Abstract
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease characterized by selective loss of motoneurons. Recently we studied glycine receptors (GlyRs) in motoneurons in an ALS mouse model expressing a mutant form of human superoxide dismutase-1 with a Gly93→Ala substitution (G93A-SOD1). Living motoneurons in dissociated spinal cord cultures were identified by using transgenic mice expressing eGFP driven by the Hb9 promoter. We showed that GlyR-mediated currents were reduced in large-sized (diameter >28 µm) Hb9-eGFP+ motoneurons from G93A-SOD1 embryonic mice. Here we analyze GlyR currents in a morphologically distinct subgroup of medium-sized (diameter 10–28 µm) Hb9-eGFP+ motoneurons, presumably gamma or slow-type alpha motoneurons. We find that glycine-induced current densities were not altered in medium-sized G93A-SOD1 motoneurons. No significant differences in glycinergic mIPSCs were observed between G93A-SOD1 and control medium-sized motoneurons. These results indicate that GlyR deficiency early in the disease process of ALS is specific for large alpha motoneurons.
Key words: Hb9-eGFP, mutant SOD1, motoneuron culture, patch clamp, mIPSC, gamma motoneuron, alpha motoneuron
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease characterized by the loss of motoneurons.1 The majority of the ALS cases are sporadic, but approximately 10% of ALS patients have a familial form of ALS (fALS). Approximately 20% of fALS patients have mutations in copper/zinc superoxide dismutase-1 (SOD1), an antioxidant enzyme.2 Transgenic mice carrying a mutant form of human SOD1 with a Gly93→Ala substitution (G93A-SOD1) have been generated and they develop phenotypes and histopathology similar to human ALS.3
The mechanisms underlying motoneuron dysfunction and degeneration in ALS have not been fully investigated. The long-held glutamatergic hyperexcitability-excitotoxicity theory emphasizes mostly the contribution of excessive synaptic excitation of motoneurons leading to their death.4–7 However, the possibility of insufficient synaptic inhibition has been largely ignored. Previously, we found that transgenic ALS mice develop an age-related loss of glycinergic innervation of motoneurons while GABAergic innervation is mostly spared.8 Recently we examined functionality of glycine receptors (GlyRs) and GABAA receptors (GABAARs) in spinal motoneurons from G93A-SOD1 transgenic mice.9 We developed a dissociated spinal cord culture model using embryonic double transgenic mice expressing enhanced green fluorescent protein (eGFP) driven by the Hb9 promoter and human mutant SOD1. We showed that Hb9-eGFP labels a subset of living neurons that had morphological characteristics of motoneurons in dissociated spinal cord cultures. We identified three subgroups of Hb9-eGFP+ neurons based on their morphology. We found that glycine-induced currents and glycinergic mIPSCs were reduced in large-sized (diameter >28 µm) Hb9-eGFP+ motoneurons prepared from G93A-SOD1 embryonic mice.9 However other Hb9-eGFP+ motoneurons were not examined electrophysiologically in the earlier study. Here we analyze GlyR currents in medium-sized (diameter of 10–28 µm) Hb9-eGFP+ motoneurons.
Results
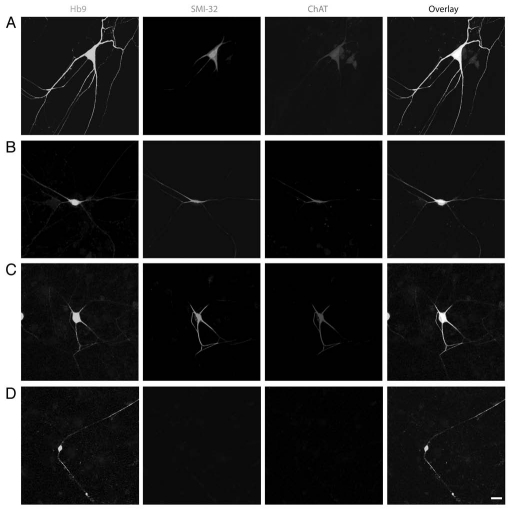
As described previously in reference 9, Hb9-eGFP+ neurons in dissociated spinal cord cultures are divided into three groups according to their somal sizes at 12–16 days in vitro (DIV): large(diameter >28 µm) (Fig. 1A), medium-(diameter 10–28 µm) (Fig. 1B and C) and small- (diameter <10 µm) (Fig. 1D) sized cells. We examined more closely the morphological classification of these cells. The average soma diameters of large-, medium- and small-sized Hb9-eGFP+ cells are 39.32 ± 1.61 µm (n = 41), 24.26 ± 1.52 µm (n = 45) and 9.54 ± 1.02 µm (n = 38), respectively. Compared to large-sized motoneurons, medium-sized Hb9-eGFP+ cells have a significantly smaller size of cell body. Cultured cells were double-stained with motoneuron markers SMI-32 (for neurofilament protein) and acetylcholine-synthesizing enzyme choline acetyltransferase (ChAT). The percentage of medium-sized Hb9-eGFP cells that were SMI-32/ChAT-positive was 42.7 ± 3.2% (n = 224), and these cells had characteristic morphologies that mimic the large-sized Hb9-eGFP+ motoneurons,9 but the dendritic trees of medium-sized Hb9-eGFP+ cells are less branched and are much simpler than those of large-sized motoneurons (Fig. 1). These morphological characteristics of medium-sized Hb9-eGFP+ cells suggest they could be gamma motoneurons or S (slow-twitch fatigue-resistant) type alpha motoneurons.10,11
Figure 1.
Three groups of Hb9-eGFP+ cells in dissociated mouse spinal cord cultures. Large-diameter >28 µm, (A), medium- [diameter 10–28 µm (B) and (C)], and small- [diameter <10 µm, (D)] sized Hb9-eGFP+ neurons in DIV 12–16 dissociated spinal cord cultures double-stained for SMI-32 (red) and ChAT (blue) are shown. Medium-sized eGFP+ cells (B and C) show simpler and less branched dendritic trees than those of large Hb9-eGFP+ motoneurons (A), but both populations are ChAT-positive. Small-sized Hb9-eGFP+ neurons (D) are ChAT-negative interneurons. Scale bar = 20 µm.
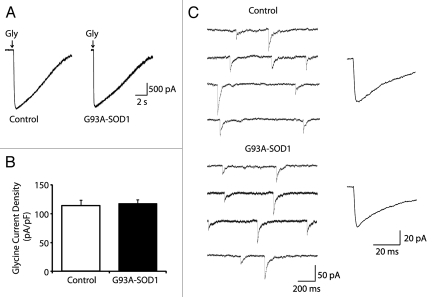
Whole-cell patch clamp recordings were performed in the presence of an antagonist mixture containing the voltage-dependent sodium channel blocker tetrodotoxin (TTX; 0.5 µM), the GABAAR antagonist bicuculline (5 µM) and non-NMDA and NMDA ionotropic glutamate receptor antagonists 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 5 µM) and DL-2-amino-5-phosphonovalerate (APV; 50 µM). Pressure ejection of 1 mM glycine (100 ms, 1–2 psi) onto cell bodies induced large inward currents in 17 of 18 medium-sized Hb9-eGFP+ motoneurons. The peak amplitude of glycine-induced currents in medium-sized Hb9-eGFP+ motoneurons was much smaller than those in large-sized Hb9-eGFP+ motoneurons (mean amplitude for large- and medium-sized cells were 5,129 ± 379 pA, n = 20 and 2,548 ± 151 pA, n = 17, respectively). However, no significant differences were observed in the current densities between large- and medium-sized motoneurons (mean current densities for large- and medium-sized cells were 121.2 ± 9.5 pA/pF, n = 20 and 115.4 ± 8.9 pA/pF, n = 17, respectively). As anticipated, membrane capacitance of medium-sized Hb9-eGFP+ motoneurons (21.7 ± 1.2, n = 17) is significantly smaller than that of large-sized Hb9-eGFP+ motoneurons (43.5 ± 2.4, n = 20). No significant differences were observed in glycine-induced current densities between control and G93A-SOD1 medium-sized Hb9-eGFP+ motoneurons (mean current densities for control and G93A-SOD1 cells were 114.1 ± 9.6 pA/pF, n = 11 and 117.9 ± 6.6 pA/pF, n = 10, respectively; Fig. 2A and B). No significant difference was observed in the rise and decay time of the glycine-evoked currents between control and G93A-SOD1 medium-sized Hb9-eGFP+ motoneurons (data not shown).
Figure 2.
Glycine receptor-mediated currents are unaltered in medium-sized G93A-SOD1 motoneurons. (A) Representative recordings of glycine-evoked currents in medium-sized control and G93A-SOD1 motoneurons. (B) No significant differences in glycine-induced current densities are observed between G93A-SOD1 (n = 10) and control motoneurons (n = 11). Data represent the mean ± SEM (Student's t test). (C) Representative traces and averaged glycinergic mIPSCs in medium-sized control and G93A-SOD1 motoneurons.
Glycinergic mIPSCs were detected in 17 of 18 Hb9-eGFP motoneurons in the presence of the antagonist mixture as described above. The mean amplitude of glycinergic mIPSCs in medium-sized Hb9-eGFP+ motoneurons (83.5 ± 6.8 pA, n = 17) was similar with that of glycinergic mIPSCs in large-sized Hb9-eGFP+ motoneurons (87.2 ± 5.1 pA, n = 20). The frequency and the rise and decay time of mIPSC events in medium-sized Hb9-eGFP+ motoneurons (frequency was 0.19 ± 0.03 Hz, rise time was 3.7 ± 0.3 ms, decay time was 23.5 ± 2.18 ms, n = 17) were similar to those of glycinergic mIPSCs in large-sized Hb9-eGFP+ motoneurons (frequency was 0.21 ± 0.03 Hz, rise time was 3.5 ± 0.4 ms, decay time was 25.5 ± 2.59 ms, n = 20). The amplitude of glycinergic mIPSCs in medium-sized Hb9-eGFP+ motoneurons was not different between G93A-SOD1 and control groups (Fig. 2C). The mean amplitudes of mIPSCs for control and G93A-SOD1 motoneurons were 83.3 ± 6.6 pA (n = 11) and 75.6 ± 7.2 pA (n = 10), respectively. No significant differences in the frequency and the rise and decay times of glycinergic mIPSC events in medium-sized Hb9-eGFP+ motoneurons were observed between control and G93ASOD1 groups (data not shown).
Discussion
We found that GlyR-mediated currents are differentially affected in specific subsets of motoneurons in a mouse model expressing human ALS-linked mutant SOD1. Large-sized alpha motoneurons have reduced GlyR currents,9 whereas medium-sized gamma or S type alpha motoneurons have normal GlyR currents.
Most motor pools are composed of a mixture of alpha and gamma motoneurons which innervate different types of muscle fibers.11 Alpha motoneurons predominate within motor pools and innervate the extrafusal skeletal muscle and drive muscle contraction.11 Gamma motoneurons constitute approximately one third of all motoneurons within a pool and innervate the intrafusal muscle fibers found in muscle spindles, where they modulate the sensitivity of muscle spindles to stretch.11 Gamma and alpha motoneurons also differ in morphology and connectivity profile within the spinal cord. Alpha motoneurons have large cell bodies and most receive direct monosynaptic group Ia-derived proprioceptive sensory input,12 whereas gamma motoneurons have cell bodies that are smaller compared to alpha motoneurons13 and lack direct input from proprioceptive sensory afferents.14 Alpha motoneurons can be further classified into subtypes according to the contractile properties of the motor units that they form with target muscle fibers: fast-twitch fatigable (FF), fast-twitch fatigue-resistant (FR) and slow-twitch fatigue resistant (S).15 The size of the cell bodies and the dendritic structure of the medium-sized Hb9-eGFP+ motoneurons from which we recorded suggest that they could be gamma or S alpha motoneurons. Another possibility is a less well-defined population called beta motoneuron that innervates both intra- and extrafusal fibers. Analyses of molecular markers and connectivity are required to further confirm the identity of this subset of motoneurons.
This study in combination with our earlier report in reference 9, demonstrates that large putative FF alpha motoneurons, but not gamma or S alpha motoneurons, in an ALS mouse model have deficient GlyR currents. This finding is not due to differences in human mutant SOD1 transgene expression in the different types of motoneurons because transgene is expressed robustly in all types of motoneurons in G93A-SOD1 mice.16,17 It remains to be determined if the abnormalities in GlyR currents in large alpha motoneurons in ALS mice reflect a primary disease process or a secondary change resulting from the disease. Henneman18,19 reported that the size or surface area of a motoneuron determines its excitability and the muscle units that they innervate. Our results are consistent with the hypothesis that implicates soma size and metabolic demands in the preferential susceptibility of motoneurons.20 Larger soma-sized motoneurons are more susceptible to excitability and inhibitability18 and more vulnerable to neurodegeneration in ALS.20 The selective vulnerability of large alpha motoneurons is consistent with the observations in human ALS studies21 and in ALS animal models22,23 showing that FF motor units decay early, while slow motor units tend to be spared in the disease. Our study shows specific subtypes of spinal motoneurons differing in their vulnerability to GlyR channel deficits, suggesting that differences among these neurons are critically important to disease progression.
Methods
Transgenic mice.
Transgenic mice expressing a human mutant SOD1 gene encoding the glycine/alanine substitution at codon 93 (G93A) driven by the human SOD1 promoter3 and B6.Cg-Tg (Hlxb9-gfp)1Tmj/j transgenic mice expressing eGFP driven by the mouse Hlxb9 (Hb9) promoter24 were originally obtained from Jackson Laboratories (Bar Harbor, Maine) and then housed in our animal facilities. The G93A-SOD1 transgenic mice with a high copy number of mutant allele (∼20 copies) and a rapid disease onset were used. The mice first show signs of spasticity at about 10 weeks of age, and then unilateral or bilateral hind limb paresis at around 11–12 weeks of age; the disease then progresses to end-stage when the mice are quadriplegic at around 16 weeks of age.3,17 The institutional Animal Care and Use Committee approved the animal protocols. Every effort was made to minimize the number of animals used and their suffering.
Cell culture.
To obtain embryos for spinal cord culture, G93A-SOD1 mice were mated with Hb9-eGFP mice. Breeder pairs were screened for the presence of the transgenes by PCR on tail DNA. On gestational day 12–14 (E12-14), female mice with potential double transgenic G93A-SOD1/Hb9-eGFP embryos and single transgenic Hb9-eGFP or G93A-SOD1 embryos were anesthetized with isoflurane and all embryos were harvested by caesarian section. Hb9-eGFP expression in embryos was confirmed under a fluorescence microscope. Primary cultures were obtained from total spinal cords of male and female Hb9-eGFP+ embryos. Each spinal cord was cultured individually and each embryo was genotyped by PCR for the human SOD1 gene using genomic DNA isolated from the body. Mixed spinal cord cultures were prepared and maintained as described in reference 9.
Immunocytochemistry.
Spinal cord cultures grown on glass coverslips were fixed with 4% paraformaldehyde/PBS for 20 min, washed with PBS, permeabilized with 0.2% Triton X-100, blocked with 10% donkey serum, and then incubated in a primary antibody mixture containing mouse anti-SMI-32 (monoclonal, 1:1,000; Covance, Princeton, NJ) and goat anti-ChAT (polyclonal, 1:200; Millipore) diluted in PBS containing 2% donkey serum and 0.05% Triton X-100 overnight at 4°C. After four washes with PBS, coverslips were incubated for 2 h at room temperature in a mixture of species-specific secondary antibodies (all raised in donkey) conjugated to Alexa Fluor 594 and Alexa Fluor 647 (Invitrogen). Coverslips were washed again and mounted using anti-fade mounting solution (Vectashield; Vector Laboratories, Burlingame, CA) and viewed using confocal microscopy as described in reference 9.
Electrophysiology.
The Hb9-eGFP+ neurons were identified under fluorescent microscope and then recorded under differential interference contrast (DIC) optics. Whole-cell patch clamp recordings were made with glass pipettes pulled on a P-97 electrode puller (Sutter Instruments, Novato, CA). The resistance of the pipettes was 2–4 MΩ when filled with an intracellular solution (in mM: 120 CsCl, 21 tetraethylammonium chloride, 2 MgCl2, 10 HEPES, 10 EGTA, 2 Mg-ATP, 0.3 NaGTP, 10 phosphocreatine, pH 7.25 adjusted with CsOH). Cells were super-fused at a rate of 2 ml/min with an external bath solution containing the following (in mM): 150 NaCl, 2.5 KCl, 10 HEPES, 10 D-glucose, 2 CaCl and 1 MgCl2, pH 7.3–7.4. Experiments were performed at room temperature (22–24°C) and in the presence of TTX (0.5 µM).
Currents from neurons were monitored with an Axopatch 200B amplifier (Molecular Devices, Palo Alto, CA), acquired through Digidata 1440A (Molecular Devices) onto a computer using pClamp 10 software (Molecular Devices). Peak amplitudes were measured using Mini Analysis software (Synaptosoft, Decatur, GA). To assess the differences between motoneurons independently of cell size, the glycine current recorded for each motoneuron was normalized with respect to the whole cell surface area derived from measurements of the membrane capacitance. At least four cultures were used for the measurements of each parameter given in the results, and four to six motoneurons were recorded from each culture. All recordings were made blinded of human SOD1 genotype.
Glycine was applied through a small tipped (∼1 µm) pipette that was moved under visual control to within ∼50 µm of the soma of the neuron under study. The glycine solution was pressure ejected (100 ms, 1∼2 psi) directly onto the cell body and proximal dendrites using a Picospritzer (General Valve, Fairfield, NJ). The pipette solution contained 1 mM glycine dissolved in extracellular solution as described above.
For the recording of glycinergic miniature postsynaptic currents (mIPSCs), 0.5 µM TTX, 5 µM CNQX, 50 µM APV and 5 µM bicuculline were added to the bath solution to block action potential, glutamatergic and GABAergic activities. Recordings were carried out for enough time to observe at least 100 events. Currents were analyzed using Mini Analysis software. mIPSCs were detected with a threshold amplitude of 5 pA (Mini Analysis) and verified by eye. Peak amplitude, rise time from 10 to 90% of the peak amplitude and decay time from peak to 37% of the peak amplitude were measured for each event.
All chemicals used for electrophysiological recordings were purchased from Sigma. All drugs and solutions were made fresh from drug stock solutions.
Acknowledgments
This work was supported by grants NIH-NS034100 and NIH-NS065895.
Abbreviations
- ALS
amyotrophic lateral sclerosis
- fALS
familial ALS
- GlyR
glycine receptor
- GABAR
GABA receptor
- SOD1
copper/zinc superoxide dismutase-1
- eGFP
enhanced green fluorescent protein
- DIV
days in vitro
- ChAT
choline acetyltransferase
- TTX
tetrodotoxin
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- APV
DL-2-amino-5-phosphonovalerate
- FF
fast-twitch fatigable
- FR
fast-twitch fatigue-resistant
- S
slow-twitch fatigue resistant
References
- 1.Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344:1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- 2.Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 3.Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 4.van Zundert B, Peuscher MH, Hynynen M, Chen A, Neve RL, Brown R, Jr, et al. Neonatal neuronal circuitry shows hyperexcitable disturbance in a mouse model of the adult-onset neurodegenerative disease amyotrophic lateral sclerosis. J Neurosci. 2008;28:10864–10874. doi: 10.1523/JNEUROSCI.1340-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heath PR, Shaw PJ. Update on the glutamatergic neurotransmitter system and the role of excitotoxicity in amyotrophic lateral sclerosis. Muscle Nerve. 2002;26:438–458. doi: 10.1002/mus.10186. [DOI] [PubMed] [Google Scholar]
- 6.Pieri M, Albo F, Gaetti C, Spalloni A, Bengtson CP, Longone P, et al. Altered excitability of motor neurons in a transgenic mouse model of familial amyotrophic lateral sclerosis. Neurosci Lett. 2003;351:153–156. doi: 10.1016/j.neulet.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Pieri M, Gaetti C, Spalloni A, Cavalcanti S, Mercuri N, Bernardi G, et al. alpha-Amino-3-hydroxy-5-methylisoxazole-4-propionate receptors in spinal cord motor neurons are altered in transgenic mice overexpressing human Cu,Zn superoxide dismutase (Gly93→Ala) mutation. Neuroscience. 2003;122:47–58. doi: 10.1016/j.neuroscience.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Chang Q, Martin LJ. Glycinergic innervation of motoneurons is deficient in amyotrophic lateral sclerosis mice: a quantitative confocal analysis. Am J Pathol. 2009;174:574–585. doi: 10.2353/ajpath.2009.080557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang Q, Martin LJ. Glycine receptor channels in spinal motoneurons are abnormal in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurosci. 2011;31:2815–2827. doi: 10.1523/JNEUROSCI.2475-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westbury DR. A comparison of the structures of alpha and gamma-spinal motoneurones of the cat. J Physiol. 1982;325:79–91. doi: 10.1113/jphysiol.1982.sp014137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanning KC, Kaplan A, Henderson CE. Motor neuron diversity in development and disease. Annu Rev Neurosci. 33:409–440. doi: 10.1146/annurev.neuro.051508.135722. [DOI] [PubMed] [Google Scholar]
- 12.Eccles JC, Eccles RM, Lundberg A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957;137:22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burke RE, Strick PL, Kanda K, Kim CC, Walmsley B. Anatomy of medial gastrocnemius and soleus motor nuclei in cat spinal cord. J Neurophysiol. 1977;40:667–680. doi: 10.1152/jn.1977.40.3.667. [DOI] [PubMed] [Google Scholar]
- 14.Eccles JC, Eccles RM, Iggo A, Lundberg A. Electrophysiological studies on gamma motoneurones. Acta Physiol Scand. 1960;50:32–40. doi: 10.1111/j.1748-1716.1960.tb02070.x. [DOI] [PubMed] [Google Scholar]
- 15.Burke RE, Levine DN, Tsairis P, Zajac FE., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973;234:723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong M, Martin LJ. Skeletal muscle-restricted expression of human SOD1 causes motor neuron degeneration in transgenic mice. Hum Mol Genet. 2010;19:2284–2302. doi: 10.1093/hmg/ddq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin LJ, Liu Z, Chen K, Price AC, Pan Y, Swaby JA, Golden WC. Motor neuron degeneration in amyotrophic lateral sclerosis mutant superoxide dismutase-1 transgenic mice: mechanisms of mitochondriopathy and cell death. J Comp Neurol. 2007;500:20–46. doi: 10.1002/cne.21160. [DOI] [PubMed] [Google Scholar]
- 18.Henneman E, Somjen G, Carpenter DO. Excitability and inhibitability of motoneurons of different sizes. J Neurophysiol. 1965;28:599–620. doi: 10.1152/jn.1965.28.3.599. [DOI] [PubMed] [Google Scholar]
- 19.Henneman E, Mendell LM. Functional organization of the motoneurone pool and its inputs. In: Brooks V, editor. Handbook of Physiology, Vol.II. The Nervous System. Bethesda, MD: American Physiological Society; 1981. pp. 345–442. [Google Scholar]
- 20.Shaw PJ, Eggett CJ. Molecular factors underlying selective vulnerability of motor neurons to neurodegeneration in amyotrophic lateral sclerosis. J Neurol. 2000;247:17–27. doi: 10.1007/BF03161151. [DOI] [PubMed] [Google Scholar]
- 21.Dengler R, Konstanzer A, Kuther G, Hesse S, Wolf W, Struppler A. Amyotrophic lateral sclerosis: macro-EMG and twitch forces of single motor units. Muscle Nerve. 1990;13:545–550. doi: 10.1002/mus.880130612. [DOI] [PubMed] [Google Scholar]
- 22.Pun S, Santos AF, Saxena S, Xu L, Caroni P. Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat Neurosci. 2006;9:408–419. doi: 10.1038/nn1653. [DOI] [PubMed] [Google Scholar]
- 23.Hegedus J, Putman CT, Gordon T. Time course of preferential motor unit loss in the SOD1 G93A mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2007;28:154–164. doi: 10.1016/j.nbd.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]