Abstract
Male Syrian hamsters are naturally aggressive animals that reliably defend their home territory against intruding conspecifics. Hamsters that lose agonistic encounters subsequently exhibit a striking change in their agonistic behavior, however, expressing no aggression and instead becoming highly submissive, a behavioral change that we have termed conditioned defeat. We have generally employed an inescapable defeat training protocol when studying conditioned defeat. The purpose of the present study was to determine if conditioned defeat is an epiphenomenon of the inescapable defeat experience by comparing the behavior of hamsters exposed to inescapable versus escapable defeat. In the conditioned defeat model, defeated hamsters subsequently generalize their submission and social avoidance to a novel, non-aggressive opponent, suggesting that hamsters subjected to inescapable defeat may not form a specific memory of their aggressive opponent. Thus, a secondary purpose of the present study was to determine whether hamsters subjected to our defeat protocol have the ability to recognize a familiar opponent following defeat. Our results provide evidence that conditioned defeat is not solely a by-product of inescapable defeat because all experimental animals, regardless of the type of defeat, expressed conditioned defeat during testing. We also found that animals experiencing an inescapable defeat avoided a familiar aggressor significantly more than they did an unfamiliar aggressor, demonstrating that these animals have the ability to recognize their previous attacker. Thus, we maintain that a variety of social defeat models, and conditioned defeat in particular, represent generalizable and ethologically valid models with which to study the effects of social stress on physiology and behavior.
Keywords: social stress, controllability, anxiety, defensive behavior
1. Introduction
Social stress, stemming from any number of causes such as bullying or abuse, can be a contributing factor to the onset of a variety of psychiatric disorders, including post-traumatic stress disorder and major depression [1–3]. A variety of animal models have been developed to study the behavioral changes associated with social stress. Many of these models use social defeat, which involves an aggressive encounter between two animals and the subsequent creation of a dominant-subordinate (winner-loser) relationship [4,5]. Golden hamsters (also referred to as Syrian hamsters) are solitary animals and are excellent candidates for examining social stress because they are naturally aggressive and will reliably defend their home territory against an intruding conspecific without any need for complex social housing conditions. Hamsters also display ritualized agonistic behaviors and brief encounters rarely result in injury, thereby providing a valid animal representation of social or psychological stress rather than stress resulting from a physically harmful encounter. In fact, we have demonstrated that the hormonal stress response to social defeat in hamsters is largely psychological as it occurs in subordinate hamsters even in the absence of any physical contact with the dominant opponent [6]. Our lab generally uses a model in which an experimental animal is placed in the home cage of a larger, more aggressive animal, wherein it is reliably and quickly defeated. After this brief social defeat, the subject exhibits high levels of submission even toward a much smaller and younger opponent that would usually be non-threatening. This phenomenon has been termed conditioned defeat [7,8]. Social defeat models are becoming increasingly popular in studying mental illness, especially depression and anxiety disorders, and are important in understanding the etiology, mechanisms and treatment options for clinical populations [9,10].
Most defeat models of social stress use an inescapable defeat experience in which the experimental animal is not able to leave the cage of the aggressive opponent until the trial is over; in our studies this is typically a single, 15-min period [11]. In many laboratories, however, animals are paired repeatedly and the experimental animal is often left in the cage with its opponent, at times separated by a barrier, for several weeks [9,12]. Thus, it is possible that the pronounced and generalized social avoidance displayed by a variety of species during subsequent testing is a by-product of the inescapable defeat experience and would not occur if the subject had the ability to escape its attacker. In fact, there are data to suggest this might be the case because hamsters that are paired with an aggressive opponent three times but retain the ability to escape the cage do not subsequently avoid a familiar, socially neutral male [13]. In order to address this issue, we compared the agonistic behavior of hamsters subjected to an escapable model of defeat to the behavior of hamsters subjected to our standard, acute inescapable defeat model. In addition, following social defeat, hamsters were tested with caged opponents that were familiar (the previous dominant opponent) or unfamiliar (a novel aggressor) in order to determine whether a specific social memory was formed of the aggressive opponent during inescapable and escapable defeat. We hypothesized that conditioned defeat would be present during testing regardless of the type of defeat experience and that animals experiencing an escapable defeat would avoid only the familiar aggressor, while animals experiencing inescapable defeat would generalize their avoidance to all subsequent opponents.
2. Material and Methods
2.1 Subjects
Adult male Syrian hamsters were obtained from Charles River Laboratories. Subjects, approximately 3 months of age and weighing between 120g–140g, were individually housed in polycarbonate cages (24 × 33 × 20 cm) for two weeks prior to the beginning of the experiment. The animals were handled daily for five days preceding the study in order to habituate them to the stress of being handled. Resident aggressors (RA) were larger hamsters, weighing between 160g–175g, which were also individually housed. All cages contained corncob bedding and cotton nesting material, and cages were not changed for one week before the experiment so that hamsters could scent mark their cages. Food and water were available ad libitum. All behavioral manipulations were done in a dedicated hamster testing suite within the vivarium. All procedures and protocols were approved by the Georgia State University Institutional Animal Care and Use Committee and are in accordance with the standards outlined in the National Institutes of Health Guide for Care and Use of Laboratory Animals.
2.2 Behavioral Procedures
On Day 1 of the experiment, subjects were randomly assigned to one of three weight-matched groups: inescapable defeat, escapable defeat, or no defeat control. On Day 2, subjects were further divided into one of five weight-matched groups: inescapable defeat tested with familiar RA; inescapable defeat tested with an unfamiliar RA; escapable defeat tested with familiar RA; escapable defeat tested with an unfamiliar RA; no defeat control group paired with a randomly assigned RA during testing. No defeat control animals were brought to the testing suite but were left undisturbed in their home cage during defeat training on Day 1. Subjects were allowed to habituate to the testing suite for 30 min before all manipulations. All behavioral testing was performed during the first 3 hours of the dark phase of the light-dark cycle under dim red illumination to minimize circadian variation in the behavioral measures and because this is the time when hamsters produce the majority of their agonistic behavior [14]. All training and testing trials were recorded using an infrared CCD camera for later analysis.
2.2.1 Inescapable Defeat Training
Subjects were placed into the home cage of a randomly assigned RA, and a clear plastic lid was placed on top of the cage to keep the subject in the RA’s cage for the duration of the trial. Defeat training lasted 15 min, during which time the RA reliably attacked and defeated the subjects. The pairings were recorded for later behavioral analysis, and animals were monitored throughout defeat training to ensure that no injuries occurred to either of the animals.
2.2.2 Escapable Defeat Training
The escapable defeat procedure followed the protocol used by Johnston et al. [13,15]. Briefly, subjects in the escapable defeat group were placed into the home cage of the RA. No cage top was used during the defeats in order to give the subject the opportunity to jump out of the cage and flee the attack. These defeats consisted of three trials with a three min intertrial interval. The end of each trial was declared when the subject animal jumped out of the RA’s cage, and this latency was recorded. The subject was placed in its own home cage between trials.
2.2.3 Testing
Twenty-four hr following defeat, subjects from both defeat groups as well as the no-defeat controls were tested with a caged RA in a novel arena for five min (Figure 1). The RA was confined in a plastic mesh cage to allow subjects to see, hear, and smell the RA, but the animals were not able to physically contact each other. The plastic mesh cage (13.5 × 13.5 × 7 cm) used to enclose the RA during testing was also placed in the cage during all defeats. The caged RA was either the same RA that had defeated the subject on the prior day (familiar) or a RA from the defeat of another animal (unfamiliar). No-defeat control animals were tested against a randomly assigned RA. The plastic mesh cage was cleaned with a 70% alcohol solution and thoroughly dried between each change in RA. At the beginning of testing, the subject was placed in the opposite end of the testing arena from the caged RA, and the behavior and location of the subject were observed during the five-min testing period. A lid was placed on the testing arena during all testing sessions. The subject was allowed to freely move about the testing arena and an observer blind to testing condition scored the duration of time spent in each area of the arena using Noldus Observer. The movements of the no-defeat control animals served as a baseline for normal exploratory behavior around the testing arena. Time spent in the far half of the cage (see Figure 1) was defined as avoidance behavior. The frequency of submissive behavior, including the number of flights and risk assessments, were also measured (Table 1). Flight was defined as a quick and sudden locomotion away from the RA, and risk assessment was defined as a slow, cautious approach towards the confined RA with the body fully extended and a flat-back posture.
Figure 1.
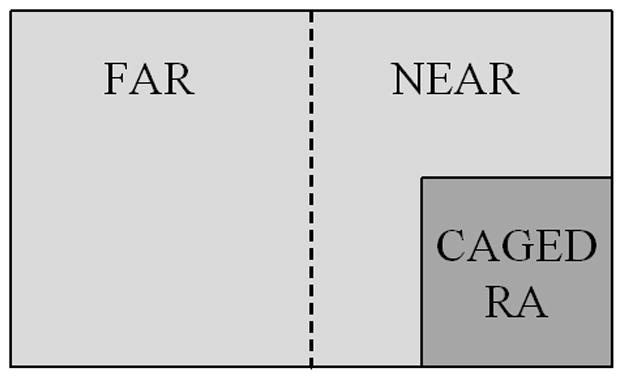
Testing Arena. The duration of time (sec) spent in each section (near vs. far) of the arena during the 5 min testing period was measured using Noldus Observer.
Table 1.
Average frequency of submissive behaviors (mean ± S.E.M) recorded during testing.
| Flights | Risk Assessments | Total Frequency | |
|---|---|---|---|
| Inescapable | 1.72 ± 0.42 | 4.06 ± 0.64 | 5.78 ± 0.81 |
| Familiar | 2.44 ± 0.60 | 4.00 ± 0.73 | 6.44 ± 0.65 |
| Unfamiliar | 1.00 ± 0.50 | 4.11 ± 1.11 | 5.11 ± 1.49 |
| Escapable | 2.43 ± 0.72 | 3.79 ± 0.87 | 6.21 ± 1.51 |
| Familiar | 1.43 ± 0.81 | 3.71 ± 1.12 | 5.14 ± 1.78 |
| Unfamiliar | 3.43 ± 1.13 | 3.87 ± 1.44 | 7.29 ± 2.52 |
| Control | 0.00 ± 0.00 | 2.38 ± 0.53 | 2.38 ± 0.53 |
2.3 Statistical Analysis
The effect of defeat was analyzed using a one-way between-subjects analysis of variance (ANOVA) and post hoc analysis was conducted using Fisher’s Least Significant Difference (LSD). Planned comparisons between time spent in avoidance of familiar and unfamiliar opponents were made with independent t-tests to assess potential significant differences. All data was analyzed using SPSS for Windows (PASW Statistics 18.0). All group data are reported as the mean ± standard error of the mean.
3. Results
3.1 Defeat Training
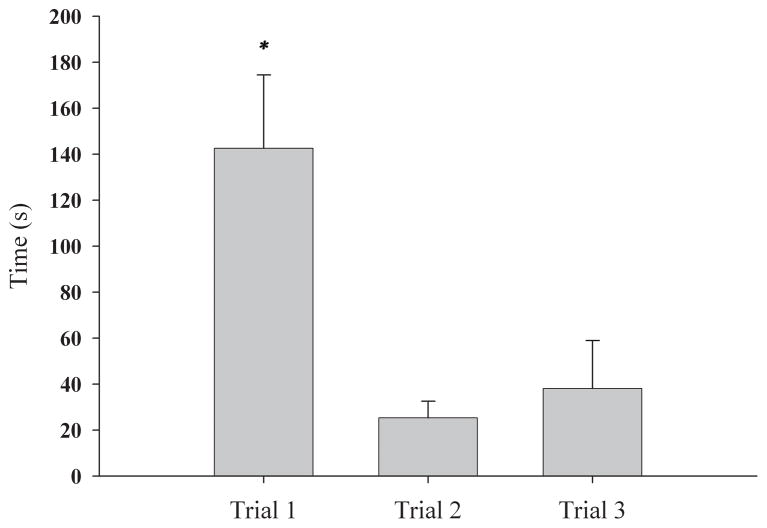
No animals were injured during the defeat training. In the escapable defeat group, the latency of the subject to escape the cage of the RA was recorded for each trial (Figure 2). On average, these animals were exposed to the RA for a total of 206 sec (±43.85 sec) across all three trials. In contrast, animals in the inescapable defeat group were exposed to the RA for 900 sec, of which an average of 357.5 sec (±30 sec) was spent in contact (social behavior or agonistic interaction) with the RA. On Day 2, all animals were tested in an enclosed arena, from which they could not escape, for 5 min with either a familiar or unfamiliar confined RA. Three animals had to be removed from the analysis for the following reasons: one non-defeated animal was removed from the analysis because it had extremely high avoidance behavior and was deemed an outlier due to a z-score of 2, one animal was removed because it defeated the RA during Day 1 defeat training, and the final animal was excluded because the RA escaped the plastic mesh cage during testing on Day 2. These animals were from control, inescapable/unfamiliar, and escapable/unfamiliar groups, respectively.
Figure 2.
Latency to jump out of the RA’s cage during escapable defeat training (mean ± S.E.M). Subjects took significantly longer to escape the cage on Trial 1 than they did on Trials 2 and 3 (F(2,39) = 8.260, p < 0.05).
* p < 0.05
3.2 Question 1: Does the type of defeat experience affect the expression of conditioned defeat?
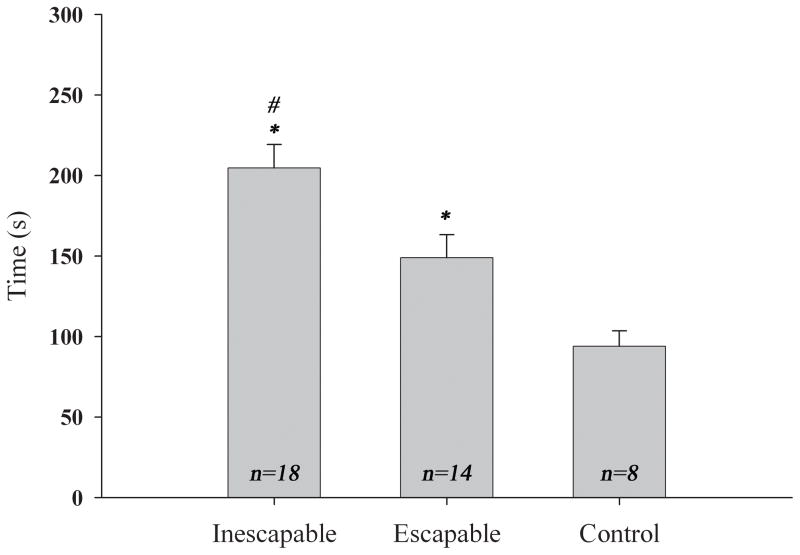
All defeated animals showed significant avoidance of the confined opponent as compared to the baseline exploratory behavior of the no-defeat controls (F(2,37) = 12.536, p < 0.05, Figure 3), suggesting that after a defeat experience all defeated hamsters will express conditioned defeat regardless of their ability to terminate the interaction. Post-hoc analysis revealed that there was also a significant difference in duration of avoidance between the two types of defeat, with animals in the inescapable defeat condition (n=18) avoiding the caged opponent significantly more than did animals in the escapable defeat (n=14). In addition, animals in both defeat groups avoided the caged opponents more than did animals in the no-defeat (n=8) group (p < 0.05). The animals in the inescapable defeat group avoided the caged opponent for an average of 205 sec (±14.54 sec) out of the 300 sec of testing compared to 149 sec (±14.22 sec) of avoidance by the animals in the escapable model. By contrast, the no-defeat animals spent only 94 sec (±9.58 sec) on the far side of the testing cage. Subjects from both defeat groups also showed overt submissive behaviors, including flights and risk assessments. Animals from the inescapable defeat group expressed these submissive behaviors significantly more than did no-defeat controls (t(24) = 2.676, p < 0.05). Animals from the escapable defeat group exhibited significantly more flights than did the no defeat controls (t(20) = 2.509, p < 0.05). Table 1 shows the frequency of flights and risk assessments for each group during testing on Day 2.
Figure 3.
Time spent in the far half of the arena during testing (mean ± S.E.M.). Animals in the inescapable defeat group spent significantly more time in the far half of the testing arena from the caged RA than did the escapable group and the control group. The escapable defeat group also spent significantly more time in the far side than did controls.
# p < 0.05 significantly greater than escapable defeat
* p < 0.05 significantly greater than controls
3.3 Question 2: Does the type of defeat affect the ability of hamsters to recognize a previous opponent?
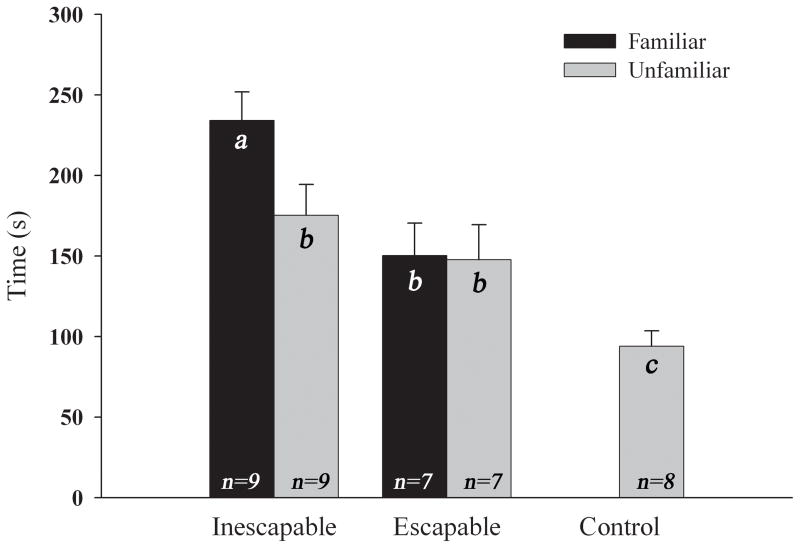
Subjects from the inescapable defeat group paired with a familiar opponent (n=9) exhibited significantly more avoidance behavior than did subjects paired with an unfamiliar (n=9) caged opponent during testing on Day 2 (t(16) = 2.256, p < 0.05). By contrast, animals from the escapable defeat showed no difference in their avoidance of familiar (n=7) versus unfamiliar (n=7) caged opponents on Day 2 (t(12) = 0.087, p > 0.05). Figure 4 shows the amount of time (mean ± standard error of the mean in sec) subjects from each experimental group spent in the far half of the testing arena.
Figure 4.
Time spent in the far half of arena when exposed to familiar and unfamiliar caged opponents (mean ± S.E.M). The animals in the inescapable defeat group tested against a familiar RA avoided significantly more than did animals tested against an unfamiliar RA. There were no differences between avoidance of a familiar versus an unfamiliar RA in the escapable defeat group. Bars with different letters are significantly different from one another (p < 0.05).
4. Discussion
The results of the present study provide clear evidence that conditioned defeat is not just an epiphenomenon of an inescapable defeat experience. All defeated animals expressed conditioned defeat regardless of their ability to escape the defeat, and even though animals in the escapable defeat group were exposed to a shorter duration of agonistic behavior than were animals in the inescapable defeat group, these animals still showed significant avoidance of the confined opponent during testing. Thus, we maintain that conditioned defeat (as defined by social avoidance in the current study) is a robust and reproducible response to a variety of social defeat experiences.
While both modes of defeat resulted in significant social avoidance of subsequent opponents, there were also significant differences between the defeat groups (Figures 3 and 4), with animals from the inescapable defeat group avoiding opponents significantly more than did animals exposed to escapable defeat. It is possible that this difference resulted simply from the longer contact time experienced by the animals in the inescapable defeat group. Importantly, the two defeat models also differed in terms of controllability of the social stressor in that the animals in the escapable model had the ability to flee their attacker and thereby terminate the defeat. It has long been known and current research continues to support the finding that behavioral stress responses can be mediated by controllability of the original stressor in a variety of designs, including drug addiction models and stress models of depression and anxiety [16–19]. In fact, the ability to control and escape a previous tailshock can actually block the stress-related behavioral changes associated with social defeat in subsequent testing, suggesting control as an “immunizing” agent against future social stress [20]. Controllability, therefore, may explain, at least in part, why escapable defeat produced less avoidance behavior than did inescapable defeat. It is important to note, however, that even with the ability to control their environment, animals from the escapable defeat group were not completely resilient and still showed conditioned defeat. This finding clearly illustrates that the stress of a presumably mild and controllable social defeat experience can still produce a lasting psychological impact on the animal. Future experiments should utilize a yoked design, in which the animals in both the escapable and inescapable defeat groups have an equal amount of exposure to the aggressor, to determine if exposure time or controllability has a stronger effect on the expression of conditioned defeat during testing.
Given that hamsters are not physically injured during social defeat encounters, we assert that conditioned defeat is a valid animal model of psychological stress. The uncontrollable nature of the inescapable defeat may provide a clinically relevant model for the psychological impact experienced by humans subjected to bullying, abuse, or trauma. Relatedly, a number of studies of posttraumatic stress disorder have demonstrated that there is a positive correlation between a person ranking a traumatic experience as mentally or psychologically defeating and the subsequent development of PTSD [for a review, see 21]. It has also been suggested that perceived defeat can actually be used as a predictor of PTSD following trauma [22]. In addition, patients diagnosed with PTSD often generalize their fear to non-threatening stimuli even when they are aware that the current situation is not actually a risk to their safety [23]. Similarly, all defeated hamsters in the current study exhibited a degree of generalized avoidance in that they not only avoided the familiar aggressor but also an unfamiliar opponent, and animals from the uncontrollable, inescapable defeat avoided opponents more than did any other group regardless of familiarity of the opponents. Interestingly, however, animals from the inescapable defeat group avoided their familiar attacker significantly more than they did an unfamiliar RA, providing clear evidence that while the stress of the inescapable defeat experience evoked a generalized fear response to all subsequent opponents, hamsters were still able to recognize their attacker and to avoid it more. These results clearly demonstrate that the social avoidance exhibited by hamsters following social stress does not simply represent a non-specific response to a severe, uncontrollable stressor but is, in fact, a nuanced strategy exhibited by all defeated animals.
Based on previous work demonstrating that hamsters subjected to escapable social defeat later avoid only familiar opponents [13,24], we were surprised that animals in the escapable defeat condition in our study seemed to generalize their fear and did not demonstrate a specific memory of their attacker as defined by greater avoidance. Unlike the studies mentioned above, however, our testing apparatus did not allow the subject another area to explore besides the main testing arena (see Figure 1). The previous studies used either multi-chambered arenas in which the animal could choose different compartments to explore or a Y-maze in which the animal also had multiple options [13,15,24]. Thus, it is possible that the combination of the very mild defeat experienced by the escapable defeat group and the subsequent testing of these subjects in a single, novel chamber led to the generalization of social avoidance to any RA, familiar and unfamiliar. It is also possible that while the escapable defeat animals may have been able to recognize their attacker, the lower overall expression of conditioned defeat may have resulted in a type of floor effect representing a baseline avoidance caused by a previous defeat. Additionally, we also only tested our subjects with RAs, whereas a previous study compared familiar aggressors with familiar neutral animals, with which the experimental animal had previous contact but had not had an agonistic encounter [13]. This procedural difference could also account for the inconsistency in the findings.
Using a caged opponent is a new testing procedure for our laboratory as we have previously used freely moving, non-aggressive, smaller animals (i.e., “non-aggressive intruders”) to test for the presence of conditioned defeat. While the use of the non-aggressive intruders is a powerful tool that allows us to observe a full range of behavioral responses to the opponent following defeat, this procedure also introduces additional variability because the behavior of the intruder can influence the encounter. The use of the caged-opponent method in this experiment allowed us to reduce the variability introduced by the actions or movements of the stimulus animal during testing and to focus more on the behavior of the experimental animal. It also provided a way to test for social recognition without the risk of the subject being re-defeated. Using this protocol, we were able to show that animals subjected to inescapable defeat do have the ability to recognize their previous opponent. Furthermore, despite the fact that the stimulus opponent was confined during testing, subjects in this study still exhibited overt submissive behaviors in reaction to the caged animal (Table 1), demonstrating that it may still be possible using this procedure to observe treatment effects on avoidance, defense and submission. One potential disadvantage to the caged opponent method is that we would be unable to examine some other behaviors, such as aggression, that require direct interaction of the subject with the stimulus animal during testing. Thus, pharmacological studies in which we might predict a possible increase in aggression would still require us to use a testing procedure with a freely moving, non-aggressive intruder.
The results of this study provide evidence that the expression of conditioned defeat is not solely a consequence of inescapable defeat. While an uncontrollable defeat appears to have a more profound behavioral impact than a more controllable one, firm conclusions about this relationship are difficult to make as there were important differences in the overall duration of contact between opponents in the two defeat conditions, as well. Nevertheless, it is apparent that a controllable social stressor (escapable defeat) still leads to the expression of conditioned defeat. Thus, we maintain that conditioned defeat is an extremely robust and reproducible model of social stress-induced behavioral changes that can be stimulated by a wide variety of social defeat procedures and represents a powerful tool with which to study the mechanisms of and possible treatments for these stress-induced changes.
Highlights.
Conditioned defeat is not an epiphenomenon of inescapable defeat.
Hamsters from an inescapable defeat have the ability to recognize their attacker.
Conditioned defeat is a robust model and can be seen in multiple testing methods.
Acknowledgments
We thank Alisa Norvelle, M.S. and Chris Markham, Ph.D. for their expert advice and assistance. This work was supported by NIH RO1 MH62044 awarded to KLH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Katharine E. McCann, Email: kmccann3@student.gsu.edu.
Kim L. Huhman, Email: khuhman@gsu.edu.
References
- 1.Agid O, Kohn Y, Lerer B. Environmental stress and psychiatric illness. Biomed Pharmacother. 2000;54:135–41. doi: 10.1016/S0753-3322(00)89046-0. [DOI] [PubMed] [Google Scholar]
- 2.Huhman KL. Social conflict models: can they inform us about human psychopathology? Horm Behav. 2006;50:640–6. doi: 10.1016/j.yhbeh.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 3.Kelleher I, Harley M, Lynch F, Arseneault L, Fitzpatrick C, Cannon M. Associations between childhood trauma, bullying and psychotic symptoms among a school-based adolescent sample. Br J Psychiatry. 2008;193:378–82. doi: 10.1192/bjp.bp.108.049536. [DOI] [PubMed] [Google Scholar]
- 4.Berton O, Durand M, Aguerre S, Mormede P, Chaouloff F. Behavioral, neuroendocrine and serotonergic consequences of single social defeat and repeated fluoxetine pretreatment in the Lewis rat strain. Neuroscience. 1999;92:327–41. doi: 10.1016/s0306-4522(98)00742-8. [DOI] [PubMed] [Google Scholar]
- 5.Meerlo P, Sgoifo A, Turek FW. The effects of social defeat and other stressors on the expression of circadian rhythms. Stress. 2002;5:15–22. doi: 10.1080/102538902900012323. [DOI] [PubMed] [Google Scholar]
- 6.Huhman KL, Moore TO, Mougey EH, Meyerhoff JL. Hormonal responses to fighting in hamsters: separation of physical and psychological causes. Physiol Behav. 1992;51:1083–6. doi: 10.1016/0031-9384(92)90097-l. [DOI] [PubMed] [Google Scholar]
- 7.Potegal M, Huhman K, Moore T, Meyerhoff J. Conditioned defeat in the Syrian golden hamster (Mesocricetus auratus) Behav Neural Biol. 1993;60:93–102. doi: 10.1016/0163-1047(93)90159-f. [DOI] [PubMed] [Google Scholar]
- 8.Huhman KL, Solomon MB, Janicki M, Harmon AC, Lin SM, Israel JE, Jasnow AM. Conditioned defeat in male and female Syrian hamsters. Horm Behav. 2003;44:293–9. doi: 10.1016/j.yhbeh.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–8. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 10.Blanchard RJ, McKittrick CR, Blanchard DC. Animal models of social stress: effects on behavior and brain neurochemical systems. Physiol Behav. 2001;73:261–71. doi: 10.1016/s0031-9384(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 11.Markham CM, Huhman KL. Is the medial amygdala part of the neural circuit modulating conditioned defeat in Syrian hamsters? Learn Mem. 2008;15:6–12. doi: 10.1101/lm.768208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, McEwen B, Sakai RR. Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology. 1995;20:117–34. doi: 10.1016/0306-4530(94)e0045-b. [DOI] [PubMed] [Google Scholar]
- 13.Lai WS, Ramiro LL, Yu HA, Johnston RE. Recognition of familiar individuals in golden hamsters: a new method and functional neuroanatomy. J Neurosci. 2005;25:11239–47. doi: 10.1523/JNEUROSCI.2124-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landau IT. Light-dark rhythms in aggressive behavior of the male golden hamster. Physiol Behav. 1975;14:767–74. doi: 10.1016/0031-9384(75)90068-2. [DOI] [PubMed] [Google Scholar]
- 15.Lai WS, Chen A, Johnston RE. Patterns of neural activation associated with exposure to odors from a familiar winner in male golden hamsters. Horm Behav. 2004;46:319–29. doi: 10.1016/j.yhbeh.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Jackson RL, Maier SF, Coon DJ. Long-term analgesic effects of inescapable shock and learned helplessness. Science. 1979;206:91–3. doi: 10.1126/science.573496. [DOI] [PubMed] [Google Scholar]
- 17.Will MJ, Watkins LR, Maier SF. Uncontrollable stress potentiates morphine’s rewarding properties. Pharmacol Biochem Behav. 1998;60:655–64. doi: 10.1016/s0091-3057(98)00027-6. [DOI] [PubMed] [Google Scholar]
- 18.Amat J, Paul E, Zarza C, Watkins LR, Maier SF. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J Neurosci. 2006;26:13264–72. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rozeske RR, Der-Avakian A, Bland ST, Beckley JT, Watkins LR, Maier SF. The medial prefrontal cortex regulates the differential expression of morphine-conditioned place preference following a single exposure to controllable or uncontrollable stress. Neuropsychopharmacology. 2009;34:834–43. doi: 10.1038/npp.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amat J, Aleksejev RM, Paul E, Watkins LR, Maier SF. Behavioral control over shock blocks behavioral and neurochemical effects of later social defeat. Neuroscience. 2010;165:1031–38. doi: 10.1016/j.neuroscience.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor PJ, Gooding P, Wood AM, Tarrier N. The role of defeat and entrapment in depression, anxiety, and suicide. Psychol Bull. 2011;137:391–420. doi: 10.1037/a0022935. [DOI] [PubMed] [Google Scholar]
- 22.Ehlers A, Maercker A, Boos A. Posttraumatic stress disorder following political imprisonment: the role of mental defeat, alienation and perceived permanent change. J Abnorm Psychol. 2000;109:45–55. [PubMed] [Google Scholar]
- 23.Ehlers A, Clark DM. A cognitive model of posttraumatic stress disorder. Behav Res Ther. 2000;38:319–45. doi: 10.1016/s0005-7967(99)00123-0. [DOI] [PubMed] [Google Scholar]
- 24.Petrulis A, Weidner M, Johnston RE. Recognition of competitors by male golden hamsters. Physiol Behav. 2004;81:629–38. doi: 10.1016/j.physbeh.2004.03.001. [DOI] [PubMed] [Google Scholar]