Abstract
Rats are able to associate a flavor with the delayed presentation of a food, but the obtained flavor preferences are often weak. The present studies evaluated the effect of delay between a flavor CS and a post-oral nutrient US on the expression of conditioned flavor preferences. In Experiment 1, rats were trained with two CS flavors: one was followed after a delay by intragastric infusion of 8% glucose, and the other was followed after the same delay by intragastric water. Rats trained with 2.5, 10, and 30-min delays expressed significant (84 – 68%) preferences for the glucose-paired flavor whereas rats trained with 60-min delays were in different (51%). Experiment 2 examined flavor conditioning over a 60-min delay using 8 or 16% Polycose based on findings that orally consumed Polycose conditions preferences at this delay interval. The 8 and 16% Polycose infusions produced significant preferences which peaked at 62% and 73%, respectively. The ability to bridge these delays may allow animals to learn about slowly digested foods.
Keywords: flavor learning, glucose, Polycose, gastric infusion
1. Introduction
Animals learn to prefer flavors that have been paired with foods with attractive tastes and/or positive post-oral consequences. We and others have demonstrated this using both simple procedures (mixing the target flavor with the attractive taste) and more elaborate ones (pairing the flavor with intragastric (IG) nutrient infusions) [reviewed in 42]. These techniques mimic the essential elements of learning to choose foods that are nutritious and not harmful.
The importance of conditioning processes in the acquisition of flavor preferences has been known for some time, but the temporal limits of this form of learning are uncertain. Although the post-oral reinforcing stimuli for flavor preference learning have not been identified, the available evidence points to an intestinal signal, at least in the case of glucose [4,17]. Because the digestion and absorption of food is a gradual process, with stomach metering of nutrient passage to the intestines [e.g., 31], the learning mechanism must be able to span the elapsed time between initial orosensory experience and post-oral stimulation. Yet there are few studies that have examined delays between oral and post-oral stimuli in flavor preference learning.
One common method is the oral-delay procedure: oral access to a target flavor (the conditioned stimulus, CS+) is followed after a delay by oral access to the nutrient unconditioned stimulus (US). An alternate flavor, the CS−, is presented in other sessions without a subsequent US. The obtained preferences were often weak or unreliable, particularly when com pared to the strong (>90%) preferences conditioned by a simultaneous procedure in which the CS and US were presented as a mixture. For example, with a glucose US, a 20-min CS-US delay yielded a 56% preference [48] or no preference [9], and a 30-min delay produced a 63% preference for the glucose-paired flavor in one study [23] and a nonsignificant 58% in another [48]. The flavors in these studies were cinnamon and wintergreen extracts, which can be less effective cues than fruit-flavored drink mixes [18]. More robust CS+ preferences of 74% to 81% were obtained in our laboratory using 10-min delays between CS flavors (sweetened Kool-Aid flavors) and carbohydrate (glucose and the hydrolyzed starch Polycose) US solutions [18,41]. The many differences among these studies, and the lack of a clear relationship between the strength of the preference and the length of the delay, preclude any specification of a delay gradient.
The oral-delay procedure has the draw back that the flavor of the nutrient may interfere with learning about the intended CS flavor, both because it is temporally closer to the post-oral US and because it is often more palatable and therefore a more salient stimulus that overshadows the CS flavor [10,18]. In our previous study of delay gradient in flavor learning, we minimized overshadowing by the flavor of Polycose by pre-exposing rats to a 16% Polycose solution adulterated with acarbose [18, Exp. 6]. The drug acarbose retards the digestion and absorption of carbohydrates [36], which would degrade the temporal relationship between the flavor of Polycose and its norm ally rapid nutritive effects, so that the rats would not form a strong association between Polycose flavor and Polycose post-oral effects. Subsequent training with a CS flavor followed by delayed access to Polycose without acarbose, permitted preferential association of the CS+ flavor with the post-oral effects of Polycose. The use of this pre-exposure method allowed us to test the effects of various delays between CS flavor and US access. The pre-exposed rats acquired similar CS+ preferences (77–79%) with delays of 10, 30 and 60 min between presentation of the CS and US. This study is the only one that has compared delay durations; it provided the first demonstration that robust preferences can be conditioned by delays longer than 30 min.
Another way to eliminate the influence of nutrient flavor when studying CS-US delays is to provide oral access to the CS solution and, following the delay, to infuse the nutrient post-orally. An early study [7] assessed rats’ ability to acquire preferences with a 10-min delay between CS access and the onset of IG nutrient infusions. Despite a complicated training procedure, rats trained with delayed carbohydrate or protein infusions exhibited 73% and 79% CS+ preferences, respectively, after only six training/test sessions. More recently our lab has used 15-min delays between the oral CS and an IG ad ministered maltodextrin US to evaluate subtle differences in learning ability due to specific brain lesions [52–54] or differential food experience [35]. None of these studies, however, compared the effects of different CS-US delays.
The present experiments were designed to evaluate the effect of CS-US delay on the strength of preferences conditioned by post-oral infusions of carbohydrate. Experiment 1 compared four intervals (2.5, 10, 30 and 60 min) between intake of a CS solution and intragastric infusion of an 8% glucose solution. The 2.5-min delay interval approximated a minimal delay, and reflected the average time to transfer a squad of animals from drinking cage to infusion cage. The remaining intervals were chosen to match those of our previous oral-delay study [18]. The 60-min group did not acquire a CS+ preference, suggesting that the post-oral effects of an 8% glucose solution were too weak to serve as an effective US after that delay. Experiment 2 examined the possibility that increasing the US intensity might extend the effective interval for flavor preference conditioning to 60 min, as previously observed with the oral-delay procedure [18]. That study used 16% Polycose, which has markedly lower osmotic effects than 16% glucose [39]; Polycose was used here to avoid the potentially aversive effects of a hyperosmotic 16% glucose solution. To provide a comparison to Experiment 1, a second group of animals was trained with 8% Polycose as the US.
2. General Method
2.1 Subjects
Adult fem ale Sprague-Dawley rats obtained from Charles River Laboratories (Wilmington, MA) (Experiment 1) or bred in the laboratory from Charles River stock (Experiment 2) were individually housed in stainless steel cages in a temperature-controlled vivarium maintained at 21 degrees C under a 12:12 h light:dark cycle. Unless otherwise noted, the rats were maintained at 85% to 90% of their free-feeding body weights on powdered chow meal (no. 5001, PMI Nutrition International, Brentwood, MO). Food rations were adjusted daily and were provided about 2 h after each daily test session. Except during test sessions, tap water was freely available.
2.2 Surgery
Following pre-training (described in Section 2.5), rats were returned to ad lib chow. They were then anesthetized with an ip administration of ketamine (63 mg/kg) and xylazine (9.4 mg/kg) and fitted with in dwelling gastric catheters using a technique modified from Davis and Campbell [16]. Briefly, a silastic tube (1.02 mm. i.d., 2.16 mm o.d.) was inserted into the fundic region of the stomach. The tube was routed under the skin to the back of the neck and was connected to 20-gauge L-shaped stainless steel hypodermic tubing fixed to the skull with dental cement and stainless steel screws.
2.3 Apparatus
Eight plexiglas cages (22.5 cm long × 22.5 cm wide × 26.5 cm high) were kept in an isolated room maintained at 21 degrees C. A slot (1 cm wide × 16 cm long) in the top of the cage allowed passage of the infusion tubes. Tygon microbore plastic tubing (0.76 mm i.d. × 2.29 mm o.d.) attached to 30 ml syringes in variable rate syringe pumps (Razel, mod el A-99) were connected to the input port of a swivel (Instech Laboratories, Horsham, PA) on a counterbalanced lever above the cage. A 50 cm length of the same tubing, protected by a stainless-steel spring, connected the swivel’s output port to the rat’s cannula.
2.4 Test Solutions
All solutions were prepared on a weight/volume basis using tap water. The conditioned stimulus solutions consisted of 0.2% sodium saccharin (Sigma Chemical Company, St. Louis, MO) and 0.05% cherry or grape Kool-Aid (unsweetened mix; Kraft Food s, White Plains, NY). For half the rats in each group, the CS+ was cherry and the CS− was grape; for the other half the CS flavors were reversed. The infusion solutions consisted of 8% glucose or 8% or 16% Polycose (hydrolyzed corn starch; Abbott Nutrition, Columbus OH). Infusions were delivered at room temperature at a fixed volume of 10 ml, infused at a rate of 1.3 ml/min over 7.7 min. Pilot work demonstrated that this volume of IG 8% glucose conditioned strong (91%) flavor preferences when there was no delay between CS consumption and IG infusion. The 8% and 16% solutions delivered 0.32 kcal/ml and 0.64 kcal/ml, respectively. Oral intakes and infusion volumes were measured to the nearest 0.5 ml.
2.5 Procedure
Pre-surgery training
The rats were familiarized with 0.2% saccharin solution prior to training. This was accomplished by first giving rats adlibitum access to the saccharin solution in their home cages (1 day with a second bottle containing water, and another day with saccharin only). Next the rats were trained to drink the saccharin in short sessions in their home cages, which were brought into the test room. To enhance consumption during the daily sessions the rats were first trained to drink 2% sucrose-0.2% saccharin solution (2 days) during 30-min sessions. For the next 2 days they were given 30-min access to plain 0.2% saccharin solution; then daily access was limited to 10 min for another 1 to 3 days. Drinking sessions were limited to 10 min in order to control the delay interval between offset of the CS and onset of the US.
Post-surgery training
Rats were given approximately 1 week to recover from surgery before food restriction and training recommenced. Rats were given some additional post-surgery pretraining to re-acclimate them to drinking saccharin during 10-min sessions (2–3 days) and to adapt them to being connected to the infusion system and infused with 10 ml of water following their specified delay interval (3 days).
2.6 Statistical Analysis
The primary data included the amount of fluid orally consumed during training (one-bottle) and testing (two-bottle) sessions. The data were averaged over two-day or three-day periods and were analyzed using repeated measures analysis of variance followed by simple main effects tests and Newman-Keuls tests, where appropriate. The amount of fluid consumed in two-bottle tests was also expressed as percent CS+ preference ((CS+ intake/total intake) × 100).
3. Experiment 1: delay gradient for CS flavor preference learning with intragastric 8% glucose US
3.1 Subjects and infusions
Four groups of rats were studied at one of four delay intervals: 2.5 min (n=7), 10 min (n=8), 30 min (n=10) and 60 min (n=7). The 2.5 and 60 min groups were studied concurrently, so that some animals from each group were run in each squad of 7 animals. The 10- and 30-min groups were obtained from the supplier at the same time but studied in separate replications. The 30-min group was thus 16 weeks old at the start of training, whereas the others were 12 weeks old. This difference did not affect the training intakes, as reported in section 3.3. The infusions were 8% glucose on CS+ days and water on CS− days.
3.2 Procedure
Flavor conditioning occurred over two 8-day cycles. On days 1, 3 and 5 the rats were given 10-min access to the CS+; on days 2, 4, and 6 they were given 10-min access to the CS−. The CS solutions were consumed in the home cage to ensure that there were no CS cues present in the infusion cage. The rats in the 2.5-min delay group were immediately transferred to the infusion cages to receive the associated IG infusions. For the 2.5-min group, the nominal delay interval of 2.5 min reflected the average delay interval, which varied from 1 to 5 min. Specifically, as soon as the 10-min CS drinking period ended, the rats were transferred, one at a time, into the infusion cages and as soon as all rats in the group were transferred, the infusions were started. The rats in the longer delay groups waited in their home cages until approximately 5 min before the specified delay interval elapsed at which time cage transferring began. This ensured that all animals had equivalent time in the infusion cages. When the time interval elapsed, the pumps were activated and infusions occurred. Although the 10 ml infusions were completed in 7.7 min, the rats were left in the infusion cages undisturbed for a total of 20 min to allow some gastric emptying to occur before they were detached from the infusion system. No fluids were available during the delay interval. On days 7 and 8 of each cycle, the rats were given 30-min CS+ vs. CS− two-bottle preference tests in their home cages without infusions.
3.3 Results
One-bottle training
During training CS solution intake did not differ as a function of paired infusion or delay until infusion. However, intake of the CS solutions increased from cycle 1 to cycle 2 (from 6.7 to 10.1 ml; F(1,28) = 231.5, p < 0.0001).
Two-bottle preference tests
Average intakes in the two-bottle tests are shown in Figure 1. Between-groups analysis of the two-bottle intake data revealed that the rats consumed more of the CS+ than the CS− overall (F(1,28) = 40.6, p < 0.0001) and average CS solution intakes increased from cycle 1 to cycle 2 (8.1 vs. 9.0 ml; F(1,28) = 16.0, p = 0.001). The comparison of primary interest, the effect of US delay on CS intakes, showed that the relative intakes of the CS+ and CS− differed among the groups (group × flavor interaction: F(3,28) = 4.3, p < 0.05). Because there was a group × cycle interaction (F(3,28) = 10.6, p < 0.001), the cycles were analyzed separately.
Figure 1.
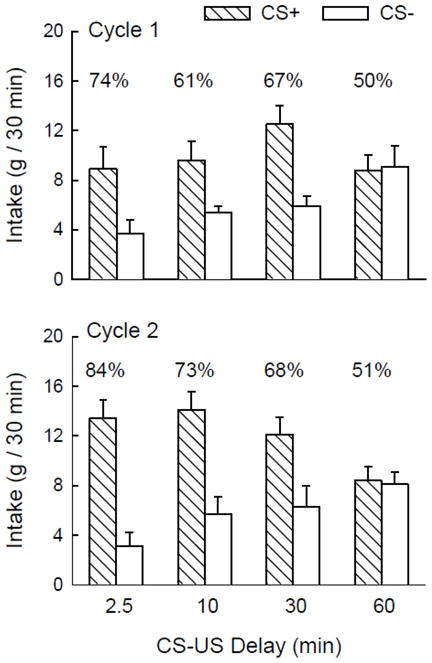
Mean (+SEM) intakes of CS+ and CS− solutions by the 2.5-, 10-, 30- and 60-min groups during two-bottle testing in cycles 1 (top panel) and 2 (bottom panel) of Experiment 1. The group names refer to the delay between CS intake and 8% glucose US infusion in training sessions. Numbers atop the columns indicate the percentage preference for the CS+ solution.
The CS flavor effect was significant in both cycles (F(1,28) = 16.0, 32.2, ps < 0.001). The overall group × cycle interaction reflected the group difference in CS intakes in cycle 2; the interaction was not significant in cycle 1. Rats in the 2.5-, 10- and 30-min groups consumed reliably more of the CS+ than the CS− in cycle 2 (group × flavor interaction F(1,28) = 3.60, p < 0.05), whereas CS intakes of the 60-min group did not differ. Analysis of percent CS+ intakes in cycle 2 tests showed a group effect, F(3,28) = 5.88, p < 0.01; in agreement with the intake analysis, the CS+ preferences of the 2.5-, 10- and 30-min groups (84, 73, 68%) did not significantly differ but were stronger than that of the 60-min group (51%).
3.4 Discussion
The present findings confirm prior reports that preferences can be conditioned with delays between the CS flavor and postingestive consequences of the US. The new finding is that preference conditioning decreases as the delay interval increases. Although delay gradients have been obtained in several other conditioning paradigms (i.e., conditioned flavor aversions), these results are the first to demonstrate a learning gradient in preference conditioning.
Only one other study has compared flavor preferences conditioned at different delays [18]. In that study, three separate groups of rats that were trained to associate a CS+ with consumption of a Polycose solution after delays of 10, 30 or 60 min acquired equivalent preferences of 77%, 79%, and 78% respectively. In contrast, the final preferences of the 10-, 30- and 60-min groups of the present study were 73%, 68% and 51%. The incompatible findings may be due to procedural differences such as the route of nutrient delivery (oral versus IG), the particular carbohydrate used (Polycose versus glucose), and/or differences in the amount of nutrient in the US. The rats in the Elizalde and Sclafani [18] study received more than four times the amount of nutrient than the rats in the present study (~20 ml of a 16% polycose solution versus 10 ml of an 8% glucose solution).
The prediction that a larger US dose may be capable of sustaining conditioning over long delays has been borne out in the conditioned taste aversion paradigm [5,30,34,38]. That is, aversions were noted at longer delays when the dose of the illness-inducing US was increased. Capaldi et al. [13] also obtained some evidence to suggest that substantial energy delivery is required for preference conditioning with delays. Flavor preferences were conditioned by 8% carbohydrate solutions following a 5-min delay but were not conditioned by 1% carbohydrate solutions using the same procedures. Greater nutrient loads, up to a point, condition stronger preferences without delays as well [1,11,22,28,51].
Based on the results of the present study and those of Elizalde and Sclafani [18] we predicted that a greater amount of nutrient or greater nutrient density may be necessary to sustain preference conditioning with extended delays. Another possibility is that Polycose retains its reinforcing strength with greater delays than does glucose. These issues were further addressed in Experiment 2.
4. Experiment 2:60-min delay between CS and intragastric 8% and 16% Polycose US
Experiment 1 indicated that significant preferences can be conditioned with delays up to 30 min between the offset of cue flavor consumption and onset of IG delivery of 8% glucose. The 60-min delay group, however, did not acquire a preference. With an oral-US procedure, Elizalde and Sclafani [18] obtained preferences with a 60-min delay between consumption of a CS+ flavor and a 16% Polycose solution. The ability of oral 16% Polycose, but not IG 8% glucose, to condition preferences with a 60-min delay is likely to be due primarily to the amount of nutrient but the route of delivery may also be important.
The influence of carbohydrate form is harder to predict. Because rats learn to prefer flavors mixed into Polycose solutions, but not Polycose + acarbose solutions [18, Exp. 2], Polycose-based flavor preferences require digestion to glucose. Although Polycose is rapidly digested to glucose in the intestine, suggesting the two carbohydrates should have equivalent effects, an in vitro study found that the glucose released from infused Polycose was more rapidly absorbed by the mucosa than infused glucose [15]. The lower osmolality of Polycose than glucose may also be important in the preferences obtained in the Elizalde and Sclafani [18] study; a 16% glucose solution would be hyperosmotic and might therefore have aversive consequences.
The goal of Experiment 2 was to assess the effect of the amount of nutrient infused on the strength of conditioning with 60-min delays. Given that rats learned a flavor preference with 60-min delayed presentation of oral 16% Polycose in the earlier study [18], we infused 16% Polycose to one group, and predicted that they would acquire a preference for the CS+ flavor consumed 60 min earlier. Another group of animals was infused with 8% Polycose, providing two kinds of comparison: the effect of providing only half the carbohydrate given to the 16% group, and the effect of carbohydrate form by comparison with the 60-min 8% glucose group of Experiment 1.
4.1 Method
Eighteen 12-week old rats were fitted with gastric catheters and trained and tested according to the same procedures described for Experiment 1 using nine identical infusion cages. Following post-surgery pre-training, the rats were divided into two groups (n = 9 each) matched on 10-min saccharin intake. One group received 10 ml infusions of 8% Polycose while the other group received 10 ml infusions of 16% Polycose on CS+ days following a 60-min delay. The groups were studied concurrently, so that 4 or 5 animals from each group were run in each squad. Three 8-day cycles were conducted.
4.2 Results
One-bottle training
During training CS solution intakes did not differ as a function of paired infusion or Polycose concentration. Overall, the rats increased their intake of the CS solutions over cycles (F(2,32) = 103.5, p < 0.0001). Newman-Keuls pairwise comparisons indicated a reliable increase in CS solution intake from each cycle to the next (7.2, 9.8, and 11.6 g in cycle 1, 2 and 3, p < 0.0001).
Two-bottle preference tests
Average intakes in the two-bottle tests are shown in Figure 2. During two-bottle preference tests, the rats consumed more CS+ than CS− overall (flavor F(1,16) = 21.1, p < 0.001), but this difference varied over cycles (cycle × flavor interaction: F(2,32) = 4.1, p < 0.05). Simple main effects tests showed that the difference between CS+ and CS− intake was significant in cycles 2 and 3 but marginal in the first cycle (p = 0.058), and that intake of the CS+, but not the CS−, differed across cycles. The main effect of cycle (F(2,32) = 9.6, p < 0.01) was due to greater intake in cycles 2 and 3 than in cycle 1. Overall, there were no significant group differences in CS intakes. The 8% and 16% groups also did not significantly differ in their CS+ preferences and their peak CS+ preferences were 62% and 73%, respectively. .
Figure 2.
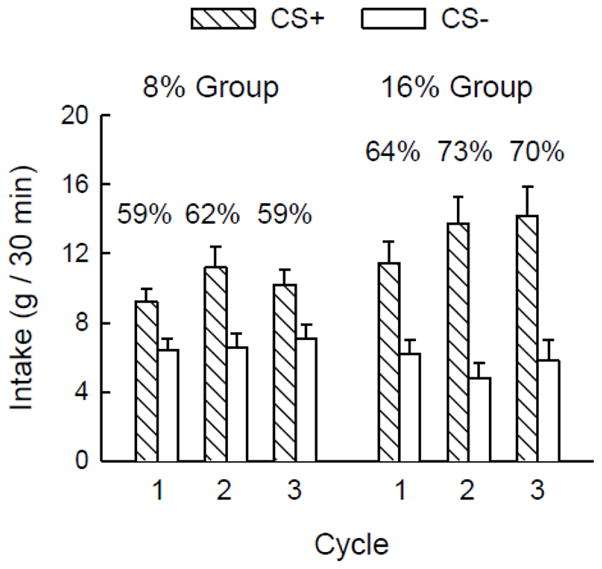
Mean (+SEM) intakes of CS+ and CS− solutions by the 8% and 16% Polycose groups during two-bottle testing in cycles 1, 2, and 3 of Experiment 2. Both groups were trained with a 60-min delay between CS intake and Polycose infusion. Numbers atop the columns indicate the percentage preference for the CS+ solution.
4.3 Discussion
The present findings confirm that preferences can be conditioned with a 60-min delay between the flavor and postingestive consequences of Polycose [18]. The finding that relatively strong (~70%) preferences were obtained with a 60-min delay between flavor consumption and IG nutrient infusion suggests that even longer delays are possible. Thus, whether the nutrient is consumed by mouth or introduced post-orally, preference conditioning is possible with long delays between the flavor and postingestive effects of nutrients. However, the results appear to be highly sensitive to variations in the training procedures employed.
Intragastric infusion of 8% Polycose conditioned a flavor preference at a 60-min delay whereas 8% glucose (Experiment 1) did not. The reasons for this difference are not certain, but are not likely to reflect differences in the conduct of the two experiments. Although the animals differed in provenance (obtained directly from a supplier or bred in our laboratory from animals obtained from the supplier), this is unlikely to account for the difference, because all the animals were derived from the same stock (Charles River Sprague-Dawley). The same experimenter conducted both studies within the span of a few months, and carefully replicated the procedure. Therefore the difference is probably in the post-oral response to the two forms of carbohydrate. One possibility is that the 60-min delay is at the limits of associability, and that Polycose digestion delivers the postingestive stimulus more quickly than glucose [15].
5. General Discussion
These studies provide new information about the impact of delay between CS flavor and US nutrient in flavor preference learning. Using a uniform stimulus (8% glucose), we showed that increasing the time between experience of a flavor and a post-oral load weakened the resulting flavor preference learning. Preferences were obtained with up to 30-min intervals between the CS and an 8% glucose US. A larger carbohydrate load of 16% Polycose was capable of producing a flavor preference even at 60 min post-flavor; a parallel group given 8% Polycose was not statistically different. These data suggest that the animals could remember a flavor that preceded postingestive effects by as much as an hour, and certainly after 30 min for the less concentrated infusion. US concentration, and presumably its detected intensity, has an effect on its associability with a less-recent CS flavor.
5.1 The delay gradient
In Experiment 1 conditioning with delays of 2.5, 10, 30 and 60 min was examined. As the delay interval between flavor consumption and IG nutrient infusion increased from 2.5 to 60 min, the strength of flavor conditioning decreased. With a 60-min delay preferences were not obtained. Although delay gradients have been obtained in many other conditioning paradigms [29], this is the first demonstration in flavor preference conditioning based on positive post-oral effects.
The delay gradient obtained in Experiment 1 differed from the previous oral study in two important ways. Elizalde and Sclafani [18, Exp. 6] observed preferences with a 60-min delay that were equivalent to the preferences conditioned with delays of 10 and 30 min. Thus, the oral-delay procedure did not obtain a learning gradient.
Unlike the oral-delay procedure, the IG-delay procedure used in Experiment 1 completely dissociated orosensory stimulation from nutrient delivery. This is desirable from an associative learning perspective because it eliminates potential interference by the nutrient’s flavor. However, the lack of oral processing may also diminish the full metabolic effects of the nutrient. When nutrients are placed directly into the stomach in the absence of orosensory stimulation, metabolic responses that normally occur in response to the sight, smell and taste of food are eliminated. The absence of these cephalic phase preparatory responses has been shown to alter digestion and metabolism in rats [32,37] and may also serve to diminish the postingestive reinforcing effect.
There is some indication from early operant conditioning studies, that IG nutrient infusions do not reinforce lever pressing responses unless an oral stimulus is also provided [8,24]. Generalizing from these findings, it is quite conceivable that in the IG-delay conditioning procedure, the absence of concurrent orosensory stimulation at the time of gastric nutrient arrival may have weakened the reinforcing effect of the nutrient. Hence, as the delay between cue flavor consumption and delivery of the nutrient increased, the potential for the maximum reinforcing effect may have decreased due to a diminution of the cephalic phase reflexive responses. This explanation could account for the finding of Elizalde and Sclafani [18] that orally consumed Polycose maintained its associative strength over increasing delays whereas a decrement in conditioning with delays was observed in the present IG-delay experiment.
Aversive flavor-postingestive conditioning can withstand quite long CS-US delays. Flavor aversion has been observed with delays of 1, 6, 12 and even 24 hours between the CS and delivery of the illness-producing US [20,21,33,38,50]. Although conditioning based on positive nutritional effects has not been obtained with delays as long, the two paradigms are not directly comparable because the two ends of the preference-aversion spectrum are not symmetrical [60]; that is, the intensity of aversive stimuli can far exceed the intensity of reinforcing stimuli. In addition, oversatiation by nutrients can produce an aversive state referred to as nimiety [12]. Furthermore, the physiological relevance of post-oral nutritional information occurring several hours after consumption is questionable. It is worth noting, however, that in one study weak preferences were observed with a delay of five hours between the CS and the chocolate milk US after extensive training [14].
The effect of CS intensity was not tested in the present studies. Perhaps a stronger CS (e.g., more concentrated cherry and grape flavors) could also enhance the association with delayed nutrient, so that 8% glucose would be effective at a 1-h delay. Several of our studies suggest that CS salience plays a role in flavor preference conditioning with IG nutrients. The flavors used here were sweetened with saccharin, primarily to ensure that animals would consume them readily in short sessions. In other work with long sessions, in which the CS flavor was the only fluid available to the animals, we have used the same flavors without saccharin. These support flavor preference learning with IG Polycose and sucrose [6,19,40], but we have found that for some nutrients (fructose, oil, ethanol) preferences established in long sessions are markedly improved with sweetened compared to unsweetened flavors [2,3,27]. These observations suggest that increasing the salience of CS flavors by increasing their concentration might also be effective. Furthermore, delay learning may be more sensitive to CS quality than simultaneous procedures; the cinnamon and wintergreen flavors used in the earlier work were less effective than Kool-Aid flavors when tested under the same conditions [18]. Comparison to long-delay aversion learning suggests that pure taste stimuli, such as the commonly used saccharin CS in aversion studies, may be more effective than odors (the primary difference between Kool-Aid flavors), but some studies have found flavor aversion learning with pure odor (amyl acetate with a 4-h delay) or an odorous nutrient (casein hydrolysate at 2 or 4 h) [25,26,49].
5.2 US intensity
The selection of 8% glucose for the IG infusion in Experiment 1 was guided in part by our previous work that paired ingestion of the CS solution with concurrent infusion of the US. Many of our IG studies have successfully used 16% carbohydrate infusions, which when infused concurrently are diluted in the stomach to 8% by the equal volume of oral CS intake. The 8% concentration was therefore adequately associable with flavors experienced simultaneously. The volume was chosen to avoid excessive gastric fill while still providing an adequate stimulus.
In the Elizalde and Sclafani [18] study using oral US delivery, the rats consumed four times the amount of carbohydrate in every CS+ training session than that infused to the animals in Experiment 1. Because those animals developed a CS+ preference even after a 60-min delay, Experiment 2 examined the role of magnitude of reinforcement in IG conditioning with this delay interval. By changing the carbohydrate source from glucose to Polycose, we avoided possibly aversive hyperosmotic effects. This also allowed a comparison to the earlier oral study and a test of the generality of our findings concerning US intensity, since the Polycose stimulus in flavor preference conditioning requires its digestion to glucose [18]. The preferences expressed by the 16% Polycose group were not stronger than those of the 8% Polycose group, suggesting that doubling the amount of carbohydrate does not increase its reinforcing potency at this delay. Perhaps the use of an even higher concentration, such as 32% Polycose, would have generated a stronger preference for the CS+. However, when trained with Polycose infused concurrently with CS intake, rats prefer the flavor paired with 16% Polycose over the 32% Polycose-paired flavor [28], indicating that the more concentrated infusion might be less potent as a reinforcer.
Under these conditions the limit for effective association of 8% glucose-based loads with prior flavor experience appears to be somewhere between 30 and 60 min. By doubling the US concentration, we obtained clear preferences despite a 60-min delay, suggesting that this amount of carbohydrate might reinforce a flavor preference when delivered after even longer delays.
5.3 Mechanism(s) for CS-US association
In the kind of delayed learning situation examined here, the problem for the rat is to remember what was ingested some time ago (a task made somewhat easier because nothing else was available in the interim) and associate it with a post-oral reinforcer. While we cannot yet specify how this occurs, we can rule out (or discount) some processes based on previous work. In particular, we have determined that the positive food-based signals that condition the flavor preferences are not necessarily the same signals that contribute to satiety [46].
The locus of the US detection remains unknown but prior work suggests that the upper intestine is an important site of action. In particular, preferences for a non-nutritive flavor CS+ are conditioned by gastric, duodenal, and jejunal glucose infusions but not ileal or hepatic-portal infusions or by infusions limited to the stomach [4,17]. The presence of T1R2+T1R3 sweetener receptors in the intestines allows for the possibility that they mediate carbohydrate reinforcement, but this is not supported by the recent report that T1R3 knockout mice are similar to wild type mice in their preference conditioning response to IG sucrose infusions [47]. Instead, a glucose-specific sensor is implicated by the differential conditioning effects of IG glucose, fructose, and galactose infusions in rats [44]. Much remains to be learned about the central neural processes that mediate CS-US associations involved in preference learning over a delay. Several brain sites are implicated in preference learning including, the parabrachial nucleus, amygdala, nucleus accumbens, medial prefrontal cortex, and lateral hypothalamus (LH) [45,52,55–59]. The lateral hypothalamus is of particular interest for CS-US delay conditioning because LH lesions, while they partially attenuated CS+ preference conditioning by concurrent IG nutrient infusions, completely blocked conditioning when there was a 15-min delay between the CS intake and US infusion [54].
6. Significance
The ability to associate flavors with the delayed onset of post-oral reinforcement clearly has functional significance. Preferences for flavors associated with potent but slowly digested nutrients could still be learned, which in turn could expand the array of potential foods in an animal’s diet. The present experiments used rapidly digested and absorbed carbohydrates infused at different delays to control the time between flavor experience and post-oral stimulation. Variations of this method could be used to explore other nutrients and expand our understanding of the temporal limits of learning to associate positive oral and post-oral stimuli.
Highlights.
Rats learned to prefer a CS flavor with an IG 8% glucose infusion delivered 2.5, 10, and 30 min but not 60 min later.
Other rats learned a CS flavor preference based on 8% or 16% Polycose infusions delayed by 60 min.
The greater effectiveness of Polycose than glucose may reflect osmotic or absorptive differences.
The ability to associate oral stimuli with delayed post-oral effects may enhance diet selection processes.
Acknowledgments
This work was supported by grant NIH/NIDDK 031135 from the National Institute of Diabetes and Digestive and Kidney Diseases. Portions of the data were presented at the 1998 meeting of the Society for the Study of Ingestive Behavior [43].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ackroff K, Sclafani A. Flavor preferences conditioned by intragastric infusions of dilute Polycose solutions. Physiol Behav. 1994;55:957–62. doi: 10.1016/0031-9384(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 2.Ackroff K, Sclafani A. Flavor quality and ethanol concentration affect ethanol-conditioned flavor preferences. Pharmacol Biochem Behav. 2002;74:229–40. doi: 10.1016/s0091-3057(02)00987-5. [DOI] [PubMed] [Google Scholar]
- 3.Ackroff K, Sclafani A. Fructose conditioned flavor preferences in male and female rats: effects of sweet taste and sugar concentration. Appetite. 2004;42:287–97. doi: 10.1016/j.appet.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Ackroff K, Yiin Y-M, Sclafani A. Post-oral infusion sites that support glucose-conditioned flavor preferences in rats. Physiol Behav. 2010;99:402–11. doi: 10.1016/j.physbeh.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrews EA, Braveman NS. The combined effects of dosage level and interstimulus interval on the formation of one-trial poison-based aversions in rats. Anim Learn Behav. 1975;3:287–9. [Google Scholar]
- 6.Azzara AV, Sclafani A. Flavor preferences conditioned by intragastric sugar infusions in rats: Maltose is more reinforcing than sucrose. Physiol Behav. 1998;64:535–41. doi: 10.1016/s0031-9384(98)00113-9. [DOI] [PubMed] [Google Scholar]
- 7.Baker BJ, Booth DA. Preference conditioning by concurrent diets with delayed proportional reinforcement. Physiol Behav. 1989;46:585–90. doi: 10.1016/0031-9384(89)90336-3. [DOI] [PubMed] [Google Scholar]
- 8.Berkun MM, Kessen ML, Miller NE. Hunger-reducing effects of food by stomach fistula versus food by mouth measured by a consummatory response. J Comp Physiol Psychol. 1952;45:550–4. doi: 10.1037/h0061931. [DOI] [PubMed] [Google Scholar]
- 9.Boakes RA, Rossi-Arnaud C, Garcia-Hoz V. Early experience and reinforcer quality in delayed flavour-food learning in the rat. Appetite. 1987;9:191–206. doi: 10.1016/s0195-6663(87)80013-2. [DOI] [PubMed] [Google Scholar]
- 10.Boakes RA, Lubart T. Enhanced preference for a flavour following reversed flavour glucose pairing. Q J Exp Psychol. 1988;40:49–62. [PubMed] [Google Scholar]
- 11.Bolles RC, Hayward L, Crandall C. Conditioned taste preferences based on caloric density. J Exp Psychol Anim Behav Process. 1981;7:59–69. doi: 10.1037//0097-7403.7.1.59. [DOI] [PubMed] [Google Scholar]
- 12.Cabanac M. Sensory pleasure. Q Rev Biol. 1979;54:1–29. doi: 10.1086/410981. [DOI] [PubMed] [Google Scholar]
- 13.Capaldi ED, Campbell DH, Sheffer JD, Bradford JP. Conditioned flavor preferences based on delayed caloric consequences. J Exp Psychol Anim Behav Process. 1987;13:150– 5. [PubMed] [Google Scholar]
- 14.Capaldi ED, Sheffer JD. Contrast and reinforcement in consumption. Learn Motiv. 1992;23:63–79. [Google Scholar]
- 15.Daum F, Cohen MI, McNamara H, Finberg L. Intestinal osmolality and carbohydrate absorption in rats treated with polymerized glucose. Pediatr Res. 1978;12:24–6. doi: 10.1203/00006450-197801000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Davis JD, Campbell CS. Chronic intrajugular, intraportal, gastric, and duodenal cannulae for the rat. In: Singh D, Avery DD, editors. Physiological techniques in behavioral research. Monterey: Brooks Cole; 1975. pp. 163–77. [Google Scholar]
- 17.Drucker DB, Sclafani A. The role of gastric and post-gastric sites in glucose-conditioned flavor preferences in rats. Physiol Behav. 1997;61:351–8. doi: 10.1016/s0031-9384(96)00414-3. [DOI] [PubMed] [Google Scholar]
- 18.Elizalde G, Sclafani A. Starch-based conditioned flavor preferences in rats: Influence of taste, calories, and CS-US delay. Appetite. 1988;11:179–200. doi: 10.1016/s0195-6663(88)80002-3. [DOI] [PubMed] [Google Scholar]
- 19.Elizalde G, Sclafani A. Flavor preferences conditioned by intragastric Polycose: A detailed analysis using an electronic esophagus preparation. Physiol Behav. 1990;47:63– 77. doi: 10.1016/0031-9384(90)90043-4. [DOI] [PubMed] [Google Scholar]
- 20.Etscorn F, Stephens R. Establishment of conditioned taste aversions with a 24-hour CSUS interval. Physiol Psychol. 1973;1:251–3. [Google Scholar]
- 21.Garcia J, Ervin FR, Koelling RA. Learning with prolonged delay of reinforcement. Psychon Sci. 1966;5:121–2. [Google Scholar]
- 22.Hayward L. The role of oral and postingestional cues in the conditioning of taste preferences based on differing caloric density and caloric outcome in weanling and mature rats. Anim Learn Behav. 1983;11:325–31. [Google Scholar]
- 23.Holman EW. Immediate and delayed reinforcers for flavor preferences in rats. Learn Motiv. 1975;6:91–100. [Google Scholar]
- 24.Holman GL. Intragastric reinforcement effect. J Comp Physiol Psychol. 1968;69:432–41. doi: 10.1037/h0028233. [DOI] [PubMed] [Google Scholar]
- 25.Kalat JW, Rozin P. Role of interference in taste-aversion learning. J Comp Physiol Psychol. 1971;77:53–8. doi: 10.1037/h0031585. [DOI] [PubMed] [Google Scholar]
- 26.Kalat JW, Rozin P. “Learned safety” as a mechanism in long-delay taste-aversion learning in rats. J Comp Physiol Psychol. 1973;83:198–207. doi: 10.1037/h0034424. [DOI] [PubMed] [Google Scholar]
- 27.Lucas F, Sclafani A. Flavor preferences conditioned by intragastric fat infusions in rats. Physiol Behav. 1989;46:403–12. doi: 10.1016/0031-9384(89)90011-5. [DOI] [PubMed] [Google Scholar]
- 28.Lucas F, Azzara AV, Sclafani A. Flavor preferences conditioned by intragastric Polycose in rats: More concentrated Polycose is not always more reinforcing. Physiol Behav. 1998;63:7–14. doi: 10.1016/s0031-9384(97)00364-8. [DOI] [PubMed] [Google Scholar]
- 29.Mackintosh NJ. Conditioning and associative learning. New York: Oxford University Press; 1983. [Google Scholar]
- 30.Martin GM, Timmins WK. Taste-sickness associations in young rats over varying delays, stimulus, and test conditions. Anim Learn Behav. 1980;8:529–33. [Google Scholar]
- 31.McHugh PR, Moran TH. Calories and gastric emptying: a regulatory capacity with implications for feeding. Am J Physiol. 1979;236:R254–R60. doi: 10.1152/ajpregu.1979.236.5.R254. [DOI] [PubMed] [Google Scholar]
- 32.Molina F, Thiel T, Deutsch JA, Puerto A. Comparison between some digestive processes after eating and gastric loading in rats. Pharmacol Biochem Behav. 1977;7:347–50. doi: 10.1016/0091-3057(77)90230-1. [DOI] [PubMed] [Google Scholar]
- 33.Nachman M. Learned taste and temperature aversions due to lithium chloride sickness after temporal delays. J Comp Physiol Psychol. 1970;73:22–30. doi: 10.1037/h0029807. [DOI] [PubMed] [Google Scholar]
- 34.Nachman M, Ashe JH. Learned taste aversions in rats as a function of dosage, concentration, and route of administration of LiCl. Physiol Behav. 1973;10:73–8. doi: 10.1016/0031-9384(73)90089-9. [DOI] [PubMed] [Google Scholar]
- 35.Pérez C, Fanizza L, Sclafani A. Flavor preferences conditioned by intragastric nutrient infusions in rats fed chow or a cafeteria diet. Appetite. 1999;32:155–70. doi: 10.1006/appe.1998.0182. [DOI] [PubMed] [Google Scholar]
- 36.Puls W, Krause HP, Muller L, Schott H, Sitt R, Thomas G. Pharmacology of a glucosidase inhibitor. Front Horm Res. 1980;7:125–46. [Google Scholar]
- 37.Ramirez I. Oral stimulation alters digestion of intragastric oil meals in rats. Am J Physiol. 1985;248:R459–R63. doi: 10.1152/ajpregu.1985.248.4.R459. [DOI] [PubMed] [Google Scholar]
- 38.Revusky SH. Aversion to sucrose produced by contingent x-irradiation: temporal and dosage parameters. J Comp Physiol Psychol. 1968;65:17–22. doi: 10.1037/h0025416. [DOI] [PubMed] [Google Scholar]
- 39.Ross Laboratories. Polycose. Columbus, OH: Ross Laboratories; 1984. [Google Scholar]
- 40.Sclafani A, Nissenbaum J. Robust conditioned flavor preference produced by intragastric starch infusion in rats. Am J Physiol. 1988;255:R672–R5. doi: 10.1152/ajpregu.1988.255.4.R672. [DOI] [PubMed] [Google Scholar]
- 41.Sclafani A, Ackroff K. Glucose- and fructose-conditioned flavor preferences in rats: Taste versus postingestive conditioning. Physiol Behav. 1994;56:399–405. doi: 10.1016/0031-9384(94)90213-5. [DOI] [PubMed] [Google Scholar]
- 42.Sclafani A. How food preferences are learned –laboratory animal models. Proc Nutr Soc. 1995;54:419–27. doi: 10.1079/pns19950011. [DOI] [PubMed] [Google Scholar]
- 43.Sclafani A, Pérez C, Drucker DB. Temporal dimensions of flavor-nutrient conditioning. Appetite. 1998;31:282. [Google Scholar]
- 44.Sclafani A, Fanizza LJ, Azzara AV. Conditioned flavor avoidance, preference and indifference produced by intragastric infusion of galactose, glucose, and fructose in rat s. Physiol Behav. 1999;67:227–34. doi: 10.1016/s0031-9384(99)00053-0. [DOI] [PubMed] [Google Scholar]
- 45.Sclafani A, Azzara A, Touzani K, Grigson PS, Norgren R. Parabrachial nucleus lesions block taste and attenuate flavor preference and aversion conditioning in rats. Behav Neurosci. 2001;115:920–33. doi: 10.1037/0735-7044.115.4.920. [DOI] [PubMed] [Google Scholar]
- 46.Sclafani A, Ackroff K. The relationship between food reward and satiation revisited. Physiol Behav. 2004;82:89–95. doi: 10.1016/j.physbeh.2004.04.045. [DOI] [PubMed] [Google Scholar]
- 47.Sclafani A, Glass D, Margolskee RF, Glendinning JI. Gut T1R3 sweet taste receptors do not mediate sucrose-conditioned flavor preferences in mice. Am J Physiol. 2010;299:R1643–R50. doi: 10.1152/ajpregu.00495.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simbayi LC, Boakes RA, Burton MJ. Can rats learn to associate a flavour with the delayed delivery of food? Appetite. 1986;7:41–53. doi: 10.1016/s0195-6663(86)80040-x. [DOI] [PubMed] [Google Scholar]
- 49.Slotnick BM, Westbrook F, Darling FMC. What the rat’s nose tells the rat’s mouth: long delay aversion conditioning with aqueous odors and potentiation of taste by odors. Anim Learn Behav. 1997;25:357–69. [Google Scholar]
- 50.Smith JC, Roll DL. Trace conditioning with X-rays as an aversive stimulus. Psychon Sci. 1967;9:11–2. [Google Scholar]
- 51.Toth P, Shaw C, Perlanski E, Grupp LA. Cholecystokinin octapeptide reduces ethanol intake in food- and water- sated rats. Pharmacol Biochem Behav. 1990;35:493–5. doi: 10.1016/0091-3057(90)90193-l. [DOI] [PubMed] [Google Scholar]
- 52.Touzani K, Sclafani A. Conditioned flavor preference and aversion: role of the lateral hypothalamus. Behav Neurosci. 2001;115:84–93. doi: 10.1037/0735-7044.115.1.84. [DOI] [PubMed] [Google Scholar]
- 53.Touzani K, Sclafani A. Area postrema lesions impair flavor-toxin aversion learning but not flavor-nutrient preference learning. Behav Neurosci. 2002;116:256–66. [PubMed] [Google Scholar]
- 54.Touzani K, Sclafani A. Lateral hypothalamic lesions impair flavour-nutrient and flavour-toxin trace learning in rats. Eur J Neurosci. 2002;16:2425–33. doi: 10.1046/j.1460-9568.2002.02404.x. [DOI] [PubMed] [Google Scholar]
- 55.Touzani K, Sclafani A. Critical role of amygdala in flavor but not taste preference learning in rats. Eur J Neurosci. 2005;22:1767–74. doi: 10.1111/j.1460-9568.2005.04360.x. [DOI] [PubMed] [Google Scholar]
- 56.Touzani K, Bodnar R, Sclafani A. Activation of dopamine D1-like receptors in nucleus accumbens is critical for the acquisition, but not the expression, of nutrient-conditioned flavor preferences in rats. Eur J Neurosci. 2008;27:1525–33. doi: 10.1111/j.1460-9568.2008.06127.x. [DOI] [PubMed] [Google Scholar]
- 57.Touzani K, Bodnar RJ, Sclafani A. Dopamine D1-like receptor antagonism in amygdala impairs the acquisition of glucose-conditioned flavor preference in rats. Eur J Neurosci. 2009;30:289–98. doi: 10.1111/j.1460-9568.2009.06829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Touzani K, Bodnar RJ, Sclafani A. Lateral hypothalamus dopamine D1-like receptors and glucose-conditioned flavor preferences in rats. Neurobiol Learn Mem. 2009;92:464–7. doi: 10.1016/j.nlm.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Touzani K, Bodnar RJ, Sclafani A. Acquisition of glucose-conditioned flavor preference requires the activation of dopamine D1-like receptors within the medial prefrontal cortex in rats. Neurobiol Learn Mem. 2010;94:214–9. doi: 10.1016/j.nlm.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zahorik DM. Learned changes in preferences for chemical stimuli: Asymmetrical effects of positive and negative consequences, and species differences in learning. In: Kroeze JHA, editor. Preference behaviour and chemoreception. London: Information Retrieval Ltd; 1979. pp. 233–45. [Google Scholar]


