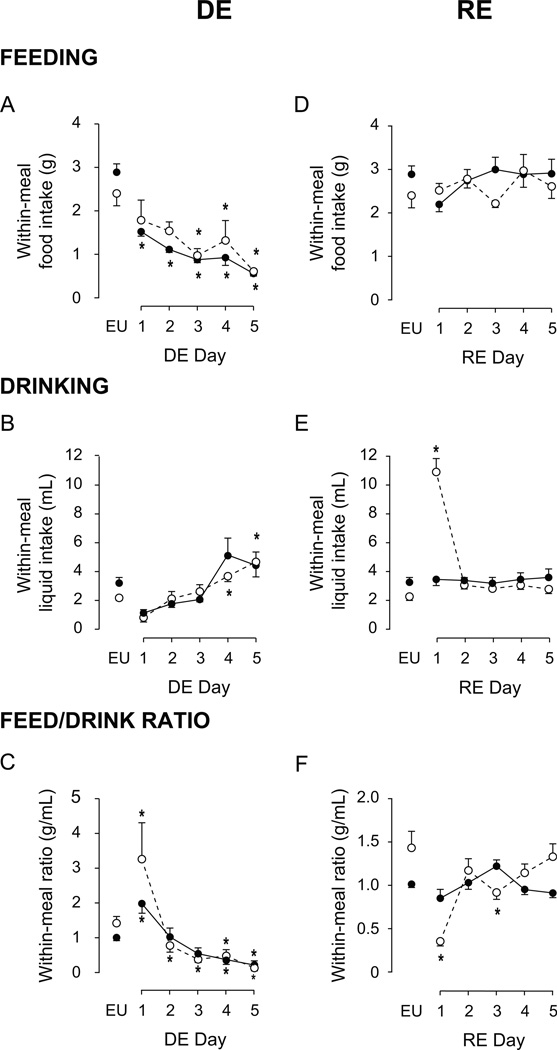
The development of DE-anorexia led to significant alterations of within-meal characteristics compared to EU animals (Fig. 3). These were evident from the first day of drinking HS, which progressively reduced the average amount of food consumed within a meal and the duration of feeding (Figs. 3A and 3D; F [df 5, 9]=69.75 and 28.51, respectively; both P<0.001).
Figure 3. Effects of DE progression on within-meal characteristics.
Mean (± SEM) within-meal food intake (A), within-meal liquid intake (B), within-meal food:liquid intake ratio (C), within-meal feeding duration (D), within-meal drinking duration (E), within-meal feeding:drinking duration ratio (F), as measured daily over the course of five days of DE. Water was replaced with 2.5% saline at 1000 hours on Day 1, and remained the only fluid available for the subsequent five days. EU represents baseline values as shown in Table 1. Significant differences across days were determined using one-way ANOVA, see text for results; Symbols denote significant individual differences between EU and subsequent days of DE, where *P<0.05.
The number of days spent drinking HS significantly affected both within-meal liquid intake (Fig. 3B; F [df 5, 21.25]=24.13, P<0.001) and drinking duration (Fig. 3E; F [df 5, 22.42]=5.78, P<0.01). However, the changes in drinking values from baseline EU were neither unidirectional nor universal as DE progressed. Within-meal liquid intake was significantly less than EU intake only on the first two days of DE. While no individual differences were detected between Days 3–5 and EU baseline, there was a marked upward trend such that within-meal drinking was more than double on Days 4 and 5 compared to Day 3. Within-meal drinking duration significantly increased from EU on Days 4 and 5 of DE. This variability of within-meal drinking was also revealed in the within-meal food-to-liquid ratios, with a significant effect of DE progression on both within-meal food-to-liquid intake ratio (Fig. 3C; F [df 5, 22.48]=29.91, P<0.001) and duration ratio (Fig. 3F; F [df 5, 21.30]=11.20, P<0.001). See Figure 3 for individual differences.
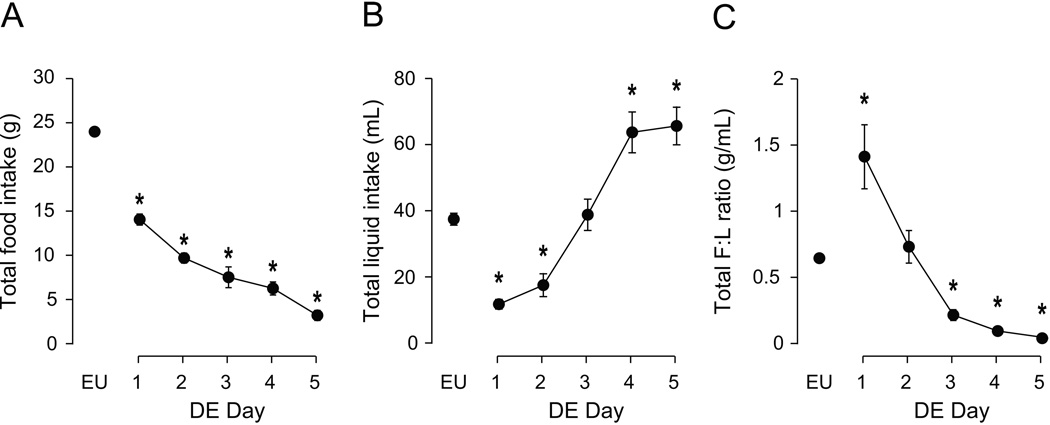
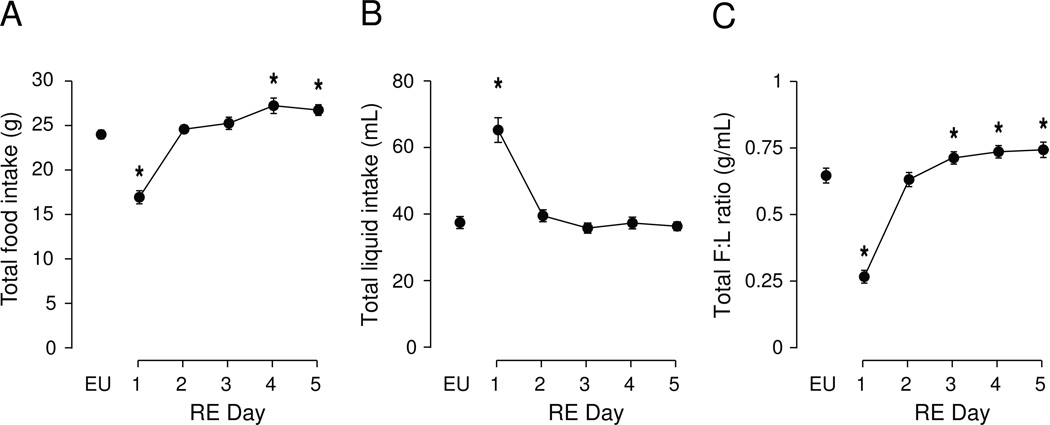
As we have previously reported [2], drinking HS had clear effects on total daily intake of food and HS (Fig. 4). There was a significant effect of time on total food intake (Fig. 4A; F [df 5, 9]=132.7, P<0.001), total HS intake (Fig. 4B; F [df 5, 9]=33.02, P<0.001), and on the ratio of total food-to-total HS intake (Fig. 4C; F [df 5, 9]=7.10, P<0.001). There were progressive daily decreases in total food intake compared to EU, and bidirectional effects over the 5 days of drinking HS for total HS intake and total intake ratio (see Fig. 4 for post-hoc analyses).
Figure 4. Effects of DE progression on total daily intake of food, hypertonic saline, and food:saline intake ratio.
Mean (± SEM) total daily food intake (A), saline intake (B), and the food:saline intake ratio (C) as measured daily over the course of five days of DE. Water was replaced with 2.5% saline at 10.00h on Day 1, and remained the only fluid available for the subsequent five days. EU represents baseline values as shown in Table 1. Significant differences across days were determined using one-way ANOVA, see text for results; Symbols denote significant individual differences between EU and subsequent days of RE, where *P<0.05.
3.3 Expt 3) How Does Composite Meal Structure Change After Drinking Water Is Returned?
Table 2 shows how composite and within-meal characteristics changed in the five days following the return of drinking water. Meal number gradually and significantly increased following water back (F [df 5, 9]=4.99, P<0.001), although none of these values were significantly different from the EU control meal number. IMI gradually declined and was indistinguishable from EU baseline values by Day 4 (F [df 5, 9]=3.62, P<0.01).
TABLE 2.
The mean (± SEM) effects of returning drinking water (RE) on the composite meal and within-meal structure for the subsequent 5 days. Significant differences across days were determined using one-way ANOVA, see text for results.
| Mean (± SEM) |
Dunnett’s post-hoc vs EU |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RE Day | |||||||||||
| Measure | EU | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 |
| Number of composite meals | 9.0 (0.5) | 7.6 (0.5) | 9.3 (0.4) | 9.8 (0.7) | 10.0 (0.6) | 10.3 (1.0) | ns | ns | ns | ns | ns |
| Inter-meal Interval (min) | 87.7 (5.7) | 116.8 (8.0) | 120.5 (5.2) | 113.9 (6.0) | 104.2 (6.1) | 97.0 (9.0) | * | * | * | ns | ns |
| Composite meal duration (min) | 24.6 (1.6) | 25.4 (1.7) | 22.0 (1.3) | 20.5 (1.5) | 21.2 (1.9) | 20.5 (2.8) | ns | ns | * | ns | * |
| Within-meal feeding (min) | 18.1 (1.6) | 15.7 (1.4) | 15.8 (1.2) | 14.7 (1.6) | 15.1 (1.7) | 14.9 (2.5) | ns | ns | ns | ns | ns |
| Within-meal drinking (min) | 3.7 (0.5) | 7.3 (0.6) | 3.7 (0.3) | 3.6 (0.1) | 3.7 (0.2) | 3.9 (0.6) | * | ns | ns | ns | ns |
| Food:Liquid ratio (min/min) | 5.5 (0.8) | 2.2 (0.2) | 4.5 (0.5) | 4.1 (0.4) | 4.1 (0.4) | 4.1 (0.4) | * | ns | * | * | * |
| Within-meal size | |||||||||||
| Within-meal feeding (g) | 2.8 (0.2) | 2.3 (0.1) | 2.7 (0.1) | 2.7 (0.2) | 2.8 (0.2) | 2.8 (0.3) | * | ns | ns | ns | ns |
| Within-meal drinking (mL) | 3.1 (0.2) | 6.8 (0.6) | 3.2 (0.2) | 3.0 (0.3) | 3.3 (0.4) | 3.4 (0.5) | * | ns | ns | ns | ns |
| Food:Liquid ratio (g/mL) | 1.1 (0.0) | 0.6 (0.1) | 1.1 (0.1) | 1.1 (0.0) | 1.0 (0.1) | 1.0 (0.0) | * | ns | ns | ns | ns |
| Percentage of meals in dark phase | 82.9 (1.7) | 54.1 (2.7) | 49.4 (4.3) | 54.7 (3.6) | 56.3 (3.5) | 61.2 (3.8) | * | * | * | * | * |
Symbols denote significant individual differences between EU and subsequent days of RE where *P<0.05.
There was a significant effect of time on composite meal duration and on the percentage of daily meals occurring in the dark. Significantly shorter meals compared to EU controls were seen on days 3 and 5 (F [df 5, 9]=8.12, P<0.001), while the percentage of daily meals occurring in the dark remained significantly lower that EU controls for all 5 days after the return of water (F [df 5, 9]=15.73 P<0.001).
From the second day following the return of water, both within-meal food intake and feeding duration returned to and remained at baseline values, with no detectable significant differences for the remaining 4 days (F [df 5, 9]=2.24 and 1.84, respectively; P=0.067 and 0.124, respectively). A significant reduction in within-meal food intake was present on the first day of drinking water.
After the return of drinking water there was a significant effect of time on both within-meal water intake and drinking duration (F [df 5, 9]=30.44 and 13.77, respectively; P<0.001 for both). There was also a significant effect of time on both within-meal food-to-liquid intake ratio (F [df 5, 9]=11.78, P<0.001) and duration ratio (F [df 5, 9]=6.05, P=0.001). However, it is clear from the results of the individual post-hoc comparisons that the significant differences of within-meal drinking-related and ratio parameters were skewed by the robust drinking that occurred immediately following the return of water. When all within-meal drinking-related data sets were re-analyzed after excluding data from the first 24h following the return of water, no significant differences for any parameter across the remaining 4 days were detected.
As we have previously noted [2], returning water to DE-anorexic rats significantly altered the total 24h intake of food and water for several subsequent days (Fig. 5). There was a significant effect of time on total food intake (Fig. 5A. F [df 5, 9]=46.31, P<0.001). During the first day after water-back, food intake was significantly suppressed from EU levels, but recovered to baseline levels by Day 2, and exceeded this amount by Day 4. A significant effect of RE progression on total water intake was also detected (F [df 5, 9]=51.75, P<0.001), due primarily to the robust drinking that occurs in the first 24 hours following water-back; by Day 2, drinking volumes returned to baseline. Total food-to-total water intake ratio were also altered by RE (F [df 5, 9]=101.8, P<0.001). Following an initial disruption in the ratio caused by the water-back drinking in the first 24 hours, the ratio between feeding and drinking was transiently restored to baseline on Day 2, and then remained elevated from Day 3 to 5. See Figure 5 for statistical results from comparison of individual days.
Figure 5. Total daily intake of food, water, and food:water intake ratio during the five days following water-back.
Mean (± SEM) total daily food intake (A), water intake (B), and the food:water intake ratio (C) as measured daily for five days following the return of water to DE rats at 12.00h on Day 1. EU represents baseline values as shown in Table 1. Significant differences across days were determined using one-way ANOVA (see text for results). Symbols denote significant individual differences between EU and subsequent days of RE, where *P<0.05.
3.4 Summary of changes to meal structure during periods of DE and RE
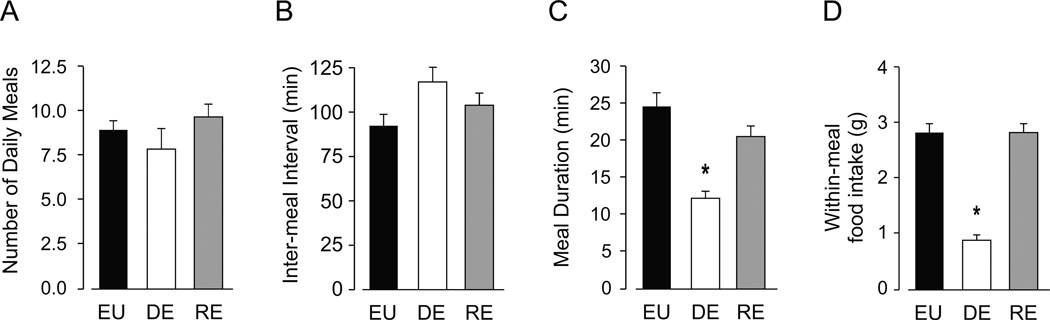
To summarize the main findings from Experiments 1–3, representative data from the four main meal parameters from each phase are shown in Figure 6. The comparison of baseline EU data, and data collected on the third day of the DE phase and the fourth day of the RE phase, demonstrates the effects of DE-anorexia and the return of water on composite meal patterning. Day 3 of DE was chosen to reflect the initial anorexic response to drinking HS, and Day 4 of RE was chosen to illustrate the meal parameters after they had all returned to baseline. While neither DE nor RE had a significant effect on daily meal number (Fig. 6A) or IMI (Fig 6B), DE significantly reduced both the duration of a composite meal (Fig. 6C) and the amount of food consumed within a meal (Fig. 6D). The return of water restored meal duration and meal size to EU values (Figs. 6C and 6D).
Figure 6. Summary of the effects of DE and RE on composite meal structure.
The comparison of baseline EU data (black bars), and data collected on the third day of the DE phase (white bars) and the fourth day of the RE phase (gray bars), demonstrates the effects of DE-anorexia and the return of water on daily meal number (A), duration of the IMI (B), duration of a composite meal (C), and amount of food consumed within a meal (D). Symbols denote significant differences from EU values, where *P<0.05; see sections 3.2 and 3.3 for detailed results.
3.5 Expt 4) Is the Temporal Distribution of Composite Meals between the Light and Dark Phases of the Day modified during and after Dehydration-Anorexia?
3.5.1 Meal Parameters in Euhydrated Animals
There were no significant differences in the five-day composite meal parameters for EU animals when they were examined within the light and dark phases. The values for each of the five days were used to calculate a mean for the composite meal parameters (Table 1). These values were then used to determine the effects of drinking HS and the return of water on the distribution of composite meal characteristics between the light and dark phases (Figs. 6 & 7).
Figure 7. Diurnal variations of composite meal patterns during DE and RE.
Effects of the daily progression of DE (A–C) and RE (D–F) on mean (± SEM) number of meals (A, D), IMI (B, E), and composite meal duration (C, F), during the dark phase (closed circles, solid lines) and light phase (open circles, dashed lines). In each graph, EU represents baseline values as shown in Table 1; Day 1 indicates the first morning of water replacement with HS for DE, or return of water for RE. Significant differences across days, for each phase, were determined using one-way ANOVA (see text for results). Symbols denoting individual differences from EU phase baseline, where *P<0.05.
3.5.2 Composite Meal Parameters in Dehydrated-Anorexic Animals Before and After the Return of Drinking Water
As DE-anorexia developed, there was a significant decline in number of nocturnal meals (Fig. 7A; F [df 5, 24.84]=10.06, P<0.001), while the number of diurnal meals increased through Day 4 (F [df 5, 24.74]=4.87, P<0.01). A significant increase in IMI was only seen during the nocturnal phase (Fig. 7B; nocturnal: F [df 5, 21.99]=3.89, P<0.05; diurnal: F [df 5, 17.76]=2.23, P=0.097), although high variability in diurnal values may have influenced these results. Significant decreases in composite meal duration were detected for both the nocturnal (Fig. 7C; F [df 5, 23.89]=6.34, P<0.001) and diurnal (F [df 5, 18.66]=3.31, P<0.05) phases. After water was returned, the significant decrease in the number of nocturnal meals (Fig. 7D; F [df 5, 9]=9.30, P<0.001), and increase in the number of diurnal meals (F [df 5, 9]=9.69, P<0.001) persisted for up to five days. A small increase in IMI was only detectable nocturnally after the return of water (Fig. 7E; F [df 5, 9]=2.69, P<0.05), while there was no significant change in diurnal IMI duration (F [df 5, 9]=0.83, P=0.536). With the exception of diurnal RE Day 1, composite meal duration immediately returned to EU values for both dark (Fig. 7F; F [df 5, 9]=1.15, P=0.3507), and light (F [df 5, 9]=13.50, P<0.001) phases.
3.5.3 Within-Meal Parameters in Dehydrated-Anorexic Animals Before and After the Return of Drinking Water
How the diurnal/nocturnal within-meal parameters change as DE-anorexia develops and after water is returned is shown in Figure 8. Drinking HS led to a significant and immediate decrease in nocturnal within-meal food intake (Fig. 8A; F [df 5, 23.72]=33.16, P<0.001). A significant effect on diurnal within-meal food intake was delayed and emerged 2 days later (F [df 5, 18.45]=33.16, P<0.001). Drinking HS also significantly altered diurnal (Fig. 8B; F [df 5, 13.60]=7.22, P<0.01) and nocturnal (F [df 5, 20.07]=17.47, P<0.001) within-meal liquid intake. Changes in within-meal food and water intake, in turn, altered meal food-to-liquid intake ratio during the light (Fig. 8C; F [df 5, 11.33]=11.96, P<0.001), and dark phases (F [df 5, 20.54]=15.14, P<0.001). After water was returned, there were no significant differences in diurnal nor nocturnal within-meal food intake (Fig. 8D; F [df 5, 9]= 1.65 and 1.38, respectively; both P>0.05). The return to drinking water had no effect on nocturnal within-meal drinking (Fig. 8E; F [df 5, 9]=0.21, P=0.96), but as would be expected, had a robust and significant effect on diurnal within-meal drinking immediately following the return of water (F [df 5, 9]=73.09, P<0.001). The return of water significantly affected the diurnal (Fig. 8F; F [df 5, 9]=11.34, P<0.001) and nocturnal (F [df 5, 9]=3.58, P<0.01) within-meal food-to-water intake ratio. (See Fig. 8 for post-hoc statistical comparisons of individual DE and RE days.)
Figure 8. Diurnal variations of within-meal food and liquid intake during DE and RE.
Effects of the daily progression of DE (A–C) and RE (D–F) on mean (± SEM) within-meal food intake (A, D), within-meal liquid intake (B, E), and the food:liquid intake ratio (C, F), during the dark phase (closed circles, solid lines) and light phase (open circles, dashed lines). In each graph, EU represents baseline values as shown in Table 1; Day 1 indicates the first morning of water replacement with hypertonic saline for DE, or return of water for RE. Significant differences across days, for each phase, were determined using one-way ANOVA, see text for results; symbols denoting individual differences from EU phase baseline, where *P<0.05
4. DISCUSSION
Here we have explored how the development and reversal of DE-anorexia affects specific components of ingestive behavior. Our results demonstrate that DE-anorexia derives from a reduction in meal size while the number of daily meals is virtually unchanged. This pattern is consistent with the hypothesis we have previously proposed where meal initiation is unimpeded, but meals are terminated prematurely [1, 15]. We also find that as drinking HS continues fewer meals are taken during the dark period and more are taken during the light period, an adaptation that persists for some days following the return of drinking water.
4.1 DE-Anorexia: Meal Size and Inter-meal Interval
Our study demonstrates a clear, rapid, and dramatic effect of drinking HS on meal size. This was manifest on the first day of drinking HS as a reduction in composite meal duration, together with reductions in both the amount of food consumed within a meal and feeding duration. Together, these results suggest that drinking HS increases either the levels of factors that regulate satiation, the brain’s sensitivity to these factors, or both.
Pretel and Piekut [16] reported that drinking HS increased oxytocin (OT) mRNA in neurons in the caudal parvicellular part of the PVH that project to the spinal cord and dorsal vagal complex (DVC), including the nucleus of the solitary tract (NTS) [17, 18]. Both cholesystokinin (CCK) and OT decrease food intake [19], perhaps by way of convergent forebrain and hindbrain mechanisms [20, 21]. Furthermore, DE-anorexia is attenuated in OT-knock out mice [22], although this study used a somewhat different model than the one we employ. Therefore, it seems reasonable to speculate that one mechanism that decreases meal size after drinking HS involves projections from OT neurons in the PVH to CCK-sensitive neurons in the NTS.
In addition to the rapid decrease in meal size, we also observed a significant but more delayed increase in IMI, which became evident by the fourth day of drinking HS. Increased IMI derives from prolonged post-prandial satiety [23], suggesting that as DE-anorexia develops, additional inhibitory mechanisms are recruited to potentiate the suppression of feeding. Several studies show that satiety factors such as glucagon-like peptide-1 (GLP-1) [24, 25] work in combination with CCK to promote meal termination and prolong onset of the subsequent meal. GLP-1-containing neurons in the DVC are innervated by the same OT neurons of the PVH that project to CCK-sensitive neurons in the NTS [26, 27]. Since OT-dependent inhibition of food intake requires functional GLP-1 receptors in the DVC [26], altered function within an OT-CCK-GLP-1 network may contribute to DE-anorexia by decreasing meal size and increasing IMI.
4.2 DE-Anorexia: Meal Number
The fact that the number of meals taken during each 24h period remains unchanged for at least 4 days of drinking HS while meal size decreases significantly within the first 24h suggest that DE-anorexia results from dysfunction in the ability to maintain the duration of a meal once it is initiated. This conclusion is further supported by examining the way that neuropeptide Y (NPY) mechanisms function during DE-anorexia. NPY is an important factor that regulates both meal initiation and meal size. Central NPY administration increases food intake using two mechanisms: by stimulating the appetitive rather than the consummatory phase of feeding behavior [28–30], and by delaying the onset of satiation [30, 31].
Two findings from our group suggest that NPY-dependent meal initiation is intact in DE-anorexic animals. First, NPY and AgRP mRNAs are upregulated in the ARH of DE-anorexic rats [3, Salter-Venzon & Watts, unpublished findings], which is consistent with an increased drive to feed mediated by the reduced circulating leptin seen in these animals [3]. Second, NPY given at low doses to either the PVH or the LHA of DE-anorexic animals is unable to sustain food intake at the levels seen in EU rats. Importantly however, these animals show no difference in the latency to begin feeding following NPY compared to euhydrated controls [15], meaning that NPY’s ability to initiate feeding remains intact in DE-anorexic rats. Collectively, these results suggest that only NPY’s ability to delay satiation onset is dysregulated in DE-anorexia.
A recent study using viral-mediated over-expression of NPY in the PVH or LHA found that NPY actions on meal initiation and termination are dissociable and are mediated by different sets of hypothalamic neurons [32]. Thus, NPY over-expression in the PVH or LHA both increase food intake, but have differential effects on meal structure; over-expression in the PVH increased meal number, while over-expression in LHA increased meal size. Taken together with Tiesjema et al [32] and Salter-Venzon & Watts [15], our finding that meal number is unaffected by drinking HS suggests that NPY’s actions in the PVH to initiate feeding remains intact, whereas decreased meal size may result from dysregulated NPY function in the LHA.
Decreased meal size is usually compensated by an increase in the daily meal number. For example, CCK administration alone typically does not decrease overall food intake, because its suppressive effect on meal size is usually accompanied by an increase in meal number [33]. However, drinking HS does not markedly alter the number of daily meals initiated over the course of the day, despite the reduction in meal size. Therefore, our results demonstrate that DE-anorexia develops because the dramatic decrease in meal size is not offset by an increase in meal number.
4.3 DE-Anorexia: The Distribution of Meals between the Dark and Light Phases of the Day
A previous study from our group measured total food and liquid intake during the day and night, and concluded that the mechanisms responsible for organizing circadian timing patterns of ingestive behaviors were unaffected by drinking HS [2]. However, when we examine the detailed temporal distribution of all meals rather than just total intake, we now find much more striking and complex effects that emphasize that understanding how, not just how much is consumed greatly improves our understanding of feeding behavior [8].
First, there is a gradual but significant shift in the temporal distribution of meals from the dark to the light period as DE-anorexia develops. By the fourth day of drinking HS the number of diurnal meals is significantly increased, while nocturnal meal number steadily declines to about 50% of control values. In contrast, at least 80% of daily meals occur during the dark phase of control EU animals. Second, although drinking HS reduces nocturnal within-meal food intake as early as the first dark phase, it is not until the third day that diurnal within-meal food intake is significantly attenuated. Third, once drinking water is resumed significant numbers of meals continue to be taken during the light period meaning that the ability to distribute meals appropriately throughout a 24h period is surprisingly slow to recover. This contrasts with the (satiety) mechanisms that regulate the duration and IMI of individual meals, which rapidly recover.
These findings show the significant impact of drinking HS on the mechanisms that temporally distribute meals relative to circadian timing signals, which contrasts to the more resilient mechanisms that initiate individual meals. The surprising number of diurnal meals seen long after drinking water is resumed may be a favorable adaptation that allows animals to rapidly correct the negative energy balance accrued during DE-anorexia using meals with normally structured satiety mechanisms.
4.4 Thirst and Fluid Balance
Although the suppression of feeding is a major part of the physiological adaptation to cellular dehydration, drinking HS for up to 5 days is a potent stimulus for thirst, meaning that the drive to find and consume water becomes increasingly strong. In this context, a drinking-explicit meal pattern analysis [11] provides a powerful way to explore the within-meal relationship between eating and drinking as cellular dehydration develops. With this method we find that the gradually developing cellular dehydration induced by drinking HS modifies both the within-meal and 24h food-to-liquid intake, and that this altered ratio varies as dehydration becomes exacerbated.
Euhydrated rats consistently maintain an approximately one-to-one within-meal, and a slightly more than one-to-two food-to-liquid intake ratio over a 24h period [2, 10, 11]. This tight interaction between food and water intake is maintained during periods of imposed food restriction [Watts, A.G., unpublished observations], showing that the relationship between hunger and thirst mechanisms is maintained when food intake is reduced but access to water is retained. However, during the first day of drinking HS, rats decrease their drinking to a significantly greater extent than their within-meal food intake, leading to a markedly elevated food-to-fluid intake ratio. This most likely derives from an initial strong aversion to drinking HS. But by the third day HS intake increases significantly—presumably driven by elevated thirst—so that both the within-meal and the 24h food-to-liquid intake ratios were significantly reduced compared to euhydrated controls.
These findings show that as HS is consumed there are significant modifications to the neural networks coordinating eating and drinking that are not seen with food restriction alone. The neural substrates of these modifications are not known but may involve changes in neuropeptide gene expression in the bed nuclei of the stria terminalis, amygdala, and LHA that show complex temporal expression patterns as DE-anorexia develops [34] or are not seen with paired food restriction [3].
4.5 Conclusion
We show that the most important factor underlying the development of DE-anorexia is not the ability to initiate a meal (Fig. 6A), but a failure to maintain feeding behavior once a meal has started (Figs. 6C & 6D). This outcome most likely results from of an increased sensitivity to satiation signals, and possibly post-prandial satiety mechanisms. Furthermore, DE-anorexia also significantly disrupts the temporal distribution of meals across the day so that the number of nocturnal meals is gradually reduced while diurnal meal number increases. Our high-resolution meal pattern analysis shows that not all aspects of food intake are inhibited during DE-anorexia. Instead, drinking HS selectively and differentially targets those neural networks controlling meal initiation and meal termination.
These results support a functional neural network model where drinking HS upregulates the activity of both stimulatory and inhibitory networks [1, 35]. While stimulatory mechanisms preserve a relatively normal frequency of meal initiation, elevated activity within inhibitory networks leads to premature meal termination and a net decrease in food intake. The rapid reinstatement of feeding that follows the return of water reveals a third network that disinhibits feeding. While this disinhibitory network is likely responsible for the initial drive to feed, the 24 hour delay we see in meal pattern normalization after the return of water suggests that full attenuation of up-regulated inhibitory networks requires significant time to dissipate once drinking water is resumed [1, 36].
Highlights.
Meal pattern analysis reveals how food intake is disrupted in dehydration-anorexia
Anorexia develops because meals are smaller and shorter than controls
The number of meals decreases during the night and increases during the day
Total daily food intake quickly recovers when anorexia is reversed
Diurnal meal number remains elevated for up to five days after anorexia is reversed
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Susan Melhorn, Eric Krause, and Randall Sakai for their contribution to the analysis of these data. This work was supported by PHS grant MH-066168 to AGW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Watts AG, Boyle CN. The functional architecture of dehydration-anorexia. Physiol Behav. 2010;100:472–477. doi: 10.1016/j.physbeh.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watts AG. Dehydration-associated anorexia: development and rapid reversal. Physiol Behav. 1999;65:871–878. doi: 10.1016/s0031-9384(98)00244-3. [DOI] [PubMed] [Google Scholar]
- 3.Watts AG, Sanchez-Watts G, Kelly AB. Distinct patterns of neuropeptide gene expression in the lateral hypothalamic area and arcuate nucleus are associated with dehydration-induced anorexia. J Neurosci. 1999;19:6111–6121. doi: 10.1523/JNEUROSCI.19-14-06111.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watts AG, Sanchez-Watts G. Rapid and preferential activation of Fos protein in hypocretin/orexin neurons following the reversal of dehydration-anorexia. J Comp Neurol. 2007;502:768–782. doi: 10.1002/cne.21316. [DOI] [PubMed] [Google Scholar]
- 5.Kissileff HR. Ingestive behavior microstructure, basic mechanisms and clinical applications. Neurosci Biobehav Rev. 2000;24:171–172. doi: 10.1016/s0149-7634(99)00071-8. [DOI] [PubMed] [Google Scholar]
- 6.Geary N. A new way of looking at eating. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1444–R1446. doi: 10.1152/ajpregu.00066.2005. [DOI] [PubMed] [Google Scholar]
- 7.Brobeck JR. Neural regulation of food intake. Ann N Y Acad Sci. 1955;63:44–55. doi: 10.1111/j.1749-6632.1955.tb36544.x. [DOI] [PubMed] [Google Scholar]
- 8.Smith JC. Microstructure of the rat's intake of food, sucrose and saccharin in 24-hour tests. Neurosci Biobehav Rev. 2000;24:199–212. doi: 10.1016/s0149-7634(99)00073-1. [DOI] [PubMed] [Google Scholar]
- 9.Kissileff HR. Food-associated drinking in the rat. J Comp Physiol Psychol. 1969;67:284–300. doi: 10.1037/h0026773. [DOI] [PubMed] [Google Scholar]
- 10.Fitzsimons TJ, Le Magnen J. Eating as a regulatory control of drinking in the rat. J Comp Physiol Psychol. 1969;67:273–283. doi: 10.1037/h0026772. [DOI] [PubMed] [Google Scholar]
- 11.Zorrilla EP, Inoue K, Fekete EM, Tabarin A, Valdez GR, Koob GF. Measuring meals: structure of prandial food and water intake of rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1450–R1467. doi: 10.1152/ajpregu.00175.2004. [DOI] [PubMed] [Google Scholar]
- 12.Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Monnikes H, et al. Activation of brain somatostatin 2 receptors stimulates feeding in mice: analysis of food intake microstructure. Physiol Behav. 2010;101:614–622. doi: 10.1016/j.physbeh.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis JD, Smith GP. Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behav Neurosci. 1992;106:217–228. [PubMed] [Google Scholar]
- 14.Wilcox RR. Applying contemporary statistical techniques. Amsterdam ; Boston: Academic Press; 2003. [Google Scholar]
- 15.Salter-Venzon D, Watts AG. Site-specific attenuation of food intake but not the latency to eat after hypothalamic injections of neuropeptide Y in dehydrated-anorexic rats. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1813–R1821. doi: 10.1152/ajpregu.00116.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pretel S, Piekut DT. Mediation of changes in paraventricular vasopressin and oxytocin mRNA content to the medullary vagal complex and spinal cord of the rat. J Chem Neuroanat. 1989;2:327–334. [PubMed] [Google Scholar]
- 17.Swanson LW, McKellar S. The distribution of oxytocin- and neurophysin-stained fibers in the spinal cord of the rat and monkey. J Comp Neurol. 1979;188:87–106. doi: 10.1002/cne.901880108. [DOI] [PubMed] [Google Scholar]
- 18.Sawchenko PE, Swanson LW. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol. 1982;205:260–272. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- 19.Olson BR, Drutarosky MD, Chow MS, Hruby VJ, Stricker EM, Verbalis JG. Oxytocin and an oxytocin agonist administered centrally decrease food intake in rats. Peptides. 1991;12:113–118. doi: 10.1016/0196-9781(91)90176-p. [DOI] [PubMed] [Google Scholar]
- 20.Blevins JE, Eakin TJ, Murphy JA, Schwartz MW, Baskin DG. Oxytocin innervation of caudal brainstem nuclei activated by cholecystokinin. Brain Res. 2003;993:30–41. doi: 10.1016/j.brainres.2003.08.036. [DOI] [PubMed] [Google Scholar]
- 21.Blevins JE, Schwartz MW, Baskin DG. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol. 2004;287:R87–R96. doi: 10.1152/ajpregu.00604.2003. [DOI] [PubMed] [Google Scholar]
- 22.Rinaman L, Vollmer RR, Karam J, Phillips D, Li X, Amico JA. Dehydration anorexia is attenuated in oxytocin-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1791–R1799. doi: 10.1152/ajpregu.00860.2004. [DOI] [PubMed] [Google Scholar]
- 23.Chapelot D, Aubert R, Marmonier C, Chabert M, Louis-Sylvestre J. An endocrine and metabolic definition of the intermeal interval in humans: evidence for a role of leptin on the prandial pattern through fatty acid disposal. Am J Clin Nutr. 2000;72:421–431. doi: 10.1093/ajcn/72.2.421. [DOI] [PubMed] [Google Scholar]
- 24.Vilsboll T, Krarup T, Sonne J, Madsbad S, Volund A, Juul AG, et al. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2003;88:2706–2713. doi: 10.1210/jc.2002-021873. [DOI] [PubMed] [Google Scholar]
- 25.Chelikani PK, Haver AC, Reidelberger RD. Intravenous infusion of glucagon-like peptide-1 potently inhibits food intake, sham feeding, and gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1695–R1706. doi: 10.1152/ajpregu.00870.2004. [DOI] [PubMed] [Google Scholar]
- 26.Rinaman L, Rothe EE. GLP-1 receptor signaling contributes to anorexigenic effect of centrally administered oxytocin in rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R99–R106. doi: 10.1152/ajpregu.00008.2002. [DOI] [PubMed] [Google Scholar]
- 27.Baskin DG, Kim F, Gelling RW, Russell BJ, Schwartz MW, Morton GJ, et al. A New Oxytocin-Saporin Cytotoxin for Lesioning Oxytocin-Receptive Neurons in the Rat Hindbrain. Endocrinology. 2010 doi: 10.1210/en.2010-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seeley RJ, Payne CJ, Woods SC. Neuropeptide Y fails to increase intraoral intake in rats. Am J Physiol. 1995;268:R423–R427. doi: 10.1152/ajpregu.1995.268.2.R423. [DOI] [PubMed] [Google Scholar]
- 29.Ammar AA, Nergardh R, Fredholm BB, Brodin U, Sodersten P. Intake inhibition by NPY and CCK-8: A challenge of the notion of NPY as an "Orexigen". Behav Brain Res. 2005;161:82–87. doi: 10.1016/j.bbr.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Baird JP, Gray NE, Fischer SG. Effects of neuropeptide Y on feeding microstructure: dissociation of appetitive and consummatory actions. Behav Neurosci. 2006;120:937–951. doi: 10.1037/0735-7044.120.4.937. [DOI] [PubMed] [Google Scholar]
- 31.Lynch WC, Hart P, Babcock AM. Neuropeptide Y attenuates satiety: evidence from a detailed analysis of patterns ingestion. Brain Res. 1994;636:28–34. doi: 10.1016/0006-8993(94)90171-6. [DOI] [PubMed] [Google Scholar]
- 32.Tiesjema B, Adan RA, Luijendijk MC, Kalsbeek A, la Fleur SE. Differential effects of recombinant adeno-associated virus-mediated neuropeptide Y overexpression in the hypothalamic paraventricular nucleus and lateral hypothalamus on feeding behavior. J Neurosci. 2007;27:14139–14146. doi: 10.1523/JNEUROSCI.3280-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.West DB, Fey D, Woods SC. Cholecystokinin persistently suppresses meal size but not food intake in free-feeding rats. Am J Physiol. 1984;246:R776–R787. doi: 10.1152/ajpregu.1984.246.5.R776. [DOI] [PubMed] [Google Scholar]
- 34.Watts AG, Kelly AB, Sanchez-Watts G. Neuropeptides and thirst: the temporal response of corticotropin-releasing hormone and neurotensin/neuromedin N gene expression in rat limbic forebrain neurons to drinking hypertonic saline. Behav Neurosci. 1995;109:1146–1157. doi: 10.1037//0735-7044.109.6.1146. [DOI] [PubMed] [Google Scholar]
- 35.Watts AG. Understanding the neural control of ingestive behaviors: helping to separate cause from effect with dehydration-associated anorexia. Horm Behav. 2000;37:261–283. doi: 10.1006/hbeh.2000.1581. [DOI] [PubMed] [Google Scholar]
- 36.Boyle CN, Watts AG. Evidence for multiple inhibitory feeding signals in dehydration anorexia. Appetite. 2009;52:821. [Google Scholar]