Abstract
The R- and S-enantiomers of racemic 3,4-methylenedioxymethamphetamine (MDMA) exhibit different dose-concentration curves. In plasma, S-MDMA was eliminated at a higher rate, most likely due to stereoselective metabolism. Similar data were shown in various in vitro experiments. The aim of the present study was the in vivo investigation of stereoselective elimination of MDMA's phase I and phase II metabolites in human urine following controlled oral MDMA administration. Urine samples from 10 participants receiving 1.0 and 1.6 mg/kg MDMA separated by at least one week were analyzed blind by liquid chromatography-high resolution-mass spectrometry and gas chromatography-mass spectrometry after chiral derivatization with S-heptafluorobutyrylprolyl chloride. R/S ratios at Cmax were comparable after low and high doses with ratios >1 for MDMA, free DHMA, and HMMA sulfate, and with ratios <1 for MDA, free HMMA, DHMA sulfate and HMMA glucuronide. In the five days after the high MDMA dose, a median of 21% of all evaluated compounds were excreted as R-stereoisomers and 17% as S-stereoisomers. Significantly greater MDMA, DHMA, and HMMA sulfate R-enantiomers and HMMA and HMMA glucuronide S-stereoisomers were excreted. No significant differences were observed for MDA and DHMA sulfate stereoisomers. Changes in R/S ratios could be observed over time for all analytes, with steady increases in the first 48 h. R/S ratios could help to roughly estimate time of MDMA ingestion and therefore, improve interpretation of MDMA and metabolite urinary concentrations in clinical and forensic toxicology.
Keywords: MDMA, phase I metabolites, phase II metabolites, urine, chiral, controlled administration
1. Introduction
3,4-Methylenedioxymethamphetamine (MDMA) or Ecstasy, is a recreational drug of abuse popular among young people. Recently, the Substance Abuse and Mental Health Services Administration reported increasing MDMA consumption in the United States [1]. MDMA is usually consumed as a 1:1 racemate of R- and S-enantiomers. The S-enantiomer is more potent than the R-enantiomer in producing euphoria, energy and a desire to socialize [2,3]. MDMA also can induce severe acute toxic symptoms, such as tachycardia, hypertension, hyperthermia, and hepatotoxicity [3], severe and fatal intoxications also have been described [3]. Concerning chronic toxicity, animal data suggest that MDMA causes irreversible damage to serotonergic nerve terminals in the central nervous system [3-6]. In humans, chronic MDMA toxicity is still controversial, as some recent publications suggest that animal doses may be too high compared to human pharmacokinetics [7,8], while others indicate that humans are susceptible to brain serotonin neurotoxicity [9]. Admittedly, direct MDMA injection into rat brain failed to reproduce neurotoxic effects seen after systemic administration [10]. Furthermore, alteration of cytochrome P450 (CYP)-mediated MDMA metabolism influenced MDMA-induced neurotoxicity [10,11]. Therefore, MDMA metabolism may be an important contributor to neurotoxicity [12-15].
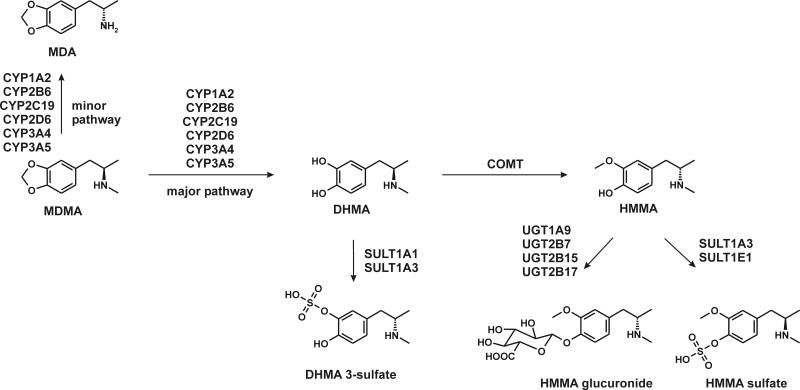
In vivo and in vitro MDMA studies revealed two main metabolic pathways. One includes multiple CYP enzyme-catalyzed O-demethylenation of MDMA to 3,4-dihydroxymeth-amphetamine (DHMA), followed by catechol-O-methyltransferase (COMT)-catalyzed O-methylation, primarily to 4-hydroxy-3-methoxymethamphetamine (HMMA). DHMA and HMMA also may be conjugated by uridine diphosphate glucuronyltransferases (UGT) to DHMA 3-glucuronide, DHMA 4-glucuronide, and HMMA glucuronide, or by sulfotransferases (SULT) to DHMA 3-sulfate, DHMA 4-sulfate, and HMMA sulfate. A minor pathway includes demethylation to 3,4-methylendioxyamphetamine (MDA) followed by demethylenation to 3,4-dihydroxyamphetamine (DHA), O-methylation to 4-hydroxy-3-methoxyamphetamine (HMA), and conjugation [5,16-21].
During the first 48 h after racemic MDMA ingestion, the S-enantiomer was eliminated from plasma at a faster rate than the R-enantiomer [2,3,22-25]. Enantioselective metabolism is the most likely explanation for different MDMA enantiomer pharmacokinetics in humans. In vitro experiments mediated by CYP, COMT, SULT and UGT enzymes documented preferential metabolism of S-enantiomers [19,26-28].
Enantioselective metabolism and elimination data in human blood or urine following controlled MDMA administration are available [2,24,25]; however, in all of these, DHMA, HMMA, and/or HMA data were obtained after conjugate cleavage either by acidic or enzymatic hydrolysis (Helix pomatia). There are no data available following direct measurement of intact glucuronides and sulfates that also might show different enantiomeric ratios. The aim of the present study was to investigate in vivo excretion of chiral MDMA phase I and phase II metabolites in human urine following controlled oral MDMA administration.
2. Materials and Methods
2.1 Chemicals and reagents
Racemic MDA, HMA, DHA, MDMA, HMMA, and DHMA hydrochlorides were obtained from Lipomed (Bad Saeckingen, Germany). Methanolic 1 mg/mL MDA-d5 and MDMA-d5 were purchased from LGC Promochem (Wesel, Germany), 4-hydroxymethamphetamine (pholedrine), 3,4-dihydroxybenzylamine (DHBA), and morphine 6-glucuronide (M6G), hexamethyldisilazane (HMDS) and sulfatases (EC no. 3.1.6.1) from Aerobacter aerogenes were from Sigma Aldrich (Steinheim, Germany). R/S-DHMA sulfates, R/S-HMMA sulfate, and single diastereomers of HMMA glucuronides that are not commercially available were synthesized in the author's laboratory [19,20], as well as the derivatization reagent S-heptafluorobutylprolyl chloride (S-HFBPrCl) [29]. Isolute Confirm C18 cartridges (500 mg, 3 mL) were obtained from Biotage (Grenzach-Wyhlen, Germany). Water was purified with a Millipore filtration unit and acetonitrile of HPLC grade was obtained from VWR Prolabo (Darmstadt, Germany). All other chemicals (analytical grade) were from Merck (Darmstadt, Germany).
2.2 Controlled MDMA Administration
The study design, study population, drug administration, and sample collection were described elsewhere [30]. Briefly, participants were administered placebo, 1.0 mg/kg (low), and 1.6 mg/kg (high) MDMA at least one week apart in this double blind, randomized, placebo-controlled study. Urine specimens from 10 participants were collected in Institutional Review Board-approved protocols between 2005 and 2006 at the National Institute on Drug Abuse (NIDA), Baltimore, MD. Specimens were measured for total volume, aliquotted into multiple tubes, and frozen within hours at -20°C until GC-MS analysis at NIDA. Separate aliquots of the same specimens (756 samples) were shipped frozen (never thawed and refrozen) for blind analysis in 2010 by liquid chromatography-high resolution mass spectrometry (LC-HRMS) and gas chromatography- negative-ion chemical ionization- mass spectrometry (GC-NICI-MS) for this research.
2.3 Creatinine determination
Urine creatinine was determined by the Jaffé reaction (Cobas Creatinine, Roche Diagnostics) on a Roche Hitachi 917 (Roche Diagnostics, Mannheim, Germany).
2.4 Sample preparation and analysis
Urine sample preparation was performed as previously described [31]. Urine samples were analyzed by three different approaches. Method A: LC-HRMS for stereoselective glucuronide and achiral sulfate analysis. Method B: GC-NICI-MS for chiral sulfate analysis after cleavage, and method C: GC-NICI-MS for chiral MDMA and unconjugated metabolite analysis. For method A, 100 μl urine were submitted to C18 solid phase extraction and analyzed on a Thermo Fisher (TF, Dreieich, Germany) Accela LC system equipped with a heated electrospray ionization II source. The stationary phase was a Phenomenex (Aschaffenburg, Germany) Chirex3012 (250 × 4.6 mm, 5 μm) column and the mobile phase consisted of ammonium formate buffer and acetonitrile, both containing 0.1% of formic acid. For method B, 100 μl urine were incubated with 30 μl Aerobacter aerogenes solution for 2h at 37°C in a water bath prior to a combined liquid-liquid extraction with ethyl acetate/cyclohexane (1:1, v:v) and chiral derivatization with S-HFBPrCl approach. After a second derivatization with HMDS, extracts were analyzed by GC-NICI-MS on an Agilent Technologies (AT, Waldbronn, Germany) 6890 Series GC system combined with an AT 5973 network mass selective detector. Samples containing sulfate concentrations within the calibration range of method A (DHMA sulfates 0.5-75 μM, HMMA sulfate 0.2-67 μM) were reanalyzed using method B. For method C, 100 μl of urine were processed and analyzed as described for method B without enzymatic conjugate cleavage. All three methods were fully validated including selectivity, recovery, matrix effects, process efficiency, bias and precision, stabilities, and limits of quantification and detection as described previously [31].
2.5 Pharmacokinetic analysis
Maximum concentration (Cmax) and times of maximum concentrations (tmax) without and with normalization to 100 mg/dL creatinine (Cmax100, tmax100) were determined for each R- and S-stereoisomer. R/S ratios were calculated for MDMA, MDA, DHMA, DHMA sulfate, HMMA, HMMA sulfate, and HMMA glucuronide. The time interval after dosing for the first positive sample was designated as the time of first detection (tonset); the interval after dosing for the last positive sample was designated as the time of last detection (tlast detection).
2.6 Cut offs for estimation of ingestion time
R/S ratios of all analytes were plotted versus time after administration. Different R/S cut-offs were evaluated to divide data into two groups: time of ingestion earlier or later than 24 h. Percentages of samples fitted correctly to these groups at a chosen R/S ratio were calculated.
2.7 Percentage of total excretion in human urine
Percentages of dose excreted as R- or S-stereoisomer in urine for different time intervals (each 12 h) and total dose excreted over 5 days were calculated by summing amounts (μmol) excreted in individual urine samples for each subject and divided by the administered dose.
2.8 Statistical analysis
Statistical analysis was performed with a nonparametric Wilcoxon matched pairs test with p<0.05 (*), p<0.01 (**), p<0.001 (***) with GraphPad Prism 5.00 software (GraphPad Software, San Diego, CA).
3. Results
3.1. Pharmacokinetic analysis
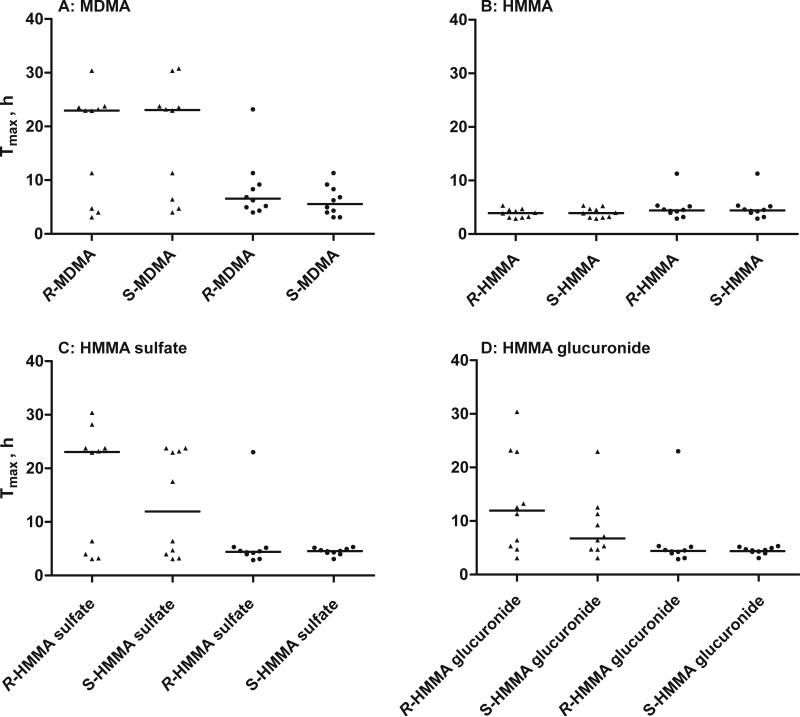
Tables 1 and 2 summarize Cmax, tmax, Cmax100, and tmax100 for each R- and S-enantiomer. Statistically significant differences were observed for the enantiomers of all compounds except HMMA sulfate after creatinine normalization. Higher median maximum concentrations were noted for the R-stereoisomers of MDMA, DHMA and HMMA sulfate with and without creatinine normalization. Conversely, median Cmax and Cmax100 were higher for the S-enantiomers of all other analytes. The S-enantiomer median tmax were significantly earlier following the high dose for DHMA sulfate (p<0.05) and HMMA sulfate (p<0.05). As shown in Fig. 1, normalization to 100 mg/dL creatinine lead to less variation and significantly decreased median tmax100 for all analytes except HMMA, and eliminated significant differences in tmax for all phase II metabolites.
Table 1.
Median (range) maximum urine concentrations (Cmax) and times of maximum urine concentrations (tmax) of R- and S-stereoisomers in urine after low (1.0 mg/kg) and high (1.6 mg/kg) oral MDMA.
| Cmax (μM) | t max (h) | ||||
|---|---|---|---|---|---|
| R | S | R | S | ||
| MDMA | low | 37.9 (18.4-66.9) | 19.1 (11.7-39.0) | 12.6 (6.0 -16.6) | 12.6 (6.0-16.6) |
| high | 61.0 (24.2-179) | 43.1 (11.8-104) | 22.9 (3.1-30.4) | 23.1 (4.0-30.8) | |
| DHMA | low | 0.8 (0.2-1.6) | 0.4 (0.2-0.8) | 12.8 (2.6-23.3) | 13.2 (3.2-23.3) |
| high | 0.9 (0.4-2.0) | 0.4 (0.1-1.2) | 23.2 (3.1-30.4) | 22.9 (3.1-23.8) | |
| DHMA sulfate | low | 17.6 (7.6-30.7) | 21.4 (8.4-38.9) | 13.7 (7.5-25.5) | 7.9 (1.5-23.3) |
| high | 29.1 (16.4-44.6) | 38.3 (20.1-62.1) | 8.9 (3.1-30.4) | 4.4 (3.1-23.0) | |
| HMMA | low | 1.4 (0.5-8.8) | 2.1 (0.5-11.7) | 4.5 (1.5-9.2) | 4.5 (1.5-9.2) |
| high | 3.6 (0.6-11.6) | 4.6 (1.1-19.6) | 3.9 (2.9-5.3) | 3.9 (2.9-5.3) | |
| HMMA sulfate | low | 21.5 (8.1-37.2) | 15.9 (6.5-33.8) | 14.5 (5.4-23.3) | 7.9 (2.1-23.3) |
| high | 33.8 (14.5-48.4) | 25.0 (9.3-37.9) | 23.1 (3.1-30.4) | 12.0 (3.1-23.8) | |
| HMMA glucuronide | low | 11.4 (2.5-27.0) | 18.9 (2.0-56.4) | 11.5 (4.9-23.3) | 7.3 (2.1-16.6) |
| high | 12.1 (2.8-22.2) | 19.6 (2.7-39.2) | 11.9 (3.1-30.4) | 6.8 (3.1-23.0) | |
| MDA | low | 1.8 (0.9-5.4) | 2.1 (1.2-6.9) | 23.0 (10.6-25.5) | 13.4 (1.8-25.5) |
| high | 4.2 (1.5-9.2) | 4.6 (1.6-12.5) | 23.4 (11.3-30.4) | 23.4 (11.3-30.4) | |
Table 2.
Median (range) maximum urine concentrations (Cmax100) and times of maximum urine concentrations (tmax100) normalized to 100 mg/dL creatinine of R- and S-stereoisomers in urine after low (1.0 mg/kg) and high (1.6 mg/kg) oral MDMA administration.
| Cmax 100 (μmol/g creatinine) | t max 100 (h) | ||||
|---|---|---|---|---|---|
| R | S | R | S | ||
| MDMA | low | 49.0 (15.2-277) | 34.9 (9.5-144) | 8.4 (3.2-13.7) | 7.4 (3.2-13.4) |
| high | 140.8 (68.4-264) | 111.0 (46.6-163) | 6.5 (4.0-23.2) | 5.6 (3.1-11.3) | |
| DHMA | low | 1.1 (0.5-5.7) | 0.7 (0.2-5.3) | 13.7 (2.6-87.8) | 9.4 (2.6-87.8) |
| high | 1.8 (0.4-4.3) | 0.7 (0.3-4.4) | 8.8 (1.2-62.4) | 7.7 (0.9-23.2) | |
| DHMA sulfate | low | 57.0 (21.8-115) | 85.9 (18.7-185) | 7.9 (2.1-17.3) | 5.7 (2.1-17.3) |
| high | 81.7 (28.3-178) | 112.1 (49.3-338) | 4.5 (3.1-6.3) | 4.8 (4.0-6.3) | |
| HMMA | low | 8.5 (0.3-21.5) | 9.5 (0.4-28.0) | 3.2 (1.5-7.3) | 3.7 (1.5-8.5) |
| high | 17.6 (2.3-61.6) | 23.8 (4.2-96.6) | 4.4 (2.9-11.3) | 4.4 (2.9-11.3) | |
| HMMA sulfate | low | 49.9 (23.6-122) | 46.0 (12.8-137) | 5.7 (2.1-17.3) | 5.7 (2.1-17.3) |
| high | 63.5 (27.2-105) | 53.8 (27.4-128) | 4.40 (2.9-23.0) | 4.5 (3.1-5.3) | |
| HMMA glucuronide | low | 31.2 (10.7-151) | 51.3 (24.6-240) | 4.7 (3.2-10.0) | 4.7 (3.2-10.0) |
| high | 39.5 (9.7-107) | 84.3 (19.3-189) | 5.2 (4.3-7.3) | 5.1 (3.1-7.3) | |
| MDA | low | 2.5 (1.0-13.1) | 3.0 (1.1-17.5) | 13.5 (6.0-34.3) | 12.9 (6.0-56.4) |
| high | 3.8 (2.1-10.1) | 6.3 (3.8-12.0) | 10.3 (5.0-35.2) | 7.8 (5.0-35.2) | |
Fig. 1.
Median tmax (lines) of R- and S-stereoisomers of MDMA, HMMA, HMMA sulfate, and HMMA glucuronide after a 1.6 mg/kg MDMA oral dose. Triangles and circles represent tmax without and with creatinine normalization, respectively.
3.2 Percentage of total excretion in human urine
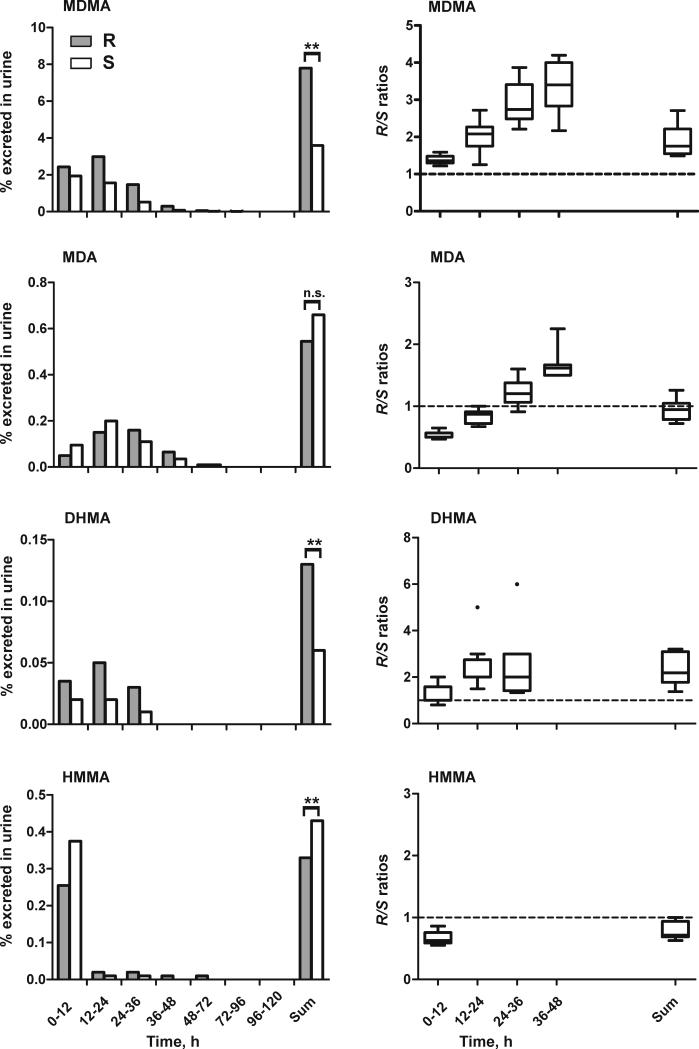
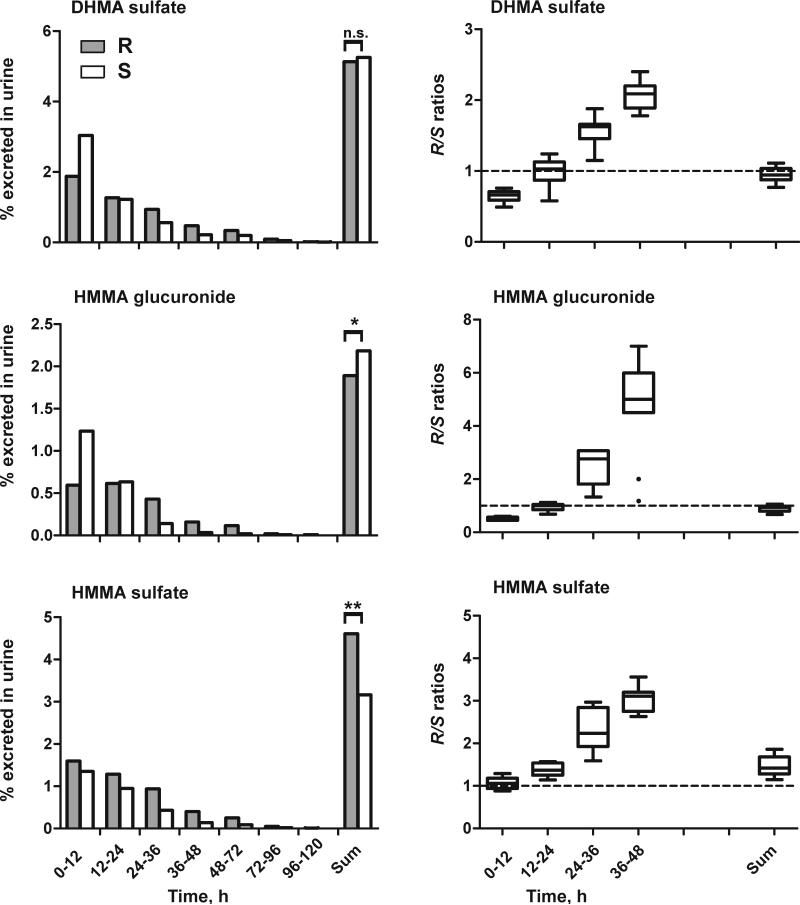
Total urinary recovery over 5 days revealed significant differences for MDMA, DHMA, HMMA, and HMMA conjugates (Fig. 2). Total urinary recoveries were higher for R-enantiomers of MDMA, DHMA and HMMA sulfate, and for S-enantiomers of HMMA and HMMA glucuronide, but MDA and DHMA sulfate enantiomers were close to unity and not significantly different.
Fig. 2.
Median percent dose excreted as R- (grey bars) or S- (white bars) stereoisomer for different time intervals (each 12 h) and total dose excreted in urine over 5 days following the high (1.6 mg/kg) MDMA dose (left). Statistical analysis was performed with a paired, two-tailed t-test with p<0.05 (*), p<0.01 (**), p<0.001 (***). R/S ratios for different time intervals (each 12 h) or over 5 days following the high (1.6 mg/kg) MDMA dose (right). The broken line represents equal amounts of R- and S-isomers.
Changes in enantiomeric disposition were observed over time. MDA, HMMA, DHMA sulfate, and HMMA glucuronide were mainly excreted as S-enantiomers within the first 24 h after ingestion, but higher percentages of R-enantiomers were noted after 24 h (Fig. 2 left). Percentages excreted in urine showed large inter-subject variation for all analytes, while R/S ratios over time revealed less variation, with steadily increasing R/S ratios within the 48 h after ingestion (Fig. 2, right part).
Initial R/S ratios were >1 for MDMA and DHMA, <1 for MDA, HMMA, DHMA sulfate, and HMMA glucuronide, with no enantioselectivity for HMMA sulfate. After 24 h, R/S ratios were >1 for all analytes. No differences in enantiomeric disposition were observed between low and high MDMA doses. In total, a significantly greater percentage (p<0.01) of MDMA dose was excreted in human urine over 5 days as the R-stereoisomer (median 21% vs. 17%).
3.3 Estimation of ingestion time
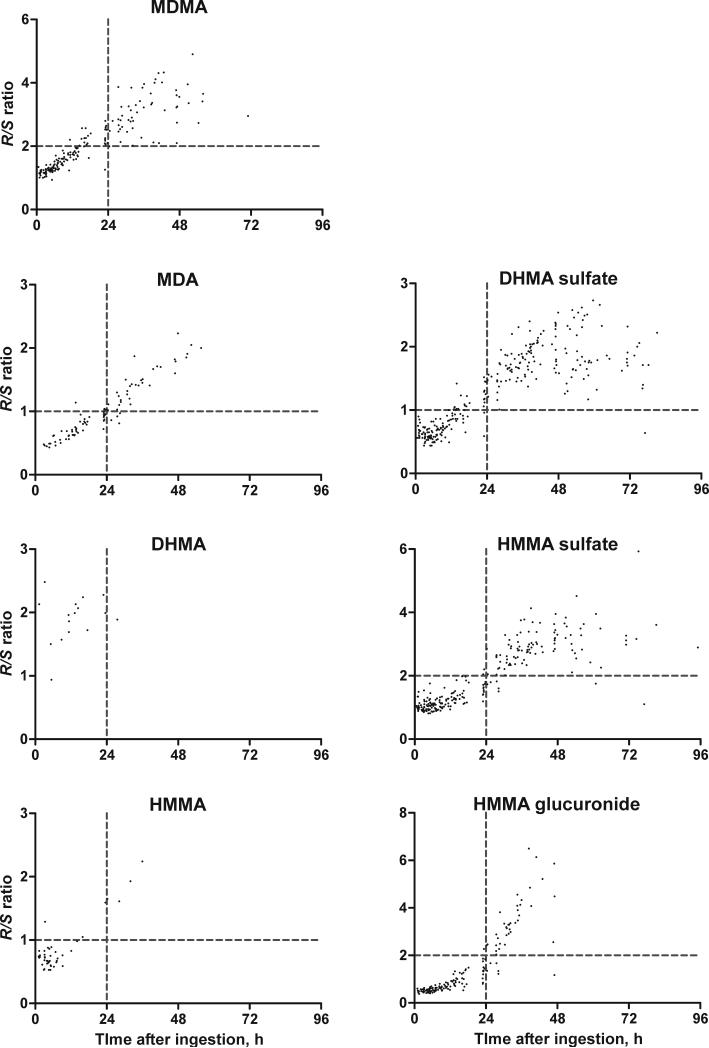
R/S ratios for all analytes with concentrations >0.5 μM were plotted versus time after administration (Fig. 3). No differences in enantiomeric disposition were observed between low and high MDMA doses. The following R/S cut-offs were chosen to divide data into time of ingestion earlier or later than 24 h: R/S of 2 for MDMA, HMMA sulfate, and HMMA glucuronide and R/S of 1 for MDA, HMMA, and DHMA sulfate. Percentages of samples fitted correctly to these groups at a chosen R/S ratio were 87% (MDMA), 91% (MDA), 93% (HMMA), 91% (DHMA sulfate), 96% (HMMA sulfate), and 96% (HMMA glucuronide).
Fig. 3.
R/S ratios in specimens with concentrations of analytes above 0.5 μM following 1.0 and 1.6 mg/kg MDMA doses. The vertical broken line represents 24 h; horizontal broken lines represent R/S cut-offs for each analyte (R/S of 2 for MDMA, HMMA sulfate, and HMMA glucuronide; R/S of 1 for MDA, HMMA, and DHMA sulfate).
4. Discussion
Few data on enantioselective urinary excretion kinetics of MDMA and its metabolites in human urine are available [32-35]. None determined enantiomeric ratios of all major metabolites after controlled oral MDMA administration, and phase II metabolite data are rare. The present study is the first to monitor enantioselective excretion of phase I and II glucuronides and sulfates after controlled oral MDMA administration to humans. Direct analysis of intact conjugates was a prerequisite for assessing the impact of glucuronidation and sulfation as individual metabolic steps on a stereoselective excretion of MDMA. The different conjugates represent individual chemical entities with their own stereoselectivity and risk of toxicity or drug interactions. All urine samples of the previous study [30] were stored at -20°C prior to analysis. A critical point might be the stability of the analytes under these conditions. Long-term stability experiments for phase II metabolites were previously conducted and showed no instability over 6 months [31] and 13 months [21]. Long-term stability of phase I metabolites was shown over at least 6 [36] and 10 months (data not shown). Furthermore, good correlation was observed between the first analysis in 2009 [30] and the current analysis indicating stability of the free analytes. Concerning the phase II metabolites, instability should result in the formation of the respective free drugs. However, concentrations of free drugs were shown to be less than 5% of total DHMA and HMMA [21] which further indicates a sufficient stability of the phase II metabolites.
R- and S-enantiomer concentrations were determined for all analytes; however, as previously shown [21], only MDMA, MDA, DHMA, DHMA sulfates, HMMA, HMMA sulfates, and HMMA glucuronides were excreted in human urine in substantial amounts. Therefore, enantioselective data were focused on these metabolites.
R- and S-enantiomer Cmax were determined with and without creatinine normalization. Statistically significant differences between the two enantiomers were observed for all compounds, except HMMA sulfate after creatinine normalization. As previously described, creatinine normalization did not reduce inter-subject Cmax variation [21], and had no impact on R/S ratios.
Higher R-enantiomer Cmax were observed for MDMA, DHMA and HMMA sulfate, whereas S-enantiomers were higher for DHMA sulfate, HMMA, HMMA glucuronide, and MDA (Table 1, Fig. 4). Although in vitro data with human liver microsomes observed preferences for S-DHMA formation, at Cmax in vivo, unconjugated R-DHMA exceeded unconjugated SDHMA. However, DHMA should be considered an intermediate metabolite with only low concentrations. The majority of DHMA is further metabolized, either by SULT1A3 to DHMA sulfates or COMT to HMMA, as shown in Fig. 4. Both pathways revealed preferences for S-enantiomers in vitro [26,28], which could explain the higher Cmax for the remaining RDHMA. HMMA conjugation to HMMA sulfate in vitro showed no enantioselectivity [28], in line with the in vivo excretion data at Cmax.
Fig. 4.
Main MDMA metabolic steps in humans with stereo preferences for metabolites at Cmax. Solid bonds represent R-configuration, broken bonds S-configuration.
Although Cmax differed between the enantiomers, no significant differences in tmax were observed after normalization to creatinine. As shown in Fig. 1 for MDMA, HMMA, HMMA sulfate, and HMMA glucuronides, creatinine normalization led to significantly decreased median tmax and less variation. Furthermore, differences in median R- and S-enantiomer tmax for HMMA phase II metabolites without creatinine normalization were not observed after creatinine normalization. Concerning the time of first detection, no differences were observed for the two enantiomers. Generally, detectability tended to be longer for R-enantiomers, although for MDA, DHMA and HMMA no significant differences were observed. For DHMA and HMMA sulfates, first and last detection times were not determined, as only samples with sulfate concentrations within the calibration range of the achiral measurement [21], were reanalyzed after sulfate cleavage.
In racemic MDMA, molar amounts of R- and S-MDMA are the same, therefore, the sum of all R- and S-stereoisomers excreted should be equivalent with passage of enough time. However, regarding total urinary recovery in human urine over 5 days, a greater percentage of the MDMA dose was excreted in urine as the R-stereoisomer (median 21% vs. 17%). Significant differences were observed for MDMA, DHMA, HMMA, and HMMA conjugates (Fig. 2). Enantiomeric preferences were the same as observed at Cmax, with higher amounts of R-MDMA and R-DHMA and more S-HMMA and S-HMMA glucuronide. Although, initially higher concentrations of S-MDA and S-DHMA sulfate were observed, there were no significant enantiomer differences in total urinary recoveries. HMMA sulfate was not enantioselective at Cmax, but the R-enantiomer predominated in total urinary recovery. Pizarro et al. reported similar findings concerning MDA, with initial R/S ratios <1, but comparable amounts of R- and S-enantiomers in total urinary excretion over 72 h [25]. Furthermore, R/S HMMA ratios determined after conjugate cleavage were about 1 after 24 h, and about 2 in total [25]. They concluded that the lack of enantioselectivity at the beginning and increasing R-HMMA excretion later might be explained by enantioselectivity of COMT [25]. However, as shown in the present study with independent analyses of HMMA, HMMA sulfate, and HMMA glucuronide, only HMMA sulfation, as the major phase II metabolic step, showed no enantioselectivity at the beginning and increasing R/S ratios in the later excretion phase.
In general, all metabolites exhibited changes in enantiomeric disposition over time. For example, MDA was mainly excreted as its S-enantiomer within the first 24 h after ingestion, transitioning to higher percentages of R-enantiomer after 24 h. The same was true for HMMA, DHMA sulfate, and HMMA glucuronide, with more S-isomer within the first 12 h and more R-enantiomer over the remaining excretion period (Fig. 2, left part). Generally, initial stereoisomer preferences (0-12 h) mimicked those observed in previous in vitro experiments [19,26-28]. In the later excretion phase (after 24 h), R/S ratios were >1 for all compounds. This is quite remarkable, as the enantiomeric ratios of at least one metabolite should be reversed from that of MDMA. However, it must be considered that urinary analysis reflects not only metabolite formation, but also distribution and elimination processes. Metabolism is represented mainly within the first 12 to 24 h, whereas later on, elimination is more relevant. One explanation for the observed time-dependency could be substrate availability. With increasing time, the amount of R- relative to S-enantiomers could increase, leading to increased metabolism of R-enantiomers, although affinity for S-enantiomers is higher. However, this only applies for analytes with initial preferences for S-enantiomers. On the other hand, distribution processes, including transport protein availability, could play a major role in enantioselective disposition and metabolite excretion.
Percentages excreted in urine showed large inter-subject variability for all analytes, for example S-DHMA sulfate excreted in the first 12 h ranged from 0.7 to 3.4%. However, R/S ratios over time revealed less variation and a dependence on time after ingestion, but not on MDMA doses (1.0 and 1.6 mg/kg) administered (Fig. 3). Cut-off R/S values may distinguish between recent (within 24 h) MDMA consumption and earlier ingestion. R/S cut-offs ≥2 for MDMA, HMMA sulfate, and HMMA glucuronide, and ≥1 for MDA, HMMA, and DHMA sulfate correctly predicted time of ingestion in more than 87% of all samples. The best results were obtained with MDA and HMMA phase II metabolites; more than 95% of all samples accurately predicted the time of MDMA ingestion. R/S ratios for DHMA and HMMA, as only minor metabolites, were generally detectable only in the first 24 h after ingestion, thereby providing further indication of recent MDMA consumption. Fallon et al. [2] proposed a model for prediction of MDMA ingestion time in human plasma, based on MDMA R/S ratios and multiple regression analysis. These data are the first allowing similar calculations in human urine. Of all metabolites, MDA R/S ratios provided the best linear correlation to time after ingestion within the first 48 h. However, for all estimations, only a single low or high MDMA dose was administered. Further studies are necessary to evaluate R/S ratios after multiple oral MDMA doses.
These are the first human urine data evaluating stereoselective elimination of phase II MDMA metabolites. Differences in urinary elimination of R- and S-enantiomers were observed for MDMA and all metabolites, depending upon the time after administration. R/S ratios could predict time after MDMA ingestion, thereby, improving urinary MDMA and metabolite interpretation in clinical and forensic toxicology.
5. Acknowledgements
The authors would like to thank Carsten Schröder, Armin Weber, and Gabriele Ulrich from the Department of Experimental and Clinical Toxicology, Institute of Experimental and Clinical Pharmacology and Toxicology, Saarland University and Frank T. Peters from Schiller University Jena for their assistance and helpful discussions and Thermo Fisher for their support. Funding was provided by HOMFOR 2010, the research fund of the Medical Faculty, Saarland University, Homburg and the Intramural Research Program of the National Institutes of Health, National Institute on Drug Abuse.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- 1.U.S.Department of Health and Human Services - Substance Abuse and Mental Health Services Administration Office of Applied Studies 2010 ( http://oas.samhsa.gov/NSDUH/2k9NSDUH/2k9ResultsP.pdf) Results from the 2009, National Survey on Drug Use and Health: Volume I. Summary of National Findings
- 2.Fallon JK, Kicman AT, Henry JA, Milligan PJ, Cowan DA, Hutt AJ. Stereospecific analysis and enantiomeric disposition of 3, 4-methylenedioxymethamphetamine (Ecstasy) in humans. Clin Chem. 1999;45:1058–69. [PubMed] [Google Scholar]
- 3.Kalant H. The pharmacology and toxicology of “ecstasy” (MDMA) and related drugs [review]. Can Med Assoc J. 2001;165:917–28. [PMC free article] [PubMed] [Google Scholar]
- 4.Monks TJ, Jones DC, Bai F, Lau SS. The role of metabolism in 3,4-(+)-methylenedioxyamphetamine and 3,4-(+)-methylenedioxymethamphetamine (ecstasy) toxicity [review]. Ther Drug Monit. 2004;26:132–6. doi: 10.1097/00007691-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 5.de la Torre R, Farre M, Roset PN, Pizarro N, Abanades S, Segura M, et al. Human pharmacology of MDMA: pharmacokinetics, metabolism, and disposition [review]. Ther Drug Monit. 2004;26:137–44. doi: 10.1097/00007691-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Easton N, Marsden CA. Ecstasy: are animal data consistent between species and can they translate to humans? [review]. J Psychopharmacol. 2006;20:194–210. doi: 10.1177/0269881106061153. [DOI] [PubMed] [Google Scholar]
- 7.Baumann MH, Zolkowska D, Kim I, Scheidweiler KB, Rothman RB, Huestis MA. Effects of dose and route of administration on pharmacokinetics of (+ or -)-3,4-methylenedioxymethamphetamine in the rat. Drug Metab Dispos. 2009;37:2163–70. doi: 10.1124/dmd.109.028506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueller M, Kolbrich EA, Peters FT, Maurer HH, McCann UD, Huestis MA, et al. Direct comparison of (+/-) 3,4-methylenedioxymethamphetamine (“ecstasy”) disposition and metabolism in squirrel monkeys and humans. Ther Drug Monit. 2009;31:367–73. doi: 10.1097/FTD.0b013e3181a4f6c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCann UD, Szabo Z, Vranesic M, Palermo M, Mathews WB, Ravert HT, et al. Positron emission tomographic studies of brain dopamine and serotonin transporters in abstinent (+/-)3,4-methylenedioxymethamphetamine (“ecstasy”) users: relationship to cognitive performance. Psychopharmacology (Berl) 2008;200:439–50. doi: 10.1007/s00213-008-1218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esteban B, O'Shea E, Camarero J, Sanchez V, Green AR, Colado MI. 3,4-Methylenedioxymethamphetamine induces monoamine release, but not toxicity, when administered centrally at a concentration occurring following a peripherally injected neurotoxic dose. Psychopharmacology (Berl) 2001;154:251–60. doi: 10.1007/s002130000645. [DOI] [PubMed] [Google Scholar]
- 11.Gollamudi R, Ali SF, Lipe G, Newport G, Webb P, Lopez M, et al. Influence of inducers and inhibitors on the metabolism in vitro and neurochemical effects in vivo of MDMA. Neurotoxicology. 1989;10:455–66. [PubMed] [Google Scholar]
- 12.Bai F, Lau SS, Monks TJ. Glutathione and N-acetylcysteine conjugates of alpha-methyldopamine produce serotonergic neurotoxicity: possible role in methylenedioxyamphetamine-mediated neurotoxicity. Chem Res Toxicol. 1999;12:1150–7. doi: 10.1021/tx990084t. [DOI] [PubMed] [Google Scholar]
- 13.Hiramatsu M, Kumagai Y, Unger SE, Cho AK. Metabolism of methylenedioxymethamphetamine: formation of dihydroxymethamphetamine and a quinone identified as its glutathione adduct. J Pharmacol Exp Ther. 1990;254:521–7. [PubMed] [Google Scholar]
- 14.Miller RT, Lau SS, Monks TJ. 2,5-Bis-(glutathion-S-yl)-alpha-methyldopamine, a putative metabolite of (+/-)-3,4-methylenedioxyamphetamine, decreases brain serotonin concentrations. Eur J Pharmacol. 1997;323:173–80. doi: 10.1016/s0014-2999(97)00044-7. [DOI] [PubMed] [Google Scholar]
- 15.Mueller M, Yuan J, Felim A, Neudörffer A, Peters FT, Maurer HH, et al. Further studies on the role of metabolites in (+/-)-3,4-methylenedioxymethamphetamine-induced serotonergic neurotoxicity. Drug Metab Dispos. 2009;37:2079–86. doi: 10.1124/dmd.109.028340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maurer HH. On the metabolism and the toxicological analysis of methylenedioxyphenylalkylamine designer drugs by gas chromatography-mass spectrometry. Ther Drug Monit. 1996;18:465–70. doi: 10.1097/00007691-199608000-00027. [DOI] [PubMed] [Google Scholar]
- 17.Maurer HH, Bickeboeller-Friedrich J, Kraemer T, Peters FT. Toxicokinetics and analytical toxicology of amphetamine-derived designer drugs (“Ecstasy”). Toxicol Lett. 2000;112:133–42. doi: 10.1016/s0378-4274(99)00207-6. [DOI] [PubMed] [Google Scholar]
- 18.Shima N, Katagi M, Kamata H, Zaitsu K, Kamata T, Nishikawa M, et al. Urinary excretion of the main metabolites of 3,4-methylenedioxymethamphetamine (MDMA), including the sulfate and glucuronide of 4-hydroxy-3-methoxymethamphetamine (HMMA), in humans and rats. Xenobiotica. 2008;38:314–24. doi: 10.1080/00498250701802506. [DOI] [PubMed] [Google Scholar]
- 19.Schwaninger AE, Meyer MR, Zapp J, Maurer HH. The role of human UDP-glucuronyltransferases on the formation of the methylenedioxymethamphetamine (ecstasy) phase II metabolites R- and S-3-methoxymethamphetamine 4-O-glucuronides. Drug Metab Dispos. 2009;37:2212–20. doi: 10.1124/dmd.109.029215. [DOI] [PubMed] [Google Scholar]
- 20.Schwaninger AE, Meyer MR, Zapp J, Maurer HH. Sulfation of the 3,4-methylenedioxymethamphetamine (MDMA) metabolites 3,4-dihydroxymethamphetamine (DHMA) and 4-hydroxy-3-methoxymethamphetamine (HMMA) and their capability to inhibit human sulfotransferases. Toxicol Lett. 2011;202:120–8. doi: 10.1016/j.toxlet.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 21.Schwaninger AE, Meyer MR, Barnes AJ, Kolbrich-Spargo EA, Gorelick DA, Goodwin RS, et al. Human MDMA and phase I and phase II metabolite urinary excretion kinetics following controlled MDMA administration. Clin Chem. 2011 doi: 10.1373/clinchem.2011.172254. in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraemer T, Maurer HH. Toxicokinetics of amphetamines: Metabolism and toxicokinetic data of designer drugs, of amphetamine, methamphetamine and their N-alkyl derivatives [review]. Ther Drug Monit. 2002;24:277–89. doi: 10.1097/00007691-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Peters FT, Samyn N, Wahl M, Kraemer T, de Boeck G, Maurer HH. Concentrations and ratios of amphetamine, methamphetamine, MDA, MDMA, and MDEA enantiomers determined in plasma samples from clinical toxicology and driving under the influence of drugs cases by GC-NICI-MS. J Anal Toxicol. 2003;27:552–9. doi: 10.1093/jat/27.8.552. [DOI] [PubMed] [Google Scholar]
- 24.Peters FT, Samyn N, Lamers C, Riedel W, Kraemer T, de Boeck G, et al. Drug testing in blood: validated negative-ion chemical ionization gas chromatographic-mass spectrometric assay for enantioselective measurement of the designer drugs MDEA, MDMA, and MDA and its application to samples from a controlled study with MDMA. Clin Chem. 2005;51:1811–22. doi: 10.1373/clinchem.2005.052746. [DOI] [PubMed] [Google Scholar]
- 25.Pizarro N, Farre M, Pujadas M, Peiro AM, Roset PN, Joglar J, et al. Stereochemical analysis of 3,4-methylenedioxymethamphetamine and its main metabolites in human samples including the catechol-type metabolite (3,4-dihydroxymethamphetamine). Drug Metab Dispos. 2004;32:1001–7. [PubMed] [Google Scholar]
- 26.Meyer MR, Maurer HH. Enantioselectivity in the methylation of the catecholic phase I metabolites of methylenedioxy designer drugs and their capability to inhibit catechol-O-methyltransferase-catalyzed dopamine 3-methylation. Chem Res Toxicol. 2009;22:1205–11. doi: 10.1021/tx900134e. [DOI] [PubMed] [Google Scholar]
- 27.Meyer MR, Peters FT, Maurer HH. The role of human hepatic cytochrome P450 isozymes in the metabolism of racemic 3,4-methylenedioxy-methamphetamine and its enantiomers. Drug Metab Dispos. 2008;36:2345–54. doi: 10.1124/dmd.108.021543. [DOI] [PubMed] [Google Scholar]
- 28.Schwaninger AE, Meyer MR, Maurer HH. Investigation on the enantioselectivity of the sulfation of the methylenedioxymethamphetamine (MDMA) metabolites 3,4-dihydroxymethamphetamine (DHMA) and 4-hydroxy-3-methoxymethamphetamine (HMMA) using the substrate depletion approach. Drug Metab Dispos. 2011 doi: 10.1124/dmd.111.041129. DOI: 10.1124/dmd.111.041129. [DOI] [PubMed] [Google Scholar]
- 29.Peters FT, Kraemer T, Maurer HH. Drug testing in blood: validated negative-ion chemical ionization gas chromatographic-mass spectrometric assay for determination of amphetamine and methamphetamine enantiomers and its application to toxicology cases. Clin Chem. 2002;48:1472–85. [PubMed] [Google Scholar]
- 30.Abraham TT, Barnes AJ, Lowe RH, Kolbrich Spargo EA, Milman G, Pirnay SO, et al. Urinary MDMA, MDA, HMMA, and HMA excretion following controlled MDMA administration to humans. J Anal Toxicol. 2009;33:439–46. doi: 10.1093/jat/33.8.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwaninger AE, Meyer MR, Huestis MA, Maurer HH. Development and validation of LC-HRMS and GC-NICI-MS methods for stereoselective determination of methylenedioxymethamphetamine (MDMA) and its phase I and II metabolites in human urine. J Mass Spectrom. 2011;46:603–14. doi: 10.1002/jms.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pizarro N, Llebaria A, Cano S, Joglar J, Farre M, Segura J, et al. Stereochemical analysis of 3,4-methylenedioxymethamphetamine and its main metabolites by gas chromatography/mass spectrometry. Rapid Commun Mass Spectrom. 2003;17:330–6. doi: 10.1002/rcm.919. [DOI] [PubMed] [Google Scholar]
- 33.de Boer D, Tan LP, Gorter P, van-de-Wal RM, Kettenes-van den Bosch JJ, de-Bruijn EA, et al. Gas chromatographic/mass spectrometric assay for profiling the enantiomers of 3,4-methylenedioxymethamphetamine and its chiral metabolites using positive chemical ionization ion trap mass spectrometry. J Mass Spectrom. 1997;32:1236–46. doi: 10.1002/(SICI)1096-9888(199711)32:11<1236::AID-JMS584>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 34.Moore KA, Mozayani A, Fierro MF, Poklis A. Distribution of 3,4-methylenedioxymethamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA) stereoisomers in a fatal poisoning. Forensic Sci Int. 1996;83:111–9. doi: 10.1016/s0379-0738(96)02025-7. [DOI] [PubMed] [Google Scholar]
- 35.Lanz M, Brenneisen R, Thormann W. Enantioselective determination of 3,4-methylene-dioxymethamphetamine and two of its metabolites in human urine by cyclodextrin-modified capillary zone electrophoresis. Electrophoresis. 1997;18:1035–43. doi: 10.1002/elps.1150180628. [DOI] [PubMed] [Google Scholar]
- 36.Wan Raihana WA, Gan SH, Tan SC. Stereoselective method development and validation for determination of concentrations of amphetamine-type stimulants and metabolites in human urine using a simultaneous extraction-chiral derivatization approach. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:8–16. doi: 10.1016/j.jchromb.2010.10.037. [DOI] [PubMed] [Google Scholar]