Abstract
The molting hormone 20-hydroxyecdysone (20E) is an active metabolite of ecdysone and plays vital roles during ontogeny of the fruit fly Drosophila, coordinating critical developmental transitions such as molting and metamorphosis. Although 20E is known to exist throughout life in both male and female flies, its functions in adult physiology and behavior remain largely elusive. Notably, findings from previous studies suggest that this hormone may be involved in adult stress responses. Consistent with this possibility, we have found that ecdysone signaling in adult flies is activated by “stressful” social interactions and plays a role in the formation of long-term courtship memory.1 In addition, we recently reported that ecdysone signaling contributes to the regulation of sleep, affecting transitions between sleep and wakefulness.2 Here we first summarize our findings on the unconventional roles of 20E in regulating memory and sleep in adult flies. We then discuss speculative ideas concerning the stress hormone-like features of 20E, as well as the possibility that ecdysone signaling contributes to remodeling of the adult nervous system, at both the functional and structural levels, through epigenetic mechanisms.
Key words: Drosophila, ecdysone, sleep, memory, steroid, stress, chromatin modification, neuronal remodeling, non-genomic steroid action
The Steroid Molting Hormone and Stress Responses in Adult Flies
The molting hormone 20-hydroxyecdysone (20E), an active metabolite of ecdysone, is the major steroid hormone in the fruit fly Drosophila melanogaster. It is well established that precisely controlled pulses of 20E trigger and coordinate critical developmental events, including embryonic morphogenesis,3 larval molting and metamorphosis.4 Additionally, in the ovaries of mature adult females, ecdysone signaling is essential for particular aspects of oogenesis such as follicle development5 and the timing of border-cell migration.6,7 Although a large body of literature describes the functions of 20E in development and reproduction, little attention has been devoted to its roles in the physiology and behavior of adult flies, despite the fact that it is present in both males and females throughout life.8
Several notable observations suggest that ecdysone signaling plays a role in adult stress responses. While 20E levels in embryos, larvae and pupae are dictated mainly by genetically controlled developmental programs, those in mature adult flies are highly dependent on the external environment. For example, 20E concentrations in the ovaries and hemolymph rise when adult females are transferred onto a sugar diet without a yeast supplement.9 This elevation of the 20E level apparently serves as a signal of nutritional shortage, leading to an arrest of oogenesis and the induction of apoptosis. As a result, females can use the limited energy and nutrients that are available for their own survival in exchange for reduced reproduction.9 Likewise, exposing Drosophila virilis to thermal stress (38°C, 60 min) results in an increase in 20E levels.10 These results are consistent with the idea that stressful conditions induce the production and secretion of 20E, altering the physiological and behavioral states of adult flies so that they can acutely cope with the unfavorable environment. Thus, in adult flies, 20E may have stress hormone-like properties.
A connection between the molting hormone and the stress response in adult flies is further supported by several intriguing phenotypes in mutants for ecdysone signaling. The actions of ecdysone are primarily mediated by ecdysone receptors (EcRs), which are members of an evolutionarily conserved family of nuclear hormone receptors.11 Reflecting the indispensable nature of nuclear receptor-mediated ecdysone signaling during development, homozygosity for loss-of-function mutations in EcR causes developmental lethality.12 Although EcR heterozygous mutants (EcR/+) are fully viable and display no obvious abnormality in development, fertility or general activity, Simon et al.13 discovered that EcR/+ adult flies are significantly more resistant to various stresses (e.g., heat, dry starvation and oxidative stress) and exhibit a remarkable 50% life-span extension compared to appropriate genetic controls. Consistent with these findings, flies heterozygous for DTS-3—a mutant allele of molting defective (mld) (personal communication, Maroy P, University of Szeged, Szeged, Hungary) characterized by lower 20E titers—show stress response and longevity phenotypes similar to those observed in EcR/+ flies. The increased stress resistance and the extension of lifespan in DTS-3/+ adults are more likely caused by the low 20E titers in adults than by disturbed development, because the mutant phenotypes are readily reversed by feeding adults 20E.13 These results suggest that frequent or chronic activation of ecdysone signaling in adult flies is intrinsically harmful to their overall health, even though the same signaling may have beneficial, short-term effects, under certain circumstances, e.g., in the acute management of unfavorable environmental conditions.
Ecdysone Signaling Plays a Role in Formation of Long-Term Courtship Memory in Adult Flies
In humans and mammalian model animals, steroid hormones released in response to a stressful experience are critical for the consolidation of memory.14–16 Intriguingly, such a steroid-mediated strategy for memory consolidation seems to be conserved in Drosophila. We have found that in adult males, 20E levels are significantly elevated after these flies are paired with non-virgin females for an extended period of time (7 hr). Interestingly, the elevation of 20E levels is associated with activation of the cAMP response element binding protein (CREB), an essential regulator of long-term memory formation.1 Presumably, this interaction is “stressful” to male flies because they repeatedly receive courtship rejection from non-receptive mating partners, and it could result in experience-dependent long-lasting courtship suppression. This courtship suppression in flies is a representation of associative long-term memory, which lasts at least five days.17,18 We also found that exogenous administration of 20E to male flies has context-dependent effects on courtship long-term memory, and that EcR/+ males are normal with respect to short-term (30 min) but defective for long-term (five days) courtship memory.
During Drosophila ontogeny, pulses of 20E induce transitions between distinct developmental states. Our finding of a role for 20E in memory consolidation suggests that ecdysone signaling in adult flies may play a role in transitions between different brain states in response to stressful conditions, so that memories acquired in the presence of high levels of 20E are preferentially stabilized and lasts longer.
Ecdysone Signaling Promotes Sleep in Adult Flies
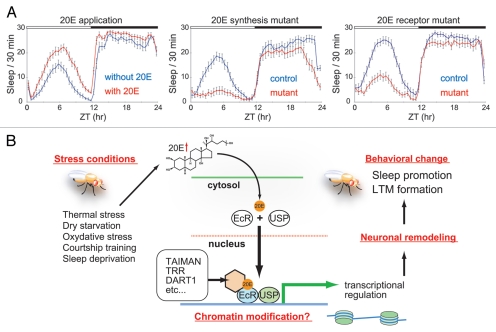
Sleep is conserved among evolutionarily diverse animal species,19–22 and is thought to be fundamental to survival.23,24 Although the biological functions of sleep are not well understood, one possible role is to restore physiological conditions that progressively disintegrate during the waking period.25,26 According to this view, and considering that the activation of ecdysone signaling seems to have destructive effects on the physiology of the adult fly brain, substantial increases of 20E levels in adult flies should be associated with an increase in the need for sleep. As expected, we found that feeding adult flies 20E increases the total sleep time, in a dose-dependent manner2 (Fig. 1A). Consistent with the sleep-promoting effect of 20E, mutants in which ecdysone signaling is reduced display a short-sleep phenotype (Fig. 1A). Furthermore, these mutants fail to exhibit adequate sleep rebound following sleep deprivation. In wild-type flies, the endogenous levels of 20E tend to increase during the light period, possibly due to higher activity during the day. We also found that the levels of 20E are elevated after sleep deprivation, an experience that is likely to be highly stressful to animals.2
Figure 1.
(A) Sleep is enhanced by feeding adult flies the steroid molting hormone 20-hydroxyecdysone (20E). In contast, sleep is suppressed when ecdysone signaling is reduced by a loss-of-function mutation in either molting defective (mld) or in Ecdysone receptor (EcR). mld encodes a nuclear zinc finger protein required for ecdysone biosynthesis (personal communication, Maroy P) and EcR encodes an ecdysone-dependent transcription factor (modified from the figures in ref. 2). (B) The levels of 20E in mature adult flies are dependent on the external and internal environments. Different unfavorable environmental stimuli increase the 20E levels and trigger ecdysone signaling. Ecdysone signal is mediated mainly by EcRs, which form heterodimers with the retinoid X receptor homologue Ultraspiracle (USP) and act as ligand-activated transcription factors. The EcR/USP complex recruits various co-activators and co-repressors. Some of these co-regulators are capable of modifying the chromatin structure, which leads to epigenetic changes in patterns of gene expression. Ecdysone-induced changes in gene expression may reinitiate some of the molecular and cellular processes that are employed for normal neural development, and enhance structural and functional reorganization of the adult nervous system. Such neuronal remodeling could result in stable or dynamic alterations in behavioral outputs.
It is generally thought that reduced sleep has adverse effects on the overall health of animals and results in reduced lifespan. Contrary to this prevailing notion, mutants with suboptimal ecdysone signaling sleep less2 and live longer.13 This might be explained by assuming that in wild-type flies, normal daily activity causes low-grade chronic activation of ecdysone signaling, which is intrinsically detrimental and may contribute to the generation of potentially harmful by-products. Suppressed ecdysone signaling due to a reduction in the level of either ligand (20E) or receptor (EcR) may lead to reduced accumulation of such damaging materials, and consequently to a reduced need for sleep and an extension of lifespan—at least under standard laboratory conditions.
We found that ecdysone signaling positively regulates sleep by increasing and decreasing the length of sleep and wake bouts, respectively, without significantly affecting waking activity. These results again indicate that 20E controls the transition between the distinct physiological and behavioral states of adult flies, specifically sleep and wakefulness in this case. It is reasonable to hypothesize that shared mechanisms underlie both the transitions between different developmental stages and the transitions between behavioral states in adults, both of which are regulated by ecdysone signaling. A link between the developmental transitions and the sleep-wake regulation is further supported by a recent finding in the nematode Caenorhabditis elegans. Raizen et al.27 have shown that lethargus—the developmentally regulated behavioral quiescence that precedes larval molting—has sleep-like properties including reversibility, reduced responsiveness and homeostasis. Developmentally programmed quiescence featuring sleep-like properties may be a common phenomenon; it has been observed in species other than flies and worms. For example, people have long known that, prior to each molt, silkworm larvae stop eating and become immobilized, displaying a characteristic posture in which the anterior portion extends vertically. Interestingly, this developmental period of behavioral quiescence in the silkworm has been termed “Min” in Japanese, which literally means “sleep”. It is likely that “Min” in the silkworm has sleep-like features similar to those of lethargus in the nematode.
Because the major developmental transitions (e.g., molting and metamorphosis) involve massive metabolic alterations and drastic tissue reorganization, they are expected to increase the amount of potentially harmful by-products. Although such functional and structural changes are necessary for normal development, they must have significant adverse effects on animal physiology. Entry into a sleep-like state during development may help maintain or reestablish normal physiological conditions prior to, during or after the major developmental transitions. In this sense, developmentally programmed behavioral quiescence and adult sleep may share not only regulatory mechanisms, but also a role in homeostatic regulation.
Global Modulation of the Adult Nervous System by Ecdysone
What are the neuronal consequences of ecdysone signaling activation in the adult brain? How does ecdysone activity influence the functional properties of the nervous system? We speculate that ecdysone signaling may be involved in the tuning of neuronal circuits in the brain. One of several provocative theories about sleep is the synaptic homeostasis hypothesis, which argues that the function of sleep is to down-scale brain synapses that are stimulated and potentiated through the neural activity during the waking period.28,29 In support of this theory, Gilestro et al. have found that the levels of several synaptic marker proteins in the Drosophila brain change (with some increasing and others decreasing) during waking and sleeping, respectively. Notably, these changes correlate with sleep-wake states rather than with time of day, and are observed in fairly widespread areas of the brain.30 Such global modifications of the nervous system in association with sleep-wake transitions could well be regulated by hormones, and our findings suggest that specifically 20E is a good candidate for such a regulator. With regard to this possibility, it is noteworthy that developmental studies have demonstrated a significant role for EcR-mediated ecdysone signaling in neuronal remodeling during formation of the adult nervous system.31–34 For example, when EcR-mediated ecdysone signaling is blocked during the remodeling of neurosecretory Tv neurons, filipodial activity and axonal sprouting are severely inhibited, resulting in adult Tv neurons with a significantly reduced, misshapen axonal arbor.35 Thus, 20E is able to trigger and coordinate the molecular processes necessary for proper neuronal remodeling during development. It is tempting to speculate that some neurons in the mature adult brain remain sensitive to ecdysone signaling and undergo fine neuronal remodeling in response to 20E during sleep and wakefulness. Although ecdysone signaling for larval-adult neuronal remodeling during development is controlled by genetically determined programs, it may be that the ecdysone signaling that leads to remodeling in the adult nervous system is dictated by environmental cues.
As mentioned above, previous work in our laboratory demonstrated that ecdysone signaling is required for long-term courtship memory. That study also suggested that the activation of ecdysone signaling during experience-dependent courtship conditioning causes functional modifications of brain neurons, and that these contribute to the formation of long-term courtship memory.1 Such functional modifications are likely accompanied by structural reorganization of the relevant neurons. An attractive hypothesis is that ecdysone signaling is involved in the neuronal reorganization related to memory formation, as well as to that involved in sleep regulation. The molecular and cellular mechanisms underlying these processes may be at least partly shared. In this context, it is intriguing that sleep increases in flies exposed to social conditions under which long-term courtship memory is induced.36 This finding implies that sleep regulation and memory consolidation, both of which are influenced by ecdysone signaling, are linked both functionally and anatomically.
Possible Involvement of Chromatin Modifications in Ecdysone-Mediated Behavioral Regulation in Adult Flies
Microarray analyses have demonstrated that in Drosophila, as in vertebrate animals, the transitions between sleep and wake states are accompanied by widespread changes in gene expression in the brain.37,38 EcRs, which form heterodimers with the retinoid X receptor homologue Ultraspiracle (USP), act as ligand-activated transcription factors.39 Although it is unknown exactly which genes are activated or suppressed through the action of ecdysone signaling in the mature adult brain, the EcR/USP complex must have a significant role in gene transcription during memory formation, as well as in the regulation of sleep and wake states.
As in the case of most transcription factors, EcRs activate or repress target-gene expression by recruiting various co-regulators to the EcR/USP complex, and recent studies have revealed that many of the EcR-specific co-activators and co-repressors serve as chromatin-modifying proteins during development. The developmental co-regulators of EcRs include TAIMAN (histone acetylase),7 Trithorax-related (TRR) (histone lysine methyltransferase),40 DART1 (histone arginine methyltransferases),41 BRAHMA (ATP-dependent chromatin remodeler),42 NURF (ATP-dependent chromatin remodeler),43 and DEK (histone chaperone).44 It will be interesting to examine whether these epigenetic factors, which influence EcR-mediated transcription in the nervous system during development, are likewise involved in transcriptional regulation in the nervous system in mature adult flies.
The notion that epigenetic mechanisms play a critical role in regulating synaptic plasticity and memory is strongly supported by accumulating evidence.45 Particularly intriguing is the recent finding in mice that epigenetic alterations mediated by histone acetylases and DNA methylases are critical for the beneficial effects of the steroid hormone estrogen on memory consolidation.46 If the mechanisms underlying steroid-induced memory enhancement are evolutionarily conserved, it is possible that chromatin modifications also play a significant role in the ecdysone-mediated formation of long-term courtship memory in adult flies.
While the epigenetic changes that lead to formation of long-term memory must be fairly stable, recent reports demonstrated that chromatin modifications can also be very dynamic—undergoing cyclical changes within hours to minutes.47 Therefore, it is reasonable to speculate that dynamic epigenetic regulation contributes to the maintenance of, or transitions between, sleep and wake states. Ecdysone may also influence such a regulatory process through various co-activator or co-repressor of EcRs that have different chromatin modifying activities.
Non-Genomic Actions of the Steroid Hormones in Regulating Adult Behaviors
DTS-3/+ flies (reduced ecdysone levels) and EcR mutants (reduced receptor activity) both sleep less than their control counterparts. Although both mutants suppress ecdysone signaling, their phenotypes are not exactly same. In particular, the average daytime wake-bout duration is drastically increased in DTS-3/+ flies, but is unaltered in EcR mutants, and administration of 20E significantly reverses the increased wake-bout duration in the DTS-3/+ flies.2 These results imply that wake-bout durations may be controlled by an EcR-independent ecdysone signaling pathway. A molecular component that could potentially mediate such an EcR-independent ecdysone signaling pathway is a recently characterized G protein-coupled receptor, DopEcR.48 Using in vitro cell culture systems, Srivastava et al.48 showed that DopEcR responds to both dopamine and ecdysteroids (ecdysone and 20E) to stimulate cAMP and mitogen-activated protein kinase pathways, respectively. Particularly interesting is this group's finding that the response of DopEcR to dopamine can be inhibited by ecdysteroids.
We recently identified a hypomorphic mutation in the Drosophila DopEcR gene, and found that reduced DopEcR activity leads to a significant increase in locomotor activity (unpublished observation). Because dopamine is a positive regulator of arousal in Drosophila and increased dopamine signaling enhances locomotor activity,49 our new finding is consistent with the idea that DopEcR serves as a negative regulator of dopamine signaling in a steroid hormone-dependent manner. It is possible that the drastic increase in the daytime wake-bout duration occurs in DTS-3/+ flies but not in EcR mutant flies because dopamine signaling is disinhibited when ecdysteroid levels are low (DTS-3/+ flies) but not when nuclear-receptor activity is low (EcR mutants).
Our recent unpublished results have also demonstrated that DopEcR plays a role in regulating both associative and non-associative learning. Specifically, DopEcR hypomorphic mutants display defects in short-lasting courtship memory and in habituation of a jump-and-flight escape reflex. Interestingly, genetic analysis of the DopEcR mutant suggests that the ecdysone and cAMP signaling pathways interact functionally in the nervous system. In fact, our imaging analysis has revealed that cAMP levels in brain neurons are significantly altered after flies are fed 20E (manuscript in preparation). Taken together, these results emphasize the importance of the unconventional non-genomic actions of 20E in regulating adult behavior.
Future Perspectives
One interesting hypothesis that has emerged regarding neural plasticity is that the Drosophila molting steroid hormone, 20E, has stress hormone-like features in adult flies and induces structural and functional modifications of the mature nervous system through the mechanisms that involve epigenetic regulation. The recently identified non-genomic actions of 20E may also play a role in neural plasticity. Such modifications in the nervous system may lead to significant changes in behavioral outputs (Fig. 1B). It is expected that the advanced genetic tools available in Drosophila will contribute to a better understanding of steroid-mediated regulation of behavior in mature adults. Considering the functional importance of steroid-mediated behavioral regulation, the underlying molecular and cellular processes must be well conserved evolutionarily. Thus, the study of this regulation in Drosophila would provide invaluable insight into our understanding of the relationship between steroid hormones and behavior in higher vertebrates, including humans. Because dysfunction in steroid hormone signaling is closely related to a number of neurological and psychiatric diseases including depression, schizophrenia and post-traumatic stress disorder, having a basic understanding of how steroid hormones function in regulating the nervous system would also be clinically significant. With new roles of the molting hormone 20E becoming recognized, this long known steroid has shed its old cuticle and opened a new field of research in Drosophila.
Acknowledgements
This study was supported by grants from the National Institute of Health (MH62684 and MH085081) and the University of Iowa (Biological Sciences Funding Program Grant) to Toshihiro Kitamoto. Hiroshi Ishimoto was partly supported by an Uehara Memorial Foundatioin Fellowship.
Extra View to: Ishimoto H, Sakai T, Kitamoto T. Ecdysone signaling regulates the formation of long-term courtship memory in adult Drosophila melanogaster. Proc Natl Acad Sci USA. 2009;106:6381–6386. doi: 10.1073/pnas.0810213106. and Ishimoto H, Kitamoto T. The steroid molting hormone ecdysone regulates sleep in adult Drosophila melanogaster. Genetics. 2010;185:269–281. doi: 10.1534/genetics.110.114587..
References
- 1.Ishimoto H, Sakai T, Kitamoto T. Ecdysone signaling regulates the formation of long-term courtship memory in adult Drosophila melanogaster. Proc Natl Acad Sci USA. 2009;106:6381–6386. doi: 10.1073/pnas.0810213106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishimoto H, Kitamoto T. The steroid molting hormone Ecdysone regulates sleep in adult Drosophila melanogaster. Genetics. 2010;185:269–281. doi: 10.1534/genetics.110.114587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kozlova T, Thummel CS. Essential roles for ecdysone signaling during Drosophila mid embryonic development. Science. 2003;301:1911–1914. doi: 10.1126/science.1087419. [DOI] [PubMed] [Google Scholar]
- 4.Riddiford LM. Hormones and Drosophila development. In: Bate M, Arias AM, editors. The Development of Drosophila melanogaster. New York: Cold Spring Harbor Press; 1993. pp. 899–939. [Google Scholar]
- 5.Carney GE, Bender M. The Drosophila ecdysone receptor (EcR) gene is required maternally for normal oogenesis. Genetics. 2000;154:1203–1211. doi: 10.1093/genetics/154.3.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jang AC, Chang YC, Bai J, Montell D. Border-cell migration requires integration of spatial and temporal signals by the BTB protein Abrupt. Nat Cell Biol. 2009;11:569–579. doi: 10.1038/ncb1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bai J, Uehara Y, Montell DJ. Regulation of invasive cell behavior by taiman, a Drosophila protein related to AIB1, a steroid receptor coactivator amplified in breast cancer. Cell. 2000;103:1047–1058. doi: 10.1016/s0092-8674(00)00208-7. [DOI] [PubMed] [Google Scholar]
- 8.Handler AM. Ecdysteroid titers during pupal and adult development in Drosophila melanogaster. Dev Biol. 1982;93:73–82. doi: 10.1016/0012-1606(82)90240-8. [DOI] [PubMed] [Google Scholar]
- 9.Terashima J, Takaki K, Sakurai S, Bownes M. Nutritional status affects 20-hydroxyecdysone concentration and progression of oogenesis in Drosophila melanogaster. J Endocrinol. 2005;187:69–79. doi: 10.1677/joe.1.06220. [DOI] [PubMed] [Google Scholar]
- 10.Hirashima A, Rauschenbach IY, Sukhanova MJ. Ecdysteroids in stress responsive and nonresponsive Drosophila virilis lines under stress conditions. Biosci Biotech Bioch. 2000;64:2657–2662. doi: 10.1271/bbb.64.2657. [DOI] [PubMed] [Google Scholar]
- 11.Koelle MR, Talbot WS, Segraves WA, Bender MT, Cherbas P, Hogness DS. The Drosophila EcR gene encodes an ecdysone receptor, a new member of the steroid receptor superfamily. Cell. 1991;67:59–77. doi: 10.1016/0092-8674(91)90572-g. [DOI] [PubMed] [Google Scholar]
- 12.Bender M, Imam FB, Talbot WS, Ganetzky B, Hogness DS. Drosophila ecdysone receptor mutations reveal functional differences among receptor isoforms. Cell. 1997;91:777–788. doi: 10.1016/s0092-8674(00)80466-3. [DOI] [PubMed] [Google Scholar]
- 13.Simon AF, Shih C, Mack A, Benzer S. Steroid control of longevity in Drosophila melanogaster. Science. 2003;299:1407–1410. doi: 10.1126/science.1080539. [DOI] [PubMed] [Google Scholar]
- 14.Luine VN. Sex steroids and cognitive function. J Neuroendocrinol. 2008;20:866–872. doi: 10.1111/j.1365-2826.2008.01710.x. [DOI] [PubMed] [Google Scholar]
- 15.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 16.Parducz A, Hajszan T, Maclusky NJ, Hoyk Z, Csakvari E, Kurunczi A, et al. Synaptic remodeling induced by gonadal hormones: neuronal plasticity as a mediator of neuroendocrine and behavioral responses to steroids. Neuroscience. 2006;138:977–985. doi: 10.1016/j.neuroscience.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 17.McBride SM, Giuliani G, Choi C, Krause P, Correale D, Watson K, et al. Mushroom body ablation impairs short-term memory and long-term memory of courtship conditioning in Drosophila melanogaster. Neuron. 1999;24:967–977. doi: 10.1016/s0896-6273(00)81043-0. [DOI] [PubMed] [Google Scholar]
- 18.Sakai T, Tamura T, Kitamoto T, Kidokoro Y. A clock gene, period, plays a key role in long-term memory formation in Drosophila. Proc Natl Acad Sci USA. 2004;101:16058–16063. doi: 10.1073/pnas.0401472101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell SS, Tobler I. Animal sleep—a review of sleep duration across phylogeny. Neurosci Biobehav R. 1984;8:269–300. doi: 10.1016/0149-7634(84)90054-x. [DOI] [PubMed] [Google Scholar]
- 20.Hendricks JC, Finn SM, Panckeri KA, Chavkin J, Williams JA, Sehgal A, Pack AI. Rest in Drosophila is a sleep-like state. Neuron. 2000;25:129–138. doi: 10.1016/s0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 21.Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 22.Greenspan RJ, Tononi G, Cirelli C, Shaw PJ. Sleep and the fruit fly. Trends Neurosci. 2001;24:142–145. doi: 10.1016/s0166-2236(00)01719-7. [DOI] [PubMed] [Google Scholar]
- 23.Rechtschaffen A, Gilliland MA, Bergmann BM, Winter JB. Physiological correlates of prolonged sleep-deprivation in rats. Science. 1983;221:182–184. doi: 10.1126/science.6857280. [DOI] [PubMed] [Google Scholar]
- 24.Shaw PJ, Tononi G, Greenspan RJ, Robinson DF. Stress response genes protect against lethal effects of sleep deprivation in Drosophila. Nature. 2002;417:287–291. doi: 10.1038/417287a. [DOI] [PubMed] [Google Scholar]
- 25.Siegel JM. Clues to the functions of mammalian sleep. Nature. 2005;437:1264–1271. doi: 10.1038/nature04285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho KS, Sehgal A. Drosophila melanogaster: An Insect Model for Fundamental Studies of Sleep. Methods Enzymol. 2005;393:772–793. doi: 10.1016/S0076-6879(05)93041-3. [DOI] [PubMed] [Google Scholar]
- 27.Raizen DM, Zimmerman JE, Maycock MH, Ta UD, You YJ, Sundaram MV, Pack AI. Lethargus is a Caenorhabditis elegans sleep-like state. Nature. 2008;451:569–572. doi: 10.1038/nature06535. [DOI] [PubMed] [Google Scholar]
- 28.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62:143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Gilestro GF, Tononi G, Cirelli C. Widespread changes in synaptic markers as a function of sleep and wakefulness in Drosophila. Science. 2009;324:109–112. doi: 10.1126/science.1166673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown HL, Truman JW. Fine tuning of secondary arbor development: the effects of the ecdysone receptor on the adult neuronal lineages of the Drosophila thoracic CNS. Development. 2009;136:3247–3256. doi: 10.1242/dev.039859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee T, Marticke S, Sung C, Robinow S, Luo L. Cell autonomous requirement of the USP/EcR-B ecdysone receptor for mushroom body neuronal remodeling in Drosophila. Neuron. 2000;28:807–818. doi: 10.1016/s0896-6273(00)00155-0. [DOI] [PubMed] [Google Scholar]
- 33.Zheng X, Wang J, Haerry TE, Wu AY, Martin J, O'Connor MB, et al. TGFbeta signaling activates steroid hormone receptor expression during neuronal remodeling in the Drosophila brain. Cell. 2003;112:303–315. doi: 10.1016/s0092-8674(03)00072-2. [DOI] [PubMed] [Google Scholar]
- 34.Kraft R, Levine RB, Restifo LL. The steroid hormone 20-hydroxyecdysone enhances neurite growth of Drosophila mushroom body neurons isolated during metamorphosis. J Neurosci. 1998;18:8886–8899. doi: 10.1523/JNEUROSCI.18-21-08886.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown HL, Cherbas L, Cherbas P, Truman JW. Use of time-lapse imaging and dominant negative receptors to dissect the steroid receptor control of neuronal remodeling in Drosophila. Development. 2006;133:275–285. doi: 10.1242/dev.02191. [DOI] [PubMed] [Google Scholar]
- 36.Donlea JM, Ramanan N, Shaw PJ. Use-dependent plasticity in clock neurons regulates sleep need in Drosophila. Science. 2009;324:105–108. doi: 10.1126/science.1166657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmerman JE, Rizzo W, Shockley KR, Raizen DM, Naidoo N, Mackiewicz M, et al. Multiple mechanisms limit the duration of wakefulness in Drosophila brain. Physiol Genomics. 2006;27:337–350. doi: 10.1152/physiolgenomics.00030.2006. [DOI] [PubMed] [Google Scholar]
- 38.Cirelli C, LaVaute TM, Tononi G. Sleep and wakefulness modulate gene expression in Drosophila. J Neurochem. 2005;94:1411–1419. doi: 10.1111/j.1471-4159.2005.03291.x. [DOI] [PubMed] [Google Scholar]
- 39.Aranda A, Pascual A. Nuclear hormone receptors and gene expression. Physiol Rev. 2001;81:1269–1304. doi: 10.1152/physrev.2001.81.3.1269. [DOI] [PubMed] [Google Scholar]
- 40.Sedkov Y, Cho E, Petruk S, Cherbas L, Smith ST, Jones RS, et al. Methylation at lysine 4 of histone H3 in ecdysone-dependent development of Drosophila. Nature. 2003;426:78–83. doi: 10.1038/nature02080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kimura S, Sawatsubashi S, Ito S, Kouzmenko A, Suzuki E, Zhao Y, et al. Drosophila arginine methyltransferase 1 (DART1) is an ecdysone receptor co-repressor. Biochem Biophys Res Commun. 2008;371:889–893. doi: 10.1016/j.bbrc.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Zraly CB, Middleton FA, Dingwall AK. Hormone-response genes are direct in vivo regulatory targets of Brahma (SWI/SNF) complex function. J Biol Chem. 2006;281:35305–35315. doi: 10.1074/jbc.M607806200. [DOI] [PubMed] [Google Scholar]
- 43.Badenhorst P, Xiao H, Cherbas L, Kwon SY, Voas M, Rebay I, et al. The Drosophila nucleosome remodeling factor NURF is required for ecdysteroid signaling and metamorphosis. Genes Dev. 2005;19:2540–2545. doi: 10.1101/gad.1342605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sawatsubashi S, Murata T, Lim J, Fujiki R, Ito S, Suzuki E, et al. A histone chaperone, DEK, transcriptionally coactivates a nuclear receptor. Genes Dev. 24:159–170. doi: 10.1101/gad.1857410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roth TL, Sweatt JD. Regulation of chromatin structure in memory formation. Curr Opin Neurobiol. 2009;19:336–342. doi: 10.1016/j.conb.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao Z, Fan L, Frick KM. Epigenetic alterations regulate estradiol induced enhancement of memory consolidation. Proc Natl Acad Sci USA. 2010;107:5605–5610. doi: 10.1073/pnas.0910578107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Metivier R, Gallais R, Tiffoche C, Le Peron C, Jurkowska RZ, Carmouche RP, et al. Cyclical DNA methylation of a transcriptionally active promoter. Nature. 2008;452:45–50. doi: 10.1038/nature06544. [DOI] [PubMed] [Google Scholar]
- 48.Srivastava DP, Yu EJ, Kennedy K, Chatwin H, Reale V, Hamon M, et al. Rapid, nongenomic responses to ecdysteroids and catecholamines mediated by a novel Drosophila G-protein-coupled receptor. J Neurosci. 2005;25:6145–6155. doi: 10.1523/JNEUROSCI.1005-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kume K, Kume S, Park SK, Hirsh J, Jackson FR. Dopamine is a regulator of arousal in the fruit fly. J Neurosci. 2005;25:7377–7384. doi: 10.1523/JNEUROSCI.2048-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]