Abstract
Seizures may directly cause brain injury by disrupting the structure and function of synapses. Previous studies using in vivo time-lapse imaging have demonstrated an acute beading of dendrites and loss of dendritic spines immediately following status epilepticus, but the effects of brief seizures and the long-term evolution of this dendritic injury are unknown. Here, we examined the effects of seizures of varying durations on dendritic structure over several weeks using in vivo multiphoton imaging with kainate-induced seizures in mice. The degree of dendritic injury was directly dependent on the duration of the seizures, with seizures lasting more than 30 minutes (status epilepticus) resulting in a greater than 75% spine loss. However, even brief seizures (<5 minutes) induced moderate dendritic beading and spine loss. The dendritic injury from brief seizures usually recovered within two weeks, whereas status epilepticus-induced injury only partially reversed. These studies demonstrate that seizures of all durations may trigger at least transient neuronal injury.
Keywords: epilepsy, seizure, dendrite, dendritic spine, mice
Introduction
Seizures may directly induce brain injury and potentially contribute to neurological and cognitive deficits that frequently occur in epilepsy patients. While seizures can cause neuronal death in some situations, they may also have deleterious effects on neuronal structure and function via a variety of “non-lethal” mechanisms. Dendritic spines represent the main anatomic sites of contact for excitatory, glutamatergic synaptic inputs onto cortical neurons and are strongly implicated in mechanisms of synaptic plasticity and learning. A loss of dendritic spines in neocortex or hippocampus have been observed in pathological specimens from human epilepsy patients (Schiebel et al., 1974; Isokawa and Levesque, 1991; Multani et al., 1994) and animal seizure models (Olney et al. 1983; Muller et al., 1993; Drakew et al., 1996; Isokawa, 1998; Jiang et al., 1998), suggesting that dendritic spine loss could represent a pathological substrate of memory deficits and other cognitive dysfunction in epilepsy (Swann et al., 2000; Wong, 2005). However, it is difficult to determine the direct contribution of seizures to dendritic injury, as well as the time course of these changes, based on conventional pathological studies of fixed tissue alone. Recently modern cellular imaging techniques have assessed the direct effects of seizures (Mizrahi et al., 2004; Rensing et al., 2005; Zeng et al., 2007) and ischemia (Zhang et al., 2005; Risher et al., 2010) on dendritic structure in living mice on a very rapid time scale. In particular, kainate-induced status epilepticus can trigger an acute beading of dendrites and loss of dendritic spines of neocortical neurons within minutes (Zeng et al., 2007). However, whether brief seizures produce similar dendritic changes is unknown, and the long-term consequences of this acute dendritic injury have not been investigated.
In this study, we utilized in vivo multiphoton imaging to examine the dependence of dendritic injury on seizure duration and follow the long-term evolution of seizure-induced dendritic changes over several weeks. Interestingly, even brief seizures, lasting less than 5 minutes, caused some degree of dendritic spine loss, although this was reversible over time. In contrast, status epilepticus produced severe dendritic injury that persisted for several weeks through the duration of the study.
Materials and methods
Animals
Two to three-month-old transgenic mice with a C57BL/6 background expressing enhanced green fluorescent protein (GFP) under a thy1 promoter (line GFP-M) were used for all experiments (Feng et al., 2000). In neocortex, GFP-M mice exhibit expression of GFP in a subpopulation of pyramidal neurons, primarily in cortical layer 5 and, to a lesser extent, layer 2/3. The C57BL/6 genetic background was advantageous for these studies, as this strain of mice is resistant to kainate-induced neuronal death and epileptogenesis (Schauwecker and Steward, 1997; Yang et al., 2005; Zeng et al., 2007), which otherwise could represent confounding factors in interpretation of our experiments. Care and use of animals conformed to a protocol approved by the Washington University School of Medicine Animal Studies Committee.
Surgery
Animal surgery was performed by similar methods as previously reported (Rensing et al., 2005; Zeng et al., 2007). Briefly, mice were anesthetized with isoflurane anesthesia and held in a custom-made stereotaxic device, which could be mounted to the microscope stage. A heating pad was used to maintain body temperature while under anesthesia. A round cranial window (approximately 2.5 mm diameter) was first drilled in the skull with the center of the window approximately 3 mm posterior to bregma and 2 mm lateral to midline. Three screw electrodes were placed adjacent to the cranial window to record electroencephalography (EEG). A glass coverslip (#1.5, 8 mm) was centered over the cranial window and attached to the skull with dental acrylic, which also stabilized the EEG electrodes.
Multiphoton Imaging
Control, baseline images of dendrites and dendritic spines of neocortical neurons expressing GFP were obtained through the cranial window with a multiphoton microscope (LSM 510; Zeiss, Thornwood, NY) and a water immersion objective (Zeiss, 40×, 0.8 numerical aperture (NA), IR-adjusted, Zeiss). A Titanium-Sapphire pulsed infrared laser (Coherent, Santa Clara, CA) was used to stimulate GFP at 900 nm. Low-magnification images approximately 50 to 100 μm below the neocortical surface were first obtained to identify regions with GFP-expressing dendrites. At higher magnification (3× digital zoom), z-stacks of 6 to 10 images separated by 1 μm steps were taken of dendrites and accompanying spines. Individual images were acquired at 12 bits with frame averaging (2–4 times). Following seizures, surface blood vessels and other landmarks were used to identify the same dendrites for post-seizure time-lapse imaging.
Seizure Induction and Electroencephalogram Recording
After obtaining baseline images, the mice were allowed to recover from anesthesia. EEG signals were amplified and filtered (1–100Hz) using standard amplifiers (Grass P-511; Astro-Med, West Warwick, RI) and digitized (200Hz) with commercial hardware and software (Axon Digidata 1322 and Axoscope; Molecular Devices, Sunnyvale, CA). Mice were then injected with kainate (Sigma, St. Louis, MO) (20 mg/kg, i.p.). Control mice received saline injection instead of kainate. Electrographic seizures were recorded by EEG and the cumulative duration of individual seizures was monitored. An individual seizure was defined as a discrete epoch of repetitive spikes or spike-and-wave discharges lasting at least 10 seconds. The behavioral correlate of seizures was noted using a modified Racine scale (Racine, 1972; Zeng et al., 2007): stage 1 – behavioral arrest with mouth/facial movements, stage 2 – head nodding, stage 3 – forelimb clonus, stage 4 – rearing, stage 5 – rearing and falling, stage 6 – loss of posture and generalized convulsive activity.
In one set of experiments to determine the effect of seizure duration, seizures were terminated after various estimated cumulative durations of electrographic seizures (<5 min, 5–10 min, 10–20 min, 20–30 min, and 30–45 min) by isoflurane anesthesia induction for subsequent post-seizure imaging for 4 hours following seizure termination. In a second set of experiments to determine the long-terms effects of seizures, two groups with cumulative electrographic seizure duration of <5 min or 30–45 min were followed with post-seizure time-lapse imaging for 6 weeks following seizure termination. In both sets of experiments, seizures were terminated by isoflurane by rapid induction in a gas anesthesia induction chamber. Mice were then transferred to the stereotaxic device for subsequent imaging, during which isoflurane via mask was administered continuously for four hours, after which animals were sacrificed (first set of experiments) or allowed to recover (second set of experiments). In chronic experiments, EEG was also continued for six weeks to confirm the lack of seizure recurrence after stopping anesthesia and to monitor for the subsequent development of spontaneous seizures.
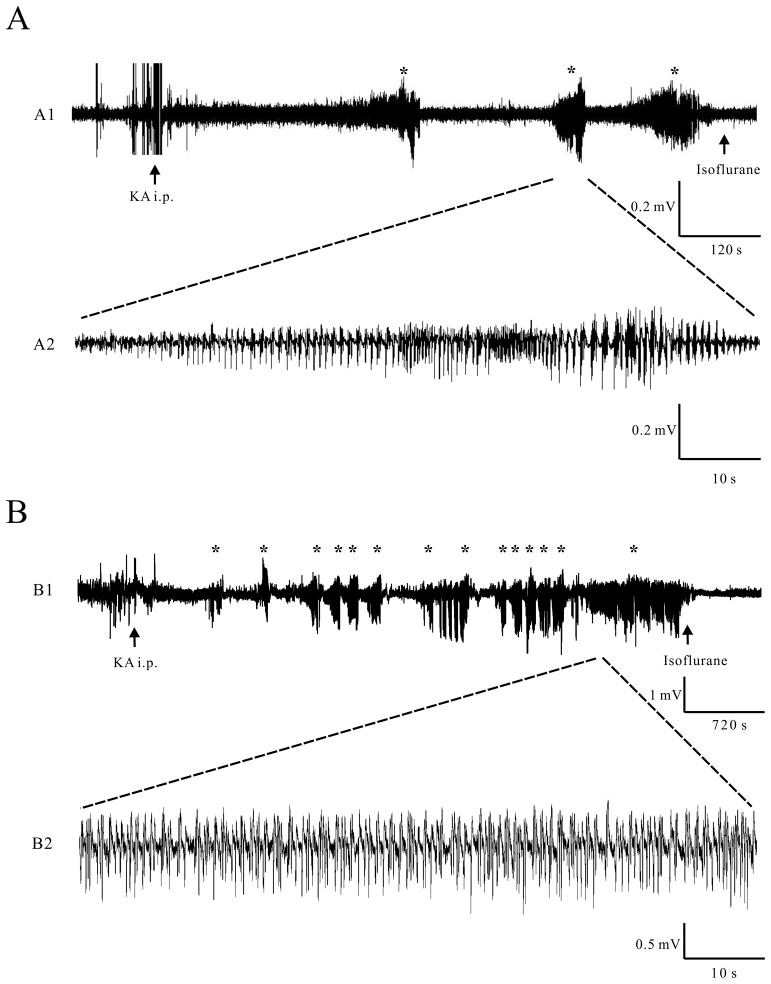
Post-hoc analysis of the EEG confirmed termination of the seizures and allowed calculation of a precise cumulative seizure duration for each animal. In the early stages following kainate injection, seizures initially are brief and intermittent and then become longer and more continuous in later stages of status epilepticus (Fig. 1). Thus, the shorter duration seizure groups (e.g. <5 min) usually consisted of several brief seizures, rather than one continuous seizure.
Figure 1.
Representative examples of kainate-induced EEG seizures of different cumulative seizure durations. A) In mice experiencing brief seizures (<5 min), a series of intermittent, individual seizures with a cumulative seizure duration of <5 min occurred within several minutes of kainate (KA) injection and was terminated by isoflurane after the end of the last seizure. B) In mice that were allowed to progress into status epilepticus (30–45 min cumulative seizure duration), the initial brief intermittent seizures transitioned into more frequent longer seizures and finally continuous seizure activity, which was eventually terminated by isoflurane. The asterisks mark individual seizures. Note the difference in time base between A1 and B1.
Post hoc image analysis
Post hoc image analysis was performed using LSM 5 Image Examiner software (Zeiss) to evaluate changes in dendrites over time, as described previously (Rensing et al., 2005; Zeng et al., 2007). Briefly, spines were defined as perpendicular projections out of the main axis of the dendrite that were narrower than the dendrite from which they arose and could progressively taper, maintain their width, or form “caps.” The numbers of spines at different time points after the seizures were normalized to those at baseline before the seizures in each group. In addition to spine counting, a qualitative scoring system was also used to grade the degree of beading that frequently occurred after seizures: no beading; mild beading (visible beads with diameter of beads <3 times the diameter of the original dendrite with normal intervening segments of dendrite); severe beading (visible beading with diameter of beads >3 times the diameter of the original dendrite without normal intervening segments of dendrite). Both summed projections of z-stacks and individual images (typically 6–10) of the z-stack were analyzed, allowing more accurate identification of spines, mild beading, and artifacts (such as turns in dendrites, mimicking beading). Two separate blinded people analyzed the imaging data independently to confirm interobserver reliability of the analysis method. For the chronic imaging studies over six weeks, mice that developed obvious technical complications (e.g. bleeding or severe clouding over of the cranial window) at any point during the period were excluded from analysis. All remaining mice included for analysis were successfully followed for the six week duration without any loss or “drop-out” of imaged dendrites.
Statistics
Repeated one-way ANOVA with Tukey-Kramer post-tests for multiple comparisons was used to compare changes in dendritic spine number between different groups. Chi-square test of independence was used to compare the distribution of dendritic beading severity as a function of seizure duration. All data are expressed as mean ± SEM. Statistical significance was defined as p<0.05.
Results
Acute dendritic injury occurs following brief seizures and depends on seizure duration
To determine the effect of seizure duration on dendritic injury, we first examined acute dendritic changes between 0 and 4 hours after kainate-induced seizures of various cumulative durations (<5 min, 5–10 min, 10–20 min, 20–30 min and 30–45 min) based on EEG recordings. Following kainate injection, seizures exhibit a typical pattern of development and progression, usually starting with brief, intermittent individual seizures (Fig. 1A) and then quickly transitioning into longer, more frequent seizures that eventually become continuous (Fig. 1B). Thus, mice in which seizures were terminated after shorter cumulative durations (e.g. <5 min) exhibited a series of brief seizures, whereas mice that were allowed to progress into status epilepticus (30–45 min) transitioned from brief intermittent seizures into continuous seizure activity (Fig. 1; Table 1). Most EEG seizures had a behavioral correlate, the severity of which was dependent on the cumulative seizure duration; seizures in mice with cumulative seizure duration of <5 min were usually subtle, consisting of behavioral arrest (stage 1) or head nodding (stage 2), whereas mice that progressed into status epilepticus (30–45 min) exhibited rearing (stage 4) or rearing and falling (stage 5) (Table 1). EEG confirmed that electrographic seizure activity stops after isoflurane induction to terminate the seizures.
Table 1.
Characteristics of Kainate-induced Seizures of Different Cumulative Durations
| Group (cumulative seizure duration) | Number of seizures | Single seizure duration (min) | Cumulative seizure duration (min) | Highest behavioral seizure stage |
|---|---|---|---|---|
| <5 min | 3.1 ± 0.7 | 0.7 ± 0.1 | 2.1 ± 0.4 | 1.3 ± 0.2 |
| 30–45 min | 12.8 ± 3.3 | 2.7 ± 0.7 | 34.9 ± 2.2 | 4.8 ± 0.2 |
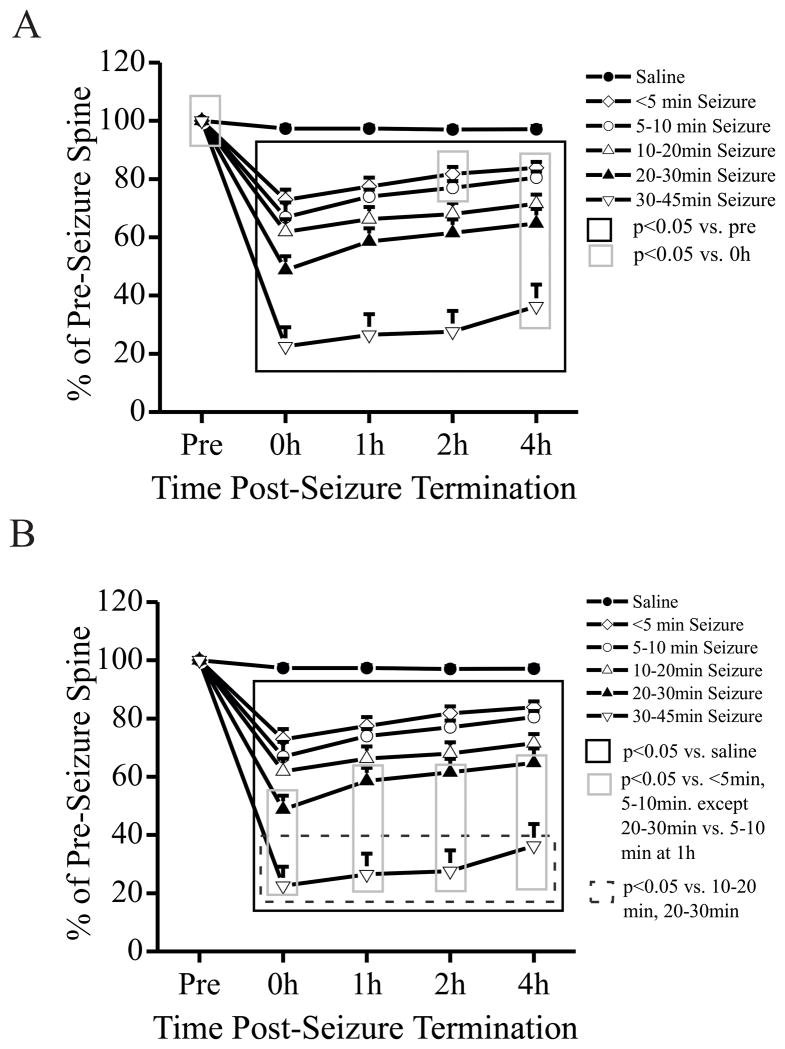
Dendrites of saline-injected control mice showed no significant changes in spine number and no evidence of dendritic beading over 4 hours. In contrast, seizures of all examined durations led to acute spine loss compared with the baseline dendrites before the seizures (Figs. 2A, 3). Moreover, the degree of spine loss was dependent on the duration of the electrographic seizures (Fig. 2B). Interestingly, even brief seizures (<5 min) caused an immediate ~25% spine loss, whereas seizures lasting 30–45 minutes led to ~75% spine loss (Figs. 2B, 3). There was a partial, but incomplete, recovery of spines over 4 hours following the seizures. Spine number recovered to 80% of baseline with brief seizures (<5 min) and to 40% of baseline with 30–45 min seizures. Parallel with the dendritic spine loss, seizures of all durations produced acute dendritic beading, with the incidence and severity of beading correlating with the duration of the seizures (Table 2). Brief seizures (<5 min) produced mild beading in about 30% of dendrites, but almost never severe beading. Seizures lasting 30–45 minutes caused severe beading in about 70% of dendrites. Similar to spine loss, dendritic beading showed partial, but not complete, resolution over the 4 hour period. Seizures of intermediate durations (5–10 min, 10–20 min, 20–30 min) exhibited correspondingly intermediary spine loss (Fig. 2B, 3) and dendritic beading (Table 2). Overall, these studies demonstrate that even brief kainate-induced seizures can cause acute dendritic injury, including spine loss and dendritic beading, and the severity of dendritic injury is dependent on the seizure duration.
Figure 2.
Kainate-induced seizures cause acute dendritic spine loss which partially recovers within 4 hours and is dependent on seizure duration. (A) Saline-injected control mice had no significant dendritic spine loss over a 4 h period (n = 602 total spines from 43 dendrites of 8 mice). In contrast, kainate-induced seizures of all examined durations caused acute loss of spines immediately after termination of the seizures (0 h), compared to the pre-seizure baseline. This spine loss partially recovered in 4 hours following termination of the seizures. Spine number was normalized to those at baseline (Pre). (B) The degree of spine loss was dependent on the duration of electrographic seizures. Even brief seizures with a cumulative duration of less than 5 minutes caused ~25% spine loss (n = 467 total spines from 34 dendrites of 8 mice). Seizures lasting 30–45 minutes caused ~75% spine loss (n = 310 total spines from 23 dendrites of 6 mice), with intermediate seizure durations having correspondingly intermediary spine loss. For clarity, A and B present different statistical comparisons of the same data/experiments (by ANOVA).
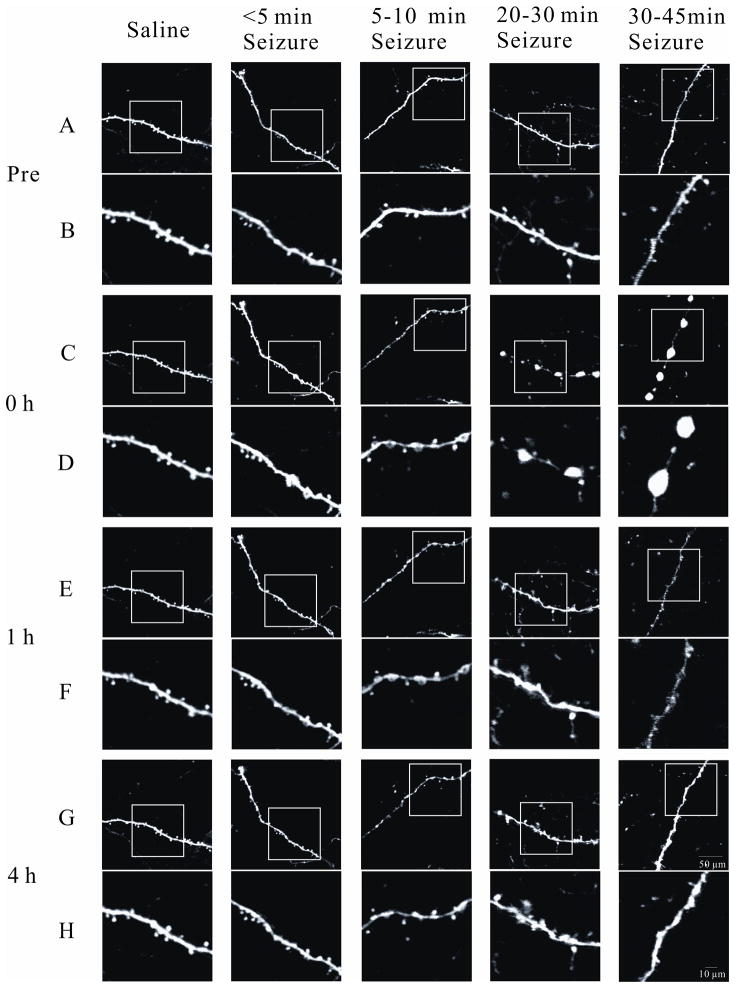
Figure 3.
Representative images of acute dendritic changes following kainate seizures of various durations. No obvious spine loss or dendritic beading was observed in saline-injected control mice. Brief seizures caused no or mild dendritic beading with only modest spine loss. In contrast, longer seizures induced more severe beading and extensive spine loss. The lower panels (B, D, F, and H) in each group are enlargements of the images in the frame in the upper panels (A, C, E, and G), respectively. Scale bars: 50 μm in the upper (A, C, E, and G), and 10 μm in the lower (B, D, F, and H) panels in each group.
Table 2.
Severity of kainate seizure-induced dendritic beading is dependent on seizure duration.
| Group/Time After Seizure | Total Dendrites | No beading | Mild Beading | Severe Beading |
|---|---|---|---|---|
| Saline (no seizure) | ||||
| Baseline | 43 | 43 (100%) | 0 (0%) | 0 (0%) |
| 0 h | 43 | 43 (100%) | 0 (0%) | 0 (0%) |
| 1 h | 43 | 43 (100%) | 0 (0%) | 0 (0%) |
| 2 h | 43 | 43 (100%) | 0 (0%) | 0 (0%) |
| 4 h | 43 | 43 (100%) | 0 (0%) | 0 (0%) |
| < 5min seizure | ||||
| Pre-seizure | 34 | 34 (100%) | 0 (0%) | 0 (0%) |
| 0 h | 34 | 22 (64.7%) | 11 (32.4%) | 1 (2.9%) |
| 1 h | 34 | 23 (67.6%) | 10 (29.5%) | 1 (2.9%) |
| 2 h | 34 | 23 (67.6%) | 11 (32.4%) | 0 (0%) |
| 4 h | 34 | 24 (70.6%) | 10 (29.4%) | 0 (0%) |
| 5–10 min seizure | ||||
| Pre-seizure | 29 | 29 (100%) | 0 (0%) | 0 (0%) |
| 0 h | 29 | 14 (48.3%) | 12 (41.4%) | 3 (10.3%) |
| 1 h | 29 | 14 (48.3%) | 15 (51.7%) | 0 (0%) |
| 2 h | 29 | 14 (48.3%) | 15 (51.7%) | 0 (0%) |
| 4 h | 29 | 14 (48.3%) | 15 (51.7%) | 0 (0%) |
| 10–20 min seizure | ||||
| Pre-seizure | 38 | 38 (100%) | 0 (0%) | 0 (0%) |
| 0 h | 38 | 11 (28.9%) | 23 (60.6%) | 4 (10.5%) |
| 1 h | 38 | 11 (28.9%) | 24 (63.2%) | 3 (7.9%) |
| 2 h | 38 | 11 (28.9%) | 24 (63.2%) | 3 (7.9%) |
| 4 h | 38 | 11 (28.9%) | 26 (68.5%) | 1 (2.6%) |
| 20–30 min seizure | ||||
| Pre-seizure | 34 | 34 (80%) | 0 (0%) | 0 (0%) |
| 0 h | 34 | 3 (8.8%) | 23 (67.6%) | 8 (23.5%) |
| 1 h | 34 | 1 (2.9%) | 28 (82.4%) | 5 (14.7%) |
| 2 h | 34 | 1 (2.9%) | 29 (85.3%) | 4 (11.8%) |
| 4 h | 34 | 1 (2.9%) | 29 (85.3%) | 4 (11.8%) |
| 30–45 min seizure | ||||
| Pre-seizure | 23 | 23 (0%) | 0 (0%) | 0 (0%) |
| 0 h | 23 | 0 (0%) | 7 (30.4%) | 16 (69.6%) |
| 1 h | 23 | 0 (0%) | 9 (39.1%) | 14(60.9%) |
| 2 h | 23 | 0 (0%) | 10 (43.5%) | 13 (56.5%) |
| 4 h | 23 | 0 (0%) | 13 (56.5%) | 10 (43.5%) |
Saline-injected control mice had no beading over 4 hours. Kainate seizures of all durations induced acute dendritic beading, with minimal recovery over 4 hours. The incidence and severity of beading was dependent on the duration of the seizures. p<0.05 by chi-square test of independence for distribution of beading categories at all examined time points for all seizure durations.
Dendritic injury with brief seizures, but not status epilepticus, fully recover over several weeks
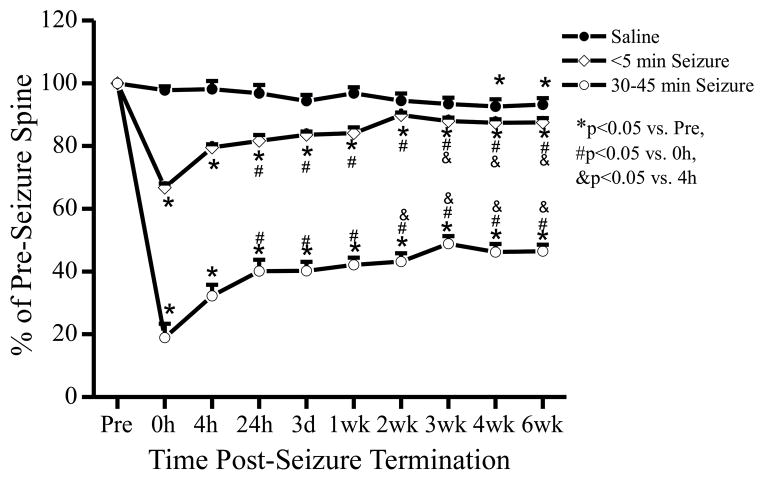
As seizure-induced dendritic injury did not recover completely over 4 hours in the initial studies, a separate set of experiments was performed to monitor the evolution and long-term recovery of this dendritic injury over a 6 week period, focusing on the two extreme seizure groups: brief seizures (<5 min) and status epilepticus (30–45 min). In saline-injected control mice, there was no significant change in spine number over 3 weeks; however, spine number decreased by a small, but significant, amount at 4 and 6 weeks compared to baseline. Similar to the first set of studies monitoring 4 hours after seizures, kainate-induced seizures of both <5 min and 30–45 min durations led to acute spine loss immediately following seizure termination which recovered partially over 4 hours (Figs. 4, 5). Upon extending the period of observation to 6 weeks, the spine loss from brief seizures (<5 min) recovered to control levels of saline-injected mice within 2 weeks and stayed stable for the following 4 weeks. However, seizures lasting 30–45 minutes caused more severe spine loss that only partially recovered over 6 weeks of observation, despite the absence of EEG-recorded spontaneous seizures. After termination of the initial kainate-induced seizures by isoflurane, long-term EEG recordings demonstrated interictal spike discharges that decreased over a couple days, but no acute recurrence of seizures or chronic development of spontaneous seizures over the 6 week period. Correspondingly, there was some additional gradual recovery of spines between 4 hours and 2 weeks following seizure termination, but no further recovery between 2 weeks and 6 weeks.
Figure 4.
Evolution and long-term recovery of acute seizure-induced dendritic injury. Saline-injected mice had no significant change in spine number over 3 weeks, but a modest spine loss after 4–6 weeks of observation (n = 352 total spines from 37 dendrites of 6 mice). In contrast, kainate-induced seizures of both <5 min and 30–45 min led to an acute spine loss immediately after termination of the seizures (0 h), compared to the pre-seizure baseline. Acute spine loss from brief seizures (< 5 min) (n = 363 total spines from 35 dendrites of 6 mice) recovered in 2 weeks to control levels. However, seizures lasting 30–45 minutes caused spine loss (n = 261 total spines from 25 dendrites of 6 mice) that only partially recovered over 6 weeks of observation. Statistical comparisons by ANOVA.
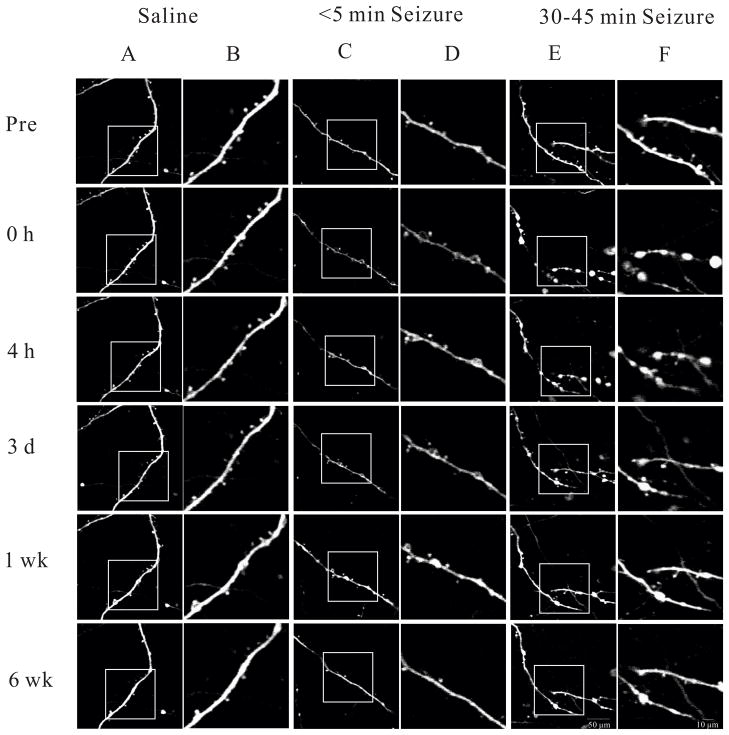
Figure 5.
Representative images of long term dendritic changes in control (saline-injected), brief seizure (<5 min), and long lasting seizure (30–45 min) groups. Minimal spine loss of dendritic beading was observed in saline-injected mice over 6 weeks. Brief (<5 min) seizures caused no or mild dendritic beading with only modest spine loss, and the spine loss completely recovered after 2 weeks. In contrast, more severe beading and extensive spine loss occurred with long lasting (30–45 min) seizures and started to recover within 3 days, but did not completely recover even after 6 weeks. The right panels (B, D, and F) in each group are enlargements of the images in the frame in the left panels (A, C, and E), respectively. Scale bar: 50 μm in the left (A, C, and E), and 10 μm in the right (B, D, and F) panels in each group.
The recovery of dendritic beading over 6 weeks showed a similar pattern as spine loss (Table 3). In saline-injected control mice, no severe beading and minimal mild beading was observed over 6 weeks. With brief seizures (<5 min), mild beading occurred in 40% of all dendrites and severe beading in 3% of dendrites immediately after termination of seizures; and the dendritic beading recovered almost completely after 6 weeks. In parallel, long lasting seizures (30–45 min) caused beading in all dendrites, with severe beading in most dendrites (72%) immediately after termination of seizure. By 1 week, all dendrites with severe beading improved to at least mild beading, but there was minimal additional recovery over the following 5 weeks. Therefore, dendritic beading, as well as spine loss, due to brief seizures can almost completely recover after several weeks, but dendritic injury following status epilepticus appeared to be largely irreversible.
Table 3.
Reversibility of kainate seizure-induced dendritic beading over 6 weeks.
| Group/Time after Seizure | Total Dendrites | No beading | Mild Beading | Severe Beading |
|---|---|---|---|---|
| Saline (no seizure) | ||||
| Baseline | 37 | 37 (100%) | 0 (0.00%) | 0 (0%) |
| 0 h | 37 | 37 (100%) | 0 (0.00%) | 0 (0%) |
| 4 h | 37 | 36 (97%) | 1 (3%) | 0 (0%) |
| 24 h | 37 | 35 (95%) | 2 (5%) | 0 (0%) |
| 3d | 37 | 35 (95%) | 2 (5%) | 0 (0%) |
| 1 wk | 37 | 36 (97%) | 1 (3%) | 0 (0%) |
| 2 wk | 37 | 36 (97%) | 1 (3%) | 0 (0%) |
| 3 wk | 37 | 36 (97%) | 1 (3%) | 0 (0%) |
| 4 wk | 37 | 35 (95%) | 2 (5%) | 0 (0%) |
| 6 wk | 37 | 35 (95%) | 2 (5%) | 0 (0%) |
| <5min Seizure | ||||
| Pre-Seizure | 35 | 35 (100%) | 0 (0%) | 0 (0%) |
| 0 h | 35 | 20 (57%) | 14 (40%) | 1 (3%) |
| 4 h | 35 | 24 (69%) | 11 (31%) | 0 (0%) |
| 24 h | 35 | 25 (71%) | 10 (29%) | 0 (0%) |
| 3 d | 35 | 26 (74%) | 9 (26%) | 0 (0%) |
| 1 wk | 35 | 28 (80%) | 7 (20%) | 0 (0%) |
| 2 wk | 35 | 28 (80%) | 7 (20%) | 0 (0%) |
| 3 wk | 35 | 28 (80%) | 7 (20%) | 0 (0%) |
| 4 wk | 35 | 31 (89%) | 4 (11%) | 0 (0%) |
| 6 wk | 35 | 31 (89%) | 4 (11%) | 0 (0%) |
| 30–45 min Seizure | ||||
| Pre-Seizure | 25 | 25 (100%) | 0 (0%) | 0 (0%) |
| 0 h | 25 | 0 (0%) | 7 (28%) | 18 (72%) |
| 4 h | 25 | 0 (0%) | 13 (52%) | 12 (48%) |
| 24 h | 25 | 0 (0%) | 18 (72%) | 7 (28%) |
| 3 d | 25 | 1 (4%) | 20 (80%) | 4 (16%) |
| 1 wk | 25 | 2 (8%) | 23 (92%) | 0 (0%) |
| 2 wk | 25 | 4 (16%) | 21 (84%) | 0 (0%) |
| 3 wk | 25 | 4 (16%) | 21 (84%) | 0 (0%) |
| 4 wk | 25 | 5 (20%) | 20 (80%) | 0 (0%) |
| 6 wk | 25 | 5 (20%) | 20 (80%) | 0 (0%) |
Saline-injected control mice had no severe beading and minimal mild beading over 6 weeks. Brief seizures (0–5 min) caused primarily mild beading in more than a third of dendrites and rare severe beading immediately after the termination of seizure; and after 6 weeks, the mild dendritic beading remained in only about 10% of dendrites. In parallel, long lasting seizures (30–45 min) caused beading in all examined dendrites with severe beading in most dendrites (72%) immediately after the termination of the seizures. By 1 week after long lasting seizures, severe dendritic beading changed to mild beading, which showed minimal additional recover over 6 weeks. p<0.05 by chi-square test of independence for distribution of beading categories at all examined time points for both seizure durations.
Discussion
Recent studies utilizing in vivo multiphoton imaging have demonstrated very rapid structural changes in dendrites, including dendritic beading and spine loss, which occur immediately following status epilepticus and in the absence of neuronal death (Zeng et al., 2007). However, the effects of shorter seizures and the long-term evolution of this dendritic injury are not known. In the present study, we demonstrated the surprising and important finding that even brief seizures, less than 5 minutes in duration, may cause acute dendritic changes. However, compared to status epilepticus, the severity of this dendritic injury with brief seizures was less, and the injured dendrites appeared to recover back to baseline within a couple weeks. In contrast, prolonged seizures lasting more than 30 minutes (status epilepticus) produced more severe dendritic damage that was mostly irreversible over several weeks.
While these structural effects of seizures on dendrites appear quite obvious and direct, a number of potential caveats and confounding factors should be considered. First, it is important to distinguish between effects of the seizures themselves and other factors, such as direct neurotoxic effects of kainate. Our previous work demonstrated that administration of kainate, with seizures suppressed by pentobarbital, has no acute effects on dendrites, indicating that the seizures are necessary to cause dendritic injury (Zeng et al. 2007). Furthermore, C57 mice, as used in this study, have been shown to be resistant to kainate-induced neuronal death (Schauwecker and Steward, 1997; Zeng et al., 2007). Our previous studies also demonstrated that there were no significant changes in systemic blood oxygenation or pH, suggesting that local effects of seizure activity within the brain are more critical than any indirect systemic effects of seizures (Zeng et al., 2007). However, due to technical challenges, we have not verified that the neurons imaged in this study directly participated in the seizures. Despite this limitation, if we assume that some of the imaged neurons were not actively involved in the seizures, this would suggest that the observed dendritic injury may be an underestimate of direct seizure-induced injury and would further strengthen the conclusion that seizures cause significant dendritic damage.
The finding that even brief seizures caused some dendritic injury is probably the most remarkable, novel result in this study. However, the validity of this result is dependent on having accurate measurements of seizure duration. In this regard, it is important that EEG monitoring confirmed that the seizures were successfully terminated immediately upon initiation of isoflurane anesthesia and did not recur during the initial four hour imaging session. It should also be noted that following kainate injection, seizures initially are brief and intermittent and then become longer and more continuous in later stages of status epilepticus. Thus, the shorter duration seizure groups (e.g. <5 min) usually consisted of several brief seizures, which might have different effects than one continuous seizure of the same cumulative duration. As neurons may be able to recover partially between a series of brief seizures, we suspect that our results may again represent an underestimate of the injurious effects of seizures.
The mechanism of this acute dendritic injury is not entirely known, but likely involves a combination of massive osmotic shifts, causing swelling of the dendrites, and breakdown of the actin cytoskeleton (Zeng et al., 2007; Kurz et al., 2008). The apparent recovery of mild dendritic injury following brief seizures indicates that these mechanisms are potentially reversible. However, the long-lasting dendritic changes that result from status epilepticus indicate at some point these processes may become irreversible, such as long-term reorganization of the actin cytoskeleton. The lack of complete recovery of dendritic injury following status epilepticus could also be related to associated neuronal death or the emergence of spontaneous seizures (with recurrent seizure-induced dendritic injury). However, the use of mice with a C57BL/6 genetic background, which are resistant to kainate-induced neuronal death and epileptogenesis (Schauwecker and Steward, 1997; Yang et al., 2005; Zeng et al., 2007), the use of a relatively low dose of kainate, and the absence of seizures recorded during the chronic phase of this study make these possibilities unlikely. Despite the absence of recurrent seizures or neuronal death, interictal spike discharges were observed for at least a couple days after the initial kainate-induced seizures, which could potentially exacerbate dendritic injury or delay recovery. Determining the mechanistic basis for this conversion from reversible to permanent structural changes with longer seizures may have important implications for understanding normal synaptic plasticity and therapeutic applications for preventing seizure-induced brain injury (Wong, 2008).
The clinical implications of this seizure-induced dendritic injury may be quite direct and dramatic. Given the role of dendritic spines in mechanisms of synaptic plasticity and learning, the long-lasting loss of spines following status epilepticus could be a structural basis for cognitive deficits in epilepsy patients (Wong, 2005). The reversible, milder dendritic changes observed with brief seizures could account for transient cognitive impairment or the “postictal” state that is common following seizures. Conversely, it is possible that synaptic failure associated with seizure-induced dendritic changes may paradoxically help terminate the seizure. Future studies examining associated effects of seizures on the presynaptic elements and synaptic physiology itself will help further delineate the functional and clinical implications of this acute dendritic injury.
In conclusion, this study finds that seizure of all durations may cause at least transient dendritic injury. Even brief seizures, less than 5 minutes in duration, cause some dendritic beading and spine loss, but this recovers over a couple weeks. In contrast, status epilepticus produce more severe dendritic injury that only partially reverses over several weeks. These findings have strong clinical implications related to the detrimental effects of seizures and the therapeutic approach to patient with epilepsy.
Highlights.
Seizures cause acute dendritic beading and loss of dendritic spines of cortical neurons in mice.
The severity of dendritic injury is dependent on the duration of the seizures.
Even brief (<5 minutes) seizures cause some moderate dendritic injury.
Dendritic injury from brief seizures recovers over two weeks, but status epileptus may cause irreversible dendritic damage.
Acknowledgments
This work was supported by the National Institutes of Health (R01 NS056872 to MW; NIH Neuroscience Blueprint Core Grant NS057105 to Washington University), Citizens United for Research in Epilepsy, and the Alafi Family Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Drakew A, Muller M, Gahwiler BH, Thompson SM, Frotscher M. Spine loss in experimental epilepsy: quantitative light and electron microscopic analysis of intracellularly stained CA3 pyramidal cells in hippocampal slice cultures. Neuroscience. 1996;70:31–45. doi: 10.1016/0306-4522(95)00379-w. [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Isokawa M. Remodeling dendritic spines in the rat pilocarpine model of temporal lobe epilepsy. Neurosci Lett. 1998;258:73–76. doi: 10.1016/s0304-3940(98)00848-9. [DOI] [PubMed] [Google Scholar]
- Isokawa M, Levesque MF. Increased NMDA responses and dendritic degeneration in human epileptic hippocampal neurons in slices. Neurosci Lett. 1991;132:212–216. doi: 10.1016/0304-3940(91)90304-c. [DOI] [PubMed] [Google Scholar]
- Jiang M, Lee CL, Smith KL, Swann JW. Spine loss and other persistent alterations of hippocampal pyramidal cell dendrites in a model of early-onset epilepsy. J Neurosci. 1998;18:8356–8368. doi: 10.1523/JNEUROSCI.18-20-08356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz JE, Moore BJ, Henderson SC, Campbell JN, Churn SB. A cellular mechanism for dendritic spine loss in the pilocarpine model of status epilepticus. Epilepsia. 2008;49:1696–1710. doi: 10.1111/j.1528-1167.2008.01616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi A, Crowley JC, Shtoyerman E, Katz LC. High-resolution in vivo imaging of hippocampal dendrites and spines. J Neurosci. 2004;24:3147–3151. doi: 10.1523/JNEUROSCI.5218-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Gahwiler BH, Rietschin L, Thompson SM. Reversible loss of dendritic spines and altered excitability after chronic epilepsy in hippocampal slice cultures. Proc Natl Acad Sci USA. 1993;90:257–261. doi: 10.1073/pnas.90.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multani P, Myers RH, Blume HW, Schomer DL, Sotrel A. Neocortical dendritic pathology in human partial epilepsy: a quantitative Golgi study. Epilepsia. 1994;35:728–736. doi: 10.1111/j.1528-1157.1994.tb02503.x. [DOI] [PubMed] [Google Scholar]
- Olney JW, deGubareff T, Sloviter RS. “Epileptic” brain damage in rats induced by sustained electrical stimulation of the perforant path. II. Ultrastructural analysis of acute hippocampal pathology. Brain Res Bull. 1983;10:699–712. doi: 10.1016/0361-9230(83)90038-2. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroenceph Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rensing NR, Ouyang Y, Yang XF, Yamada KA, Rothman SM, Wong M. In vivo imaging of dendritic spines during electrographic seizures. Ann Neurol. 2005;58:888–898. doi: 10.1002/ana.20658. [DOI] [PubMed] [Google Scholar]
- Risher WC, Ard D, Yuan J, Kirov SA. Recurrent spontaneous spreading depolarizations facilitate acute dendritic injury in the ischemic penumbra. J Neurosci. 2010;30:9859–9868. doi: 10.1523/JNEUROSCI.1917-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauwecker PE, Steward O. Genetic determinants of susceptibility to excitotoxic cell death: implications for gene targeting approaches. Proc Natl Acad Sci USA. 1997;94:4103–4108. doi: 10.1073/pnas.94.8.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibel ME, Crandall PH, Scheibel AB. The hippocampal-dentate complex in temporal lobe epilepsy. Epilepsia. 1974;15:55–80. doi: 10.1111/j.1528-1157.1974.tb03997.x. [DOI] [PubMed] [Google Scholar]
- Swann JW, Al-Noori S, Jiang M, Lee CL. Spine loss and other dendritic abnormalities in epilepsy. Hippocampus. 2000;10:617–625. doi: 10.1002/1098-1063(2000)10:5<617::AID-HIPO13>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Wong M. Modulation of dendritic spines in epilepsy: cellular mechanisms and functional implications. Epilepsy Behav. 2005;7:569–577. doi: 10.1016/j.yebeh.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Wong M. Stabilizing dendritic structure as a novel therapeutic approach for epilepsy. Expert Rev Neurotherapeutics. 2008;8:907–915. doi: 10.1586/14737175.8.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Houk B, Shah J, Hauser KF, Luo Y, Smith G, Schauwecker E, Barnes GN. Genetic background regulate semaphoring gene expression and epileptogenesis in mouse brain after kainic acid status epilepticus. Neuroscience. 2005;131:853–869. doi: 10.1016/j.neuroscience.2004.09.064. [DOI] [PubMed] [Google Scholar]
- Zeng LH, Xu L, Rensing NR, Sinatra PM, Rothman SM, Wong M. Kainate seizures cause acute dendritic spine loss and actin depolymerization in vivo. J Neurosci. 2007;27:11604–11613. doi: 10.1523/JNEUROSCI.0983-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Boyd J, Delaney K, Murphy TH. Rapid reversible changes in dendritic spine structure in vivo gated by the degree of ischemia. J Neurosci. 2005;25:5333–5338. doi: 10.1523/JNEUROSCI.1085-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]