Abstract
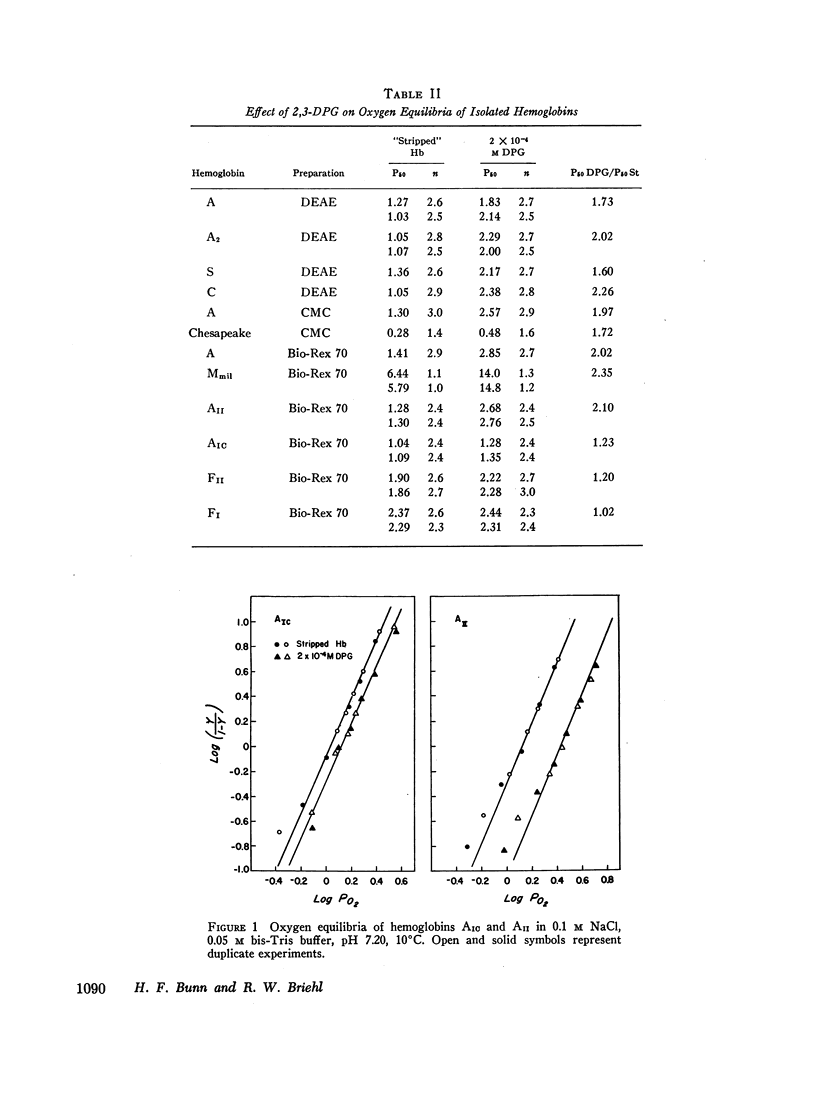
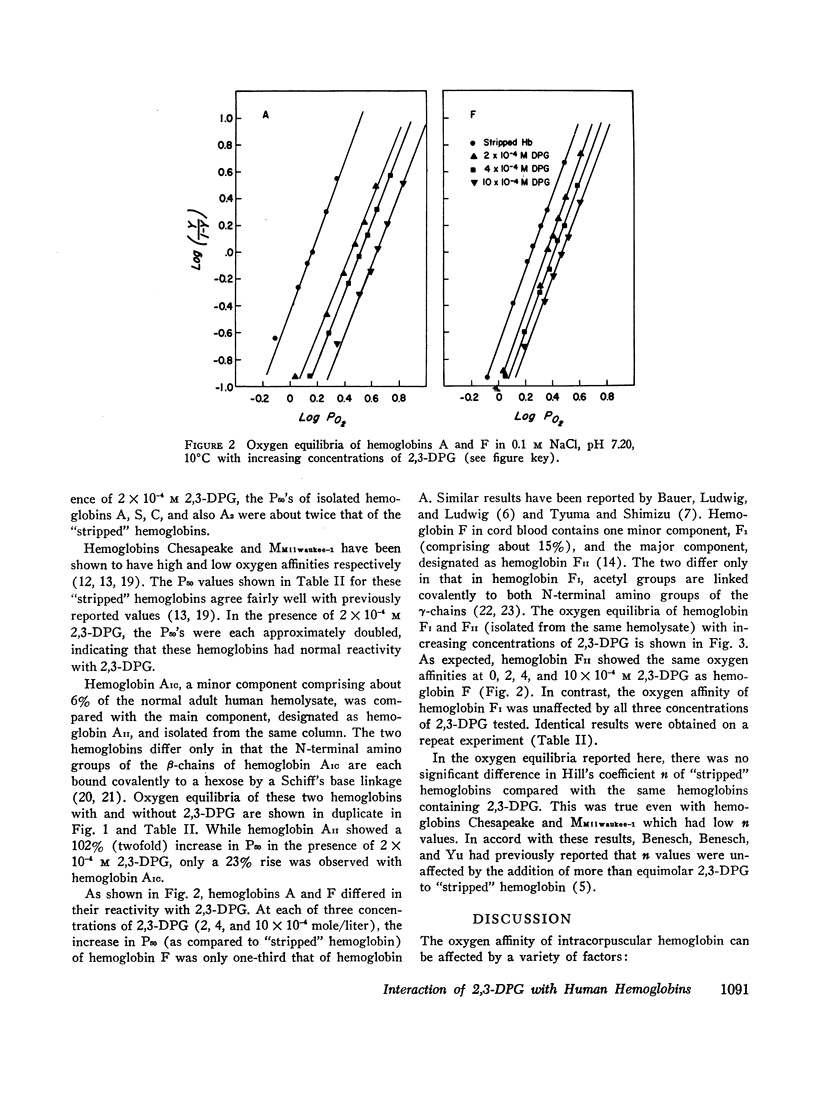
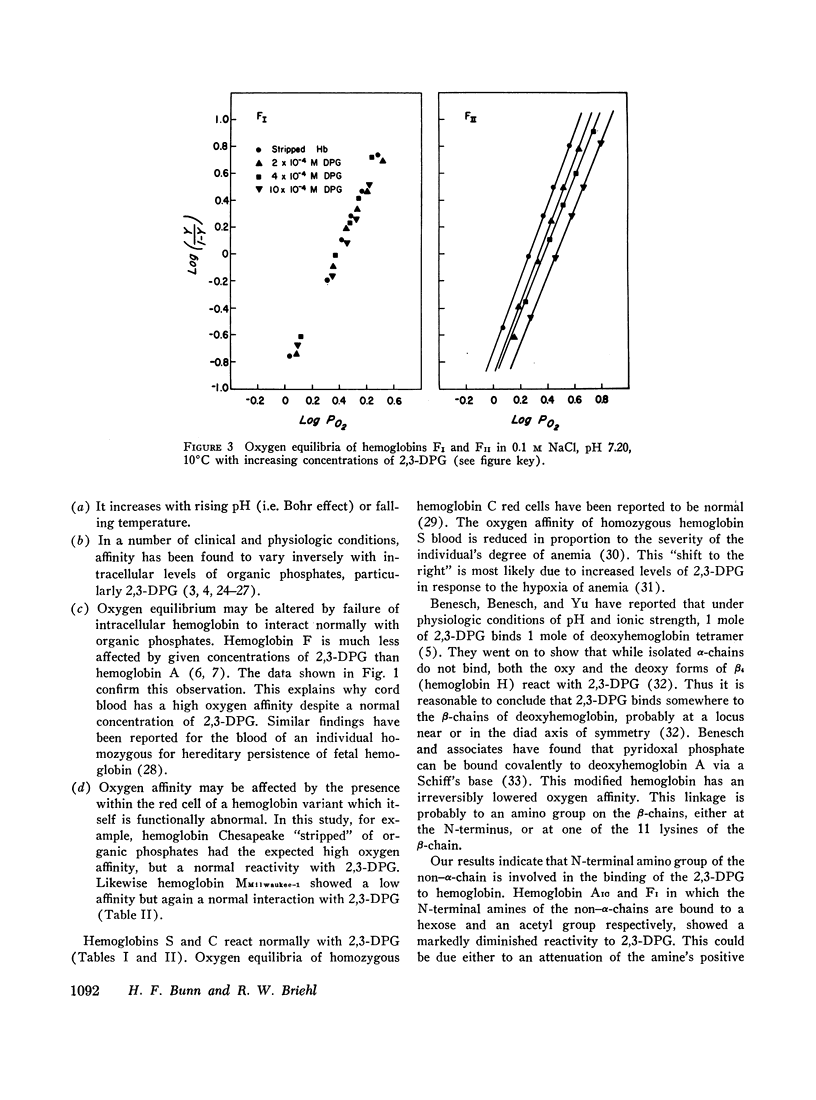
Oxygen equilibria were measured on a number of human hemoglobins, which had been “stripped” of organic phosphates and isolated by column chromatography. In the presence of 2 × 10-4 M 2,3-diphosphoglycerate (2,3-DPG), the P50 of hemoglobins A, A2, S, and C increased about twofold, signifying a substantial and equal decrease in oxygen affinity. Furthermore, hemoglobins Chesapeake and MMilwaukee-1 which have intrinsically high and low oxygen affinities, respectively, also showed a twofold increase in P50 in the presence of 2 × 10-4 M 2,3-DPG. In comparison to these, hemoglobins AIC and F were less reactive with 2,3-DPG while hemoglobin FI showed virtually no reactivity. The N-terminal amino of each β-chain of hemoglobin AIC is linked to a hexose. In hemoglobin FI the N-terminal amino of each γ-chain is acetylated. These results suggest that the N-terminal amino groups of the non-α-chains are involved in the binding of 2,3-DPG to hemoglobin.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN D. W., GUTHE K. F., WYMAN J., Jr Further studies on the oxygen equilibrium of hemoglobin. J Biol Chem. 1950 Nov;187(1):393–410. [PubMed] [Google Scholar]
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Akerblom O., de Verdier C. H., Garby L., Högman C. Restoration of defective oxygen-transport function of stored red blood cells by addition of inosine. Scand J Clin Lab Invest. 1968;21(3):245–248. doi: 10.3109/00365516809076991. [DOI] [PubMed] [Google Scholar]
- Bellingham A. J., Huehns E. R. Compensation in haemolytic anaemias caused by abnormal haemoglobins. Nature. 1968 Jun 8;218(5145):924–926. doi: 10.1038/218924a0. [DOI] [PubMed] [Google Scholar]
- Benesch R. E., Benesch R., Yu C. I. The oxygenation of hemoglobin in the presence of 2,3-diphosphoglycerate. Effect of temperature, pH, ionic strength, and hemoglobin concentration. Biochemistry. 1969 Jun;8(6):2567–2571. doi: 10.1021/bi00834a046. [DOI] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E., Enoki Y. The interaction of hemoglobin and its subunits with 2,3-diphosphoglycerate. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1102–1106. doi: 10.1073/pnas.61.3.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E. The effect of organic phosphates from the human erythrocyte on the allosteric properties of hemoglobin. Biochem Biophys Res Commun. 1967 Jan 23;26(2):162–167. doi: 10.1016/0006-291x(67)90228-8. [DOI] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E., Yu C. I. Reciprocal binding of oxygen and diphosphoglycerate by human hemoglobin. Proc Natl Acad Sci U S A. 1968 Feb;59(2):526–532. doi: 10.1073/pnas.59.2.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookchin R. M., Gallop P. M. Structure of hemoglobin AIc: nature of the N-terminal beta chain blocking group. Biochem Biophys Res Commun. 1968 Jul 11;32(1):86–93. doi: 10.1016/0006-291x(68)90430-0. [DOI] [PubMed] [Google Scholar]
- Briehl R. W. Relations between aggregation of subunits and the oxygen equilibrium of human hemoglobin. J Biol Chem. 1970 Feb 10;245(3):538–543. [PubMed] [Google Scholar]
- Bromberg P. A., Jensen W. N. Blood oxygen dissociation curves in sickle cell disease. J Lab Clin Med. 1967 Sep;70(3):480–488. [PubMed] [Google Scholar]
- Bunn H. F., Jandl J. H. Exchange of heme among hemoglobins and between hemoglobin and albumin. J Biol Chem. 1968 Feb 10;243(3):465–475. [PubMed] [Google Scholar]
- Bunn H. F., May M. H., Kocholaty W. F., Shields C. E. Hemoglobin function in stored blood. J Clin Invest. 1969 Feb;48(2):311–321. doi: 10.1172/JCI105987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanutin A., Curnish R. R. Effect of organic and inorganic phosphates on the oxygen equilibrium of human erythrocytes. Arch Biochem Biophys. 1967 Jul;121(1):96–102. doi: 10.1016/0003-9861(67)90013-6. [DOI] [PubMed] [Google Scholar]
- Chanutin A., Curnish R. R. Effect of organic phosphates on the oxygen equilibrium of carboxypeptidase digests of human hemoglobin. Arch Biochem Biophys. 1968 Jan;123(1):163–165. doi: 10.1016/0003-9861(68)90114-8. [DOI] [PubMed] [Google Scholar]
- Charache S., Grisolia S., Fiedler A. J., Hellegers A. E. Effect of 2,3-diphosphoglycerate on oxygen affinity of blood in sickle cell anemia. J Clin Invest. 1970 Apr;49(4):806–812. doi: 10.1172/JCI106294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charache S., Weatherall D. J., Clegg J. B. Polycythemia associated with a hemoglobinopathy. J Clin Invest. 1966 Jun;45(6):813–822. doi: 10.1172/JCI105397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delivoria-Papadopoulos M., Oski F. A., Gottlieb A. J. Oxygen-hemoglobulin dissociation curves: effect of inherited enzyme defects of the red cell. Science. 1969 Aug 8;165(3893):601–602. doi: 10.1126/science.165.3893.601. [DOI] [PubMed] [Google Scholar]
- Engel K., Duc G. Effect of iodoacetate and fluoride on the position of the haemoglobin oxygen dissociation curve of human whole blood. Nature. 1968 Aug 31;219(5157):936–938. doi: 10.1038/219936a0. [DOI] [PubMed] [Google Scholar]
- Garby L., Gerber G., De Verdier C. H. Binding of 2,3-diphosphoglycerate and adenosine triphosphate to human haemoglobin A. Eur J Biochem. 1969 Aug;10(1):110–115. doi: 10.1111/j.1432-1033.1969.tb00662.x. [DOI] [PubMed] [Google Scholar]
- HUISMAN T. H., MEYERING C. A. Studies on the heterogeneity of hemoglobin. I. The heterogeneity of different human hemoglobin types in carboxymethylcellulose and in amberlite IRC-50 chromatography qualitative aspects. Clin Chim Acta. 1960 Jan;5:103–123. doi: 10.1016/0009-8981(60)90098-x. [DOI] [PubMed] [Google Scholar]
- Hamilton H. B., Iuchi I., Miyaji T., Shibata S. Hemoglobin Hiroshima (beta-143 histidine--aspartic acid): a newly identified fast moving beta chain variant associated with increased oxygen affinity and compensatory erythremia. J Clin Invest. 1969 Mar;48(3):525–535. doi: 10.1172/JCI106010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmquist W. R., Schroeder W. A. A new N-terminal blocking group involving a Schiff base in hemoglobin AIc. Biochemistry. 1966 Aug;5(8):2489–2503. doi: 10.1021/bi00872a002. [DOI] [PubMed] [Google Scholar]
- Huehns E. R. The properties and reactions of haemoglobin F(1) and their bearing on the dissociation equilibrium of haemoglobin. Biochem J. 1966 Dec;101(3):852–860. doi: 10.1042/bj1010852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman T. H., Dozy A. M. Studies on the heterogeneity of hemoglobin. IX. The use of Tris(hydroxymethyl)aminomethanehcl buffers in the anion-exchange chromatography of hemoglobins. J Chromatogr. 1965 Jul;19(1):160–169. doi: 10.1016/s0021-9673(01)99434-8. [DOI] [PubMed] [Google Scholar]
- Imai K. Oxygen-equilibrium characteristics of abnormal hemoglobin Hiroshima (alpha-2 beta-2 143 Asp). Arch Biochem Biophys. 1968 Sep 20;127(1):543–547. doi: 10.1016/0003-9861(68)90260-9. [DOI] [PubMed] [Google Scholar]
- Lenfant C., Torrance J., English E., Finch C. A., Reynafarje C., Ramos J., Faura J. Effect of altitude on oxygen binding by hemoglobin and on organic phosphate levels. J Clin Invest. 1968 Dec;47(12):2652–2656. doi: 10.1172/JCI105948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara K., Hayashi N., Kikuchi G., Shibata S. Oxygen equilibrium of hemoglobin Hiroshima. Biochem Biophys Res Commun. 1968 Sep 6;32(5):763–769. doi: 10.1016/0006-291x(68)90305-7. [DOI] [PubMed] [Google Scholar]
- Miwa I., Erdös E. G., Seki T. Presence of three peptides in urinary kinin (substance Z) preparations. Life Sci. 1968 Dec 15;7(24):1339–1343. doi: 10.1016/0024-3205(68)90265-8. [DOI] [PubMed] [Google Scholar]
- Muirhead H., Cox J. M., Mazzarella L., Perutz M. F. Structure and function of haemoglobin. 3. A three-dimensional fourier synthesis of human deoxyhaemoglobin at 5.5 Angstrom resolution. J Mol Biol. 1967 Aug 28;28(1):117–156. doi: 10.1016/s0022-2836(67)80082-2. [DOI] [PubMed] [Google Scholar]
- Nagel R. L., Gibson Q. H., Charache S. Relation between structure and function in Hemoglobin Chesapeake. Biochemistry. 1967 Aug;6(8):2395–2402. doi: 10.1021/bi00860a015. [DOI] [PubMed] [Google Scholar]
- Oski F. A., Gottlieb A. J., Delivoria-Papadopoulos M., Miller W. W. Red-cell 2,3-diphosphoglycerate levels in subjects with chronic hypoxemia. N Engl J Med. 1969 May 22;280(21):1165–1166. doi: 10.1056/NEJM196905222802108. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Muirhead H., Mazzarella L., Crowther R. A., Greer J., Kilmartin J. V. Identification of residues responsible for the alkaline Bohr effect in haemoglobin. Nature. 1969 Jun 28;222(5200):1240–1243. doi: 10.1038/2221240a0. [DOI] [PubMed] [Google Scholar]
- RIGGS A. The metamorphosis of hemoglobin in the bullfrog. J Gen Physiol. 1951 Sep;35(1):23–40. doi: 10.1085/jgp.35.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHROEDER W. A., CUA J. T., MATSUDA G., FENNINGER W. D. Hemoglobin F1, an acetyl-containing hemoglobin. Biochim Biophys Acta. 1962 Oct 8;63:532–534. doi: 10.1016/0006-3002(62)90125-7. [DOI] [PubMed] [Google Scholar]
- Tyuma I., Shimizu K. Different response to organic phosphates of human fetal and adult hemoglobins. Arch Biochem Biophys. 1969 Jan;129(1):404–405. doi: 10.1016/0003-9861(69)90192-1. [DOI] [PubMed] [Google Scholar]
- de Verdier C. H., Garby L. Low binding of 2,3-diphosphoglycerate to haemoglobin F. A contribution to the knowledge of the binding site and an explanation for the high oxygen affinity of foetal blood. Scand J Clin Lab Invest. 1969 Apr;23(2):149–151. doi: 10.3109/00365516909077018. [DOI] [PubMed] [Google Scholar]



