Abstract
Objective
This study was conducted to examine the safety and efficacy of pioglitazone, a thiazolidinedione insulin sensitizer, in adult outpatients with major depressive disorder.
Method
In a 12-week, open-label, flexible-dose study, 23 patients with major depressive disorder received pioglitazone monotherapy or adjunctive therapy initiated at 15mg daily. Subjects were required to meet criteria for abdominal obesity (waist circumference >35 in. in women and >40 in. in men) or metabolic syndrome. The primary efficacy measure was the change from baseline to Week 12 on the Inventory of Depressive Symptomatology (IDS) total score. Partial responders (≥25% decrease in IDS total score) were eligible to participate in an optional extension phase for an additional three months.
Results
Pioglitazone decreased depression symptom severity from a total IDS score of 40.3 ± 1.8 to 19.2 ± 1.8 at week 12 (p<.001). Among partial responders (≥ 25% decrease in IDS total score), an improvement in depressive symptoms was maintained during an additional 3-month extension phase (total duration = 24 weeks) according to IDS total scores (p<.001). Patients experienced a reduction in insulin resistance from baseline to Week 12 according to the log homeostasis model assessment (−0.8 ± 0.75; p<.001) and a significant reduction in inflammation as measured by log highly- sensitive C-reactive protein (−0.87 ± 0.72; p<.001). During the current episode, the majority of participants (74%, n=17), had already failed at least one antidepressant trial. The most common side effects were headache and dizziness; no patient discontinued due to side effects.
Limitations
These data are limited by a small sample size and an open-label study design with no placebo control.
Conclusion
Although preliminary, pioglitazone appears to reduce depression severity and improve several markers of cardiometabolic risk, including insulin resistance and inflammation. Larger, placebo-controlled studies are indicated.
INTRODUCTION
Although the monoamine theory has contributed to understanding the pathophysiology of mood disorders, monoamine-based treatments remain limited in fully addressing the needs of patients with MDD. Thus, the identification of non-catecholamine neurotransmitter systems as the point of intervention for patients with mood disorders has become the focus of neuroscience research over recent years, and includes such targets as neurotrophic factors, extracellular receptor-coupled kinases, and inhibitors of glycogen synthase kinase-3 (Mathew et al., 2008). Modulation of insulin signaling pathways has likewise been proposed as an alternative approach to relieving depression, as insulin and related peptides are hypothesized to play a critical role in neuroplasticity and neuroprotection within the central nervous system (Burgdorf et al., 2010; Eissa Ahmed et al., 2009; McIntyre et al., 2008; Rasgon et al., 2007).
In clinical practice, a high rate of obesity and other cardiometabolic disorders is frequently observed among individuals seeking treatment for mood disorders (McElroy et al., 2004). For instance, elevated visceral fat mass is associated with a greater likelihood of becoming depressed (Voegelzangs et al., 2010), suggesting that the biological mechanisms associated with increased cardiometabolic risk may contribute to the development of depression. Further substantiating this theory, prospective studies show that patients with the metabolic syndrome or insulin resistance syndrome experience a significantly elevated risk of developing depression (Almeida et al., 2009; Koponen et al., 2008).
Pioglitazone is an oral hypoglycemic agent of the thiazolidinedione class (Davidson, 2005). Its primary action is to enhance insulin sensitivity in adipose tissue, skeletal muscle, and the liver. Although its mechanisms of action are not fully understood, pioglitazone is a highly selective and potent agonist for the peroxisome proliferator-activated receptor gamma (PPAR-gamma) that regulates a transcription factor responsible for glucose and fat metabolism. Pioglitazone effectively lowers fasting blood glucose levels and also reduces glycosylated hemoglobin, but is associated with a low likelihood of hypoglycemia (Jain et al., 2006). In patients with type-2 diabetes, pioglitazone treatment results in a shift of fat distribution from visceral to subcutaneous depots, thereby improving hepatic and peripheral tissue sensitivity to insulin (Miyazaki et al., 2002). Thiazolidinediones also exert anti-inflammatory effects on a variety of cell types, and for this reason are being considered for the treatment of diseases with an inflammatory etiology, such as inflammatory bowel disease (Saubermann et al., 2002), psoriasis (Mittal et al., 2009), and atherosclerosis (Igrashi et al., 2008). Pioglitazone is also being tested in autism (Boris et al., 2007), Alzheimer’s Disease (Pershadsingh et al., 2004), and multiple sclerosis (Kaiser et al., 2009), where its ability to reduce microglial activation (Heneka et al., 2005), decrease neuronal damage (Zhao et al., 2006), and enhance brain glucose utilization through increased neuronal mitochondrial biogenesis (Strum et al., 2007) holds promise for treating neuropsychiatric disorders.
A case report of an individual with treatment refractory depression suggests that pioglitazone may be useful in reducing depression severity and improving the insulin resistance associated with metabolic syndrome (Kemp et al., 2009). Rosiglitazone, another insulin sensitizer of the thiazolidinedione class, has been shown to have antidepressant-like activity using the mouse tail suspension test and the rat forced swimming test, two models sensitive to the effects of antidepressants (Eissa Ahmed et al., 2009). In humans, a pilot trial found rosiglitazone to be associated with significant depressive symptom decline over 12 weeks when administered to patients with insulin resistance and unipolar or bipolar depression (Rasgon et al., 2010). From a mechanistic standpoint, a reduction in depression severity with pioglitazone may occur due to a decrease in visceral adiposity, decreased inflammation, and/or an improvement in insulin sensitivity. Pioglitazone is also recognized to cross the blood brain barrier (Maeshiba et al., 1997) and may mediate a reduction in depressive symptoms by increasing neuronal survival (Fuenzalida et al., 2007; Zhao et al., 2006), increasing glial uptake of excitotoxic molecules (Romera et al., 2007), or modulating Ca-dependent pathways in the brain (Pancani et al., 2009). A better understanding of the mechanisms linking insulin resistance with depression outcomes could inform interventions to not only reverse cardiometabolic risk factors in this vulnerable population, but also uncover novel mechanisms of antidepressant action. To that end, we evaluated pioglitazone as monotherapy or adjunctive therapy for the treatment of major depressive episodes that co-occurred with abdominal obesity. The study’s primary objective was to evaluate the antidepressant efficacy of 12 weeks of open label treatment with pioglitazone. Secondary objectives were to assess whether pioglitazone could effectively improve the parameters associated with metabolic syndrome and insulin resistance.
METHOD
This 12-week, open-label, prospective trial in major depressive disorder (MDD) was conducted at the Mood & Metabolic Clinic of University Hospitals Case Medical Center at Case Western Reserve University after approval by the Institution Review Board. Written informed consent was obtained from all subjects prior to participation. Participants were enrolled from April 2008 to November 2009. Subjects were treated to evaluate the safety and efficacy of pioglitazone in the treatment of major depressive episodes in adult patients with MDD. Participants demonstrating at least a partial antidepressant response (≥ 25% reduction in depression severity) to pioglitazone were offered treatment for an additional 12 weeks during an extension phase.
Patient population
Outpatients aged 18 to 70 years who met the Diagnostic and Statistical Manual of Mental Disorders 4th Edition Text Revision (DSM-IV-TR) criteria for the diagnosis of MDD without psychotic features were eligible for inclusion in the study. Baseline depression symptom-severity in this sample was high moderate to severe. A current diagnosis of MDD was confirmed using the Mini-International Neuropsychiatric Interview (MINI) (Sheehan et al., 1998). Patients were required to have a Quick Inventory of Depressive Symptoms (QIDS) (Trivedi et al., 2004) score ≥ 11 and either the presence of abdominal obesity or metabolic syndrome as established by the National Cholesterol Education Program’s Adult Treatment Panel III (2001). Patients were excluded if they had an unstable medical condition, were taking another glucose-lowering agent, or met criteria for dependence on alcohol or drugs within 3 months prior to enrollment.
Study medication
Open-label pioglitazone was administered as monotherapy or adjunctive therapy over 12 weeks and initiated at 15mg daily in a single dose. Dose increases were individually titrated according to patient response and tolerability at four week intervals, up to a maximum of 45 mg daily. If patients were already taking concomitant antidepressant medication, dose changes were not permissible either within 4 weeks of receiving pioglitazone or during the study itself. Receipt of lorazepam or zolpidem for anxiety or insomnia was permitted.
Efficacy evaluations
Clinical assessments were conducted at baseline and then at weeks 1, 2, 3, 4, 6, 8, 10, and 12. For patients entering the extension phase, additional assessments were conducted at weeks 16, 20, and 24. The primary efficacy variable was the mean change in the Inventory of Depressive Symptomatology (IDS) (Trivedi et al., 2004) from baseline to Week 12. Secondary analyses included an assessment of anxiety symptoms by the Structured Interview Guide for the Hamilton Anxiety Scale (Shear et al., 2001), global symptom severity by the Clinical Global Impressions (CGI) scales for change and severity of illness (Guy, 1976), self-assessment of depression symptom severity by the QIDS (Trivedi et al., 2004), and disability by the Sheehan Disability Scale (Leon et al., 1997).
Medical and laboratory assessments
A detailed medical and psychiatric history was performed, including a physical examination, where anthropometric measurements were obtained such as height, weight and waist circumference. Laboratory assessments included a basic chemistry panel, complete blood count, thyroid and liver function tests, fasting lipid profile, and urine toxicology for drugs of abuse. Women of childbearing potential had urine pregnancy tests at study inception and used two forms of medically accepted birth control throughout the trial. Adverse events were elicited by both spontaneous report and direct verbal query. Insulin resistance was estimated using the homeostasis model assessment of insulin resistance (HOMA-IR), a widely used surrogate measure that is calculated by: (fasting insulin (µU/ml)) × (fasting glucose (mg/dL)) ÷ 22.5 (Matthews et al., 1985).
Statistical analyses
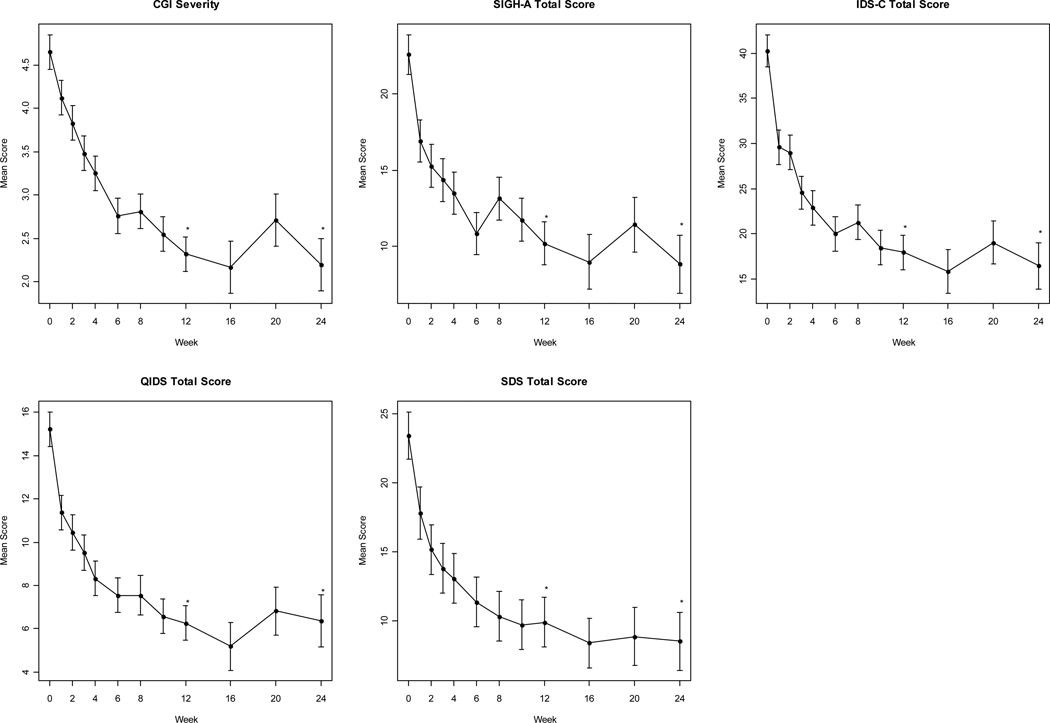
Simple descriptive statistics (mean ± SD) were obtained for the patients' demographic and baseline clinical characteristics. The data were log-transformed for insulin, HOMA-IR, triglycerides/HDL-C ratio, triglycerides, highly sensitive C-reactive protein, IL-1 and IL-6 to satisfy the normality assumption required by Student’s t-test. The primary and secondary efficacy analyses were performed on the intent-to-treat (ITT) population, which included all patients who took at least one dose of study medication and had at least one post-baseline efficacy assessment. The primary a priori endpoint was symptom severity as assessed by the change from baseline to final assessment in the IDS total scores. The outcome measures CGI severity, SIGH-A total score, IDS-C total score, QIDS total score and SDS total score were analyzed using mixed-model repeated-measures (MMRM) regression analysis. In the MMRM models, patient was treated as a random effect and visit week as a fixed effect. A compound symmetry matrix was specified as the appropriate structure for the variance-covariance matrix used to describe the relationship among the time (visit week) data points. Means and standard errors are reported. Twelve-week and 24-week changes (Figure 2) from baseline were assessed using linear contrasts on the MMRM models. Overall mean change in cardiometabolic and inflammatory biomarkers were calculated for each patient and analyzed for statistical significance using the paired t-test. A two-tailed p-value < 0.05 was considered statistically significant; p-values were not adjusted for multiplicity of comparisons. A positive response was defined as a ≥ 50% reduction in IDS total score. Remission was defined as an IDS total score ≤ 15. Data were analyzed using SAS software (SAS for Windows, version 9.2, SAS Institute Inc., Cary, NC).
Figure 2. Change in Outcome Measures with Pioglitazone over the 24-week Study Period.
Data are mean changes (± SEM) in scores from baseline at subsequent visits and at the end of the acute phase (week 12) and extension phase (week 24). *p<.001. p values are from MMRM analyses.
CGI=Clinical Global Impressions; IDS-C=Inventory of Depressive Symptoms; QIDS=Quick Inventory of Depressive Symptoms; SDS=Sheehan Disability Scale; SIGH-A=Structured Interview Guide for the Hamilton Anxiety Scale
RESULTS
Patients and disposition
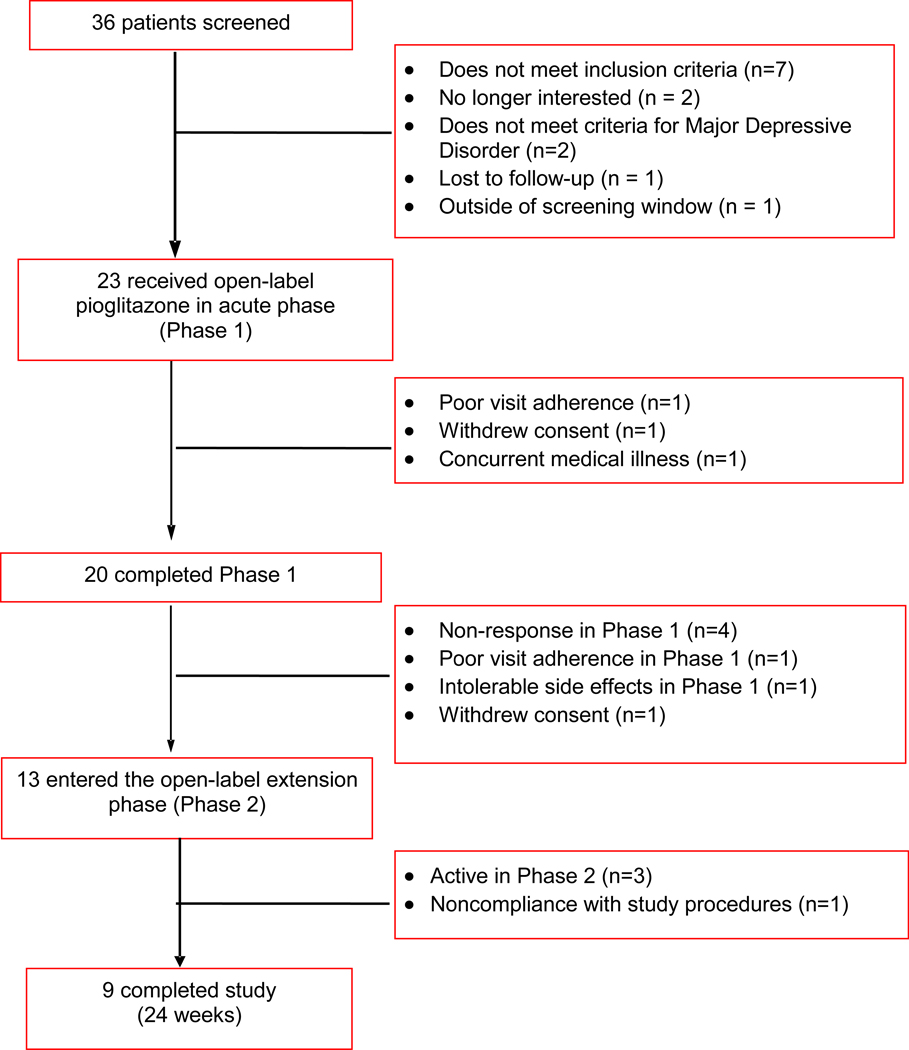
Thirty-six patients consented for participation in the study. Thirteen patients completed screening but were either ineligible or chose not to participate (Figure 1). Twenty-three patients were enrolled and received open-label pioglitazone; the mean daily dose of pioglitazone was 32.7 mg (range 15–45mg/day). A summary of baseline demographic and clinical characteristics is presented in Table 1. Eighty-seven percent (n=20) were female, 57% (n=13) were Caucasian, and 78% (n=18) had at least some college education. Lifetime generalized anxiety disorder was diagnosed in 57% (n=13) of patients and 44% (n=10) had a lifetime history of an alcohol use disorder. Seventy-four percent (n=17) of subjects had failed at least one antidepressant during the current depressive episode and 39% (n=9) had failed 3 or more antidepressants.
Figure 1. Patient Disposition During the Study Evaluating Pioglitazone as Antidepressant Monotherapy or Adjunctive Therapy.
Table 1.
Baseline Clinical Characteristics of Patients Treated with Pioglitazone for Major Depressive Disorder (N=23)
| Mean | SD | |
|---|---|---|
| Age (yr) | 44.6 | 10.2 |
| Age of depression onset (yr) | 18.2 | 12.4 |
| Age first treated for depression (yr) | 34.3 | 7.2 |
| Number of prior depressive episodes | 23.9 | 28.0 |
| Number of prior hospitalizations | 2.0 | 4.9 |
| Number of prior suicide attempts | 1.7 | 6.2 |
| N | % | |
| Gender | ||
| Male | 3 | 13.0 |
| Female | 20 | 87.0 |
| Ethnicity | ||
| White | 13 | 56.6 |
| Black | 9 | 39.1 |
| Hispanic | 1 | 4.3 |
| Employed | 13 | 56.6 |
| Education level | ||
| Less than high school degree | 2 | 8.7 |
| High school degree | 3 | 13.0 |
| Some college | 9 | 39.1 |
| College degree | 9 | 39.1 |
| Number of failed antidepressant trials during the current episode | ||
| 0 | 6 | 26.1 |
| 1 | 5 | 21.7 |
| 2 | 3 | 13.0 |
| >=3 | 9 | 39.1 |
| Prior electroconvulsive therapy | 4 | 13.8 |
| Comorbid diagnoses, lifetime | ||
| Generalized anxiety disorder | 13 | 56.5 |
| Panic disorder | 6 | 26.1 |
| Post-traumatic stress disorder | 5 | 21.7 |
| Obsessive-compulsive disorder | 5 | 21.7 |
| Alcohol use disorder | 10 | 43.5 |
| History of physical abuse | 6 | 26.1 |
| History of verbal abuse | 11 | 47.8 |
| History of sexual abuse | 8 | 34.8 |
| Metabolic syndrome | 15 | 65.2 |
| Abdominal obesity | 23 | 100.0 |
Three patients in the intent-to-treat sample discontinued before completing the 12-week study period. One patient each discontinued prematurely due to withdrawal of consent, study non-compliance, and development of an exclusionary medical illness. Twenty (87%) patients completed the entire 12 weeks of the study’s acute phase.
Efficacy at 12 weeks
Pioglitazone significantly reduced symptoms of depression across both clinician- and patient-rated assessments of depression severity (Table 2). The total mean IDS score decreased from 40.3 ± 1.8 at baseline to 19.2 ± 1.8 (p<.001). Self-reported assessments of depression symptom severity reflected a similar improvement with mean QIDS scores decreasing from 15.2 ± 0.8 at baseline to 7.1 ± 0.8 (p<.001). Anxiety symptoms decreased significantly according to the SIGH-A total score, decreasing from a mean of 22.6 ± 1.4 at baseline to 11.7 ± 1.4 (p<.001). The mean CGI-S scores also decreased from 4.7 ± 0.2 to 2.5 ± 0.2 (p<.001). The categorical response criteria were met by 65% (N=15) of patients on both the IDS and QIDS at the acute phase endpoint. Remission of depressive symptoms was achieved by 22% (N=5) of patients on both the IDS and QIDS.
Table 2.
Psychiatric Outcome Measures Among Patients with Major Depressive Disorder and Abdominal Obesity Treated with Pioglitazone
| Outcome measure | Baseline | Week 12 | P value | Week 24 | P value |
|---|---|---|---|---|---|
| (N=23) | (N=22)* | (N=11)* | |||
| Depressive symptoms | |||||
| IDS total score | 40.3 (1.8) | 19.2 (1.8) | <.001 | 15.3 (2.4) | <.001 |
| QIDS total score | 15.2 (0.8) | 7.1 (0.8) | <.001 | 6.6 (1.1) | <.001 |
| Anxiety symptoms | |||||
| SIGH-A total score | 22.6 (1.4) | 11.7 (1.4) | <.001 | 8.5 (1.8) | <.001 |
| Global severity of illness | |||||
| CGI-Severity | 4.7 (0.2) | 2.5 (0.2) | <.001 | 2.1 (0.3) | <.001 |
| Sheehan Disability Scale | |||||
| Total score | 23.4 (1.7) | 10.0 (1.7) | <.001 | 8.0 (2.1) | <.001 |
| Work | 5.1 (0.6) | 3.0 (0.6) | <.001 | 2.4 (0.7) | <.001 |
| Social | 6.7 (0.5) | 3.1 (0.5) | <.001 | 2.5 (0.6) | <.001 |
| Family | 6.2 (0.5) | 3.0 (0.5) | <.001 | 2.5 (0.6) | <.001 |
Mean (SEM) refers to score at that time point. Between group least square means and p values are from MMRM.
CGI=Clinical Global Impressions scale; IDS=Inventory of Depressive Symptomatology; QIDS=Quick Inventory of Depressive Symptoms; SIGH-A=Structured Interview Guide for the Hamilton Anxiety Scale
Includes 2 LOCF observations
The presence or absence of metabolic syndrome did not appear to affect the change in depression severity. Similar reductions in IDS scores were observed in patients with metabolic syndrome (−20.4 ±2.5; p<.001) as compared to patients without metabolic syndrome (−22.4 ± 3.3; p<.001). When stratified by use of concomitant antidepressant therapy, patients receiving pioglitazone monotherapy experienced a similar decrease in mean IDS total scores (−20.7 ± 3.2; p=.001) as did patients using pioglitazone as an adjunctive therapy (−21.3 ± 2.7; p<.001).
Glucose metabolism
Effects of pioglitazone on anthropometric measurements, glucose, lipoproteins, and inflammatory biomarkers are shown in Table 3. Patients experienced a significant decrease from baseline in fasting plasma glucose (−8.2 mg/dl ± 13.3 mg/dl; p=.01). Likewise, fasting log insulin levels (mg/dL) and insulin resistance (as assessed by log HOMA-IR) decreased significantly among pioglitazone-treated subjects from baseline to Week 12 (−0.72 mg/dl ±0.70; p=.001) and (−0.81 ±0.75; p<.001), respectively. In the overall sample, the correlation between the change in insulin resistance and change in depression severity was not significant. Two participants had extremely elevated basal insulin levels, believed to be due to noncompliance with fasting procedures and a lab error, respectively. When these outliers were excluded, the reduction in insulin resistance was significantly correlated with improvement in depression severity (r=0.46, p=0.048).
Table 3.
Cardiometabolic Outcome Measures in a Study of Pioglitazone-Treated Subjects with Major Depressive Disorder and Abdominal Obesity
| Baseline | Endpoint (Week 12) | Change: Week 12 - Baseline a (N=20) | Endpoint (Week 24) | Change: Week 24 - Baseline a (N=9) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | t | df | p-value | Mean | SD | Mean | SD | t | df | p-value | |
| Anthropometric measurements | ||||||||||||||||
| Systolic blood pressure (mmHg) | 130.5 | 14.6 | 128.9 | 16.5 | −1.6 | 11.9 | −0.604 | 19 | 0.533 | 122.0 | 8.8 | −2.7 | 9.5 | −0.843 | 8 | 0.424 |
| Diastolic blood pressure (mmHg) | 87.8 | 12.1 | 86.2 | 11.4 | −1.6 | 7.5 | −0.921 | 19 | 0.369 | 83.300 | 6.2 | 1.0 | 7.3 | 0.412 | 8 | 0.691 |
| Body mass index (kg/m2) | 40.381 | 7.1 | 40.729 | 7.3 | 0.348 | 1.176 | 1.324 | 19 | 0.201 | 39.536 | 8.6 | 0.519 | 1.928 | 0.807 | 8 | 0.443 |
| Waist circumference (in) | 45.475 | 5.8 | 43.725 | 5.4 | −1.750 | 1.372 | −5.706 | 19 | <0.001 | 42.917 | 6.1 | −2.444 | 1.230 | −5.964 | 8 | <0.001 |
| Weight (lb) | 241.090 | 44.6 | 243.110 | 45.8 | 2.020 | 7.352 | 1.229 | 19 | 0.234 | 228.511 | 45.3 | 2.420 | 12.053 | 0.603 | 8 | 0.563 |
| Glucose metabolism measurements | ||||||||||||||||
| Glucose (mg/dL) | 98.850 | 11.1 | 90.650 | 9.6 | −8.200 | 13.324 | −2.752 | 19 | 0.013 | 92.222 | 15.9 | −4.222 | 9.536 | −1.328 | 8 | 0.221 |
| log(Insulin) | 2.722 | 0.577 | 1.998 | 0.663 | −0.724 | 0.695 | −4.649 | 19 | <0.001 | 1.694 | 0.801 | −0.946 | 0.784 | −3.619 | 8 | 0.007 |
| log(HOMA-IR) | 1.295 | 0.619 | 0.485 | 0.713 | −0.810 | 0.748 | −4.846 | 19 | <0.001 | 0.191 | 0.859 | −0.996 | 0.824 | −3.628 | 8 | 0.007 |
| log(Triglycerides/HDL-C ratio) | 0.633 | 0.741 | 0.289 | 0.517 | −0.344 | 0.664 | −2.315 | 19 | 0.032 | 0.461 | 0.624 | −0.435 | 0.677 | −1.930 | 8 | 0.090 |
| Lipid Panel | ||||||||||||||||
| LDL-cholesterol (mg/dL) | 128.450 | 33.2 | 115.500 | 25.2 | −12.950 | 26.518 | −2.184 | 19 | 0.042 | 121.222 | 21.7 | 4.333 | 43.327 | 0.300 | 8 | 0.772 |
| HDL-cholesterol (mg/dL) | 57.400 | 13.1 | 63.100 | 9.9 | 5.700 | 10.001 | 2.549 | 19 | 0.020 | 61.778 | 10.3 | 11.889 | 6.827 | 5.224 | 8 | <0.001 |
| log(Triglycerides) | 4.656 | 0.630 | 4.423 | 0.485 | −0.233 | 0.564 | −1.852 | 19 | 0.080 | 4.573 | 0.568 | −0.208 | 0.648 | −0.965 | 8 | 0.363 |
| Total cholesterol (mg/dL) | 211.750 | 46.9 | 197.250 | 32.300 | −14.500 | 36.709 | −1.767 | 19 | 0.093 | 204.667 | 29.4 | 7.889 | 63.699 | 0.372 | 8 | 0.720 |
| Inflammatory biomarker measurements | ||||||||||||||||
| log(Highly sensitive C-reactive protein) | 1.990 | 0.779 | 1.118 | 1.031 | −0.872 | 0.724 | −4.346 | 12 | <0.001 | 0.725 | 0.753 | −0.299 | 1.432 | −0.417 | 3 | 0.705 |
| log(Interleukin-1β) | −4.231 | 2.224 | −5.371 | 2.206 | −1.140 | 3.251 | −1.313 | 13 | 0.212 | −4.042 | 1.642 | −0.698 | 0.708 | −2.413 | 5 | 0.061 |
| log(Interleukin-6) | 0.760 | 0.489 | 0.575 | 0.509 | −0.185 | 0.613 | −1.132 | 13 | 0.278 | 0.656 | 0.404 | −0.231 | 0.559 | −1.010 | 5 | 0.359 |
| Tumor necrosis factor-α (pg/ml) | 6.807 | 1.7 | 6.671 | 1.7 | −0.136 | 1.019 | −0.501 | 13 | 0.625 | 7.258 | 1.5 | −0.988 | 1.845 | −1.312 | 5 | 0.246 |
Paired t-test
HDL=High density lipoprotein; HOMA-IR=Homeostasis Model Assessment of Insulin Resistance; LDL=Low density lipoprotein
Lipids
The fasting lipid profile analyses showed a significant reduction in LDL cholesterol (−13.0 mg/dl ± 26.5 mg/dl; p=.04) and a trend for reduced log triglycerides (−0.23 ± 0.56; p=.08) from baseline to the Week 12 endpoint. HDL-C levels significantly increased over 12 weeks of pioglitazone treatment (5.7 mg/dl ± 10.0 mg/dl; p=.02).
Three month extension phase
During the optional extension period, patients continued to show a gradual improvement in depressive symptoms. The mean reduction from baseline in IDS total score was 27.0 points at Week 12 and 27.8 points at Week 24. Likewise, the mean reduction from baseline in QIDS total score was 11.2 points at Week 12 and 9.9 points at Week 24. Ninety percent (n=9) of patients entering the extension phase remained antidepressant responders to pioglitazone. The change from baseline in log HOMA-IR (−1.0 ± 0.82; p<.01) and log fasting insulin (−0.95 mg/dl ± 0.78 mg/dl; p<.01) remained significant at Week 24, as did the change in HDL-C (11.9 mg/dl ± 6.8 mg/dl; p<.001) and waist circumference (−2.4 in. ± 1.2 in.; p<.001).
Inflammatory biomarkers
Levels of inflammatory cytokines appeared to decrease over 12 weeks of treatment with pioglitazone, including a significant reduction in log highly-sensitive C-reactive protein (−0.87 ± 0.72; p<.001). Mean decreases in log IL-6 levels also occurred from baseline to Week 12, although statistical significance was only observed at the Week 4 time point (−0.22 ± 0.38; p=0.04). Among the treated sample, no significant changes were observed in tumor necrosis factor (TNF)-α levels.
Participants experiencing an antidepressant response (≥ 50% decrease in IDS total score) to pioglitazone experienced a trend reduction in log IL-6 levels at week 4 (−0.26 pg/ml ± .44 pg/ml; p=0.08). Similarly, a trend for reduced levels of IL-1β occurred from baseline to Week 4 among antidepressant responders (−2.08 pg/ml ± 1.78 pg/ml; p=0.06). No significant changes were observed in TNF-α levels among pioglitazone responders or non-responders at any point during the study.
Treatment-emergent adverse events
Adverse events occurring in ≥ 5% of pioglitazone-treated subjects are shown in Table 4. The most common adverse events were headache and dizziness. No patient discontinued because of side effects. There were no serious adverse events. One patient discontinued due to development of shortness of breath and findings suggestive of mild congestive heart failure on a chest radiograph. However, on further examination the patient was found to have developed infection with the H1N1 influenza virus, which accounted for the respiratory findings. One participant developed peripheral edema and no participants developed clinically significant weight gain (≥ 7% body weight). The mean body weight of patients increased non-significantly by 2.0 ± 7.2 pounds (p=.21) over 12 weeks.
Table 4.
Incidence of Adverse Events Reported in ≥5% of Pioglitazone-Treated Subjects
| Adverse event | N | % |
|---|---|---|
| Headache | 6 | 26.1 |
| Dizziness/lightheaded | 5 | 21.7 |
| Increased appetite | 4 | 17.4 |
| Weight gain | 4 | 17.4 |
| Musculoskeletal pain | 4 | 17.4 |
| Blurred vision | 3 | 13.0 |
| Nausea | 2 | 8.7 |
| Sedation | 2 | 8.7 |
| Hot Flashes | 2 | 8.7 |
| Insomnia | 2 | 8.7 |
| Pruritis | 2 | 8.7 |
DISCUSSION
The present open-label study is the first clinical trial to evaluate the efficacy and safety of pioglitazone in patients with MDD and co-occurring abdominal obesity. The results indicate significant improvement in depressive symptoms following pioglitazone treatment over an acute 12-week period. For patients continuing on pioglitazone over 24 weeks, a consistent antidepressant effect was maintained according to both clinician- and patient-rated assessments of depression severity. Moreover, efficacy results were similar in patients treated with monotherapy pioglitazone or when administered as an adjunctive therapy with a conventional antidepressant(s).
A protocol defined response (≥ 50% decrease in IDS total score) occurred in 65% (n=15) of the sample, and 22% (n=5) of patients met criteria for remission. These outcomes are comparable to the STAR*D trial, in which response and remission rates were 49% and 37%, respectively (Rush et al., 2006). Representing the first large-scale trial to compare the effectiveness of different antidepressant strategies for patients who did not become symptom free after initial treatment with citalopram, STAR*D suggests that lower remission rates should be expected when additional treatment steps are required. As 52% (n=12) of patients enrolled in the present trial had failed 2 or more antidepressant treatments during the current episode, the observed response and remission rates are striking, but may be inflated as an inadequate response to past antidepressant trials was not observed prospectively.
Approximately 57% (n=13) had a lifetime co-occurring generalized anxiety disorder. Among all patients the mean SIGH-A scores at study entry were approximately 23, indicating the presence of a moderate level of anxiety severity. The significant improvement in symptoms of anxiety with pioglitazone suggests that therapeutic benefit may extend beyond a potential ability to acutely relieve depressive symptoms.
Overall pioglitazone was very well tolerated, with no serious adverse events reported. None of the patients discontinued prematurely due to an adverse event during the acute 12-week phase. Consistent with pioglitazone’s side effect profile in type-2 diabetes, a non-significant mean weight gain was observed (Shah and Mudaliar, 2010). The increase in body weight was presumably due to an increase in subcutaneous as opposed to visceral adiposity, given that measurements of waist circumference decreased significantly. Pioglitazone has been observed to remodel abdominal fat tissue, differentiating preadipocytes into small fat cells within subcutaneous depots and redistributing large fat cells from visceral into subcutaneous fat depots (Miyazaki et al., 2002). These changes are accompanied by improved glycemic control and improved hepatic and peripheral tissue sensitivity to insulin.
In this study, pioglitazone also produced improvement on measures of glucose homeostasis and dyslipidemia. In type-2 diabetes, pioglitazone has well documented effects for reducing HbA1c and has been shown to reduce all-cause mortality, non-fatal myocardial infarction, and stroke in patients at high risk for macrovascular events (Dormandy et al., 2005). In the present study, treatment with pioglitazone significantly improved the atherogenic profile of patients with abdominal obesity or metabolic syndrome. Significant improvements occurred in LDL-C and HDL-C levels, while a trend occurred for a decrease in triglycerides and total cholesterol. These findings are in concert with long-term results of pioglitazone therapy in patients with diabetic dyslipidemia, where durable improvements in triglyceride and high-density lipoprotein cholesterol levels occurred independent of baseline statin therapy (Spanheimer et al., 2009). Pioglitazone was also associated with a significant reduction in levels of highly sensitive C-reactive protein, a biomarker of inflammation implicated in the risk of both first incident and recurrent cardiovascular events (Zacho et al., 2008).
Converging lines of clinical and pre-clinical research support the rationale for studying thiazolidinediones in the treatment of depressive disorders. Not only can depression precipitate the occurrence of insulin resistance and states of increased cardiometabolic risk, but the metabolic syndrome (Koponen et al., 2008), type-2 diabetes (Pan et al., 2010), and abdominal obesity (Vogelzangs et al., 2010) have all been identified as risk factors for the development of depression. Population-based studies have found that men and women with metabolic syndrome are twice as likely to develop depressive symptoms as compared to individuals without metabolic syndrome at baseline (Koponen et al., 2008). Similarly, in a large cohort of community-based older men aged 65–84 years old, metabolic syndrome at the time of recruitment was associated with a 137% increase in the adjusted risk of incident depression (Almeida et al., 2009). These findings suggest that reducing the prevalence of metabolic syndrome could potentially lead to a decline in the prevalence of depression.
Alterations in insulin signaling pathways may also represent part of the underlying pathophysiology of mood disorders. For instance, insulin signaling has been shown to regulate dopamine-mediated neurotransmission in animal models (Williams et al., 2010). By depleting insulin levels, amphetamine-induced dopamine release can be severely attenuated. Related lines of research suggest that circulating levels of insulin can influence the function of the norepinephrine and serotonin transporter, and in turn, extracellular levels of norepinephrine and serotonin (Daws et al., 2009). In animal models, pioglitazone has been shown to reduce the numbers of activated microglia and may mitigate the inflammation associated with chronic and acute neurological insults (Kapadia et al., 2008). Activation of inflammatory cytokines is known to stimulate indoleamine-2,3-dioxygenase. Stimulation of the indoleamine-2,3-dioxygenase enzyme promotes the formation of kynurenine and quinolinic acid, which have been correlated with depressive symptoms (Raison et al., 2010). In animals, PPAR-gamma activation by pioglitazone has been shown to attenuate quinolinic acid induced neurotoxicity (Kalonia et al., 2010).
A small pilot study involving the drug rosiglitazone, another member of the thiazolidinedione class, has recently been reported to reduce depression severity in 12 patients with MDD and insulin resistance (Rasgon et al., 2010). In that trial, patients showed a mean 39% reduction in Hamilton Depression Rating Scale scores, with 50% (n=4) of study completers showing ≥ 50% reduction in severity. Although insulin resistance improved significantly, no correlations were observed between change in depression severity and change in insulin resistance. In contrast, change in insulin resistance in the present trial was significantly correlated with change in depression severity, such that patients with greater improvement in insulin sensitivity also experienced the greatest reductions in depressive symptoms. Positive correlations between insulin resistance and severity of depressive symptoms have been identified in cross-sectional studies of adults and adolescents (Shomaker et al., 2010; Timonen et al., 2005), but whether insulin-resistant states lead to depressed mood or vice versa is unclear. For the first time, we prospectively show that intervention with a thiazolidinedione intended to reduce insulin resistance was correlated with clinically meaningful reductions in depression severity. Although causality cannot be determined, the finding does suggest that insulin sensitivity may be one biological mediator of depression, and that efforts to increase insulin sensitivity through any number of mechanisms (e.g. medications, exercise, diet) may generate sustainable antidepressant effects.
Strengths of the present study include the use of objective and validated measures of depressive symptomatology and use of a structured clinical interview to diagnose MDD. The patient population was broadly representative and did not exclude patients with comorbid anxiety disorders, substance abuse, or a variety of concurrent medical illnesses. The 12-week duration was longer than several antidepressant trials which typically last only 6 to 8 weeks, serving to limit the potential for the observed improvement to be only a transient phenomenon.
The results of this study should also be interpreted in the context of several limitations. These data are uncontrolled and open-label. Response rates in open-label studies are typically higher than those observed in studies employing a placebo-controlled design (Adam et al., 2005). The sample size was small and may have led to underestimation of potential side effects that could emerge when pioglitazone is administered to a population with major depression. Some patients received pioglitazone as augmentation to existing psychotropic medications, potentially obscuring the full effect of the drug. Use of the HOMA-IR technique to measure insulin resistance is not ideal because the model assumes that insulin sensitivity values from hepatic and peripheral sources are equivalent. Future studies may benefit from a more precise measure of approximating whole-body insulin sensitivity, such as the Matsuda Index as derived from an oral glucose tolerance test (Matsuda et al., 1999). The results reported for this prospective pilot study of pioglitazone are encouraging, but are not definitive. These data support the conduct of larger, placebo-controlled trials to fully delineate the role of pioglitazone in the treatment of patients with depression and metabolic risk factors.
Metabolic abnormalities in patients with mood disorders present clinicians with a challenging situation, as several medications newly approved for the adjunctive treatment of acute major depressive episodes (i.e. aripiprazole and quetiapine extended-release) are associated with an increased risk for weight gain, hyperglycemia and dyslipidemia (Chen et al., 2011; Fava et al., 2009; Weisler et al., 2009). At present, pioglitazone and potentially other agents that promote insulin sensitivity appear worthy of further study as they may reduce depressive symptoms and address the cardiometabolic risks associated with mood disorders. The preliminary research findings suggest that by treating underlying metabolic defects, both the core mood symptoms and cardiometabolic abnormalities may improve.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Previous presentation: Part of these data has been presented previously at the American College of Neuropsychopharmacology Annual Meeting, December 6–10, 2009, Hollywood, FL.
Disclosures:
Dr. Calabrese has received grant support, lecture honoraria, or has participated in advisory boards with Abbott, AstraZeneca, Bristol-Myers Squibb/Otsuka, Cephalon, Dainippon Sumitomo, Forest, GlaxoSmithKline, Janssen, Lilly, Lundbeck, Pfizer, Schering-Plough, Servier, Solvay, Sanofi, Synosia, Supernus Pharmaceuticals, Takeda and Wyeth.
Dr. Findling receives or has received research support, acted as a consultant, and/ or served on a speakers bureau for Abbott, Addrenex, Alexza, AstraZeneca, Biovail, Bristol-Myers Squibb, Forest, GlaxoSmithKline, Johnson & Johnson, Kempharm, Eli Lilly & Co., Lundbeck, Merck, Neuropharm, Novartis, Noven, Organon, Otsuka, Pfizer, Rhodes Pharmaceuticals, Sanofi-aventis, Schering-Plough, Seaside Therapeutics, Sepracore, Shire, Solvay, Sunovion, Supernus Pharmaceuticals, Transcept Pharmaceuticals, Validus, and Wyeth.
Dr. Ganocy receives grant support from AstraZeneca and Eli Lilly.
Dr. Kemp has acted as a consultant to Bristol-Myers Squibb and is on the speaker’s bureau for AstraZeneca and Pfizer.
Dr. Ismail-Beigi has acted as a consultant to Eli Lilly. Ms. Conroy, Ms. Fein, and Ms. Obral have no disclosures to report.
REFERENCES
- Adam D, Kasper S, Möller HJ, Singer EA. 3rd European Expert Forum on Ethical Evaluation of Placebo-Controlled Studies in Depression. Placebo-controlled trials in major depression are necessary and ethically justifiable: how to improve the communication between researchers and ethical committees. Eur. Arch. Psychiatry Clin. Neurosci. 2005;255:258–260. doi: 10.1007/s00406-004-0555-5. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Calver J, Jamrozik K, Hankey GJ, Flicker L. Obesity and metabolic syndrome increase the risk of incident depression in older men: the health in men study. Am. J. Geriatr. Psychiatry. 2009;17:889–898. doi: 10.1097/JGP.0b013e3181b047e3. [DOI] [PubMed] [Google Scholar]
- Boris M, Kaiser CC, Goldblatt A, Elice MW, Edelson SM, Adams JB, Feinstein DL. Effect of pioglitazone treatment on behavioral symptoms in autistic children. J. Neuroinflammation. 2007;4:3. doi: 10.1186/1742-2094-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Kroes RA, Beinfeld MC, Panksepp J, Moskal JR. Uncovering the molecular basis of positive affect using rough-and-tumble play in rats: a role for insulin-like growth factor I. Neuroscience. 2010;168:769–777. doi: 10.1016/j.neuroscience.2010.03.045. [DOI] [PubMed] [Google Scholar]
- Chen J, Gao K, Kemp DE. Second-generation antipsychotics in major depressive disorder: update and clinical perspective. Curr. Opin. Psychiatry. 2011;24:10–17. doi: 10.1097/YCO.0b013e3283413505. [DOI] [PubMed] [Google Scholar]
- Davidson MB. Thiazolidinediones. N. Engl. J. Med. 2005;352:205–207. doi: 10.1056/NEJM200501133520222. [DOI] [PubMed] [Google Scholar]
- Daws LW, Owens A, Campos P, Gould G, Galli A, France C. Regulation of biogenic amine transporters by insulin: Implications for antidepressant drug efficacy. Presented at the 48th Annual Meeting of the American College of Neuropsychopharmacology; December 6–10, 2009; Hollywood, FL. [Google Scholar]
- Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefèbvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Korányi L, Laakso M, Mokán M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J. PROactive investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- Eissa Ahmed AA, Al-Rasheed NM, Al-Rasheed NM. Antidepressant-like effects of rosiglitazone, a PPARγ agonist, in the rat forced swim and mouse tail suspension tests. Behav. Pharmacol. 2009;20:635–642. doi: 10.1097/FBP.0b013e328331b9bf. [DOI] [PubMed] [Google Scholar]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of The National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) J.A.M.A. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- Fava M, Wisniewski SR, Thase ME, Baker RA, Tran QV, Pikalov A, Yang H, Marcus RN, Berman RM. Metabolic assessment of aripiprazole as adjunctive therapy in major depressive disorder: a pooled analysis of 2 studies. J. Clin. Psychopharmacol. 2009;29:362–367. doi: 10.1097/JCP.0b013e3181ac9b0b. [DOI] [PubMed] [Google Scholar]
- Fuenzalida K, Quintanilla R, Ramos P, Piderit D, Fuentealba RA, Martinez G, Inestrosa NC, Bronfman M. Peroxisome proliferator-activated receptor gamma up-regulates the Bcl-2 anti-apoptotic protein in neurons and induces mitochondrial stabilization and protection against oxidative stress and apoptosis. J. Biol. Chem. 2007;282:37006–37015. doi: 10.1074/jbc.M700447200. [DOI] [PubMed] [Google Scholar]
- Guy W. US Dept Health, Education, and Welfare publication (ADM) 76–338. Rockville, Md: National Institute of Mental Health; 1976. ECDEU Assessment manual for psychopharmacology; pp. 218–222. [Google Scholar]
- Heneka MT, Sastre M, Dumitrescu-Ozimek L, Hanke A, Dewachter I, Kuiperi C, O’Banion K, Klockgether T, Van Leuven F, Landreth GE. Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1-42 levels in APPV717I transgenic mice. Brain. 2005;128:1442–1453. doi: 10.1093/brain/awh452. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Hirata A, Yamaguchi H, Jimbu Y, Tominaga M. Pioglitazone reduces atherogenic outcomes in type 2 diabetic patients. Atheroscler. Thromb. 2008;15:34–40. doi: 10.5551/jat.e528. [DOI] [PubMed] [Google Scholar]
- Jain R, Osei K, Kupfer S, Perez AT, Zhang J. Long-term safety of pioglitazone versus glyburide in patients with recently diagnosed type 2 diabetes mellitus. Pharmacotherapy. 2006;26:1388–1395. doi: 10.1592/phco.26.10.1388. [DOI] [PubMed] [Google Scholar]
- Kaiser CC, Shukla DK, Stebbins GT, Skias DD, Jeffery DR, Stefoski D, Katsamakis G, Feinstein DL. A pilot test of pioglitazone as an add-on in patients with relapsing remitting multiple sclerosis. J. Neuroimmunol. 2009;211:124–130. doi: 10.1016/j.jneuroim.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Kalonia H, Kumar P, Kumar A. Pioglitazone ameliorates behavioral, biochemical and cellular alterations in quinolinic acid induced neurotoxicity: possible role of peroxisome proliferator activated receptor-Upsilon (PPARUpsilon) in Huntington's disease. Pharmacol. Biochem. Behav. 2010;96:115–124. doi: 10.1016/j.pbb.2010.04.018. [DOI] [PubMed] [Google Scholar]
- Kapadia R, Yi JH, Vemuganti R. Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Front. Biosci. 2008;13:1813–1826. doi: 10.2741/2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp DE, Ismail-Beigi F, Calabrese JR. Antidepressant response associated with pioglitazone: support for an overlapping pathophysiology between major depression and metabolic syndrome. Am. J. Psychiatry. 2009;166:619. doi: 10.1176/appi.ajp.2008.08081195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koponen H, Jokelainen J, Keinänen-Kiukaanniemi S, Kumpusalo E, Vanhala M. Metabolic syndrome predisposes to depressive symptoms: a population-based 7-year follow-up study. J. Clin. Psychiatry. 2008;69:178–182. doi: 10.4088/jcp.v69n0202. [DOI] [PubMed] [Google Scholar]
- Leon AC, Olfson M, Portera L, Farber L, Sheehan DV. Assessing psychiatric impairment in primary care with the Sheehan Disability Scale. Int. J. Psychiatry Med. 1997;27:93–105. doi: 10.2190/T8EM-C8YH-373N-1UWD. [DOI] [PubMed] [Google Scholar]
- Maeshiba Y, Kiyota Y, Yamashita K, Yoshimura Y, Motohashi M, Tanayama S. Disposition of the new antidiabetic agent pioglitazone in rats, dogs, and monkeys. Arzneimittelforschung. 1997;47:29–35. [PubMed] [Google Scholar]
- Mathew SJ, Manji HK, Charney DS. Novel drugs and therapeutic targets for severe mood disorders. Neuropsychopharmacology. 2008;33:2080–2092. doi: 10.1038/sj.npp.1301652. [DOI] [PubMed] [Google Scholar]
- Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Kotwal R, Malhotra S, Nelson EB, Keck PE, Nemeroff CB. Are mood disorders and obesity related? A review for the mental health professional. J. Clin. Psychiatry. 2004;65:634–651. doi: 10.4088/jcp.v65n0507. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Vagic D, Swartz SA, Soczynska JK, Woldeyohannes HO, Voruganti LP, Konarski JZ. Insulin, insulin-like growth factors and incretins: neural homeostatic regulators and treatment opportunities. CNS Drugs. 2008;22:443–453. doi: 10.2165/00023210-200822060-00001. [DOI] [PubMed] [Google Scholar]
- Mittal R, Malhotra S, Pandhi P, Kaur I, Dogra S. Efficacy and safety of combination Acitretin and Pioglitazone therapy in patients with moderate to severe chronic plaque-type psoriasis: a randomized, double-blind, placebo-controlled clinical trial. Arch. Dermatol. 2009;145:387–393. doi: 10.1001/archdermatol.2009.5. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Mahankali A, Matsuda M, Mahankali S, Hardies J, Cusi K, Mandarino LJ, DeFronzo RA. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J. Clin. Endocrinol. Metab. 2002;87:2784–2791. doi: 10.1210/jcem.87.6.8567. [DOI] [PubMed] [Google Scholar]
- Pan A, Lucas M, Sun Q, van Dam RM, Franco OH, Manson JE, Willett WC, Ascherio A, Hu FB. Bidirectional association between depression and type 2 diabetes mellitus in women. Arch. Intern. Med. 2010;170:1884–1891. doi: 10.1001/archinternmed.2010.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancani T, Phelps JT, Searcy JL, Kilgore MW, Chen KC, Porter NM, Thibault O. Distinct modulation of voltage-gated and ligand-gated Ca2+ currents by PPAR-gamma agonists in cultured hippocampal neurons. J. Neurochem. 2009;109:1800–1811. doi: 10.1111/j.1471-4159.2009.06107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pershadsingh HA, Heneka MT, Saini R, Amin NM, Broeske DJ, Feinstein DL. Effect of pioglitazone treatment in a patient with secondary multiple sclerosis. J Neuroinflammation. 2004;1:3. doi: 10.1186/1742-2094-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, Spivey JR, Saito K, Miller AH. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol. Psychiatry. 2010;15:393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasgon NL, Kenna HA, Williams KE, Powers B, Wroolie T, Schatzberg AF. Rosiglitazone add-on in treatment of depressed patients with insulin resistance: a pilot study. ScientificWorldJournal. 2010;10:321–328. doi: 10.1100/tsw.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasgon N, Jarvik L. Insulin resistance, affective disorders, and Alzheimer's disease: review and hypothesis. J. Gerontol. A. Biol. Sci. Med. Sci. 2007;59:178–183. doi: 10.1093/gerona/59.2.m178. [DOI] [PubMed] [Google Scholar]
- Romera C, Hurtado O, Mallolas J, Pereira MP, Morales JR, Romera A, Serena J, Vivancos J, Nombela F, Lorenzo P, Lizasoain I, Moro MA. Ischemic preconditioning reveals that GLT1/EAAT2 glutamate transporter is a novel PPARgamma target gene involved in neuroprotection. J. Cereb. Blood Flow Metab. 2007;27:1327–1338. doi: 10.1038/sj.jcbfm.9600438. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am. J. Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Saubermann LJ, Nakajima A, Wada K, Zhao S, Terauchi Y, Kadowaki T, Aburatani H, Matsuhashi N, Nagai R, Blumberg RS. Peroxisome proliferator-activated receptor gamma agonist ligands stimulate a Th2 cytokine response and prevent acute colitis. Inflamm. Bowel Dis. 2002;8:330–339. doi: 10.1097/00054725-200209000-00004. [DOI] [PubMed] [Google Scholar]
- Shah P, Mudaliar S. Pioglitazone: side effect and safety profile. Expert Opin. Drug Saf. 2010;9:347–354. doi: 10.1517/14740331003623218. [DOI] [PubMed] [Google Scholar]
- Shear MK, Vander Bilt J, Rucci P, Endicott J, Lydiard B, Otto MW, Pollack MH, Chandler L, Williams J, Ali A, Frank DM. Reliability and validity of a structured interview guide for the Hamilton Anxiety Rating Scale (SIGH-A): Depress. Anxiety. 2001;13:166–178. [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59 suppl 20:22–33. [PubMed] [Google Scholar]
- Shomaker LB, Tanofsky-Kraff M, Young-Hyman D, Han JC, Yanoff LB, Brady SM, Yanovski SZ, Yanovski JA. Psychological symptoms and insulin sensitivity in adolescents. Pediatr. Diabetes. 2010;11:417–423. doi: 10.1111/j.1399-5448.2009.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanheimer R, Betteridge DJ, Tan MH, Ferrannini E, Charbonnel B PROactive Investigators. Long-term lipid effects of pioglitazone by baseline anti-hyperglycemia medication therapy and statin use from the PROactive experience (PROactive 14) Am. J. Cardiol. 2009;104:234–239. doi: 10.1016/j.amjcard.2009.03.023. [DOI] [PubMed] [Google Scholar]
- Strum JC, Shehee R, Virley D, Richardson J, Mattie M, Selley P, Ghosh S, Nock C, Saunders A, Roses A. Rosiglitazone induces mitochondrial biogenesis in mouse brain. J. Alzheimers Dis. 2007;11:45–51. doi: 10.3233/jad-2007-11108. [DOI] [PubMed] [Google Scholar]
- Timonen M, Laakso M, Jokelainen J, Rajala U, Meyer-Rochow VB, Keinänen-Kiukaanniemi S. Insulin resistance and depression: cross sectional study. BMJ. 2005;330:17–18. doi: 10.1136/bmj.38313.513310.F71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Ibrahim HM, Carmody TJ, Biggs MM, Suppes T, Crismon ML, Shores-Wilson K, Toprac MG, Dennehy EB, Witte B, Kashner TM. The Inventory of Depressive Symptomatology, Clinician Rating (IDS) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol. Med. 2004;34:73–82. doi: 10.1017/s0033291703001107. [DOI] [PubMed] [Google Scholar]
- Vogelzangs N, Kritchevsky SB, Beekman AT, Brenes GA, Newman AB, Satterfield S, Yaffe K, Harris TB, Penninx BW for the Health ABC Study. Obesity and onset of significant depressive symptoms: results from a prospective community-based cohort study of older men and women. J. Clin. Psychiatry. 2010;71:391–399. doi: 10.4088/JCP.08m04743blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisler R, Joyce M, McGill L, Lazarus A, Szamosi J, Eriksson H Moonstone Study Group. Extended release quetiapine fumarate monotherapy for major depressive disorder: results of a double-blind, randomized, placebo-controlled study. CNS Spectr. 2009;14:299–313. doi: 10.1017/s1092852900020307. [DOI] [PubMed] [Google Scholar]
- Williams JM, Owens WA, Turner GH, Saunders C, Dipace C, Blakely RD, France CP, Gore JC, Daws LC, Avison MJ, Galli A. Hypoinsulinemia regulates amphetamine-induced reverse transport of dopamine. PLoS Biol. 2010;5:e274. doi: 10.1371/journal.pbio.0050274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N. Engl. J. Med. 2008;359:1897–1908. doi: 10.1056/NEJMoa0707402. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Patzer A, Herdegen T, Gohlke P, Culman J. Activation of cerebral peroxisome proliferator-activated receptors gamma promotes neuroprotection by attenuation of neuronal cyclooxygenase-2 overexpression after focal cerebral ischemia in rats. FASEB J. 2006;20:1162–1175. doi: 10.1096/fj.05-5007com. [DOI] [PubMed] [Google Scholar]