Abstract
We recently found that ethanol-induced long-term facilitation (LTF) of NMDAR activity is mediated by NR2B-NMDARs and is observed in the dorsomedial striatum (DMS) but not in the dorsolateral striatum (DLS).9 We also showed that repeated administration of ethanol causes a long-lasting increase in NMDAR activity in the DMS, resulting from ethanol-mediated Fyn phosphorylation of NR2B subunits.9 In this addendum, we report that the different sensitivity of NMDARs to ethanol between the DMS and DLS is not attributed to the abundance of synaptic NR2B-NMDARs or differences in Fyn levels. We further show that LTF is specific for NR2B-, but not NR2A-NMDARs, and that the duration of the in vivo ethanol-mediated increase in NMDAR activity is associated with the period of ethanol exposure, but not with alteration in NR1 or NR2A protein levels. Together, these results suggest that upregulation of NR2B-NMDAR activity by ethanol is selective and that ethanol's effect on NMDAR activity is gradual and cumulative.
Key words: NMDA receptor, fyn, dorsal striatum, alcohol, addiction
Introduction
Recent studies suggest that development of compulsive drug-seeking and -taking depends on molecular neuroadaptations in the dorsal striatum.1–3 We previously found that acute ex vivo ethanol exposure and withdrawal results in long-term facilitation (LTF) of NMDAR activity in the dorsal striatum, which depends on Fyn-mediated phosphorylation of NR2B subunits and plays a key role in ethanol consumption.4 The dorsal striatum can be divided into the DMS and the DLS, which differ in connectivity, receptor distribution, synaptic plasticity and behavioral function.1,5–8 Recently, we showed that LTF of NMDAR activity is centered in the DMS, and that ethanol-mediated activation of Fyn in the DMS correlates to increased phosphorylation levels of NR2B subunits at synaptic membranes, leading to a long-lasting enhancement of channel activity in response to ethanol.9 Similarly, excessive ethanol consumption results in a remarkable long-lasting upregulation of synaptic NMDAR activity and NR2B phosphorylation in the DMS.9 Finally, we showed that inhibition of the Fyn/NR2B-NMDAR pathway specifically in the DMS decreases operant ethanol self-administration and reinstatement of ethanol seeking in rats.9 The results presented herein expand the role of NR2B subunits in the long-lasting upregulation of NMDAR activity in the DMS in respond to ethanol.
Results
Differential sensitivity of NMDARs to ethanol is not attributed to the abundance of synaptic NR2B-NMDARs.
We previously showed that acute exposure of striatal slices to, and withdrawal from, ethanol induces LTF of NMDAR activity in the DMS but not DLS.9 We further showed that the brain region specificity is not due to differential total levels of the NR2B or NR2A subunit protein in the two brain regions.9 Heterogeneity in synaptic NR2 subunits of NMDARs in the DMS and DLS may account for the region-specific sensitivity of NMDARs to ethanol.10 Thus, we tested whether inhibition of NR2B-NMDARs in the DMS and DLS produce distinct synaptic responses. We found that the degree of inhibition of NMDAR-mediated excitatory postsynaptic currents (NMDAR-EPSCs) in the presence of an NR2B-NMDAR-specific antagonist, Ro 25-6981 (0.25 µM), is similar in both subregions (Fig. 1), suggesting that the abundance of synaptic NR2B-NMDARs is not responsible for the different sensitivity of NMDARs to ethanol in the DMS and DLS.
Figure 1.
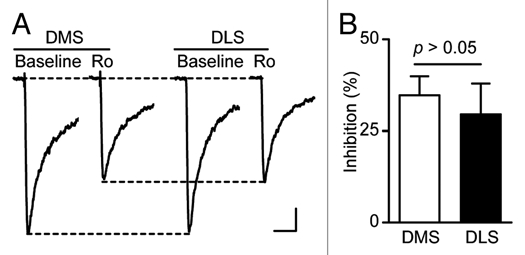
The abundance of synaptic NR2B-NMDARs does not differ between the DMS and DLS in naïve rats. Striatal slices were prepared from 3- to 4-week-old Sprague Dawley rats. NMDAR-mediated EPSCs were measured in the presence of 0.05 mM Mg2+, 0.1 mM picrotoxin, 0.01 mM NBQX and with neurons clamped at −70 mV. Inhibition of the EPSCs in the presence of a NR 2B-NMDAR specific antagonist, Ro 25-6981, was similar in DMS and DLS neurons. (A) Representative traces of NMDAR-mediated EPSCs in the absence (Baseline) and presence (Ro) of Ro 25-6981 (0.25 µM) in DMS (left) and DLS (right) neurons. Scale bars: 100 ms, 20 pA. (B) Bar graph showing no difference in the extent of inhibition of EPSCs by Ro 25-6981 between DMS and DLS neurons. p > 0.05 by t-test, n = 6 for DMS and DLS.
Differential sensitivity of NMDARs to ethanol is not attributed to differential protein levels of Fyn.
We previously showed that acute ex vivo or in vivo exposure to ethanol increases Fyn activity in the hippocampus and dorsal striatum,4,9,11 leading to increased NR2B phosphorylation.4,9,11 To test whether the differential sensitivity of NMDARs to ethanol in the DMS and DLS is associated with distinct expression of Fyn, we measured protein levels of Fyn. Since the electrophysiological and behavioral experiments were performed in juvenile and adult rats respectively, we determined the level of Fyn in the DLS and DMS in both ages. We found that Fyn levels do not differ in these subregions (Fig. 2) suggesting that the protein level of Fyn is not responsible for the differential sensitivity of NMDARs to ethanol.
Figure 2.
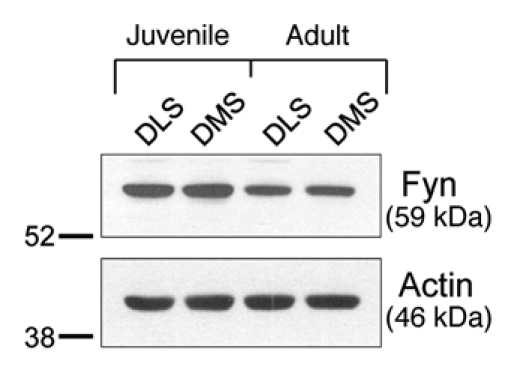
Fyn protein levels do not differ between the DLS and DMS in naïve rats. Western blot analysis was used to examine the total protein levels of Fyn kinase in the DLS and DMS of juvenile (21 days) and adults (90 days) Sprague-Dawley rats. Actin was used as a loading control.
Ethanol-mediated LTF is observed in the DMS of NR2A knockout (KO) mice.
We previously showed that the NR2B subunit is required for ethanol-mediated LTF of NMDAR activity in the dorsal striatum.4,9 To determine whether the NMDAR-containing NR2A subunit (NR2A-NMDAR), another major NR2 subunit in the dorsal striatum,12,13 also contributes to LTF, we compared the level of LTF upon acute ex vivo ethanol exposure and withdrawal in the DMS of NR2A KO14 and wild-type (WT) littermate mice. We reasoned that if NR2A-NMDARs are required for LTF, we should observe less or no LTF in brain slices from NR2A KO mice compared to WT littermate mice. We found that LTF of NMDAR-EPSCs following acute ethanol withdrawal was not smaller but actually greater in DMS neurons from NR2A KO mice compared to WT (Fig. 3). These results suggest that NR2A subunits are not required for LTF in response to acute ethanol exposure, and strongly suggest a specific contribution of NR2B-NMDARs to ethanol-mediated LTF in the DMS.
Figure 3.
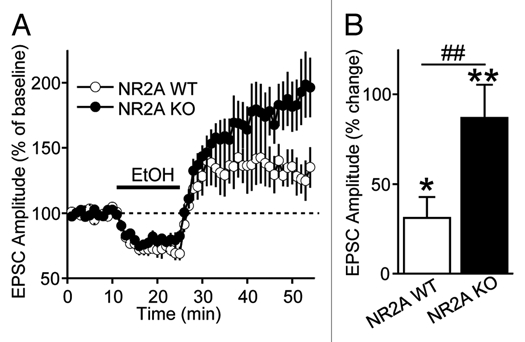
The NR2A subunit-containing NMDAR does not contribute to ethanol-mediated LTF in the DMS. (A) Time-course of NMDAR-EPSCs in DMS neurons from NR2A knockout (KO) and wild-type (WT) mice before, during and after bath application of ethanol (80 mM). n = 7 slices for each group. (B) Bar graph comparing the magnitude of LTF in the DMS from NR2A WT and NR2A KO mice. LTF is calculated as the magnitude of averaged EPSCs from 21–30 min after ethanol was washed out. *p < 0.05 vs. baseline, **p < 0.01 vs. baseline, ##p < 0.05 WT vs. KO. Two-way ANOVA with repeated measures. n = 7 (NR2A KO mice). n = 7 (WT mice).
The duration of ethanol-mediated increases in NMDAR activity is associated with the period of ethanol exposure.
Next, we examined whether in vivo ethanol exposure and withdrawal cause an increase in NMDAR activity in the DMS. We found that a single systemic administration of ethanol did not alter NMDAR activity in the DMS when measured 16 hrs after the ethanol treatment (Fig. 4A). These findings lead to the hypothesis that repeated ethanol exposure and withdrawal is required to obtain a gradual and cumulative increase in NMDAR activity. As predicted, we observed that once-daily administration of ethanol for seven days increases NMDA-elicited currents and NMDAR-EPSCs9 but not the frequency of miniature EPSCs (mEPSCs) in DMS neurons (Fig. 4B), suggesting that repeated ethanol administration increases postsynaptic NMDAR function. The increases were observed 16 hrs,9 but not 40 hrs after the last of seven ethanol administrations (Fig. 4C). However, prolonged 14 daily administrations of ethanol caused similar increases in NMDAR function 40 hrs after the last ethanol administration.9 These results suggest that repeated ethanol exposure and withdrawal results in a cumulative long-lasting increase in NMDAR activity in the DMS, supporting the model described by Wang et al.9
Figure 4.
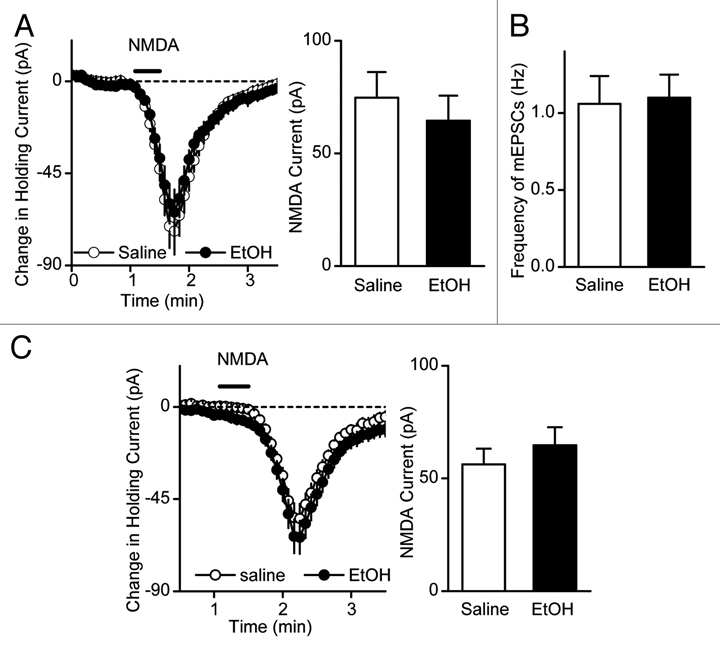
Duration of the ethanol-mediated increase in NMDAR activity is associated the period of ethanol exposure. (A) Single administration of ethanol did not lead to a detectable change in NMDA-induced current. Rats were systemically administrated with 2 g/kg of ethanol or saline and striatal slices were prepared 16 hrs after the administration to measure NMDA-induced currents. Left, Changes in holding currents were measured after NMDA (10 µM, 30 s) was applied to the slices. n = 16 (saline) and 16 (EtOH). Right, Bar graph summarizing the peak amplitudes of NMDA-induced currents in DMS neurons from ethanol- and saline-treated rats. p > 0.05 by t-test. (B) Repeated ethanol administration does not alter presynaptic release of neurotransmitter. The frequency of miniature EPSCs (mEPSCs) were compared in DMS neurons from saline- and ethanol-treated animals. n = 25 (saline) and 29 (ethanol) slices. p > 0.05, Mann-Whitney Rank Sum test. (C) Seven administrations of ethanol did not alter NMDA-induced currents 40 hrs later after the last treatment. Rats were systemically administered with 2 g/kg of ethanol or saline daily for seven days and striatal slices were prepared 40 hrs after the last administration to measure NMDA-induced currents. Left, Changes in holding currents were measured after NMDA (10 µM, 30 s) was applied to the slices. n = 14 (saline) and 14 (EtOH). Right, Bar graph summarizing the peak amplitudes of NMDA-induced currents in DMS neurons from ethanol- and saline-treated rats. p > 0.05 by t-test.
Repeated administration of ethanol or excessive ethanol consumption does not alter protein levels of NR1 or NR2A subunits in the DMS.
We previously reported that repeated systemic administration of ethanol or high levels of voluntary ethanol drinking results in a long-lasting increase in NMDAR activity.9 Moreover, we found that the NR2B-NMDAR contribution to the overall channel activity was associated with increased phosphorylation and membrane localization of NR2B subunits in the DMS.9 Here, we report that repeated daily administration of ethanol did not alter total protein levels (Fig. 5A) or membrane localization (Fig. 5B) of NR2A or NR1 subunits, and that excessive ethanol-drinking which results in long-lasting increase in NR2B phosphorylation,9 does not alter total protein levels of NR1 or NR2A subunits (Fig. 5C). Together, these data suggest that ethanol exposure causes a selective long-lasting alteration in NR2B-NMDARs in the DMS resulting in an increase in channel function.
Figure 5.

Repeated ethanol administration did not alter the protein levels of NR1 or NR2A subunits in the DMS. (A and B) Sprague-Dawley rats were treated with saline or ethanol (2 g/kg, i.p.) once a day for seven days. DMS was dissected 16 hrs after the last injection. (A) Repeated ethanol administration did not alter the protein levels of NR1 or NR2A subunits in total DMS homogenates. Left, image is representative of n = 3 for NR1 (top) and n = 9 for NR2A (bottom). p > 0.05 vs. saline-treated rats (two-tailed t-test). (B) Repeated ethanol administration did not alter the protein levels of synaptosomal NR1 or NR2A subunits in the DMS. Left, image is representative of n = 6 (saline) and n = 6 (ethanol) for NR1 (top). n = 6 (saline) and n = 5 (ethanol) for NR2A (bottom). (A and B) Middle and right, bar graphs summarizing the averaged changes in protein levels of NR1 (middle) and NR2A subunits (right). Western blot data was normalized to GAPDH and plotted as percentage of saline treatment. p > 0.05 vs. saline-treated rats (two-tailed t-test). (C) Intermittent access to ethanol did not alter the protein levels of NR1 or NR2A in total DMS homogenates. Long Evans rats were exposed to 18 ethanol-drinking sessions under an intermittent access to 20% ethanol in a two-bottle-choice paradigm. One day after the last drinking session, DMS tissue was dissected out and protein levels of NR1 and NR2A subunits in homogenates were measured. Left, Image is representative of n = 7 (water). n = 8 (ethanol). Middle and right, bar graphs summarizing the averaged changes in protein levels of NR1 (middle) and NR2A subunits (right). Western blot data was normalized to GAPDH and plotted as percentage of water treatment. p > 0.05 vs. control (two-tailed t-test).
Materials and Methods
All materials and methods used in the current study, except for the information regarding the NR2A KO mice, are described in Wang et al.9 Briefly, NR2A KO mice and their corresponding WT littermates were generated by in-house breeding of male and female NR2A heterozygous mice. Mice genotype was determined by polymerase chain reaction (PCR) analysis of products derived from tail DNA, and were used for experiments at three to five weeks of age. For electrophysiological recordings, NMDAR-mediated EPSCs were recorded at −70 mV and in the presence of 0.05 mM Mg2+, 0.01 mM NBQX and 0.1 mM picrotoxin in the bath solution. To measure NMDA-induced currents, NMDA (10 µM) was bath applied for 30 s and holding currents were measured every 5 s.
Conclusions
We show that neither the abundance of synaptic NR2B-NMDARs nor Fyn protein level accounts for the difference in NMDAR sensitivity to ethanol between the DMS and DLS. We also report that NR2A-NMDARs are not required for LTF of NMDAR activity. Finally, we found that the duration of ethanol-mediated increase in NMDAR activity is determined by the period of ethanol exposure and is associated with neither protein levels nor membrane localization of NR1 and NR2A subunits. Together, these results suggest that ethanol exposure causes a selective long-lasting alteration in NR2B-NMDARs in the DMS resulting in an increase in channel function.
We recently demonstrated that inhibition of NR2B-NMDAR/Fyn pathway in the DMS reduces operant ethanol self-administration and reinstatement of ethanol seeking.9 These data, together with the data presented herein, strongly suggest that the specific long-term activation of the Fyn/NR2B-NMDAR pathway specifically within the DMS is an important player in the mechanism underlying excessive ethanol-drinking behavior. Facilitation of NMDAR activity following repeated cycles of ethanol bouts and withdrawal,4 may lead to alteration of AMPAR-LTP in the DMS, which is NMDAR-dependent.15 Such aberrant plasticity may be part of the mechanism involved in the transition from social drinking to excessive and compulsive ethanol intake and relapse.
Acknowledgements
This work was supported by the National Institute on Alcohol Abuse and Alcoholism (R01AA/MH13438-O1A1, Dorit Ron), by the funds provided by the state of California for medical research on alcohol and substance abuse through the University of California, San Francisco (Dorit Ron), and by ABMRF/The Foundation for Alcohol Research (Jun Wang). We would like to thank Dr. David M. Lovinger for providing the NR2A KO mice for our studies.
Abbreviations
- DLS
dorsolateral striatum
- DMS
dorsomedial striatum
- LTF
long-term facilitation
- mEPSCs
miniature excitatory postsynaptic currents
- NMDAR
N-methyl D-aspartate receptor
- NR2A-NMDAR
NR2A subunit-containing NMDAR
- NR2A KO mice
NR2A knockout mice
- NR2B-NMDAR
NR2B subunit-containing NMDAR
- NMDAR-EPSCs
NMDAR-mediated excitatory postsynaptic currents
References
- 1.Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behav Brain Res. 2009;199:89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 2.Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Carnicella S, Phamluong K, Jeanblanc J, Ronesi JA, Chaudhri N, et al. Ethanol induces long-term facilitation of NR2B-NMDA receptor activity in the dorsal striatum: implications for alcohol drinking behavior. J Neurosci. 2007;27:3593–3602. doi: 10.1523/JNEUROSCI.4749-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joel D, Weiner I. The connections of the dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience. 2000;96:451–474. doi: 10.1016/s0306-4522(99)00575-8. [DOI] [PubMed] [Google Scholar]
- 6.Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- 8.Gerdeman GL, Partridge JG, Lupica CR, Lovinger DM. It could be habit forming: drugs of abuse and striatal synaptic plasticity. Trends Neurosci. 2003;26:184–192. doi: 10.1016/S0166-2236(03)00065-1. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Lanfranco MF, Gibb SL, Yowell QV, Carnicella S, Ron D. Long-lasting adaptations of the NR2B-containing NMDA receptors in the dorsomedial striatum play a crucial role in alcohol consumption and relapse. J Neurosci. 2010;30:10187–10198. doi: 10.1523/JNEUROSCI.2268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol. 2007;7:39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Yaka R, Phamluong K, Ron D. Scaffolding of Fyn kinase to the NMDA receptor determines brain region sensitivity to ethanol. J Neurosci. 2003;23:3623–3632. doi: 10.1523/JNEUROSCI.23-09-03623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, et al. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- 13.Standaert DG, Testa CM, Young AB, Penney J., Jr Organization of N-methyl-D-aspartate glutamate receptor gene expression in the basal ganglia of the rat. J Comp Neurol. 1994;343:1–16. doi: 10.1002/cne.903430102. [DOI] [PubMed] [Google Scholar]
- 14.Kiyama Y, Manabe T, Sakimura K, Kawakami F, Mori H, Mishina M. Increased thresholds for long-term potentiation and contextual learning in mice lacking the NMDA-type glutamate receptor epsilon1 subunit. J Neurosci. 1998;18:6704–6712. doi: 10.1523/JNEUROSCI.18-17-06704.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Partridge JG, Tang KC, Lovinger DM. Regional and postnatal heterogeneity of activity-dependent long-term changes in synaptic efficacy in the dorsal striatum. J Neurophysiol. 2000;84:1422–1429. doi: 10.1152/jn.2000.84.3.1422. [DOI] [PubMed] [Google Scholar]


