Abstract
There are few effective therapeutic options for metastatic renal cell carcinoma (RCC). Conventional chemotherapeutic agents are ineffective since these tumors are unusually resistant to DNA damage, likely due to an exuberant DNA repair response. Sorafenib, as one of the few available effective therapeutic options for metastatic RCC, has been shown to inhibit cell proliferation by inhibition of tyrosine kinases. We have recently shown that sorafenib inhibits soluble epoxide hydrolase, which catalyzes metabolism of the anti-inflammatory epoxyeicosatrienoic acids. Given previous work demonstrating the anti-apoptotic role of p21 in RCC as a potential mechanism for its drug resistance, we asked whether sorafenib signals through this pathway. We now show that sorafenib markedly decreases p21 levels in several RCC and hepatocellular carcinoma cells. Neither the MEK inhibitor PD98059 nor the sEH inhibitor t-AUCB, which represent known sorafenib-targeted signaling pathways, alter p21 levels, demonstrating that the p21 inhibitory effect of sorafenib is independent of these signaling cascades. In cells treated with doxorubicin to augment p21, sorafenib markedly decreases this protein, and the combinations of paclitaxel or doxorubicin with sorafenib show additive cytotoxicity as a function of the VHL status of the cells, suggesting that lower doses of each agent could be used in the clinical setting. In summary, we show a novel signaling pathway by which sorafenib exerts its salutary effects in RCC; future work will focus on the use of these drug combinations in the context of conventional therapeutics, and novel compounds and protocols targeting p21 in conjunction with sorafenib should be pursued.
Keywords: sorafenib, p21, kidney cancer, apoptosis, DNA damage, soluble epoxide hydrolase
Introduction
Renal cell carcinoma (RCC) is the sixth most common cancer in the United States and one of the few cancers whose incidence is increasing, and survival of patients with metastatic RCC is dismal (26% 5-y survival of TNM Stage IV based on 2005 statistics).1 For the one-third of patients who present with metastatic disease, there are few therapeutic options available since conventional chemotherapeutic and immunomodulatory approaches are ineffective. An important area of research in our and other laboratories relates to the mechanism of RCC chemotherapy resistance, which is a serious clinical problem and likely due to an exuberant DNA repair mechanism mediated by the p53 tumor suppressor pathway, and subsequent induction of the downstream antiapoptotic molecule p21.2,3
We have previously shown that p21, a cyclin-dependent kinase inhibitor intimately involved in p53 signaling, can direct cells into the growth suppressive or anti-apoptotic pathways.3 Consistent with this finding, p21 has been shown to be a prognostic marker indicating worse survival when cytosolically located in both RCC4 and breast cancer.5 In addition, forced cytosolic localization of p21 results in anti-apoptosis6,7 and growth promotion8 in different cell types. Indeed, and likely for this reason, p21 induction has been shown to be an early event in oncogenesis.9
Sorafenib is a multi-kinase inhibitor which targets both angiogenic and non-angiogenic targets in cancer. This agent is in current clinical use to treat advanced RCC10 as well as unresectable hepatocellular carcinoma.11 However, there are severe, although rare, substantial adverse events associated with the use of this drug, such as cardiac ischemia, left ventricular dysfunction, neutropenia and hypertension. Thus, novel mechanisms of sorafenib are being evaluated in order to narrow the molecular targets associated with its therapeutic application thereby decreasing adverse events. Sorafenib has not previously been shown to have a specific effect upon p21, which lies downstream of p53 and which conveys the survival effect necessary for DNA repair. Due to the likely pivotal role of p21 induction in chemotherapy failure in RCC as well as other cancers, we asked whether one of the mechanisms by which sorafenib exerts its beneficial therapeutic effect is via inhibition of p21.
The soluble epoxide hydrolase (sEH) converts epoxyeicosatrienoic acids (EETs) to the less active dihydroxyeicosatrienoic acids (DHETs).12 The EETs have been demonstrated to be vasodilators in various animal models and play an important role in regulation of blood pressure as well as control and prevention of heart disease.13–16 The sEH inhibitors have been shown to stabilize the EET levels and thus have beneficial effects on hypertension,17 nociception,18 atherosclerosis19 and inflammation20 through increasing endogenous levels of EETs and other lipid epoxides. Previous work in our laboratory has demonstrated that sorafenib is a potent inhibitor (KI = 17 ± 4 nM) of the soluble epoxide hydrolase (sEH), an enzyme with pleiotropic effects on inflammation and vascular disease,21 this finding suggests that sEH inhibition may account for at least part of the effect of sorafenib on RCC. In our ongoing studies of unexpected mechanisms of sorafenib signaling in RCC, we now show that p21 is markedly inhibited by sorafenib in both kidney and liver cancer cell lines, and that this result is independent of this drug's known effects on both MEK/ERK and sEH pathways. Furthermore, despite the substantial induction of p21 by the doxorubicin, sorafenib is still able to markedly downregulate p21 and thereby contribute to the cytotoxicity of DNA-damaging chemotherapy in this combination therapy.
Our finding of the unexpected attenuation of a major antiapoptotic protein which is induced by chemotherapy by a currently available drug suggests the need for further exploration of combination therapy with sorafenib, or inhibitors of p21, and DNA-damaging agents in RCC, both in vitro and in vivo. In light of this data, additional studies directed at novel and specific methods of p21 attenuation as a therapeutic manipulation in kidney and other cancers are currently underway in our laboratory.
Results
Sorafenib decreases levels of p21 independently from MEK/ERK and sEH pathways.
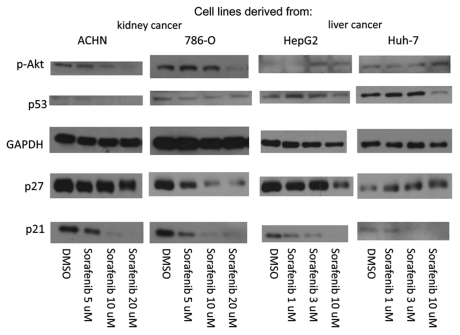
The cyclin kinase inhibitor p21 is a p53-pathway protein induced by DNA damage or cellular stress and possesses differential effects on apoptosis depending on cell lines and conditions (reviewed in ref. 2 and 3); we have previously demonstrated an anti-apoptotic effect of p21 in RCC cell lines.22,23 This survival function of p21 likely maintains cell viability during the DNA repair process,3 such that attenuation of p21 can subvert this repair process and thereby sensitize RCC23 and other cancer cells24–27 to conventional chemotherapy. Due to sorafenib's efficacy in metastatic RCC, a disease with substantial resistance to conventional chemotherapy, we asked whether at least part of this drug's salutary effect is mediated by p21 modulation. Given the fact that steady-state plasma levels of sorafenib of up to 10 mg/l (16 µM) can be achieved by oral administration,28,29 we incubated two separate RCC and hepatocellular carcinoma cell lines for 24 h with sorafenib at concentrations ranging from 1–20 µM. Substantial decrease in p21 was seen in all cell lines tested, and occurred in a dose-dependent manner and at pharmacological plasma levels30 (Fig. 1).
Figure 1.
Sorafenib attenuates p21 in RCC and HCC cell lines. RCC (ACHN and 786-O) and HCC (HepG2 and Huh-7) cells were grown to confluence in 10% serum-containing media and treated with sorafenib or vehicle (DMSO) at the indicated concentrations for 24 h. The cells were harvested and immunoblotted with the antibodies indicated. GAP DH is a loading control. The experiment shown is representative of at least three separate experiments.
To determine specificity of sorafenib for p21 downregulation, we examined the levels of several other proteins relevant p21 signaling, including p53, the cyclin kinase inhibitor p27, and p-Akt which has been shown to phosphorylate and thereby stabilize p21 to promote cell survival.31 Despite the fact that p21 was markedly attenuated in all four cell lines, both p-Akt and p27 showed inconsistent levels of decrease or induction when all cell lines were evaluated in aggregate (Fig. 1), suggesting that the sorafenib effect on p21 is specific and independent of the Akt/PKB signaling pathway. In addition, despite the parallel attenuation of p53 to p21 observed in some of the cell lines, the mechanism of p21 downregulation by sorafenib is unlikely to be solely through p53, since p27, which also lies downstream of p53, is not consistently decreased after sorafenib treatment among all cell lines (i.e., it is increased in Huh-7 cells).
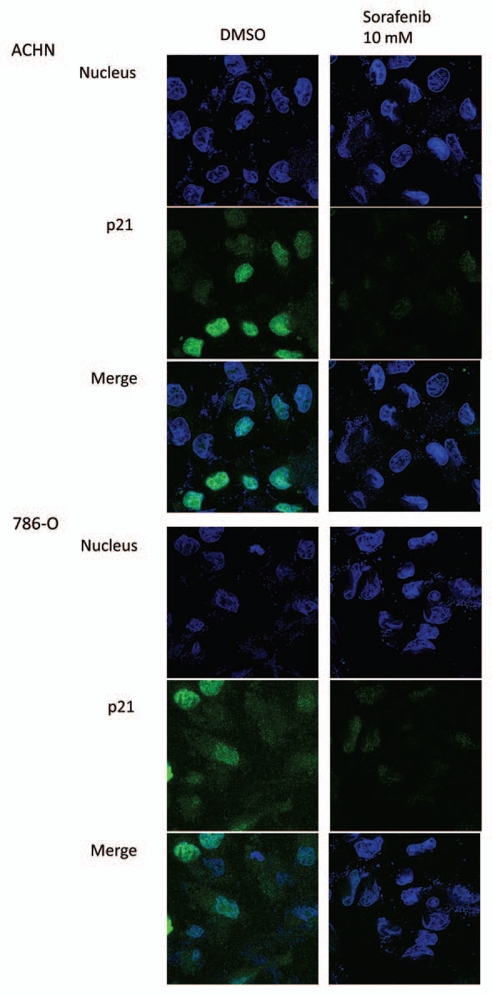
To confirm the immunoblotting data showing that p21 is decreased by sorafenib in RCC cells, we performed immunofluorescence in both of the RCC cell lines used in this study. Basal levels of p21 were seen in both cytosolic and nuclear compartments in ACHN cells, with higher levels in the nucleus as expected since p21 was not induced by cellular stress. When incubated with sorafenib at 10 µM, there was a marked decrement of p21 in both compartments (Fig. 2), consistent with the immunoblotting data (Fig. 1). In addition, this finding is consistent with at least partial p53 independence of sorafenib-induced p21 attenuation, since 786-O cells are mutant for the von Hippel-Lindau tumor suppressor gene (which is seen in most sporadic cases of clear cell RCC and contributes to its pathogenesis32–34), hence have unstable p53,35 and appear to have quantitatively less p21 using similar confocal microscopy settings.
Figure 2.
Sorafenib inhibits both cytosolic and nuclear p21 in RCC cells. ACHN and 786-O cells were grown to confluence on 8 well chamber slides and treated with sorafenib or vehicle (DMSO) at the indicated concentrations for 24 h. The cells were subjected to immunofluorescence visualization by confocal microscopy as described in Materials and Methods. p21 is green and the nuclear dye (DAP I) is blue.
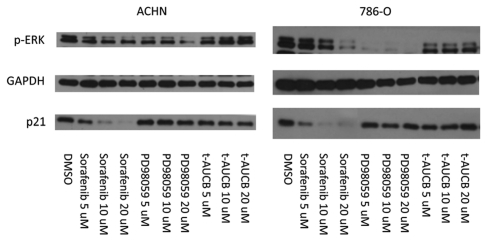
Sorafenib is well known as a multi-kinase inhibitor of Raf, VEGFR and PDGFR kinases and has growth inhibitory effects attributed to its effects on these signaling cascades through MEK/ERK pathway attenuation.36 Thus, in light of this fact and our earlier findings that sorafenib possesses sEH inhibitory effects,21 we next asked whether the mechanism of sorafenib's inhibition of p21 expression is related to alteration of these signaling pathways. Both p53-wild type ACHN and VHL mutant 786-O cells were treated with the specific MEK inhibitor, PD98059 or a specific sEH inhibitor, t-AUCB.21,37 While p-ERK, which lies downstream of MEK, was decreased by both sorafenib and PD98059 in both cell lines, p21 was decreased only by sorafenib and not by either PD98059 or t-AUCB (Fig. 3). Thus, downregulation of p21 levels by sorafenib occurs independently of the MEK/ERK and sEH pathways.
Figure 3.
Sorafenib attenuates p21 independently from p-MEK and sEH inhibition. ACHN cells were grown to confluence and treated with sorafenib, PD98059, t-AUCB or vehicle (DMSO) at the indicated concentrations for 24 h. The cells were harvested and immunoblotted with the antibodies indicated. The experiment shown is representative of at least three separate experiments.
The cytotoxicity of sorafenib is greater than that of MEK/ERK and sEH inhibitors.
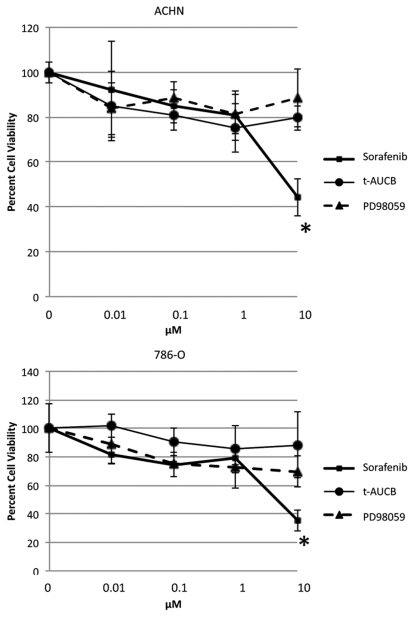
As a multi-kinase inhibitor, sorafenib has profound effects on cell viability, but it is not clear which kinases or other signaling proteins are actually responsible for its cytotoxic effect in RCC cells. As we have already shown that p21 inhibition was independent of MEK/ERK pathway proteins and sEH inhibition, we next asked whether these pathways are required for sorafenib's cytotoxic effects in RCC by performing MTT assays of sorafenib in parallel with inhibitors of these individual signaling pathways. When ACHN cells were incubated with PD98059, t-AUCB, or sorafenib at the same concentrations, sorafenib alone showed greater than 50% cell death at 10 µM, while, surprisingly, PD98059 and t-AUCB showed non-significant cytotoxicity at this dose (Fig. 4). Thus, sorafenib causes cytotoxicity either independently of the MEK/ERK and sEH signaling pathways, or via a combination of these and other pathways; these data, in light of our previous work showing that p21 attenuation shows cytotoxicity in these cell lines,23 suggest that the decrease in p21 protein is at least in part responsible for the profound cytotoxicity of sorafenib.
Figure 4.
Sorafenib demonstrates greater cytotoxicity than p-MEK or sEH inhibition. ACHN and 786-O cells grown to confluence in complete growth media (10% serum) and treated with sorafenib, t-AUCB, PD98059 or vehicle (DMSO) for 72 h. Subsequently, an MTT assay was performed as described in Materials and Methods. Error bars are standard deviations in quadruplicate. The experiment shown is representative of at least three separate experiments. *p < 0.05 of sorafenib compared with both other additions.
Sorafenib attenuates p21 despite chemotherapeutic induction by doxorubicin and leads to additive cytotoxicity.
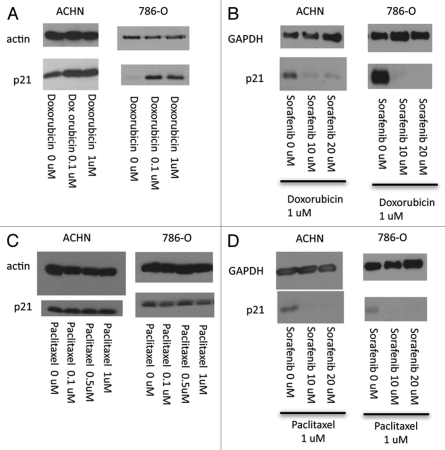
Both doxorubicin and paclitaxel cause DNA damage which is presumed to contribute to their anticancer effects.38,39 However, in order for this mechanism to be effective, the DNA repair process has to be subverted or overwhelmed, or DNA damage will be repaired and the cancer cells will survive due to lack of apoptosis.3 In addition, given the frequency and/or severity of adverse events using high doses of most chemotherapeutic agents when used alone, it is often desirable to reduce the dose by means of combination chemotherapy. Thus, we next asked whether p21 can remain attenuated by sorafenib even when it is augmented as a result of DNA damage from chemotherapy. When ACHN and 786-O cells were incubated with doxorubicin, p21 was increased as expected due to DNA damage (or other cellular stress) (Fig. 5A), yet despite this marked increase, p21 was still markedly decreased by sorafenib in both cell lines (Fig. 5B). Interestingly, there was no induction of p21 by paclitaxel at the same concentrations (Fig. 5C), possibly due to less quantitative DNA damage by this agent at given concentrations as compared with doxorubicin. As with doxorubicin, however, p21 was similarly reduced by the combination of sorafenib and paclitaxel (Fig. 5D).
Figure 5.
Sorafenib attenuates p21 in RCC cells treated with doxorubicin or paclitaxel. ACHN and 786-O cells grown to confluence in media containing 10% serum and treated with sorafenib or vehicle (DMSO) without (A and C) or with (B and D) doxorubicin or paclitaxel at the indicated doses for 24 h. The cells were harvested and immunoblotted with the antibodies indicated. The experiment shown is representative of at least three separate experiments.
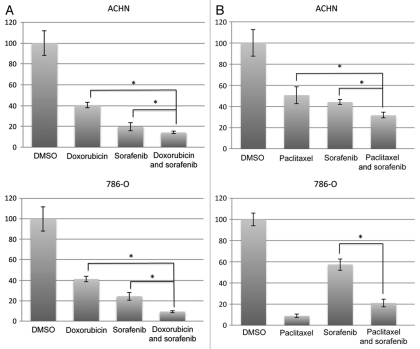
Given our finding that p21 is decreased by sorafenib, we next asked whether sorafenib capitalizes on the anti-apoptotic effect of this protein for its in vitro cytotoxicity in the face of DNA-damaging chemotherapeutics. Such a finding would be evidenced by increasing cytotoxicity with the combination of DNA damaging chemotherapy and p21-attenuating sorafenib, and would be expected to be at least partially dependent on the p53 status of the cell lines investigated. Doxorubicin alone at 0.1 µM caused the expected cytotoxic effect in both ACHN and 786-O cells as assessed by an MTT assay (Fig. 6A). Upon incubation with the combination of doxorubicin (0.1 µM) and sorafenib (at 10 µM), there was an additive effect on cytoxicity of doxorubicin in both cell lines (Fig. 6A). Lower concentrations of doxorubicin did not elicit an additive effect with sorafenib (data not shown). At 1 µM of doxorubicin, toxicity was too great to observe an additive effect with sorafenib (data not shown).
Figure 6.
Sorafenib has additive effect to chemotherapy treatment. ACHN and 786-O cells were plated in 10% serum-containing media and treated with (A) doxorubicin (0.1 µM) or (B) paclitaxel (10 µM) in the presence or absence of sorafenib (10 µM) in complete (10% serum) growth media for 72 h. Subsequently, an MTT assay was performed as described in Materials and Methods. The experiment shown is representative of at least three separate experiments. * p < 0.05 comparing single vs. double treatment.
In contrast, in the case of paclitaxel, which did not augment p21 (Fig. 5), there was an additive effect of the combination of this drug (10 µM) and sorafenib (10 µM) in ACHN cells, but there was no additive effect at any concentration tested in the VHL-mutant 786-O cells (Fig. 6B). As in the case of doxorubicin, lower concentrations of paclitaxel did not elicit additive effects with sorafenib (10 µM; data not shown). Thus, the combination of sorafenib and doxorubicin led to a consistent additive cytotoxic effect in p53 wild-type ACHN cells, whereas the combination of sorafenib and paclitaxel, which did not increase p21, resulted in cytotoxicity in just one RCC cell line (p53-wt ACHN), consistent with the necessity of p21 downregulation for the full cytotoxic effect of sorafenib. In addition, in the VHL mutant and hence p53 unstable cell line,35 786-O, there was no additive at any concentrations in response to paclitaxel. Thus, the additive effect of sorafenib and DNA-damaging therapeutics in RCC cells is a function of the p21 inducing property of the chemotherapy as well as of the p53 status of the cells examined; furthermore, this data support the necessity of p21 attenuation for the full apoptotic effect of sorafenib in RCC cells.
Discussion
For many cancers, treatment with DNA damaging agents, at doses required for clinical efficacy, are associated with unacceptable adverse effects as well as inadequate cure rates. Kidney cancer is notoriously resistant to chemotherapy as well as conventional immunotherapy, although recent work with kinase inhibitors has shown promise for late-stage disease.1 A possible reason for chemotherapy resistance is failure of DNA damaging agents, when used alone, to cause cancer cell apoptosis, since inactivation of apoptosis pathways after cellular stress is essential for cancer development.40,41 The mechanism by which a cell escapes apoptosis after being subjected to stresses leading, for example, to DNA strand breakage is assumed to be through induction of the anti-apoptotic protein p21. This p21 induction, either dependent or independent of p53, functions to arrest growth in G1 such that DNA damage can be repaired.3,42 By exploiting the anti-apoptotic function of p21 to therapeutic advantage in several previous studies in RCC cells using both antisense oligodeoxynucleotides23,25 and small molecule inhibitors,43 we have identified another paradigm with which to attack therapy-resistant kidney cancers at their Achilles' heel. Attenuation of p21 causing increase cell killing, and for which the paradigm described here would be expected to hold, is not limited to kidney cancer, as this finding also been shown in colon44,45 and breast cancer,25 and in malignant mesothelioma.46 The importance of the current work lies not only in our demonstration of a novel and unexpected mechanism of action of sorafenib, but also in the fact that we now show that an already approved and currently utilized drug has p21 attenuating effects and could therefore be rapidly moved to the clinic.
Sorafenib was originally described as a multi-kinase inhibitor with specificity for the Raf, VEGFR and PDGFR kinases,36 and we have recently shown that this drug also inhibits activity of sEH, an enzyme which catalyzes metabolism of the anti-inflammatory epoxyeicosatrienoic acids.21 The affected growth factor receptors activate the Raf/MEK/ERK pathway which induces cell proliferation. Furthermore, in clear cell RCC (ccRCC), which is by far the most common RCC histology type in humans,47 loss of VHL function, which occurs in the majority of ccRCCs, results in constitutive activation of the HIF-1α transcript leading to abnormal activation of VEGFR. Thus the mechanism of sorafenib's action as targeted therapy for ccRCC has been assumed to involve mainly inhibition of Raf and VEGFR and thereby reduction of cell proliferation. In this study, we provide a novel mechanism by which sorafenib influences the apoptotic state of RCC cells: through attenuation of p21. To our knowledge, this is the first comprehensive study of sorafenib or any approved drug as a p21 inhibitor.
Sorafenib, at doses that attenuate p21, is considerably more cytotoxic than the MEK/ERK pathway and sEH inhibitors, both of which when added separately did not show a decrease in p21. In light of previous work showing RCC cell cytotoxicity using antisense and small molecule inhibitor approaches to p21 attenuation,23,43 these results strongly suggest that downregulation of p21, independent of MEK/ERK or sEH inhibition, confers a substantial part of the cytotoxicity, and hence the clinical efficacy, of sorafenib. These findings are supported by earlier reports that cytosolic p21 interferes with the activities of caspases,48–50 thereby interfering with the apoptotic process, and that higher cytosolic expression of p21 is associated with a worse outcome in patients with metastatic RCC.4
In RCC, but not in hepatoma cells, p-Akt was decreased by sorafenib in a dose dependent fashion. As it has been shown that p-Akt stabilizes p21 and causes it to remain p21 in the cytosol,31 this finding suggests that a possible mechanism of p21 inhibition by sorafenib, at least in RCC, is indirect and occurs via p-Akt inhibition by sorafenib. In an attempt to address this concern, an Akt inhibitor (Akt Inhibitor V, Triciribine51) was evaluated but failed to decrease p-Akt without substantial toxicity in the RCC cell lines used in this study. So it remains possible that p21 lies at the distal end of several signaling cascades impacted by sorafenib in RCC, and we are currently evaluating other possible indirect mechanisms of p21 inhibition by sorafenib, but, based on the hepatocellular carcinoma data (Fig. 1), it is clear that p-Akt attenuation is not universally required for the downregulation of p21.
Others have examined combinations of sorafenib and conventional chemotherapies, although not DNA-damaging agents, in RCC. An unpublished Phase II report with gemcitabine52 showed that this combination showed no significant side effects, but efficacy was not reported. Sorafenib and doxorubicin showed advantages as compared with each agent separately in HCC patients,53 yet sorafenib and cisplatin showed decreased cellular cytotoxicity in human colorectal carcinoma cell lines.54 Interestingly, the latter study also demonstrated decreased levels of p21 in these cells without comment on the potential significance of this finding or any further experiments related to it. We could find no published studies evaluating the combination of a DNA intercalating agent (such as doxorubicin) or a microtubule inhibiting agent (such as paclitaxel) in RCC. RCCs treated with doxorubicin, by virtue of the DNA damage seen with this agent, would be expected to rely heavily on p21 induction to escape an apoptotic fate, and surprisingly, paclitaxel has been shown to have similar properties.39 This reliance on p21 by both of these agents is consistent with the additive effect of sorafenib and both doxorubicin and paclitaxel in the p53 wild-type ACHN cells seen in our study, with less of an effect in the VHL mutant 786-O cells.
In summary, our data provide a plausible argument that sorafenib can act as a sensitizing agent for conventional chemotherapeutics in the treatment of RCC by attenuating the cyclin-dependent kinase inhibitor p21 which has anti-apoptotic properties in this cancer. Given the high levels of resistance of RCC to conventional chemotherapies, such that these have been largely abandoned in the clinic, the efficacy of the compounds and combinations shown here in vitro should lead to further evaluations of p21 attenuation, by sorafenib as well as other p21-inhibitry methods, in animal and human trials.
Materials and Methods
Materials.
Sorafenib (free base) was purchased from LC Laboratories. Paclitaxel, doxorubicin, and the p-MEK inhibitor (PD98059) were purchased from Sigma. The sEH inhibitor trans-4-[4-(3-adamantan-1-yl-ureido)-cyclo-hexyloxy]-benzoic acid (t-AUCB) was synthesized according to our previously described method.37 Paclitaxel, doxorubicin, PD98059, sorafenib and t-AUCB were dissolved in dimethyl sulfoxide (DMSO). Mouse monoclonal anti-p21WAF1/Cip antibody and anti-GAPDH antibody were obtained from Millipore. Mouse monoclonal anti-phosphor Akt antibody and rabbit monoclonal anti-phospho extracellular signal-regulated kinase (ERK) antibody were obtained from Cell Signaling Technology, Inc. Goat anti-mouse and goat anti-rabbit HRP conjugated IgG were obtained from Bio-Rad. ECL Plus Western Blotting Detection Reagents was obtained from GE Healthcare. Normal goat serum, rabbit monoclonal antip21WAF1/Cip antibody, and Anti-rabbit IgG (H+L), F(ab')2 Fragment (Alexa Fluor® 488 Conjugate) were purchased from Cell Signaling Technology, Inc., VECTASHIELD HardSet Mounting Medium with DAPI was purchased from Vector Laboratories.
Cell lines.
Two human proximal tubule epithelial cancer cell lines, ACHN and 786-O, and the human liver cancer cell line, HepG2, were obtained from the American Type Culture Collection. The human liver cancer cell line, Huh-7, was generously provided by Dr. Jian Wu (Division of Hepatology and Gastroenterology, UC, Davis). ACHN cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (FBS), and 100 units/mL streptomycin and 100 mg/mL penicillin. 786-O cells were maintained in RPMI supplemented with 10% FBS and 100 units/mL streptomycin, and 100 mg/mL penicillin. HepG2 and Huh-7 cells were cultured in Eagle's Minimum Essential Medium supplemented with 10% FBS, and 100 units/mL streptomycin and 100 mg/mL penicillin. Cells were maintained at 5% CO2 at 37°C.
Immunoblotting.
Cells were washed with phosphate buffered saline (PBS) and lysed in lysis buffer composed of 50 mM HEPES, 1% Triton X-100, 10 mM sodium pyrophosphate, 100 mM sodium fluoride and 4 mM EDTA at 4°C. Cell lysates were pelleted. Supernatants were electrophoresed and immunoblotted. Membranes were blocked in 5% nonfat dry milk for 30 min at room temperature and probed with appropriate antibodies. Membranes were then probed with horseradish peroxidase tagged anti-mouse or anti-rabbit IgG antibodies (diluted 3:20,000 and 3:10,000 in 5% nonfat dry milk, respectively) for 2 h at room temperature. Signal was detected using enhanced chemiluminescence (ECL) solutions.
Immunofluorescence.
After appropriate treatments in 8-well chamber slides, the cells were washed with PBS and fixed in 2% paraformaldehyde for 1 h at room temperature. The cells were washed with PBS and blocked in the blocking buffer, 5% normal goat serum and 0.3% Triton X-100 in PBS, for 1 h at room temperature. After blocking, the cells were incubated with rabbit monoclonal anti-p21WAF1/Cip antibody overnight at 4°C. The cells were washed with PBS and incubated with anti-rabbit IgG (H+L), F(ab')2 Fragment Alexa Fluor® 488 Conjugate, diluted 1:1,000 in antibody dilution buffer (1% BSA and 0.3% Triton X-100 in PBS) for two hours in the dark at room temperature. The cells were washed with PBS and coverslipped with vectashield with DAPI. The specimens were examined by confocal microscopy. Three to four randomly selected fields were examined in each of three separate experiments.
MTT assay.
Five × 104 cells were plated in 96-well plates and incubated for 16 h at 5% CO2 at 37°C. After appropriate treatments, the cells were incubated in 20 µl of thiazolyl blue tetrazolium bromide (MTT) solution (5 mg/ml in PBS) with 180 µl of the growth media for 3 h. Then, the MTT solution was removed and the blue crystalline precipitate in each well was dissolved in DMSO (200 µl). Visible absorbance of each well at 540 nm was quantified using a microplate reader.
Statistical analysis.
Comparisons of mean values were performed using the independent samples t-test. A p value of less than 0.05 was considered significant.
Acknowledgments
This work was supported by NIEHS grant ES02710, NIEHS Superfund grant P42 ES04699, and NIHLB grant HL059699 (B.D.H.) T32CA108459 (A.T.W.), and NIH grants 5UO1CA86402 (Early Detection Research Network), 1R01CA135401-01A1, and 1R01DK082690-01A1 (R.H.W.), and the Medical Service of the US Department of Veterans' Affairs (R.H.W.).
Abbreviations
- RCC
renal cell carcinoma
- sEH
soluble epoxide hydrolase
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Weiss RH, Lin PY. Kidney cancer: identification of novel targets for therapy. Kidney Int. 2006;69:224–232. doi: 10.1038/sj.ki.5000065. [DOI] [PubMed] [Google Scholar]
- 2.Gartel AL, Tyner AL. The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther. 2002;1:639–6549. [PubMed] [Google Scholar]
- 3.Weiss RH. p21Waf1/Cip1 as a therapeutic target in breast and other cancers. Cancer Cell. 2003;4:425–429. doi: 10.1016/S1535-6108(03)00308-8. [DOI] [PubMed] [Google Scholar]
- 4.Weiss RH, Borowsky AD, Seligson D, Lin PY, Dillard-Telm L, Belldegrun AS, et al. p21 is a prognostic marker for renal cell carcinoma: implications for novel therapeutic approaches. J Urol. 2007;177:63–68. doi: 10.1016/j.juro.2006.08.073. [DOI] [PubMed] [Google Scholar]
- 5.Winters ZE, Hunt NC, Bradburn MJ, Royds JA, Turley H, Harris AL, et al. Subcellular localisation of cyclin B, Cdc2 and p21(WAF1/CIP1) in breast cancer. association with prognosis. Eur J Cancer. 2001;37:2405–2412. doi: 10.1016/S0959-8049(01)00327-6. [DOI] [PubMed] [Google Scholar]
- 6.Asada M, Yamada T, Ichijo H, Delia D, Miyazono K, Fukumuro K, et al. Apoptosis inhibitory activity of cytoplasmic p21(Cip1/WAF1) in monocytic differentiation. EMBO J. 1999;18:1223–1234. doi: 10.1093/emboj/18.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka H, Yamashita T, Asada M, Mizutani S, Yoshikawa H, Tohyama M. Cytoplasmic p21(Cip1/WAF1) regulates neurite remodeling by inhibiting Rho-kinase activity. J Cell Biol. 2002;158:321–329. doi: 10.1083/jcb.200202071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong Y, Chi SL, Borowsky AD, Fan Y, Weiss RH. Cytosolic p21Waf1/Cip1 increases cell cycle transit in vascular smooth muscle cells. Cell Signal. 2004;16:263–269. doi: 10.1016/S0898-6568(03)00136-0. [DOI] [PubMed] [Google Scholar]
- 9.Biankin AV, Kench JG, Morey AL, Lee CS, Biankin SA, Head DR, et al. Overexpression of p21(WAF1/CIP1) is an early event in the development of pancreatic intraepithelial neoplasia. Cancer Res. 2001;61:8830–8837. [PubMed] [Google Scholar]
- 10.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 11.Keating GM, Santoro A. Sorafenib: a review of its use in advanced hepatocellular carcinoma. Drugs. 2009;69:223–240. doi: 10.2165/00003495-200969020-00006. [DOI] [PubMed] [Google Scholar]
- 12.Newman JW, Morisseau C, Hammock BD. Epoxide hydrolases: their roles and interactions with lipid metabolism. Prog Lipid Res. 2005;44:1–51. doi: 10.1016/j.plipres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Seubert JM, Zeldin DC, Nithipatikom K, Gross GJ. Role of epoxyeicosatrienoic acids in protecting the myocardium following ischemia/reperfusion injury. Prostaglandins Other Lipid Mediat. 2007;82:50–59. doi: 10.1016/j.prostaglandins.2006.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imig JD, Zhao X, Capdevila JH, Morisseau C, Hammock BD. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension. 2002;39:690–694. doi: 10.1161/hy0202.103788. [DOI] [PubMed] [Google Scholar]
- 15.Zhao X, Yamamoto T, Newman JW, Kim IH, Watanabe T, Hammock BD, et al. Soluble epoxide hydrolase inhibition protects the kidney from hypertension-induced damage. J Am Soc Nephrol. 2004;15:1244–1253. [PubMed] [Google Scholar]
- 16.Imig JD, Zhao X, Zaharis CZ, Olearczyk JJ, Pollock DM, Newman JW, et al. An orally active epoxide hydrolase inhibitor lowers blood pressure and provides renal protection in salt-sensitive hypertension. Hypertension. 2005;46:975–981. doi: 10.1161/01.HYP.0000176237.74820.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung O, Brandes RP, Kim IH, Schweda F, Schmidt R, Hammock BD, et al. Soluble epoxide hydrolase is a main effector of angiotensin II-induced hypertension. Hypertension. 2005;45:759–765. doi: 10.1161/01.HYP.0000153792.29478.1d. [DOI] [PubMed] [Google Scholar]
- 18.Schmelzer KR, Inceoglu B, Kubala L, Kim IH, Jinks SL, Eiserich JP, et al. Enhancement of antinociception by coadministration of nonsteroidal anti-inflammatory drugs and soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci USA. 2006;103:13646–13651. doi: 10.1073/pnas.0605908103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ulu A, Davis BB, Tsai HJ, Kim IH, Morisseau C, Inceoglu B, et al. Soluble epoxide hydrolase inhibitors reduce the development of atherosclerosis in apolipoprotein e-knockout mouse model. J Cardiovasc Pharmacol. 2008;52:314–323. doi: 10.1097/F°C.0b013e318185fa3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, Hammock BD. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci USA. 2005;102:9772–9777. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu JY, Park SH, Morisseau C, Hwang SH, Hammock BD, Weiss RH. Sorafenib has soluble epoxide hydrolase inhibitory activity, which contributes to its effect profile in vivo. Mol Cancer Ther. 2009;8:2193–2203. doi: 10.1158/1535-7163.MCT-09-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin PY, Fosmire SP, Park SH, Park JY, Baksh S, Modiano JF, et al. Attenuation of PTEN increases p21 stability and cytosolic localization in kidney cancer cells: A potential mechanism of apoptosis resistance. Mol Cancer. 2007;6:16. doi: 10.1186/1476-4598-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park SH, Park JY, Weiss RH. Antisense attenuation of p21 sensitizes kidney cancer to apoptosis in response to conventional DNA damaging chemotherapy associated with enhancement of phospho-p53. J Urol. 2008;180:352–360. doi: 10.1016/j.juro.2008.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu XF, Xia YF, Li MZ, Wang HM, He YX, Zheng ML, et al. The effect of p21 antisense oligodeoxynucleotides on the radiosensitivity of nasopharyngeal carcinoma cells with normal p53 function. Cell Biol Int. 2006;30:283–287. doi: 10.1016/j.cellbi.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Fan Y, Borowsky AD, Weiss RH. An antisense oligodeoxynucleotide to p21(Waf1/Cip1) causes apoptosis in human breast cancer cells. Mol Cancer Ther. 2003;2:773–782. [PubMed] [Google Scholar]
- 26.Tian H, Wittmack EK, Jorgensen TJ. p21WAF1/CIP1 antisense therapy radiosensitizes human colon cancer by converting growth arrest to apoptosis. Cancer Res. 2000;60:679–684. [PubMed] [Google Scholar]
- 27.Johnson KR, Fan W. Reduced expression of p53 and p21WAF1/CIP1 sensitizes human breast cancer cells to paclitaxel and its combination with 5-fluorouracil. Anticancer Res. 2002;22:3197–3204. [PubMed] [Google Scholar]
- 28.Awada A, Hendlisz A, Gil T, Bartholomeus S, Mano M, de VD, et al. Phase I safety and pharmacokinetics of BAY 43-9006 administered for 21 days on/7 days off in patients with advanced, refractory solid tumours. Br J Cancer. 2005;92:1855–1861. doi: 10.1038/sj.bjc.6602584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strumberg D, Richly H, Hilger RA, Schleucher N, Korfee S, Tewes M, et al. Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23:965–972. doi: 10.1200/°CO.2005.06.124. [DOI] [PubMed] [Google Scholar]
- 30.Minami H, Kawada K, Ebi H, Kitagawa K, Kim YI, Araki K, et al. Phase I and pharmacokinetic study of sorafenib, an oral multikinase inhibitor, in Japanese patients with advanced refractory solid tumors. Cancer Sci. 2008;99:1492–1498. doi: 10.1111/j.1349-7006.2008.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Dowbenko D, Lasky LA. AKT/PKB phosphorylation of p21Cip1/WAF1 enhances protein stability of p21Cip1/WAF1 and promotes cell survival. J Biol Chem. 2002;277:11352–11361. doi: 10.1074/jbc.M109062200. [DOI] [PubMed] [Google Scholar]
- 32.Gnarra JR, Tory K, Weng Y, Schmidt L, Wei MH, Li H, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet. 1994;7:85–90. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- 33.Clifford SC, Prowse AH, Affara NA, Buys CH, Maher ER. Inactivation of the von Hippel-Lindau (VHL) tumour suppressor gene and allelic losses at chromosome arm 3p in primary renal cell carcinoma: evidence for a VHL-independent pathway in clear cell renal tumourigenesis. Genes Chromosomes Cancer. 1998;22:200–209. doi: 10.1002/(SICI)1098-2264(199807)22:3<200::AID-GCC5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 34.Bindra RS, Vasselli JR, Stearman R, Linehan WM, Klausner RD. VHL-mediated hypoxia regulation of cyclin D1 in renal carcinoma cells. Cancer Res. 2002;62:3014–3019. [PubMed] [Google Scholar]
- 35.Roe JS, Kim H, Lee SM, Kim ST, Cho EJ, Youn HD. p53 stabilization and transactivation by a von Hippel-Lindau protein. Mol Cell. 2006;22:395–405. doi: 10.1016/j.molcel.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Motzer RJ, Bukowski RM. Targeted therapy for metastatic renal cell carcinoma. J Clin Oncol. 2006;24:5601–5608. doi: 10.1200/°CO.2006.08.5415. [DOI] [PubMed] [Google Scholar]
- 37.Hwang SH, Tsai HJ, Liu JY, Morisseau C, Hammock BD. Orally bioavailable potent soluble epoxide hydrolase inhibitors. J Med Chem. 2007;50:3825–3840. doi: 10.1021/jm070270t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee S, Baek M, Kim H, Ha J, Jeoung D. Mechanism of doxorubicin-induced cell death and expression profile analysis. Biotechnol Lett. 2002;24:1147–1151. doi: 10.1023/A:1016174800956. [DOI] [Google Scholar]
- 39.Branham MT, Nadin SB, Vargas-Roig LM, Ciocca DR. DNA damage induced by paclitaxel and DNA repair capability of peripheral blood lymphocytes as evaluated by the alkaline comet assay. Mutat Res. 2004;560:11–17. doi: 10.1016/j.mrgentox.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 40.Brown JM, Attardi LD. The role of apoptosis in cancer development and treatment response. Nat Rev Cancer. 2005;5:231–237. doi: 10.1038/nrc1560. [DOI] [PubMed] [Google Scholar]
- 41.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–348. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 42.Waldman T, Kinzler KW, Vogelstein B. p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res. 1995;55:5187–5190. [PubMed] [Google Scholar]
- 43.Park SH, Wang X, Liu R, Lam KS, Weiss RH. High throughput screening of a small molecule one-bead-one-compound combinatorial library to identify attenuators of p21 as chemotherapy sensitizers. Cancer Biol Ther. 2008;7:2015–2022. doi: 10.4161/cbt.7.12.7069. [DOI] [PubMed] [Google Scholar]
- 44.Sharma RR, Ravikumar TS, Raimo D, Yang WL. Induction of p21(WAF1) expression protects HT29 colon cancer cells from apoptosis induced by cryoinjury. Ann Surg Oncol. 2005;12:743–752. doi: 10.1245/ASO.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 45.Waldman T, Lengauer C, Kinzler KW, Vogelstein B. Uncoupling of S phase and mitosis induced by anticancer agents in cells lacking p21 [see comments] Nature. 1996;381:713–716. doi: 10.1038/381713a0. [DOI] [PubMed] [Google Scholar]
- 46.Lazzarini R, Moretti S, Orecchia S, Betta PG, Procopio A, Catalano A. Enhanced antitumor therapy by inhibition of p21waf1 in human malignant mesothelioma. Clin Cancer Res. 2008;14:5099–5107. doi: 10.1158/1078-0432.CCR-08-0255. [DOI] [PubMed] [Google Scholar]
- 47.Linehan WM, Zbar B. Focus on kidney cancer. Cancer Cell. 2004;6:223–228. doi: 10.1016/j.ccr.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y, Fujita N, Tsuruo T. Caspase-mediated cleavage of p21Waf1/Cip1 converts cancer cells from growth arrest to undergoing apoptosis. Oncogene. 1999;18:1131–1138. doi: 10.1038/sj.onc.1202426. [DOI] [PubMed] [Google Scholar]
- 49.Sohn D, Essmann F, Schulze-Osthoff K, Janicke RU. p21 blocks irradiation-induced apoptosis downstream of mitochondria by inhibition of cyclin-dependent kinase-mediated caspase-9 activation. Cancer Res. 2006;66:11254–11262. doi: 10.1158/0008-5472.CAN-06-1569. [DOI] [PubMed] [Google Scholar]
- 50.Tang JJ, Shen C, Lu YJ. Requirement for pre-existing of p21 to prevent doxorubicin-induced apoptosis through inhibition of caspase-3 activation. Mol Cell Biochem. 2006;291:139–144. doi: 10.1007/s11010-006-9206-7. [DOI] [PubMed] [Google Scholar]
- 51.Kim D, Cheng GZ, Lindsley CW, Yang H, Cheng JQ. Targeting the phosphatidylinositol-3 kinase/Akt pathway for the treatment of cancer. Curr Opin Investig Drugs. 2005;6:1250–1258. [PubMed] [Google Scholar]
- 52.Tomasello L, Sertoli MR, Rubagotti A, Guglielmini P, Tacchini L, Bedognetti D, et al. Combination of sorafenib and weekly gemcitabine in patients (pts) with metastatic renal cell Cancer (MRCC): A phase II study, preliminary results. J Clin Oncol. 2008:26. [Google Scholar]
- 53.Abou-Alfa GK, Johnson P, Knox JJ, Capanu M, Davidenko I, Lacava J, et al. Doxorubicin plus sorafenib vs. doxorubicin alone in patients with advanced hepatocellular carcinoma: a randomized trial. JAMA. 2010;304:2154–2160. doi: 10.1001/jama.2010.1672. [DOI] [PubMed] [Google Scholar]
- 54.Heim M, Scharifi M, Zisowsky J, Jaehde U, Voliotis D, Seeber S, et al. The Raf kinase inhibitor BAY 43-9006 reduces cellular uptake of platinum compounds and cytotoxicity in human colorectal carcinoma cell lines. Anticancer Drugs. 2005;16:129–136. doi: 10.1097/00001813-200502000-00003. [DOI] [PubMed] [Google Scholar]