1. INTRODUCTION
Schizophrenia is a chronic and potentially disabling disorder with widespread neuroanatomical abnormalities thought to be caused by progressive brain changes (Andreasen, 2010), and an equally wide variety of impairments in cognitive functioning (Palmer et al., 2009). In general, individuals with schizophrenia demonstrate significantly impaired performance on a full range of neuropsychological tasks, often reaching greater than one standard deviation below the norm (Dickinson et al., 2007). One particularly puzzling issue is that approximately 15–30% of schizophrenia patients have been found to perform in the normal range of neuropsychological functioning (Kremen et al., 2000; Palmer et al., 1997).
Given hypothesized relationships between brain structure and cognitive function in both health and disease, the question arises whether schizophrenia subjects with neuropsychological performance in the normal range also demonstrate “normal” brain structure commensurate with their cognitive abilities. Brain abnormalities are a hallmark of schizophrenia, with widespread cortical thinning across various regions (Kuperberg et al., 2003; Schultz et al., 2010). Relatively few studies have examined the brain structure of cognitive subgroups, including neuropsychologically normal subjects, and to date, no detailed examination of cortical thinning patterns have been performed to compare these subgroups. In an early computerized tomography (CT) study, Allen and colleagues (2000) found no difference in ventricular size between schizophrenia subgroups clustered by cognitive performance, but greater global sulcal widening was evident in the severely impaired group. Recently, Wexler and coworkers (2009) assessed magnetic resonance imaging (MRI)-based measures in “neuropsychologically near-normal” (NPNN) and “neuropsychologically impaired” (NPI) schizophrenia subjects, and reported smaller cortical gray matter and larger third ventricular volumes for NPI and NPNN groups relative to healthy subjects. Furthermore, NPI subjects demonstrated additional reductions in white matter volumes and enlargements in lateral ventricle volumes as compared to the NPNN subgroup. Interestingly, NPNN subjects exhibited significantly smaller orbitofrontal volumes in relation to the other groups.
In this study, we sought to identify neuropsychologically near-normal (i.e., NPNN) schizophrenia subjects from a large sample of participants through use of a series of unsupervised clustering techniques. It was hypothesized that a stable classification of subjects would emerge, with one or more clusters containing subjects with few neuropsychological deficits (NPNN), while subjects with typical schizophrenia profiles of broad neuropsychological impairment (i.e., NPI) being contained in other clusters. We then tested the hypothesis that NPI subjects would demonstrate widespread cortical thinning in regions similar to those found in previous studies, while NPNN subjects would exhibit a more limited pattern, with perhaps small regions of thinning corresponding to a milder form of the disease. Finally, we examined whether selfreported duration of illness was associated with group membership, the severity of cognitive deficits, or the degree of cortical thinning. We hypothesized that the absence of such associations would suggest that the presence or absence of widespread neuropsychological impairments in schizophrenia is a stable trait of the disorder, and therefore potentially valuable in defining and understanding disease heterogeneity.
2. METHODS
2.1 Participants
The schizophrenia (SCZ, n=79) and healthy comparison participants (COM, n=65) in this study were drawn from a larger population of individuals recruited as part of a longitudinal study of brain structure in schizophrenia. Participants were excluded if they met DSM-IV criteria for mental retardation, substance abuse (moderate or severe) or dependence (any type) at present time or during the past 6 months. Duration of illness (DOI) was calculated based on self-reported date of first treatment or hospitalization. Informed consent was obtained from each subject after a complete description of the study was given.
2.2 Clinical and Neuropsychological Assessment
Psychopathology and neuropsychological functioning were assessed as previously described (Delawalla et al., 2006; Harms et al., 2007). Subjects were administered a battery of neuropsychological measures that correspond with broad domains of verbal IQ, working memory, episodic memory, and executive functioning that have shown performance deficits in schizophrenia populations (Heaton et al., 2001); see Supplementary material for specific tests used in the neuropsychological and clinical battery. Raw and scaled scores for all measures were transformed to z-scores using data from a previously published and overlapping reference group (Smith et al., 2009).
2.3 MRI Acquisition Parameters and Data Processing
MR scans were acquired on a 1.5T Vision scanner (Siemens Medical Systems) and collected using an MPRAGE sequence (TR = 10ms, TE = 4ms, Flip angle = 30° ACQ – 1, Matrix = 256 X 256, Scanning time = 5.6 minutes) with 1 mm×1 mm×1.25mm isotropic resolution. Scans were then analyzed and processed using FreeSurfer (FS) release 3.0.5 (Dale et al., 1999). A 2-dimensional smoothing kernel was applied along the cortical surface with a 20mm full-width/half-maximum window. Spherical maps for each subject were morphed into a common spherical atlas using a nonlinear surface-registration procedure that allows for high-registration, surface-based averaging, and comparison of cortical measurements across subjects.
2.4 Statistical Analyses
A hierarchical cluster analysis using Ward’s method (Ward, 1963) was conducted using z-scores from neuropsychological measures in only schizophrenia subjects. Inspection of the dendrogram revealed the overall structure of the data to suggest the best cluster solutions. Finally, a k-means algorithm was utilized as the primary clustering technique to asses stability of the final solution based on its iterative properties (Hartigan & Wong, 1979). See Supplementary material for details regarding the specific clustering process.
Surfaced-based analysis of cortical thickness data involved generation of statistical surface maps using a general linear model that displayed differences in thickness between groups for each vertex. Key demographic variables were not controlled, as these variables did not statistically differ between groups. False Discovery Rate (FDR), which controls for the expected proportion of false positives in a statistical test, was applied to adjust for multiple comparisons at a value of 0.05. Vertex-wise Cohen’s d cortical thickness effect-size maps were also generated for each contrast in support of significance comparisons.
Independent sample t-tests and Chi-square statistics were used to examine differences in demographic variables. Group comparisons on neuropsychological performance were conducted using analysis of variance (ANOVA) models, followed by Tukey post hoc comparisons. The influence of DOI on neuropsychological performance was examined using bivariate Pearson correlations; multiple comparison correction was set at p < 0.005. Cortical thinning areas that emerged from group comparisons were selected as an overall region of interest (ROI) and then parcellated into lobular subdivisions based on anatomical boundary schemes from FS. These ROIs were mapped across all subjects and mean per hemisphere thickness values were derived. The ROI means were then entered as dependent variables in analysis of variance (ANOVA) models with COM and SCZ cluster group status as a main effect. An additional ROI analysis of covariance (ANCOVA) model was run with only SCZ cluster groups to determine the effect of DOI as a covariate. All statistics were analyzed using SPSS Statistics 19 (SPSS, Chicago IL).
3. RESULTS
3.1 Cluster Analysis
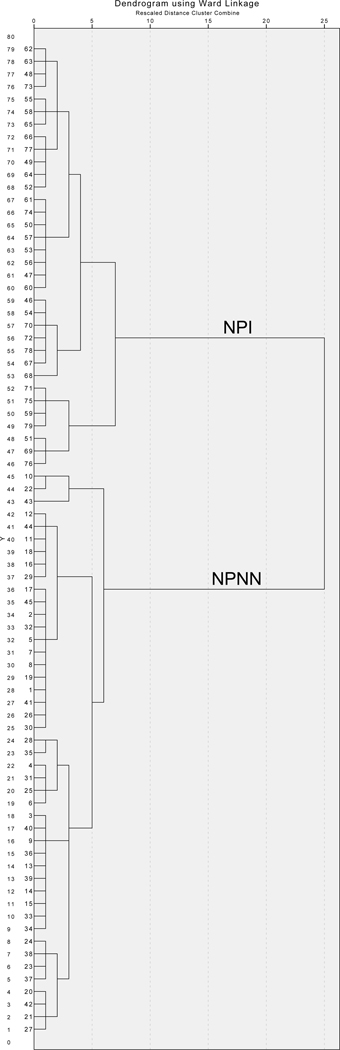
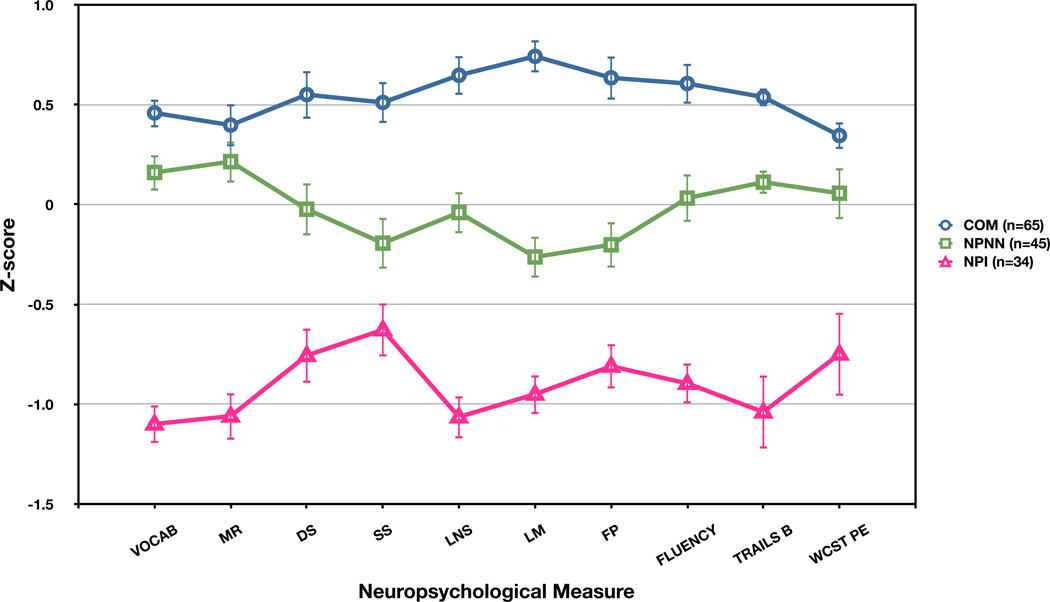
Inspection of the dendrogram generated by Ward’s method revealed strong support for a 2-group solution (Figure 1). Through the iterative k-means clustering process, a NPNN cluster group (n=45), with scores in the average to low average range of functioning was readily identified (Table 1 and Figure 2). Mean scores in the second cluster were in the mildly to moderately impaired range, and it was labeled as the NPI group (n=34). The 2-group solution remained highly stable across alternative clustering methods, see Supplementary material for details.
Figure 1.
Dendogram of SCZ clustering using Ward’s method
Table 1.
Mean scaled and raw neuropsychological test scores for cluster and comparison groups
| Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Measure | NPI n = 34 |
NPNN n = 45 |
COM n = 65 |
ANOVA | |||||
| Mean (SD) Scaled/Raw Scores |
F | df | p | ||||||
| WAIS-III VOCAB | 5.4 | (2.2) *† | 10.4 | (2.4) * | 11.6 | (2.3) | 82.12 | 2 | <0.001 |
| WAIS-III MR | 6.7 | (2.5) *† | 11.2 | (2.5) | 11.9 | (3.1) | 42.17 | 2 | <0.001 |
| WMS-III DS | 7.1 | (2.5) *† | 9.5 | (2.8) * | 11.3 | (3.1) | 23.77 | 2 | <0.001 |
| WMS-III LNS | 5.2 | (2.3) *† | 8.9 | (2.5) * | 11.4 | (2.9) | 62.60 | 2 | <0.001 |
| WMS-III SS | 6.0 | (2.8) * | 7.6 | (3.1) * | 10.1 | (3.0) | 23.37 | 2 | <0.001 |
| WMS-III LM I | 4.7 | (2.4) *† | 7.6 | (2.9) * | 11.9 | (2.7) | 82.73 | 2 | <0.001 |
| WMS-III FP I | 5.4 | (2.4) *† | 7.7 | (2.9) * | 10.9 | (3.4) | 39.33 | 2 | <0.001 |
| Verbal Fluency (total) | 22.2 | (5.4) *† | 30.8 | (7.4) * | 36.1 | (7.5) | 43.28 | 2 | <0.001 |
| Trails B (seconds) | 170.7 | (74.0) *† | 90.9 | (27.1) * | 61.4 | (25.7) | 74.25 | 2 | <0.001 |
| WCST PE (total) | 31.1 | (19.3) *† | 18.3 | (13.8) | 12.7 | (10.0) | 19.70 | 2 | <0.001 |
= differs from COM at p < 0.05,
= differs from NPNN at p < 0.05; based on post-hoc analyses
Figure 2.
Mean (std error) neuropsychological z-scores for schizophrenia cluster (NPNN, NPI) and healthy comparison (COM) groups; VOCAB = WAIS-III Vocabulary, MR = WAIS-III Matrix Reasoning, DS = WMS-III Digit Span, SS = WMS-III Spatial Span, LNS = WMS-III Letter-Number Sequencing, LM = WMS-III Logical Memory, FP = WMS-III Family Pictures, FLUENCY = Lexical + Semantic fluency, TRAILS B = Trail Making Test Part B, WCST = perseverative errors on the Wisconsin Card Sorting Test
3.2 Cluster Differences in Demographic and Neuropsychological Variables
As noted in Table 2, there were no demographic differences between NPI and COM, and NPI and NPNN, with the exception of race and parental SES. Comparisons between NPNN and COM revealed no significant demographic differences. A trend difference was noted in DOI between the SCZ cluster groups, with NPI subjects having been diagnosed with schizophrenia for a slightly longer period (t77 = 1.89, p = 0.06). Regarding clinical symptoms, t-tests between NPI and NPNN revealed no mean difference in positive (t77 = -0.73, p = 0.47) or disorganized (t77 = 1.47, p = 0.14) symptoms, but did show a statistically significant difference for negative symptoms (t77 = 2.06, p = .04), with greater severity for the NPI cluster group.
Table 2.
Demographic characteristics of cluster and comparison groups
| Group | ||||||
|---|---|---|---|---|---|---|
| Characteristic | NPI (n = 34) |
NPNN (n = 45) |
COM (n = 65) |
|||
| Mean | (SD) | Mean | (SD) | Mean | (SD) | |
| Age | 36.57 | (10.84) | 32.38 | (13.37) | 34.15 | (12.75) |
| Parental SES (1–5) | 3.85 | (.784) *† | 3.18 | (.912) | 3.18 | (.917) |
| Duration of illness (years) | 15.91 | (12.3) | 10.86 | (11.40) | - | - |
| Chlorpromazine Equivalent | ||||||
| 1st generation (dose years) | 2.27 | (5.15) | 1.67 | (3.13) | - | - |
| 2nd generation (dose years) | 2.51 | (2.98) | 4.76 | (9.18) | - | - |
| Clinical Symptom Domains | ||||||
| Positive | 2.82 | (2.83) | 3.18 | (2.39) | - | - |
| Negative | 8.15 | (3.89) † | 6.55 | (3.47) | - | - |
| Disorganized | 3.97 | (2.13) | 3.20 | (2.00) | - | - |
| N | (%) | N | (%) | N | (%) | |
| Male Gender | 21 | (61.8) | 30 | (66.7) | 34 | (52.3) |
| Right-handed | 25 | (73.5) | 34 | (75.6) | 52 | (80.0) |
| Race | ||||||
| Caucasian | 10 | (29.4) | 32 | (71.1) | 41 | (63.1) |
| African-American | 24 | (70.6) *† | 13 | (28.9) | 24 | (36.9) |
= differs from COM at p < 0.05,
= between SCZ cluster group difference at p < 0.05
Significant ANOVA group effects were noted across all neuropsychological tasks (Table 1). Tukey HSD post-hoc analyses revealed both the NPI and NPNN groups performed significantly poorer compared to COM on the majority of measures, with the exception of NPNN and COM groups performing similar on two tests. Performance between NPI and NPNN significantly differed on all but one test.
3.3 Cluster Differences in Vertex-Based Cortical Thickness
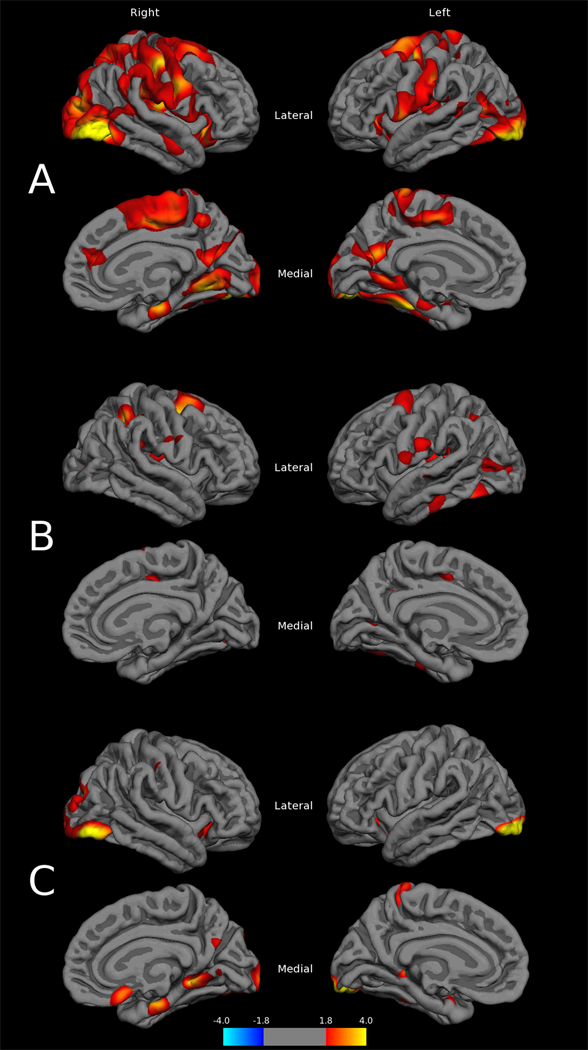
Statistical cortical thickness maps revealed prominent thinning of the cortex in NPI when compared to COM subjects (Figure 3A). Regions included posterior superior frontal, primary and association sensorimotor cortices, lateral occipital, superior parietal, insular, and supramarginal areas, as well as additional involvement of superior and middle temporal gyri. In addition, there was bilateral thinning in the inferior frontal gyrus, particularly of the pars orbitalis, and lateral orbital gyrus. On the medial aspects of the surface, paracentral, cuneus, lingual, parahippocampal, and fusiform thinning was present, with some mild involvement of the anterior cingulate. Severity of thinning appeared asymmetric, with a right greater than left spread in posterior regions. These results met FDR correction for multiple comparisons at a rate of 0.05. Examination of effect-size maps for this comparison (Figure 4A) revealed medium to large magnitudes of effect within, and extending beyond, the cortical thinning pattern described above.
Figure 3.
Cortical thickness difference maps between A) COM – NPI, B) COM – NPNN, and C) NPNN – NPI groups (p values are -log10p)
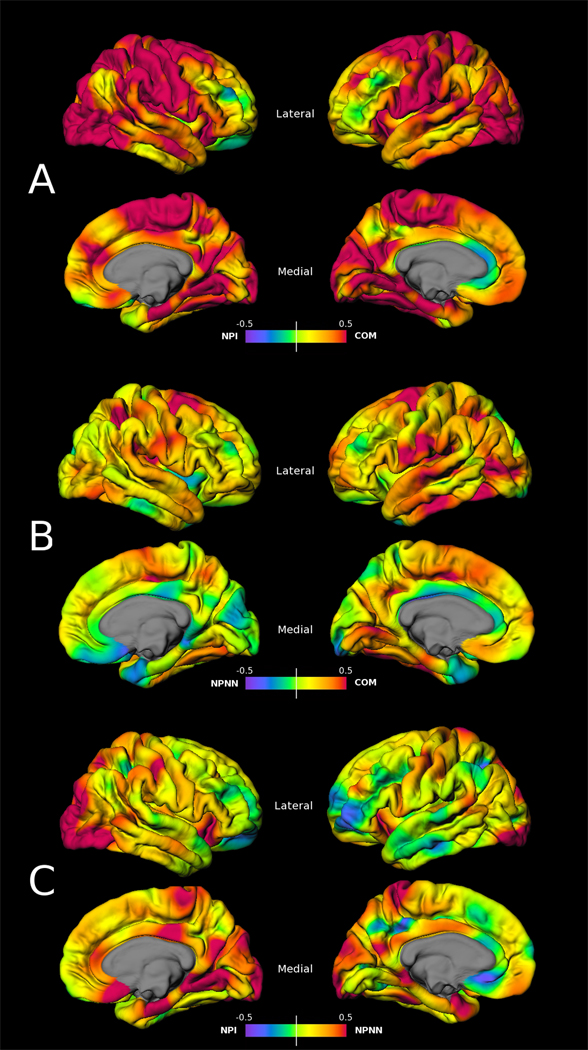
Figure 4.
Cohen’s d effect-size maps based on vertex-wise cortical thickness values between A) COM – NPI, B) COM – NPNN, and C) NPNN – NPI groups (maximum amplitude d = 0.5)
Initial examination of cortical thickness mapping in NPNN, contrasted with COM subjects (Figure 3B), revealed mild thinning in left-sided fusiform, lateral occipital, superior temporal, and subcentral regions, with right-sided superior frontal and parietal areas, as well as bilateral posterior insula. None of these findings survived FDR correction for multiple comparisons, indicating a lack of significant vertex-wise cortical thinning between these two groups. However, examination of the magnitude of difference between these groups (Figure 4B) revealed moderate effect sizes in these regions, with small effects extending beyond.
Cortical thickness comparisons between NPNN and NPI groups (Figure 3C), revealed a thinning pattern mainly posterior in distribution that overlapped with COM/NPI results. Regions most significantly affected were lateral occipital and medial temporal cortices. This pattern also did not survive multiple comparison correction, but moderate to strong effects were observed in regions overlapping the thinning distribution in both this and the COM/NPI comparison (Figure 4C).
3.4 Cluster Differences in ROI-based Cortical Thickness
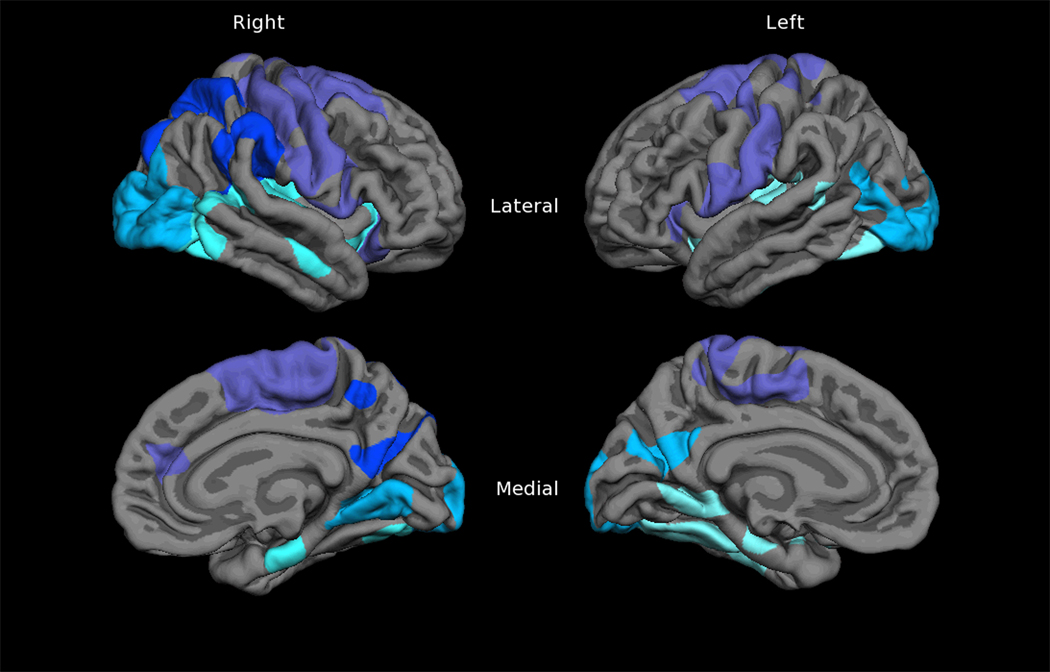
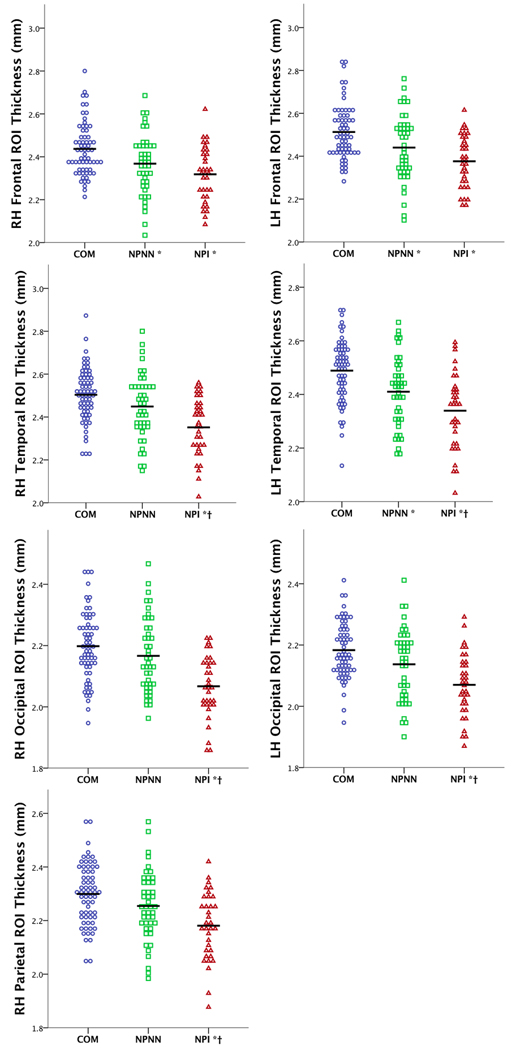
The widespread thinning pattern in NPI subjects (Figure 3A) was used as the basis for an ROI that was divided in to lobular regions: frontal, temporal (including insula), parietal, and occipital (Figure 5), and mapped across subjects. Comparison of thickness values within the thinning pattern ROIs revealed a consistent step-wise pattern of loss between the three groups. ANOVA results revealed significant differences between NPI and COM in all areas, but only differences in left frontal, temporal, and right frontal regions between NPNN and COM (Figure 6). For NPNN and NPI, significant differences were evident in left temporal, occipital, and right temporal, occipital, and parietal regions. When controlling for DOI, significant differences in the right temporal and parietal areas were attenuated to non-significance.
Figure 5.
Mapped cortical thinning-based regions of interest (ROI) subdivided according to lobe; purple = frontal, pale green = temporal, blue = parietal, pale blue = occipital
Figure 6.
Dot plots of mean cortical thickness values from lobe-specific ROIs for each group; * = differs from COM at p < 0.05, † = differs from NPNN at p < 0.05; based on post-hoc analyses
3.5 Relationships Between Duration of Illness and Neuropsychological Performance
Bivariate Pearson correlations between DOI and neuropsychological variables revealed significant negative correlations with Trail Making Test Part B (r = -0.411, p = 0.01), as well as perseverative errors on the Wisconsin Card Sorting Test (r = -0.410, p = 0.01) in the NPNN group. The level of significance is attenuated when applying the p < 0.005 multiple comparison correction. No significant correlations between DOI and neuropsychological performance were noted for NPI subjects.
4. DISCUSSION
In this study, neuropsychologically defined schizophrenia subtypes were identified through unsupervised clustering processes similar to those used in previous studies (Allen et al., 1998; Bell et al., 2010; Hill et al., 2002). The rationale for the current study was to take into account a broader spectrum of neuropsychological performance to define impairment and “normality,” as it was believed these measures in aggregate best related to global evaluation of underlying neuroanatomic abnormalities in cortical thickness. Ultimately it was determined that a 2-group clustering solution was the best fit for the data; however, this is not to imply that only two cognitive schizophrenia subtypes exist. Indeed, previous studies have found upwards of four to five subtypes, depending on neuropsychological variables and methods used (Goldstein et al., 1998a; Heinrichs & Awad, 1993; Horan & Goldstein, 2003). The intent of this study was to effectively identify NPNN subjects, without particular regard to the separation and degrees of impairment within NPI participants. It is believed that this 2-cluster method was fairly robust as NPNN classification remained highly intact (93- 100% overlap) across various algorithms and when forcing a different number of solutions. Further support for the method is that our resultant cognitive subtypes had neuropsychological and demographic profiles remarkably similar to those found in previous studies (Allen et al., 1998; Goldstein et al., 1998a; Hill et al., 2002; Horan & Goldstein, 2003; Palmer et al., 1997; Seaton et al., 1999).
Regarding the primary cortical thickness analysis, the NPI group displayed a somewhat atypical pattern of strong posterior changes, but one that has been noted in other work (Narr et al., 2005). With the exception of partial involvement in the superior and inferior frontal gyrus and anterior cingulate, there was a general lack of characteristic frontal lobe thinning, which, while surprising, is not without precedent (Narr et al., 2005; Wiegand et al., 2004). The absence of significant thinning in the NPNN – COM and NPNN – NPI vertex-wise analyses was unexpected as initially it was hypothesized an attenuated pattern would emerge that matched their cognitive profile. However, effect-size and uncorrected contrast maps for these comparisons suggest trend differences, indicating subtle characteristics unique to this group may exist, reinforcing the notion that “near-normal” status is not synonymous with “unaffected.” Indeed, follow-up ROI analyses revealed comparable thinning in NPNN and NPI from COM in frontal areas, but significantly differing for each other in temporal, occipital and parietal regions. This suggests that despite having a neurobiological illness, a subset of schizophrenia patients with “near-normal” cognitive functioning may also have relatively “near-normal” cortical gray matter thickness, which is intermediate between healthy and severely impaired states (Figure 6). That the NPNN group demonstrates few neurobiological differences from COM subjects, yet significantly differs from them on most cognitive measures is interesting in that it highlights certain aspects of the illness still affect this group. While these individuals may reside in the lower cognitive band of “normality,” there appears to exist some neurobiological compensatory mechanism preventing them from functioning similar to their NPI counterparts.
The finding also has implications for the influence of cortical thickness on the expression of various clinical aspects of the illness. Consistent with our results, it is common for impaired cluster groups to experience more negative symptoms compared to their less impaired counterparts, but show similar positive and disorganized symptomatology (Kremen et al., 2000; Palmer et al., 1997). Hitherto, these findings have yet to be associated with anatomical results; hence future work would focus on both the structural and functional aspects of cognitive subtypes in an effort to clarify the influence of these factors. For example, if cortical gray matter is not contributing to the clinical expression of the schizophrenic condition in NPNN subjects, then investigations of localized changes in other regions, such as subcortical nuclei (Wang et al., 2008) and white matter pathways (Voineskos et al., 2010), or unified networks of these structures, could provide alternative explanations.
Several possibilities for this “near-normal” phenomenon in schizophrenia are postulated: first, NPNN subjects may be experiencing a different form of the disease, with separate underlying neurobiological processes mild in their expression of schizophrenia-like structural and functional deficits, similar to that proposed by Murray and associates (1987); second, NPNN may represents an early transitory state, where individuals eventually progress to, and remain neuropsychologically impaired as disease effects persist. This second explanation seems less likely given that, with the exception of peri-onset changes, neuropsychological deficits appear relatively stable over the course of the illness (Albus et al., 2002; Heaton et al., 2001; Hill et al., 2004). Indeed, subjects in our study were more chronic, suggesting neuropsychological profiles less amenable to progressive deterioration. One final theory is that the observed differences in cluster groups may be explained by current concepts of brain and cognitive reserve (Satz et al., 2011; Stern, 2002). It is possible that a latent factor related to the separation of schizophrenia participants into subgroups is one associated with cognitive reserve, where NPNN subjects are those who experience greater advantages in variables underlying this concept.
The literature on illness severity and duration suggests that the degree of cognitive performance is not related to these variables (Holthausen et al., 2002). Our analysis of DOI favors the model of separate disease entities, but also indicates that it wields some influence in NPNN cluster groups. Only for NPNN subjects were correlations found between DOI and select neuropsychological measures related to executive functioning, whereas no relationships were found in NPI subjects; however, these relationships were attenuated when corrected for multiple comparisons. This is similar to Goldstein and colleagues (1998b), who also found low correlations between age, as a proxy for illness duration, and neuropsychological performance in an impaired cognitive cluster, but significant correlations in a near-normal cognitive cluster. Also, in their study on the effect of age on cortical thickness, Kubota and coworkers (2011) found that illness duration did not influence changes in regional thickness for their schizophrenia group. Despite the attenuation of some region-specific effects when controlling for DOI, our results were similar in that differences in cortical thickness between NPI and NPNN subjects still remained. Overall, our result argues against a general effect of disease severity on the neuroanatomical differences between subgroups, but still leaves room for further investigation of factors contributing to neuropsychological heterogeneity.
In conclusion, our study adds insight into the cortical signature of neuropsychologically near-normal schizophrenia. Future work would include examining white matter and functional measures, accompanied by longitudinal change estimates. Increased characterization of this population will continue to elucidate the relationship between cognitive and neurobiological features in schizophrenia, and assist in exploration of disease heterogeneity.
Supplementary Material
Supplementary Figure. Dendogram of SCZ + COM clustering using Ward’s method
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
John G. Csernansky, Email: jgc@northwestern.edu.
Lei Wang, Email: leiwang1@northwestern.edu.
References
- Albus M, Hubmann W, Scherer J, Dreikorn B, Hecht S, Sobizack N, et al. A prospective 2-year follow-up study of neurocognitive functioning in patients with first-episode schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2002;252(6):262–267. doi: 10.1007/s00406-002-0391-4. [DOI] [PubMed] [Google Scholar]
- Allen DN, Huegel SG, Seaton BE, Goldstein G, Gurklis JA, van Kammen DP. Confirmatory factor analysis of the WAIS-R in patients with schizophrenia. Schizophr Res. 1998;34(1–2):87–94. doi: 10.1016/s0920-9964(98)00090-5. [DOI] [PubMed] [Google Scholar]
- Allen DN, Seaton BE, Goldstein G, Sanders RD, Gurklis JA, Jr., Peters JL, et al. Neuroanatomic differences among cognitive and symptom subtypes of schizophrenia. J Nerv Ment Dis. 2000;188(6):381–384. doi: 10.1097/00005053-200006000-00010. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. The lifetime trajectory of schizophrenia and the concept of neurodevelopment. Dialogues in clinical neuroscience. 2010;12(3):409–415. doi: 10.31887/DCNS.2010.12.3/nandreasen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MD, Johannesen JK, Greig TC, Wexler BE. Memory profiles in schizophrenia: categorization validity and stability. Schizophr Res. 2010;118(1–3):26–33. doi: 10.1016/j.schres.2009.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Delawalla Z, Barch DM, Fisher Eastep JL, Thomason ES, Hanewinkel MJ, Thompson PA, et al. Factors mediating cognitive deficits and psychopathology among siblings of individuals with schizophrenia. Schizophr Bull. 2006;32(3):525–537. doi: 10.1093/schbul/sbj082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: A metaanalytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry. 2007;64(5):532–542. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- Goldstein G, Allen DN, Seaton BE. A comparison of clustering solutions for cognitive heterogeneity in schizophrenia. J Int Neuropsychol Soc. 1998a;4(4):353–362. [PubMed] [Google Scholar]
- Goldstein G, Allen DN, van Kammen DP. Individual differences in cognitive decline in schizophrenia. Am J Psychiatry. 1998b;155(8):1117–1118. doi: 10.1176/ajp.155.8.1117. [DOI] [PubMed] [Google Scholar]
- Harms MP, Wang L, Mamah D, Barch DM, Thompson PA, Csernansky JG. Thalamic shape abnormalities in individuals with schizophrenia and their nonpsychotic siblings. J Neurosci. 2007;27(50):13835–13842. doi: 10.1523/JNEUROSCI.2571-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartigan J, Wong M. A K-Means Clustering Algorithm. Journal of the Royal Statistical Society. Series C (Applied Statistics) 1979;28(1):100–108. [Google Scholar]
- Heaton RK, Gladsjo JA, Palmer BW, Kuck J, Marcotte TD, Jeste DV. Stability and course of neuropsychological deficits in schizophrenia. Arch Gen Psychiatry. 2001;58(1):24–32. doi: 10.1001/archpsyc.58.1.24. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Awad AG. Neurocognitive subtypes of chronic schizophrenia. Schizophr Res. 1993;9(1):49–58. doi: 10.1016/0920-9964(93)90009-8. [DOI] [PubMed] [Google Scholar]
- Hill SK, Ragland JD, Gur RC, Gur RE. Neuropsychological profiles delineate distinct profiles of schizophrenia, an interaction between memory and executive function, and uneven distribution of clinical subtypes. J Clin Exp Neuropsychol. 2002;24(6):765–780. doi: 10.1076/jcen.24.6.765.8402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SK, Schuepbach D, Herbener ES, Keshavan MS, Sweeney JA. Pretreatment and longitudinal studies of neuropsychological deficits in antipsychotic-naive patients with schizophrenia. Schizophr Res. 2004;68(1):49–63. doi: 10.1016/S0920-9964(03)00213-5. [DOI] [PubMed] [Google Scholar]
- Holthausen EA, Wiersma D, Sitskoorn MM, Hijman R, Dingemans PM, Schene AH, et al. Schizophrenic patients without neuropsychological deficits: Subgroup, disease severity or cognitive compensation? Psychiatry Res. 2002;112(1):1–11. doi: 10.1016/s0165-1781(02)00184-1. [DOI] [PubMed] [Google Scholar]
- Horan WP, Goldstein G. A retrospective study of premorbid ability and aging differences in cognitive clusters of schizophrenia. Psychiatry Res. 2003;118(3):209–221. doi: 10.1016/s0165-1781(03)00078-7. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Seidman LJ, Faraone SV, Toomey R, Tsuang MT. The paradox of normal neuropsychological function in schizophrenia. J Abnorm Psychol. 2000;109(4):743–752. doi: 10.1037//0021-843x.109.4.743. [DOI] [PubMed] [Google Scholar]
- Kubota M, Miyata J, Yoshida H, Hirao K, Fujiwara H, Kawada R, et al. Age-related cortical thinning in schizophrenia. Schizophr Res. 2011;125(1):21–29. doi: 10.1016/j.schres.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60(9):878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Murray RM, Lewis SW. Is schizophrenia a neurodevelopmental disorder? Br Med J (Clin Res Ed) 1987;295(6600):681–682. doi: 10.1136/bmj.295.6600.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr KL, Toga AW, Szeszko P, Thompson PM, Woods RP, Robinson D, et al. Cortical thinning in cingulate and occipital cortices in first episode schizophrenia. Biol Psychiatry. 2005;58(1):32–40. doi: 10.1016/j.biopsych.2005.03.043. [DOI] [PubMed] [Google Scholar]
- Palmer BW, Dawes SE, Heaton RK. What do we know about neuropsychological aspects of schizophrenia? Neuropsychol Rev. 2009;19(3):365–384. doi: 10.1007/s11065-009-9109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer BW, Heaton RK, Paulsen JS, Kuck J, Braff D, Harris MJ, et al. Is it possible to be schizophrenic yet neuropsychologically normal? Neuropsychology. 1997;11(3):437–446. doi: 10.1037//0894-4105.11.3.437. [DOI] [PubMed] [Google Scholar]
- Satz P, Cole MA, Hardy DJ, Rassovsky Y. Brain and cognitive reserve: mediator(s) and construct validity, a critique. J Clin Exp Neuropsychol. 2011;33(1):121–130. doi: 10.1080/13803395.2010.493151. [DOI] [PubMed] [Google Scholar]
- Schultz CC, Koch K, Wagner G, Roebel M, Schachtzabel C, Gaser C, et al. Reduced cortical thickness in first episode schizophrenia. Schizophr Res. 2010;116(2–3):204–209. doi: 10.1016/j.schres.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Seaton BE, Allen DN, Goldstein G, Kelley ME, van Kammen DP. Relations between cognitive and symptom profile heterogeneity in schizophrenia. J Nerv Ment Dis. 1999;187(7):414–419. doi: 10.1097/00005053-199907000-00004. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Barch DM, Csernansky JG. Bridging the gap between schizophrenia and psychotic mood disorders: Relating neurocognitive deficits to psychopathology. Schizophr Res. 2009;107(1):69–75. doi: 10.1016/j.schres.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8(3):448–460. [PubMed] [Google Scholar]
- Voineskos AN, Lobaugh NJ, Bouix S, Rajji TK, Miranda D, Kennedy JL, et al. Diffusion tensor tractography findings in schizophrenia across the adult lifespan. Brain : a journal of neurology. 2010;133(Pt 5):1494–1504. doi: 10.1093/brain/awq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Mamah D, Harms MP, Karnik M, Price JL, Gado MH, et al. Progressive deformation of deep brain nuclei and hippocampal-amygdala formation in schizophrenia. Biol Psychiatry. 2008;64(12):1060–1068. doi: 10.1016/j.biopsych.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. Hierarchical grouping to optimize an objective function. Journal of the American statistical association. 1963;58(301):236–244. [Google Scholar]
- Wexler BE, Zhu H, Bell MD, Nicholls SS, Fulbright RK, Gore JC, et al. Neuropsychological near normality and brain structure abnormality in schizophrenia. Am J Psychiatry. 2009;166(2):189–195. doi: 10.1176/appi.ajp.2008.08020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand LC, Warfield SK, Levitt JJ, Hirayasu Y, Salisbury DF, Heckers S, et al. Prefrontal cortical thickness in first-episode psychosis: A magnetic resonance imaging study. Biol Psychiatry. 2004;55(2):131–140. doi: 10.1016/j.biopsych.2003.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure. Dendogram of SCZ + COM clustering using Ward’s method