Abstract
We assessed neonatal diethylstilbestrol (DES)-induced disruption at various endocrine levels in the hamster. In particular, we used organ transplantation into the hamster cheek pouch to determine whether abnormalities observed in the post-pubertal ovary are due to: a) a direct (early) mechanism; or b) an indirect (late) mechanism that involves altered development and function of the hypothalamus and/or pituitary. Of the various disruption endpoints and attributes assessed: 1) some were consistent with the direct mechanism (altered uterine and cervical dimensions/organization, ovarian polyovular follicles, vaginal hypospadius, endometrial hyperplasia/dysplasia); 2) some were consistent with the indirect mechanism (ovarian/oviductal salpingitis, cystic ovarian follicles); 3) some were consistent with a combination of the direct and indirect mechanisms (altered endocrine status); and 4) the mechanism(s) for one (lack of corpora lutea) was uncertain. This study also generated some surprising observations regarding vaginal estrous assessments as a means to monitor periodicity of ovarian function in the hamster.
Keywords: female reproductive tract, diethylstilbestrol, endocrine disruption, ovary, hamster
1. INTRODUCTION
The synthetic estrogen, diethylstilbestrol (DES), was synthesized in 1938 [1] and prescribed starting in 1947 for pregnant women at high risk for miscarriage [2]. Then in 1971, Herbst et al. reported the occurrence of vaginal clear cell adenocarcinoma in a group of women whose mothers received that treatment [3]. Subsequent studies confirmed that many women exposed to DES in utero developed a range of structural reproductive tract abnormalities including neoplasia, and that they were prone to infertility and adverse pregnancy outcomes [4-11]. Additionally, numerous experimental animal studies on the effects of perinatal DES exposure document teratogenic and neoplastic lesions in both the male and female reproductive tracts [12-19]. Deciphering the mechanism(s) of this prototypical endocrine disruption phenomenon must include determination of how and at what level(s) of the control axis (hypothalamic – pituitary – gonadal – terminal hormone target tissue) perinatal DES exposure alters key endocrine signalling and response systems. Here we investigated this topic at various endocrine levels but with special scrutiny at the ovarian level.
Part of the rationale for that special scrutiny is that a number of studies in mice demonstrate that perinatal DES exposure induces extensive ovarian disruption consequences including cystic structures, medullary tubule-like structures, inflammation, increased interstitial compartment size, absence of corpora lutea, enlarged vacuolated interstitial cells, reduced overall mass, and polyovular follicles (POF) [20-28]. Furthermore, we determined that neonatal DES treatment results in a similar spectrum of ovarian abnormalities in the hamster [15]. However, it remains uncertain whether the abnormalities observed in the post-pubertal rodent ovary are due to: a) a direct (early) mechanism that involves immediate DES-induced alterations in development of the perinatal ovary; or b) an indirect (late) mechanism that involves altered development and function of the hypothalamus and/or pituitary which, in turn, results in altered gonadotropin-regulated function of the mature ovary. For instance, of those mouse studies that used in vitro or tissue transplantation approaches to test the above alternative mechanistic hypotheses, some generated results consistent with the direct mechanism [21, 22, 25] whereas others generated results consistent with the indirect mechanism [20, 27]. To further test those alternative direct vs. indirect hypotheses, here we again exploited the unique hamster cheek pouch as a tissue transplantation site because: 1) An early study reported reestablishment of ovarian function after transplantation to that site [29]; 2) Our initial experience using that site demonstrated its simplicity and convenience and showed that neonatal uteri transplanted into it grew, differentiated, and followed an endocrine-responsive morphogenetic program matching that of the host’s in situ uterus [30]; and 3) Using that site to swap control and neonatally DES-exposed donor uteri between control and neonatally DES-exposed host animals, we proved that neonatal DES exposure does disrupt the hamster uterus by a direct mechanism [31]. Results from both the analyses of basic reproductive endocrine status (circulating gonadotropin and ovarian steroid levels) and the ovarian transplantation procedure yielded new insight into the mechanisms by which neonatal DES exposure disrupts development and regulated function at various levels of the hypothalamic – pituitary – gonadal control axis in the female hamster.
2. MATERIALS AND METHODS
2.1. Reagents
Sigma Chemical Co. (St Louis, MO) was the source of diethylstilbestrol, estradiol-17β, HEPES, paraformaldehyde, and Dulbecco’s phosphate-buffered saline. Cellgro/Mediatech, Inc. (Manassas, VA) was the source of penicillin and streptomycin. BioWhittaker, Inc. (Walkersville, MD) was the source of DMEM/F-12 culture media.
2.2. General Animal Information
Animals were maintained and treated in an AAALAC-accredited facility as authorized by the Wichita State University Institutional Animal Care and Use Committee (IACUC). All procedures including neonatal treatment, anesthesia, ovariectomy, cheek pouch transplantation, chronic estrogenic stimulation, sacrificing, and tissue/blood collections followed IACUC-approved and established protocols [29-32]. In all cases, tissue/blood collections were performed at mid-day.
2.3. Neonatal Animal Treatment
Timed pregnant Syrian golden hamsters (Mesocricetus auratus) from Charles River Breeding Laboratories (Wilmington, MA) or Harlan Sprague Dawley, Inc. (Indianapolis, IN) were caged singly under a 14 hr light:10 hr dark photoperiod at 23-25°C with laboratory chow and water provided ad libitum. The chow was a 2:1 mixture of #5001 rodent diet and #5015 mouse diet from LabDiet (PMI Nutrition Int. Brentwood, MO). Within 6 hrs of birth (day-0), litter size was adjusted to eight neonates/litter by eliminating males and all animals in a litter were treated with a single s.c. injection of 50 μl corn oil vehicle either alone (control, CON) or containing 100 μg of DES. As acknowledged previously [15], that dose level is high but not unreasonable considering that DES ingestion levels by women were as much as 150 mg daily and 18.2 g total during their pregnancy [33]; with the median total dose being 10.7 g [12]. It is also the dose level we previously used to establish, assess, and compare neonatal DES-induced disruption in various regions of both the hamster female [31, 32, 34-37] and male [38-40] reproductive tract.
2.4. Prepubertal Procedures
On day 21 of life (~7 days prior to puberty), groups of control and neonatally DES-treated animals underwent the following procedures: 1) The d-21 groups did not undergo surgery but were anesthetized with CO2, decapitated, trunk blood was collected for serum separation, and reproductive tracts were isolated for fixation and histological processing (see below); 2) The 2-mo/I groups were left intact (I); 3) The 2-mo/O groups were bilaterally ovariectomized (O) only; 4) The 2-mo/O+E2 groups were bilaterally ovariectomized and began chronic estrogen stimulation by the s.c. insertion (between the shoulder blades) of a plugged Silastic (Dow Corning Corp., Midland, MI) tube (open lumen length, 1.0 cm; inner diameter, 1.57 mm; outer diameter, 2.41 mm) filled with crystalline estradiol-17β (O+E2); 5) The ovarian transplantation groups are described in the next section. Tissue harvesting (see below) from the group 2-5 animals listed above was performed when they were 2 months (2-mo) of age.
2.5. Ectopic Ovarian Transplantation
On day 21 of life, ovaries and adjacent oviducts were removed from both control and neonatally DES-treated groups of animals (donors) as for the 2-mo/O and 2-mo/O+E2 groups described above. Each ovary was dissected out of its bursa and immersed in sterile tissue culture media (DMEM/F-12 [1:1], 33mM HEPES, 100U/ml penicillin, 100 μg/ml streptomycin). While the ovariectomized animals were still under anesthesia, they became transplantation hosts (one isolated ovary per cheek pouch, both from the same donor animal) as depicted in Table 1.
Table 1.
Ovarian transplantation groups.
|
Host Neonatal Treatment |
Donor Neonatal Treatment |
Transplantation Type |
Group Name |
|---|---|---|---|
| CON | CON | Homo | C/C |
| DES | DES | Homo | D/D |
| CON | DES | Cross | C/D |
| DES | CON | Cross | D/C |
Neonatal treatments consisted of a single s.c. injection per animal of 50 μl corn oil vehicle either alone (control, CON) or containing 100 μg of DES.
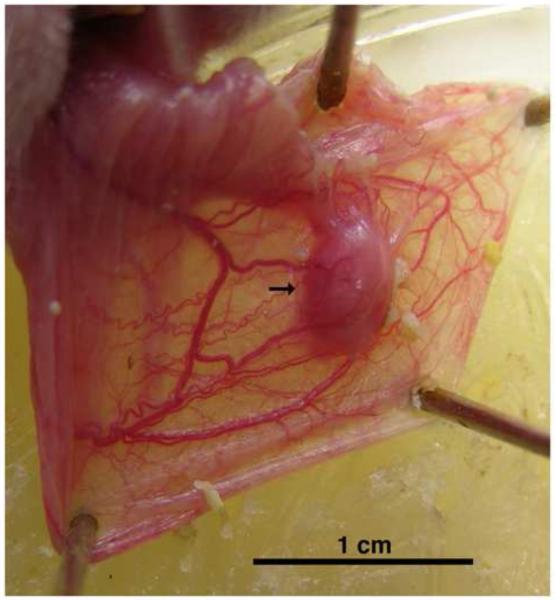
Each host animal’s cheek pouches (randomized with regard to order of left vs. right sides) were gently everted using forceps, spread on a paraffin plate, and held with T-pins. An incision (~2mm) was then made in the epithelium of the cheek pouch and the opening of the incision was widened slightly with forceps to make a pocket between the two epithelial layers of the pouch. An isolated ovary was then inserted into the pocket using microforceps and massaged about 5mm beyond the incision site. Finally, the incision site was sealed using a liquid suturing agent (Vetbond Tissue Adhesive, 3M Animal Care Products, St. Paul, MN). Weekly post-transplantation inspections were conducted by lightly anesthetizing the animals, gently everting their cheek pouches, and assessing the presence, dimensions, general appearance, and vascularization state of the ovarian transplant masses. Shown in Fig. 1 is an example of what was judged a successful and viable ovarian transplant site at such an inspection. The overall transplant success rates for all four groups, as also confirmed by histological analysis (see Figs. 8, 9), are shown in Table 2.
Figure 1. Appearance of a viable ovarian transplant in an everted cheek pouch.
Shown is the everted and pinned cheek pouch with a viable and well-vascularized ovarian transplant mass (arrow). It is representative of those transplant sites judged successful during weekly post-surgical assessments. Scale bar at bottom represents 1 cm.
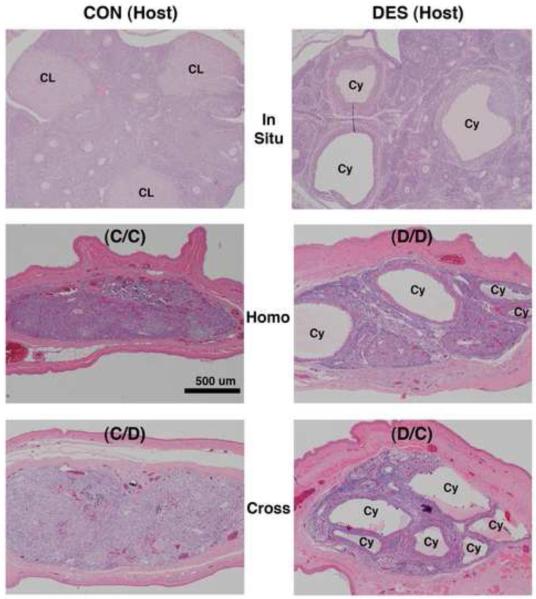
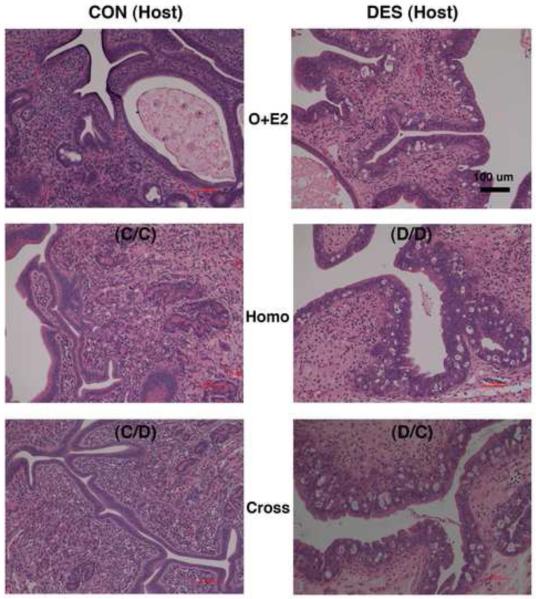
Figure 8. Effects of neonatal DES treatment on ovarian histomorphology in mature hamsters as located in situ and after prepubertal transplantation into the cheek pouch.
Shown are hematoxylin and eosin-stained ovarian tissue sections (all taken at the same magnification) from mature (2-mo) animals that: 1) on the day of birth (d-0) were not (control or CON, left panels) or were treated with DES (right panels) and then were 2) left intact (In Situ, upper panels) or were prepubertally (d-21) ovariectomized and received cheek pouch transplants of ovaries from 3) the same (Homo, middle panels) or 4) the other (Cross, lower panels) neonatal treatment group of donor animals (see Table 1 for further transplant group descriptions). Scale bar in middle left panel represents 500 μm.
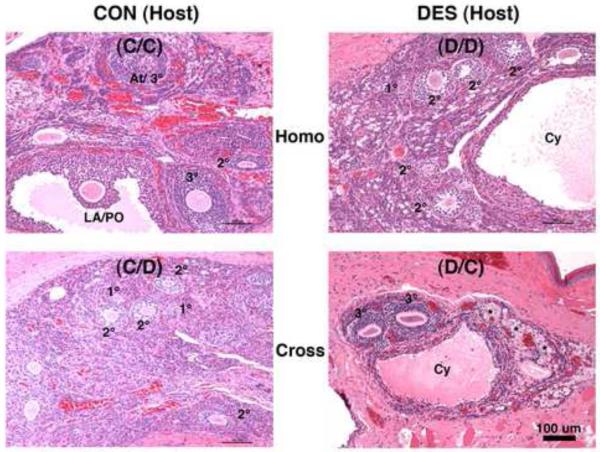
Figure 9. Ovarian follicle histomorphology in mature hamsters after transplantation into the cheek pouch.
Shown are hematoxylin and eosin-stained ovarian tissue sections (all taken at the same magnification, but higher than that used in Fig. 8) from mature (2-mo) animals that: 1) on the day of birth (d-0) were not (control or CON, left panels) or were treated with DES (right panels) and then were prepubertally (d-21) ovariectomized and received cheek pouch transplants of ovaries from 2) the same (Homo, upper panels) or 3) the other (Cross, lower panels) neonatal treatment group of donor animals (see Table 1 for further transplant group descriptions). Indicated are primary (1°), secondary (2°), tertiary (3°), atretic (At), late antral/preovulatory (LA/PO), and Cystic (Cy) follicles along with oogonial nests (*). Scale bar in lower right panel represents 100 μm.
Table 2. Ovarian Transplant Viability.
|
Transplant Groupa: |
C/C | D/D | C/D | D/C |
|---|---|---|---|---|
|
Total Transplant
Site Attemptsb: |
18 | 16 | 26 | 26 |
|
Total Viable
Sitesc: |
12 (67) | 13 (81) | 16 (62) | 19 (73) |
|
Both Left/Right
Sites Viablec: |
8 (44) | 10 (63) | 14 (54) | 14 (54) |
|
Left Site Only
Viablec: |
2 (11) | 0 (0) | 1 (4) | 2 (8) |
|
Right Site Only
Viablec: |
2 (11) | 3 (19) | 1 (4) | 3 (12) |
|
Neither Sites
Viablec: |
1 (6) | 0 (0) | 4 (15) | 1 (4) |
See Materials and Methods section and Table 1 for further details/descriptions of the ovarian transplant groups.
Results are expressed as the total number of indicated results observed in mature (2-mo) hostsc and, in parentheses, the percentage that number represents of the total transplant sites attempted prepubertally (d-21) for that transplant groupb.
2.6. Assessment of Vaginal Estrous
To determine if post-pubertal animals (caged individually) exhibit the very regular four-day estrous cycle characteristic of normal hamsters, we routinely check daily at 9 AM for appearance of the distinctive post-estrous vaginal discharge (vaginal estrous) signaling the morning of cycle day 1 [41]. For most of the animals in the transplant groups described above, we performed that assessment for at least two weeks prior to the two-month time point at which they were sacrificed.
2.7. Tissue Harvesting, Processing, and Analysis
As for the day-21 prepubertal animals (see above), all mature (1 and 2-month) animals were anesthetized with CO2, decapitated, and trunk blood was collected for serum separation. Reproductive tract regions remaining in situ at the time of sacrifice were excised and immediately immersed in fixative (4% paraformaldehyde in PBS, pH 7.2). After cheek pouches with viable ovarian transplant masses were everted, spread, and pinned to a paraffin plate, they were covered in fixative (to stiffen the double cheek-pouch membranes enveloping the ovarian transplant mass) and, ~10min later, a small region with the enveloped mass was excised and immersed in fixative. Ultimately, the excised tracts and ovarian transplant masses underwent two changes (24 h each) of fixative and then were transferred to 70% ethanol before they were carefully trimmed of fat and mesentery, sometimes photographed, paraffin embedded, sectioned (4-5 μm-thick sections cut transversely: 1) from the mid-region of uterine horns and in situ oviduct/ovarian regions; and 2) as indicated by the arrow [following the plane perpendicular to that of the image] in Fig. 1 for the ectopic ovarian transplant masses), and processed for standard hematoxylin and eosin (H&E) staining.
2.8. Histomorphology
All photomicroscopy images were taken using a Nikon DS-Fi1 digital color camera and acqusition software. Images shown were captured using a Nikon Eclipse E800 microscope using Infinity Optics and Plan Apo objectives. For cross-section area analysis of uterine horns and ovarian transplant masses, digital images of H&E-stained tissue sections were processed using a MetaMorph Basic analysis system (Universal Imaging Corp., Downington, PA).
2.9. Hormone Radioimmunoassays
Serum levels of the sex steroid estradiol (E2) and the gonadotropins luteinizing hormone (LH) and follicle-stimulating hormone (FSH) were measured using established assay protocols [42]. Assay sensitivities were 19 pg/ml for E2, 0.78 ng/ml for LH, and 15.6 ng/ml for FSH.
2.10. Statistics
Student’s t-tests were performed to determine if significant differences (p values ≤0.05) existed between mean values for control vs. DES-exposed animals where n ≥3 for all six treatment groups shown in Figs. 4 - 6. Using the Statistical Analysis System (SAS) PC Version 9.2 Level 2M2 X-64 Platform (SAS Institute Inc., Cary, North Carolina), a 2×6 factorial ANOVA followed by Tukey’s multiple contrast test was also performed to distinguish significance (p values ≤0.05) among the mean values for all twelve treatment combinations shown in Figs. 4 - 6.
Figure 4. Effects of neonatal DES treatment on serum estradiol levels in prepubertal hamsters and in mature hamsters that were subjected or not to various prepubertal surgical procedures.
| d21 | 2-mo/I | 2-mo/O | 2-mo/O+E2 | HOMO | CROSS | ||||||
| C | D | C | D | C | D | C | D | C | D | C | D |
| 3 | 3 | 3 | 3 | 11 | 3 | 8 | 8 | 8 | 8 | 10 | 12 |
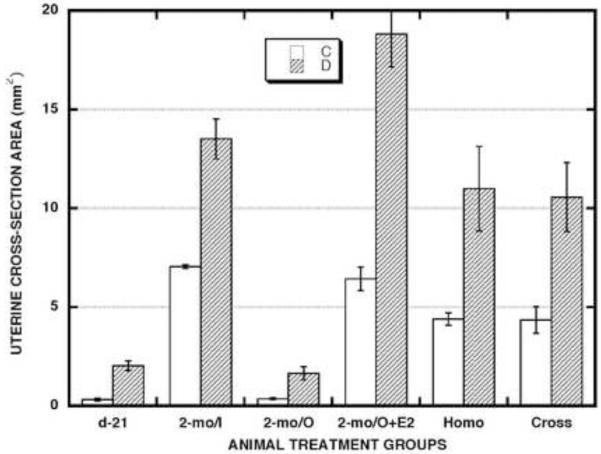
Figure 6. Effects of neonatal DES treatment on the cross-sectional area of uterine horns in prepubertal hamsters and in mature hamsters that were subjected or not to various prepubertal surgical procedures.
| d-21 | 2-mo/I | 2-mo/O | 2-mo/O+E2 | HOMO | CROSS | ||||||
| C | D | C | D | C | D | C | D | C | D | C | D |
| 5 | 5 | 3 | 3 | 6 | 4 | 3 | 3 | 5 | 5 | 9 | 10 |
3. RESULTS
3.1. Morphology of Reproductive Tract Regions in Intact Prepubertal and Mature Hamsters
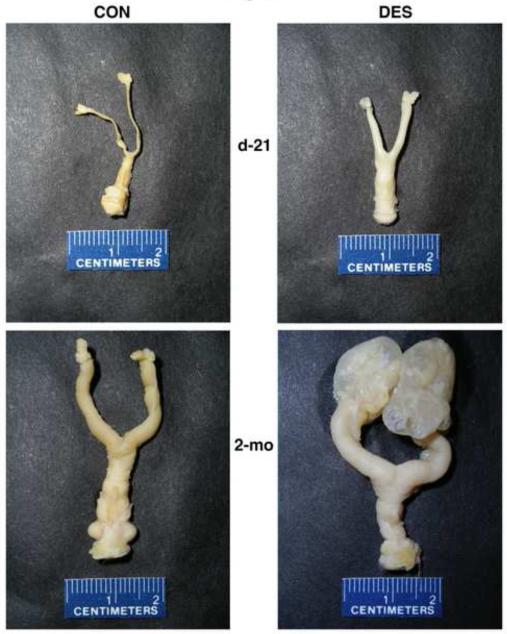
Figure 2 shows representative examples of control and neonatally DES-exposed complete tracts (vagina to ovaries) from prepubertal (d-21) and mature (2-mo) animals. At both ages, cervical and uterine horn dimensions were dramatically enhanced in DES-exposed animals. While gross appearance of the oviduct and ovary following neonatal DES treatment was not obviously affected in prepubertal animals, it was profoundly abnormal in mature animals due to severe inflammatory lesions of the oviduct and ovarian bursa (salpingitis).
Figure 2. Effects of neonatal DES treatment on complete reproductive tracts from prepubertal and mature hamsters.
The paraformaldehyde-fixed and trimmed tracts (vagina at the bottom to ovary/oviduct at the top of each panel) were removed from control (left panels) and neonatally DES-exposed (right panels) 21-day old prepubertal (upper panels) and 2-month old mature (lower panels) hamsters.
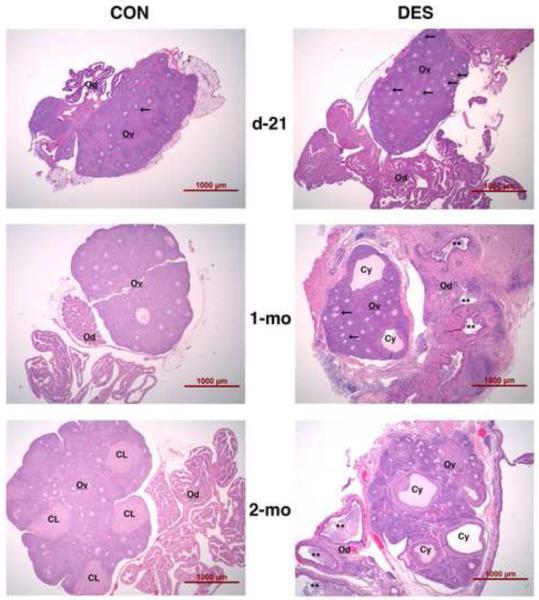
Figure 3 shows how the oviduct and ovary disruption phenomenon progresses at the histomorphological level. In prepubertal (day-21) animals, POF were more prevalent in the DES-exposed group but then less so in pubertal (1-month) animals. Thereafter, POF were rare in both groups. Beginning at puberty (see middle, right panel of Fig. 3), cystic follicles were always found in DES-exposed animals. While vaginal estrous and multiple corpora lutea were observed in control animals at 2-months of age, neither were observed in DES-exposed animals at any age. Instead (see lower, right panel of Fig. 3), a continued presence of cystic follicles and progressive disorganization of both follicle and stromal tissue compartments were observed in the ovaries of all neonatally DES-exposed animals.
Figure 3. Effects of neonatal DES treatment on the histomorphology of oviducts and ovaries in prepubertal and mature hamsters.
Shown are hematoxylin and eosin-stained sections of ovary (Ov) and oviduct (Od) regions from control (left panels) and neonatally DES-exposed (right panels) hamsters at day-21 (upper panels), 1-month (middle panels), and 2-months (lower panels) of age. Indicated are polyovular follicles (arrows), cystic follicles (Cy), oviductal luminal regions exhibiting salpingitis often with massive infiltration of inflammatory cells (**), and corpora lutea (CL). Scale bars in each panel represent 1 mm.
Figure 3 also illustrates the severe inflammatory and tissue/organ-distorting impact of salpingitis that fulminates postpubertally in DES-exposed animals. Indeed, the extent of that condition at the gross level (as exhibited in Fig. 2) often prevented us from finding the ovary in the massively inflamed tissue mass and/or it induced marked morbidity (abdominal distension, hunched/unstable gait, general muscular atrophy) and very early mortality in many of the DES-exposed animals.
3.2. Ovarian Transplantation Success
To test the alternative (direct vs. indirect) mechanisms of perinatal DES-induced ovarian disruption, we used the hamster cheek pouch site for ectopic organ transplantation. General performance of that site according to assessment of ovarian transplant mass viability at the gross inspection level (example in Fig. 1) is presented in Table 2. Overall success rates ranged from 62% to 81% with no significant difference among the four transplant groups. Most of the successful transplant sites (~77%) were bilateral and, for those cases of unilateral success, there was no significant right vs. left bias with regard to neonatal treatment status of either the host or donor animals.
3.3. Vaginal Estrous Assessments
Hamster estrous cycles are monitored by a distinctive vaginal discharge (vaginal estrous) [41] due to the action of ovarian progesterone secreted in response to the preovulatory LH surge. Strikingly, we observed vaginal estrous in all of the 16 control host animals (CON Homo or C/C and CON Cross or C/D groups) assessed but not in any of the 14 DES-exposed host animals (DES Homo or D/D and DES Cross or D/C groups) assessed, regardless of whether the cheek pouch transplants came from control or DES-exposed donors. Consistent with our unpublished but longstanding observations in DES-exposed intact animals, all of the DES-exposed host animals (DES Homo or D/D and DES Cross or D/C groups) also exhibited clear evidence of hypospadias [data not shown].
3.4. Endocrine Status
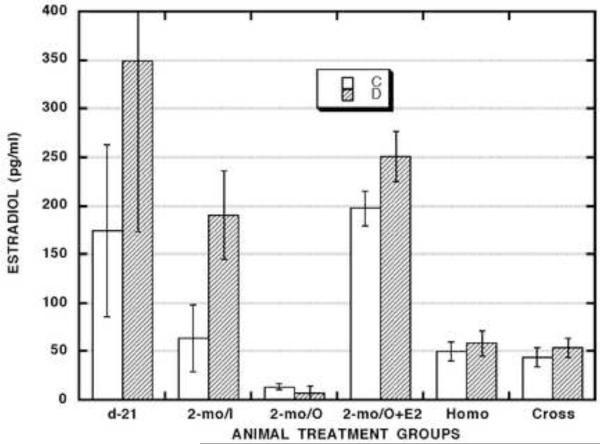
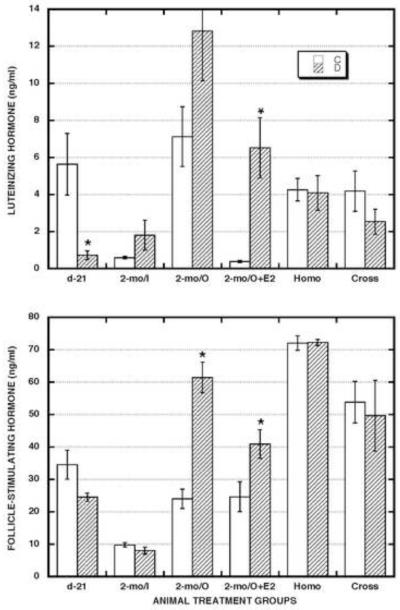
To assess general endocrine status in both control and neonatally DES-exposed groups of intact and surgically manipulated animals, we measured serum levels of estradiol (E2) (Fig. 4) and luteinizing hormone (LH) plus follicle-stimulating hormone (FSH) (Fig. 5). In prepubertal animals (d-21), there was a trend of higher (2.0x) E2 levels and lower (0.7x) FSH levels while LH levels were significantly lower (0.1x) in the DES-exposed compared to the control group.
Figure 5. Effects of neonatal DES treatment on serum gonadotropin levels in prepubertal hamsters and in mature hamsters that were subjected or not to various prepubertal surgical procedures.
| d-21 | 2-mo/I | 2-mo/O | 2-mo/O+E2 | HOMO | CROSS | ||||||
| C | D | C | D | C | D | C | D | C | D | C | D |
| 3 | 3 | 3 | 3 | 5 | 5 | 6 | 5 | 3 | 3 | 6 | 6 |
In mature, intact animals (2-mo/I), serum E2 levels tended to be higher (3.0x) in the DES-exposed compared to the control group. Serum gonadotropin levels in such animals were generally low and not significantly different between the control and DES-exposed groups.
In mature, ovariectomized (2-mo/O) animals, serum E2 levels were barely detectable in both control and DES-exposed groups. Serum gonadotropin levels in such animals were: 1) generally elevated (LH/C - 12.3x; LH/D - 7.1x; FSH/C - 2.4x; FSH/D - 7.7x) compared to those in mature, intact (2-mo/I) animals, and 2) higher (LH - 1.8x; FSH - 2.6x) in DES-exposed compared to control animals, but that difference was significant only for the FSH assays.
In mature, ovariectomized, and estrogen-replaced (2-mo/O+E2) animals, E2 levels were: 1) equivalent in the control and DES-exposed groups, and 2) equivalent to those measured in the DES-exposed group of mature, intact animals (2-mo/I). In contrast, both LH (17.2x) and FSH (1.7x) levels were significantly higher in the DES-exposed group. This trend was similar to that noted for the 2-mo/O groups (but the enhanced LH levels in DES-exposed animals were more dramatic (9.6x) in the 2-mo/O+E2 group than in the 2-mo/O group).
In the mature host animals with viable ovarian masses in their cheek pouch transplantation sites, E2 levels in both the Homo- and Cross-transplant groups were: 1) almost identical in control vs. DES-exposed animals; and 2) equivalent to those in the control group of mature, intact animals (2-mo/I). Gonadotropin levels in both transplant groups were quite similar in control vs. DES-exposed animals and comparable between the two groups but differed in various ways from those measured in the other mature animal groups. For LH, levels in the transplant host animals were: 1) generally higher than those in both control (6.4x) and DES-exposed (2.1x) groups of mature, intact (2-mo/I) animals; 2) generally lower than those in the mature, ovariectomized (2-mo/O) animals and much more so in the DES-exposed (0.2x) than the control group (0.6x) of such animals; and 3) higher than in the control group (11.2x) but lower than in the DES-exposed group (0.5x) of mature, ovariectomized, and estrogen-replaced (2-mo/O+E2) animals. For FSH, levels in the transplant host animals were: 1) much higher than those in both control (6.1x) and DES-exposed (7.2x) groups of mature, intact (2-mo/I) animals; and 2) much higher than those in the control groups of both mature, ovariectomized (2-mo/O - 2.5x) and mature, ovariectomized, and estrogen-replaced (O+E2 − 2.4x) animals. Lastly, the similarity in serum E2, LH, and FSH levels between the control and DES-exposed groups of transplant host animals contrasts sharply with the differences observed between those groups in terms of vaginal estrous assessments presented above.
According to ANOVA evaluation among the mean values for all twelve treatment combinations shown in Figs. 4 and 5: 1) E2 levels were significantly higher in all the 2-mo/O+E2 group animals than in all the animals in the 2-mo/O group and in both the Homo- and Cross-transplant groups; 2) LH levels were significantly higher in all the 2-mo/O group animals than in all the animals in the 2-mo/I group and in both the homo- and cross-transplant groups; and 3) FSH levels were significantly lower in all the 2-mo/I group animals than in all the animals in both the Homo- and Cross-transplant groups.
3.5. Uterine Dimensions and Histomorphology
To evaluate how and to what extent the normal and altered endocrine environments described above affected a terminal hormone target tissue, we assessed uterotrophic status, a well-established end-point of estrogenic responsiveness. Organ dimensions as determined here by uterine horn cross-sectional area measurements (Fig. 6) were significantly enhanced (1.9 - 6.4x) in neonatally DES-exposed vs. control animals for all six treatment groups. In other words, regardless of whether circulating E2 levels were low (2-mo/O), high (2-mo/O+E2), or intermediate (Homo- and Cross-transplant hosts) but equivalent between the control and neonatally DES-exposed animals (Fig. 4), uterine dimensions were consistently enhanced in the DES-exposed animals. According to ANOVA evaluation among the mean values for all the twelve treatment combinations shown in Fig. 6, uterine dimensions were significantly less in all the d-21 and 2-mo/O group animals than in all the 2-mo/I and 2-mo/O+E2 group animals. We also noted (data not shown) that, in control and neonatally DES-exposed groups of mature (2-mo) host animals found not to have viable ovarian tissue transplants in either of their cheek pouches, their uteri mimicked the very atrophic state observed in control and neonatally DES-exposed groups of mature, ovariectomized (2-mo/O) animals, respectively, and is clearly indicative of estrogen withdrawal.
At the histopathological level, uterine aberrations in neonatally DES-exposed animals were strikingly similar among the mature, ovariectomized, and estrogen-replaced (2-mo/O+E2) group (Fig. 7, top panels) and both the Homo (Fig. 7, middle panels) and the Cross (Fig. 7, bottom panels) transplant host groups. Those aberrations were most dramatic in the endometrial epithelial compartment and consisted of extremely hyperplastic cells in a very chaotic pseudostratified organization that was riddled with cavities containing apoptotic cells (all three right-side panels in Fig. 7). It is also noteworthy that the pattern of uterine hyperplasia/dysplasia that was manifest in a neonatal DES exposure-specific manner parallels that for the differences described above in terms of vaginal estrous assessments.
Figure 7. Effects of neonatal DES treatment on uterine histomorphology in mature, ovariectomized, and estrogen-replaced hamsters and in both groups of host hamsters with viable ovarian transplant tissue masses.
Shown are hematoxylin and eosin-stained uterine cross sections (all taken at the same magnification) from mature (2-mo) animals that: 1) on the day of birth (d-0) were not (control or CON, left panels) or were treated with DES (right panels) and then were prepubertally (d-21) ovariectomized and either 2) received an estradiol-releasing implant (O+E2, upper panels) or received cheek pouch transplants of ovaries from 3) the same (Homo, middle panels) or 4) the other (Cross, lower panels) neonatal treatment group of donor animals (see Table 1 for further transplant group descriptions). Scale bar in upper right panel represents 100 μm.
3.6. In Situ and Transplant Ovary Histomorphology
Shown in Fig. 8 are characteristic views of in situ ovaries (upper panels) and ectopic/transplanted ovaries (middle and lower panels) in mature (2-mo) hamsters. Complementing the evidence in Fig. 3, the upper panels in Fig. 8 depict our consistent observations that: 1) multiple corpora lutea develop postpubertally within the in situ ovaries of control animals but never within those of neonatally DES-exposed animals and 2) cystic follicles are always found postpubertally within the in situ ovaries of DES-exposed animals. According to cross-section area analysis of the ovarian tissue regions present in the excised cheek pouch specimens (data not shown), mean dimensions of the transplant masses were not statistically different among the four treatment groups shown in the middle and lower panels of Fig. 8.
Histomorphological analysis of the viable transplant masses in all four treatment groups revealed the presence of ovarian tissue-containing follicles at various stages of development (Fig. 9); but none of the analyzed masses exhibited the normal ovarian histomorphology as depicted in the upper left panel of Fig. 8. In general, stromal compartments within the ovarian transplant masses were disorganized and most of the follicles appeared distorted. Furthermore, cells with the characteristic polyhedral morphology of healthy luteal cells were not observed in any of the transplant masses from the four treatment groups. However, structures reminiscent of the cystic follicles observed postpubertally within the in situ ovaries of DES-exposed animals (Fig. 8, upper right panel) were often observed within the transplant masses from both groups (5 of 11 D/D and 6 of 16 D/C) of neonatally DES-exposed hosts (Fig. 8, middle and lower right panels and Fig. 9, both right panels) but were seldom observed in both groups (1 of 11 C/C and 1 of 12 C/D) of control hosts (Fig. 8, middle and lower left panels and Fig. 9, both left panels). In other words, the ovarian disruption phenomenon of cystic follicle development at the cheek pouch transplant site depended on neonatal treatment history of the host animal rather than the donor animal.
4. DISCUSSION
Despite the wealth of both clinical [3-11, 33] and experimental [12-19] evidence establishing DES as the prototypical perinatal endocrine disruptor, important questions remain regarding its mechanism of action at various endocrine regulatory and target organ levels [8, 12, 43]. In the hamster for instance, neonatal treatment with DES (but not with the natural ovarian estrogen, E2) elicits distinct disruption processes in various regions of both the female (vagina, cervix, uterus, oviduct, ovary) and male (testis, epididymis, seminal vesicle) reproductive tract [15, 32, 34, 40]. Furthermore, the timing and apparent hormone dependency of those DES-induced disruption endpoints differs among the various tract sites. Thus, the objectives of the present study were to: 1) further define the extent of neonatal DES-induced disruption of general reproductive endocrine status and target tissue sites with particular interest in ovarian structure/function and 2) more specifically probe the mechanism(s) responsible for the ovarian disruption phenomenon. Our findings from this and other related studies are summarized in Table 3 and discussed below.
Table 3. Summary of Neonatal DES-Induced Disruption Endpoints and Attributes.
| Mechanistic Attributes | |||
|---|---|---|---|
| Disruption Endpoint | Early (E) or Late (L)a |
Transient (T) or Persistent (P)b |
Direct (D) or Indirect (I)c |
| Uterine and Cervical Dimensions |
E | P | D |
| Salpingitis | L | P | I |
| Polyovular Follicles (POF) |
E | T | D |
| Cystic Follicles | L | P | I |
| Hypospadias | L | P | D |
| Endocrine Status | E & L | P | D & I |
| Endometrial Hyperplasia/Dysplasia |
E | P | D |
| Lack of Corpora Lutea |
L | P | ? |
Indicates whether the disruption endpoint emerged either prepubertally (early, E) or postpubertally (late, L).
Indicates whether presence of the disruption endpoint waned after it first emerged (transient, T) or continued and/or progressed thereafter (persistent, P).
The characterization of whether the disruption endpoint is due either to a direct (D) mechanism that involves immediate DES-induced alterations in development of the perinatal tissue/organ or to an indirect (I) mechanism that involves altered development and function of the hypothalamus and/or pituitary which, in turn, results in altered gonadotropin-regulated function of the mature ovary.
4.1. Morphology of Reproductive Tract Regions in Intact Prepubertal and Mature Hamsters
In neonatally DES-exposed hamsters, disrupted morphology occurs very early and then is sustained in the uterus and cervix [32, 34] but, as preliminarily noted [15] and confirmed here, the ovary responds differently. For instance, the enhanced POF phenomenon was transient. Enhanced POF incidence also occurs in neonatally DES-exposed mice [23, 24] and is induced by a direct mechanism [22] that depends on the beta form of the estrogen receptor (ERß) [44] and involves reduced oocyte apoptosis [45]. However, contrary to the timescale we observe in the hamster, dramatically enhanced POF incidence is maintained to at least two months of age in neonatally DES-exposed mice [44]. Thus in the hamster, neonatally DES-induced POF formation may also be the result of a direct (early) mechanism but is more transient than in mice. Perhaps that transience results from recovery of an oocyte:somatic cell ratio-targeted mechanism that is normally involved in breakdown of germ cell cysts during perinatal folliculogenesis [46-48] and is possibly perinatally targeted by estrogenic endocrine disruptors in general [49]. Another ovarian disruption endpoint with a different timescale is the development of cystic follicles. Such abnormal ovarian structures also develop in perinatally DES-exposed mice [25, 26] and women [50]. This phenomenon in mice appears to depend specifically on the alpha form of the estrogen receptor (ERα) [44], also emerges peripubertally, and persists thereafter [26]. Such a delayed onset but then persistent phenomenon is consistent with cystic follicles being the result of an indirect (late) mechanism. Also consistent with the endocrine trends reported here, it might involve enhanced levels of LH [51] and steroidogenesis [52].
A delayed but then progressive disruption endpoint reported here and previously in hamsters neonatally treated with DES (but not with E2) [15, 53] was severe inflammatory lesions of the oviduct and ovarian bursa (salpingitis), an endpoint also reported in prenatally DES-exposed mice [26, 54], rats [55], and women [56]. Consistent with the timescale observed here in the hamster, evidence of salpingitis in DES-exposed mice emerged at 1-month of age and intensified thereafter [26]. This pattern suggests that salpingitis is also the result of an indirect (late) mechanism.
4.2. Ovarian Transplantation Success
Prompted by previous reports of the hamster cheek pouch’s utility as an ectopic site for reproductive organ transplantation [29, 57, 58] and by our success using it to study the mechanism of neonatal DES-induced disruption at the uterine level [30, 31], we used it here to further study the mechanism of disruption at the ovarian level. In the original report of ovarian transplantation to the hamster cheek pouch [29], the success rate was ~53 % according to estrous cycle assessments using the characteristic lordosis reflex behavior as evidence of cycle day 1. In comparison, our overall success rate was somewhat better (range of 62-81% among the four transplant groups) according to viable transplant mass assessments at both the gross and histological level. Likely reasons for the unsuccessful transplants in this study include donor organ damage during the transplantation procedure and/or inadequate neovascularization at the host transplantation site.
4.3. Endocrine Status
The endocrine status assessments presented here complement and extend those in some of our prior studies [16, 53, 59]. However, an endocrine aspect included here but not in other studies of perinatal DES-induced disruption (assessment of circulating gonadotropin levels) provided important new information relevant to the direct vs. indirect mechanism question. The general pattern of gonadotropin profile disruption in the neonatally DES-exposed groups consisted of suppressed levels in prepubertal (d-21) animals but then enhanced levels in mature animals that had either been ovariectomized only (2-mo/O) or had also received estrogen replacement (2-mo/O+E2). That pattern is consistent with a direct (early) mechanism of disruption at the peptide hormone production (hypothalamo/pituitary function) and/or clearance level. However, the presence of ovarian tissues, either in situ or ectopic, appears to mitigate the phenomenon of gonadotropin profile disruption in DES-exposed animals. The latter observation suggests that ovarian products, perhaps members of the inhibin/activin peptide family [60], can blunt the consequences of direct neonatal DES-induced disruption at the hypothalamo/pituitary level.
In animals with in situ ovaries, reconciling E2 levels with the gonadotropin levels is complicated. For instance, the trend of enhanced E2 levels in the DES-exposed groups of prepubertal (d-21) and mature (2-mo/I) animals could be the result of altered gonadotropin stimulation of ovarian steroidogenesis, altered steroid negative feed back at the hypothalamo/pituitary level, and/or altered hormone (steroid and gonadotropin) clearance. Furthermore, such dynamics were differentially altered in prepubertal vs. mature animals.
Also as previously reported [16, 53], for both control and neonatally DES-exposed animals, ovariectomy reduced circulating E2 nearly to undetectable levels and the estrogen replacement regimen restored levels to those attained in the DES-exposed group of mature (2-mo/I) animals that exhibit an apparent persistent estrous state. As noted above, those similar E2 levels failed to exert similar feedback effects in terms of gonadotropin levels in control vs. DES-exposed animals. In all the transplant host animals, it is also notable that circulating levels of all the hormones assessed were almost identical in the control vs. DES-exposed groups despite the striking differences observed between them in terms of vaginal estrous status (see below).
4.4. Uterine Dimensions and Histomorphology
Both the uterine dimensions and histomorphology assessments presented here confirm previous conclusions [15, 16, 31, 32, 36, 53] that neonatal DES exposure directly and permanently disrupts early development of the hamster uterus such that it responds abnormally later in life to estrogenic stimulation. They also demonstrate that the donor-type (control vs. DES-exposed) of transplanted ovarian tissue does not influence the uterine disruption phenomenon in terms of estrogen-dependent hyperplasia/dysplasia/neoplasia.
4.5. In Situ and Transplant Ovary Histomorphology
In the in situ ovarian tissue sections from mature animals (2-mo/I), histomorphological assessments further defined our preliminary observations [15] of structural disruption in neonatally DES-exposed animals. In the viable ectopic ovarian tissue sections from the same-aged hosts, the general histomorphology profiles did not closely match either that of normal in situ ovaries in control animals or that of the characteristically disrupted in situ ovaries in neonatally DES-exposed animals. Nevertheless, in the viable ovarian transplants, we did observe histomorphological traits consistent with a mechanism wherein neonatal DES exposure directly alters early development and function of the hypothalamus and/or pituitary that later/indirectly induces disrupted structure and function of the mature ovary. In contrast, it is uncertain whether the lack of luteal cells/tissue in all four transplant groups is consistent with a direct mechanism, an indirect mechanism, or a combination thereof.
4.6. Vaginal Estrous Assessments
Another host-dependent rather than ovary donor-dependent phenomenon was the striking difference in vaginal estrous assessments between the control and DES-exposed groups of transplant host animals. However, the implications of those assessments in the DES-exposed hosts must take into account that, as is observed in the mouse [61], we also consistently observe in the hamster [unpublished] that perinatal DES exposure induces female hypospadias. The consequences of that anomaly is formation of a urethro-vaginal cloaca such that urinary products empty into the vagina and often result in the deposit of vaginal concretions or “stones” [62, 63] that can be irritating [64] and thereby contribute to vaginal abnormalities/dysfunction. Thus, we are uncertain whether the observed lack of a distinctive post-estrous vaginal discharge in mature, DES-exposed hamsters (either intact animals or the host groups used in this study) is due to a disrupted endocrine environment, vaginal epithelial exposure to urinary products, or a combination thereof. However, the fact that the endocrine parameters evaluated in this study (E2, LH, FSH) did not differ between control and DES-exposed animals in either host group suggests that the vaginal disruption phenomenon may also depend on a direct effect of DES at this level.
Implications of the vaginal estrous observations in the control groups of transplant host animals are also enigmatic. Based on the appearance over regular four-day periods of vaginal discharges considered to be post-estrous signal events in vivo, we were surprised by what we found in the serum and ovarian transplants subsequently harvested from those animals. We expected to observe a pattern of general endocrine status (E2, LH, and FSH levels) and ovarian histomorphology that was similar to that in the control group of same-aged, intact (2mo/I) animals but that was not the case. The original description of ovarian transplantation in the hamster cheek pouch also reported evidence of reestablished estrous cycles [29]. However, that very brief report used a different cyclicity assessment protocol (lordosis reflex behavior and mating-induced pseudopregnant cycles vs. vaginal estrous) and provided minimal histomorphological data. For instance, the single tissue section shown in that report does display an apparently normal late antral/preovulaory follicle (as is also displayed in the top left panel of our Fig. 9) but the authors do not comment on the presence or absence of luteal cells/tissue in their ectopic ovaries. Since neither the original report [29] nor the present study provides direct evidence of actual ovulation events in the hamster ovary after transplantation into the hamster cheek pouch, this experimental approach deserves further evaluation. For instance, due to the number of treatment groups in the present study, we were compelled to restrict our tissue/blood harvesting protocol to a single time point (mid-day) rather than several time points for each group. Consequently, we could not fully evaluate the dynamics of circulating gonadotropin and steroid levels known to occur over the hamster’s normal 4-day estrous cycle, especially the most dramatic ones occurring just before and after ovulation on the morning of cycle day 1 [59, 65-67]. Clearly, more comprehensive and specific characterization of hormone secretion patterns is needed to conclusively define endocrine status within the context of the ovarian transplantation system used here.
5. SUMMARY AND CONCLUSIONS
In terms of the alternative mechanism hypotheses and the various neonatal DES-induced disruption endpoints and attributes assessed in this study (Table 3): 1) some were consistent with the direct mechanism (altered uterine and cervical dimensions/organization, ovarian polyovular follicles, vaginal hypospadius, endometrial hyperplasia/dysplasia); 2) some were consistent with the indirect mechanism (ovarian/oviductal salpingitis, cystic ovarian follicles); 3) some were consistent with a combination of the direct and indirect mechanisms (altered endocrine status [i.e., circulating E2, LH, and FSH levels]); and 4) the mechanism(s) for one (lack of corpora lutea) was uncertain. Also striking was the appearance in control host animals of regular four-day cycles of vaginal estrous despite the presence of distinctly abnormal endocrine environments and the fact that transplant masses derived from both control and DES-exposed donors did not retain normal ovarian morphology and exhibited no clear evidence of ovulation events (complete absence of luteal cells).
-
-
We assessed neonatal DES-induced disruption at various endocrine levels in hamsters.
-
-
We used the cheek pouch transplantation site to analyze the ovarian disruption.
-
-
Disruption at the ovarian level occurs via both direct and indirect mechanisms.
ACKNOWLEDGMENTS
We thank Dr. Karen L. Brown-Sullivan for valuable guidance in performing statistical analyses. This work was supported in part by United States Public Health Service grant P20 RR016475 from the BRIN/INBRE program of the National Center for Research Resources and by the Flossie E. West Memorial Foundation. Portions of this work appeared in the theses submitted by I. D. Alwis and D. M. Maroni in partial fulfillment of the MS degree in Biological Sciences and at the 37th & 40th Annual Meetings of the Society for the Study of Reproduction.
Abbreviations
- DES
diethylstilbestrol
- E2
estradiol-17β
- ERα
estrogen receptor alpha
- ERβ
estrogen receptor beta
- FSH
follicle-stimulating hormone
- LH
luteinizing hormone
- POF
polyovular follicles
- P4
progesterone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Dodds EC, Goldberg L, Lawson W, Robinson R. Estrogenic activity of certain synthetic compounds. Nature. 1938;141:247–248. [Google Scholar]
- 2.Swan SH. Intrauterine exposure to diethylstilbestrol: long-term effects in humans. Apmis. 2000;108:793–804. doi: 10.1111/j.1600-0463.2000.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 3.Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med. 1971;284:878–881. doi: 10.1056/NEJM197104222841604. [DOI] [PubMed] [Google Scholar]
- 4.Blunt E. Diethylstilbestrol exposure: it’s still an issue. Holist Nurs Pract. 2004;18:187–191. doi: 10.1097/00004650-200407000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman RH, Adam E. Findings in female offspring of women exposed in utero to diethylstilbestrol. Obstet Gynecol. 2002;99:197–200. doi: 10.1016/s0029-7844(01)01682-9. [DOI] [PubMed] [Google Scholar]
- 6.Kruse K, Lauver D, Hanson K. Clinical implications of DES. Nurse Pract. 2003;28:26–32. 35. doi: 10.1097/00006205-200307000-00005. table of contents; quiz 35-27. [DOI] [PubMed] [Google Scholar]
- 7.Lauver D, Nelles KK, Hanson K. The health effects of diethylstilbestrol revisited. J Obstet Gynecol Neonatal Nurs. 2005;34:494–499. doi: 10.1177/0884217505278293. [DOI] [PubMed] [Google Scholar]
- 8.Newbold RR. Lessons learned from perinatal exposure to diethylstilbestrol. Toxicol Appl Pharmacol. 2004;199:142–150. doi: 10.1016/j.taap.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 9.Rubin MM. Antenatal exposure to DES: lessons learned...future concerns. Obstet Gynecol Surv. 2007;62:548–555. doi: 10.1097/01.ogx.0000271138.31234.d7. [DOI] [PubMed] [Google Scholar]
- 10.Schrager S, Potter BE. Diethylstilbestrol exposure. Am Fam Physician. 2004;69:2395–2400. [PubMed] [Google Scholar]
- 11.Veurink M, Koster M, Berg LT. The history of DES, lessons to be learned. Pharm World Sci. 2005;27:139–143. doi: 10.1007/s11096-005-3663-z. [DOI] [PubMed] [Google Scholar]
- 12.Anderson LM. Predictive values of traditional animal bioassay studies for human perinatal carcinogenesis risk determination. Toxicol Appl Pharmacol. 2004;199:162–174. doi: 10.1016/j.taap.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Bern HA. Diethylstilbestrol (DES) syndrome: Present status of animal and human studies. In: Li JJ, Nandi S, editors. Hormonal Carcinogenesis. Springer-Verlag; New York: 1991. pp. 1–8. [Google Scholar]
- 14.Greco TL, Duello TM, Gorski J. Estrogen receptors, estradiol, and diethylstilbestrol in early development: the mouse as a model for the study of estrogen receptors and estrogen sensitivity in embryonic development of male and female reproductive tracts. Endocr Rev. 1993;14:59–71. doi: 10.1210/edrv-14-1-59. [DOI] [PubMed] [Google Scholar]
- 15.Hendry WJ, 3rd, Sheehan DM, Khan SA, May JV. Developing a laboratory animal model for perinatal endocrine disruption: the hamster chronicles. Exp Biol Med. 2002;227:709–723. doi: 10.1177/153537020222700904. [DOI] [PubMed] [Google Scholar]
- 16.Leavitt WW, Evans RW, Hendry WJ., 3rd Etiology of DES-induced uterine tumors in the Syrian hamster. Adv Exp Med Biol. 1982;138:63–86. doi: 10.1007/978-1-4615-7192-6_4. [DOI] [PubMed] [Google Scholar]
- 17.Newbold R. Cellular and molecular effects of developmental exposure to diethylstilbestrol: implications for other environmental estrogens. Environ Health Perspect. 1995;103(Suppl 7):83–87. doi: 10.1289/ehp.95103s783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rustia M, Shubik P. Transplacental effects of diethylstilbestrol on the genital tract of hamster offspring. Cancer Lett. 1976;1:139–146. [Google Scholar]
- 19.Rustia M, Shubik P. Effects of transplacental exposure to diethylstilbestrol on carcinogenic susceptibility during postnatal life in hamster progeny. Cancer Res. 1979;39:4636–4644. [PubMed] [Google Scholar]
- 20.Halling A, Forsberg JG. Ovarian reproductive function after exposure to diethylstilbestrol in neonatal life. Biol Reprod. 1990;43:472–477. doi: 10.1095/biolreprod43.3.472. [DOI] [PubMed] [Google Scholar]
- 21.Haney AF, Newbold RR, McLachlan JA. Prenatal diethylstilbestrol exposure in the mouse: effects on ovarian histology and steroidogenesis in vitro. Biol Reprod. 1984;30:471–478. doi: 10.1095/biolreprod30.2.471. [DOI] [PubMed] [Google Scholar]
- 22.Iguchi T, Fukazawa Y, Uesugi Y, Takasugi N. Polyovular follicles in mouse ovaries exposed neonatally to diethylstilbestrol in vivo and in vitro. Biol Reprod. 1990;43:478–484. doi: 10.1095/biolreprod43.3.478. [DOI] [PubMed] [Google Scholar]
- 23.Iguchi T, Takasugi N. Polyovular follicles in the ovary of immature mice exposed prenatally to diethylstilbestrol. Anat Embryol (Berl) 1986;175:53–55. doi: 10.1007/BF00315455. [DOI] [PubMed] [Google Scholar]
- 24.Iguchi T, Takasugi N, Bern HA, Mills KT. Frequent occurrence of polyovular follicles in ovaries of mice exposed neonatally to diethylstilbestrol. Teratology. 1986;34:29–35. doi: 10.1002/tera.1420340105. [DOI] [PubMed] [Google Scholar]
- 25.McLachlan JA, Newbold RR, Bullock BC. Long-term effects on the female mouse genital tract associated with prenatal exposure to diethylstilbestrol. Cancer Res. 1980;40:3988–3999. [PubMed] [Google Scholar]
- 26.Newbold RR, Bullock BC, Mc Lachlan JA. Exposure to diethylstilbestrol during pregnancy permanently alters the ovary and oviduct. Biol Reprod. 1983;28:735–744. doi: 10.1095/biolreprod28.3.735. [DOI] [PubMed] [Google Scholar]
- 27.Tenenbaum A, Forsberg JG. Structural and functional changes in ovaries from adult mice treated with diethylstilboestrol in the neonatal period. J Reprod Fertil. 1985;73:465–477. doi: 10.1530/jrf.0.0730465. [DOI] [PubMed] [Google Scholar]
- 28.Wordinger RJ, Highman B. Histology and ultrastructure of the adult mouse ovary following a single prenatal exposure to diethylstilbestrol. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;45:241–253. doi: 10.1007/BF02889867. [DOI] [PubMed] [Google Scholar]
- 29.Caldwell BV, Pawling RS, Wright PA. Reestablishment of ovarian periodicity after transplantation to the Syrian hamster cheek pouch. Proc Soc Exp Biol Med. 1966;123:551–553. doi: 10.3181/00379727-123-31540. [DOI] [PubMed] [Google Scholar]
- 30.Hendry WJ, III, Branham WS, Sheehan DM. The hamster cheek pouch as a convenient ectopic site for studies of uterine morphogenesis and endocrine responsiveness. Differentiation. 1992;51:49–54. doi: 10.1111/j.1432-0436.1992.tb00679.x. [DOI] [PubMed] [Google Scholar]
- 31.Hendry WJ, 3rd, Zheng X, Leavitt WW, Branham WS, Sheehan DM. Endometrial hyperplasia and apoptosis following neonatal diethylstilbestrol exposure and subsequent estrogen stimulation in both host and transplanted hamster uteri. Cancer Res. 1997;57:1903–1908. [PubMed] [Google Scholar]
- 32.Hendry WJ, 3rd, DeBrot BL, Zheng X, Branham WS, Sheehan DM. Differential activity of diethylstilbestrol versus estradiol as neonatal endocrine disruptors in the female hamster (Mesocricetus auratus) reproductive tract. Biol Reprod. 1999;61:91–100. doi: 10.1095/biolreprod61.1.91. [DOI] [PubMed] [Google Scholar]
- 33.Herbst AL, Scully RE, Robboy SJ. Prenatal diethylstilbestrol exposure and human genital tract abnormalities. Natl Cancer Inst Monogr. 1979:25–35. [PubMed] [Google Scholar]
- 34.Hendry WJ, 3rd, Branham WS, Sheehan DM. Diethylstilbestrol versus estradiol as neonatal disruptors of the hamster (Mesocricetus auratus) cervix. Biol Reprod. 2004;70:1306–1316. doi: 10.1095/biolreprod.103.024992. [DOI] [PubMed] [Google Scholar]
- 35.Hendry WJ, III, Leavitt WW. Binding and retention of estrogen in the uterus of hamsters treated neonatally with diethylstilbestrol. Journal of Steroid Biochemistry. 1982;17:479–487. doi: 10.1016/0022-4731(82)90005-x. [DOI] [PubMed] [Google Scholar]
- 36.Hendry WJ, III, Leavitt WW. Altered morphogenesis of the immature hamster uterus following neonatal exposure to diethylstilbestrol. Differentiation. 1993;52:221–227. doi: 10.1111/j.1432-0436.1993.tb00634.x. [DOI] [PubMed] [Google Scholar]
- 37.Zheng X, Hendry WJ., III Neonatal diethylstilbestrol treatment alters the estrogen-regulated expression of both cell proliferation and apoptosis-related protooncogenes (c-jun, c-fos, c-myc, bax, bcl-2 and bcl-x) in the hamster uterus. Cell Growth and Differentiation. 1997;8:425–434. [PubMed] [Google Scholar]
- 38.Hendry WJ, 3rd, Weaver BP, Naccarato TR, Khan SA. Differential progression of neonatal diethylstilbestrol-induced disruption of the hamster testis and seminal vesicle. Reprod Toxicol. 2006;21:225–240. doi: 10.1016/j.reprotox.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 39.Karri S, Johnson H, Hendry WJ, 3rd, Williams SC, Khan SA. Neonatal exposure to diethylstilbestrol leads to impaired action of androgens in adult male hamsters. Reprod Toxicol. 2004;19:53–63. doi: 10.1016/j.reprotox.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 40.Khan SA, Ball RB, Hendry WJ., 3rd Effects of neonatal administration of diethylstilbestrol in male hamsters: disruption of reproductive function in adults after apparently normal pubertal development. Biol Reprod. 1998;58:137–142. doi: 10.1095/biolreprod58.1.137. [DOI] [PubMed] [Google Scholar]
- 41.Siegal HI. Appendix-Characteristics of Mesocricetus auratus. In: Siegel HI, editor. The Hamster: Reproduction and Behavior. Plenum Press; New York: 1985. pp. 435–436. [Google Scholar]
- 42.Terranova PF, Greenwald GS. Steroid and gonadotropin levels during the luteal-follicular shift of the cyclic hamster. Biol Reprod. 1978;18:170–175. doi: 10.1095/biolreprod18.2.170. [DOI] [PubMed] [Google Scholar]
- 43.Miller KP, Borgeest C, Greenfeld C, Tomic D, Flaws JA. In utero effects of chemicals on reproductive tissues in females. Toxicol Appl Pharmacol. 2004;198:111–131. doi: 10.1016/j.taap.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 44.Kirigaya A, Kim H, Hayashi S, Chambon P, Watanabe H, Lguchi T, Sato T. Involvement of estrogen receptor beta in the induction of polyovular follicles in mouse ovaries exposed neonatally to diethylstilbestrol. Zoolog Sci. 2009;26:704–712. doi: 10.2108/zsj.26.704. [DOI] [PubMed] [Google Scholar]
- 45.Kim H, Nakajima T, Hayashi S, Chambon P, Watanabe H, Iguchi T, Sato T. Effects of diethylstilbestrol on programmed oocyte death and induction of polyovular follicles in neonatal mouse ovaries. Biol Reprod. 2009;81:1002–1009. doi: 10.1095/biolreprod.108.070599. [DOI] [PubMed] [Google Scholar]
- 46.Jefferson W, Newbold R, Padilla-Banks E, Pepling M. Neonatal genistein treatment alters ovarian differentiation in the mouse: inhibition of oocyte nest breakdown and increased oocyte survival. Biol Reprod. 2006;74:161–168. doi: 10.1095/biolreprod.105.045724. [DOI] [PubMed] [Google Scholar]
- 47.Pepling ME, Spradling AC. Female mouse germ cells form synchronously dividing cysts. Development. 1998;125:3323–3328. doi: 10.1242/dev.125.17.3323. [DOI] [PubMed] [Google Scholar]
- 48.Pepling ME, Spradling AC. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol. 2001;234:339–351. doi: 10.1006/dbio.2001.0269. [DOI] [PubMed] [Google Scholar]
- 49.Guillette LJ, Jr., Moore BC. Environmental contaminants, fertility, and multioocytic follicles: a lesson from wildlife? Semin Reprod Med. 2006;24:134–141. doi: 10.1055/s-2006-944419. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt G, Fowler WC., Jr. Gynecologic operative experience in women exposed to DES in utero. South Med J. 1982;75:260–263. doi: 10.1097/00007611-198203000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Sinkevicius KW, Burdette JE, Woloszyn K, Hewitt SC, Hamilton K, Sugg SL, Temple KA, Wondisford FE, Korach KS, Woodruff TK, Greene GL. An estrogen receptor-alpha knock-in mutation provides evidence of ligand-independent signaling and allows modulation of ligand-induced pathways in vivo. Endocrinology. 2008;149:2970–2979. doi: 10.1210/en.2007-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monniaux D, Clemente N, Touze JL, Belville C, Rico C, Bontoux M, Picard JY, Fabre S. Intrafollicular steroids and anti-mullerian hormone during normal and cystic ovarian follicular development in the cow. Biol Reprod. 2008;79:387–396. doi: 10.1095/biolreprod.107.065847. [DOI] [PubMed] [Google Scholar]
- 53.Hendry WJ, III, Zheng X, Leavitt WW, Branham WS, Sheehan DM. In: Tilly JL, Strauss JF III, Tenniswood M, editors. Synthetic estrogen-mediated alterations in uterine cell fate; Serono International Symposium on Cell Death in Reproductive Physiology; New York: Springer-Verlag, Inc.. 1997.pp. 272–291. [Google Scholar]
- 54.Newbold RR, Bullock BC, McLachlan JA. Diverticulosis and salpingitis isthmica nodosa (SIN) of the fallopian tube. Estrogen-induced diverticulosis and SIN of the mouse oviduct. Am J Pathol. 1984;117:333–335. [PMC free article] [PubMed] [Google Scholar]
- 55.Rothschild TC, Calhoon RE, Boylan ES. Genital tract abnormalities in female rats exposed to diethylstilbestrol in utero. Reprod Toxicol. 1987;1:193–202. doi: 10.1016/s0890-6238(87)80033-3. [DOI] [PubMed] [Google Scholar]
- 56.Balasch J, Coll O, Jove I, Moreno V, Mulet J, Vanrell JA. Diethylstilbestrol-induced mullerian abnormalities, septate uterus, genital tuberculosis and twin pregnancy with term delivery after in-vitro fertilization. Hum Reprod. 1991;6:690–693. doi: 10.1093/oxfordjournals.humrep.a137409. [DOI] [PubMed] [Google Scholar]
- 57.Caldwell BV, Mazer RS, Wright PA. Luteolysis as affected by uterine transplantation in the Syrian hamster. Endocrinology. 1967;80:477–482. doi: 10.1210/endo-80-3-477. [DOI] [PubMed] [Google Scholar]
- 58.Duby RT, McDaniel JW, Black DL. Homo-transplantation of the hamster uterus. Nature. 1965;205:720. doi: 10.1038/205720a0. [DOI] [PubMed] [Google Scholar]
- 59.Evans RW, Chen TJ, Hendry WJ, Leavitt WW. Progesterone regulation of estrogen receptor in the hamster uterus during the estrous cycle. Endocrinology. 1980;107:383–390. doi: 10.1210/endo-107-2-383. [DOI] [PubMed] [Google Scholar]
- 60.Ying SY. Inhibins and activins: chemical properties and biological activity. Proc Soc Exp Biol Med. 1987;186:253–264. doi: 10.3181/00379727-186-42611a. [DOI] [PubMed] [Google Scholar]
- 61.Miyagawa S, Buchanan DL, Sato T, Ohta Y, Nishina Y, Iguchi T. Characterization of diethylstilbestrol-induced hypospadias in female mice. Anat Rec. 2002;266:43–50. doi: 10.1002/ar.10033. [DOI] [PubMed] [Google Scholar]
- 62.Dunn TB. Cancer and other lesions in mice receiving estrogens. Recent Results Cancer Res. 1979;66:175–192. doi: 10.1007/978-3-642-81267-5_6. [DOI] [PubMed] [Google Scholar]
- 63.Iguchi T, Takasugi N. Occurrence of permanent changes in vaginal and uterine epithelia in mice treated neonatally with progestin, estrogen and aromatizable or non-aromatizable androgens. Endocrinol Jpn. 1976;23:327–332. doi: 10.1507/endocrj1954.23.327. [DOI] [PubMed] [Google Scholar]
- 64.Warner MR, Warner RL, Clinton CW. Reproductive tract calculi, their induction, age incidence, composition and biological effects in Balb/c Crgl mice injected as newborns with estradiol-17 beta. Biol Reprod. 1979;20:310–322. doi: 10.1095/biolreprod20.2.310. [DOI] [PubMed] [Google Scholar]
- 65.Bast JD, Greenwald GS. Serum profiles of follicle-stimulating hormone, luteinizing hormone and prolactin during the estrous cycle of the hamster. Endocrinology. 1974;94:1295–1299. doi: 10.1210/endo-94-5-1295. [DOI] [PubMed] [Google Scholar]
- 66.Ridley K, Greenwald GS. Progesterone levels measured every two hours in the cyclic hamster. Proc Soc Exp Biol Med. 1975;149:10–12. doi: 10.3181/00379727-149-38733. [DOI] [PubMed] [Google Scholar]
- 67.Saidapur SK, Greenwald GS. Peripheral blood and ovarian levels of sex steroids in the cyclic hamster. Biol Reprod. 1978;18:401–408. doi: 10.1095/biolreprod18.3.401. [DOI] [PubMed] [Google Scholar]