Abstract
This magnetic resonance imaging study demonstrates increased lateral ventricle volume (LVV) in adolescents and adults with bipolar disorder (BD) with psychotic symptoms, but not without psychosis, compared to healthy adolescents and adults. This suggests LVV is a morphologic feature associated with psychosis in BD, present by adolescence.
Keywords: mood disorder, MRI, ventricles
1. Introduction
Varying reports of increases or no differences in lateral ventricle volume (LVV) in adults with bipolar disorder (BD) (Andreasen et al., 1990; Ali et al., 2001; Brambilla et al., 2001; McDonald et al., 2006; Kempton et al.; 2008; Rosa et al., 2010) could result from clinical heterogeneity (Strasser et al., 2005). Increased LVV is a consistent finding in psychotic disorders (Wright et al., 2000), long implicated in psychosis development (Johnstone et al., 1976). This study tested the hypothesis LVV is increased in adolescents and adults with BD with psychotic symptoms (PBD), but not in those who have not experienced psychosis (NPBD).
2. Methods
Thirty-six individuals with PBD (ages 14–56yrs, 61% female, 33% adolescents ≤21years), 48 with NPBD (10–59yrs, 58% female, 35% adolescents) and 79 healthy comparison (HC) participants without personal history or first-degree relative with an Axis I disorder (10–57yrs, 54% female, 42% adolescents) (non-LVV data on 64 included in Kalmar et al., 2009; Womer et al., 2009) were recruited from Yale University and Veterans Affairs medical centers and advertisement in the community. Written informed consent was obtained from parents/guardians of minors and participants ≥18yrs, and written assent from minors, in accordance with institutional review boards of the Yale School of Medicine and Department of Veterans Affairs.
The presence or absence of DSM IV Axis I Disorders and mood state at scanning were confirmed by administration of the revised Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime to participants ≤18yrs and their parents/guardians (Kaufman et al., 1997), or Structured Clinical Interview for DSM-IV Axis I Disorders for participants over 18yrs (First et al., New York State Psychiatric Institute, 2002). PBD was defined by history of hallucinations or delusions determined by consensus of subject self-report, structured and clinical interviews, and discussion with treaters. No participant had head trauma with loss of consciousness over 5min, or a major neurological or medical disorder, except 5 BD participants with treated hypothyroidism.
Twenty-one percent (7 PBD, 11 NPBD) of BD participants were unmedicated at scanning; the remainder was prescribed lithium carbonate (10 PBD, 10 NPBD), anticonvulsants (16 PBD, 22 NPBD), atypical antipsychotics (17 PBD, 19 NPBD), antidepressants (11 PBD, 19 NPBD) or benzodiazepines (5 PBD, 7 NPBD). One BD subject was never-medicated. Sixty-one percent of BD participants were euthymic (21 PBD, 30 NPBD), 25% were experiencing an elevated (manic/mixed/hypomanic) mood episode (7 PBD, 14 NPBD) and 14% were depressed (8 PBD, 4 NPBD). Forty-eight percent of BD subjects had rapid-cycling or chronic mood symptoms (17 PBD, 23 NPBD). Thirty-five percent of BD participants (15PBD, 14NPBD) had a history of alcohol or other substance abuse or dependence in remission. Five BD subjects had comorbid post traumatic stress disorder [4PBD (1 also with social phobia and 1 specific phobia), 1 NPBD], 2 panic disorder (1PBD, 1 NPBD), 2 generalized anxiety disorder (1PBD, 1NPBD) and 2PBD a specific phobia. Attention deficit hyperactivity disorder and oppositional defiant disorder were assessed in adolescents and present in 2PBD/4NPBD and 1 NPBD participant respectively.
MRI scans were obtained on a 3T Trio MR Scanner (Siemens, Erlangen, Germany) using a three-dimensional Magnetization Prepared Rapid Acquisition Gradient Echo T1-weighted sequence (TR=1500ms, TE=2.83ms, FOV=256 × 256 mm2, matrix=256 × 256, 160 1.0mm contiguous sagittal slices, NEX=2). Images were aligned along the anterior commissure-posterior commissure plane. Gray matter, white matter, and cerebrospinal fluid segmentation were performed using the Statistical Parametric Mapping 99 (SPM99) (www.fil.ion.ucl.ac.uk) tissue classification algorithm. The lateral ventricles were delineated by hand on axial slices and confirmed in orthogonal planes using BioImage Suite software (www.bioimagesuite.org) by operators (EEE, EH, LS) blind to participant characteristics: interrater intraclass reliability coefficient >0.99, intrarater intraclass reliability coefficient >0.97. Gray and white matter were summed for total brain volume (TBV) (Womer et al., 2009).
Analyses were conducted using an analysis of covariance (ANCOVA) model that included LVV as the dependent measure, diagnosis (HC, PBD, NPBD) as a between-subjects factor, and age and TBV as covariates. The distributions of LVVs were skewed (McDonald et al., 2006); values were logarithmically transformed successfully before analyses. Hypothesis-testing was performed by pairwise comparison between the HC and PBD group; additional posthoc pairwise comparisons were also performed. All significant main effects and 2-way interactions (p<0.05) are reported below.
3. Results
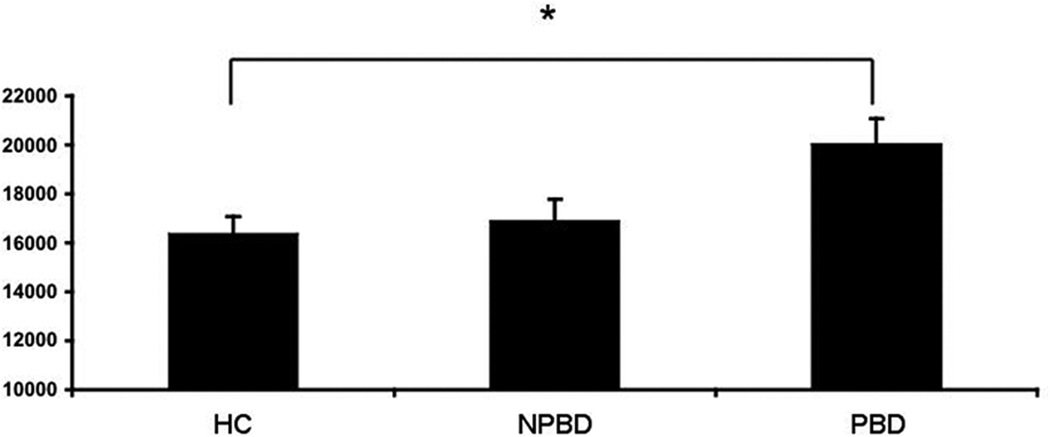
Groups did not differ significantly in age (HCmean27.5yrs±SD 14.1, NPBD 29.7±13.7, PBD 29.0±12.7, p=0.69). Age and TBV were both positively associated with LVV (p<0.0001). LVV was larger in the PBD group, compared to the HC group (p=0.034) (Figure), but did not differ between the NPBD and either the HC or PBD groups (unadjusted p=0.66, p=0.12, respectively). There was no significant interaction of age and group on LVV (p=0.75). No significant effects of mood state, rapid-cycling or medication subclasses (lithium/anticonvulsants/antipsychotics/antidepressants) were detected.
Figure 1.
Lateral Ventricle Volume in Psychotic Bipolar Disorder (PBD), Nonpsychotic BD (NPBD) and Healthy Comparison (HC) Groups
Least square means adjusted for age and total brain volume±standard errors *p<0.05.
4. Discussion
This is the first report of which we are aware of increased LVV in adolescents and adults with PBD, but not NPBD, relative to HC participants. The findings suggest LVV may be a morphologic feature associated with psychotic symptoms in BD present by adolescence. Increased LVV has been reported previously in one study of adults with PBD (Strasser et al., 2005). Its presence in adolescents and adults could suggest a developmental mechanism. Alternatively, increased ventricle size might reflect the presence of a degenerative mechanism, early brain insult or progressive periventricular volume losses resulting from mood episodes (Strakowski et al., 2002). However, longitudinal studies of larger samples are needed to assess age-related effects. Combined imaging and genetic studies may help elucidate etiologies, particularly given reports relating risk alleles in catechol-O-methyl transferase and neuregulin 1 both to susceptibility to PBD and schizophrenia and to increases in LVV in these disorders (Crespo-Facorro et al., 2007; Mata et al., 2009).
Effects of medication subclasses were not detected but power was limited; studies of medication-naive individuals would provide more definitive evidence. Though there were comparable rates of alcohol/substance comorbidity, as for medication use, retrospective reporting was not considered sufficiently reliable to investigate effects of duration and severity of use. An effect of rapid-cycling was not detected, suggesting LVV is not associated with mood episodes; however, analyses for episode numbers were not performed as these could not be reliably assessed, especially in a sample with high rapid-cycling rates. Future studies that employ longitudinal designs with larger samples of adolescents and adults and examine clinical factors, such as number of mood episodes, psychosis symptom severity, duration and severity of alcohol/substance comorbidity, amount and duration of medication exposure, as well as functional outcomes, may elucidate the mechanisms and effects of increased LVV in PBD.
Acknowledgements
The authors were supported by grants from the NIMH Nos. R01MH69747(HPB), R01MH070902(HPB), R25MH071240(FYW), K01MH086621(FW) and T32MH14276(JHK,LGC), NIH Clinical and Translational Science Award UL1 RR0249139, NIH/NIBIB R01EB006494(XP), Veterans Affairs Career Development(HPB), Merit Review(HPB) and Research Enhancement Award Program(HPB, LGC) programs, the National Alliance for Research in Schizophrenia and Depression (Great Neck, New York)(HPB,FW,JHK), the Attias Family Foundation(HPB), Marcia Simon Kaplan(JHK), Women's Health Research at Yale (New Haven, Connecticut)(HPB), and the Klingenstein Foundation (FW, JHK).
The authors thank Cheryl Lacadie, Karen Martin, Terry Hickey, and Hedy Sarofin, for technical expertise, Kathleen Colonese, Susan Quatrano, Philip Markovich, Allison McDonough and Lindsay Warren for their aid with the study, and the research subjects for their participation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ali SO, Denicoff KD, Altshuler LL, Hauser P, Li X, Conrad AJ, Smith-Jackson EE, Leverich GS, Post RM. Relationship between prior course of illness and neuroanatomic structures in bipolar disorder: a preliminary study. Neuropsychiatry Neuropsychology and Behavioral Neurology. 2001;14:227–232. [PubMed] [Google Scholar]
- Andreasen NC, Swayze V, 2nd, Flaum M, Allinger R, Cohen G. Ventricular abnormalities in affective disorder: clinical and demographic correlates. American Journal of Psychiatry. 1990;147:893–900. doi: 10.1176/ajp.147.7.893. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Harenski K, Nicoletti M, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC. MRI study of posterior fossa structures and brain ventricles in bipolar patients. Journal of Psychiatry Research. 2001;35:313–322. doi: 10.1016/s0022-3956(01)00036-x. [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro B, Roiz-Santiáñez R, Pelayo-Terán JM, Pérez-Iglesias R, Carrasco-Marín E, Mata I, Gonzalez-Mandly A, Jorge R, Vazquez-Barquero JL. Low-activity allele of Catechol-O-Methyltransferase (COMTL) is associated with increased lateral ventricles in patients with first episode non-affective psychosis. Progress in Neuropsychopharmacology and Biological Psychiatry. 2007;31:1514–1518. doi: 10.1016/j.pnpbp.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Johnstone EC, Crow TJ, Frith CD, Husband J, Kreel L. Cerebral ventricular size and cognitive impairment in chronic schizophrenia. Lancet. 1976;2:924–926. doi: 10.1016/s0140-6736(76)90890-4. [DOI] [PubMed] [Google Scholar]
- Kalmar JH, Wang F, Chepenik LG, Womer FY, Jones MM, Pittman B, Shah MP, Martin A, Constable RT. Relationship between amygdala structure and function in adolescents with bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:636–642. doi: 10.1097/CHI.0b013e31819f6fbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kempton MJ, Geddes JR, Ettinger U, Williams SCR, Grasby PM. Meta-analysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder. Archives of General Psychiatry. 2008;65:1017–1032. doi: 10.1001/archpsyc.65.9.1017. [DOI] [PubMed] [Google Scholar]
- Mata I, Perez-Iglesias R, Roiz-Santiañez R, Tordesillas-Gutierrez D, Gonzalez-Mandly A, Vazquez-Barquero JL, Crespo-Facorro B. A neuregulin 1 variant is associated with increased lateral ventricle volume in patients with first-episode schizophrenia. Biological Psychiatry. 2009;65:535–540. doi: 10.1016/j.biopsych.2008.10.020. [DOI] [PubMed] [Google Scholar]
- McDonald C, Marshall N, Sham PC, Bullmore ET, Schulze K, Chapple B, Bramon E, Filbey F, Quraishi S, Walshe M, Murray RM. Regional brain morphometry in patients with schizophrenia or bipolar disorder and their unaffected relatives. American Journal of Psychiatry. 2006;163:478–487. doi: 10.1176/appi.ajp.163.3.478. [DOI] [PubMed] [Google Scholar]
- Rosa PG, Schaufelberger MS, Uchida RR, Duran FLS, Lappin JM, Menezes PR, Scazufca M, McGuire PK, Murray RM, Busatto GF. Lateral ventricle differences between first-episode schizophrenia and first-episode psychotic bipolar disorder: a population-based morphometric MRI study. The World Journal of Biological Psychiatry. 2010;11:873–887. doi: 10.3109/15622975.2010.486042. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Zimmerman ME, Getz GE, Mills NP, Ret J, Shear P, Adler CM. Ventricular and periventricular structural volumes in first-versus multiple-episode bipolar disorder. American Journal of Psychiatry. 2002;159:1841–1847. doi: 10.1176/appi.ajp.159.11.1841. [DOI] [PubMed] [Google Scholar]
- Strasser HC, Lilyestrom J, Ashby ER, Honeycutt NA, Schretlen DJ, Pulver AE, Hopkins RO, Depaulo JR, Potash JB, Schweizer B, Yates KO, Kurian E, Barta PE, Pearlson GD. Hippocampal and ventricular volumes in psychotic and nonpsychotic bipolar patients compared with schizophrenia patients and community control subjects: a pilot study. Biological Psychiatry. 2005;57:633–639. doi: 10.1016/j.biopsych.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Womer FY, Wang F, Chepenik LG, Kalmar JH, Spencer L, Edmiston E, Pittman BP, Constable RT, Papademetris X, Blumberg HP. Sexually dimorphic features of vermis morphology in bipolar disorder. Bipolar Disorder. 2009;11:753–758. doi: 10.1111/j.1399-5618.2009.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. American Journal of Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]