Abstract
Interactions between calmodulin (CaM) and voltage-gated calcium channels (Cavs) are crucial for Cav activity-dependent feedback modulation. We recently reported an X-ray structure that shows two Ca2+/CaM molecules bound to the Cav1.2 C-terminal tail, one at the PreIQ region and one at the IQ domain. Surprisingly, the asymmetric unit of the crystal showed a dimer in which Ca2+/CaM bridged two PreIQ helixes to form a 4:2 Ca2+/CaM:Cav C-terminal tail assembly. Contrary to previous proposals based on a similar crystallographic dimer, extensive biochemical analysis together with subunit counting experiments of full-length channels in live cell membranes failed to find evidence for multimers that would be compatible with the 4:2 crossbridged complex. Here, we examine this possibility further. We find that CaM overexpression has no functional effect on Cav1.2 inactivation or on the stoichiometry of full-length Cav1.2. These data provide further support for the monomeric Cav1.2 stoichiometry. Analysis of the electrostatic surfaces of the 2:1 Ca2+/CaM:Cav C-terminal tail assembly reveals notable patches of electronegativity. These could influence various forms of channel modulation by interacting with positively charged elements from other intracellular channel domains.
Key words: voltage-gated calcium channel, Cav1.2, calmodulin, calcium-dependent inactivation (CDI), stoichiometry
Introduction
High-voltage activated voltage-gated calcium channels (Cav1s and Ca v2s) couple membrane depolarization dependent calcium entry to many physiological processes.1,2 These channels are multi-subunit complexes that include a Cav α1 pore-forming subunit, the auxiliary proteins Cavβ, Cavα2δ and calmodulin (CaM).1,3,4 Calcium-calmodulin (Ca2+/CaM) has a central role in two distinct Cav feedback mechanisms, calcium-dependent inactivation (CDI) and calcium-dependent facilitation (CDF),5 that involve direct interactions with the Cavα1 C-terminal cytoplasmic tail IQ domain.6–12 Understanding the molecular basis for CaM-Cav interactions and how these interactions affect channel function remains an important challenge that requires structural definition and biochemical analysis of key regulatory CaM binding sites.
Results and Discussion
We recently reported the structure of a complex of Ca2+/CaM and a segment of the Cav1.2 C-terminal tail that encompasses the PreIQ and IQ domains (residues 1,561–1,637).8 Ca2+/CaM has two lobes, N-lobe and C-lobe, which can each engage target-binding partners. Consequently, a number of diverse Ca2+/CaM-target binding modes have been reported.13,14 The Ca2+/CaM:Cav1.2 C-terminal tail complex defines two Ca2+/CaM binding sites that are occupied by two separate Ca2+/CaMs. Each interacts with the channel in distinct ways (Fig. 1A). One Ca2+/CaM engages the IQ domain using both lobes in a manner identical to prior structures of Ca2+/CaM-Cav1.2 IQ domain complex.12,15 In contrast, the second Ca2+/CaM binds the C-terminal segment of the long PreIQ helix using only Ca2+/C-lobe. Notably, the association of CaM with the PreIQ-IQ segment creates a number of electronegative patches (Fig. 1B and C). These patches raise the possibility that positively charged residues elsewhere in the channel form key interactions with this segment.
Figure 1.
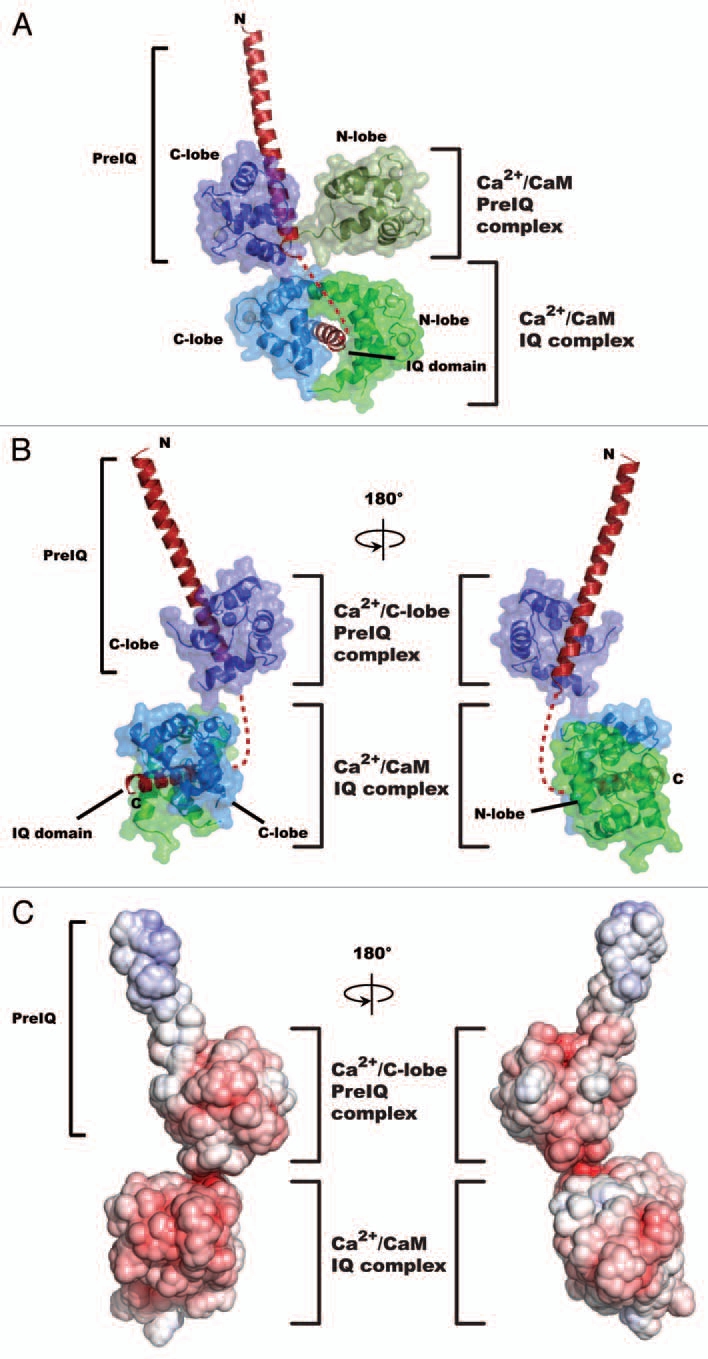
Structure of the 2:1 Ca2+/CaM:Cav1.2 C-terminal tail complex. (A) Cartoon diagram of the Ca2+/CaM:Cav1.2 C-terminal tail complex. The Cav1.2 C-terminal tail is shown in red. Ca2+/CaM molecules bound to the PreIQ and domains are indicated. In both cases Ca2+/CaM is shown using a surface representation and the Ca2+/N-lobes and Ca2+/C-lobes of the PreIQ bound and IQ bound Ca2+/CaMs are colored forest and dark blue, and green and marine, respectively. (B) Cartoon diagram as in ‘(A)’ but lacking the Ca2+/N-lobe from the PreIQ Ca2+/CaM. Colors as in (A). (C) Surface charge representation of the 2:1 Ca2+/CaM:Cav C-terminal tail shown in the same orientation as ‘(B)’. Colors indicate a scale from −10 kT/e (red) to + 10kT/e (blue).
Biochemical studies revealed that the two Ca2+/CaMs have very different properties. The Ca2+/CaM molecule bound to the PreIQ segment is labile and could be removed by passage over a phenylsepharose column in conditions of high-calcium (2 mM). In contrast, the Ca2+/CaM on the IQ domain remained bound. Further, functional studies indicated that the PreIQ site has a role in calcium-dependent facilitation (CDF).8 Extensive biophysical characterization showed that the complex maintains a 2:1 Ca2+/CaM:C-terminal tail stoichiometry even at protein concentrations that match those found in the most densely packed native Cav environment (≥100 µM). Together, these studies demonstrate that the Cav1.2 C-terminal tail can bind two Ca2+/CaMs simultaneously and suggest that the Ca2+/CaM occupying the PreIQ binding site is poised to make interactions with other elements of the channel by virtue of the free Ca2+/N-lobe.
A curious feature of the asymmetric unit of the Ca2+/CaM:PreIQ-IQ complex was that it contained two Ca2+/CaM:PreIQ-IQ complexes that interacted through a portion of the PreIQ domain and was bridged by the PreIQ-Ca2+/CaMs. A similar X-ray structure led to the proposal that Cav1.2 channels dimerize via this sort of interaction and that this interaction is functionally relevant.16 It is not straightforward to discern the relevance of protein-protein interactions from crystal lattice contacts alone as such interactions are a necessary requirement for forming a crystal lattice.17,18 Further, there are numerous examples in which crystallographic dimers do not represent biologically relevant interactions.19
Our biophysical data on the isolated Cav1.2 channel tail complex clearly indicated a 2:1 stoichiometry. Although it has not been generally thought that Cav channels function as dimers, electron microscopy studies of full-length Cav1.2 20,21 and the observation of infrequent multiple concerted Cav1.2 openings22 have suggested that under some conditions the full-length channel may form higher order assemblies. To address the question of Cav1.2 oligomerization state in live cell membranes directly, we used total internal reflection fluorescence microscopy (TIRFM) 23 and Cav1.2 bearing a C-terminal green fluorescent protein (GFP) tag for single-molecule subunit counting. Our studies showed unambiguously that the Cav1.2 channels are monomeric.8 Importantly, the Navedo et al. study showed that deletion of a large section of the C-terminal tail (D1670X), which removes all of the C-terminal tail after the IQ domain, eliminates all measurable multichannel coupling. Thus, unlike what has been proposed by Fallon et al. the Cav1.2 4:2 complex observed in the crystal structure is non-physiological and is not relevant for channel function. Further, whatever coupling mechanism underlies the observations of Navedo et al. it does not involve the PreIQ-IQ domain.
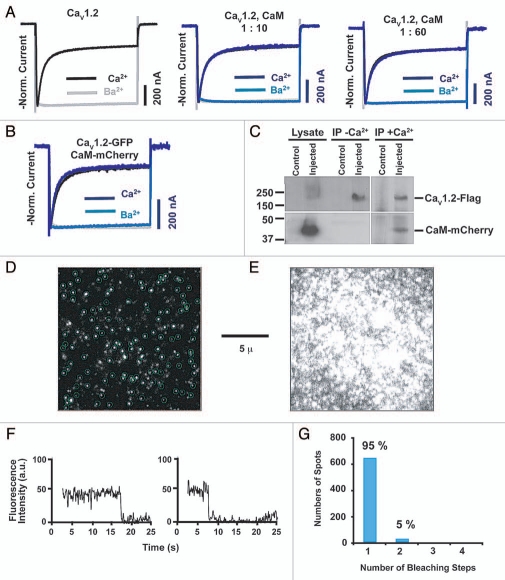
A key assumption of both our functional and subunit counting experiments is that the Xenopus oocytes that are used to overexpress Cav1.2 have sufficient endogenous CaM to make functional channels. Previous studies have shown that multiple alanine mutations on the channel that weaken CaM affinity for the IQ domain and reduce CDI can be overcome, at least in part, by CaM co-expression.24,25 Because the PreIQ-bound CaM is responsible for crossbridging interactions in the asymmetric unit and is labile, we tested whether CaM overexpression would affect Cav1.2 function or oligomerization state. Injection of Xenopus oocytes with fixed amounts of Cav1.2 mRNA and either a 10-fold or 60-fold excess of CaM mRNA gave Cav1.2 channels in which both CDI and a second form of inactivation, voltage-dependent inactivation (VDI), were indistinguishable from Cav1.2 relying on endogenous CaM (Fig. 2A and Table 1).
Figure 2.
Effect of CaM overexpression on Cav1.2 function and stoichiometry. (A) Comparison of Cav1.2 CDI and VDI as a function of CaM overexpression. Normalized Ca2+ and Ba2+ currents for channels relying on endogenous CaM (black and gray, respectively) or with excess CaM mRN A at the indicated ratios (dark blue and light blue, respectively). (B) Normalized Ca2+ and Ba2+ currents recorded from Cav1.2-GFP co-expressed with CaM? mCherry at a mRNA ratio of 1:10 (dark blue and light blue, respectively) are compared to wild type (black and gray, respectively). Experimental details are the same as in reference 8. (C) Western blot detection of whole-cell lyates and immunoprecipitation of Flag-tagged Cav1.2 from Xenopus oocytes. Detection was with an antibody against Flag or mCherry as indicated. (D) Subunit counting of Cav1.2 in live cell membranes. A representative TIRF image showing the Cav1.2-GFP fluorescent spots at the cell surface of X. laevis oocytes. Bright spots are Cav1.2-GFP single-molecules when the shutter is first opened at the beginning of the bleaching experiment. Blue circles mark the selected molecules for subunit counting. (E) TIRF image showing CaM-mCherry spots in the cell surface of X. laevis oocytes. (F) Time courses of photobleaching from single GFP fluorescent spots. Two examples are shown. (G) Distribution of fluorescent spots that bleach in one or more steps. Percentile of each bleaching step is indicated.
Table 1.
Cav1.2 inactivation parameters
| ti300 (%) | A1 (%) | τ1 (ms-1) | A2 (%) | τ2 (ms-1) | N | |
| Cav1.2 | 69.0 ± 2.2 | 55.8 ± 3.2 | 25.0 ± 3.7 | 16.4 ± 1.9 | 138 ± 21 | 12 |
| Cav1.2, CaM 1:10 | 67.7 ± 3.5 | 53.9 ± 7.3 | 26.7 ± 4.2 | 17.5 ± 5.9 | 136 ± 32 | 8 |
| Cav1.2, CaM 1:60 | 69.0 ± 2.4 | 57.2 ± 1.7 | 25.0 ± 4.8 | 15.9 ± 3.3 | 142 ± 37 | 6 |
| Cav1.2-GFP, CaM-Cherry 1:10 | 71.4 ± 7.3 | 55.2 ± 6.5 | 25.3 ± 5.3 | 20.5 ± 4.6 | 142 ± 29 | 8 |
All experiments correspond to the mean of at least two separate oocyte batches. ‘±’ values are standard deviation.
To examine whether the overexpressed CaM associated with Cav1.2, we coinjected mRNA for Flag-tagged Cav1.2 and mCherry-labeled CaM at a ratio of 1:10. This combination has identical functional properties to the untagged components (Fig. 2B and Table 1). Both components are clearly overexpressed (Fig. 2C). Immunoprecipitation using anti-Flag M2 agarose to capture the Cav1.2 subunit did not detect any interaction between Cav1.2 and CaM in the absence of Ca2+ (Fig. 2C). Importantly, similar to prior reports with untagged subunits,26 in the presence 500 µM Ca2+ the CaM-mCherry-Cav1.2 interaction was readily observed and directly demonstrated that overexpressed CaM binds the channel (Fig. 2C). These data, together with the absence of functional effects of CaM overexpression, strongly suggest that the endogenous levels of CaM present in Xenopus oocytes are sufficient to account for all Cav1.2 functional effects.
We also examined by single molecule subunit counting whether excess CaM expression influenced Cav1.2 oligomerization (Fig. 2D–G). TIRF images indicated that CaM-mCherry was present at the membrane. Examination of spots in which both Cav1.2-GFP and CaM-mCherry were present showed that the vast majority (95%) of Cav1.2-GFP fluorescent spots bleached in a single step (Fig. 2F and G). These data provide strong indication that the channels remain monomers even when excess CaM is present at the membrane and further support our interpretation that the structure of CaM bridging two PreIQ helices is not relevant for full-length channels.
Our studies demonstrate that the Cav1.2 PreIQ-IQ segment is capable of binding multiple Ca2+/CaMs simultaneously and that the PreIQ site is involved in channel function.8 The organization of multiple Ca2+/CaMs along a largely helical segment is reminiscent of how CaM and CaM-like proteins are organized on myosin27 and raises the intriguing possibility that there are previously unrecognized connections between these systems. How the Ca2+/CaM:Cav1.2 C-terminal tail complex interacts with other channel elements in both the calcium-bound and calcium-free states remains a major open question. The presence of large patches of electronegative surface on the Ca2+/CaM:Cav1.2 C-terminal tail complex raises the possibility that there may be positively charged residues elsewhere in the channel that form key interactions with this segment. Defining such interactions and their state-dependence remains an important goal for the field.
Methods
Two-electrode voltage clamp recordings.
Homo sapiens CaM (GenBank NM_006888) was subcloned into pGEMHE-mCherry plasmid28 using SacII/HindIII restriction sites. All other DNA constructs were the same as in reference 8. Generation of mRNA and electrophysiological protocols were as described previously in references 8 and 12, except where noted. In brief, Xenopus oocytes were injected with 50 nl RNA mixture of Cav1.2 (or Cav1.2-mEGFP), Cavβ2a, Cavα2δ-1 and CaM (or CaM-mCherry) at a molar ratio of 1:1:1:10 unless indicated otherwise and recorded 2–4 days after injection. All data were produced from more than one oocyte batch and analyzed with Clampfit 9.2 (Axon Instruments).
Western blot detection of whole-cell lyates and immunoprecipitation of flag-tagged Cav1.2 from Xenopus oocytes.
In each case, nineteen oocytes were injected with Cav1.2-3xFlag, Cavβ2a, Cavα2δ and CaM-mCherry at a molar ratio of 1:1:1:10 or water. After two days, oocytes were collected and homogenized in immunoprecipitation (IP) buffer 150 mM NaCl, 0.5% CHAPS, 50 mM Hepes/NaOH pH 7.4 and Complete protease inhibitors (Roche), with or without 500 µM CaCl2 (IP + Ca2+ or IP-Ca2+ respectively). Lysates were cleared by centrifugation at 13,000 rpm for 30 min and 600 µg of protein was incubated with 35 µl anti-Flag M2 agarose beads (Sigma-Aldrich) overnight at 4°C. Beads were washed four times with IP buffer and bound proteins were released by heating at 70°C for 10 min in NuPAGE sample buffer (Invitrogen). Samples were separated on a 4–12% NuPAGE Bis-Tris gel, transferred on nitrocellulose and reacted with either anti-FLAG monoclonal antibody (1:5,000, Sigma-Aldrich) or Living Colors DsRed polyclonal antibody (1:1,000, Clontech).
Subunit counting experiments.
DNA constructs for Cav1.2-mEGFP and CaM-mCherry were the same as described above and in reference 8. RNA transcription and experimental details are as described in reference 8, except where noted below. Oocytes were injected with 50 nl of a 1:1 mixture of 500 ng/µl Cav1.2-EGFP and 100 ng/µl Cavβ2a cRNA and 150 ng/µl CaM-mCherry cRNA.
References
- 1.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 2.Hille B. Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer Associates, Inc.; 2001. [Google Scholar]
- 3.Findeisen F, Minor D., Jr Progress in the structural understanding of voltage-gated calcium channel (Cav) function and modulation. Channels (Austin) 2010;4:459–474. doi: 10.4161/chan.4.6.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Petegem F, Minor DL. The structural biology of voltage-gated calcium channel function and regulation. Biochem Soc Trans. 2006;34:887–893. doi: 10.1042/BST0340887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunlap K. Calcium channels are models of self-control. J Gen Physiol. 2007;129:379–383. doi: 10.1085/jgp.200709786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeMaria CD, Soong TW, Alseikhan BA, Alvania RS, Yue DT. Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type Ca2+ channels. Nature. 2001;411:484–489. doi: 10.1038/35078091. [DOI] [PubMed] [Google Scholar]
- 7.Kim EY, Rumpf CH, Fujiwara Y, Cooley ES, Van Petegem F, Minor D., Jr Structures of Cav2 Ca2+/CaM-IQ domain complexes reveal binding modes that underlie calcium-dependent inactivation and facilitation. Structure. 2008;16:1455–1467. doi: 10.1016/j.str.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim EY, Rumpf CH, Van Petegem F, Arant RJ, Findeisen F, Cooley ES, et al. Multiple C-terminal tail Ca2+/CaMs regulate Cav1.2 function but do not mediate channel dimerization. EMBO J. 2010;29:3924–3938. doi: 10.1038/emboj.2010.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee A, Zhou H, Scheuer T, Catterall WA. Molecular determinants of Ca2+/calmodulin-dependent regulation of Cav2.1 channels. Proc Natl Acad Sci USA. 2003;12:12. doi: 10.1073/pnas.2237000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mori MX, Vander Kooi CW, Leahy DJ, Yue DT. Crystal structure of the Cav2 IQ domain in complex with Ca2+/calmodulin: high-resolution mechanistic implications for channel regulation by Ca2+ Structure. 2008;16:607–620. doi: 10.1016/j.str.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peterson BZ, DeMaria CD, Adelman JP, Yue DT. Calmodulin is the Ca2+ sensor for Ca2+-dependent inactivation of L-type calcium channels. Neuron. 1999;22:549–558. doi: 10.1016/s0896-6273(00)80709-6. [DOI] [PubMed] [Google Scholar]
- 12.Van Petegem F, Chatelain FC, Minor D., Jr Insights into voltage-gated calcium channel regulation from the structure of the Cav1.2 IQ domain-Ca2+/calmodulin complex. Nat Struct Mol Biol. 2005;12:1108–1115. doi: 10.1038/nsmb1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoeflich KP, Ikura M. Calmodulin in action: diversity in target recognition and activation mechanisms. Cell. 2002;108:739–742. doi: 10.1016/s0092-8674(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 14.Ikura M, Ames JB. Genetic polymorphism and protein conformational plasticity in the calmodulin superfamily: two ways to promote multifunctionality. Proc Natl Acad Sci USA. 2006;103:1159–1164. doi: 10.1073/pnas.0508640103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fallon JL, Halling DB, Hamilton SL, Quiocho FA. Structure of calmodulin bound to the hydrophobic IQ domain of the cardiac Cav1.2 calcium channel. Structure. 2005;13:1881–1886. doi: 10.1016/j.str.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 16.Fallon JL, Baker MR, Xiong L, Loy RE, Yang G, Dirksen RT, et al. Crystal structure of dimeric cardiac L-type calcium channel regulatory domains bridged by Ca2+* calmodulins. Proc Natl Acad Sci USA. 2009;106:5135–51940. doi: 10.1073/pnas.0807487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janin J, Bahadur RP, Chakrabarti P. Protein-protein interaction and quaternary structure. Q Rev Biophys. 2008;41:133–180. doi: 10.1017/S0033583508004708. [DOI] [PubMed] [Google Scholar]
- 18.Ponstingl H, Henrick K, Thornton JM. Discriminating between homodimeric and monomeric proteins in the crystalline state. Proteins. 2000;41:47–57. doi: 10.1002/1097-0134(20001001)41:1<47::aid-prot80>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- 19.Bahadur RP, Chakrabarti P, Rodier F, Janin J. A dissection of specific and non-specific protein-protein interfaces. J Mol Biol. 2004;336:943–955. doi: 10.1016/j.jmb.2003.12.073. [DOI] [PubMed] [Google Scholar]
- 20.Wang MC, Collins RF, Ford RC, Berrow NS, Dolphin AC, Kitmitto A. The three-dimensional structure of the cardiac L-type voltage-gated calcium channel: comparison with the skeletal muscle form reveals a common architectural motif. J Biol Chem. 2004;279:7159–7168. doi: 10.1074/jbc.M308057200. [DOI] [PubMed] [Google Scholar]
- 21.Wang MC, Velarde G, Ford RC, Berrow NS, Dolphin AC, Kitmitto A. 3D structure of the skeletal muscle dihydropyridine receptor. J Mol Biol. 2002;323:85–98. doi: 10.1016/s0022-2836(02)00890-2. [DOI] [PubMed] [Google Scholar]
- 22.Navedo MF, Cheng EP, Yuan C, Votaw S, Molkentin JD, Scott JD, et al. Increased coupled gating of L-type Ca2+ channels during hypertension and Timothy syndrome. Circ Res. 2010;106:748–756. doi: 10.1161/CIRCRESAHA.109.213363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ulbrich MH, Isacoff EY. Subunit counting in membrane-bound proteins. Nat Methods. 2007;4:319–321. doi: 10.1038/NMETH1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang H, DeMaria CD, Erickson MG, Mori MX, Alseikhan BA, Yue DT. Unified mechanisms of Ca2+ regulation across the Ca2+ channel family. Neuron. 2003;39:951–960. doi: 10.1016/s0896-6273(03)00560-9. [DOI] [PubMed] [Google Scholar]
- 25.Erickson MG, Liang H, Mori MX, Yue DT. FRET two-hybrid mapping reveals function and location of L-type Ca2+ channel CaM preassociation. Neuron. 2003;39:97–107. doi: 10.1016/s0896-6273(03)00395-7. [DOI] [PubMed] [Google Scholar]
- 26.Wang HG, George MS, Kim J, Wang C, Pitt GS. Ca2+/calmodulin regulates trafficking of Cav1.2 Ca2+ channels in cultured hippocampal neurons. J Neurosci. 2007;27:9086–9093. doi: 10.1523/JNEUROSCI.1720-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houdusse A, Gaucher JF, Krementsova E, Mui S, Trybus KM, Cohen C. Crystal structure of apocalmodulin bound to the first two IQ motifs of myosin V reveals essential recognition features. Proc Natl Acad Sci USA. 2006;103:19326–19331. doi: 10.1073/pnas.0609436103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu Y, Ulbrich MH, Li MH, Buraei Z, Chen XZ, Ong AC, et al. Structural and molecular basis of the assembly of the TRPP2/PKD1 complex. Proc Natl Acad Sci USA. 2009;106:11558–11563. doi: 10.1073/pnas.0903684106. [DOI] [PMC free article] [PubMed] [Google Scholar]