Abstract
Insects carry out essential ecological functions, such as pollination, but also cause extensive damage to agricultural crops and transmit human diseases such as malaria and dengue fever. Advances in insect transgenesis are making it increasingly feasible to engineer genes conferring desirable phenotypes, and gene drive systems are required to spread these genes into wild populations. Medea provides one solution, being able to spread into a population from very low initial frequencies through the action of a maternally-expressed toxin linked to a zygotically-expressed antidote. Several other toxin-antidote combinations are imaginable that distort the offspring ratio in favor of a desired transgene, or drive the population towards an all-male crash. We explore two such systems—Semele, which is capable of spreading a desired transgene into an isolated population in a confined manner; and Merea, which is capable of inducing a local population crash when located on the Z chromosome of a Lepidopteron pest.
Key words: dengue fever, malaria, Medea, Merea, mosquitoes, pink bollworms, population replacement, population suppression, Semele
In Greek mythology, Medea is the wife of the hero Jason, to whom she has two children. Her marriage to Jason is hard-earned, transpiring only after she supports him in his quest for the Golden Fleece. Assisting him in this task, she enables him to plough a field with fire-breathing oxen and to sow the teeth of a dragon that later sprout into an army of warriors. But despite her remarkable efforts, he leaves her when the king of Corinth offers him his daughter. As a form of revenge, Medea kills their two children, poisons the king's daughter and accidentally also the king himself.
Such a temperament would make Medea quite an unfit mother from a biological perspective; but what if this trait was genetic and the children that inherited it were able to defend themselves against the likes of such an assailant? Mathematical models predict that any gene conferring this trait (vengeful mother with murderous tendencies linked to child with impressive self-defense abilities) actually has a selective advantage and that, if present at modest levels in a population, it is expected to become present among all individuals within a matter of generations.1,2 The simple fact is that children who are able to defend themselves against a vengeful mother are more fit than those who cannot.
From Greek Mythology to Molecular Biology
The Greek analogy does sound rather obscene, but genes displaying these properties do in fact exist in nature.3–5 The first such element to be identified was in the flour beetle Tribolium castaneum3 and was given the name Medea after both the character from Greek mythology and as an acronym for “maternal-effect dominant embryonic arrest.” By crossing individuals from geographically-isolated locations, it was found that Medea-bearing males give rise to both wild-type and Medea-bearing offspring; but that Medea-bearing females only give rise to Medea-bearing offspring. It appeared that Medea-bearing mothers were selectively killing non-Medea bearing offspring; or alternatively that they were trying to kill all offspring and the Medea-bearing offspring were able to defend themselves.
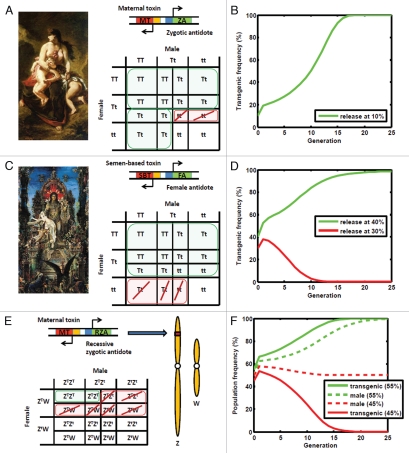
These dynamics suggest a model in which Medea consists of two tightly-linked genes—a maternally-expressed toxin gene, the product of which causes all eggs to become unviable; and a zygotically-expressed antidote gene, the product of which rescues Medea-bearing eggs from the effects of the toxin3,6 (Fig. 1A). This is exciting because it opens the possibility to create synthetic Medea elements capable of spreading into a population from low initial frequencies (Fig. 1B). In 2007, the Hay lab at Caltech succeeded in creating the first synthetic element shown to drive population replacement by applying this model.7 As a toxin, they used synthetic microRNAs designed to interfere with a pathway required for embryo development, and placed these under the expression of a strong maternal-specific promoter. As an antidote, they encoded a microRNA-insensitive version of the protein that the toxin silenced, and placed this under the expression of an early zygote-specific promoter.
Figure 1.
Novel toxin-antidote gene drive systems for controlling insect pest populations. (A) Medea, named after the character from Greek mythology, distorts the offspring ratio in its favor through the action of a maternally-expressed toxin gene and a zygotically-expressed antidote gene. (B) This enables Medea to spread into a population from low initial frequencies. (C) Semele, named after the mortal female from Greek mythology, distorts the offspring ratio in its favor through the action of a semen-based toxin and a female-specific antidote. (D) In the absence of a fitness cost, Semele spreads into a population for release frequencies exceeding ∼36%. (E) Merea distorts the offspring ratio in its favor through the action of a maternal toxin and a recessive zygotic antidote. (F) If located on the Z chromosome of a species for which females are the heterogametic sex, Merea is capable of inducing an all-male population crash for release frequencies exceeding ∼50%.
The demonstration of a synthetic gene drive system was greeted with much excitement, being selected by Scientific American as one of the top 50 technological developments of 2007.8 Although the element was originally engineered in the fruit fly Drosophila melanogaster, the potential for its application to mosquito vectors of disease was immediately obvious. Genes conferring refractoriness to rodent malaria have already been engineered in the mosquito Anopheles berghii,9 and a signaling pathway has been activated that dramatically reduces human malaria development in the mosquito Anopheles gambiae.10 Furthermore, genes have been engineered in the mosquito Aedes aegypti that have been shown to reduce dengue virus transmission.11 These genes could be attached to a Medea element and hitch-hike into a population, rendering all mosquitoes unable to transmit diseases to humans while their ecological niches remain filled.12
However, while Medea shows great promise for reducing the global mosquito-borne disease burden, it may not be ideal during the testing phase of a transgenic release. This is because, as an invasive drive system, Medea is expected to spread transgenes from one population to another and potentially into other countries, before they have agreed to their introduction.13 The Cartagena Protocol—the United Nations protocol on the international movement of transgenic organisms—prohibits such a release in the absence of a multilateral international agreement.14 Furthermore, such a release would violate the autonomy of communities15 and the principle of scientific risk management16 if conducted prior to a proper assessment of its potential risks and efficacy.
Medea only represents one possible way in which the offspring ratio can be manipulated to favor the inheritance of one allele over another. Other toxin-antidote combinations are available—for example, either the toxin or antidote gene could be placed under the control of a paternal, maternal or zygote-specific promoter, function through a recessive or dominant mechanism, and be located on a sex chromosome or autosome. With this in mind, together with members of the Hay lab at Caltech, I conducted a survey of the possible ways in which a toxin and antidote gene could be used to drive a desired gene into a population, or otherwise control a population through reducing its size, and whether such control could be achieved with less invasive tendencies.17
Semele and Confined Malaria Control
One of the first gene drive systems to come out of this analysis was a construct we named Semele, consisting of a toxin gene expressed in the semen of transgenic males (which either kills or renders infertile wild-type females), and an antidote gene expressed in females which protects them against the effects of the toxin18 (Fig. 1C). The name Semele is an acronym for “semen-based lethality” and, like Medea, also has Greek origins. In Greek mythology, Semele is a mortal female who attracts the attention of Zeus while slaughtering a bull at his altar (Zeus, at this point, is flying overhead disguised as an eagle). Zeus becomes infatuated with Semele and impregnates her, but Semele dies after witnessing his godliness because she is not herself a god. The story is entirely consistent with the hypothesis that gods have toxic semen, while only goddesses have the antidote; however, the comparison to our transgenic construct ends when Zeus sews the unborn baby into his thigh and gives birth to the god of wine.
Mathematical models predict that the Semele construct will only spread into a population if it exceeds a critical frequency in the population. This is because both wild-type and transgenic alleles are sacrificed in the process by which Semele distorts the offspring ratio, and the selective advantage of the female antidote outweighs the reproductive disadvantage conferred by toxic semen only when the allele exceeds this critical frequency. In the absence of additional fitness costs, mathematical models predict a critical population frequency of ∼36%, above which Semele is expected to spread into a population, and below which it is expected to be eliminated18 (Fig. 1D). Additionally, if only males carrying the Semele allele are released into a wild population, they are expected to reduce population size when released in large numbers, because all of the wild females that mate with Semele males are susceptible to their toxic semen.
As a population suppression system, alternatives to Semele have already been engineered that display superior qualities. Most notably, a RIDL strain of the dengue virus-transmitting A. aegypti mosquito has been developed by Oxford biotechnology company Oxitec,19 and underwent field trials in the Cayman Islands in late 2009.20 Offspring of RIDL males are viable in the larval stage but are unable to survive to adulthood. This allows them to compete for resources with wild-type larvae, further enhancing their effect on population suppression.21 Semele-induced lethality would be much earlier-acting, either killing the mother herself or cleaving zygotic DNA after fertilization.18 However, the benefit of Semele-induced population suppression is that it could be used to reduce population size preceding a super-threshold release. This would reduce the release size required to exceed the critical population frequency, and would only require approval for the release of a single transgenic strain.
The real advantage of the Semele system is that it could potentially spread desirable genes to high frequencies in a confined way. The critical release frequency may at first appear to be a disadvantage; but it has three advantages during the testing phase of population replacement, or whenever a confined release is preferred. First, accidentally released transgenic insects are unlikely to persist in the wild because they will inevitably be present at sub-threshold levels and be eliminated from the environment. Second, transgenic insects released at super-threshold frequencies at an isolated release site are expected to spread transgenes locally while they remain at sub-threshold levels at nearby locations. And third, transgenes can be eliminated from the release site by diluting them to sub-threshold frequencies through a sustained release of wild-type insects. A proper assessment of confinement will require a detailed ecological analysis, taking into account migration rates, seasonally-fluctuating population sizes and other aspects of local population structure.22 However, a preliminary analysis suggests that the Semele system and its associated transgenes can be reliably confined to release sites connected to neighboring populations by modest migration rates.23
These considerations are particularly relevant to malaria, which is proving exceptionally difficult to control in highly-endemic areas with currently-available tools.24,25 Mosquitoes engineered with malaria-refractory genes linked to gene drive systems have been proposed as a serious addition to the current repertoire of control strategies,26 but must spread to high frequencies in order to have a significant effect on disease transmission.27,28 On a regional level, invasive gene drive systems such as Medea have been proposed to achieve this;29 however, confineable systems such as Semele provide an important opportunity to test the risks, efficacy and epidemiological effect of this strategy before it is implemented on a regional scale. If malaria prevalence can first be shown to decline on a local scale, then acceptance is likely to grow for the use of invasive drive systems.
The Semele system has not yet been engineered, although several approaches have been proposed using currently-available molecular tools. As a toxin, one possibility is to use an insect-specific neurotoxin gene30 and place this under the expression of a male accessory gland-specific promoter.31 When the neurotoxin enters the female with the seminary fluid, it would interrupt the proper functioning of the female nervous system. An antidote for this toxin could consist of neutralizing antibodies secreted in the female hemolymph. An alternative approach is to use a sperm-based toxin, such as a DNAse which specifically cleaves zygotic DNA, in conjunction with an oocyte or egg-based antidote.32 An encouraging result for this approach is the observation that when the homing endonuclease I-Ppol is expressed during spermatogenesis it cleaves zygotic DNA on the X chromosome, thus causing zygote lethality in An. gambiae.33 A bias towards Y-bearing spermatozoa suggests that this cleavage also occurs during spermatogenesis, while for the Semele system, it would be necessary to silence endonuclease expression until the later stages of spermatogenesis.
Finally, the Semele system could be engineered using genes that mediate cytoplasmic incompatibility in the intracellular bacterium Wolbachia. Cytoplasmic incompatibility, in its simplest form, behaves as though sperm produce a toxin which is counteracted in the zygote by a maternally-provided antidote, thus enabling Wolbachia to spread on a regional scale.34 A Semele allele could potentially be created by linking the genes that mediate these functions and inserting them onto a nuclear chromosome.35,36 Given at least three potential approaches utilizing well-studied components, it is hopeful that, with sufficient effort, the Semele system can be engineered and tested in the coming years.
Merea and the Pink Bollworm
Another gene drive system that caught our attention during the survey of toxinantidote combinations consists of a maternally-expressed toxin gene and a zygotically-expressed antidote gene which is only functional when present on both homologous chromosomes at a given locus17 (Fig. 1E). We named this system Merea, as an acronym for “Medea with a recessive antidote.” The fact that heterozygous offspring are no longer rescued by one copy of the zygotic antidote gene (as is the case for Medea) means that both wild-type and transgenic alleles are sacrificed in the process by which Merea distorts the offspring ratio. Consequently, Merea is only expected to spread into a population if it exceeds a critical population frequency. In the absence of fitness costs, this frequency is ∼41%.17
If located on an autosome, Merea displays very similar properties to Semele. Its critical release frequency is slightly higher, and hence accidentally released transgenic insects are again unlikely to persist in the wild. Preliminary analysis also suggests that the Merea system can be confined to isolated release sites, and that it can be eliminated from these populations through dilution to sub-threshold frequencies.23 Autosomal Merea has two advantages over the Semele system—it is capable of spreading to fixation in an isolated population even if it has a fitness cost (Semele spreads to very high frequencies, but only fixes in the absence of a fitness cost); and it is capable of spreading very quickly, fixing within ten generations following a release at 50%.17 Its main disadvantage, however, is that it requires the engineering of a recessive antidote—something which has not yet been achieved.
Recessive genetic systems do exist in nature, and several mechanisms have evolved for detecting two-fold differences in gene expression and chromosome number;37–39 however, our understanding of these mechanisms is incomplete. Synthetic elements having these properties are being investigated, some utilizing the phenomenon of pair-sensitive silencing in which the presence of specific sequences near genes located at the same site on homologous chromosomes results in strong silencing of these genes in homozygotes, but much weaker silencing in heterozygotes.40 Other promising mechanisms involve multiple interacting components which, although more prone to mutational inactivation, are relatively stable because toxin-only alleles will be rapidly eliminated from any population in which they emerge.
Merea becomes especially interesting when located on the Z chromosome of a species for which males are the homogametic sex (ZZ) and females are heterogametic (ZW). In this case, females can have at most one copy of the Merea allele, and hence at most one copy of the antidote gene. Since the antidote only functions when present on two homologous chromosomes, this means that female offspring can never protect themselves against the maternal toxin; however, homozygous transgenic male offspring can. This leads to a paradoxical situation in which, above a critical population frequency, the Merea allele is favored, but females are not. For a release exceeding a critical frequency of 50% (consisting of homozygous transgenic males and hemizygous females), the Merea allele is predicted to spread to fixation within ∼25 generations, by which time the population is predicted to be entirely male (Fig. 1F). Since females are needed to produce offspring, this results in a population crash.17
Several strategies for using gene drive systems to induce a population crash have been proposed. Notably, work is ongoing towards development of an X-shredder which utilizes a homing endonuclease to create a bias towards Y-bearing spermatozoa by cleaving the X chromosome during spermatogenesis.33 Like Z-linked Merea, X-shredders favor their own inheritance at the same time as they drive the population towards and all-male crash.41,42 However, Z-linked Merea is distinct in the sense that, while X-shredders are predicted to spread from very low initial frequencies, Z-linked Merea displays threshold behavior, allowing greater control over its spatial spread. That said; X-shredders and Z-linked Merea are not competing technologies, since they apply to species for which males are heterogametic and homogametic, respectively.
Z-linked Merea is a promising new technology for the control of Lepidopteron pests, many of which are of huge economic importance, such as the pink bollworm and codling moth. The pink bollworm is of particular interest, with resistance to transgenic Bt cotton on the rise,43 and an annual cost to US cotton producers exceeding US$ 32 million (http://www.cotton.org). A transgenic pink bollworm strain expressing a fluorescent marker has been developed by Oxitec with the goal of improving monitoring of existing sterile insect programs.44 Oxitec have also developed a RIDL sterile male strain, which is now available for cage trials.45 The advantage of Z-linked Merea over sterile male strategies is that, for a super-threshold release, Z-linked Merea self-propagates until the population crashes, requiring less of a continued investment. Also, if the predictions of confinement hold true in an ecological setting, Z-linked Merea could avoid the international complications of the Cartagena Protocol, while at the same time achieving effective control over a well-chosen release area.
Novel Genetic Strategies for Pest Control
Literally hundreds of genetic constructs are imaginable that distort the offspring ratio in favor of a desired transgene or drive the population towards an all-male crash. In this commentary, we discuss three such systems—Semele, Medea and Merea—each of which is appropriate under different circumstances. Semele can be used to spread a desired transgene into a local population, Medea will spread the same transgene on a regional scale and Merea, when located on the Z chromosome, can be used to induce a local population crash. Each of these systems consists of tightly-linked toxin and antidote genes inserted at a single locus. If our survey were expanded to include two-locus systems, a myriad of new possibilities would present themselves—engineered under-dominance46 and killer-rescue constructs47 are two well-characterized examples.
Recent environmental releases of genetically-sterile male mosquitoes20 and fluorescently-labeled transgenic pink bollworms44 highlight the reality that genetic control of insect pests and disease vectors is no longer an abstract idea. Advances in insect physiology and genomics are making it increasingly feasible to engineer genes conferring desirable phenotypes, either to increase crop yields or to reduce the prevalence of insect-borne diseases. The gene drive systems described here offer a way to realize these developments at the population level, and to confront the challenges posed by the most resilient insect pest species with the strength and cunning of a Greek god.
Acknowledgments
John Marshall is grateful to members of the Hay lab at Caltech for many stimulating discussions on toxin-antidote gene drive systems—in particular, Bruce Hay, Catherine Ward, Geoffrey Pittman, Anna Buchman and Omar Akbari. This research was supported by grant number DP1 OD003878 to Bruce A. Hay from the National Institutes of Health.
References
- 1.Wade MJ, Beeman RW. The population dynamics of maternal-effect selfish genes. Genetics. 1994;138:1309–1314. doi: 10.1093/genetics/138.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward CM, Su JT, Huang Y, Lloyd AL, Gould F, Hay BA. Medea selfish genetic elements as tools for altering traits of wild populations: A theoretical analysis. Evolution. 2011;65:1149–1162. doi: 10.1111/j.1558-5646.2010.01186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beeman RW, Friesen KS, Denell RE. Maternal-effect selfish genes in flour beetles. Science. 1992;256:89–92. doi: 10.1126/science.1566060. [DOI] [PubMed] [Google Scholar]
- 4.Hurst LD. Scat+ is a selfish gene analogous to Medea of Tribolium castaneum. Cell. 1993;75:407–408. doi: 10.1016/0092-8674(93)90375-z. [DOI] [PubMed] [Google Scholar]
- 5.Weichenhan D, Kunze B, Traut W, Winking H. Restoration of the Mendelian transmission ratio by a deletion in the mouse chromosome 1 HSR. Genet Res. 1998;71:119–125. doi: 10.1017/s0016672398003206. [DOI] [PubMed] [Google Scholar]
- 6.Beeman RW, Friesen KS. Properties and natural occurrence of maternal-effect selfish genes (‘Medea’ factors) in the red flour beetle, Tribolium castaneum. Heredity. 1999;82:529–534. doi: 10.1038/sj.hdy.6885150. [DOI] [PubMed] [Google Scholar]
- 7.Chen CH, Huang H, Ward CM, Su JT, Schaeffer LV, Guo M, et al. A synthetic maternal-effect selfish genetic element drives population replacement in Drosophila. Science. 2007;316:597–600. doi: 10.1126/science.1138595. [DOI] [PubMed] [Google Scholar]
- 8.Fischetti M. Mosquitoes enlisted to beat malaria. Sci Am. 2008;298:47. [Google Scholar]
- 9.Ito J, Ghosh A, Moreira LA, Wimmer EA, Jacobs-Lorena M. Transgenic anopheline mosquitoes impaired in transmission of a malaria parasite. Nature. 2002;417:452–455. doi: 10.1038/417452a. [DOI] [PubMed] [Google Scholar]
- 10.Corby-Harris V, Drexler A, Watkins de Jong L, Antonova Y, Pakpour N, Ziegler R, et al. Activation of Akt signaling reduces the prevalence and intensity of malaria parasite infection and lifespan in Anopheles stephensi mosquitoes. PLoS Pathog. 2010;6:1001003. doi: 10.1371/journal.ppat.1001003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franz AW, Sanchez-Vargas I, Adelman N, Blair CD, Beaty BJ, James AA, et al. Engineering RNA interference-based resistance to dengue virus type 2 in genetically modified Aedes aegypti. Proc Natl Acad Sci USA. 2006;103:4198–4203. doi: 10.1073/pnas.0600479103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hay BA, Chen CH, Ward CM, Huang H, Su JT, Guo M. Engineering the genomes of wild insect populations: Challenges and opportunities provided by synthetic Medea selfish gene elements. J Insect Physiol. 2010;56:1402–1413. doi: 10.1016/j.jinsphys.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knols BG, Bossin HC, Mukabana WR, Robinson AS. Transgenic mosquitoes and the fight against malaria: managing technology push in a turbulent GMO world. Am J Trop Med Hyg. 2007;77:232–242. [PubMed] [Google Scholar]
- 14.Marshall JM. The Cartagena Protocol and genetically modified mosquitoes. Nat Biotech. 2010;28:896–897. doi: 10.1038/nbt0910-896. [DOI] [PubMed] [Google Scholar]
- 15.Marshall JM, Toure MB, Traore MM, Famenini S, Taylor CE. Perspectives of people in Mali toward genetically-modified mosquitoes for malaria control. Malaria J. 2010;9:128. doi: 10.1186/1475-2875-9-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beech CJ, Nagaraju J, Vasan SS, Rose RI, Othman RY, Pillai V, et al. Risk analysis of a hypothetical open field release of a self-limiting transgenic Aedes aegypti mosquito strain to combat dengue. AsPac J Mol Biol Biotechnol. 2009;17:99–111. [Google Scholar]
- 17.Marshall JM, Hay BA. General principles of single-construct chromosomal gene drive. doi: 10.1111/j.1558-5646.2012.01582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall JM, Pittman GW, Buchman AB, Hay BA. Semele: A killer-male, rescue-female system for suppression and replacement of insect disease vector populations. Genetics. 2011;187:535–551. doi: 10.1534/genetics.110.124479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas DT, Donnelly CA, Wood RJ, Alphey L. Insect population control using a dominant, repressible, lethal genetic system. Science. 287:2474–2476. doi: 10.1126/science.287.5462.2474. [DOI] [PubMed] [Google Scholar]
- 20.Enserink M. GM mosquito trial alarms opponents, strains ties in Gates-funded project. Science. 2010;330:1030–1031. doi: 10.1126/science.330.6007.1030. [DOI] [PubMed] [Google Scholar]
- 21.Phuc HK, Andreasen MH, Burton RS, Vass C, Epton MJ, Pape G, et al. Late-acting dominant lethal genetic systems and mosquito control. BMC Biology. 2007;5:11. doi: 10.1186/1741-7007-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tripet F, Dolo G, Lanzaro G. Multilevel analyses of genetic differentiation in Anopheles gambiae s.s. reveal patterns of gene flow important for malaria-fighting mosquito projects. Genetics. 2005;169:313–314. doi: 10.1534/genetics.104.026534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marshall JM, Hay BA. Confinement of gene drive systems to local populations: A comparative analysis. [DOI] [PMC free article] [PubMed]
- 24.Griffin JT, Hollingsworth TD, Okell LC, Churcher TS, White M, Hinsley W, et al. Reducing Plasmodium falciparum malaria transmission in Africa: A model-based evaluation of intervention strategies. PLoS Med. 2010;7:1000324. doi: 10.1371/journal.pmed.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization, author. World Malaria Report 2010. Geneva (Switzerland): WHO Press; 2010. [Google Scholar]
- 26.Alphey L, Beard CB, Billingsley P, Coetzee M, Crisanti A, Curtis C, et al. Malaria control with genetically manipulated insect vectors. Science. 2002;298:119–121. doi: 10.1126/science.1078278. [DOI] [PubMed] [Google Scholar]
- 27.Boete C, Koella JC. A theoretical approach to predicting the success of genetic manipulation of malaria mosquitoes in malaria control. Malaria J. 2002;1:3. doi: 10.1186/1475-2875-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boete C, Koella JC. Evolutionary ideas about genetically manipulated mosquitoes and malaria control. Trends Parasitol. 2003;19:32–38. doi: 10.1016/s1471-4922(02)00003-x. [DOI] [PubMed] [Google Scholar]
- 29.Marshall JM, Taylor CE. Malaria control with transgenic mosquitoes. PLoS Med. 2009;6:20. doi: 10.1371/journal.pmed.1000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicholson GM. Fighting the global pest problem: Preface to the special Toxicon issue on insecticidal toxins and their potential for insect pest control. Toxicon. 2007;49:413–422. doi: 10.1016/j.toxicon.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 31.Sirot LK, LaFlamme BA, Sitnik JL, Rubinstein CD, Avila FW, Chow CY, et al. Molecular social interactions: Drosophila melanogaster seminal fluid proteins as a case study. Adv Genet. 2009;68:23–56. doi: 10.1016/S0065-2660(09)68002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lo AS, Zhu Q, Marasco WA. Intracellular antibodies (intrabodies) and their therapeutic potential. Handb Exp Pharmacol. 2008:343–373. doi: 10.1007/978-3-540-73259-4_15. [DOI] [PubMed] [Google Scholar]
- 33.Windbichler N, Papathanos PA, Crisanti A. Targeting the X chromosome during spermatogenesis induces Y chromosome transmission ratio distortion and early dominant embryo lethality in Anopheles gambiae. PLoS Genet. 2008;4:1000291. doi: 10.1371/journal.pgen.1000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turelli M, Hoffmann AA. Rapid spread of an inherited incompatibility factor in California Drosophila. Nature. 1991;353:440–442. doi: 10.1038/353440a0. [DOI] [PubMed] [Google Scholar]
- 35.Sinkins SP, Curtis CF, O'Neill SL. The potential application of inherited symbiont systems to pest control. In: O'Neill SL, Hoffmann AA, Werren JH, editors. Influential passengers: Inherited Microorganisms and Arthropod Reproduction. Oxford (UK): Oxford University Press; 1997. [Google Scholar]
- 36.Turelli M, Hoffmann AA. Microbe-induced cytoplasmic incompatibility as a mechanism for introducing transgenes into arthropod populations. Insect Mol Biol. 1999;8:243–255. doi: 10.1046/j.1365-2583.1999.820243.x. [DOI] [PubMed] [Google Scholar]
- 37.Sanchez L. Sex-determining mechanisms in insects. Int J Dev Biol. 2008;52:837–856. doi: 10.1387/ijdb.072396ls. [DOI] [PubMed] [Google Scholar]
- 38.Keverne B. Monoallelic gene expression and mammalian evolution. BioEssays. 2009;31:1318–1326. doi: 10.1002/bies.200900074. [DOI] [PubMed] [Google Scholar]
- 39.Zakharova IS, Shevchenko AI, Zakian SM. Monoallelic gene expression in mammals. Chromosoma. 2009;118:279–290. doi: 10.1007/s00412-009-0206-8. [DOI] [PubMed] [Google Scholar]
- 40.Kassis JA. Pairing-sensitive silencing, polycomb group response elements and transposon homing in Drosophila. Adv Genet. 2002;46:421–38. doi: 10.1016/s0065-2660(02)46015-4. [DOI] [PubMed] [Google Scholar]
- 41.Burt A. Site-specific genes as tools for the control and genetic engineering of natural populations. Proc Biol Sci. 2003;270:921–928. doi: 10.1098/rspb.2002.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deredec A, Burt A, Godfray HC. The population genetics of using homing endonuclease genes in vector and pest management. Genetics. 2008;179:2013–2026. doi: 10.1534/genetics.108.089037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bagla P. Hardy cotton-munching pests are latest blow to GM crops. Science. 2010;327:1439. doi: 10.1126/science.327.5972.1439. [DOI] [PubMed] [Google Scholar]
- 44.Rose RI. A short note on the final Environmental Impact Statement—October 2008: Use of genetically engineered fruit fly and pink bollworm in APHIS plant pest control programs. AsPac J Mol Biol Biotechnol. 2009;17:87–91. [Google Scholar]
- 45.Simmons GS, Alphey L, Vasquez T, Morrison NI, Epton MJ, Miller E, et al. Potential use of a conditional lethal transgenic pink bollworm Pectinophora gossypiella in area-wide eradication or suppression programmes. Area-Wide Control of Insect Pests. 2007;2:119–123. [Google Scholar]
- 46.Davis S, Bax N, Grewe P. Engineered underdominance allows efficient and economical introgression of traits into pest populations. J Theor Biol. 2001;212:83–98. doi: 10.1006/jtbi.2001.2357. [DOI] [PubMed] [Google Scholar]
- 47.Gould F, Huang Y, Legros M, Lloyd AL. A killer-rescue system for self-limiting gene drive of anti-pathogen constructs. Proc Roy Soc B. 2008;275:2823–2829. doi: 10.1098/rspb.2008.0846. [DOI] [PMC free article] [PubMed] [Google Scholar]